Submitted:
07 October 2025
Posted:
08 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
- -
- Age between 20 and 50 years,
- -
- Presence of dentoalveolar disharmony (DAD) with mild crowding (3–7 mm).
- -
- Systemic conditions that have an impact on periodontal tissues (diabetes, immunological conditions, acute articular rheumatism, tuberculosis, etc.).
- -
- Pregnancy or breastfeeding,
- -
- Smoking,
- -
- treatment with antibiotics, vitamin D or Ca supplements in the last 3 months,
- -
- Prior use of anti-inflammatory drugs (NSAIDs).
2.3. Sample Size
2.4. Orthodontic Protocol
2.5. Laser Protocol
2.6. Immunoenzymatic Analysis
- kit sensitivity: the detection limit for osteocalcin is 0.1 ng/ml.
- kit precision: intra-assay precision has a coefficient of variation below 10%; inter-assay precision has a coefficient of variation below 15%.
2.7. Laser Therapy Evaluation
2.8. Statistical Analysis
3. Results
4. Discussion
Limitations of this Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kakali, L.; Giantikidis, I.; Sifakakis, I.; et al. Fluctuation of bone turnover markers’ levels in GCF after orthodontic stimulus: Systematic review. Syst. Rev. 2022, 11, 3. [Google Scholar] [CrossRef]
- Abbing, A.; Koretsi, V.; Eliades, T.; Papageorgiou, S.N. Duration of orthodontic treatment with fixed appliances in adolescents and adults: A systematic review with meta-analysis. Prog. Orthod. 2020, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.N.; Giannakopoulou, T.; Eliades, T.; Vandevska-Radunovic, V. Occlusal outcome of orthodontic treatment: A systematic review with meta-analyses of randomized trials. Eur. J. Orthod. 2024, 46, cjae060. [Google Scholar] [CrossRef] [PubMed]
- Muro, M.P.; Caracciolo, A.C.A.; Patel, M.P.; Feres, M.F.N.; Roscoe, M.G. Effectiveness and predictability of treatment with clear orthodontic aligners: A scoping review. Int. Orthod. 2023, 21, 100755. [Google Scholar] [CrossRef] [PubMed]
- El-Angbawi, A.; McIntyre, G.; Fleming, P.S.; Bearn, D. Non-surgical adjunctive interventions for accelerating tooth movement in patients undergoing orthodontic treatment. Cochrane Database Syst. Rev. 2023, 6, CD010887. [Google Scholar] [CrossRef]
- Fenton, G.D.; Cazaly, M.H.M.; Rolland, S.L.; Vernazza, C.R. Eliciting preferences for adult orthodontic treatment: A discrete choice experiment. JDR Clin. Transl. Res. 2022, 7, 118–126. [Google Scholar] [CrossRef]
- Yong, J.; Gröger, S.; von Bremen, J.; Martins Marques, M.; Braun, A.; Chen, X.; Ruf, S.; Chen, Q. Photobiomodulation therapy assisted orthodontic tooth movement: Potential implications, challenges, and new perspectives. J. Zhejiang Univ. Sci. B 2023, 24, 957–973. [Google Scholar] [CrossRef]
- Santhakumari, P.P.; Prathapan, V.; Varma, R.J.; et al. Impact of low-level laser therapy on orthodontic tooth movement and cytokines in GCF: Split-mouth randomized study. Cureus 2023, 15, e42809. [Google Scholar]
- Varella, A.M.; Revankar, A.V.; Patil, A.K. Low-level laser therapy increases interleukin-1β in GCF and enhances orthodontic tooth movement. Am. J. Orthod. Dentofacial Orthop. 2018, 154, 535–544.e5. [Google Scholar] [CrossRef]
- Elgadi, R.; Sedky, Y.; Franzen, R. The effectiveness of low-level laser therapy on orthodontic tooth movement: A systematic review. Lasers Dent. Sci. 2023. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, K. Clinical research: Low-level laser therapy in accelerating orthodontic tooth movement. BMC Oral Health 2021, 21, 324. [Google Scholar] [CrossRef]
- Mistry, D.; Dalci, O.; Papadopoulou, A.K.; et al. The effects of a clinically feasible application of low-level laser therapy on the rate of orthodontic tooth movement: A triple blind, split mouth, randomized controlled trial. Am. J. Orthod. Dentofacial Orthop. 2020, 157, 444–453. [Google Scholar] [CrossRef]
- Chen, H.; Li, N.; Liu, N.; Zhu, H.; Ma, C.; Ye, Y.; Shi, X.; Luo, G.; Dong, X.; Tan, T.; Wei, X.; Yin, H. Photobiomodulation modulates mitochondrial energy metabolism and ameliorates neurological damage in an APP/PS1 mouse model of Alzheimer’s disease. Alzheimers Res. Ther. 2025, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ye, Y.; Shi, X.; Li, N.; Chen, Y.; Shi, X.; Chen, H. Photobiomodulation promotes osteogenic differentiation of mesenchymal stem cells and increases P-Akt levels in vitro. Sci. Rep. 2025, 15, 17844. [Google Scholar] [CrossRef] [PubMed]
- Alfaqeeh, S.A.; Anil, S. Osteocalcin and N-telopeptides of type I collagen marker levels in gingival crevicular fluid during different stages of orthodontic tooth movement. Am. J. Orthod. Dentofacial Orthop. 2011, 139, e553–e559. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, H.S.; Ates, M.; Gun, I.O.; Kuru, B.; Cakirer, B.; Kuru, L. Osteocalcin and cross-linked C-terminal telopeptide of type I collagen in gingival crevicular fluid during piezocision accelerated orthodontic tooth movement: A randomized split-mouth study. Niger. J. Clin. Pract. 2023, 26, 470–477. [Google Scholar] [CrossRef]
- Alnazeh, A.A.; Kamran, M.A.; Aseeri, Y.; Alrwuili, M.R.; Aljabab, M.A.; Baig, E.A.; Hameed, M.S. Levels of inflammatory and bone metabolic markers in the gingival crevicular fluid of individuals undergoing fixed orthodontic treatment in comparison to those utilizing Invisalign. Medicina 2023, 59, 2107. [Google Scholar] [CrossRef]
- Tavares, L.T.R.; Saavedra-Silva, M.; López-Marcos, J.F.; Veiga, N.J.; Castilho, R.M.; Fernandes, G.V.O. Blood and salivary inflammatory biomarkers profile in patients with chronic kidney disease and periodontal disease: A systematic review. Diseases 2022, 10, 12. [Google Scholar] [CrossRef]
- Keshari, R.; Chand, P.; Singh, B.P.; Jurel, S.K.; Singh, R.; Singh, P.K.; Solanki, N. Comparison of crestal bone loss and osteocalcin release kinetics in immediately and delayed loaded implants: A randomized controlled trial. J. Prosthodont. 2022, 31, 579–584. [Google Scholar] [CrossRef]
- Soares Bonato, R.C.; Abel Mapengo, M.A.; de Azevedo-Silva, L.J.; Janson, G.; de Carvalho Sales-Peres, S.H. Tooth movement, orofacial pain, and leptin, interleukin-1β, and tumor necrosis factor-α levels in obese adolescents. Angle Orthod. 2022, 92, 95–100. [Google Scholar] [CrossRef]
- Farahani, M.; Safavi, S.M.; Dianat, O.; Khoramian Tusi, S.; Younessian, F. Acid and alkaline phosphatase levels in GCF during orthodontic tooth movement. J. Dent. 2015, 16, 237–245. [Google Scholar]
- Nassar, E.A.; Almasoud, N.N.; Al-Qurashi, M.S.; Alsulaiman, A.A.; Hassan, K.S. An evaluation of microbial flora, alkaline phosphatase and IL-8 levels in GCF of orthodontic patients with self-ligating and conventional brackets. Clin. Cosmet. Investig. Dent. 2021, 13, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Sundrani, A.; Kamble, R.; Suchak, D.; Kaiser, J.; Agarwal, N.; Toshniwal, N. A comparative analysis of alkaline phosphatase levels in gingival crevicular fluid of patients undergoing growth modulation therapy with Twin Block, Forsus Fatigue Resistant, and Clear Block appliances compared to normal individuals: An in vivo study. Cureus 2024, 16, e63374. [Google Scholar] [CrossRef] [PubMed]
- Nassrawin, N.A. Detection of osteocalcin in gingival crevicular fluid in a group of orthodontic patients. J. Int. Soc. Prev. Community Dent. 2018, 8, 168–173. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, K. Clinical research: Low-level laser therapy in accelerating orthodontic tooth movement. BMC Oral Health 2021, 21, 324. [Google Scholar] [CrossRef] [PubMed]
- Lazăr, L.; Dako, T.; Mârțu, M.A.; Bica, C.I.; Bud, A.; Suciu, M.; Păcurar, M.; Lazăr, A.P. Effects of laser therapy on periodontal status in adult patients undergoing orthodontic treatment. Diagnostics 2022, 12, 2672. [Google Scholar] [CrossRef]
- Lazăr, A.P.; Dako, T.; Bud, A.; Vlasa, A.; Ormenișan, A.; Mârțu, M.A.; Păcurar, M.; Lazăr, L. The effects of periodontal laser therapy on pain in adult patients with orthodontic treatment: A randomized clinical trial. Appl. Sci. 2022, 12, 3601. [Google Scholar] [CrossRef]
- Kumar, B.D.; Singh, N.; Verma, S.K.; Singh, S.; Thakur, S. A study to analyze the alkaline phosphatase and lactate dehydrogenase enzyme activity in gingival crevicular fluid during orthodontic tooth movements. J. Pharm. Bioallied Sci. 2022, 14 (Suppl 1), S490–S493. [Google Scholar] [CrossRef]
- Bud, A.; Lazăr, L.; Mârțu, M.A.; Dakó, T.; Suciu, M.; Vlasiu, A.; Lazăr, A.P. Challenges and perspectives regarding the determination of gingival crevicular fluid biomarkers during orthodontic treatment: A narrative review. Medicina 2024, 60, 2004. [Google Scholar] [CrossRef]
- Surlin, P.; Rauten, A.M.; Silosi, I.; Foia, L. Pentraxin-3 levels in gingival crevicular fluid during orthodontic tooth movement in young and adult patients. Angle Orthod. 2012, 82, 833–838. [Google Scholar] [CrossRef]
- Kloukos, D.; Mavrogonatou, E.; Kletsas, D.; Makras, P.; Koukos, G.; Stavropoulos, A.; Katsaros, C. Bone turnover markers in gingival crevicular fluid and blood serum of patients with fixed orthodontic appliances. Eur. J. Orthod. 2022, 44, 412–419. [Google Scholar] [CrossRef]


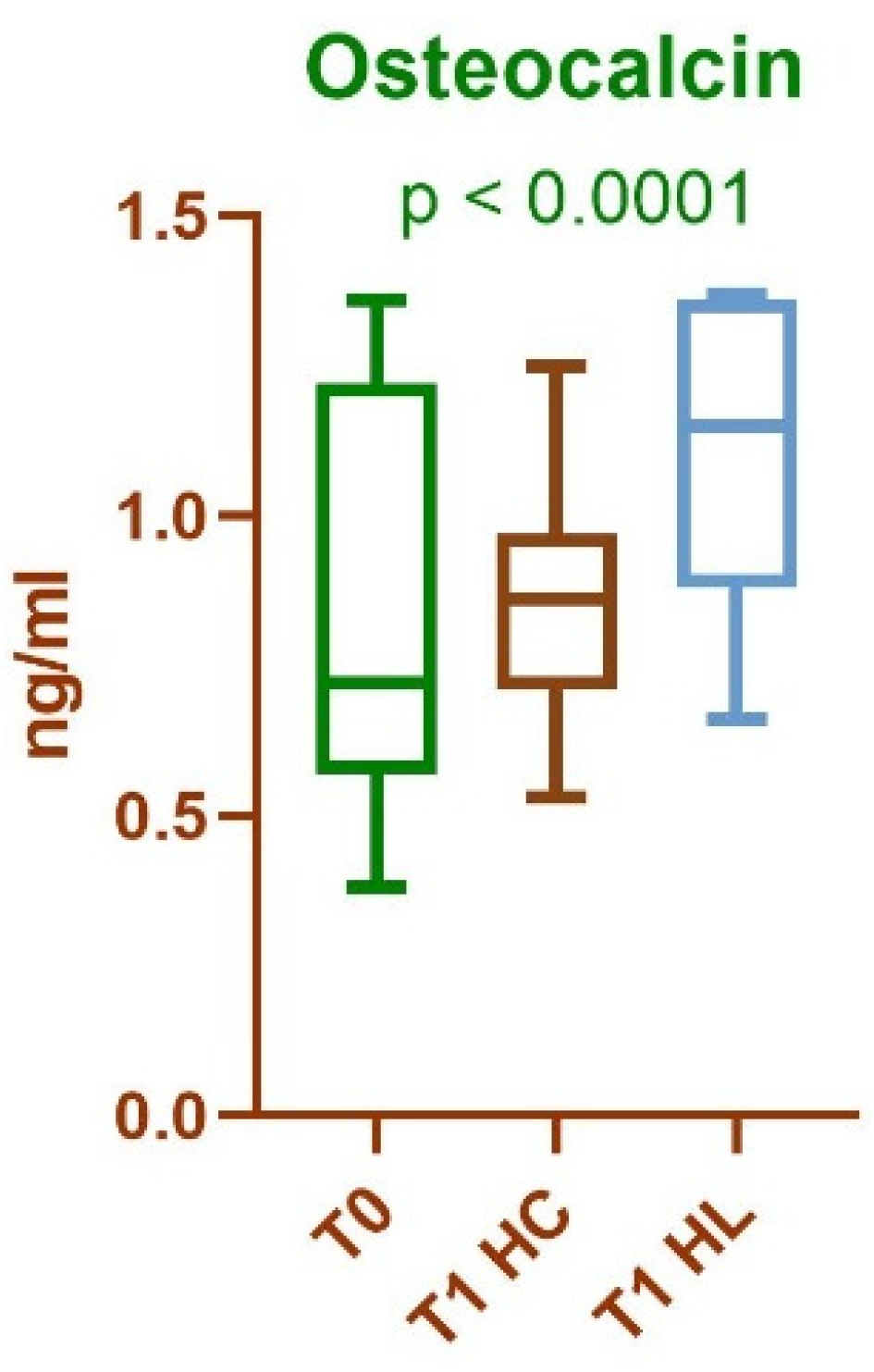
| Mean value and standard deviation for identified protein (ng/ml) T0 |
Mean value and standard deviation for identified protein (ng/ml) T1—HC |
Mean value and standard deviation for identified protein (ng/ml) T1—HL |
|---|---|---|
| 0.8507 ± 0.3237 | 0.8707 ± 0.1955 | 0.2489 ± 0.0454 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





