1. Introduction
Glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs; e.g., semaglutide, liraglutide) have reshaped treatment for higher weight and type 2 diabetes, with randomised trials demonstrating substantial and sustained effects on body weight and cardiometabolic outcomes [
1]. Beyond metabolic benefits, GLP-1RAs act on central and peripheral pathways governing hunger, satiety, gastric emptying, and reward [
2,
3]—mechanisms that directly intersect with core features of eating behaviour and eating disorders (EDs) [
4]. This convergence has prompted growing interest in how GLP-1RAs may influence appetite regulation [
2,
5], binge eating and compensatory behaviours [
6,
7], emotion-driven eating [
8,
9], and body image [
10], as well as their implications for individuals with a diagnoses or those at risk for an ED [
11,
12].
Concurrently, safety signals and regulatory reviews have raised questions about mood changes and suicidality among some GLP-1 users, although a causal link has not been established and available data are limited by short follow-up periods and the frequent exclusion of people with psychiatric comorbidity [
13,
14,
15,
16]. Emerging evidence also points to possible psychological pathways, such as rapid weight loss, disrupted interoceptive signals, and altered reward processing, which could increase susceptibility to mood disturbance, disordered eating, or suicidality in some individuals and therefore require closer examination [
17].
Beyond individual-level mechanisms, these experiences unfold within a broader cultural discourse. Media coverage and celebrity endorsements, including a recent news feature titled “
As athletes like Serena Williams promote Ozempic, experts say our obsession with weight loss is worse than ever,” frame GLP-1 use through the lens of diet culture and aesthetic performance. [
18]. Such narratives can intensify pressures to pursue thinness, reinforce stigma, and minimise the psychological complexity of pharmacologically assisted weight loss. Issues of affordability and access are relevant background considerations, but the primary focus of this review is the psychological and ED-related implications of GLP-1 use.
Taken together, these issues point to urgent questions at the heart of ED science and practice: How does pharmacological appetite suppression alter day-to-day eating patterns and cue-based regulation? Does GLP-1 usage protect against—or exacerbate—binge eating frequency, compensatory behaviours, and body-image disturbance? How do rapid changes in weight and shape influence identity and self-perception? If food becomes a less available coping tool, what happens to emotion regulation? How are expectations and anxieties about GLP-1RAs shaped by social media narratives? And are groups such as adolescents, individuals with EDs, disordered eating, or body image concerns—as well as people from diverse ethnic, cultural, and socioeconomic backgrounds—particularly vulnerable?
In this review, we synthesize emerging evidence on the psychological effects of GLP-1 treatment with a specific ED focus. We examine (1) appetite and eating behaviours (hunger/satiety, cravings, food choice, meal microstructure); (2) disordered eating and EDs, especially binge-type presentations (Binge Eating Disorder [BED]/Bulimia Nervosa [BN]) and the limited evidence in restrictive disorders (Anorexia Nervosa [AN]); (3) emotion regulation and emotional eating; (4) mental health and quality of life; (5) body image and self-perception, including interoceptive and multisensory processes; (6) social media and digital culture; and (7) considerations for special populations (e.g., adolescents, people from diverse ethnic, cultural, and socioeconomic backgrounds). We conclude with clinical implications for assessment and monitoring, and a research agenda prioritising longitudinal, mechanistic, and ED-relevant trials to inform safe, patient-centered use of GLP-1 usage.
2. Method
Narrative reviews differ from systematic reviews in that they do not follow formal reporting frameworks. Instead, they allow for a broader and more interpretative synthesis of literature, without rigid inclusion or exclusion criteria, emphasising conceptual integration over exhaustive retrieval.
This narrative review was conducted in three phases: (1) search execution, (2) screening and evaluation of relevant sources, and (3) synthesis and interpretation of key findings. Searches were conducted across major scientific databases, including PubMed, PsycINFO, Web of Science, and Google Scholar, and supplemented by manual reference checking. The search targeted studies from 2015–2025, supplemented by earlier seminal papers of theoretical or clinical importance.
Search terms combined keywords related to GLP-1 and its analogues (e.g., “GLP-1 receptor agonists,” “semaglutide,” “liraglutide,” “tirzepatide,” “Ozempic,” “Wegovy,” “Mounjaro”) with psychological and ED-related concepts (e.g., “eating behaviour,” “binge eating,” “anorexia nervosa,” “bulimia nervosa,” “body image,” “emotion regulation,” “mood,” “mental health,” “stigma,” “social media”). Eligible sources included peer-reviewed empirical studies, meta-analyses, reviews, and conceptual or qualitative papers written in English, German or Spanish.
The synthesis aimed to integrate biological, psychological, and sociocultural evidence relevant to appetite regulation, disordered eating, emotion, and body image in the context of GLP-1 use, identifying key themes, conceptual gaps, and clinical implications.
3. Appetite and Eating Behaviours
GLP-1RAs have emerged as highly effective therapies for metabolic diseases [
19]. GLP-1RAs play a central role in glucose regulation by enhancing insulin secretion, suppressing glucagon release, delaying gastric emptying, and acting on the central nervous system to regulate satiation and satiety [
2,
20].
A combination of randomized controlled trials and neuroimaging studies in humans and animals has shown that GLP-1RAs reduce hunger, increase satiety, dampen cravings, and influence food choice and eating patterns through both central and peripheral mechanisms [
2,
20,
21]. They enhance pre-ingestive satiation (fullness before and during a meal) and postprandial satiety (fullness after eating) by activating hypothalamic receptors, particularly in the dorsomedial hypothalamus, and modulating neural circuits that suppress hunger signals and downregulate food reward pathways [
5,
22]. These drugs also reduce the brain’s responsiveness to high-calorie food cues and palatable foods by altering activity in reward-related regions, such as the insula and orbitofrontal cortex [
2,
21].
Beyond appetite suppression, studies have indicated that GLP-1RAs shape eating behaviours and meal patterns [
23,
24]. They shift food preferences toward lower-calorie options, reduce the desire for energy-dense foods, slow gastric emptying, prolong satiety, reduce meal size, and extend intervals between meals [
21,
25]. Individuals taking GLP-1RAs are generally less influenced by emotional, situational, or external cues and more attuned to physiological hunger and fullness signals, leading to smaller portions, slower eating rates, and longer gaps between meals [
24,
26].
However, GLP-1RAs can cause gastrointestinal side effects through multiple interconnected mechanisms. Systematic reviews have shown that by slowing gastric emptying and reducing intestinal motility via gut receptors and enteric neurons, GLP-1RAs prolong food retention, which can lead to nausea, vomiting, bloating, and occasionally diarrhea [
27,
28]. Suppression of digestive hormones, including cholecystokinin and gastrin, can impair gallbladder contraction and gastric acid secretion, contributing to upper abdominal discomfort [
29,
30]. Central nervous system pathways, including the area postrema and nucleus of the solitary tract, further amplify gut-brain signalling that triggers nausea and vomiting. Emerging evidence also suggests potential alterations in the gut microbiome may contribute to diarrhea [
29,
31]. These side effects, alongside high costs of GLP-1RAs, limited insurance coverage, drug shortages, and social stigma, are major factors driving discontinuation, which can limit the consolidation of healthier eating behaviours and reduce long-term treatment success [
32,
33].
Despite growing evidence, several key research gaps remain. Most studies focus on short-term weight loss, leaving limited understanding of how GLP-1RAs affect hunger, satiety, cravings, and food choice during weight maintenance or prolonged use [
2,
21]. Research relies heavily on subjective self-reports, such as appetite ratings and food diaries, rather than objective, quantitative assessments like direct observation, longitudinal micro-phenotyping (e.g., ecological momentary assessment [EMA], bite metrics), or detailed meal microstructure analysis [
2]. There is also limited insight into how GLP-1RAs alter specific food preferences, meal patterns, and microstructure such as bite size, eating rate, and meal duration in real-world settings, and compensatory behaviours like grazing or liquid calorie intake are rarely captured [
21].
Mechanistic evidence of GLP-1RA effects predominantly comes from animal studies [
34,
35], with few translational studies in diverse human populations, leaving effects in non-obese, non-diabetic, or younger individuals underexplored. Finally, while some neural pathways have been identified (e.g., preingestive satiation via hypothalamic circuits [
20]), the precise cognitive and neural mechanisms by which GLP-1RAs influence anticipatory hunger, reward processing, and food-related decision-making remain incompletely understood.
4. Disordered Eating and Eating Disorders
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [
36,
37] identifies several major ED categories, all of which carry substantial medical and psychological burden. AN is characterised by severe dietary restriction, low body weight, and body image disturbance, and has the highest mortality rate of any psychiatric illness due to both medical complications and suicide risk [
36,
37,
38]. BN involves recurrent binge eating with compensatory behaviours such as vomiting or laxative misuse, leading to serious medical consequences and elevated suicidality. BED is marked by recurrent binge eating episodes without compensatory behaviours and is strongly associated with higher weight, cardiometabolic disease, and psychiatric comorbidity. Finally, Other Specified Feeding or Eating Disorders (OSFED), including atypical AN, purging disorder, night eating syndrome, and subthreshold BN and BED, carry impairment and medical risks comparable to the other EDs included in the DSM-5 [
39,
40].
The link between GLP-1RAs and EDs/disordered eating has received growing attention [
12]. Research to date has focused primarily on binge–purge disorders, particularly BED and BN, where the evidence base is strongest. The following section will review findings for BED and BN, as well as emerging evidence on restrictive EDs such as AN.
4.1. Binge Eating Behaviour and BED
Systematic reviews and meta-analyses [
6,
7,
12] show that GLP-1RAs reduce binge eating frequency and severity and improve Binge Eating Scale (BES; [
41]) scores. GLP-1RA medications such as semaglutide and liraglutide haven been found in recent studies to outperform other anti-higher weight medications. Individual trials (e.g., [
42,
43,
44]) also support these findings; for example, dulaglutide reduces binge eating, body weight, and metabolic markers more effectively than standard diabetes treatments in patients with BED and type 2 diabetes [
6,
44].
Mechanistically, as mentioned above, GLP-1RAs act on central and peripheral pathways, increasing satiety, reducing cravings, and modulating reward-related circuits. They also reduce weight, body mass index (BMI), and metabolic control [
6,
21]. GLP-1RAs are well tolerated, have a favourable psychiatric side-effect profile, and offer weekly dosing that may aid adherence [
12,
42]. Cognitive Behaviour Therapy (CBT), however, remains the gold-standard treatment for EDs, including BED [
45]. GLP-1RAs may serve only as useful adjuncts but have not yet been tested in combination with CBT treatment. In addition, concerns remain about gastrointestinal side effects and the need for clinicians to carefully evaluate the risk–benefit balance for each patient, considering individual medical, psychiatric, and treatment-related factors.
4.2. Bulimia Nervosa
Preliminary evidence, largely from case reports and small studies, suggests that GLP-1RAs may reduce binge–purge cycles and body weight in individuals with BN, with some reports of full symptom remission [
46,
47]. GLP-1RAs may also be more effective than traditional anti-higher weight medications in reducing binge behaviours, likely through their effects on appetite regulation and reward-related pathways [
12].
Supporting this, a systematic review found that individuals with BN often exhibit lower endogenous GLP-1 concentrations, providing a biological rationale for these therapeutic effects [
12]. Compared with existing pharmacotherapies, GLP-1RAs appear to have a more favourable psychiatric safety profile, with lower risks of depression, anxiety, and suicidal ideation. However, current evidence remains preliminary, and findings must be interpreted cautiously until validated in larger controlled trials [
46]. At the same time, potential adverse effects warrant consideration. Gastrointestinal symptoms such as nausea and vomiting may complicate treatment, particularly in patients with purging behaviours, where these side effects could inadvertently reinforce or exacerbate existing cycles [
48].
4.3. Restrictive Eating Disorders
Most research and clinical trials focus on binge-type EDs, not restrictive types. Research on AN and related restrictive disorders remain scarce and inconclusive. Findings on endogenous GLP-1RA levels are mixed; for example, one study in adolescent girls reported reduced fasting and postprandial concentrations, though the clinical significance is unclear [
49]. Because GLP-1RAs suppress appetite, cautions have been made that these may blunt hunger cues and complicate intuitive eating, raising theoretical concerns in at-risk for ED populations [
11]. To date, however, no clinical evidence links GLP-1RA use to the onset or worsening of AN.
Theoretical risks include restrictive behaviours being “masked” as medication adjustments (e.g., “dose working”) or compulsive reliance on pharmacological appetite suppression as a form of control [
11]. These risks may be heightened in individuals with high perfectionism or in those with presentations that shift between binge–purge and restrictive patterns, as is sometimes observed in BN and OSFED. Although sometimes overlooked, OSFED is associated with impairment and medical risk comparable to the other established EDs [
39,
40]. Across the ED spectrum, perfectionism [
50], rigidity [
51], and obsessive–compulsive traits [
52] are common, fostering inflexible rules around food and weight and a heightened drive for control, which may amplify vulnerability to medication misuse such as GLP-1RA.
Concerns are further heightened by the fact that restrictive eating often goes undetected in primary care, including GLP-1RA prescribing contexts [
11]. Patterns such as severe food restriction or rigid calorie control may emerge or be reinforced by the weight-suppressing effects of GLP-1RAs [
6,
11]. These behaviours can go unnoticed in patients with higher or “normal” BMI (e.g., atypical AN, BN or BED), where EDs are often misjudged as being defined by body weight rather than behaviours or weight loss. Without careful screening, restrictive GLP-1RA use may be misinterpreted as good adherence, masking underlying ED psychopathology. To mitigate these risks, prescribers should conduct a pre-start screen (e.g., SCOFF; [
53]), review recent ED history, assess for compensatory behaviours, and consider weight-suppression (highest–current weight difference) history, which is a key risk marker for ED severity, relapse, and medical complications [
54]. Clear red-flag triggers should also be set, including rapid weight loss, dizziness or syncope, escalating restriction, and use of purging or laxatives.
Overall, GLP-1RA usage shows promise for binge-type EDs, reducing binge eating frequency in BED and binge–purge cycles in BN, likely via modulation of appetite, satiety, and reward pathways (e.g., [
6,
7,
12]). They are generally well tolerated and have a favourable psychiatric profile, although gastrointestinal side effects may pose risks for individuals with purging behaviours [
48]. Evidence in restrictive disorders, such as AN, remains extremely limited, and theoretical concerns persist regarding appetite suppression, masked restrictive behaviours, and heightened risk in high perfectionism/obsessive compulsive or OSFED presentations [
11].
Despite these promising findings, substantial gaps remain. Most studies investigating the relationship between BN and GLP-1RAs are small, uncontrolled, or open-label, limiting confidence in efficacy, safety, optimal dosing, and long-term outcomes. Comparative effectiveness with standard treatments, including CBT and other pharmacotherapies, has not been established across disordered eating and EDs. Mechanistic and biomarker data are inconsistent, and the neurobiological pathways through which GLP-1RAs influence disordered eating remain poorly understood [
55]. Additionally, genetic and epigenetic predictors of treatment response are largely unexplored, limiting the ability to personalize treatment outcome [
56,
57].
Future research should prioritize large, randomised controlled trials in BED and BN, systematic investigation of safety in restrictive EDs, and integration of mechanistic studies using neuroimaging and endocrine assessments. Exploration of genetic, epigenetic, and biomarker predictors will be critical to identify individuals most likely to benefit and to mitigate potential risks. Such efforts will be essential to inform safe, evidence-based, and personalised use of GLP-1RAs across diverse ED populations.
5. Emotional Eating and Emotion Regulation
Research suggests that GLP-1RAs reduce emotional eating in the short term. Individuals living with in larger bodies experienced a significant decrease in emotional eating frequency after three month [
58] and six months [
59] of semaglutide treatment. Emotional eating refers to the consumption of food in response to negative emotions rather than physiological hunger [
60] that is used as an avoidant strategy to cope with emotions [
61]. GLP-1RAs reduce emotional eating by increasing satiety through the endocrine pathway or modulating reward processing in the mesolimbic reward circuitry through the neural pathway [
9,
62]. In a fMRI study, GLP-1RAs have been found to decrease brain responses to anticipatory food reward and increase consummatory food reward, which may reduce food craving and overeating [
63].
However, there is recent evidence suggesting that the effect of GLP-1RAs on reducing emotional eating may not be long-lasting [
8]. In this observational study, individuals receiving GLP-1RAs had significant reduction in emotional eating at three months post-treatment, but their emotional eating scores returned to baseline level by 12 months [
8]. A possible explanation is that individuals with pre-existing high level of emotional eating may be less sensitive to GLP-1RA treatment [
64]. Findings from this randomized trial suggested that higher emotional eating scores at baseline were related to less changes in brain areas regulating reward and satiety in responses to food cues after ten days of treatment [
64]. Hence, emotional eating could be a vulnerability factor that hinder the treatment effects of GLP-1RAs [
64]. Further research is required to study the long-term effects of GLP-1RAs in the reduction of emotional eating and whether the potential beneficial effects of GLP-1RAs on reducing emotional eating may be moderated by pre-existing risk factors such as elevated emotional eating.
While GLP-1RAs may improve emotional eating in the short term, the underlying emotion regulation deficits remain unaddressed. Emotion regulation is the process whereby individuals influence the onset, duration and intensity of emotions [
65]. Deficits in emotion regulation are implicated in emotional eating [
66,
67,
68]. Past research consistently demonstrated that emotional eating is associated with poor emotion regulation abilities [
69] and reliance on maladaptive emotion regulation strategies such as suppression and avoidance [
67,
68,
70]. The affect regulation model posits that emotional eating is a learned behaviour aiming to downregulate negative emotional states through the consumption of foods and this behaviour is maintained over time through negative reinforcement [
71,
72]. GLP-1RAs may reduce emotional eating, thus eating is less likely to be used to cope with stress and emotions [
26].
However, little is known whether GLP-1RAs can alleviate emotion regulation deficits that drive emotional eating. When experiencing distress, individuals who take GLP-1RAs may still struggle with managing their emotions, continue to rely on maladaptive emotion regulation strategies when no adaptive strategies are available, or turn to other means to regulate emotions such as engaging in compulsive behaviours. Future research could use EMA to examine real-time changes in emotions, emotion regulation difficulties and eating behaviours during GLP-1 treatment. Furthermore, GLP-1RA treatment could be integrated with emotion regulation-focused therapies, such as Enhanced CBT [
73] or Dialectical Behaviour Therapy [
74,
75], with dose titration aligned to therapy milestones.
Taken together, the current evidence suggests that GLP-1RAs may improve the regulation of eating behaviour, particularly emotional eating, in the short term, but no research has yet examined whether GLP-1RAs can improve the regulation of emotions. Examining mental health outcomes will provide insights into whether GLP-1RAs are associated with broader psychological benefits.
6. Mental Health and Quality of Life
A recent meta-analysis of eight randomized clinical trials found that GLP-1RA treatment was associated with better mental health-related, physical health-related and weight-related quality of life [
43]. Patients receiving GLP-1RA treatment reported greater quality of life, when compared with those receiving insulin treatment [
76]. However, the findings on the association between GLP-1RAs and mental health outcomes are inconsistent.
Research suggests that GLP-1RAs reduce depressive symptoms by promoting neurogenesis and synaptic plasticity [
17]. Although extant animal studies showed the anti-depressant effect of GLP-1RAs, human research yielded mixed findings [
7,
77]. A meta-analysis of five randomized controlled trials and one prospective cohort study involving 2071 individuals with type 2 diabetes mellitus or Parkinson’s disease found significant reduction in depressive scores among patients who received GLP-1RA treatment compared to controls [
78]. While this study reported the anti-depressant effect of GLP-1RAs [
78], other studies reported different findings. A pharmacovigilance analysis indicated that depression was the most reported adverse event [
79]. Similarly, another study found that patients on GLP-1RAs had a heightened risk for depression than controls [
80]. In contrast, other research reported no significant association between GLP-1RA treatment and depressive symptom change [
43,
81].
Existing evidence does not indicate an increased risk of suicidality associated with GLP-1RA treatment. A meta-analysis of randomized controlled trials found no elevated risk of suicidality among individuals with diabetes or higher weight receiving GLP-1RA treatment [
13]. However, the association between GLP-1RA usage and suicidality may vary depending on the specific medication and dimension of suicidality assessed (e.g., thoughts, behaviours). For instance, a pharmacovigilance analysis reported an increased risk of suicidal ideation associated with certain GLP-1RAs such as semaglutide, liraglutide and tirzepatide, while also identifying a decreased risk of suicidal attempts for some GLP-1RAs such as semaglutide, dulaglutide and liraglutide [
82]. In a cohort study, the association between GLP-1RA therapy and increased risk for suicidality among patients with type 2 diabetes turned non-significant, after controlling for confounding factors [
16]. To date, regulatory reviews have not confirmed a causal link between GLP-1RA use and suicidality, but ongoing clinical monitoring and further research are still warranted [
15].
Findings from semi-structured interviews indicated that participants with higher weight or type 2 diabetes perceived a positive impact of GLP-1RAs on their mental health, such as mood improvements and increased self-esteem [
83]. A study analysing comments of social media users across platforms including Reddit, YouTube and TikTok revealed mixed experiences with GLP-1RA treatment on mental health condition, with both improved and worsened mood reported [
84].
Collectively, the current research highlights the complexity of GLP-1RAs’ impact on mental health. It remains unclear whether mood improvement is due to GLP-1RA administration or instead a psychosocial reaction to rapid weight loss [
83]. Future studies using trajectory analyses are needed to distinguish the direct GLP-1RA drug effects from psychosocial factors associated with weight loss.
Moreover, the association between mood symptoms, suicidality and GLP-1RAs is often confounded by pre-existing mental health conditions, which are prevalent in individuals living with higher weight [
85]. Hence, future research should examine these associations in cohorts with psychiatric comorbidity, such as major depressive disorder, obsessive-compulsive disorder and attention-deficit/hyperactivity disorder. Finally, mixed-methods research that combines quantitative and qualitative data could provide a more comprehensive understanding of the psychological impacts of GLP-1RA treatment that may not be captured through quantitate research alone.
7. Body Image and Self Perception
Body image is a multidimensional construct encompassing perceptual, cognitive–affective, and behavioural elements [
86] and is a crucial determinant of well-being in individuals undergoing weight change. A consistent finding across ED research is that these individuals experience not only disturbance in cognitive-affective body image (e.g., dissatisfaction), but also perceptual distortions (e.g., body size overestimation) [
87]. Critically, weight loss (including that which is rapid) does not automatically resolve body dissatisfaction or perceptual distortion [
87]. Instead, ED patients often continue to experience a form of “phantom fat”, whereby internal models of body size remain resistant to updating despite substantial physical change.
Theoretical accounts help explain this dissociation: predictive coding models suggest that self-perception, from a neuro-computational lens, arises from the integration of bottom-up sensory evidence with top-down priors (beliefs) [
88]. Within this framework, distorted self-perception, such as in the case of body size overestimation in EDs, can arise when there is an overreliance on distorted priors (i.e., beliefs about one’s body shape/weight; e.g., “I am fat”) over bottom-up sensory evidence (e.g., seeing a thin body in the mirror or feeling one’s protruding ribs) [
89].
The allocentric lock hypothesis offers a complementary account, suggesting that individuals with EDs remain “locked” into maladaptive body memories that resist updating despite current (egocentric) sensory input [
90]. These frameworks suggest that rapid pharmacologically induced weight loss, such as with GLP-1RA treatments, may not straightforwardly translate into improved body satisfaction: and instead, could interact with these same mechanisms, potentially destabilizing body perception or amplifying pre-existing misperception.
Empirical evidence in this domain remains scarce. One study with U.S. undergraduates (
N = 225) found that greater interest in GLP-1RAs was associated with higher body shame, body surveillance, weight concerns, anti-fat bias, and disordered eating behaviours, alongside lower body appreciation and neutrality [
10]. While limited by its cross-sectional, non-clinical design, the findings underscore that those most attracted to GLP-1RA treatments may be the same individuals most vulnerable to body image disturbance and disordered eating risk. Notably, higher body appreciation buffered against interest in treatment despite side effects, highlighting a potential protective factor.
Indirect evidence from EDs and body image disturbance research underscores the importance of these concerns. Patients with AN and related disorders continue to overestimate body size even after significant weight change, with perceptual distortion predicting poorer prognosis. In recent decades, researchers have increasingly implicated impairments in multisensory integration—the fundamental process underlying self-perception which involves the continuous integration of sensory inputs across different modalities (e.g., exteroception [vision, touch], interoception, proprioception) [
91,
92]—in these disturbances [
89,
93].
Specifically, interoceptive dysfunction (i.e., impaired awareness of internal bodily sensations like hunger) is well documented in EDs [
94] and is believed to lead to an overreliance on external (visual) cues, rendering self-perception malleable but unstable. Research involving multisensory body illusion paradigms, such as virtual full-body illusions [
95], has provided experimental evidence that EDs are linked to such disturbances in multisensory integration (on the basis of greater susceptibility to illusion-induced distortions; [
93]).
Extrapolating from this work, GLP-1RA-induced metabolic and interoceptive changes could exacerbate or reinforce perceptual distortions (exteroceptive-interoceptive mismatches): heightening reliance on unstable external/visual cues. From a predictive coding perspective, if “priors” about body size are rigid, bottom-up evidence (weight loss) may not suffice, and consequently, GLP-1RAs may inadvertently heighten mismatches between perception and reality.
Important gaps remain. First, no longitudinal studies have tracked body image trajectories across initiation, plateau, maintenance, or discontinuation of GLP-1RA therapy. Second, future research should examine not only cognitive-affective components of body image (e.g., self-reported body dissatisfaction, body appreciation) but also perceptual components through more objective measures (e.g., body size estimation, interoceptive awareness [heart-beat counting tasks]).Third, researchers should consider qualitative exploration of lived experience and identity shifts (e.g., “medication-enabled” vs. “self-driven” change).
Finally, future research should examine how GLP-1RA therapy intersect with interoceptive processing. Multisensory body illusion paradigms, such as virtual full-body illusions [
95], offer powerful experimental tools to test how GLP-1RA users integrate interoceptive and exteroceptive cues when perceiving their body, and importantly, whether there are distortions in this domain (i.e., whether rapid weight loss recalibrates or amplifies distorted self-perception).
8. Social Media and Digital Culture
Social media has become a primary arena where narratives about GLP-1RA treatments are constructed and disseminated [
96,
97]. Hashtags such as #Ozempic, #Wegovy, and #Mounjaro frame these medications less as clinical tools and more as aesthetic enhancers, often through before/after imagery and “quick-fix” discourses [
98]. Counter-narratives of “cheating” or moralisation also circulate, underscoring the contested identity politics of pharmacologically enabled weight loss [
99]. From a body image perspective, these dynamics are particularly concerning for adolescents and individuals vulnerable to EDs, for whom appearance-focused content and transformation imagery are known to heighten social comparison, body dissatisfaction, and disordered eating risk [
100,
101].
Three empirical studies illustrate how digital culture surrounding GLP-1RA amplifies these dynamics. Propfe and Seifert [
97] analysed over 46,000 Reddit posts, identifying off-label weight loss as the dominant theme, with limited mention of health risks. Instead, peer-to-peer discourse centred on dosing, insurance denial, and side-effect management, highlighting both the popularity of off-label use and the absence of balanced information. Another study examined 100 TikTok videos (#Ozempic, approximately 70M views), finding most were consumer-generated, emphasising personal use and weight loss. Over a third portrayed the drug positively or encouraged uptake, while very few addressed off-label use, shortages, or clinical alternatives such as bariatric surgery [
96].
Finally, Fong et al. [
102] compared multiple platforms, showing semaglutide content was presented almost exclusively for weight reduction, with frequent misrepresentation of mechanism, indications, and side effects. Strikingly, common gastrointestinal problems went largely unmentioned on Instagram, where female, non-medical users dominated. They also documented AI-modified “results” videos and marketing of counterfeit products under GLP-1 hashtags. Together, these studies demonstrate that digital spaces may amplify desirability while minimising risk, with platform-specific differences in reliability (e.g., YouTube most accurate, Instagram least).
Evidence from broader ED and body image research reinforces these concerns. Studies consistently show that exposure to appearance-focused or “transformation” content on social media is linked to lower body appreciation, higher body dissatisfaction, and greater risk of disordered eating [
100,
101]. It is plausible that these risks may be amplified by algorithmic promotion of sensational content and the rise of AI-modified images. For example, when users are repeatedly exposed to highly idealised and artificially enhanced body images, paired with sensationalised narratives about GLP-1 “success stories”, they may internalise unattainable appearance standards. Subsequently, this may fuel body dissatisfaction and drive maladaptive behaviours, such as restrictive eating or pursuing cosmetic procedures, to meet these unrealistic ideals.
Yet, important gaps remain. Most existing studies have focused on describing what is posted online, rather than testing how this content affects people. For example, it is unclear whether repeated exposure to TikTok “before-and-after” videos or AI-enhanced transformation images directly increases body dissatisfaction or motivates interest in GLP-1RA use. Future research should therefore consider combining large-scale social listening with controlled experiments: for example, where participants are exposed to different types of GLP-1 content (e.g., risk-focused vs. transformation-focused) to examine causal effects on body image, self-perception, and disordered eating risk. Finally, qualitative work is also needed to capture the lived experiences of users, such as how they negotiate online narratives of “quick fixes” versus “cheating” or how reliance on medication shapes their sense of identity.
At a policy level, action is required to address misleading and potentially harmful content. Platforms could be mandated to regulate promotional claims, flag or remove fake/false GLP-1RA advertisements, and provide users with clear disclaimers when AI-modified images are used (e.g., in weight loss videos). Without such measures, digital culture may continue to amplify misinformation, normalise risky off-label use, and exacerbate body image vulnerabilities, particularly among adolescents and individuals already prone to body image and eating disturbances.
9. Weight Stigma
Weight stigma is another important issue in the conversation around GLP-1RA drugs. On the surface, these medications may help to reduce individual blame by framing higher weight as a medical condition. However, GLP-1RAs may also reinforce and perpetuate stigma in new ways, such as people being seen as “taking the easy way out,” or the judgment that weight regain after stopping treatment signals “failure.” These issues are not only personal, but also layered and intersectional, shaped by gender, race, and socioeconomic status.
The emerging empirical literature provides valuable but limited insights. Post and Persky [
103] showed that individuals who lost weight with GLP-1RAs were judged more negatively than those who used diet/exercise, mainly due to stronger beliefs that these individuals were taking a shortcut. These negative judgments applied to both larger-bodied and lean women, though lean women were sometimes judged even more strongly. Somewhat similarly, Post et al. [
104] found that GLP-1RA users were evaluated more negatively due to downward social comparison processes. Interestingly, diet/exercise narratives were not without problems either, as they sometimes led observers to report more harmful thoughts about their own eating and exercising, highlighting the broader effects of weight loss messaging.
Bachmakova et al. [
105] extended this by showing that weight loss through diet/exercise was viewed as the most effortful and praiseworthy, while Ozempic use was seen as least effortful, least admirable, and less tied to meaningful personal change. Even when combined with lifestyle changes, Ozempic use reduced how much others thought someone had “really changed.” Finally, Tomiyama [
106] offered a commentary highlighting the ambivalence within psychology and medicine about whether these drugs are a step forward or a step back for reducing stigma.
Taken together, these studies suggest that GLP-1RA users may face a “double-edged sword”. On the one hand, they are criticised for not working hard enough, while on the other hand, traditional lifestyle approaches also carry stigma and can trigger unhealthy comparison. What is missing, however, is research on lived experience: that is, how people using GLP-1RAs feel about themselves, how stigma plays out in everyday life and healthcare, and how affordability and access create new divides.
Future work should consider extending beyond experimental studies predominately involving quantitative assessments, to qualitative and longitudinal studies, such as those capturing how GLP-1RA users navigate stigma across contexts (e.g., in clinical, workplace, and digital contexts), and over time. Particular attention should be given to structural stigma, including that surrounding affordability, which risks positioning GLP-1RAs as interventions “only for the rich”. Intersectional analyses are also important, given that certain gender and racial minority groups (e.g., women and people of colour), as well as lower-socioeconomic status groups may be disproportionately stigmatised or excluded. Unless these issues are addressed, the social stigma surrounding GLP-1RAs may undermine the benefits that these drugs could bring. The next section explores these sociocultural and equity-related dimensions in greater depth, examining how stigma, access, and representation shape both the clinical and psychological impact of GLP-1RA use.
10. Costs and Inequalities
Direct-to-consumer prices of GLP-1RAs remain prohibitively high in most countries. In the United States, monthly costs typically range from USD 500–1,200 (up to ~USD 9,000 annually) when not covered by insurance [
107], while in Australia, private prescriptions for weight loss commonly cost AUD 130–600 per month [
108,
109]. Similar or higher prices are reported for newer agents such as tirzepatide, with monthly costs between AUD 345–645 [
110]. These figures indicate that, without subsidy or insurance coverage, GLP-1RAs remain largely affordable only to wealthier individuals.
This financial barrier is particularly troubling given that higher weight disproportionately affects people from lower socioeconomic backgrounds [
111]. In high-income countries, lower income, education, and occupational status are consistently linked with higher weight rates, while in low- and middle-income countries overweightness is rising fastest among disadvantaged groups as food environments and urban lifestyles change [
112,
113]. Thus, those most likely to live in larger bodies are often the very groups least able to afford these medications.
The result is a risk of entrenching a two-tiered system in which affluent or well-insured individuals access advanced pharmacological weight loss treatments, while disadvantaged groups remain excluded or reliant on less resourced interventions. This raises the critical question of whether GLP-1RAs are becoming “treatments only for the rich.” Without deliberate strategies such as subsidies, tiered pricing, or public health benefit schemes, the drugs may inadvertently deepen health inequities and reinforce structural stigma, particularly for those already marginalised by race, gender, or socioeconomic disadvantage
11. Special Populations and GLP-1 Usage
The increasing prescription of GLP-1RAs beyond adults with higher weight and type 2 diabetes highlights important questions about safety, developmental impacts, and equity of access. Two groups stand out: adolescents and young adults, and populations affected by racial, ethnic, and socioeconomic disparities.
11.1. Adolescents and Young Adults
Use of GLP-1 RAs in adolescents and young adults has risen sharply, with U.S. dispensing increasing 600% between 2020 and 2023 [
114]. Liraglutide and semaglutide are approved for individuals aged ≥12 years, while tirzepatide is approved for ≥18 years by the Food and Drug Administration. Clinical trials show weight reduction efficacy in adolescents with higher weight or type 2 diabetes [
115,
116,
117]. Meta-analyses of paediatric cohorts confirm short-term benefits [
118,
119].Yet, developmental safety remains unclear. Adolescence is a critical period for growth, bone mineralisation, and muscle accrual [
120,
121]. One trial found that GLP-1 RA therapy alone reduced bone density, while exercise combined with treatment preserved it [
122]. Data on adolescent-specific body composition are still missing.
At the same time, adolescence is also the peak risk period for EDs, disordered eating, and body image disturbance [
123]. Evidence suggests that young men using prescription weight-loss drugs, mostly GLP-1RAs, reported higher rates of binge eating, purging, and non-prescribed use [
124]. Risk factors such as weight-sensitive sports and social media exposure may heighten susceptibility [
84,
125], but have not yet been assessed systematically in relation to GLP-1 treatment.
Psychiatric safety is another concern. While regulators have reviewed GLP-1RAs against the backdrop of rising youth suicide rates, current trial and pharmacovigilance data have not demonstrated a causal link. Notably, a large cohort study found a lower risk of suicidal ideation and attempts in adolescents prescribed GLP-1RAs compared with behavioural interventions [
126]. These findings support psychiatric monitoring during treatment but do not suggest contraindication.
11.2. Racial, Ethnic, and Socioeconomic Disparities
Access to GLP-1RAs shows clear sociodemographic divides. Use is consistently lower among Asian, Black, and Hispanic adults compared with White adults, and among lower-income households compared with higher-income households [
127,
128]. This is despite higher weight prevalence and weight-related health complications among Black populations, particularly women. [
129].
Stigma compounds these inequities. Black women with higher weight report more stigmatisation than White women with similar BMIs [
130,
131]. Experiments further suggest that exposure to weight-loss information may elicit different psychological risks across groups: race/ethnicity predicts vulnerability to binge eating, while income predicts restrictive eating [
104].These disparities also appear early. Childhood overweightness disproportionately affects racial and ethnic minority groups [
132]. Socioeconomic disadvantage, food insecurity, and targeted marketing contribute to these disparities [
20], while barriers to paediatric treatment access persist for children as young as 3–10 years [
133].
Recent data show that from 2003 to 2021, perceived overweight and weight-loss attempts among U.S. high school students increased significantly among male, Black, and Hispanic youth—with steeper increases than among White or female students [
134]. These trends challenge assumptions that body image concerns predominantly affect White females and underscore the need for culturally responsive prevention strategies.
Together, these findings point to the need for equitable implementation of GLP-1RAs. Without attention to structural determinants, there is a risk that these drugs will deepen existing health inequities rather than reduce them. Importantly, these developmental, social, and structural factors reinforce that GLP-1 use cannot be understood in isolation: they must be situated within a broader biopsychosocial framework that accounts for biological, psychological, and social influences on health and illness.
12. Beyond Appetite Regulation: Biopsychosocial Pathways of GLP-1 Use
Our review has shown that GLP-1RAs exert effects across multiple domains that extend well beyond appetite regulation. Evidence (see
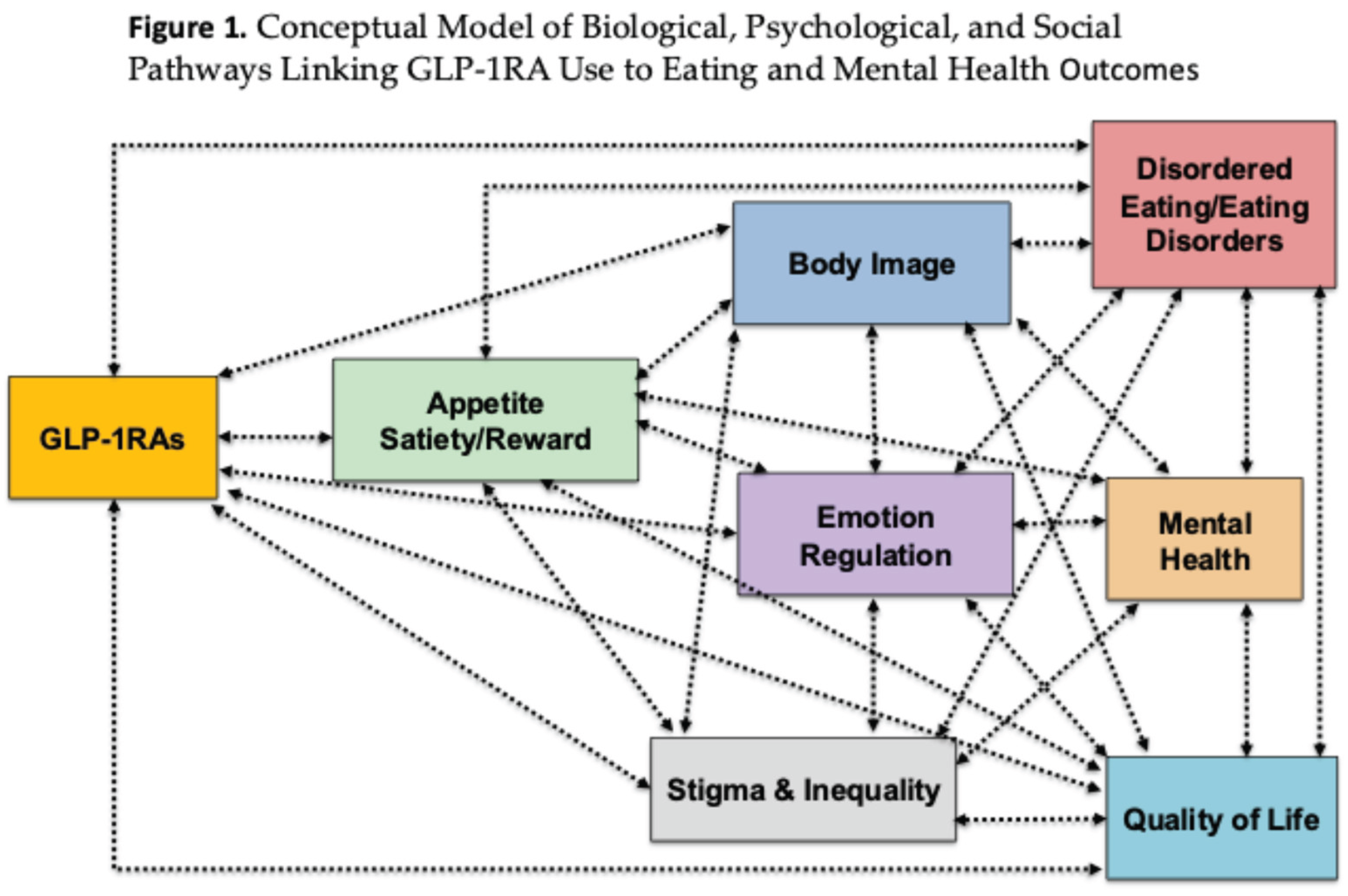
Table 1) suggests potential benefits such as reduced cravings, improvements in emotion regulation, and decreases in binge eating, alongside broader impacts on body image and quality of life. At the same time, uncertainties remain regarding long-term neural changes, risks in individuals with restrictive EDs, and broader mental health effects—including suicidality, mood, and anxiety—as well as the sociocultural consequences of use, such as stigma and inequities in access. These complexities highlight the importance of careful clinical oversight and the need for further research to clarify both the benefits and risks of GLP-1RA therapy across diverse populations.
Figure 1 provides a conceptual model of the biological, psychological, and social pathways linking GLP-1RA use to eating- and mental health–related outcomes. This figure outlines how GLP-1RAs exert effects across multiple domains. At the biological level, GLP-1RAs modulate appetite, satiety, and reward pathways, which are interdependent and jointly influence downstream processes. These biological changes extend into psychological domains, including eating behaviours, emotion regulation, and body image, each of which is known to play a central role in eating disorder risk and maintenance. Alterations in these domains can contribute to clinical outcomes such as disordered eating and EDs and mental health difficulties. These outcomes are presented as bidirectionally related, reflecting evidence that disordered eating both arises from and contributes to mental health problems. Importantly, the figure highlights that stigma and inequality permeate all stages of this pathway, shaping access to treatment, amplifying psychological vulnerabilities, and influencing both the risks and benefits associated with GLP-1RA use. Taken together, the model illustrates the need to conceptualise GLP-1RA therapy within a biopsychosocial framework, emphasising the interplay between biological mechanisms, psychological processes, and social context in determining outcomes.
13. Clinical Implications for Managing Psychological Effects of GLP-1 Use
Building on the emerging evidence, it is critical to translate research findings into clinical practice and consider how psychological effects of GLP-1RA use can be assessed, monitored, and managed. While these agents offer metabolic and behavioural benefits, their influence on mood, eating behaviours, identity, and stigma means that careful psychological oversight must be embedded in treatment pathways.
Prior to initiating therapy, clinicians should conduct comprehensive psychiatric and behavioural assessments to establish baseline vulnerabilities. These assessments should screen for mood and anxiety symptoms, suicidality, and disordered eating, while also eliciting information on compensatory strategies, body image concerns, and emotion regulation patterns. Because GLP-1RAs directly alter hunger and satiety, it is useful to explore patients’ sensitivity to internal cues of hunger and fullness, as these may shape adaptation once appetite suppression begins. Once treatment is underway, ongoing monitoring becomes essential. Follow-up consultations should evaluate changes in eating behaviours, mood, and suicidal ideation, with heightened vigilance during dose escalations or reductions, which appear to be periods of greater vulnerability [
15]. Multidisciplinary collaboration between prescribers, psychologists, psychiatrists, and dietitians is especially important to ensure early detection of adverse effects and prompt referral where needed. Attention must also be paid to the psychosocial meanings of GLP-1RA use. Rapid, medically assisted weight loss can trigger internal conflict and intensify exposure to stigma, particularly as cultural narratives often frame pharmacological treatment as “cheating” or “taking the easy way out” [
103,
105]. Clinicians can help reframe GLP-1RA therapy as a legitimate medical intervention, provide psychoeducation, and draw on compassion-focused approaches to counter shame and self-criticism. Peer networks or group-based interventions may offer valuable normalisation and support, while individual therapy can focus on developing communication strategies and resilience against unsolicited judgments or harmful social comparison [
99].
A further clinical priority is the prevention and management of disordered eating. Appetite suppression may inadvertently reinforce restrictive behaviours, while discontinuation or dose reduction can precipitate reactive bingeing. Regular screening for maladaptive food rules, compensatory behaviours, or binge urges is therefore essential [
6,
11]. When such difficulties emerge, targeted psychological interventions such as enhanced CBT (CBT-E, [
135,
136]) can be effective in challenging distorted beliefs about weight, shape, and food. Collaboration with dietitians is recommended to support balanced eating patterns that meet nutritional needs despite reduced appetite, while mindful or intuitive eating practices may be cautiously adapted to account for altered satiety signals.
Because many patients rely on food for emotion regulation, appetite suppression may leave them vulnerable to emotional distress. Clinicians should therefore introduce alternative coping strategies through skills-based interventions, such as dialectical behaviour therapy [
75], acceptance and commitment therapy, or mindfulness practices, which strengthen emotion regulation and psychological flexibility. Systematic mood tracking is advisable, as evidence suggests GLP-1RAs may have both beneficial and adverse effects on mood and cognition [
17,
78].
Given the ongoing uncertainty regarding associations between GLP-1RAs and suicidality, direct inquiry into suicidal thoughts or behaviours should form part of every assessment. Structured safety planning and urgent referral may be necessary when risk is identified, particularly around treatment initiation or dose changes, when psychological destabilisation is most likely [
13,
16].
Special consideration is also required for young adults and people from diverse ethnic/cultural backgrounds. Young adults have been found to be especially vulnerable to body image concerns [
137], and peer pressure surrounding weight loss [
138], making it important to address identity formation and self-esteem alongside physical health goals. For individuals from different cultural and ethnic groups, perceptions of weight, health, and pharmacological treatment may vary widely, and stigma can intersect with cultural expectations and healthcare inequities. Clinicians should therefore adopt a culturally responsive approach, exploring how GLP-1RA use aligns with patients’ values and lived experiences, while remaining sensitive to mistrust in medical systems and disparities in access. Incorporating culturally adapted psychoeducation, engaging family or community supports where appropriate and ensuring inclusivity in treatment models can help safeguard psychological wellbeing and promote equitable care.
Taken together, these implications highlight the importance of integrating psychological assessment and care into GLP-1RA treatment. By reframing therapy to reduce stigma, actively preventing disordered eating, supporting emotion regulation, monitoring suicidality, and tailoring care to developmental and cultural contexts, clinicians can ensure that pharmacological innovation is matched with psychological safeguards, thereby maximising both safety and long-term benefit.
14. Limitations and Future Directions
Despite an explosion of GLP-1RA research in recent years, substantial methodological gaps persist, and rigorous evidence on the drugs’ psychological impacts remains limited. First, a major limitation across the literature is the predominance of short-term and cross-sectional research. Most studies focus on weight loss during initial treatment phases, with few extending into weight maintenance or discontinuation. This narrow focus makes it difficult to determine the durability of appetite, eating behaviour, body image, or mental health changes associated with GLP-1RAs. To address this, future research should adopt longitudinal designs that capture trajectories of psychological, behavioural, and clinical outcomes over months and years rather than weeks.
Second, the measures used to evaluate appetite, eating behaviour, emotion regulation, and body image are often limited to subjective self-reports such as questionnaires and food diaries. These approaches may be prone to bias and fail to capture micro-level changes in eating behaviour, real-time emotion regulation, or perceptual aspects of body image. Future research should incorporate more rigorous and innovative methods—such as EMA, bite-by-bite micro-phenotyping, interoceptive and multisensory body illusion tasks, and neuroimaging—to provide a fuller picture of the mechanisms through which GLP-1RAs act.
Third, there is considerable inconsistency across findings, in part because of variation in populations studied. Most trials are restricted to individuals with higher weight and type 2 diabetes, with little exploration in younger cohorts, non-obese individuals, or those with psychiatric comorbidities. This limits generalisability and obscures potential moderators of response, such as baseline emotional eating or perfectionism. Large, multi-site randomized controlled trials in diverse populations are therefore needed to establish efficacy, safety, and subgroup-specific effects.
Fourth, there has been limited use of co-design or participatory approaches. Research is rarely conducted in collaboration with patients, carers, or clinicians, which means the lived experience of GLP-1RA users—including stigma, identity changes, or the psychosocial impact of digital narratives—remains underexplored. Engaging stakeholders directly through co-design will help ensure that measures capture outcomes that matter most to users and will facilitate the development of ethically and socially responsive interventions.
Fifth, while there is growing interest in the psychiatric and behavioural implications of GLP-1RAs, the field remains fragmented, with different groups studying appetite, emotion regulation, body image, stigma, or social media in isolation. This siloed approach risks duplication and missed opportunities for synthesis. Establishing an interdisciplinary steering group could provide a structured research agenda, harmonise measures across trials, and integrate biological, psychological, and social dimensions. Such a coordinated effort would accelerate progress and provide policymakers with robust evidence to guide safe and equitable implementation.
15. Conclusions
In conclusion, GLP-1RAs show clear promise in improving metabolic outcomes and short-term eating regulation, but their broader psychological effects remain insufficiently understood. Current findings highlight both potential benefits and serious risks across eating behaviour, mood, body image, stigma, and equity of access. Yet most studies are short-term, narrowly sampled, and methodologically limited. Without robust longitudinal data in diverse populations, we cannot know whether these drugs provide a safe and sustainable solution—or whether early gains mask long-term harms. Until such evidence emerges, GLP-1RAs must be approached with caution: integrated into care with rigorous monitoring, psychological support, and policies that address inequities, while remaining alert to the possibility that today’s promise could become tomorrow’s problem.
Author Contributions
I.K.—Conceptualization, original draft preparation, review and editing; A.B.D.—writing part on appetite and eating behaviours and eating disorders, review and editing; Y.W.—writing part on emotion regulation and mental health and quality of life, review and editing; J.P. —writing part on body image; stigma and social media, review and editing; K.Y.—writing part on special populations, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All available data is presented in the review.
Acknowledgments
During the preparation of this manuscript, the author(s) used ChatGPT to refine language. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
Anorexia Nervosa (AN)
Binge Eating Disorder (BED)
Binge Eating Scale (BES)
Body Mass Index (BMI)
Bulimia Nervosa (BN)
Cognitive Behaviour Therapy (CBT)
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)
Eating Disorders (EDs)
Ecological Momentary Assessment (EMA)
Glucagon-Like Peptide-1 (GLP-1)
Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RAs)
Other Specified Feeding or Eating Disorders (OSFED)
References
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, K.S.; Choi, H.J. Glucagon-Like Peptide-1 and Hypothalamic Regulation of Satiation: Cognitive and Neural Insights from Human and Animal Studies. Diabetes Metab J 2025, 49, 333–347. [Google Scholar] [CrossRef]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am J Med 2025, 138, 934–940. [Google Scholar] [CrossRef]
- Skowron, K.; Kurnik-Łucka, M.; Dadański, E.; Bętkowska-Korpała, B.; Gil, K. Backstage of Eating Disorder-About the Biological Mechanisms behind the Symptoms of Anorexia Nervosa. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Webster, A.N.; Becker, J.J.; Li, C.; Schwalbe, D.C.; Kerspern, D.; Karolczak, E.O.; Bundon, C.; Onoharigho, R.A.; Crook, M.; Jalil, M.; et al. Molecular Connectomics Reveals a Glucagon-Like Peptide 1 Sensitive Neural Circuit for Satiety. bioRxiv 2024. [Google Scholar] [CrossRef]
- Radkhah, H.; Rahimipour Anaraki, S.; Parhizkar Roudsari, P.; Arabzadeh Bahri, R.; Zooravar, D.; Asgarian, S.; Hosseini Dolama, R.; Alirezaei, A.; Khalooeifard, R. The impact of glucagon-like peptide-1 (GLP-1) agonists in the treatment of eating disorders: a systematic review and meta-analysis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity 2025, 30, 10. [Google Scholar] [CrossRef]
- Tempia Valenta, S.; Nicastri, A.; Perazza, F.; Marcolini, F.; Beghelli, V.; Atti, A.R.; Petroni, M.L. The Impact of GLP-1 Receptor Agonists (GLP-1 RAs) on Mental Health: A Systematic Review. Current Treatment Options in Psychiatry 2024, 11, 310–357. [Google Scholar] [CrossRef]
- Koide, Y.; Kato, T.; Hayashi, M.; Daido, H.; Maruyama, T.; Ishihara, T.; Nishimura, K.; Tsunekawa, S.; Yabe, D. Association between eating behavior patterns and the therapeutic efficacy of GLP-1 receptor agonists in individuals with type 2 diabetes: a multicenter prospective observational study. Frontiers in Clinical Diabetes and Healthcare, 2025. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduction and Targeted Therapy 2024, 9, 234. [Google Scholar] [CrossRef]
- Markey, C.H.; August, K.J.; Malik, D.; Richeson, A. Body image and interest in GLP-1 weight loss medications. Body Image 2025, 53, 101890. [Google Scholar] [CrossRef]
- Bartel, S.; McElroy, S.L.; Levangie, D.; Keshen, A. Use of glucagon-like peptide-1 receptor agonists in eating disorder populations. Int J Eat Disord 2024, 57, 286–293. [Google Scholar] [CrossRef]
- Aoun, L.; Almardini, S.; Saliba, F.; Haddadin, F.; Mourad, O.; Jdaidani, J.; Morcos, Z.; Al Saidi, I.; Bou Sanayeh, E.; Saliba, S.; et al. GLP-1 receptor agonists: A novel pharmacotherapy for binge eating (Binge eating disorder and bulimia nervosa)? A systematic review. Journal of Clinical & Translational Endocrinology 2024, 35, 100333. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Batlle, J.C.; Ayati, A.; Maqsood, M.H.; Long, C.; Tarabanis, C.; McGowan, N.; Liebers, D.T.; Laynor, G.; Hosseini, K.; et al. Suicide and Self-Harm Events With GLP-1 Receptor Agonists in Adults With Diabetes or Obesity: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2025, 82, 888–895. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Statement on Ongoing Review of GLP-1 Receptor Agonists. Available online: https://www.ema.europa.eu/en/news/ema-statement-ongoing-review-glp-1-receptor-agonists (accessed on 26 September 2025).
- U.S. Food and Drug Administration. Update on FDA’s ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/update-fdas-ongoing-evaluation-reports-suicidal-thoughts-or-actions-patients-taking-certain-type (accessed on 26 September 2025).
- Shapiro, S.B.; Yin, H.; Yu, O.H.Y.; Rej, S.; Suissa, S.; Azoulay, L. Glucagon-like peptide-1 receptor agonists and risk of suicidality among patients with type 2 diabetes: active comparator, new user cohort study. Bmj 2025, 388, e080679. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Rasgon, N.; Goldberg, J.; Wong, S.; Le, G.H.; Mansur, R.B.; Rosenblat, J.D.; Teopiz, K.M.; Stahl, S.M. The effect of glucagon-like peptide-1 and glucose dependent insulinotropic polypeptide receptor agonists on neurogenesis, differentiation, and plasticity (Neuro-GDP): potential mechanistically informed therapeutics in the treatment and prevention of mental disorders. CNS Spectr 2025, 30, e23. [Google Scholar] [CrossRef]
- ABC News. As athletes like Serena Williams promote Ozempic, experts say our obsession with weight loss is worse than ever. Available online: https://www.abc.net.au/news/2025-08-31/ozempic-glp1-serena-williams-diet-culture/105698136 (accessed on 26 September 2025).
- Holtrop, J.S.; Tietbohl, C.; Perreault, L.; Connelly, L.; Smith, P.C.; Williams, J. Primary care patient and practice member perspectives on weight loss medications: challenges and opportunities. Front Med (Lausanne) 2025, 12, 1584799. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, J.S.; Hwang, E.; Park, M.J.; Shin, H.Y.; Lee, Y.H.; Kim, K.M.; Gautron, L.; Godschall, E.; Portillo, B.; et al. GLP-1 increases preingestive satiation via hypothalamic circuits in mice and humans. Science 2024, 385, 438–446. [Google Scholar] [CrossRef]
- Eren-Yazicioglu, C.Y.; Kara, B.; Sancak, S.; Uysal, S.P.; Yazici, D.; Okuroglu, N.; Whitton, A.E.; Rutherford, A.V.; Yapici-Eser, H. Effect of Exenatide Use on Cognitive and Affective Functioning in Obese Patients With Type 2 Diabetes Mellitus: Exenatide Use Mediates Depressive Scores Through Increased Perceived Stress Levels. J Clin Psychopharmacol 2021, 41, 428–435. [Google Scholar] [CrossRef]
- Christensen, S.; Robinson, K.; Thomas, S.; Williams, D.R. Dietary intake by patients taking GLP-1 and dual GIP/GLP-1 receptor agonists: A narrative review and discussion of research needs. Obes Pillars 2024, 11, 100121. [Google Scholar] [CrossRef]
- Ingves, S.; Vilhelmsson, N.; Ström, E.; Fredrikson, M.; Guldbrand, H.; Nystrom, F.H. A randomized cross-over study of the effects of macronutrient composition and meal frequency on GLP-1, ghrelin and energy expenditure in humans. Peptides 2017, 93, 20–26. [Google Scholar] [CrossRef]
- Hsu, T.M.; Hahn, J.D.; Konanur, V.R.; Lam, A.; Kanoski, S.E. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 2015, 40, 327–337. [Google Scholar] [CrossRef]
- Aldawsari, M.; Almadani, F.A.; Almuhammadi, N.; Algabsani, S.; Alamro, Y.; Aldhwayan, M. The Efficacy of GLP-1 Analogues on Appetite Parameters, Gastric Emptying, Food Preference and Taste Among Adults with Obesity: Systematic Review of Randomized Controlled Trials. Diabetes Metab Syndr Obes 2023, 16, 575–595. [Google Scholar] [CrossRef] [PubMed]
- Cheney, C.; Hunter, K.; Klein, M. Impact of GLP-1 Receptor Agonists on Perceived Eating Behaviors in Response to Stimuli. Diabetes Metab Syndr Obes 2025, 18, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.; Davies, M.; Dicker, D.; Lingvay, I.; Mosenzon, O.; Rubino, D.M.; Pedersen, S.D. Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: recommendations for clinical practice. Postgrad Med 2022, 134, 14–19. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Tan, Y.; Chen, Y.; Zhou, X.; Liu, S.; Yu, J. GLP-1RAs caused gastrointestinal adverse reactions of drug withdrawal: a system review and network meta-analysis. Front Endocrinol (Lausanne) 2023, 14, 1149328. [Google Scholar] [CrossRef]
- Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol Rev 2016, 68, 954–1013. [Google Scholar] [CrossRef]
- Rehfeld, J.F.; Knop, F.K.; Asmar, A.; Madsbad, S.; Holst, J.J.; Asmar, M. Cholecystokinin secretion is suppressed by glucagon-like peptide-1: clue to the mechanism of the adverse gallbladder events of GLP-1-derived drugs. Scand J Gastroenterol 2018, 53, 1429–1432. [Google Scholar] [CrossRef]
- Brierley, D.I.; Holt, M.K.; Singh, A.; de Araujo, A.; McDougle, M.; Vergara, M.; Afaghani, M.H.; Lee, S.J.; Scott, K.; Maske, C.; et al. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab 2021, 3, 258–273. [Google Scholar] [CrossRef]
- Hamed, K.; Alosaimi, M.N.; Ali, B.A.; Alghamdi, A.; Alkhashi, T.; Alkhaldi, S.S.; Altowarqi, N.A.; Alzahrani, H.; Alshehri, A.M.; Alkhaldi, R.K.; et al. Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists: Exploring Their Impact on Diabetes, Obesity, and Cardiovascular Health Through a Comprehensive Literature Review. Cureus 2024, 16, e68390. [Google Scholar] [CrossRef]
- Unruh, M.A.; Thompson, C.M. Competing Cultural Discourses of GLP-1 Agonists: An Application of Relational Dialectics Theory. Qual Health Res 2025, 10497323251326041. [Google Scholar] [CrossRef]
- Bossart, M.; Wagner, M.; Elvert, R.; Evers, A.; Hübschle, T.; Kloeckener, T.; Lorenz, K.; Moessinger, C.; Eriksson, O.; Velikyan, I.; et al. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell Metab 2022, 34, 59–74.e10. [Google Scholar] [CrossRef] [PubMed]
- Model, J.F.A.; Rocha, D.S.; Fagundes, A.D.C.; Vinagre, A.S. Physiological and pharmacological actions of glucagon like peptide-1 (GLP-1) in domestic animals. Vet Anim Sci 2022, 16, 100245. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, ed., 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, , Text Revision ed., 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Krug, I.; Liu, S.; Portingale, J.; Croce, S.; Dar, B.; Obleada, K.; Satheesh, V.; Wong, M.; Fuller-Tyszkiewicz, M. A meta-analysis of mortality rates in eating disorders: An update of the literature from 2010 to 2024. Clin Psychol Rev 2025, 116, 102547. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.B.; Hughes, E.K.; Dang, A.B.; Lai, H.Y.; Lee, J.; Liu, S.; Portingale, J.; Fuller-Tyszkiewicz, M.; Krug, I. Taking a Deeper Dive Into OSFED Subtypes: A Meta-Analysis and Systematic Review. Int J Eat Disord 2024, 57, 2006–2040. [Google Scholar] [CrossRef] [PubMed]
- Krug, I.; Dang, A.B.; Hughes, E.K. There is nothing as inconsistent as the OSFED diagnostic criteria. Trends Mol Med 2024, 30, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Gormally, J.; Black, S.; Daston, S.; Rardin, D. The assessment of binge eating severity among obese persons. Addict Behav 1982, 7, 47–55. [Google Scholar] [CrossRef]
- Da Porto, A.; Casarsa, V.; Colussi, G.; Catena, C.; Cavarape, A.; Sechi, L. Dulaglutide reduces binge episodes in type 2 diabetic patients with binge eating disorder: A pilot study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2020, 14, 289–292. [Google Scholar] [CrossRef]
- Pierret, A.C.S.; Mizuno, Y.; Saunders, P.; Lim, E.; De Giorgi, R.; Howes, O.D.; McCutcheon, R.A.; McGowan, B.; Sen Gupta, P.; Smith, D.; et al. Glucagon-Like Peptide 1 Receptor Agonists and Mental Health: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2025, 82, 643–653. [Google Scholar] [CrossRef]
- Richards, J.; Bang, N.; Ratliff, E.L.; Paszkowiak, M.A.; Khorgami, Z.; Khalsa, S.S.; Simmons, W.K. Successful treatment of binge eating disorder with the GLP-1 agonist semaglutide: A retrospective cohort study. Obesity Pillars 2023, 7, 100080. [Google Scholar] [CrossRef]
- Linardon, J.; Wade, T.D.; de la Piedad Garcia, X.; Brennan, L. The efficacy of cognitive-behavioral therapy for eating disorders: A systematic review and meta-analysis. J Consult Clin Psychol 2017, 85, 1080–1094. [Google Scholar] [CrossRef]
- Harry, N.; Anona, K.; Obitulata-Ugwu, V.; Kuye, O.; Arubuolawe, O.; Folorunsho, I.; Busari, A.; Ibeneme, C.; Diala, A.; Afolabi, V.; et al. Potential Role of Glucagon-like Peptide-1 (GLP-1) Receptor Agonist in the Treatment of Bulimia Nervosa. Journal of Advances in Medicine and Medical Research 2024, 36, 379–389. [Google Scholar] [CrossRef]
- McElroy, S.L.; Mori, N.; Guerdjikova, A.I.; Keck, P.E., Jr. Would glucagon-like peptide-1 receptor agonists have efficacy in binge eating disorder and bulimia nervosa? A review of the current literature. Med Hypotheses 2018, 111, 90–93. [Google Scholar] [CrossRef]
- Jhe, G.B.; Egbert, A.; Ievers-Landis, C.E.; Chaves, E.; Genuario, K.; Santos, M.; Burton, E.T. GLP-1 Receptor Agonists for Treatment of Pediatric Obesity: Behavioral Health Considerations. Child Obes 2025, 21, 503–510. [Google Scholar] [CrossRef]
- Blaska, M.; Gołąb-Jenerał, K.; Ziora, K. “Satiety molecules” — nesfatin-1 and glucagon-like peptide 1 in blood serum in patients with anorexia nervosa and obesity. Endokrynologia Polska 2025, 76, 134–144. [Google Scholar] [CrossRef]
- Stackpole, R.; Greene, D.; Bills, E.; Egan, S.J. The association between eating disorders and perfectionism in adults: A systematic review and meta-analysis. Eat Behav 2023, 50, 101769. [Google Scholar] [CrossRef]
- Krug, I.; Dang, A.B.; Lu, E.; Ooi, W.L.; Portingale, J.; Miles, S. A Narrative Review on the Neurocognitive Profiles in Eating Disorders and Higher Weight Individuals: Insights for Targeted Interventions. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Pontillo, M.; Zanna, V.; Demaria, F.; Averna, R.; Di Vincenzo, C.; De Biase, M.; Di Luzio, M.; Foti, B.; Tata, M.C.; Vicari, S. Orthorexia Nervosa, Eating Disorders, and Obsessive-Compulsive Disorder: A Selective Review of the Last Seven Years. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Morgan, J.F.; Reid, F.; Lacey, J.H. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. Bmj 1999, 319, 1467–1468. [Google Scholar] [CrossRef]
- Jenkins, P.E.; Lebow, J.; Rienecke, R.D. Weight suppression as a predictor variable in the treatment of eating disorders: A systematic review. J Psychiatr Ment Health Nurs 2018, 25, 297–306. [Google Scholar] [CrossRef]
- Steward, T. Endocrinology-informed neuroimaging in eating disorders: GLP1, orexins, and psilocybin. Trends Mol Med 2024, 30, 321–323. [Google Scholar] [CrossRef]
- Himmerich, H.; Bentley, J.; Kan, C.; Treasure, J. Genetic risk factors for eating disorders: an update and insights into pathophysiology. Ther Adv Psychopharmacol 2019, 9, 2045125318814734. [Google Scholar] [CrossRef]
- Hübel, C.; Abdulkadir, M.; Herle, M.; Loos, R.J.F.; Breen, G.; Bulik, C.M.; Micali, N. One size does not fit all. Genomics differentiates among anorexia nervosa, bulimia nervosa, and binge-eating disorder. Int J Eat Disord 2021, 54, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, J.; Pujol, A.; Tofé, S.; Bonet, A.; Gil, A. Short term effects of semaglutide on emotional eating and other abnormal eating patterns among subjects living with obesity. Physiology & Behavior 2022, 257, 113967. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Ozeki, Y.; Yoshida, Y.; Okamoto, M.; Miyamoto, S.; Gotoh, K.; Shibata, H. Glucagon-Like Peptide-1 Receptor Agonist Semaglutide Improves Eating Behavior and Glycemic Control in Japanese Obese Type 2 Diabetic Patients. Metabolites 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Arnow, B.; Kenardy, J.; Agras, W.S. The Emotional Eating Scale: the development of a measure to assess coping with negative affect by eating. Int J Eat Disord 1995, 18, 79–90. [Google Scholar] [CrossRef]
- Sambal, H.; Bohon, C.; Weinbach, N. The effect of mood on food versus non-food interference among females who are high and low on emotional eating. J Eat Disord 2021, 9, 140. [Google Scholar] [CrossRef]
- Astrup, A. Reflections on the discovery GLP-1 as a satiety hormone: Implications for obesity therapy and future directions. Eur J Clin Nutr 2024, 78, 551–556. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Veltman, D.J.; Ten Kulve, J.S.; Groot, P.F.; Ruhe, H.G.; Barkhof, F.; Sloan, J.H.; Diamant, M.; Ijzerman, R.G. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes Metab 2015, 17, 878–886. [Google Scholar] [CrossRef]
- van Ruiten, C.C.; Ten Kulve, J.S.; van Bloemendaal, L.; Nieuwdorp, M.; Veltman, D.J.; RG, I.J. Eating behavior modulates the sensitivity to the central effects of GLP-1 receptor agonist treatment: a secondary analysis of a randomized trial. Psychoneuroendocrinology 2022, 137, 105667. [Google Scholar] [CrossRef]
- Gross, J.J. The Emerging Field of Emotion Regulation: An Integrative Review. Review of General Psychology 1998, 2, 271–299. [Google Scholar] [CrossRef]
- Arexis, M.; Feron, G.; Brindisi, M.C.; Billot, P.E.; Chambaron, S. A scoping review of emotion regulation and inhibition in emotional eating and binge-eating disorder: what about a continuum? J Eat Disord 2023, 11, 197. [Google Scholar] [CrossRef]
- Evers, C.; Marijn Stok, F.; de Ridder, D.T. Feeding your feelings: emotion regulation strategies and emotional eating. Pers Soc Psychol Bull 2010, 36, 792–804. [Google Scholar] [CrossRef]
- Shriver, L.H.; Dollar, J.M.; Calkins, S.D.; Keane, S.P.; Shanahan, L.; Wideman, L. Emotional Eating in Adolescence: Effects of Emotion Regulation, Weight Status and Negative Body Image. Nutrients 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Crockett, A.C.; Myhre, S.K.; Rokke, P.D. Boredom proneness and emotion regulation predict emotional eating. J Health Psychol 2015, 20, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Spoor, S.T.; Bekker, M.H.; Van Strien, T.; van Heck, G.L. Relations between negative affect, coping, and emotional eating. Appetite 2007, 48, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Macht, M. How emotions affect eating: a five-way model. Appetite 2008, 50, 1–11. [Google Scholar] [CrossRef]
- Macht, M.; Simons, G. Emotional eating. In Emotion regulation and well-being.; Springer Science + Business Media: New York, NY, US, 2011; pp. 281–295. [Google Scholar]
- Fairburn, C.G.; Cooper, Z.; O’Connor, M.E. Eating Disorder Examination (Edition 16.0D). In Cognitive Behavior Therapy and Eating Disorders, Fairburn, C.G., Ed.; Guilford Press: New York, NY, USA, 2008; pp. 265–308. [Google Scholar]
- Brown, T.A.; Cusack, A.; Anderson, L.; Reilly, E.E.; Berner, L.A.; Wierenga, C.E.; Lavender, J.M.; Kaye, W.H. Early Versus Later Improvements in Dialectical Behavior Therapy Skills Use and Treatment Outcome in Eating Disorders. Cognitive Therapy and Research 2019, 43, 759–768. [Google Scholar] [CrossRef]
- Linehan, M.M. Cognitive-behavioral treatment of borderline personality disorder; Guilford Press: New York, NY, US, 1993. [Google Scholar]
- Grant, P.; Lipscomb, D.; Quin, J. Psychological and quality of life changes in patients using GLP-1 analogues. J Diabetes Complications 2011, 25, 244–246. [Google Scholar] [CrossRef]
- Cooper, D.H.; Ramachandra, R.; Ceban, F.; Di Vincenzo, J.D.; Rhee, T.G.; Mansur, R.B.; Teopiz, K.M.; Gill, H.; Ho, R.; Cao, B.; et al. Glucagon-like peptide 1 (GLP-1) receptor agonists as a protective factor for incident depression in patients with diabetes mellitus: A systematic review. J Psychiatr Res 2023, 164, 80–89. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, P.; Wang, W.; Guo, L.; Pan, Q. The Antidepressant Effects of GLP-1 Receptor Agonists: A Systematic Review and Meta-Analysis. Am J Geriatr Psychiatry 2024, 32, 117–127. [Google Scholar] [CrossRef]
- Tobaiqy, M.; Elkout, H. Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: a pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database. Int J Clin Pharm 2024, 46, 488–495. [Google Scholar] [CrossRef]
- Kornelius, E.; Huang, J.Y.; Lo, S.C.; Huang, C.N.; Yang, Y.S. The risk of depression, anxiety, and suicidal behavior in patients with obesity on glucagon like peptide-1 receptor agonist therapy. Sci Rep 2024, 14, 24433. [Google Scholar] [CrossRef]
- Silverii, G.A.; Marinelli, C.; Mannucci, E.; Rotella, F. Glucagon-like peptide-1 receptor agonists and mental health: A meta-analysis of randomized controlled trials. Diabetes Obes Metab 2024, 26, 2505–2508. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Mansur, R.B.; Rosenblat, J.D.; Rhee, T.G.; Cao, B.; Teopiz, K.M.; Wong, S.; Le, G.H.; Ho, R.; Kwan, A.T.H. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: A replication study using reports to the World Health Organization pharmacovigilance database (VigiBase®). J Affect Disord 2025, 369, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Pierret, A.C.S.; Benton, M.; Sen Gupta, P.; Ismail, K. A qualitative study of the mental health outcomes in people being treated for obesity and type 2 diabetes with glucagon-like peptide-1 receptor agonists. Acta Diabetologica 2025, 62, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Arillotta, D.; Floresta, G.; Guirguis, A.; Corkery, J.M.; Catalani, V.; Martinotti, G.; Sensi, S.L.; Schifano, F. GLP-1 Receptor Agonists and Related Mental Health Issues; Insights from a Range of Social Media Platforms Using a Mixed-Methods Approach. Brain Sci 2023, 13. [Google Scholar] [CrossRef]
- Martins, L.B.; Monteze, N.M.; Calarge, C.; Ferreira, A.V.M.; Teixeira, A.L. Pathways linking obesity to neuropsychiatric disorders. Nutrition 2019, 66, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Cash, T.F.; Deagle, E.A. The nature and extent of body-image disturbances in anorexia nervosa and bulimia nervosa: A meta-analysis. International Journal of Eating Disorders 1997, 22, 107–126. [Google Scholar] [CrossRef]
- Prnjak, K.; Jukic, I.; Mitchison, D.; Griffiths, S.; Hay, P. Body image as a multidimensional concept: A systematic review of body image facets in eating disorders and muscle dysmorphia. Body Image 2022, 42, 347–360. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: a unified brain theory? Nat Rev Neurosci 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Brizzi, G.; Sansoni, M.; Di Lernia, D.; Frisone, F.; Tuena, C.; Riva, G. The multisensory mind: a systematic review of multisensory integration processing in Anorexia and Bulimia Nervosa. J Eat Disord 2023, 11, 204. [Google Scholar] [CrossRef]
- Riva, G. Neuroscience and eating disorders: the allocentric lock hypothesis. Med Hypotheses 2012, 78, 254–257. [Google Scholar] [CrossRef] [PubMed]
- de Vignemont, F. A Multimodal Conception of Bodily Awareness. Mind 2014, 123, 989–1020. [Google Scholar] [CrossRef]
- Tsakiris, M. The multisensory basis of the self: From body to identity to others [Formula: see text]. Q J Exp Psychol (Hove) 2017, 70, 597–609. [Google Scholar] [CrossRef]
- Portingale, J.; Krug, I.; Liu, H.; Kiropoulos, L.; Butler, D. Your body, my experience: A systematic review of embodiment illusions as a function of and method to improve body image disturbance. Clinical Psychology: Science and Practice 2024, 31, 445–458. [Google Scholar] [CrossRef]
- Cobbaert, L.; Hay, P.; Mitchell, P.B.; Roza, S.J.; Perkes, I. Sensory processing across eating disorders: A systematic review and meta-analysis of self-report inventories. Int J Eat Disord 2024, 57, 1465–1488. [Google Scholar] [CrossRef]
- Maselli, A.; Slater, M. The building blocks of the full body ownership illusion. Front Hum Neurosci 2013, 7, 83. [Google Scholar] [CrossRef]
- Basch, C.H.; Narayanan, S.; Tang, H.; Fera, J.; Basch, C.E. Descriptive analysis of TikTok videos posted under the hashtag #Ozempic. Journal of Medicine, Surgery, and Public Health 2023, 1, 100013. [Google Scholar] [CrossRef]
- Propfe, L.E.; Seifert, R. Misrepresentation of semaglutide in social media. Naunyn-Schmiedeberg’s Archives of Pharmacology, 2025. [Google Scholar] [CrossRef]
- Rad, J.; Melendez-Torres, G.J. Critical discourse analysis of social media advertisements for GLP-1 receptor agonist weight loss drugs: implications for public perceptions and health communication. BMC Public Health 2025, 25, 2996. [Google Scholar] [CrossRef]
- Ryan, N.; Savulescu, J. The Ethics of Ozempic and Wegovy. J Med Ethics 2025. [Google Scholar] [CrossRef] [PubMed]
- Dane, A.; Bhatia, K. The social media diet: A scoping review to investigate the association between social media, body image and eating disorders amongst young people. PLOS Glob Public Health 2023, 3, e0001091. [Google Scholar] [CrossRef] [PubMed]
- Dondzilo, L.; Rodgers, R.F.; Dietel, F.A. Association between engagement with appearance and eating related TikTok content and eating disorder symptoms via recommended content and appearance comparisons. Int J Eat Disord 2024, 57, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.; Carollo, A.; Lazuras, L.; Corazza, O.; Esposito, G. Ozempic (Glucagon-like peptide 1 receptor agonist) in social media posts: Unveiling user perspectives through Reddit topic modeling. Emerging Trends in Drugs, Addictions, and Health 2024, 4, 100157. [Google Scholar] [CrossRef]
- Post, S.M.; Persky, S. The effect of GLP-1 receptor agonist use on negative evaluations of women with higher and lower body weight. International Journal of Obesity 2024, 48, 1019–1026. [Google Scholar] [CrossRef]
- Post, S.M.; Stock, M.L.; Persky, S. Comparing the Impact of GLP-1 Agonists vs. Lifestyle Interventions and Weight Controllability Information on Stigma and Weight-Related Cognitions. International Journal of Behavioral Medicine 2025, 32, 528–540. [Google Scholar] [CrossRef]
- Bachmakova, M.; Bahar Buyukbabani, M.; Dranseika, V.; Brown, B.; Devolder, K.; Ryan, N.; Savulescu, J.; Everett, J.; Hannikainen, I.; Earp, B. Even with Diet and Exercise, Ozempic Use Reduces Perceived Effort and Praiseworthiness of Resulting Weight Loss; 2025.
- Tomiyama, A.J. Behavioral medicine in the GLP-1 era. Annals of Behavioral Medicine 2024, 59. [Google Scholar] [CrossRef]
- Pearson, S.D.; Whaley, C.M.; Emond, S.K. Affordable access to GLP-1 obesity medications: strategies to guide market action and policy solutions in the US. J Comp Eff Res 2025, 14, e250083. [Google Scholar] [CrossRef]
- Pyke, A. Choosing a weight loss medication – Ozempic, Wegovy, Saxenda and Mounjaro compared. Available online: https://mydr.com.au/nutrition-weight/ozempic-wegovy-saxenda-mounjaro-compared/ (accessed on 26 September 2025).
- Canales, S.B. Why isn’t Ozempic on Australia’s Pharmaceutical Benefits Scheme for weight loss? And should it be? Available online: https://www.theguardian.com/australia-news/2025/jan/28/why-isnt-ozempic-on-australia-pharmaceutical-benefits-scheme-for-weight-loss-obesity (accessed on 26 September 2025).
- Hunter Medical Research Institute. Mounjaro vs Ozempic: Are they the same? Available online: https://hmri.org.au/news-and-stories/mounjaro-vs-ozempic-are-they-the-same/ (accessed on 26 September 2025).
- Autret, K.; Bekelman, T.A. Socioeconomic Status and Obesity. J Endocr Soc 2024, 8, bvae176. [Google Scholar] [CrossRef]
- Dinsa, G.D.; Goryakin, Y.; Fumagalli, E.; Suhrcke, M. Obesity and socioeconomic status in developing countries: a systematic review. Obes Rev 2012, 13, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Reyes Matos, U.; Mesenburg, M.A.; Victora, C.G. Socioeconomic inequalities in the prevalence of underweight, overweight, and obesity among women aged 20–49 in low- and middle-income countries. International Journal of Obesity 2020, 44, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Sharifi, M.; Oshman, L.; Griauzde, D.H.; Chua, K.P. Dispensing of Glucagon-Like Peptide-1 Receptor Agonists to Adolescents and Young Adults, 2020-2023. Jama 2024, 331, 2041–2043. [Google Scholar] [CrossRef]
- Kelly, A.S.; Auerbach, P.; Barrientos-Perez, M.; Gies, I.; Hale, P.M.; Marcus, C.; Mastrandrea, L.D.; Prabhu, N.; Arslanian, S. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N Engl J Med 2020, 382, 2117–2128. [Google Scholar] [CrossRef]
- Weghuber, D.; Barrett, T.; Barrientos-Pérez, M.; Gies, I.; Hesse, D.; Jeppesen, O.K.; Kelly, A.S.; Mastrandrea, L.D.; Sørrig, R.; Arslanian, S. Once-Weekly Semaglutide in Adolescents with Obesity. N Engl J Med 2022, 387, 2245–2257. [Google Scholar] [CrossRef] [PubMed]
- Arslanian, S.A.; Hannon, T.; Zeitler, P.; Chao, L.C.; Boucher-Berry, C.; Barrientos-Pérez, M.; Bismuth, E.; Dib, S.; Cho, J.I.; Cox, D. Once-Weekly Dulaglutide for the Treatment of Youths with Type 2 Diabetes. N Engl J Med 2022, 387, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, P.; Huang, W.; Yeh, Y.-Y.; Narvaez, V.M.; Adirika, D.; Tang, H.; Bernier, A.V.; Westen, S.C.; Smith, S.M.; Bian, J.; et al. Efficacy and Safety of GLP-1 RAs in Children and Adolescents With Obesity or Type 2 Diabetes: A Systematic Review and Meta-Analysis. JAMA Pediatrics 2025. [Google Scholar] [CrossRef]
- Romariz, L.M.; de Melo, A.A.C.; Finnegan, E.; Mesquita, Y.; Janovsky, C. GLP-1 receptor agonists for the treatment of obesity in children and adolescents: a meta-analysis of randomized controlled trials. Pediatr Res 2025. [Google Scholar] [CrossRef]
- Weaver, C.M. Adolescence: the period of dramatic bone growth. Endocrine 2002, 17, 43–48. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Agarwal, M.; Aggarwal, M.; Alexander, L.; Apovian, C.M.; Bindlish, S.; Bonnet, J.; Butsch, W.S.; Christensen, S.; Gianos, E.; et al. Nutritional priorities to support GLP-1 therapy for obesity: A joint Advisory from the American College of Lifestyle Medicine, the American Society for Nutrition, the Obesity Medicine Association, and The Obesity Society. Obesity (Silver Spring) 2025, 33, 1475–1503. [Google Scholar] [CrossRef]
- Jensen, S.B.K.; Sørensen, V.; Sandsdal, R.M.; Lehmann, E.W.; Lundgren, J.R.; Juhl, C.R.; Janus, C.; Ternhamar, T.; Stallknecht, B.M.; Holst, J.J.; et al. Bone Health After Exercise Alone, GLP-1 Receptor Agonist Treatment, or Combination Treatment: A Secondary Analysis of a Randomized Clinical Trial. JAMA Network Open 2024, 7, e2416775–e2416775. [Google Scholar] [CrossRef] [PubMed]
- Breton, É.; Dufour, R.; Côté, S.M.; Dubois, L.; Vitaro, F.; Boivin, M.; Tremblay, R.E.; Booij, L. Developmental trajectories of eating disorder symptoms: A longitudinal study from early adolescence to young adulthood. J Eat Disord 2022, 10, 84. [Google Scholar] [CrossRef]
- Ganson, K.T.; Testa, A.; Lavender, J.M.; Nagata, J.M. Prescription weight loss medication use and eating disorder psychopathology among adolescent boys and young men from Canada and the United States. Eat Behav 2025, 58, 102013. [Google Scholar] [CrossRef]
- Wilksch, S.M.; O’Shea, A.; Ho, P.; Byrne, S.; Wade, T.D. The relationship between social media use and disordered eating in young adolescents. Int J Eat Disord 2020, 53, 96–106. [Google Scholar] [CrossRef]
- Kerem, L.; Stokar, J. Risk of Suicidal Ideation or Attempts in Adolescents With Obesity Treated With GLP1 Receptor Agonists. JAMA Pediatr 2024, 178, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Mittman, B.G.; Le, P.; Payne, J.Y.; Ayers, G.; Rothberg, M.B. Sociodemographic disparities in GLP-1RA and SGLT2i use among US adults with type 2 diabetes: NHANES 2005-March 2020. Curr Med Res Opin 2024, 40, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Eberly, L.A.; Yang, L.; Essien, U.R.; Eneanya, N.D.; Julien, H.M.; Luo, J.; Nathan, A.S.; Khatana, S.A.M.; Dayoub, E.J.; Fanaroff, A.C.; et al. Racial, Ethnic, and Socioeconomic Inequities in Glucagon-Like Peptide-1 Receptor Agonist Use Among Patients With Diabetes in the US. JAMA Health Forum 2021, 2, e214182–e214182. [Google Scholar] [CrossRef]
- Post, S.M. Comparing the Impact of Exposure to GLP-1 Agonists and Lifestyle Interventions on Weight Stigma Through Social Comparison Among Black and White Women. ProQuest Dissertations & Theses, 2025.
- Hebl, M.R.; Heatherton, T.F. The Stigma of Obesity in Women: The Difference is Black and White. Personality and Social Psychology Bulletin 1998, 24, 417–426. [Google Scholar] [CrossRef]
- Puhl, R.M.; Andreyeva, T.; Brownell, K.D. Perceptions of weight discrimination: prevalence and comparison to race and gender discrimination in America. Int J Obes (Lond) 2008, 32, 992–1000. [Google Scholar] [CrossRef]
- Isong, I.A.; Rao, S.R.; Bind, M.A.; Avendaño, M.; Kawachi, I.; Richmond, T.K. Racial and Ethnic Disparities in Early Childhood Obesity. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Neveux, M.; Brown, T.; Chai, L.K.; Collins, C.E.; Ells, L.J.; Nowicka, P. Social disparities in obesity treatment for children age 3-10 years: A systematic review. Obes Rev 2021, 22, e13153. [Google Scholar] [CrossRef]
- Hale, M.J.; Stancil, J.; Levinson, C.A.; Peiper, N.C. Trends and Disparities in Perceived Overweight and Weight Loss Attempts Among Adolescents in the United States: 2003-2021. Journal of Adolescent Health. [CrossRef]
- Fairburn, C.G.; Cooper, Z.; Doll, H.A.; O’Connor, M.E.; Palmer, R.L.; Dalle Grave, R. Enhanced cognitive behaviour therapy for adults with anorexia nervosa: a UK-Italy study. Behav Res Ther 2013, 51, R2-8. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Cooper, Z.; Shafran, R. Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behaviour Research and Therapy 2003, 41, 509–528. [Google Scholar] [CrossRef]
- Ooi, W.L.; Nasser, H.; Simmons, J.; Krug, I. A systematic review and meta-analysis on the temporal relationship between appearance comparisons and body dissatisfaction. Body Image 2025, 53, 101885. [Google Scholar] [CrossRef] [PubMed]
- Krug, I.; Linardon, J.; Greenwood, C.; Youssef, G.; Treasure, J.; Fernandez-Aranda, F.; Karwautz, A.; Wagner, G.; Collier, D.; Anderluh, M.; et al. A proof-of-concept study applying machine learning methods to putative risk factors for eating disorders: results from the multi-centre European project on healthy eating. Psychol Med 2023, 53, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Summary of Key Mechanisms, Psychological Effects, and Clinical Considerations of GLP-1 Treatment.
Table 1.
Summary of Key Mechanisms, Psychological Effects, and Clinical Considerations of GLP-1 Treatment.
| Domain |
Key Points |
Unclear / Risks / Questions |
| Mechanisms |
↑ satiety, ↓ hunger; ↓ gastric emptying; gut–brain axis |
Long-term neural effects? |
| Appetite & Eating Behaviours |
Short-term: ↓ cravings, smaller meals, longer intervals |
Compensation (grazing, liquid calories)? Maintenance effects? |
| Disordered Eating / EDs |
BED/BN: ↓ binge eating (possible adjunct to CBT) |
Restrictive EDs/OSFED: safety & efficacy unclear |
| Emotion Regulation |
↓ emotional eating short-term |
Enduring ER change? Risk of substitute compulsions? |
| Body Image & Self-Perception |
Rapid weight loss → identity shifts; perceptual distortion / phantom fat; interoception mismatch |
Stigma / shame risks; long-term impact on self-concept |
| Mental Health & QoL |
Possible improvement in mood and QoL |
Mixed findings for depression; suicidality: no causal link but monitoring needed |
| Social Media & Digital Culture |
↑ mainstream attention, AI / misinformation risks |
Causal effects on body image and uptake? |
| Weight Stigma |
“Shortcut” narrative; structural stigma concerns |
Intersectional stigma impacts |
| Costs & Inequalities |
High prices, coverage gaps; inequitable access |
Disadvantaged groups at higher risk of exclusion |
| Special Populations |
Adolescents: Emerging use for higher weight and T2DM; developing body image and identity increase psychological sensitivity.
Ethnic minorities: Differential access and representation in trials; unique cultural/ethnic body ideals and stigma contexts. |
Long-term safety and developmental effects in youth unclear; limited data on psychological impact and stigma across diverse ethnic/cultural groups. |
| Clinical Oversight |
Screening & monitoring required; CBT / therapy supports; vigilance with dose changes |
Lack of long-term protocols |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).






