Submitted:
11 October 2025
Posted:
13 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
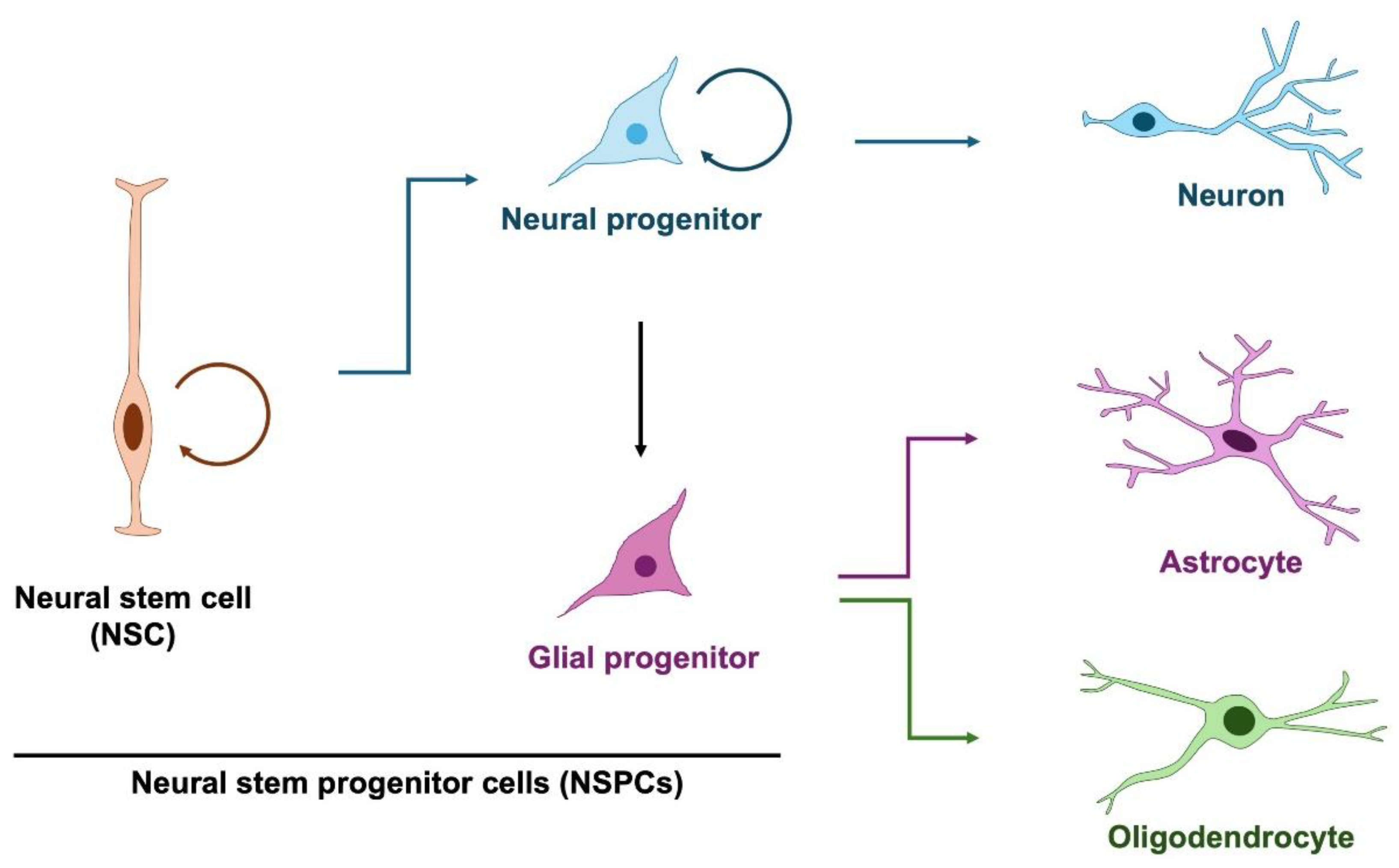
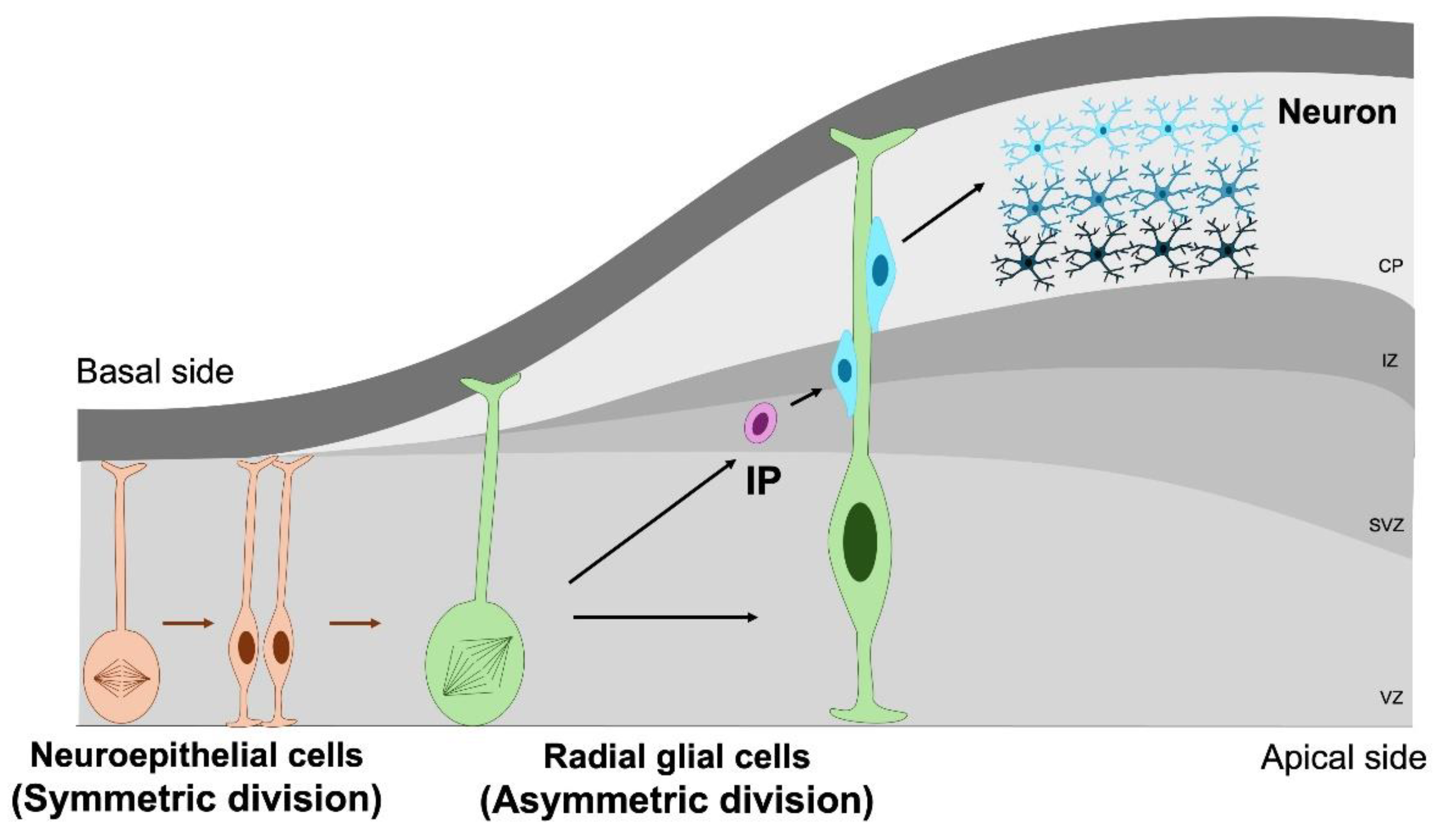
2. Neural Stem and Progenitor Cells in Normal Brain Development
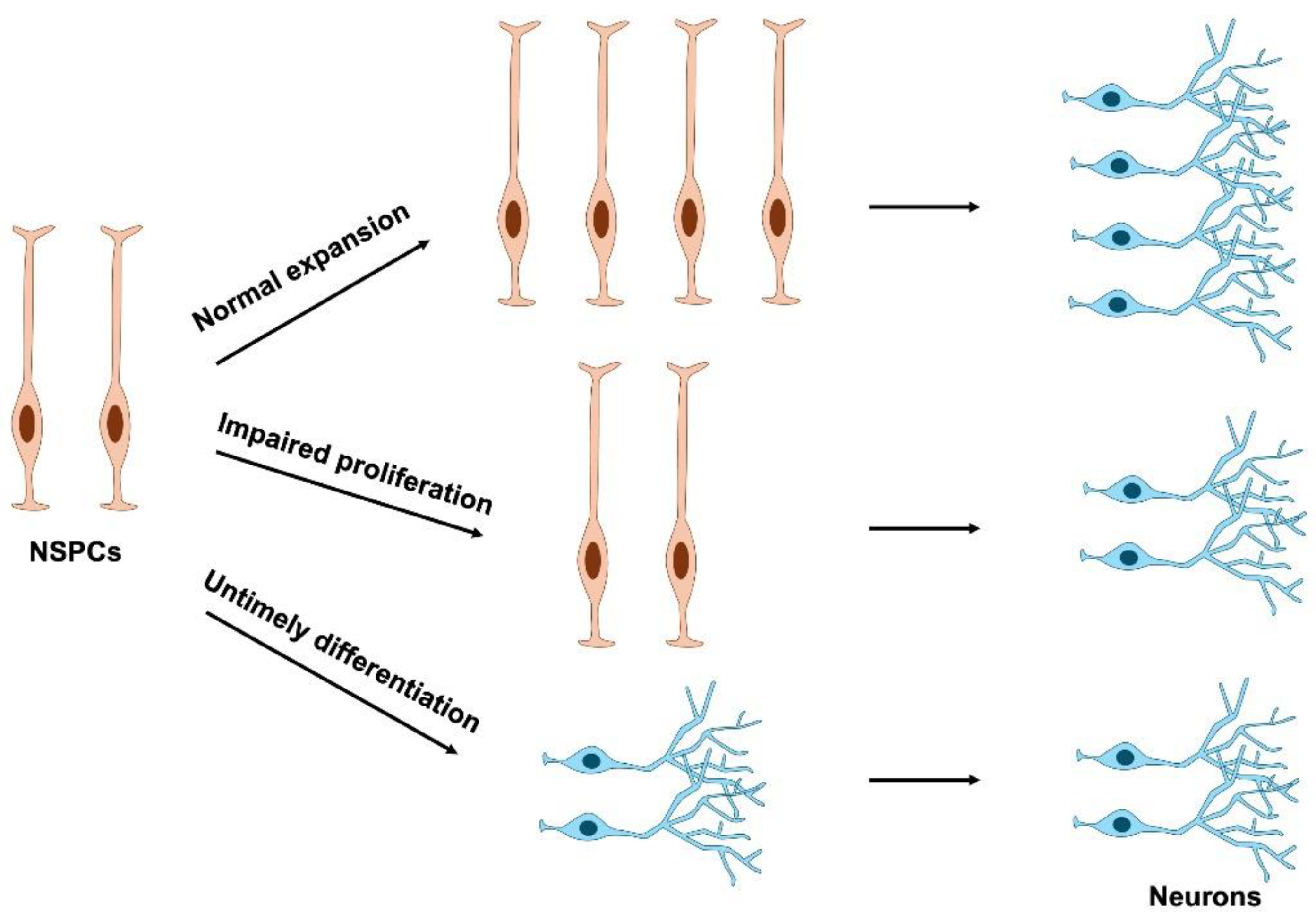
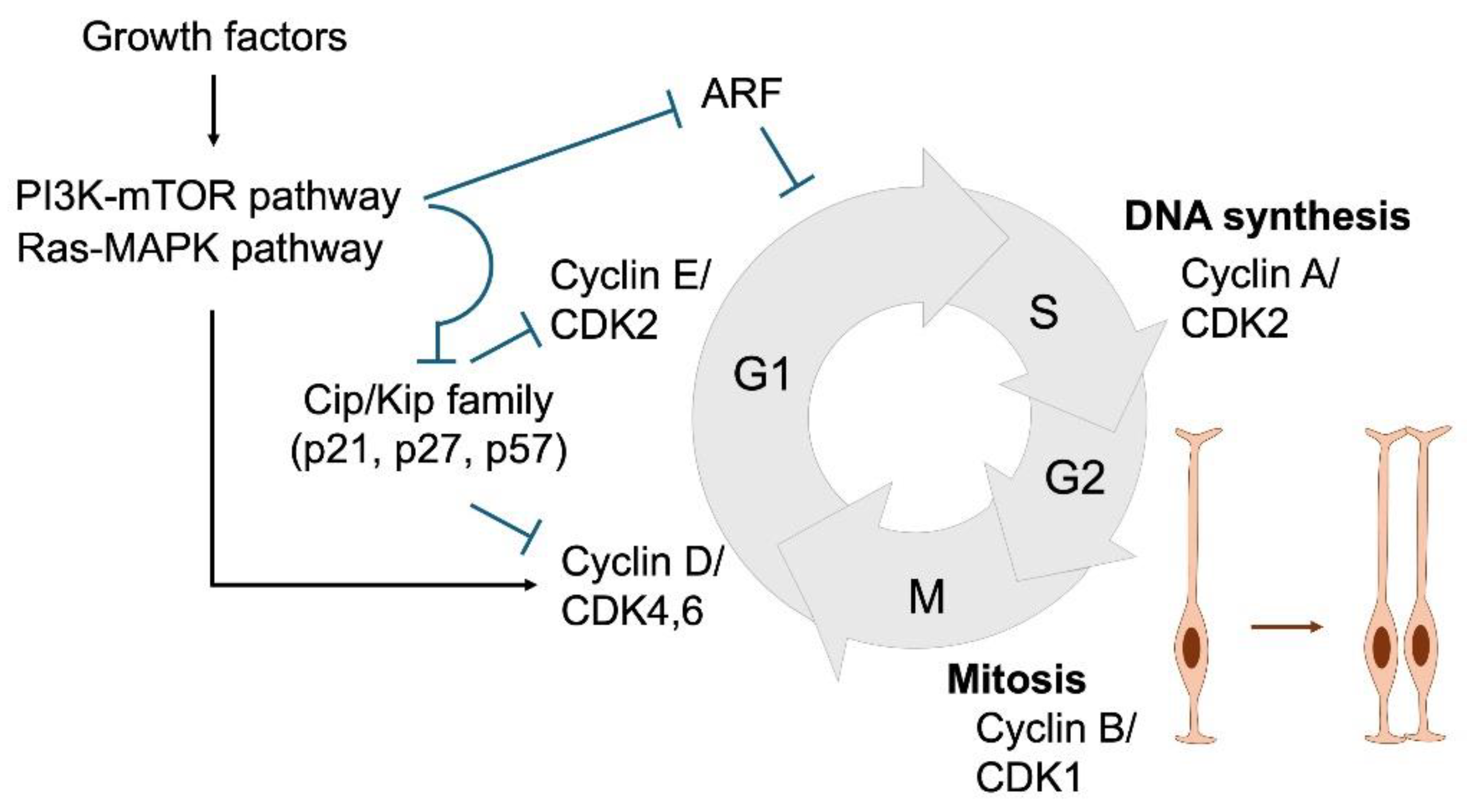
3. Cell Cycle Regulation in NSPC Proliferation
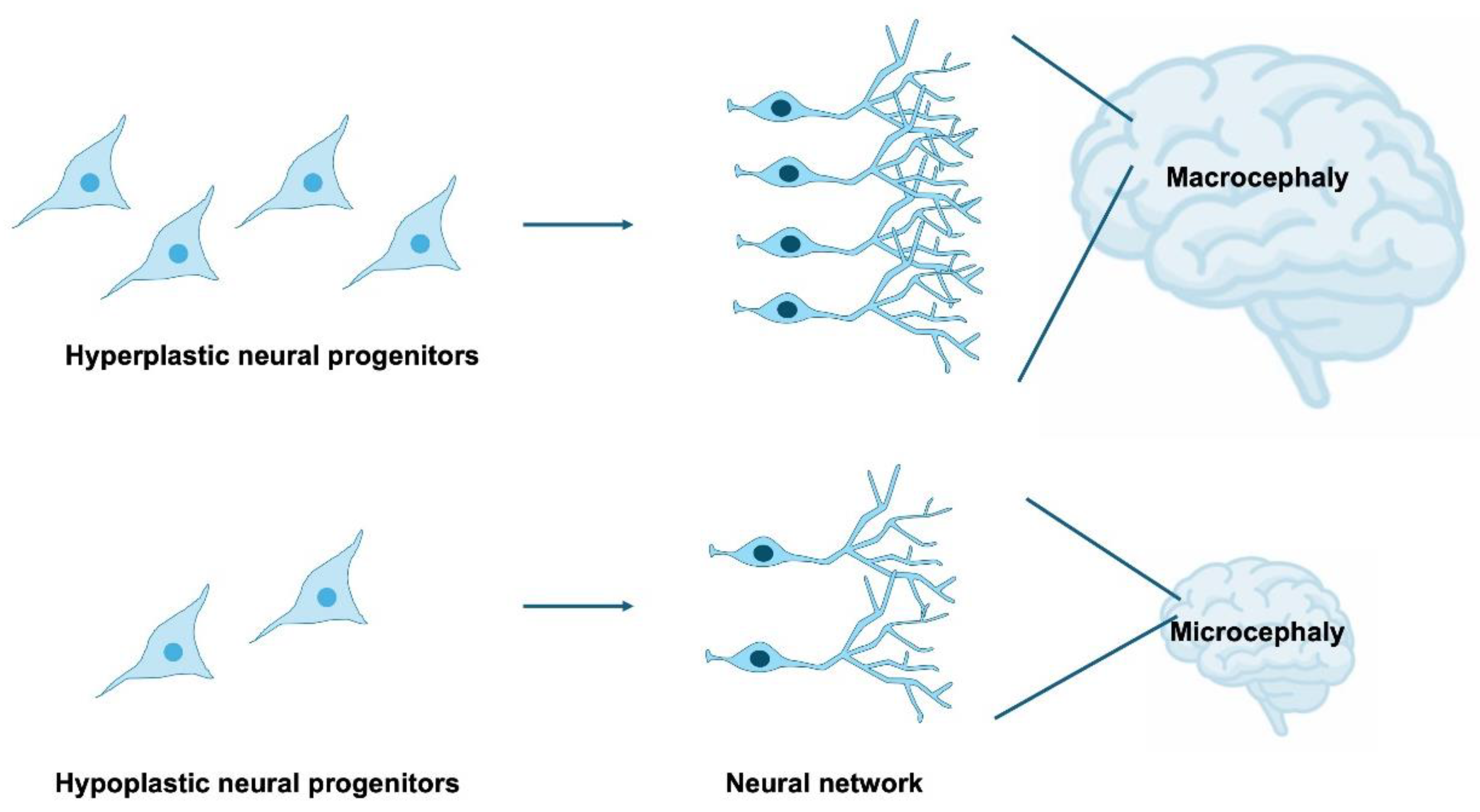
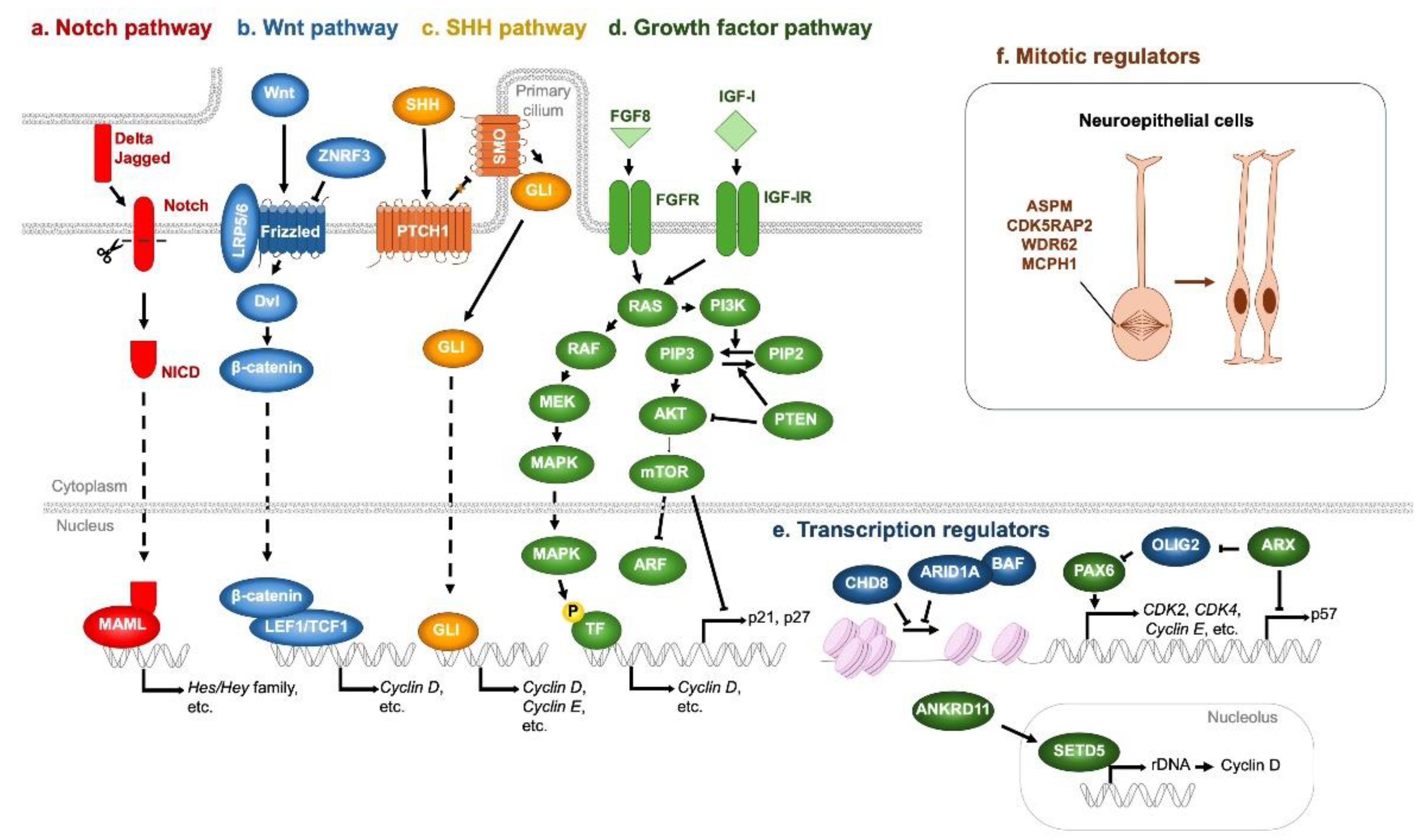
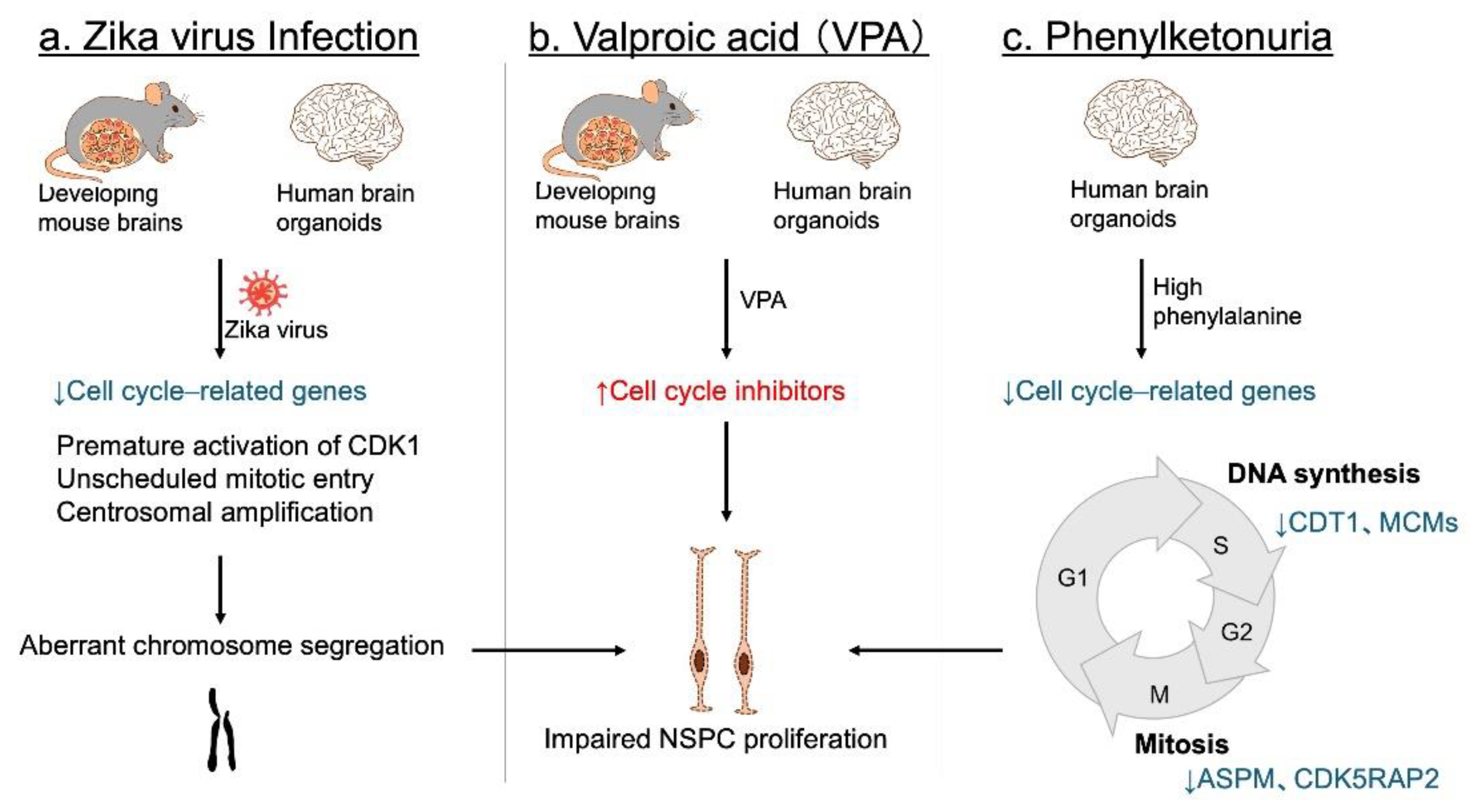
4. Impairment of NSPC Proliferation in Neurodevelopmental Disorders
4.1. Genetic Factors
4.2. Environmental Factors
5. Future Directions and Therapeutic Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris-Rosendahl, D.J. and M.A. Crocq, Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin Neurosci 2020, 22, 65–72. [Google Scholar] [CrossRef]
- Francés, L.; et al. Current state of knowledge on the prevalence of neurodevelopmental disorders in childhood according to the DSM-5: a systematic review in accordance with the PRISMA criteria, in Child Adolesc Psychiatry Ment Health. 2022, © 2022. The Author(s). England. p. 27.
- Picardi, A.; et al. Parental Burden and its Correlates in Families of Children with Autism Spectrum Disorder: A Multicentre Study with Two Comparison Groups. Clin Pract Epidemiol Ment Health 2018, 14, 143–176. [Google Scholar] [CrossRef]
- Materula, D.; et al. Needs of children with neurodevelopmental disorders and medical complexity: Caregiver perspectives. Res Dev Disabil 2024, 153, 104815. [Google Scholar] [CrossRef]
- Zhou, Y. H. Song, and G.L. Ming, Genetics of human brain development. Nat Rev Genet 2024, 25, 26–45. [Google Scholar] [CrossRef]
- Dionne, O. S. Sabatié, and B. Laurent, Deciphering the physiopathology of neurodevelopmental disorders using brain organoids. Brain 2025, 148, 12–26. [Google Scholar] [CrossRef]
- Stiles, J. and T.L. Jernigan, The basics of brain development. Neuropsychol Rev 2010, 20, 327–48. [Google Scholar] [CrossRef]
- Ladran, I.; et al. Neural stem and progenitor cells in health and disease. Wiley Interdiscip Rev Syst Biol Med 2013, 5, 701–15. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A. and A. Alvarez-Buylla, The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009, 32, 149–84. [Google Scholar] [CrossRef]
- Sojka, C. and S.A. Sloan, Gliomas: a reflection of temporal gliogenic principles. Commun Biol 2024, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Vivi, E. and B. Di Benedetto, Brain stars take the lead during critical periods of early postnatal brain development: relevance of astrocytes in health and mental disorders. Mol Psychiatry 2024, 29, 2821–2833. [Google Scholar] [CrossRef]
- Blackshaw, S. and M. Cayouette, Timing neural development and regeneration. Curr Opin Neurobiol 2025, 91, 102976. [Google Scholar] [CrossRef]
- Molofsky, A.V.; et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 2012, 26, 891–907. [Google Scholar] [CrossRef]
- Séjourné, G. and C. Eroglu, Astrocyte-neuron crosstalk in neurodevelopmental disorders. Curr Opin Neurobiol 2024, 89, 102925. [Google Scholar] [CrossRef]
- Temple, S. ; The development of neural stem cells. Nature 2001, 414, 112–7. [Google Scholar] [CrossRef]
- Götz, M. and W.B. Huttner, The cell biology of neurogenesis. Nat Rev Mol Cell Biol 2005, 6, 777–88. [Google Scholar] [CrossRef]
- Urbán, N. and F. Guillemot, Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci 2014, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Fukusumi, H.; et al. Evaluation of the susceptibility of neurons and neural stem/progenitor cells derived from human induced pluripotent stem cells to anticancer drugs. J Pharmacol Sci 2019, 140, 331–336. [Google Scholar] [CrossRef]
- Farkas, L.M. and W.B. Huttner, The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr Opin Cell Biol 2008, 20, 707–15. [Google Scholar] [CrossRef] [PubMed]
- Homem, C.C. M. Repic, and J.A. Knoblich, Proliferation control in neural stem and progenitor cells. Nat Rev Neurosci 2015, 16, 647–59. [Google Scholar] [CrossRef] [PubMed]
- Packer, A. ; Neocortical neurogenesis and the etiology of autism spectrum disorder. Neurosci Biobehav Rev 2016, 64, 185–95. [Google Scholar] [CrossRef]
- Ernst, C. ; Proliferation and Differentiation Deficits are a Major Convergence Point for Neurodevelopmental Disorders. Trends Neurosci 2016, 39, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; et al. Microcephaly gene links trithorax and REST/NRSF to control neural stem cell proliferation and differentiation. Cell 2012, 151, 1097–112. [Google Scholar] [CrossRef] [PubMed]
- Sun, T. and R.F. Hevner, Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat Rev Neurosci 2014, 15, 217–32. [Google Scholar] [CrossRef]
- Fei, J.F. C. Haffner, and W.B. Huttner, 3' UTR-dependent, miR-92-mediated restriction of Tis21 expression maintains asymmetric neural stem cell division to ensure proper neocortex size. Cell Rep 2014, 7, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.H. D.V. Hansen, and A.R. Kriegstein, Development and evolution of the human neocortex. Cell 2011, 146, 18–36. [Google Scholar] [CrossRef]
- Li, M.; et al. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 2018, 362(6420).
- Courchesne, E. V.H. Gazestani, and N.E. Lewis, Prenatal Origins of ASD: The When, What, and How of ASD Development. Trends Neurosci 2020, 43, 326–342. [Google Scholar] [CrossRef]
- Fujiki, R.; et al. A proapoptotic effect of valproic acid on progenitors of embryonic stem cell-derived glutamatergic neurons. Cell Death Dis 2013, 4, e677. [Google Scholar] [CrossRef]
- Fujimura, K.; et al. In Utero Exposure to Valproic Acid Induces Neocortical Dysgenesis via Dysregulation of Neural Progenitor Cell Proliferation/Differentiation. J Neurosci 2016, 36, 10908–10919. [Google Scholar] [CrossRef]
- Tsukada, T.; et al. Mid-pregnancy maternal immune activation increases Pax6-positive and Tbr2-positive neural progenitor cells and causes integrated stress response in the fetal brain in a mouse model of maternal viral infection. IBRO Neurosci Rep 2021, 11, 73–80. [Google Scholar] [CrossRef]
- McEwan, F. J.D. Glazier, and R. Hager, The impact of maternal immune activation on embryonic brain development. Front Neurosci 2023, 17, 1146710. [Google Scholar] [CrossRef]
- Casas Gimeno, G. and J. Paridaen, The Symmetry of Neural Stem Cell and Progenitor Divisions in the Vertebrate Brain. Front Cell Dev Biol 2022, 10, 885269. [Google Scholar] [CrossRef]
- Pontious, A.; et al. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci 2008, 30(1-3), 24-32.
- Ashitomi, H.; et al. Cullin-RING Ubiquitin Ligases in Neurodevelopment and Neurodevelopmental Disorders. Biomedicines 2025, 13(4).
- Ahmed, S.; et al. Transcription factors and neural stem cell self-renewal, growth and differentiation. Cell Adh Migr 2009, 3, 412–24. [Google Scholar] [CrossRef]
- Hardwick, L.J.; et al. Cell cycle regulation of proliferation versus differentiation in the central nervous system. Cell Tissue Res 2015, 359, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Taverna, E. M. Götz, and W.B. Huttner, The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol 2014, 30, 465–502. [Google Scholar] [CrossRef]
- Tropepe, V.; et al. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol 1999, 208, 166–88. [Google Scholar] [CrossRef]
- Arsenijevic, Y.; et al. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. J Neurosci 2001, 21, 7194–202. [Google Scholar] [CrossRef] [PubMed]
- Chenn, A. and C.A. Walsh, Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 2002, 297, 365–9. [Google Scholar] [CrossRef] [PubMed]
- Wen, S. H. Li, and J. Liu, Dynamic signaling for neural stem cell fate determination. Cell Adh Migr 2009, 3, 107–17. [Google Scholar] [CrossRef]
- Imayoshi, I.; et al. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci 2010, 30, 3489–98. [Google Scholar] [CrossRef]
- Yabut, O.R.; et al. The Neocortical Progenitor Specification Program Is Established through Combined Modulation of SHH and FGF Signaling. J Neurosci 2020, 40, 6872–6887. [Google Scholar] [CrossRef]
- Long, K.R.; et al. Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron 2018, 99, 702–719.e6. [Google Scholar] [CrossRef] [PubMed]
- Scandella, V.; et al. Neural stem cell metabolism revisited: a critical role for mitochondria. Trends Endocrinol Metab 2023, 34, 446–461. [Google Scholar] [CrossRef]
- Ohnuma, S. and W.A. Harris, Neurogenesis and the cell cycle. Neuron 2003, 40, 199–208. [Google Scholar] [CrossRef]
- Pellarin, I.; et al. Cyclin-dependent protein kinases and cell cycle regulation in biology and disease. Signal Transduct Target Ther 2025, 10, 11. [Google Scholar] [CrossRef]
- Calegari, F.; et al. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci 2005, 25, 6533–8. [Google Scholar] [CrossRef]
- Calegari, F. nd W.B. Huttner, An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci 2003, 116(Pt 24), 4947–55. [Google Scholar] [CrossRef]
- Lange, C. W.B. Huttner, and F. Calegari, Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 2009, 5, 320–31. [Google Scholar] [CrossRef]
- Pilaz, L.J.; et al. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci U S A 2009, 106, 21924–9. [Google Scholar] [CrossRef]
- Johansson, P.A. S. Cappello, and M. Götz, Stem cells niches during development--lessons from the cerebral cortex. Curr Opin Neurobiol 2010, 20, 400–7. [Google Scholar] [CrossRef]
- Solozobova, V. N. Wyvekens, and J. Pruszak, Lessons from the embryonic neural stem cell niche for neural lineage differentiation of pluripotent stem cells. Stem Cell Rev Rep 2012, 8, 813–29. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; et al. Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci 2016, 17, 537–49. [Google Scholar] [CrossRef]
- Hota, S.K. and B.G. Bruneau, ATP-dependent chromatin remodeling during mammalian development. Development 2016, 143, 2882–97. [Google Scholar] [CrossRef]
- Lim, Y. ; Transcription factors in microcephaly. Front Neurosci 2023, 17, 1302033. [Google Scholar] [CrossRef] [PubMed]
- Sueda, R. and R. Kageyama, Regulation of active and quiescent somatic stem cells by Notch signaling. Dev Growth Differ 2020, 62, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N. D.S. Castro, and F. Guillemot, Proneural genes and the specification of neural cell types. Nat Rev Neurosci 2002, 3, 517–30. [Google Scholar] [CrossRef] [PubMed]
- Fiddes, I.T.; et al. Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell 2018, 173, 1356–1369.e22. [Google Scholar] [CrossRef]
- Fischer-Zirnsak, B.; et al. Haploinsufficiency of the Notch Ligand DLL1 Causes Variable Neurodevelopmental Disorders. Am J Hum Genet 2019, 105, 631–639. [Google Scholar] [CrossRef]
- Mehta, S. S. Hingole, and V. Chaudhary, The Emerging Mechanisms of Wnt Secretion and Signaling in Development. Front Cell Dev Biol 2021, 9, 714746. [Google Scholar] [CrossRef]
- Shtutman, M.; et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A 1999, 96, 5522–7. [Google Scholar] [CrossRef]
- Liu, J.; et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 2022, 7, 3. [Google Scholar] [CrossRef]
- Boonsawat, P.; et al. Deleterious ZNRF3 germline variants cause neurodevelopmental disorders with mirror brain phenotypes via domain-specific effects on Wnt/β-catenin signaling. Am J Hum Genet 2024, 111, 1994–2011. [Google Scholar] [CrossRef]
- Hao, H.X.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef]
- Jing, J.; et al. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct Target Ther 2023, 8, 315. [Google Scholar] [CrossRef]
- Heussler, H.S.; et al. Extreme variability of expression of a Sonic Hedgehog mutation: attention difficulties and holoprosencephaly. Arch Dis Child 2002, 86, 293–6. [Google Scholar] [CrossRef]
- Derwińska, K.; et al. PTCH1 duplication in a family with microcephaly and mild developmental delay. Eur J Hum Genet 2009, 17, 267–71. [Google Scholar] [CrossRef]
- Xie, Y.; et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther 2020, 5, 181. [Google Scholar] [CrossRef]
- Pandey, P.; et al. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed Pharmacother 2023, 161, 114491. [Google Scholar] [CrossRef]
- Werner, H. ; The IGF1 Signaling Pathway: From Basic Concepts to Therapeutic Opportunities. Int J Mol Sci 2023, 24(19).
- Zhang, W. and H.T. Liu, MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E. H.J. Kim, and D.R. Kim, Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct Target Ther 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; et al. S6 Kinase- and β-TrCP2-Dependent Degradation of p19Arf Is Required for Cell Proliferation. Mol Cell Biol 2015, 35, 3517–27. [Google Scholar] [CrossRef]
- Mori, S.; et al. The mTOR pathway controls cell proliferation by regulating the FoxO3a transcription factor via SGK1 kinase. PLoS One 2014, 9, e88891. [Google Scholar] [CrossRef] [PubMed]
- Skelton, P.D. ; R.V. Stan, and B.W. Luikart, The Role of PTEN in Neurodevelopment. Mol Neuropsychiatry 2020, 5(Suppl 1), 60-71.
- Hong, S.; et al. Dominant-negative kinase domain mutations in FGFR1 can explain the clinical severity of Hartsfield syndrome. Hum Mol Genet 2016, 25, 1912–1922. [Google Scholar] [CrossRef]
- Toydemir, R.M.; et al. A novel mutation in FGFR3 causes camptodactyly, tall stature, and hearing loss (CATSHL) syndrome. Am J Hum Genet 2006, 79, 935–41. [Google Scholar] [PubMed]
- Arauz, R.F.; et al. A Hypomorphic Allele in the FGF8 Gene Contributes to Holoprosencephaly and Is Allelic to Gonadotropin-Releasing Hormone Deficiency in Humans. Mol Syndromol 2010, 1, 59–66. [Google Scholar] [CrossRef]
- McCabe, M.J.; et al. Novel FGF8 mutations associated with recessive holoprosencephaly, craniofacial defects, and hypothalamo-pituitary dysfunction. J Clin Endocrinol Metab 2011, 96, E1709–E1718. [Google Scholar] [CrossRef]
- Woods, K.A.; et al. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 1996, 335, 1363–7. [Google Scholar] [CrossRef]
- Walenkamp, M.J.; et al. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab 2005, 90, 2855–64. [Google Scholar] [CrossRef]
- Netchine, I.; et al. Partial primary deficiency of insulin-like growth factor (IGF)-I activity associated with IGF1 mutation demonstrates its critical role in growth and brain development. J Clin Endocrinol Metab 2009, 94, 3913–21. [Google Scholar]
- Abuzzahab, M.J.; et al. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med 2003, 349, 2211–22. [Google Scholar] [CrossRef]
- Yang, L.; et al. IGF1R Variants in Patients With Growth Impairment: Four Novel Variants and Genotype-Phenotype Correlations. J Clin Endocrinol Metab 2018, 103, 3939–3944. [Google Scholar] [CrossRef]
- Wang, X.; et al. The phenotypic and genetic spectrum of AKT3-related neurodevelopmental condition. Sci Rep 2025, 15 7484.
- Butler, M.G.; et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet 2005, 42, 318–21. [Google Scholar] [PubMed]
- Varga, E.A.; et al. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet Med 2009, 11, 111–7. [Google Scholar] [PubMed]
- Frazier, T.W.; et al. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol Psychiatry 2015, 20, 1132–8. [Google Scholar]
- Yeung, K.S.; et al. Identification of mutations in the PI3K-AKT-mTOR signalling pathway in patients with macrocephaly and developmental delay and/or autism. Mol Autism 2017, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Mirzaa, G.; et al. De novo CCND2 mutations leading to stabilization of cyclin D2 cause megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome. Nat Genet 2014, 46, 510–515. [Google Scholar]
- Lee, J.H.; et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet 2012, 44, 941–5. [Google Scholar]
- Rivière, J.B.; et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet 2012, 44, 934–40. [Google Scholar] [CrossRef]
- Mirzaa, G.M.; et al. Association of MTOR Mutations With Developmental Brain Disorders, Including Megalencephaly, Focal Cortical Dysplasia, and Pigmentary Mosaicism. JAMA Neurol 2016, 73, 836–845. [Google Scholar] [CrossRef]
- Kato, M.; et al. Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Hum Mutat 2004, 23, 147–159. [Google Scholar]
- Colasante, G.; et al. ARX regulates cortical intermediate progenitor cell expansion and upper layer neuron formation through repression of Cdkn1c. Cereb Cortex 2015, 25, 322–35. [Google Scholar] [CrossRef]
- Lim, Y.; et al. Arx Expression Suppresses Ventralization of the Developing Dorsal Forebrain. Sci Rep 2019, 9, 226. [Google Scholar] [CrossRef]
- Liu, W.; et al. Disruption of neurogenesis and cortical development in transgenic mice misexpressing Olig2, a gene in the Down syndrome critical region. Neurobiol Dis 2015, 77, 106–16. [Google Scholar] [CrossRef]
- Sansom, S.N.; et al. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet 2009, 5, e1000511. [Google Scholar] [CrossRef]
- Glaser, T.; et al. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet 1994, 7, 463–71. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.D.; et al. Compound heterozygosity for mutations in PAX6 in a patient with complex brain anomaly, neonatal diabetes mellitus, and microophthalmia. Am J Med Genet A 2009, 149a, 2543–6. [Google Scholar] [CrossRef]
- Warren, N.; et al. The transcription factor, Pax6, is required for cell proliferation and differentiation in the developing cerebral cortex. Cereb Cortex 1999, 9, 627–35. [Google Scholar] [CrossRef] [PubMed]
- Mi, D.; et al. Pax6 exerts regional control of cortical progenitor proliferation via direct repression of Cdk6 and hypophosphorylation of pRb. Neuron 2013, 78, 269–84. [Google Scholar] [CrossRef]
- Mi, D.; et al. Pax6 Lengthens G1 Phase and Decreases Oscillating Cdk6 Levels in Murine Embryonic Cortical Progenitors. Front Cell Neurosci 2018, 12, 419. [Google Scholar] [CrossRef]
- Ferguson, K.L.; et al. The Rb-CDK4/6 signaling pathway is critical in neural precursor cell cycle regulation. J Biol Chem 2000, 275, 33593–600. [Google Scholar] [CrossRef]
- Hussain, M.S.; et al. CDK6 associates with the centrosome during mitosis and is mutated in a large Pakistani family with primary microcephaly. Hum Mol Genet 2013, 22, 5199–214. [Google Scholar] [CrossRef]
- Coakley-Youngs, E.; et al. Autism-associated CHD8 keeps proliferation of human neural progenitors in check by lengthening the G1 phase of the cell cycle. Biol Open 2022, 11(9).
- Gompers, A.L.; et al. Germline Chd8 haploinsufficiency alters brain development in mouse. Nat Neurosci 2017, 20, 1062–1073. [Google Scholar] [CrossRef]
- Durak, O.; et al. Chd8 mediates cortical neurogenesis via transcriptional regulation of cell cycle and Wnt signaling. Nat Neurosci 2016, 19, 1477–1488. [Google Scholar] [CrossRef]
- Meert, L.; et al. A CHD8-TRRAP axis facilitates MYC and E2F target gene regulation in human neural stem cells. iScience 2025, 28, 111978. [Google Scholar] [CrossRef]
- Bidart, M.; et al. Microduplication of the ARID1A gene causes intellectual disability with recognizable syndromic features. Genet Med 2017, 19, 701–710. [Google Scholar] [CrossRef]
- Nagamani, S.C.; et al. Interstitial deletion of 6q25.2-q25.3: a novel microdeletion syndrome associated with microcephaly, developmental delay, dysmorphic features and hearing loss. Eur J Hum Genet 2009, 17, 573–81. [Google Scholar] [CrossRef] [PubMed]
- Santen, G.W.; et al. Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat Genet 2012, 44, 379–80. [Google Scholar] [CrossRef]
- Tsurusaki, Y.; et al. Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat Genet 2012, 44, 376–8. [Google Scholar] [CrossRef]
- Liu, X.; et al. Arid1a regulates neural stem/progenitor cell proliferation and differentiation during cortical development. Cell Prolif 2021, 54, e13124. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; et al. BAF250B-associated SWI/SNF chromatin-remodeling complex is required to maintain undifferentiated mouse embryonic stem cells. Stem Cells 2008, 26, 1155–65. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.C.; et al. Expanding the phenotypic spectrum of ARID1B-mediated disorders and identification of altered cell-cycle dynamics due to ARID1B haploinsufficiency. Orphanet J Rare Dis 2014, 9, 43. [Google Scholar] [CrossRef]
- Nakagawa, T.; et al. The Autism-Related Protein SETD5 Controls Neural Cell Proliferation through Epigenetic Regulation of rDNA Expression. iScience 2020, 23, 101030. [Google Scholar] [CrossRef]
- Nakagawa, T.; et al. Neurobehavioral characteristics of mice with SETD5 mutations as models of IDD23 and KBG syndromes. Front Genet 2022, 13, 1022339. [Google Scholar] [CrossRef]
- Crippa, M.; et al. SETD5 Gene Haploinsufficiency in Three Patients With Suspected KBG Syndrome. Front Neurol 2020, 11, 631. [Google Scholar] [CrossRef]
- Pascolini, G.; et al. Clinical refinement of the SETD5-associated phenotype in a child displaying novel features and KBG syndrome-like appearance. Am J Med Genet A 2022, 188, 1623–1625. [Google Scholar] [CrossRef]
- Sashiyama, S.; et al. KBG syndrome-associated protein ANKRD11 regulates SETD5 expression to modulate rRNA levels and translation. iScience 2025, 28, 112699. [Google Scholar] [CrossRef] [PubMed]
- Fokkema, I.; et al. The LOVD3 platform: efficient genome-wide sharing of genetic variants. Eur J Hum Genet 2021, 29, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Loberti, L.; et al. Natural history of KBG syndrome in a large European cohort. Hum Mol Genet 2022, 31, 4131–4142. [Google Scholar] [CrossRef]
- Ahsan, N.; et al. Expanding the Genotype and Phenotype of SETD5-Related Neurodevelopmental Syndrome. Pediatr Neurol 2023, 138, 25–26. [Google Scholar] [CrossRef]
- Jayaraman, D. B.I. Bae, and C.A. Walsh, The Genetics of Primary Microcephaly. Annu Rev Genomics Hum Genet 2018, 19, 177–200. [Google Scholar] [CrossRef]
- Saade, M.; et al. A centrosomal view of CNS growth. Development 2018, 145(21).
- Rasmussen, S.A.; et al. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med 2016, 374, 1981–7. [Google Scholar] [CrossRef]
- Wen, Z. H. Song, and G.L. Ming, How does Zika virus cause microcephaly? Genes Dev 2017, 31, 849–861. [Google Scholar] [CrossRef]
- Nielsen-Saines, K.; et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 2019, 25, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Santi, L.; et al. Zika Virus Infection Associated with Autism Spectrum Disorder: A Case Report. Neuroimmunomodulation 2021, 28, 229–232. [Google Scholar] [CrossRef]
- Wu, K.Y.; et al. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res 2016, 26, 645–54. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; et al. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 120–6. [Google Scholar] [CrossRef]
- Qian, X.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Cugola, F.R.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–71. [Google Scholar] [CrossRef]
- Onorati, M.; et al. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep 2016, 16, 2576–2592. [Google Scholar] [CrossRef]
- Souza, B.S.; et al. Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci Rep 2016, 6, 39775. [Google Scholar] [CrossRef]
- Wolf, B.; et al. Zika virus causes supernumerary foci with centriolar proteins and impaired spindle positioning. Open Biol 2017, 7(1).
- Rychlowska, M.; et al. Zika Virus Induces Mitotic Catastrophe in Human Neural Progenitors by Triggering Unscheduled Mitotic Entry in the Presence of DNA Damage While Functionally Depleting Nuclear PNKP. J Virol 2022, 96, e0033322. [Google Scholar] [CrossRef] [PubMed]
- Ardinger, H.H.; et al. Verification of the fetal valproate syndrome phenotype. Am J Med Genet 1988, 29, 171–85. [Google Scholar] [CrossRef]
- Almgren, M. B. Källén, and C. Lavebratt, Population-based study of antiepileptic drug exposure in utero--influence on head circumference in newborns. Seizure 2009, 18, 672–5. [Google Scholar] [CrossRef]
- Kataoka, S.; et al. Autism-like behaviours with transient histone hyperacetylation in mice treated prenatally with valproic acid. Int J Neuropsychopharmacol 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Nicolini, C. and M. Exp Neurol 2018, 299(Pt A), 217–227. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; et al. Aberrant induction of p19Arf-mediated cellular senescence contributes to neurodevelopmental defects. PLoS Biol 2022, 20, e3001664. [Google Scholar] [CrossRef] [PubMed]
- Elhawary, N.A.; et al. Genetic etiology and clinical challenges of phenylketonuria. Hum Genomics 2022, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Rovelli, V. and N. Longo, Phenylketonuria and the brain. Mol Genet Metab 2023, 139, 107583. [Google Scholar] [CrossRef]
- Kim, J.; et al. Neurotoxicity of phenylalanine on human iPSC-derived cerebral organoids. Mol Genet Metab 2022, 136, 132–144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




