1. Introduction
Coastal archaeological sites are consistently experiencing impacts from many probable threats: erosion, rising sea level, groundwater pollution, salinization, and climate change. The action underlain by the increased human pressures related to urbanization, agriculture, and development contributes to the existing impacts on coastal archaeological heritage.
It is noted that the expected amount of at-risk coastal archaeological sites will likely increase substantially by the years 2050 and 2100 if greenhouse gas emissions choose to remain high [
1,
2,
3]. As a result of natural or human-induced erosion, archaeological features have already been lost in areas such as Libya, New Zealand, and the Mediterranean [
4,
5]. The implications of saltwater intrusion and agricultural or urban contamination are also contributing factors for damaging subsurface remains in places such as Florida and Italy [
6,
7]. Moreover, the growth of urban centers, as well as poor land management and unsustainable agricultural practices, are compounding the risk factors noted previously, along with the increased extraction of potable groundwater, land subsidence, and the destruction of heritage sites [
3,
7,
8,
9]. Submerged and buried archaeological sites (as in the current study area of Crotone) can be especially vulnerable to as the preservation of deposits largely depends on the stability of their depositional environment [
10,
11]. For example, the ancient Greek temple of Hera Lacinia at Capo Colonna (near Crotone) may ultimately be impacted by its proximity to the coast, and the current threat of SWI which could endanger the integrity of the site.
The traditional protective materials used in archaeological sites such as geomembranes and concrete are non-biodegradable materials and would frequently conflict with ethical conservation considerations because of the environmental damage incurred and the need for manual extraction in a non-destructive manner. To address these issues, researchers have begun investigating biodegradable, eco-compatible polymers that can perform protective functions and promote environmental responsibility [
12,
13].
Polycaprolactone (PCL) is emerging as a promising candidate which is semi-crystalline, hydrophobic, and processable into thin films with relatively low water sorption [
14]. On the other hand, PCL exhibits greater durability com-pared to other biodegradable polymers such as Polylactic Acid (PLA) and Polybutylene Succinate (PBS) [
15,
16].
The barrier qualities of the biodegradable polymers depend on the ambient conditions. For instance, extreme salinity will likely increase hydrolysis and mass loss, which could compromise the protective qualities of the biodegradable polymers [
17]. Therefore, it is vital to examine the long-term survivability of biodegradable polymers in adverse saline, sub-surface conditions going forward [
15]. Functional additives could reduce the negative impact of salinity and improve the durability and effectiveness of these polymers. Addressing these gaps is crucial for the adoption of the protective barriers in heritage conservation and for advancing global sustainability goals.
2. Materials and Methods
2.1. Materials
Polycaprolactone (PCL) with an average molecular weight (Mn ≈ 80,000 g/mol) was acquired from Sigma-Aldrich without further purification. Tri-ethyl phosphate (TEP) was selected as a green solvent which supports our sustainable goals. Seawater samples from the Ionian Sea from the study location (Crotone city) were collected and analysed to confirm usability of experiments.
2.2. Film Preparation
PCL films were made with TEP as the green solvent as shown in
(Figure 1). 7 g of TEP was added to a glass flask, followed by 1 g of PCL which was added in three equal portions of 0.33 g each. Each portion was dissolved completely before the next portion was added. Once mixed, the TEP/PCL was stirring at 55 °C for 3 hrs to produce a uniform solution. The solution was left to rest for 2 hours before pouring the solution onto a flat glass plate. The films were placed in an oven at 55 °C to evaporate the solvent. Once the solvent evaporated, films were detached from the glass by immersing them in deionized water until the films were released from the glass and were then air-dried overnight at room temperature for testing.
The films were cold cut in to strips of 3cm × 3cm for weight loss experiment, and strips of 10cm × 2cm for mechanical strength tests. The average thickness of the films measured between 0.10 mm to 0.22 mm using a digital micrometer. All tests were performed in triplicate.
2.3. Ionian Seawater Immersion
For simulating condition burial of coastal artifacts, PCL films were placed in glass containers with natural seawater. Ionian seawater was used because of the similar saline conditions in the other coastal archaeological site of Crotone and similar effects to the seawater intrusion. The containers were placed in an environmental chamber at 15 °C which was selected to simulate coastal artifacts that would typically be buried beneath the earth especially in cooler stable environmental conditions. The measured temperature will provide for stabilized laboratory observations but also keep in mind the conditions with static immersion, and some wat-ant dynamic water moving effect conditions in the field of real environments.
2.4. Surface Hydrophobicity: Contact Angle Measurements
To determine the wettability of the surface, a goniometer was used, and the sessile drop method was completed. Each film surface had a droplet of two microliters of deionized water deposited, and the contact angle was measured after waiting 20 seconds. Each sample was measured three times, and the average value was calculated. Any changes in contact angle allows for interpretation of increased risk of deterioration of artifacts. A hydrophilic surface will contribute to less-desirable interactions of water with cultural heritage materials.
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
The chemical structural changes were followed by attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy (4000 - 500 cm⁻¹, 4 cm⁻¹). All spectra are the means of thirty-two scans. The observation of a diminishment in the absorption peaks related to ester carbonyl (approximately 1720 cm⁻¹) and new peaks related to hydroxyl (approximately 3300 cm⁻¹) or the arousal of new peaks for carboxylate groups (approximately 1600 cm⁻¹) can be attributed to hydrolytic degradation. FTIR data assists to demonstrate the chemical instability risk and impacts on the stability of the artifact.
2.6. Scanning Electron Microscopy (SEM)
The morphologies of the films were assessed at 0, 30, 60, and 90 days using a ZEISS Scanning Electron Microscope. All the samples were sputter coated in gold to improve conductivity of the samples. Micrographs taken at 500× to 2000× magnification were documented to record surface roughness, pitting, and cracking during the degradation of the surface films. The PM was ana-lysed via SEM to determine how the microstructure impacted any physical vulnerabilities that might jeopardize archaeological artifacts.
2.7. Seawater Permeability Testing
Seawater permeability tests are considerable methods of evaluating the performance of films to inhibit saline infiltration, which ultimately affects the amount of protection offered to the artifacts. Water permeation tests on the films used in the study were conducted using a dead-end filtration cell (UHP-25, Strelitech, Japan) with a feed pressure of 0.5 bar and medium speed of magnetic stirrer (190m) at room temperature. As time went on, mass loss was recorded as the system was stirred at continuous periods. Conductivity was also measured in the receiving chamber over a 6-hour period. The higher the permeability the greater the likelihood of salt damage occurring in the artifacts.
Where: J: Permeate flux (L/m
2.h)
Q: Volume of permeate collected (L)
A: Membrane area (m2)
t: Time (hr)
Where: P: Permeability (L/m
2.h.bar)
J: Permeate flux (L/m2.h)
ΔP: Applied pressure difference (bar)
2.8. Mechanical Strength Testing
The mechanical integrity of samples was conducted using a Zwick/Roell Z2.5 testing unit (BTC-FR2.5TN-D09, Zwick Roell Group, Ulm, Germany). Film strips were allowed to stretch, and at a constant rate of elongation (5 mm/min), were tested to rupture. The values of tensile strength (MPa), Young's modulus (MPa), and elongation at break (%) were produced, which could indicate long-term structural integrity of the polymer. An increase in loss of those mechanical properties could increase the susceptibility of the archaeological object to physical stress.
2.9. Biodegradability Assessment
Biodegradation evaluation was completed by tracking the loss of mass of samples (using the sample's dry mass) after submersion in Crotone seawater. After weighing the samples, they were submerged in the seawater for an assigned time, rinsed with freshwater, dried at ambient temperature, and weighed again. The mass loss was finally estimated as a percentage to approximate the percent of hydrolytic biodegradation during the treatment. Changes to the sample colour also provide observable qualitative data. The conceptualizations of biodegradability as a property guarantee that the selected protective materials would not produce excessive long-term detriment to the environment while preserving archaeological integrity through time.
Where:
Wo: the initial weight of the sample before biodegradation (g)
Wt: the weight of the sample after biodegradation (g)
3. Results and Discussion
The degradation behavior of unmodified polycaprolactone (PCL) films sub-merged in actual seawater for 90 days was described utilizing different analytical techniques to determine changes in physical, chemical, mechanical and permeability. The following section describes the changes observed in surface hydrophobicity, chemical structure, micro-morphology, saltwater permeability, mechanical strength, and total mass loss.
3.1. Surface Hydrophobicity
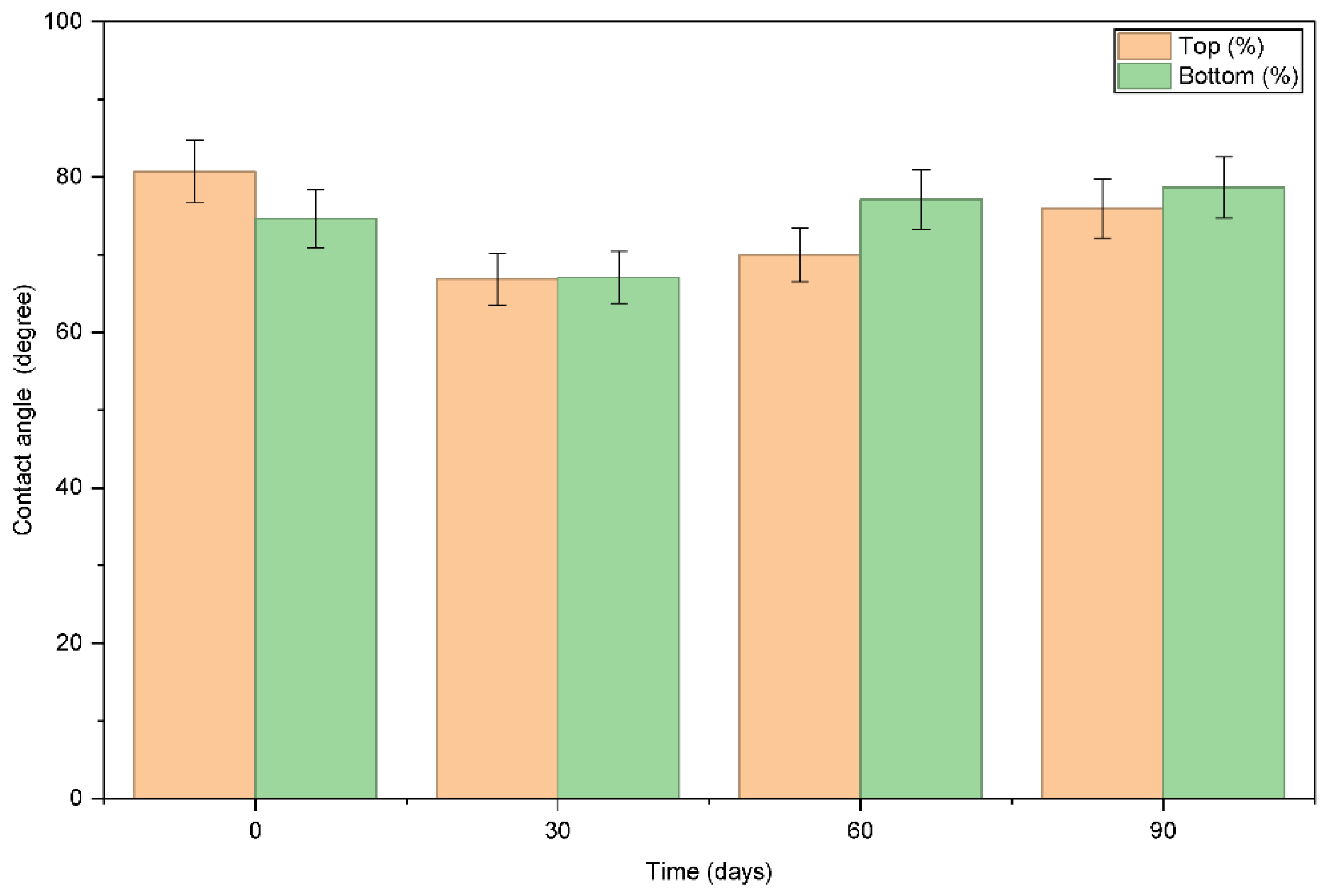
Contact angle analysis was achieved for both upper and lower surfaces of PCL films over the 90-day simulated exposure period to the salinity of seawater, demonstrating in
(Figure 2). Zero day had average contact angles of 80.69° (top) and 74.64° (bottom), denoting moderate hydrophobicity. At day 30, contact angles decreased to 66.86° (top) and 67.10° (bottom) indicating increased surface wettability due to the interaction with saline. At day 60, the upper surface partially recovered to 69.99°, while the lower surface increased to 87.11°, indicating partial surface reorganization and uneven salt deposition between the two surfaces. An increase in surface polarity will elevate the affinity for water for the material and could catalyze degradation behavior. Day 90 was stable at 75.96° (top) and 78.67° (bottom) lined in the table below, indicating partial retention of hydrophobicity, while some altered surface dynamics persist.
3.2. FTIR Spectroscopy
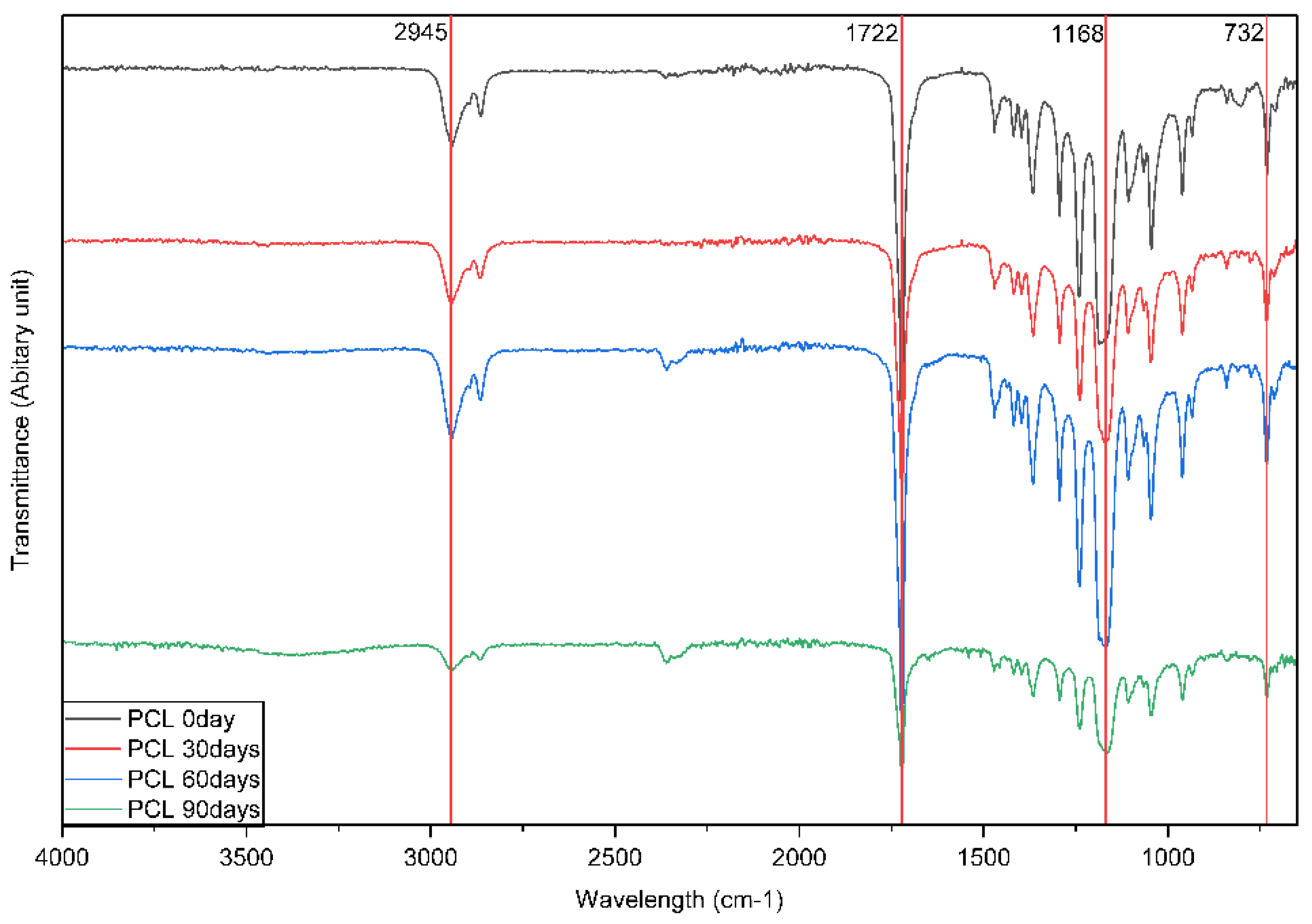
Fourier Transform Infrared (FTIR) spectroscopy was performed to confirm the chemical structure and functional groups of the synthesized polycaprolactone (PCL) through the evaluation of the PCL films at 0, 30, 60 and 90 days in natural saline as shown in
(Figure 3). FTIR spectroscopy was able to demonstrate PCL specific absorption bands including CH
2 asymmetric stretching (2946 cm⁻¹), a strong ester carbonyl (C=O) absorption band (1718 cm
-1), C–O–C stretching (1169 cm
-1), and CH₂ rocking (730 cm
-1) over time. Although the absorption bands displayed in the FTIR spectra were relatively consistent over time, significantly lower absorbance intensity was noted over the same 4 time points, especially at 1718 cm
-1 and 1169 cm
-1. The change in intensity indicated decreasing chemical stability due to hydrolytic degradation of ester groups over time. No new absorption bands were produced beyond indicating the loss of chemical stability and change in structural integrity of the polymer backbone over time. The spectral analysis of PCL highlights the degradation in intensity following immersion at 30 days for carbonyl (1722 cm
-1) and C–O–C (1168 cm
-1) as time progressed to 60 and 90 days, respectively, confirming that the entire polymer suffered some extent from hydrolytic degradation of which has previously been reported necessary for the biodegradability of PCL. The absorption bands that would have normally confirmed the establishment of secondary functional groups were not demonstrated in the FTIR measurements following the application of the saline exposure.
The C=O absorption peak at around 1725 cm
-1 is often discussed as the main indicator for the presence PCL [
18]. Additionally, the aliphatic C-H stretching vibrations identified in the range of 2945 cm
-1 and 2865 cm
-1 have also been shown to be indicative of PCL's presence in degraded composite materials [
19]. Moreover, the peaks associated with C-O-C and C-C stretching vibrations at 1240 cm
-1 and 1295 cm
-1 have been similarly documented, verifying additional impact concerning the crystalline nature of the polymer [
20].
Overall, the measured FTIR values verified continuous degradation of the chemical stability of PCL over 90 days but no alteration in its structural integrity and chemical stability was noted throughout the measurement periods.
Figure 3.
FTIR spectra of PCL films undergoing immersion at natural seawater (15 °C) at 0, 30, 60, and 90 days, indicating variations in peak heights and symmetry indicated continuous hydrolytic degradation of the polymer structure.
Figure 3.
FTIR spectra of PCL films undergoing immersion at natural seawater (15 °C) at 0, 30, 60, and 90 days, indicating variations in peak heights and symmetry indicated continuous hydrolytic degradation of the polymer structure.
3.3. SEM Morphology
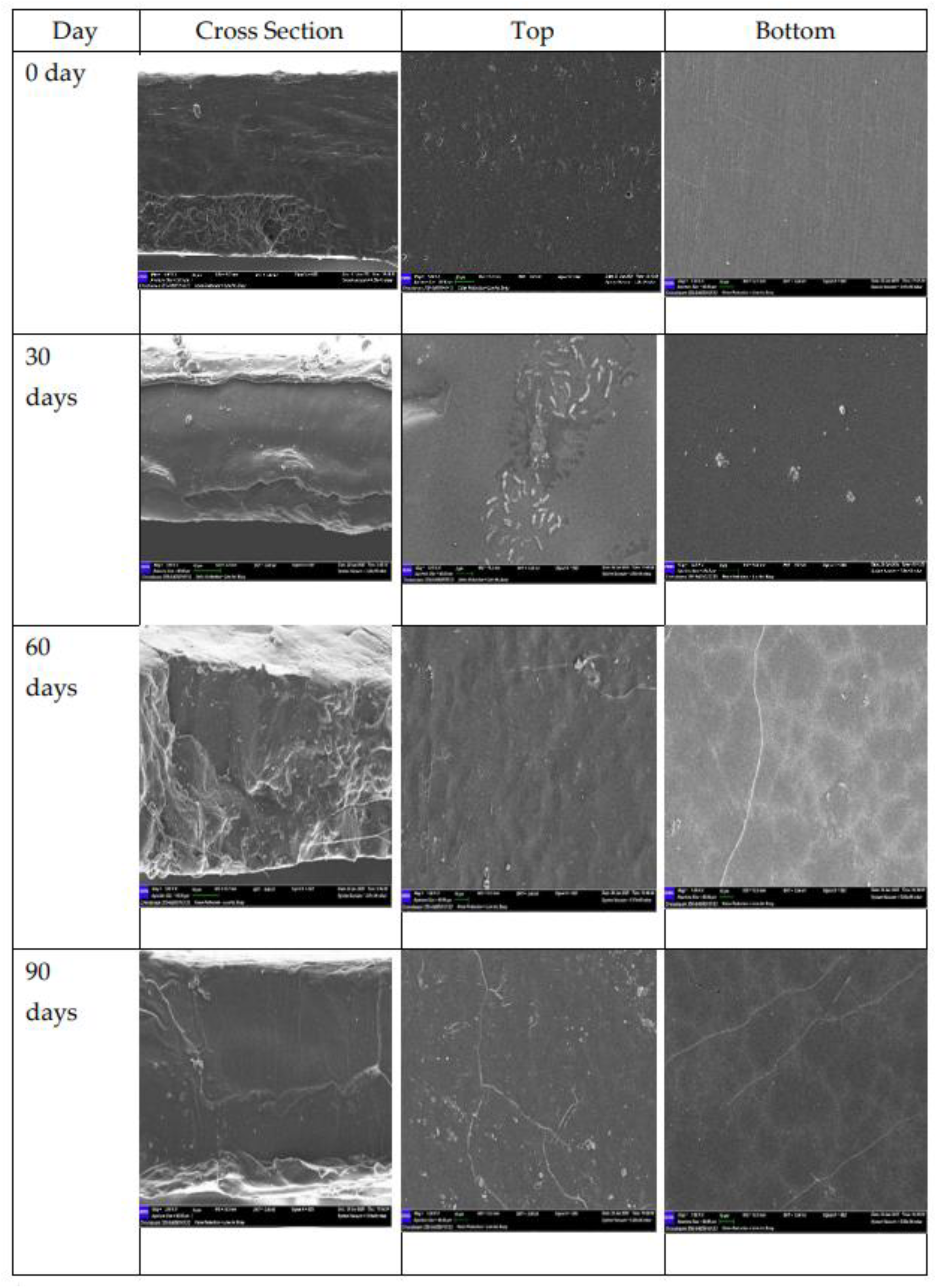
SEM images of the top, bottom, and cross-sections of PCL films (degradation time point of 0, 30, 60 days, and 90 days) are presented in
(Figure 4). In the beginning, the surfaces of the films were smooth, homogenously formed, and free of cracks or pores, both trimmed, and in cross-section appearing compact and uniform. After 30 days the cross-section began to show some roughness and slight delamination, while the top surface showed a few zealous aggregates and irregularities. The bottom surface exhibited small appearing scattered deposits which were likely due to salt crystallization. By 60 days, these features would become more pronounced. By 90 days the cross-section exhibited large cracks and layered structures, indicating that the internal structural integrity was breaking down. These changes in morphology occurred simultaneously to the changes in hydrophobicity and chemical stability, demonstrating how length of time in saline environments negatively impacts the barrier property of unmodified PCL film.
3.4. Seawater Permeability Test
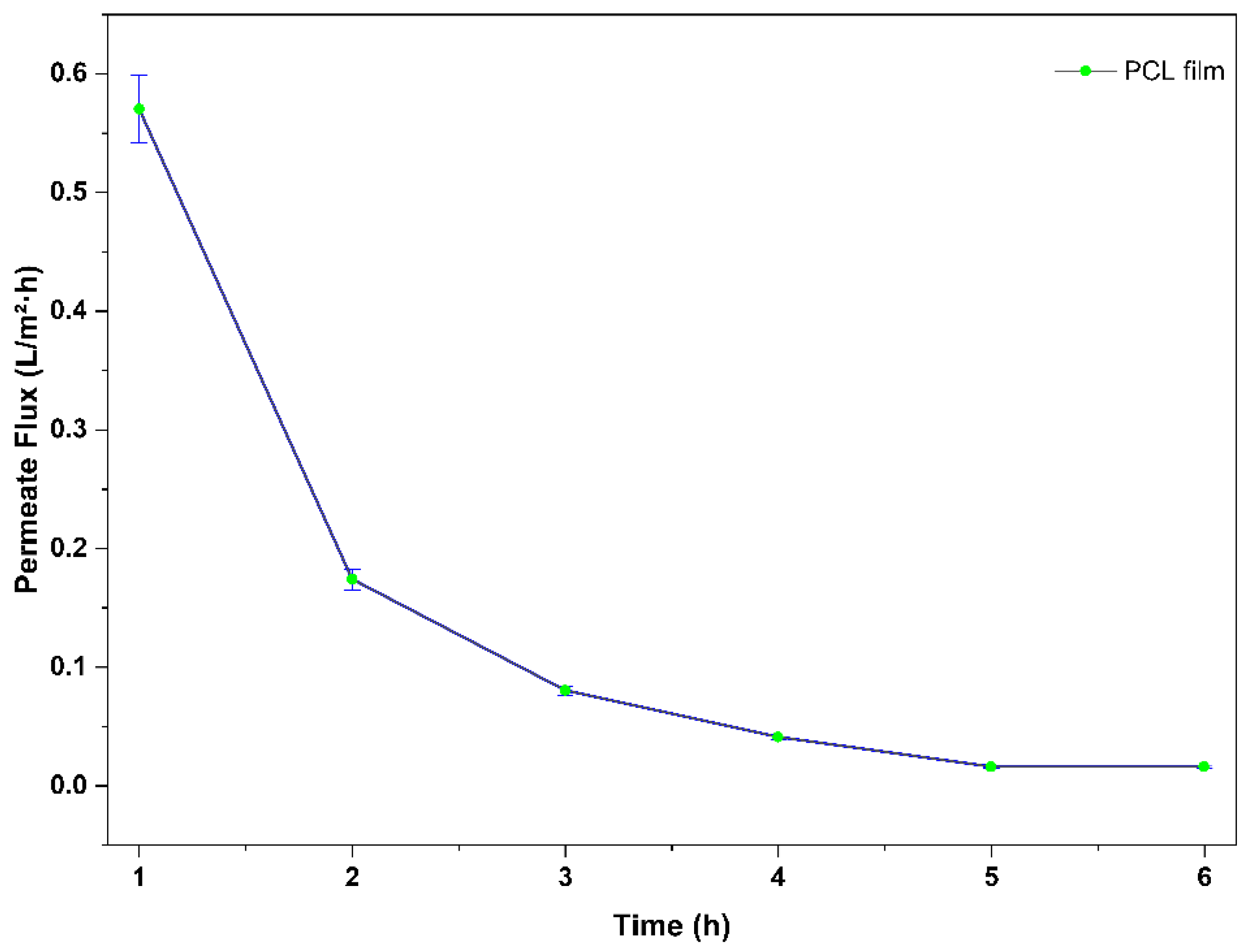
The water permeation performance of the PCL films was assessed with a dead-end filtration cell at a constant feeding pressure of 0.5 bar and room temperature. The permeate flux (J) was determined by measuring mass change over time, and the average data can be seen in
(Figure 5) with a dramatic loss in flux over the 6-hour test. The initial flux was clocked at 0.57026 L/m²·h at 1 hour, then dropped dramatically to 0.17379 L/m²·h at 2 hours and continued to tail down to 0.01584 L/m²·h by 5 hours, stabilizing thee after at 6 hours.
This trend is consistent with the behaviour of hydrophobic membranes in dead-end filtration systems, where fouling due to the accumulation of material on the membrane surface is a common challenge.
This pattern is consistent with typical behaviour of hydrophobic membranes in dead-end filtration systems, where fouling due to the accumulation of material on the membrane surface is a common challenge. For example, Wang et al., (2024), studied PCL membranes produced through electrospinning, in which hydrophobic membranes accrete and eventually block pores under dead-end pressure quickly, similar to the rapid loss of flux described in our work, as demonstrated in reference [
21]. Similarly, Jo et al., (2024) observed similar initial fluxes to the ones observed in our study, plus also films produced with PCL decreases after time [
22]. It is found that stable flux beyond the 5 hours time point demonstrates that there is a steady state whereby either incremental fouling or structural changes are negligible or not recognizable.
Figure 5.
Permeate flux of pure PCL film as a function of filtration time.
Figure 5.
Permeate flux of pure PCL film as a function of filtration time.
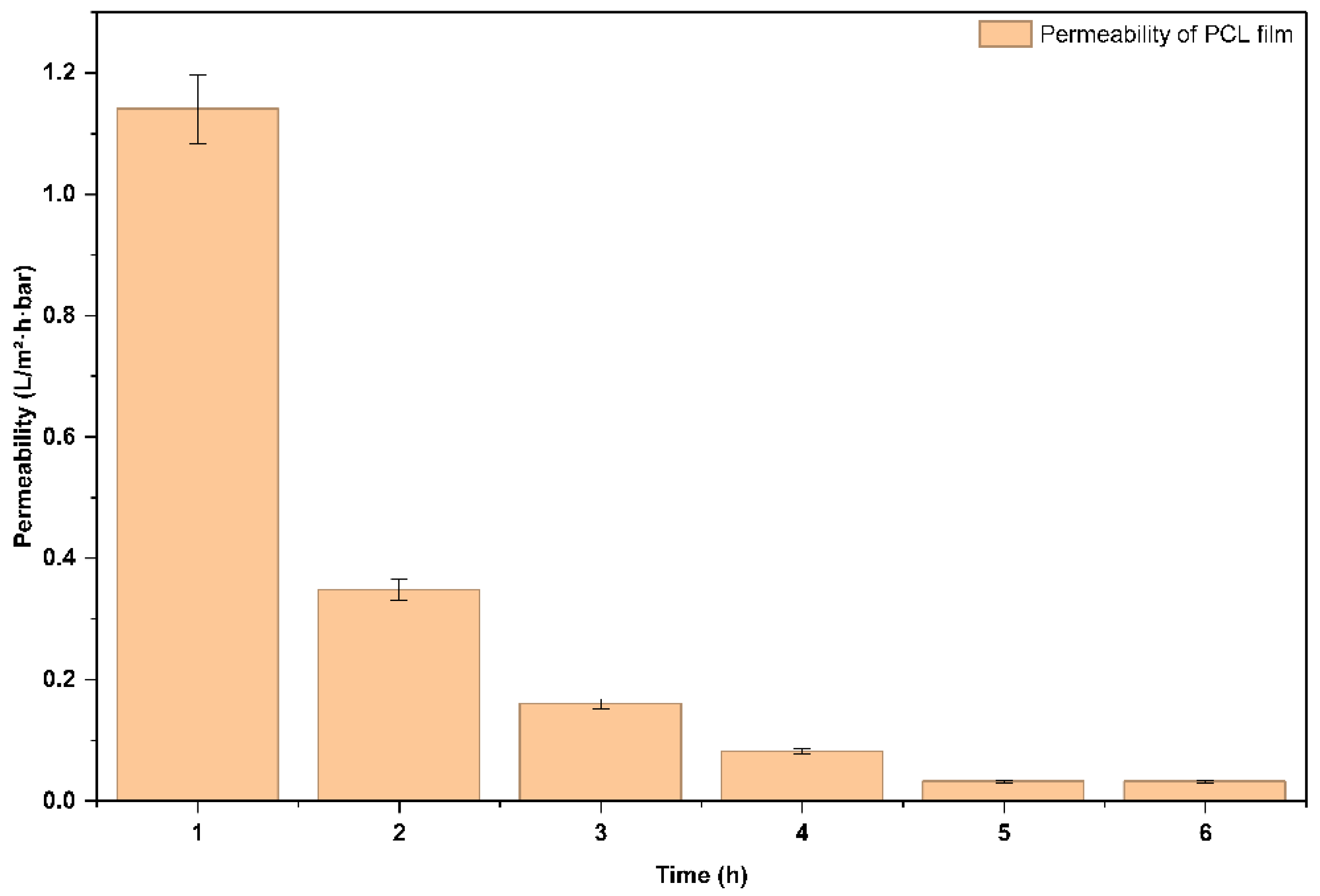
Moreover, natural Seawater Permeability (P) starting at 1.14052 L/m²·h·bar at 1 hour and declining to 0.03168 L/m²·h·bar by 5 hours, remaining constant at 6 hours as shown in
(Figure 6). This decline in both flux and permeability advocates a reduction in the film’s water transport efficiency over time, due to fouling or compaction of the PCL film under the applied pressure. This support is also confirmed by Zhang et al., (2024), where the authors conclude that stable plateau region in permeability through ultrafiltration means that the cake layer is saturated[
23].
The initial elevated permeate flux and permeability demonstrate that the PCL film has reasonable water permeability for the beginning filtration period, due to its hydrophobicity and porous structure. The consistent permeate flux and permeability beyond 5 hours (0.01584 L/m²·h and 0.03168 L/m²·h·bar, respectively) indicates that a stable condition had occurred and thus further fouling or structure change was limited. These results capture the potential of the PCL film but also highlight the limitation of the PCL for extended filtration without antifouling procedures, which could include surface modification or periodic cleaning during filtration. Future work will also include studying using higher pressures or using crossflow filtration to improve PCL performance.
3.5. Mechanical Properties
The mechanical outcomes indicate an ongoing deterioration of both stiffness and tensile strength of PCL films over time in the natural seawater. Tensile properties of PCL films were determined after 0, 30, 60 and 90 days of immersion in natural seawater and summarized in
(Table 1). Initially, PCL films had a Young’s modulus of 239.67 MPa, tensile strength of 15.3 MPa and elongation at break of 11.67% which demonstrate satisfactory mechanical integrity. After 30 days of immersion in saline (30 days), the Young’s modulus dropped to 180.90 MPa, tensile strength dropped to 9.33 MPa, and elongation at break slightly decreased to 10.30%, all indices of mechanical integrity. At 60 days, Young’s modulus dropped to 173.00 MPa, tensile strength dropped to 4.5 MPa and elongation at break dropped to 7.80%. At 90 days, Young’s modulus lowered again to 145.00 MPa, tensile strength partially recovered to 9.91 MPa and elongation at break increased to 11.00%
The decline in tensile strength from 15.3 MPa (0 d) to 4.5 MPa over the 60 d indicates the breaking down of the polymer chains in saline conditions. The slight recovery in tensile strength to 9.91 MPa on day 90 may be attributed to structural reorganization or a change in crystallinity due to some of the degraded polymer chains being realigned. The indicator of the elongation at break dropped to (7.8%) 60 days, suggesting increased brittleness. Nonetheless, the high increase in tensile strength (11.0%) at 90 days may indicate plasticization effects due to water uptake that can improve ductility even if a degree of chemical degradation is occurring. Similar studies conducted by H. Tsuji (2002) indicated that when PCL is exposed to hydrolytic (aqueous) conditions, the hydrolysis of the amorphous portion of the polymer dominated how the overall properties change [
24]. Further, for the seawater-degradable PCL films, G.X. Wang et al. (2021) provided evidence for a decline in mechanical strength due to time under immersion conditions and followed a parallel decline with both magnitudes and trends of mechanical strength observed [
25]. Similarly, the research conducted by Lyu et al. (2019) demonstrated a comparable decline in mechanical properties whereby days post-immersion resulted in notable declines in the tensile strength of the PCL films test [
26].
The overall trend of this study lines up with previous studies indicating that PCL immersed in water and saline environments undergo hydrolytic degradation and weakened mechanical properties after a certain period. The decline in average properties after 60 days and potential slight recoveries on day 90 would suggest a more complex relationship tending hydrolytic degradation, crystallinity levels, and water uptake. Therefore, the mechanical properties of film will still be limited for long-term viability unless designed.
3.6. Biodegradability of PCL Films
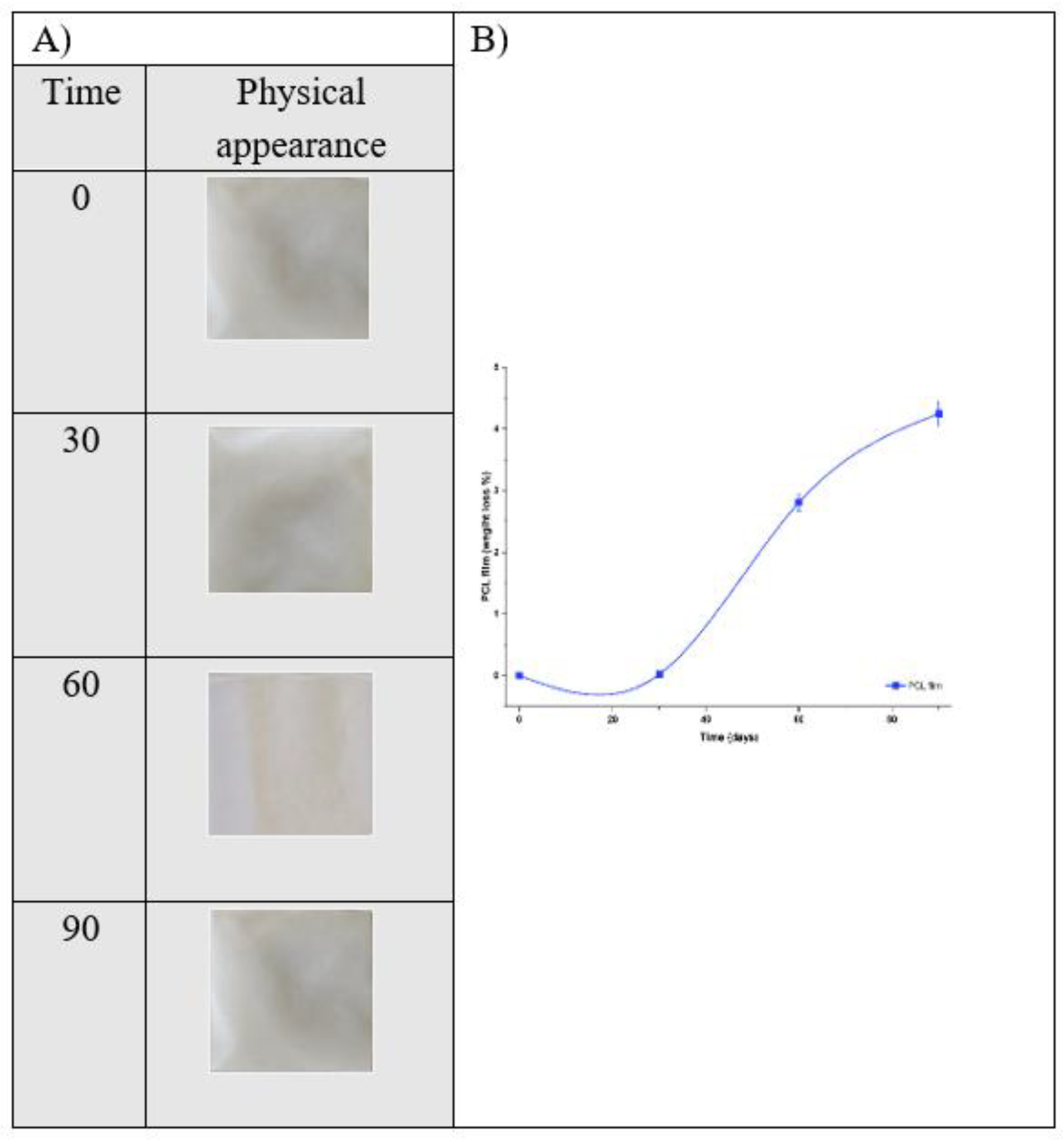
Gravimetric examination confirmed active biodegradation of the PCL films over the 90-day period of exposure to natural seawater at 15 °C, illustrating in
(Figure 7.A). As a starting point, at day 0 of the experiment, there was no weight loss at this time, indicative of polymer having good initial stability in the marine environment.
Following 30 days of immersion, the films revealed, very minimal loss of 0.02%, again, indicating limited degradation at the early stage under cold seawater conditions. The reduction of temperature (15 °C) led to reduced hydrolytic cleavage of the ester bonds within the PCL polymer and slowed microbiological activity, which can both be highly influential on the rate of biodegradation of aliphatic polyesters.
Following 60 days, mass loss had increased significantly to 2.81%, suggesting that hydrolytic processes had progressed beyond the surface of the films and started to influence the bulk polymer structure.
Finally, at day 90, the weight loss of the films indicated 4.25%, indicating that there had been some continuing breakdown of the films over the time of the test period with relatively low overall breakdown. See
(Figure 7.B).
In examining the studies from other researchers, the amount of mass loss reported in the current study (~4.25% after 90 days at 15 °C) is less than that of studies which reported the degradation of PCL at higher testing temperatures (25 – 30 °C) or in modified/seawater that are more biologically active [
27]. Heimowska et al., (2017) recorded complete degradation of some films of PCL after 6 weeks of testing in Baltic Sea water. However, the degradation of PCL films was slow when the films were tested in less aggressive waterbodies [
28]. Therefore, the results support that biodegradation of PCL takes place relatively slowly depending on temperature, microbial content, thickness of the films, and whether the films were crystalline or amorphous.
Overall, the results of our study illustrate that PCL films are degraded slowly in the influence of natural seawater at 15 °C. The slow degradation is due to the semi-crystalline structure and hydrophobic character of PCL, which prevents penetration of water and slows degradation. However, an increase in mass loss indicates that after considerable time, films may ultimately degrade in marine conditions.
Figure 7.
Biodegradability of pure PCL films in natural seawater at 15 °C. (A) Physical appearance of the films after 0, 30, 60, and 90 days of immersion. (B) Weight loss (%) of PCL films as a function of incubation time, showing gradual degradation with increasing exposure duration.
Figure 7.
Biodegradability of pure PCL films in natural seawater at 15 °C. (A) Physical appearance of the films after 0, 30, 60, and 90 days of immersion. (B) Weight loss (%) of PCL films as a function of incubation time, showing gradual degradation with increasing exposure duration.
4. Conclusions
This study assessed the performance of unmodified polycaprolactone (PCL) films as biodegradable barriers for protecting archaeological sites from saltwater intrusion. In total, the 90-day exposure period evaluated how PCL performed against saline conditions for hydrophobicity, mechanical properties, and long-term stability. The reduction in contact angle, FTIR showing hydrolytic degradation of the PCL, increases in saltwater permeability, and loss in tensile strength showed all together that unmodified PCL films can decline both structurally and functionally when exposed to saline conditions for a long duration. These results demonstrate the necessity for material changes, including the addition of nanofillers for improving hydrophobicity, chemical stability, resistance to permeability, and durability over time.
The 90-day exposure condition may not be a long enough duration to assess the long-term performance of PCL films in situ, nor did we consider chemical interactions with specific soil types or artifacts. Future research should employ field validation to further support laboratory performance under authentic environmental conditions, including varying temperature, soil conditions, and water movement, which may also include ongoing biological activity in the archaeological site. It will be important to set benchmarks for future performance, such as establishing acceptable levels of loss - less than 5% mass loss of the polymerized PCL over 12 months to protect archaeological assets. This can help future optimization studies on archaeological site protection, while meeting the regulatory standards.
Despite these limitations, this study provides initial knowledge of PCL film performance in saline conditions and could be a foundation for the development of next generation biodegradable materials that allow for environmental sustainability and preservation of cultural heritage.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- H. Hu and R. Hewitt, ‘Future climate risks to world cultural heritage sites in Spain: a systematic analysis based on Shared Socioeconomic Pathways’, International Journal of Disaster Risk Reduction, 2024. [CrossRef]
- M. Howland and V. Thompson, ‘Modeling the potential impact of storm surge and sea level rise on coastal archaeological heritage: A case study from Georgia’, PLOS ONE, vol. 19, 2024. [CrossRef]
- T. Dawson, J. Hambly, A. Kelley, W. Lees, and S. Miller, ‘Coastal heritage, global climate change, public engagement, and citizen science’, Proceedings of the National Academy of Sciences, vol. 117, pp. 8280–8286, 2020. [CrossRef]
- B. Jones et al., Regional implementation of coastal erosion hazard zones for archaeological applications’, Journal of Cultural Heritage, 2024. [CrossRef]
- A. Elfadaly et al., ‘Tracking the effects of the long-term changes on the coastal archaeological sites of the Mediterranean using remote sensing data: The case study from the northern shoreline of Nile Delta of Egypt’, Archaeological Prospection, vol. 30, pp. 369–390, 2023. [CrossRef]
- L. Stellato et al., ‘Natural and Anthropogenic Groundwater Contamination in a Coastal Volcanic-Sedimentary Aquifer: The Case of the Archaeological Site of Cumae (Phlegraean Fields, Southern Italy)’, Water, vol. 12, p. 3463, 2020. [CrossRef]
- A. Lecher and A. Watson, ‘Danger from beneath: groundwater–sea-level interactions and implications for coastal archaeological sites in the southeast US’, Southeastern Archaeology, vol. 40, pp. 20–32, 2021. [CrossRef]
- J. Mehta et al., ‘Preserving coastal environments requires an integrated natural and cultural resources management approach’, PNAS Nexus, vol. 4, 2025. [CrossRef]
- G. Mattei, A. Rizzo, G. Anfuso, P. Aucelli, and F. Gracia, ‘A tool for evaluating the archaeological heritage vulnerability to coastal processes: The case study of Naples Gulf (southern Italy)’, Ocean & Coastal Management, 2019. [CrossRef]
- B. Jones, M. Dickson, M. Ford, D. Hikuroa, and E. Ryan, ‘Aotearoa New Zealand’s coastal archaeological heritage: A geostatistical overview of threatened sites’, The Journal of Island and Coastal Archaeology, vol. 19, pp. 657–677, 2023. [CrossRef]
- K. Westley, J. Nikolaus, A. Emrage, N. Flemming, and A. Cooper, ‘The impact of coastal erosion on the archaeology of the Cyrenaican coast of Eastern Libya’, PLOS ONE, vol. 18, 2023. [CrossRef]
- Lucía Pérez-Gandarillas et. al., Conservation and Protection Treatments for Cultural Heritage: Insights and Trends from a Bibliometric Analysis.’ Accessed: Sep. 20, 2025. [Online]. Available: https://www.mdpi.com/2079-6412/14/8/1027?utm_source=chatgpt.com.
- A. Bher, P. C. Mayekar, R. A. Auras, and C. E. Schvezov, ‘Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments’, Int J Mol Sci, vol. 23, no. 20, p. 12165, Oct. 2022. [CrossRef]
- M. A. Ntrivala et al., ‘Polycaprolactone (PCL): the biodegradable polyester shaping the future of materials – a review on synthesis, properties, biodegradation, applications and future perspectives’, European Polymer Journal, vol. 234, p. 114033, Jun. 2025. [CrossRef]
- A. Samir, F. Ashour, A. Hakim, and M. Bassyouni, ‘Recent advances in biodegradable polymers for sustainable applications’, npj Materials Degradation, vol. 6, pp. 1–28, 2022. [CrossRef]
- T. Garrison, A. Murawski, and R. Quirino, Bio-Based Polymers with Potential for Biodegradability’, Polymers, vol. 8, 2016. [CrossRef]
- F. Wu, M. Misra, and A. Mohanty, Challenges, and new opportunities on barrier performance of biodegradable polymers for sustainable packaging’, Progress in Polymer Science, 2021. [CrossRef]
- P. Mosallanezhad, H. Nazockdast, Z. Ahmadi, and A. Rostami, ‘Fabrication and characterization of polycaprolactone/chitosan nanofibers containing antibacterial agents of curcumin and ZnO nanoparticles for use as wound dressing’, Front. Bioeng. Biotechnol., vol. 10, 2022. [CrossRef]
- D. Kossyvaki et al., Highly Porous Curcumin-Loaded Polymer Mats for Rapid Detection of Volatile Amines’, ACS Appl. Polym. Mater., vol. 4, no. 6, pp. 4464–4475, 2022. [CrossRef]
- S. Ali, Z. Khatri, K. W. Oh, I.-S. Kim, and S. H. Kim, Preparation, and characterization of hybrid polycaprolactone/cellulose ultrafine fibers via electrospinning’, Macromol. Res., vol. 22, no. 5, pp. 562–568, 2014. [CrossRef]
- Sara Bergamasco et. al., Electrospun PCL Filtration Membranes Enhanced with an Electrosprayed Lignin Coating to Control Wettability and Anti-Bacterial Properties.’ Accessed: Sep. 18, 2025. [Online]. Available: https://www.mdpi.com/2073-4360/16/5/674.
- Sankar Nivedita; Shiny Joseph, Performance of polycaprolactone/TiO2 composite membrane for the effective treatment of dairy effluents | Water Science & Technology | IWA Publishing’. Accessed: Sep. 18, 2025. [Online]. Available: https://iwaponline.com/wst/article/83/10/2477/81436/Performance-of-polycaprolactone-TiO2-composite.
- Jianguo Zhang et. al., ‘Reduction of Ultrafiltration Membrane Fouling by the Pretreatment Removal of Emerging Pollutants: A Review.’ Accessed: Sep. 18, 2025. [Online]. Available: https://www.mdpi.com/2077-0375/13/1/77.
- H. Tsuji and K. Suzuyoshi, Environmental degradation of biodegradable polyesters 1. Poly(ε-caprolactone), poly[(R)-3-hydroxybutyrate], and poly(L-lactide) films in controlled static seawater’, Polymer Degradation and Stability, vol. 75, no. 2, pp. 347–355, 2002. [CrossRef]
- Ge-Xia Wang et.al., ‘Seawater-Degradable Polymers—Fighting the Marine Plastic Pollution - Wang - 2021 - Advanced Science - Wiley Online Library’. Accessed: Sep. 18, 2025. [Online]. Available: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/advs.202001121?utm_source=chatgpt.com.
- J. S. Lyu, J.-S. Lee, and J. Han, Development of a biodegradable polycaprolactone film incorporated with an antimicrobial agent via an extrusion process’, Sci Rep, vol. 9, p. 20236, 2019. [CrossRef]
- L. G. Engler et al., Designing Sustainable Polymer Blends: Tailoring Mechanical Properties and Degradation Behaviour in PHB/PLA/PCL Blends in a Seawater Environment’, Polymers, vol. 15, no. 13, p. 2874, 2023. [CrossRef]
- A. Heimowska, M. Morawska, and A. Bocho-Janiszewska, ‘Biodegradation of poly(ε-caprolactone) in natural water environments’, Polish Journal of Chemical Technology, vol. 19, no. 1, pp. 120–126, 2017. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).