1. Introduction
The US healthcare landscape faces unprecedented challenges from aging populations, rising costs, and increasing complexity of chronic diseases, creating critical demands for enhanced diagnostic capabilities. Traditional diagnostic approaches, while foundational, encounter limitations in processing the exponential growth of multimodal medical data and maintaining consistent accuracy across diverse clinical scenarios [
1]. The emergence of Generative Artificial Intelligence (AI) agents represents a transformative paradigm shift, offering sophisticated computational frameworks that can autonomously perceive clinical environments, reason through complex medical data, and support diagnostic decision-making processes [
2,
3].
AI diagnostic agents distinguish themselves from conventional AI systems through their modular architectures and autonomous capabilities. As detailed in this work, these systems integrate perception modules for multimodal data ingestion, comprehensive knowledge bases encoding medical ontologies, advanced reasoning engines employing both symbolic and statistical methods, and interactive interfaces for clinical collaboration [
4,
5]. The architectural sophistication enables these agents to function not as isolated tools but as integrated diagnostic partners within clinical workflows.
The clinical impact of AI diagnostic agents spans multiple specialized domains. In medical imaging, these systems demonstrate performance comparable to human experts in detecting pathologies across radiology, histopathology, and ophthalmology [
6,
7]. For early disease detection, AI agents leverage multimodal data fusion to identify preclinical indications of conditions including cancer, neurological disorders, and cardiovascular diseases, enabling proactive intervention strategies [
8,
9]. Particularly transformative is their application in rare disease diagnosis, where AI systems significantly reduce diagnostic odysseys through pattern recognition across heterogeneous clinical data [
10,
11].
The operational benefits, systematically analyzed which demonstrate substantial improvements in diagnostic accuracy, workflow efficiency, and resource utilization. Studies document measurable enhancements in sensitivity and specificity across multiple clinical domains, while automated processing reduces diagnostic turnaround times and operational costs [
12,
13]. Furthermore, these systems enable personalized medicine approaches through integration of genomic, clinical, and lifestyle data, facilitating tailored screening and prevention strategies [
14,
15].
Despite these advancements, significant challenges persist. Critical considerations include data privacy and security in handling sensitive health information, model interpretability for clinical trust adoption, regulatory compliance for medical device certification, and ethical frameworks for responsible deployment [
1,
16]. Addressing these challenges requires multidisciplinary collaboration across computer science, clinical medicine, and regulatory domains.
2. Quantitative Findings and Performance Metrics
The integration of AI agents into medical diagnostics is supported by a growing body of evidence demonstrating their quantitative impact on scale, accuracy, and efficiency. The performance of these systems can often be formalized mathematically. Key findings from recent developments include:
These metrics and their mathematical formalisms highlight the potential of AI agents to enhance diagnostic processes by expanding the range of detectable conditions, improving the speed and precision of analysis, and leveraging large-scale data for model refinement.
3. Architecture of AI Diagnostic Agents
The efficacy of an AI diagnostic agent hinges on its underlying architecture, which is typically composed of several interconnected modules that work in concert to emulate clinical reasoning. A generalized architecture, as detailed in various industry and research publications [
3,
25,
26], can be broken down into four core components.
3.1. Perception Module
This module serves as the agent’s sensory interface with the clinical environment. It is responsible for ingesting and preprocessing heterogeneous data from multiple sources. Key data types include:
Structured Data: Laboratory results, vital signs, and demographic information from Electronic Health Records (EHRs) [
27].
Unstructured Data: Clinical notes, physician narratives, and medical literature, which are processed using Natural Language Processing (NLP) techniques [
18].
Imaging Data: X-rays, CT scans, MRIs, and histopathology slides, analyzed via computer vision algorithms [
28,
29].
Multimodal Data: Emerging agents can fuse diverse data types, such as combining medical images with patient-reported symptoms or even vocal biomarkers [
20,
23].
The perception module converts this raw data into a structured format suitable for analysis by the reasoning engine.
3.2. Knowledge Base
The knowledge base is the agent’s long-term memory, encapsulating the vast domain of medical knowledge. It is not a static database but a dynamic repository that can be updated. It typically includes:
Medical Ontologies and Terminologies: Such as the International Classification of Diseases (ICD) [
30].
Clinical Guidelines and Protocols: Evidence-based best practices for disease management.
Structured Medical Knowledge Graphs: Representing relationships between diseases, symptoms, genes, and drugs [
19,
31].
Historical Case Data: A repository of prior diagnoses and outcomes, which can be used for comparative analysis.
This component allows the agent to ground its reasoning in established medical science.
3.3. Reasoning and Decision-Making Engine
This is the core "brain" of the agent, where diagnostic inference occurs. It employs a variety of AI/ML techniques:
Machine Learning Models: Deep learning networks (e.g., CNNs for images, RNNs/LSTMs for sequential data) for pattern recognition and prediction [
21,
32].
Expert Systems and Symbolic Reasoning: Applying rule-based or logic-based systems to emulate the diagnostic reasoning of a human expert [
33].
Probabilistic Graphical Models: To handle uncertainty and compute the likelihood of various differential diagnoses [
22].
The engine processes the features extracted by the perception module, consults the knowledge base, and generates a ranked list of potential diagnoses, often accompanied by confidence scores and supporting evidence.
3.4. Action and Interaction Module
This module facilitates the agent’s interaction with the external world. Its functions include:
The seamless integration of these four modules enables the AI agent to function as a sophisticated diagnostic partner, transforming raw data into actionable clinical insights.
4. Key Application Domains
The architectural versatility of AI diagnostic agents allows them to be deployed across a wide spectrum of medical specialties. Their impact is particularly pronounced in the following domains.
4.1. Medical Imaging and Radiology
This is one of the most mature application areas for AI. AI agents are deployed to automate the analysis of medical images, leading to faster and more accurate interpretations [
7,
36].
Studies have shown that such AI-powered imaging analysis can achieve diagnostic accuracy on par with, and in some cases exceeding, that of human radiologists, especially in high-volume, repetitive tasks [
6].
4.2. Early Detection and Prediction of Diseases
A transformative promise of AI agents is their ability to identify disease risk long before traditional methods. By analyzing subtle, complex patterns across multimodal data, they can shift healthcare from a reactive to a proactive model [
38,
39].
Oncology: Agents can predict the risk of developing cancers by analyzing genetic data, family history, and lifestyle factors, enabling personalized screening schedules [
17].
Neurology: For conditions like Alzheimer’s and Parkinson’s, AI models can detect minute changes in speech patterns, motor function, or brain imaging that precede clinical diagnosis by years [
8,
20].
Chronic Diseases: By continuously monitoring data from wearables and EHRs, agents can predict exacerbations of conditions like heart failure or sepsis, allowing for preemptive intervention [
40].
4.3. Rare Disease Diagnosis
Diagnosing rare diseases is a formidable challenge due to their low prevalence and often non-specific symptoms. AI agents are uniquely suited to tackle this problem by acting as a "collective memory" of rare cases [
10,
41].
Phenotype-Driven Diagnosis: Tools like SHEPHERD use few-shot learning to match a patient’s clinical phenotype (symptoms, signs) with known rare genetic diseases, even with very few training examples [
31].
Genomic Analysis: AI agents can rapidly sequence through a patient’s genome and cross-reference variants with databases of pathogenic mutations, significantly accelerating the diagnostic process [
19].
Electronic Health Record Mining: Agents like the one developed at UCLA Health scan EHRs for patterns and clues that might be missed by a human physician, shortening the diagnostic odyssey for patients [
18].
4.4. Multimodal and Generalist Diagnostic Agents
The next frontier involves agents that can integrate multiple data types and perform a broader range of diagnostic reasoning, akin to a human general practitioner.
Conversational Diagnostics: Systems like Google’s AMIE demonstrate the potential of AI to engage in diagnostic dialogue, taking a patient’s history and reasoning through differential diagnoses in a conversational manner [
22]. The evolution of such systems to include visual data (e.g., a photo of a skin lesion) marks a significant step forward [
23].
The Path to Medical Superintelligence: Research initiatives, such as those outlined by Microsoft, envision a future where a comprehensive AI agent integrates all available patient data—from genomics and proteomics to imaging and social determinants of health—to form a holistic, longitudinal health model and provide unparalleled diagnostic and therapeutic guidance [
42,
43].
5. Benefits and Impact
The deployment of AI diagnostic agents yields significant, measurable benefits across the healthcare ecosystem, impacting clinical outcomes, operational efficiency, and patient experience.
5.1. Enhanced Diagnostic Accuracy and Reduced Errors
Human diagnosis is susceptible to fatigue, cognitive biases, and the limitations of human perception. AI agents, by contrast, can analyze vast datasets with consistent, unwavering attention to detail. They excel at identifying subtle, multivariate patterns that may be invisible to the human eye [
12,
44]. This leads to a quantifiable reduction in diagnostic errors, including both false negatives (missing a disease) and false positives (incorrectly identifying a disease) [
13]. For instance, in medical imaging, AI has been shown to improve the detection rates of early-stage cancers and micro-fractures [
7].
5.2. Increased Operational Efficiency and Cost Reduction
Healthcare systems are burdened by high costs and inefficiencies. AI agents automate labor-intensive tasks such as preliminary image analysis, data entry, and triage, freeing up highly skilled clinicians to focus on complex decision-making and patient interaction [
45,
46]. This automation leads to:
The cumulative effect is a more efficient healthcare system that can serve more patients without a proportional increase in costs.
5.3. Early Intervention and Personalized Medicine
Perhaps the most profound impact of AI diagnostics is the shift towards proactive and personalized care. By enabling early detection, AI agents create a window of opportunity for interventions that are less invasive, more effective, and less costly [
38]. Furthermore, by integrating genetic, genomic, and lifestyle data, these agents can move beyond a one-size-fits-all model. They can help predict an individual’s risk for specific diseases and recommend personalized screening and prevention strategies, tailoring healthcare to the unique biology of each patient [
14,
15].
6. Visual Architecture of AI Diagnostic Agents
This section provides a detailed explanation of the architectural diagrams that illustrate the comprehensive framework of AI diagnostic agents in healthcare. Each figure represents a critical layer in the overall system architecture, from data ingestion to clinical deployment.
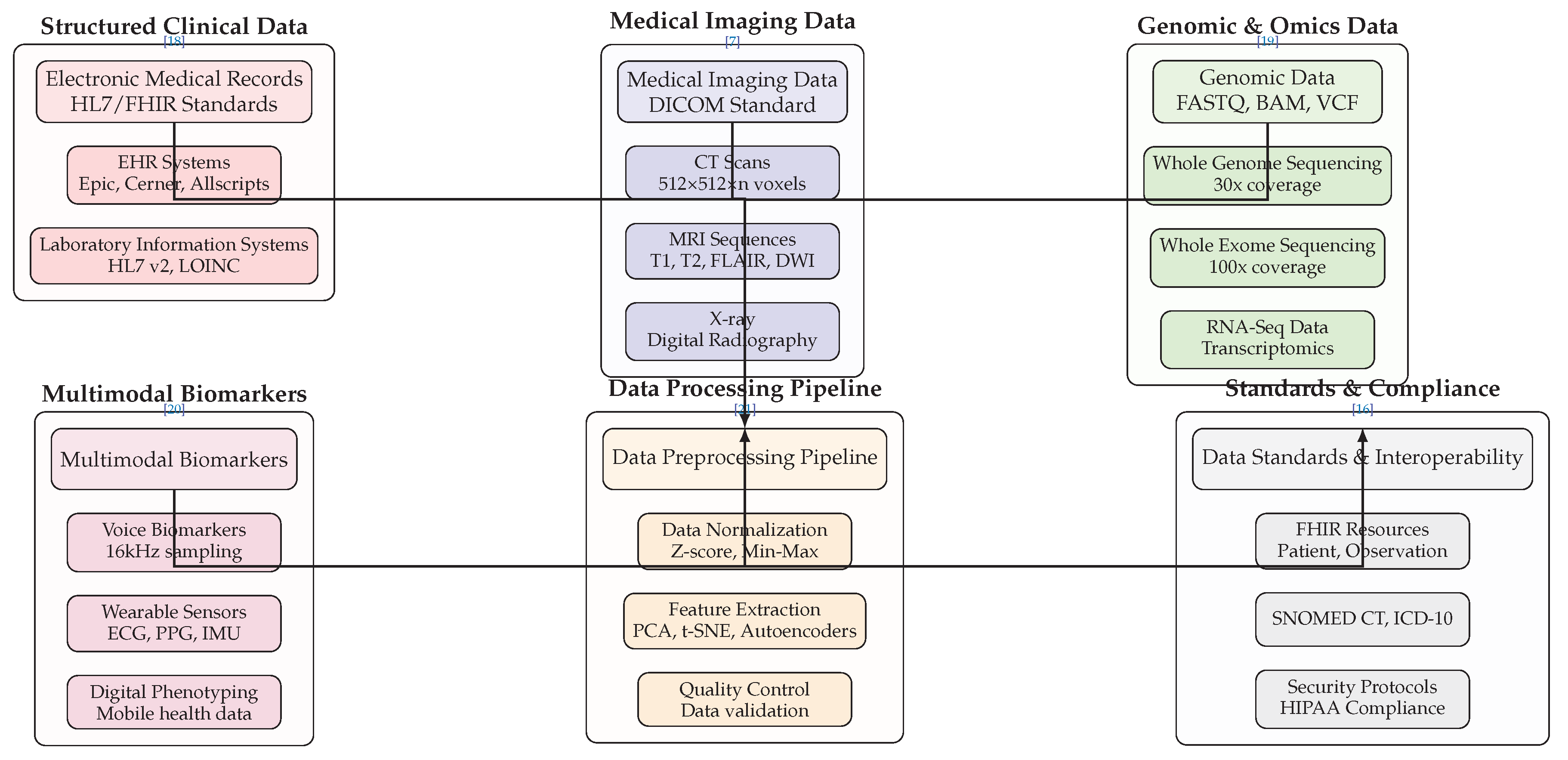
6.1. Data Sources and Preprocessing Layer
Figure 1 illustrates the multimodal data ecosystem that fuels AI diagnostic agents. The architecture encompasses six primary data categories, each with specific technical standards and processing requirements:
Structured Clinical Data includes Electronic Medical Records (EMRs) following HL7/FHIR standards, with integration capabilities for major EHR systems like Epic, Cerner, and Allscripts [
18]. Laboratory Information Systems utilize HL7 v2 messaging and LOINC coding for standardized laboratory result reporting [
30].
Medical Imaging Data operates within the DICOM standard framework, encompassing various modalities including CT scans with volumetric data representation (512×512×n voxels), multiple MRI sequences (T1, T2, FLAIR, DWI), and digital radiography systems [
7]. The technical specifications ensure compatibility with clinical imaging workflows while maintaining diagnostic quality.
Genomic and Omics Data incorporates next-generation sequencing outputs in standardized formats including FASTQ for raw sequencing data, BAM for aligned sequences, and VCF for variant calling [
19]. The architecture supports Whole Genome Sequencing at 30x coverage, Whole Exome Sequencing at 100x coverage, and RNA-Seq for transcriptomic analysis, enabling comprehensive molecular profiling [
31].
Multimodal Biomarkers represent emerging data sources including voice biomarkers sampled at 16kHz for acoustic analysis, wearable sensor data capturing ECG, PPG, and IMU signals, and digital phenotyping from mobile health applications [
8,
20]. These continuous monitoring streams enable real-time health assessment.
The
Data Preprocessing Pipeline implements essential transformation steps including data normalization using Z-score and Min-Max scaling, feature extraction through dimensionality reduction techniques (PCA, t-SNE, Autoencoders), and comprehensive quality control measures [
21]. This pipeline ensures data quality and compatibility for downstream analysis.
Standards and Compliance components ensure regulatory adherence through FHIR resources for data interoperability, standardized terminologies (SNOMED CT, ICD-10), and security protocols compliant with HIPAA requirements [
16]. The arrow flows demonstrate the sequential processing from raw data sources through standardization to compliant data products.
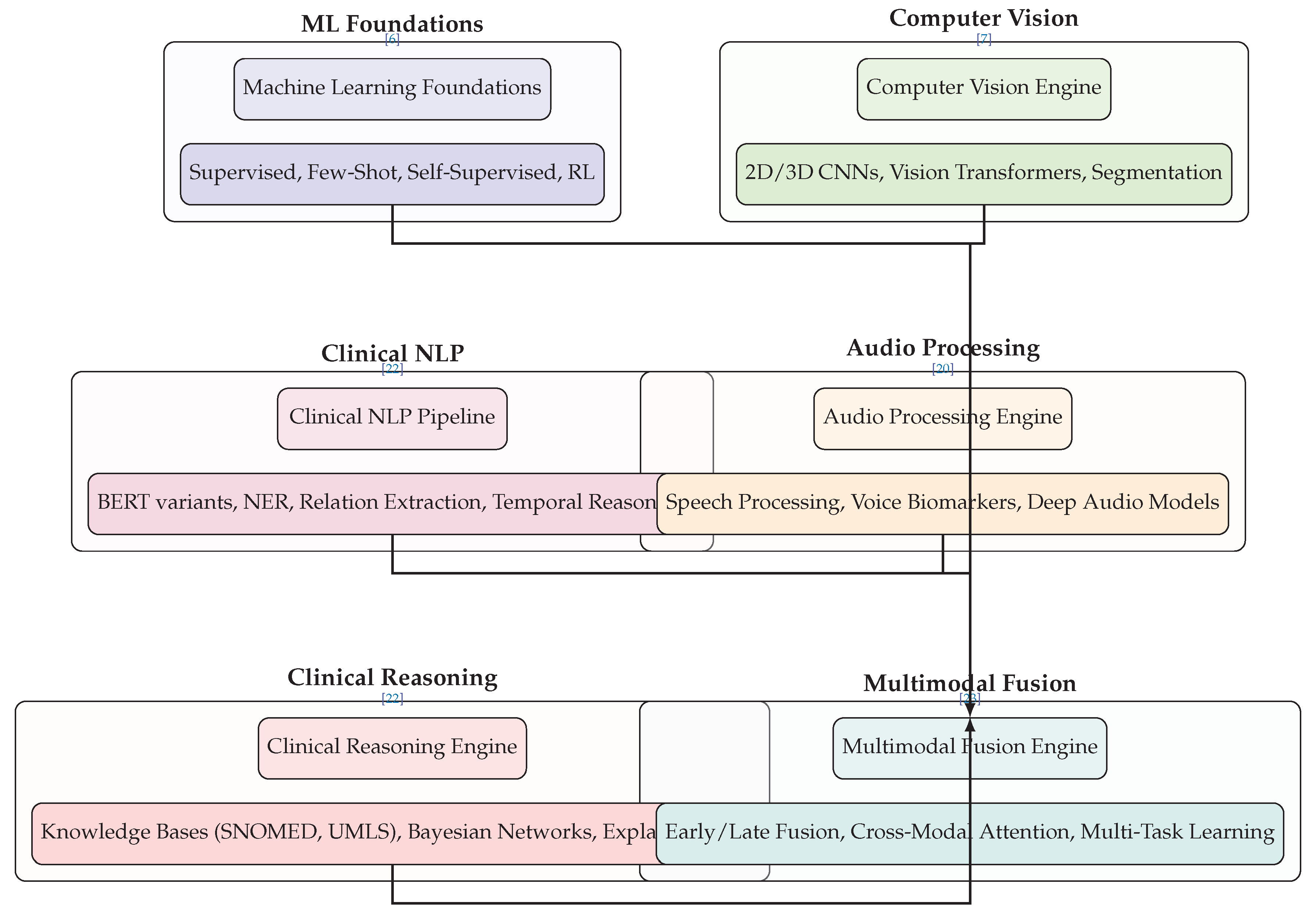
6.2. AI Core Engine Architecture
Figure 2 depicts the technical core of AI diagnostic agents, organized into six specialized processing engines that converge through multimodal fusion:
Machine Learning Foundations provide the algorithmic basis, incorporating supervised learning with architectures like ResNet and DenseNet, few-shot learning using Prototypical Networks and MAML for low-data scenarios, self-supervised learning via contrastive methods, and reinforcement learning for treatment optimization [
6,
31].
Computer Vision Engine specializes in medical image analysis through 2D CNNs (DenseNet-121, ResNet-50) for standard imaging, 3D CNNs (3D U-Net) for volumetric data processing, Vision Transformers (ViT, Swin Transformer) for advanced pattern recognition, and segmentation models (U-Net, nnU-Net) for precise anatomical delineation [
7,
37].
Clinical NLP Pipeline processes unstructured medical text using transformer models (BERT, ClinicalBERT, BioBERT) fine-tuned for medical terminology, Named Entity Recognition for clinical concept extraction, relation extraction for symptom-disease association mining, and temporal reasoning for disease progression modeling [
22].
Audio Processing Engine analyzes vocal biomarkers through speech processing techniques (MFCC, spectrogram analysis), voice biomarker extraction (jitter, shimmer, HNR measurements), audio feature analysis (prosody, articulation, phonation), and deep audio models (Wav2Vec2, HuBERT) for comprehensive acoustic assessment [
20].
Clinical Reasoning Engine implements diagnostic logic through knowledge base integration with medical ontologies (SNOMED CT, UMLS, ICD-10), chain-of-thought reasoning for step-by-step diagnosis, Bayesian networks for probabilistic inference, and explainable AI methods (SHAP, LIME, attention maps) for transparent decision support [
22,
44].
Multimodal Fusion Engine integrates diverse data streams through early fusion (feature concatenation), late fusion (model ensemble voting), cross-modal attention mechanisms, and multi-task learning for joint optimization across data types [
23]. The convergence arrows illustrate how all specialized engines feed into this unified integration layer.
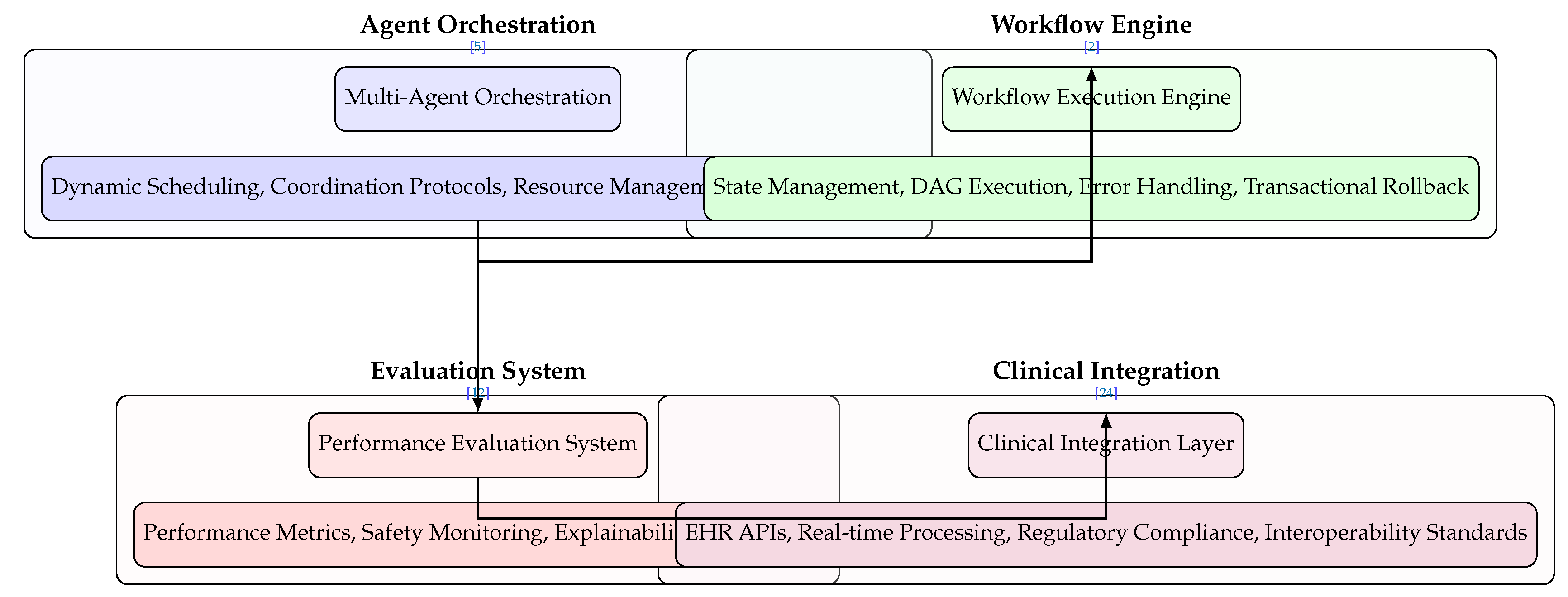
6.3. Execution and Orchestration Layer
Figure 6 illustrates the operational framework that coordinates AI agent activities within clinical environments:
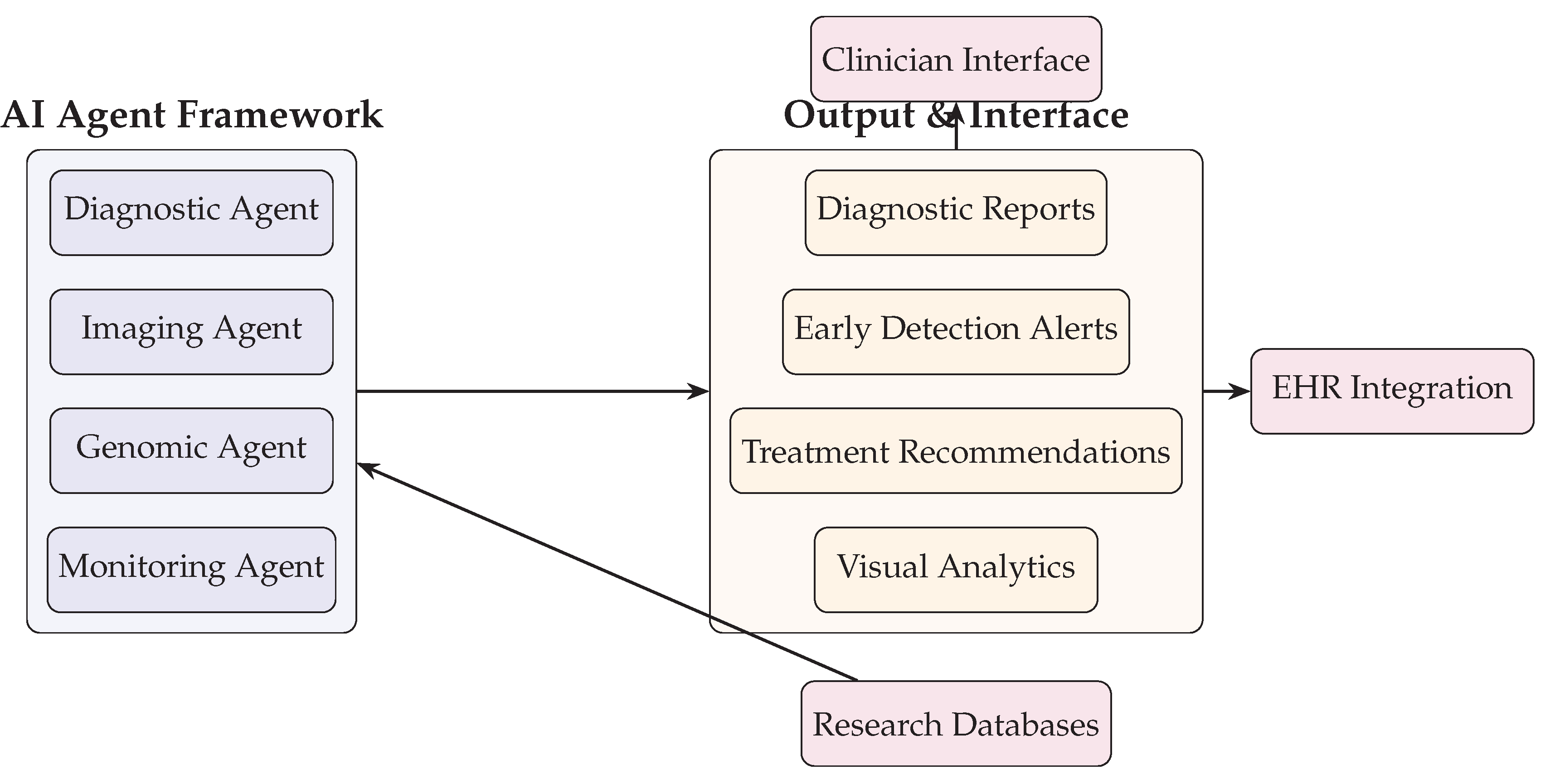
Figure 3.
AI Agent Framework and Output Interfaces including clinician interface, EHR integration, and research database feedback.
Figure 3.
AI Agent Framework and Output Interfaces including clinician interface, EHR integration, and research database feedback.
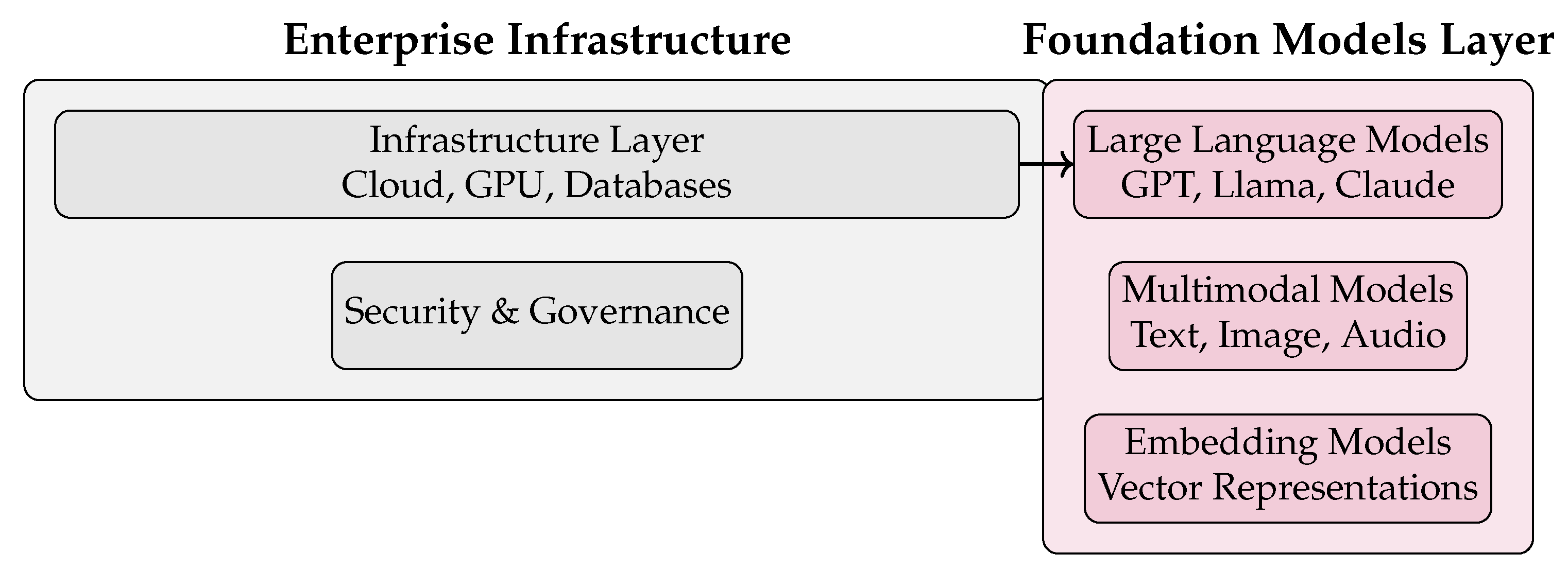
Figure 4.
Enterprise infrastructure and foundation models in a GenAI agent architecture.
Figure 4.
Enterprise infrastructure and foundation models in a GenAI agent architecture.
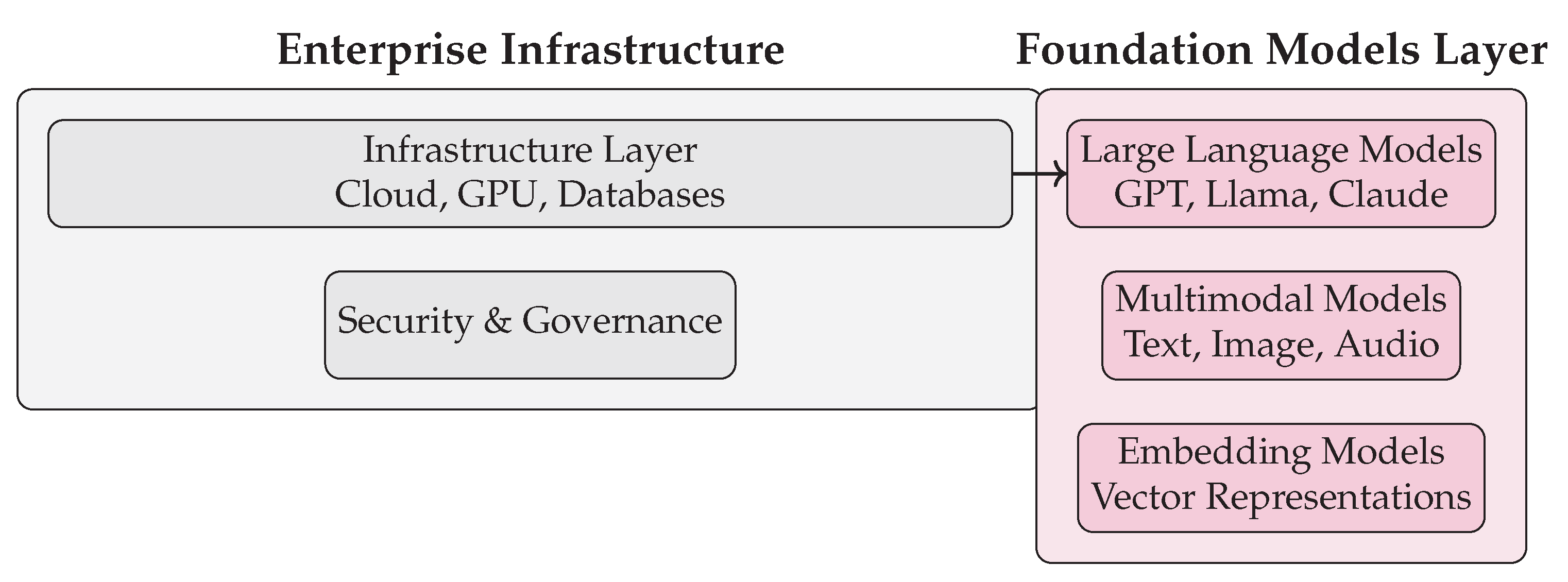
Figure 5.
Enterprise infrastructure and foundation models in a GenAI agent architecture.
Figure 5.
Enterprise infrastructure and foundation models in a GenAI agent architecture.
Figure 6.
Simplified Execution Layer architecture highlighting multi-agent orchestration, workflow engine, evaluation system, and clinical integration with essential methods and relevant citations.
Figure 6.
Simplified Execution Layer architecture highlighting multi-agent orchestration, workflow engine, evaluation system, and clinical integration with essential methods and relevant citations.
Multi-Agent Orchestration manages distributed AI components through dynamic task scheduling with priority queues and load balancing, agent coordination protocols using pub/sub and message passing architectures, resource management for GPU memory and CPU core allocation, and fault tolerance mechanisms including agent recovery and checkpointing [
5,
35].
Workflow Execution Engine coordinates clinical processes through state management using Redis and in-memory databases, DAG execution via integration with workflow systems like Airflow and Prefect, error handling with circuit breaker patterns and retry policies, and transactional rollback ensuring ACID compliance for data integrity [
2,
26].
Performance Evaluation System provides continuous assessment through performance metrics (AUC-ROC, F1-Score, precision/recall calculations), safety monitoring with drift detection and anomaly scoring, real-time explainability using SHAP and LIME interpretations, and comprehensive audit trails with version control and logging [
12,
16].
Clinical Integration Layer enables healthcare system interoperability through EHR integration using HL7 FHIR APIs and SMART on FHIR standards, real-time processing with stream processing frameworks like Kafka, regulatory compliance with HIPAA, GDPR, and FDA 21 CFR Part 11 requirements, and interoperability standards supporting DICOM, ICD-10, and SNOMED CT [
3,
24].
The sequential arrow flow demonstrates the operational pipeline from agent coordination through workflow execution, performance validation, and final clinical integration.
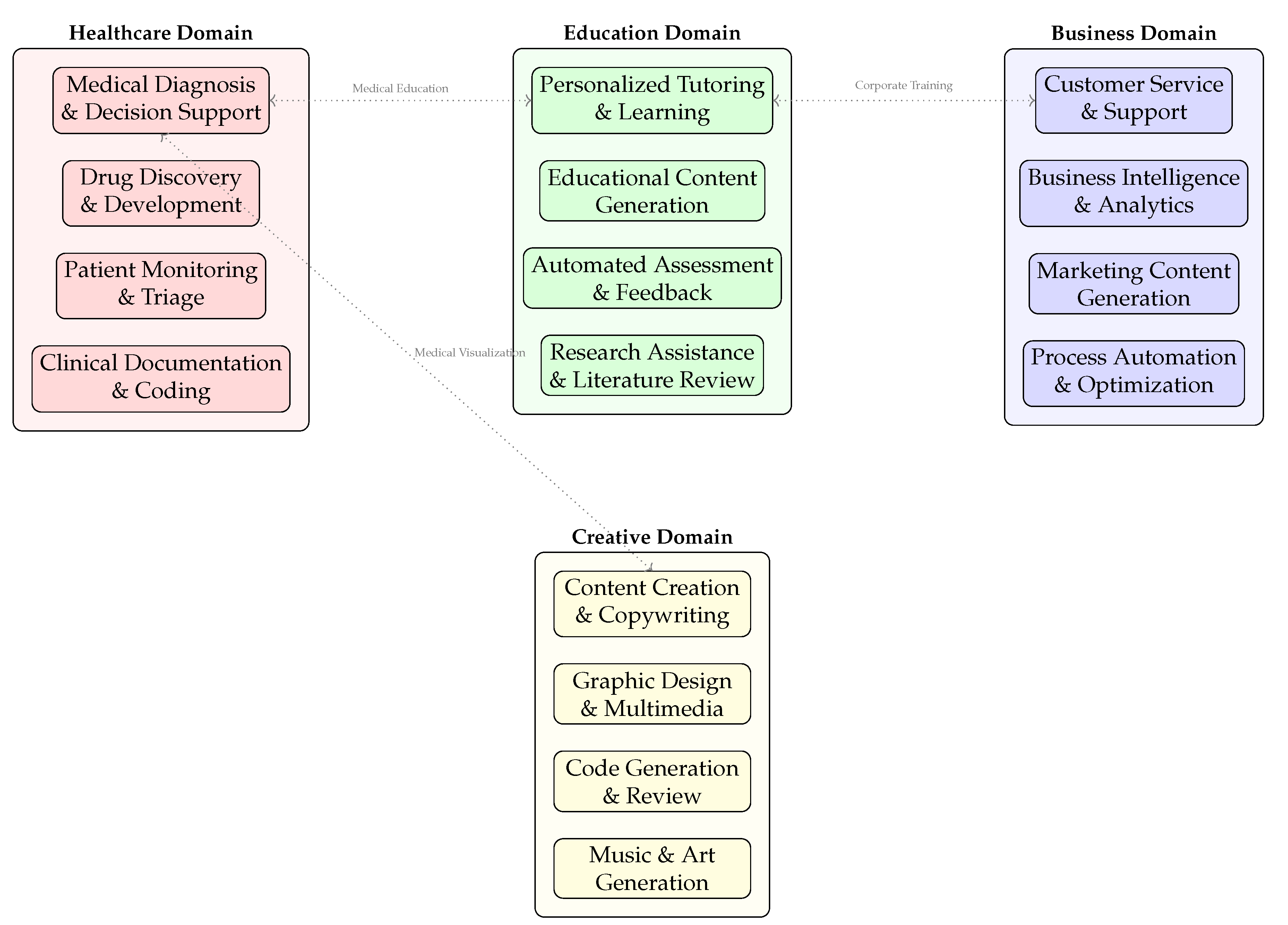
6.4. Application Domains and Deployment
Figure 7 showcases the diverse clinical implementation areas for AI diagnostic agents:
Healthcare Domain applications include medical diagnosis and decision support systems, drug discovery and development pipelines, patient monitoring and triage systems, and clinical documentation automation with medical coding [
7,
18]. These applications demonstrate the core diagnostic capabilities within clinical settings.
Cross-Domain Synergies illustrated by the connecting arrows reveal important integration points: medical education bridges healthcare and education domains through training simulation, corporate training connects education and business applications for healthcare workforce development, and medical visualization links healthcare with creative domains for enhanced clinical communication [
49,
50].
The distributed architecture allows specialized AI agents to operate within their domain expertise while maintaining interoperability through standardized interfaces and data exchange protocols.
6.5. Architectural Integration and Clinical Impact
The complete architectural framework, when integrated as depicted across all figures, enables comprehensive diagnostic capabilities that transcend traditional healthcare boundaries. The data layer provides multimodal inputs, the AI core processes these through specialized engines, the execution layer coordinates clinical workflows, and the application layer delivers domain-specific solutions.
This integrated approach addresses key healthcare challenges including diagnostic accuracy improvement through consistent multimodal analysis [
12], operational efficiency enhancement via automated workflow orchestration [
45], and personalized care enablement through adaptive learning systems [
14]. The architectural modularity ensures scalability across healthcare institutions while maintaining compliance with evolving regulatory requirements [
16].
The visual representations collectively demonstrate how AI diagnostic agents are evolving from single-purpose tools to comprehensive clinical partners capable of supporting healthcare professionals across the entire diagnostic continuum, from initial symptom presentation through final treatment recommendation and ongoing patient monitoring.
7. Policy Recommendations and Guidance for U.S. Government Stakeholders
The rapid advancement of AI agents in medical diagnostics presents both unprecedented opportunities and significant challenges for the U.S. healthcare system. Based on current developments and emerging trends, the following policy recommendations are proposed for government policymakers, regulatory agencies, and healthcare administrators.
7.1. Regulatory Framework Modernization
Establish AI-Specific Medical Device Classification: Create new FDA pathways specifically for AI diagnostic agents that can continuously learn and adapt, moving beyond the traditional static medical device paradigm [
3,
51].
Implement Tiered Approval Processes: Develop risk-based approval frameworks where AI agents for administrative tasks face lighter regulation than those for primary diagnosis of critical conditions [
26,
49].
7.2. Data Governance and Interoperability
Standardize Health Data Formats: Mandate adoption of common data standards (e.g., FHIR) to ensure AI agents can effectively learn from diverse healthcare systems while maintaining data privacy [
24,
52].
Create National AI Training Repositories: Establish secure, anonymized data repositories for training diagnostic AI agents, similar to initiatives referenced in [
7] and [
6].
7.3. Reimbursement and Payment Reform
Develop Value-Based Payment Models: Create CPT codes and reimbursement structures that reward the demonstrated diagnostic accuracy and efficiency improvements of AI agents, rather than traditional fee-for-service models [
46,
48].
Establish AI Performance Metrics: Define standardized metrics for evaluating AI diagnostic performance that can be tied to reimbursement rates and quality measurements [
12].
7.4. Workforce Development and Education
Integrate AI Literacy into Medical Education: Update medical school curricula and continuing education requirements to include training on interpreting AI-generated diagnoses and understanding AI limitations [
53,
54].
Create New Healthcare AI Roles: Support the development of certification programs for "AI Clinical Coordinators" who can bridge the gap between technical teams and clinical practitioners [
55].
7.5. Ethical Guidelines and Oversight
Establish AI Transparency Requirements: Mandate that healthcare organizations using diagnostic AI agents maintain clear documentation of training data, algorithms used, and performance limitations [
1,
2].
Create Patient Consent Protocols: Develop standardized informed consent processes that clearly explain when AI agents are involved in diagnosis and how patient data will be used [
14].
7.6. Research and Development Investment
Fund Multi-Institutional Validation Studies: Support large-scale clinical trials across multiple healthcare systems to validate AI diagnostic performance across diverse populations [
22,
23].
Promote Public-Private Partnerships: Create funding mechanisms that encourage collaboration between academic medical centers, AI developers, and community healthcare providers [
17,
45].
7.7. Implementation Timeline and Phased Approach
A phased implementation approach is recommended:
- 1.
Year 1-2: Focus on administrative AI agents and decision support tools
- 2.
Year 3-5: Expand to diagnostic assistance with human oversight
- 3.
Year 6+: Consider autonomous diagnostic agents for well-defined use cases
7.8. Conclusion
The successful integration of AI diagnostic agents into U.S. healthcare requires coordinated action across regulatory, financial, educational, and ethical dimensions. By implementing these policy recommendations, policymakers can harness the benefits of AI demonstrated in [
6] and [
17] while ensuring patient safety, equity, and the appropriate evolution of clinical practice.
8. Challenges and Future Directions
Despite the remarkable progress, the widespread clinical adoption of AI diagnostic agents faces several significant hurdles that must be addressed.
8.1. Data Privacy, Security, and Bias
AI models are only as good as the data they are trained on. The use of sensitive patient data raises critical concerns about privacy and security, governed by regulations like HIPAA [
1]. Furthermore, if training data is not representative of the broader population (e.g., skewed toward certain ethnicities or demographics), the resulting AI model can perpetuate and even amplify existing health disparities [
49]. Future work must focus on developing robust federated learning techniques (training models across decentralized data without sharing it) and rigorous bias detection and mitigation frameworks.
8.2. Interpretability and Trust
The "black box" nature of many complex AI models, particularly deep learning networks, is a major barrier to adoption. Clinicians are rightly hesitant to trust a diagnosis they cannot understand [
53]. For AI agents to be effective partners, they must be able to explain their reasoning in a way that is intuitive and clinically relevant [
34]. Research in Explainable AI (XAI) is crucial to develop agents that can provide clear, evidence-based justifications for their conclusions, thereby building trust and facilitating human-AI collaboration.
8.3. Regulatory and Ethical Hurdles
Integrating AI into clinical practice requires navigating complex regulatory landscapes. Agencies like the FDA in the US are developing frameworks for evaluating and approving AI-based SaMD (Software as a Medical Device) [
16]. Key challenges include:
Validation and Clinical Trials: Demonstrating efficacy and safety through robust, real-world clinical trials.
Continuous Learning: Regulating agents that evolve and learn after deployment without compromising safety.
Liability: Establishing clear guidelines for accountability when an AI-assisted diagnosis leads to an error.
8.4. The Path Forward: Human-AI Collaboration and Superintelligence
The future of AI in diagnostics is not about replacing physicians but about augmenting their capabilities. The most effective model is one of collaborative intelligence, where AI agents handle data-intensive tasks and generate evidence-based hypotheses, while clinicians provide oversight, contextual understanding, and empathetic patient care [
15,
54]. The long-term vision, as explored by leading research labs, is the journey toward a "medical superintelligence"—a comprehensive, multimodal AI system that serves as a powerful partner in the pursuit of global health equity and excellence [
42,
56].
9. Conclusions
This comprehensive review has systematically analyzed the architectural foundations, clinical applications, operational benefits, and implementation challenges of AI diagnostic agents in modern healthcare.
The architectural analysis reveals that effective AI diagnostic agents require sophisticated integration of multiple technical components: robust perception modules for heterogeneous data ingestion, comprehensive knowledge bases encoding medical ontologies and clinical guidelines, advanced reasoning engines combining statistical learning with symbolic AI, and interactive interfaces facilitating clinical collaboration. This modular architecture enables the scalability and adaptability necessary for diverse healthcare environments.
The examination of clinical applications demonstrates significant impact across medical specialties, with particularly transformative results in medical imaging analysis where AI agents achieve expert-level performance in detecting pathologies, early disease detection through multimodal pattern recognition that identifies preclinical conditions, and rare disease diagnosis where AI systems dramatically reduce diagnostic odysseys through sophisticated data integration and analysis.
The documented benefits substantiate substantial improvements in healthcare delivery, including enhanced diagnostic accuracy through consistent, unbiased analysis of complex datasets, increased operational efficiency via automated processing and workflow optimization, and the enabling of personalized medicine through integration of genomic, clinical, and lifestyle data for tailored interventions.
However, the path to widespread clinical adoption faces significant challenges that require careful consideration. Data privacy and security concerns necessitate robust protection mechanisms for sensitive health information, while model interpretability remains crucial for building clinical trust and facilitating human-AI collaboration. Regulatory frameworks must evolve to address the unique characteristics of continuously learning systems, and ethical guidelines need development to ensure equitable access and prevent algorithmic bias.
Future research should prioritize several key directions: advancing federated learning approaches for privacy-preserving model development, enhancing explainable AI techniques for transparent clinical decision support, developing comprehensive regulatory pathways for adaptive AI systems, and establishing frameworks for responsible human-AI collaboration that leverages the complementary strengths of computational analysis and clinical expertise.
The synthesis of current evidence indicates that AI diagnostic agents are poised to become indispensable components of modern healthcare systems. Rather than replacing clinical expertise, these systems serve as powerful augmentative tools that enhance diagnostic capabilities, expand access to specialized knowledge, and enable more proactive, personalized patient care.
References
- Takyar, A. AI in Medicine: A Comprehensive Overview, 2023.
- AI Agents in Healthcare: Use Cases, Benefits & Future Trends, 2024.
- Takyar, A. AI Agents for Healthcare: Applications, Benefits and Implementation, 2024.
- SENNI. AI Agents in Healthcare: The New Frontier of Diagnosis, 2025.
- Lee, A.G. AI Agent-Powered Multi-Medical Diagnostics:, 2025.
- News, N. AI Outpaces Humans in Rapid Disease Detection, 2024.
- Advancements in AI for Medical Imaging and Diagnostic Support: Improving Accuracy, Early Detection, and Patient Outcomes in Radiology and Neurology | Simbo AI - Blogs, 2025.
- Boon, M. AI Agents Supporting Early Detection in Neurological Diseases, 2025.
- RoX818. AI Diagnosing Diseases Before Symptoms Show. https://aicompetence.org/ai-diagnosing-diseases-before-symptoms-show/, 2025.
- Innovation, R. AI for Rare Disease Detection | 2025 Insights. https://www.rapidinnovation.io/post/ai-agent-rare-disease-diagnostic-assistant.
- A Look At AI-Driven Medtech For Rare Disease Diagnosis. https://www.meddeviceonline.com/doc/a-look-at-ai-driven-medtech-for-rare-disease-diagnosis-0001.
- The Role of Artificial Intelligence in Improving Diagnostic Accuracy and Disease Detection in Healthcare Settings | Simbo AI - Blogs, 2025.
- Revolutionizing Patient Care with AI-Driven Diagnostics | Thoughtful. https://www.thoughtful.ai/blog/revoluti onizing-patient-care-with-ai-driven-diagnostics.
- Generative AI in Healthcare: Enhancing Diagnosis and Treatment with AI-Generated Insights. https://www.arionresearch.com/blog/generative-ai-in-healthcare-enhancing-diagnosis-and-treatment-with-ai-generated-insights.
- The Future of Disease Diagnostics: AI-Powered General Practitioners Transform Healthcare. https://medistreamline.com/blog/our-blog-1/the-future-of-disease-diagnostics-ai-powered-general-practitioners-transform-healthcare-1, 2024.
- AI in Clinical Diagnostics: A Road Less Travelled, 2024.
- AstraZeneca’s New AI Technology MILTON Predicts More than 1,000 Diseases before Diagnosis. https://www.astrazeneca.com/media-centre/medical-releases/astrazeneca-new-ai-technology-milton-predicts-more-than-1000-diseases-before-diagnosis.html, 2024.
- AI-powered Tool Helps Doctors Detect Rare Diseases | UCLA Health. https://www.uclahealth.org/news/article/ai-powered-tool-rare-diseases.
- Using AI to Find Disease-Causing Genes. https://med.stanford.edu/news/insights/2022/06/using-ai-to-find-disease-causing-genes.html.
- AI and Voice Biomarkers: Transforming Disease Diagnosis | Owkin | Explainer Video. https://www.owkin.com/explainer-videos/voice-biomarkers.
- payal66. AI-Powered Disease Detection: Building a Machine Learning Model. https://www.rancholabs.com/post/ai-powered-disease-detection-building-a-machine-learning-model, 2025.
- AMIE: A Research AI System for Diagnostic Medical Reasoning and Conversations. https://research.google/blog/amie-a-research-ai-system-for-diagnostic-medical-reasoning-and-conversations/.
- AMIE Gains Vision: A Research AI Agent for Multimodal Diagnostic Dialogue. https://research.google/blog/amie-gains-vision-a-research-ai-agent-for-multi-modal-diagnostic-dialogue/.
- Digital Health: AI Agents for Healthcare. https://resources.nvidia.com/en-us-workstation/digital-health-ai-agents.
- AI Agents for Healthcare Explained: Use Cases, Applications, and How to Build One. https://www.cabotsolutions.com/blog/ai-agents-for-healthcare-explained-use-cases-applications-and-how-to-build-one.
- SmythOS - AI Agents in Healthcare: Transforming Diagnosis and Treatment, 2024.
- Prediction and Early Identification of Disease Through AI. https://www.siemens-healthineers.com/digital-health-solutions/artificial-intelligence-in-healthcare/ai-to-help-predict-disease.
- How AI Agents Improve Medical Imaging for Accurate Diagnosis. https://www.amplework.com/blog/ai-agents-for-medical-imaging-streamline-diagnosis/.
- Khurana, A. How AI Is Enhancing Early Disease Detection Through Medical Imaging?, 2025.
- International Classification of Diseases (ICD). https://www.who.int/standards/classifications/classification-of-diseases.
- Zitnik, M. Few Shot Learning for Phenotype-Driven Diagnosis of Patients with Rare Genetic Diseases. https://zitniklab.hms.harvard.edu/projects/SHEPHERD/.
- AI/ML Algorithms for Early Disease Detection and Diagnosis. https://binariks.com/blog/ai-machine-learning-for-early-disease-detection/.
- Expert Systems in, AI. https://www.geeksforgeeks.org/artificial-intelligence/expert-systems/, 00:10:58+00:00.
- Role of AI Agents in Therapy and Diagnosis: The Complete Guide. https://www.biz4group.com/blog/how-intelligent-ai-agents-transform-therapy-diagnosis.
- Incubity, A. Using AI Agents for Distributed Diagnosis in Healthcare, 2024.
- How AI Agents Are Changing Medical Imaging and Radiology | Digiqt Blog. https://digiqt.com/blog/ai-agents-in-medical-imaging-and-radiology/.
- Early Disease Detection through AI in Medical Imaging. https://www.signitysolutions.com/tech-insights/early-disease-detection-through-ai, 2023.
- AI In Early Disease Detection: Revolutionizing Healthcare. https://www.scalacode.com/blog/ai-in-early-disease-detection/, 2024.
- Chanda, D. AI in Early Disease Detection - Process, Use Cases, and More, 2024.
- The Impact of AI on Early Disease Detection. https://ycp.com/insights/article/artifical-intelligence-healthcare-southeast-asia.
- How Agentic AI Is Revolutionizing Rare Disease Identification Through Pattern Recognition. https://ciberspring.com/articles/how-agentic-ai-is-revolutionizing-rare-disease-identification-through-pattern-recognition/.
- The Path to Medical Superintelligence.
- D’Cunha, R. Microsoft’s Revolutionary Diagnostic Medical AI, Explained, 2025.
- Artificial Intelligence in the Diagnosis of Disease: An Analytical Review on the Current Trend in Research Leading to Better Outcomes - Premier Science. https://premierscience.com/pjai-24-274/, 2024.
- 6 Ways Johnson & Johnson Is Using AI to Help Advance Healthcare. https://www.jnj.com/innovation/artificial-intelligence-in-healthcare, 2025.
- Wilson, T. How AI Agents Transform the Healthcare Sector, 2025.
- AI Agents Show Promise in Transforming Healthcare. https://www.oracle.com/health/clinical-suite/ai-agents-healthcare/.
- AI Agents in Healthcare: A 2025 Guide for Hospitals | Digiqt Blog. https://digiqt.com/blog/ai-agents-in-healthcare/.
- Generative AI in Healthcare: Revolutionizing Patient Care and Diagnosis. https://www.intersystems.com/resources/generative-ai-in-healthcare-revolutionizing-patient-care-and-diagnosis/, 2024.
- Generative AI Healthcare: 15 Use Cases with Examples. https://research.aimultiple.com/generative-ai-healthcare/.
- AI Diagnostic Agents in Healthcare 2025. https://www.rapidinnovation.io/post/ai-agents-for-diagnostic-support.
- Google for Health - What Is Google for Health? https://health.google/.
- Subramanian, S. Will AI Agents Replace Humans in Medical Diagnosis Assistant Jobs? https://clouddon.ai/will-ai-agents-replace-humans-in-medical-diagnosis-assistant-jobs-552cd59e0a82, 2025.
- How These 9 AI Agents Are Transforming Patient Diagnosis and Care. https://www.solutelabs.com/blog/top-ai-agents-healthcare.
- Joshi, R. AI Agents For Healthcare: Use Cases, Benefits and Challenges, 2025.
- Genies - The Future of AI Agents // van Der Schaar Lab.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).