Submitted:
06 October 2025
Posted:
07 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Animals and Conditions and Feeding Procedure
2.3. Sample Collection
2.4. Analytical Methods
2.4.1. Growth Performance and Feed Utilization
2.4.2. Hematological Parameters Analysis
2.4.3. Immunological Parameters Analysis
2.4.4. Blood Plasma Metabolic Markers Analysis
2.4.5. Histological of Liver and Intestine Analysis
2.4.6. Carcass Composition Analysis
2.5. Digestibility Analysis
2.5.1. In Vitro Protein Digestibility of Marine Ingredients and Experimental Diets
2.5.2. In Vivo Estimation of Apparent Digestibility Coefficients
2.6. Ammonia Stress Challenge Test
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Feed Utilization
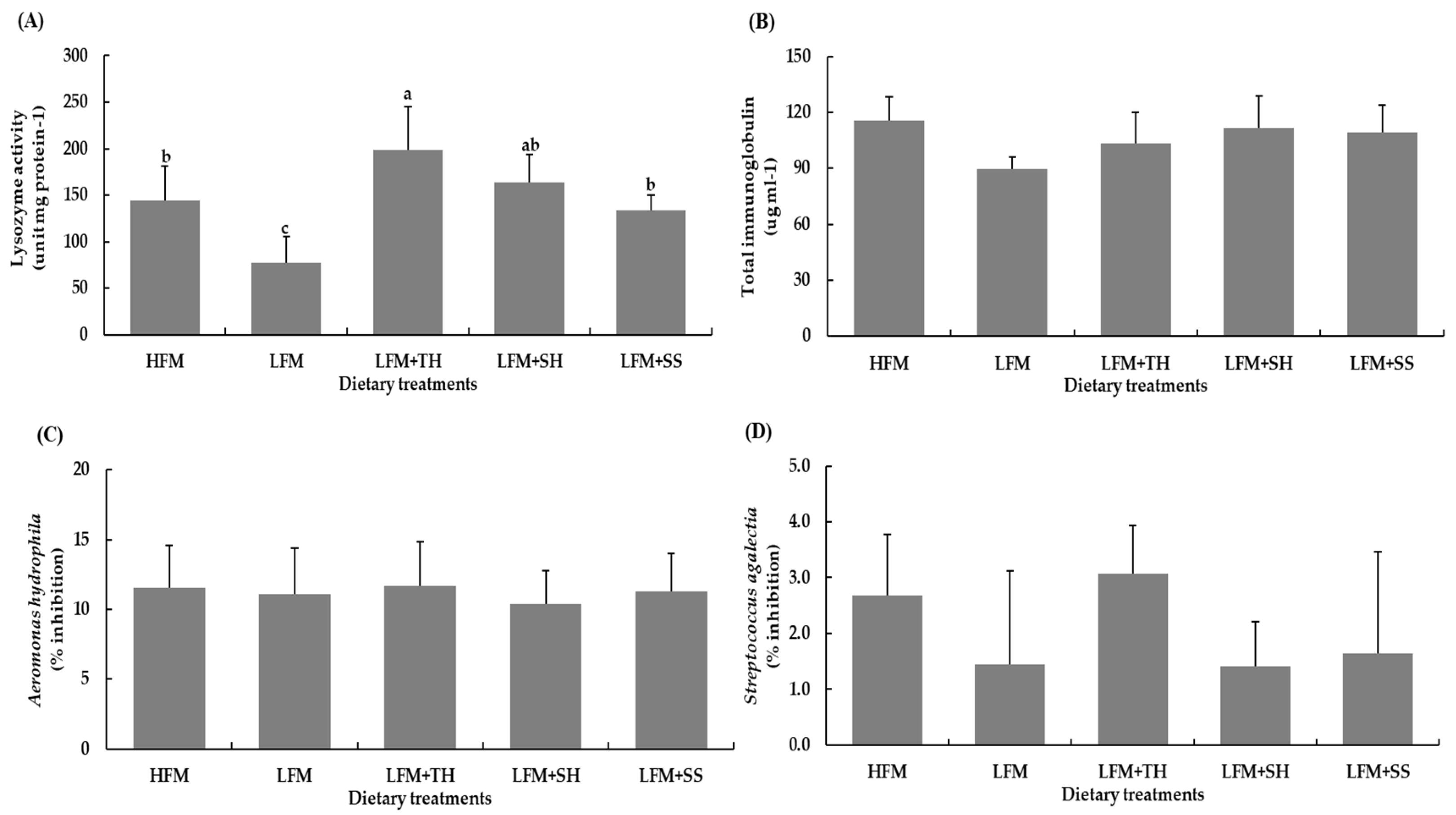
3.2. Hematological and Immunological
3.3. Blood Plasma Metabolic Markers
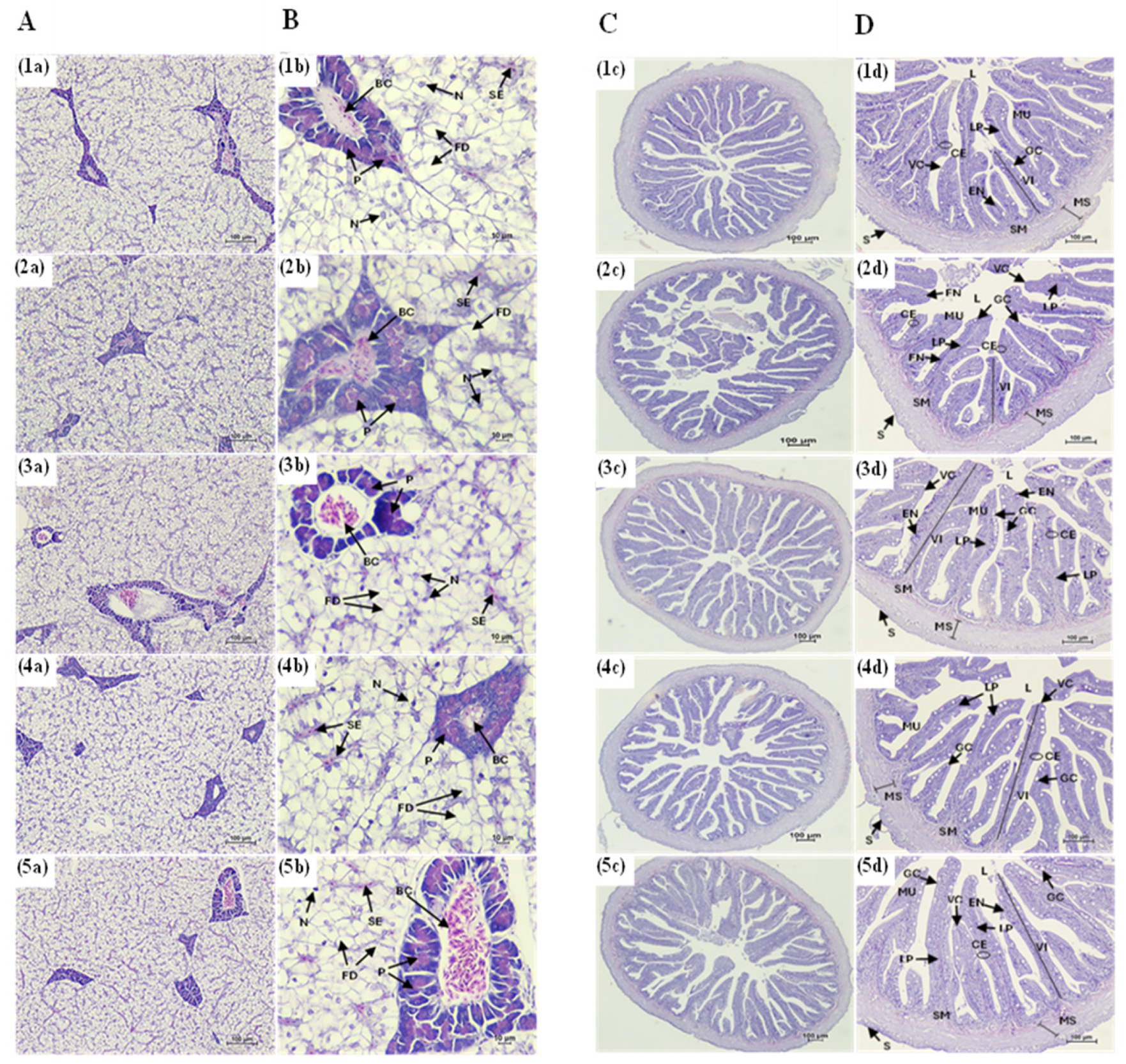
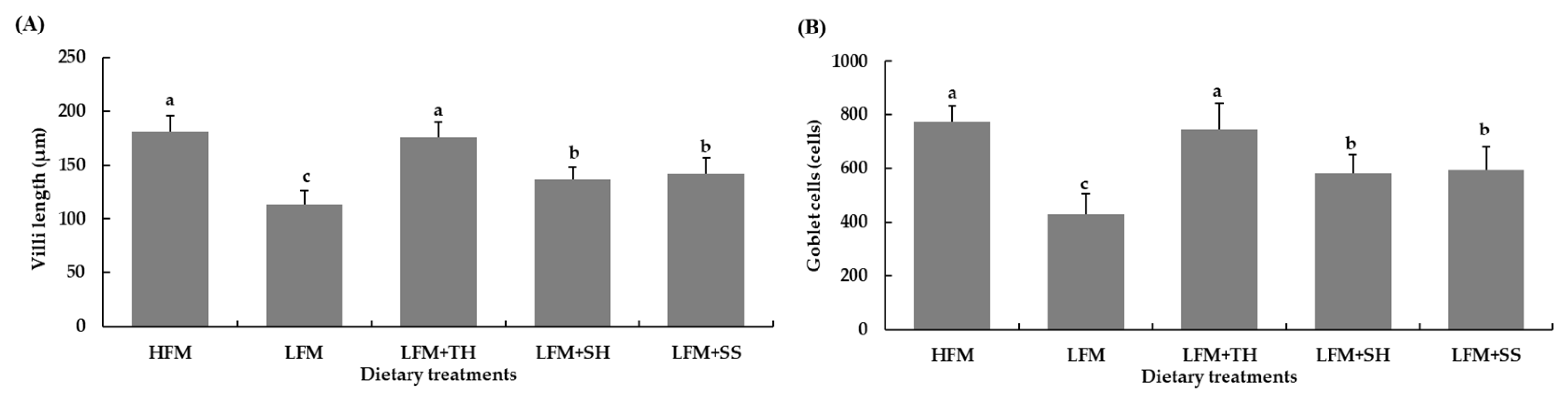
3.4. Histological of Liver and Intestine
3.5. Carcass Proximate Composition
3.6. Nutrient Digestibility
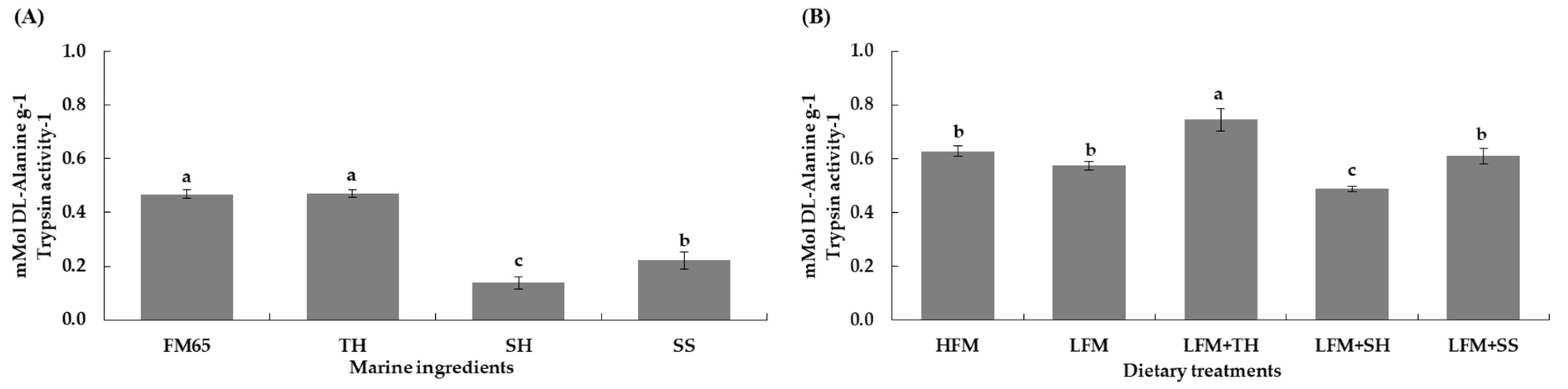
3.6.1. In Vitro Protein Digestibility of Marine Ingredients and Experimental Diets
3.6.2. Apparent Digestibility Coefficients (ADCs)
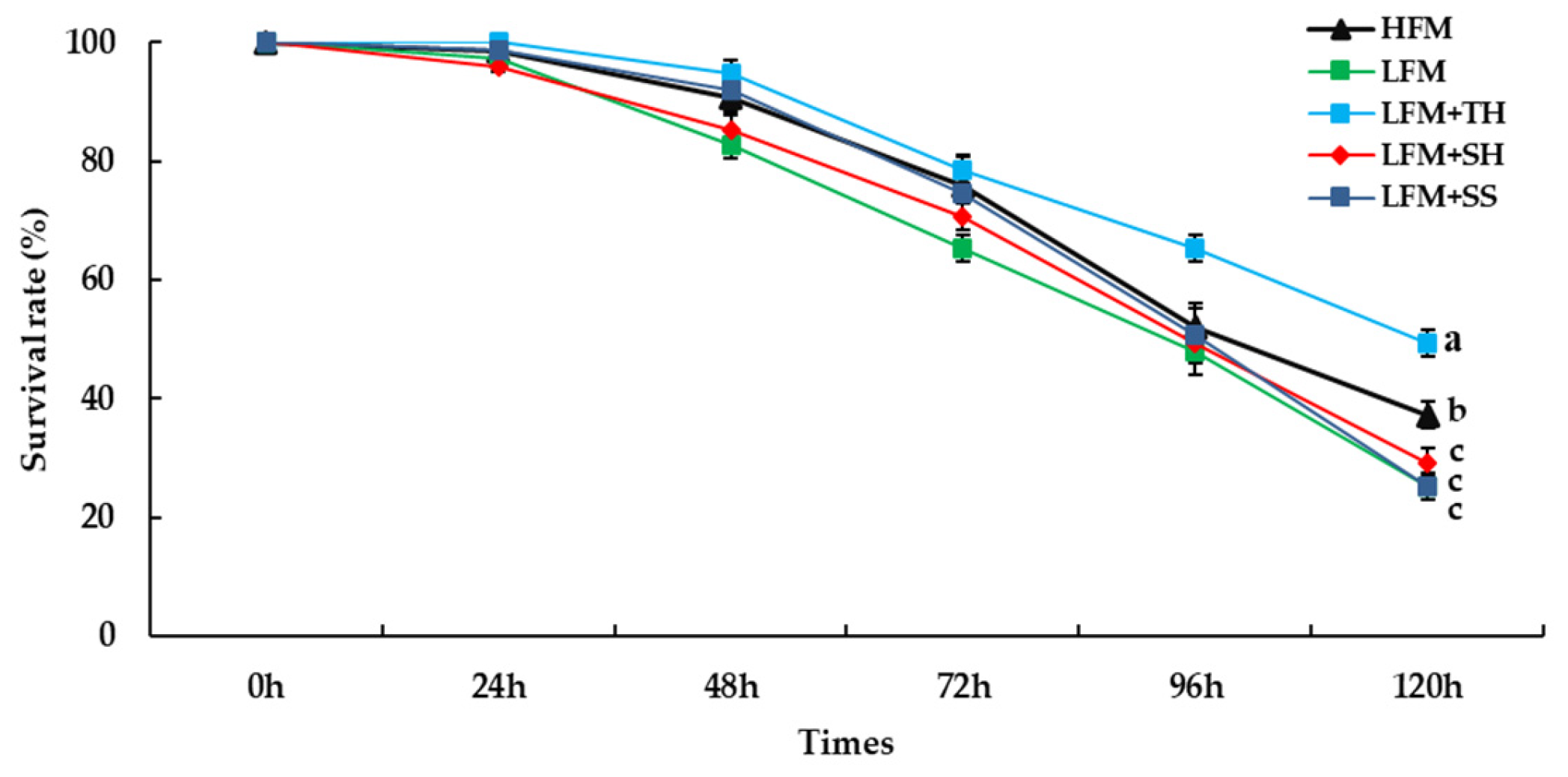
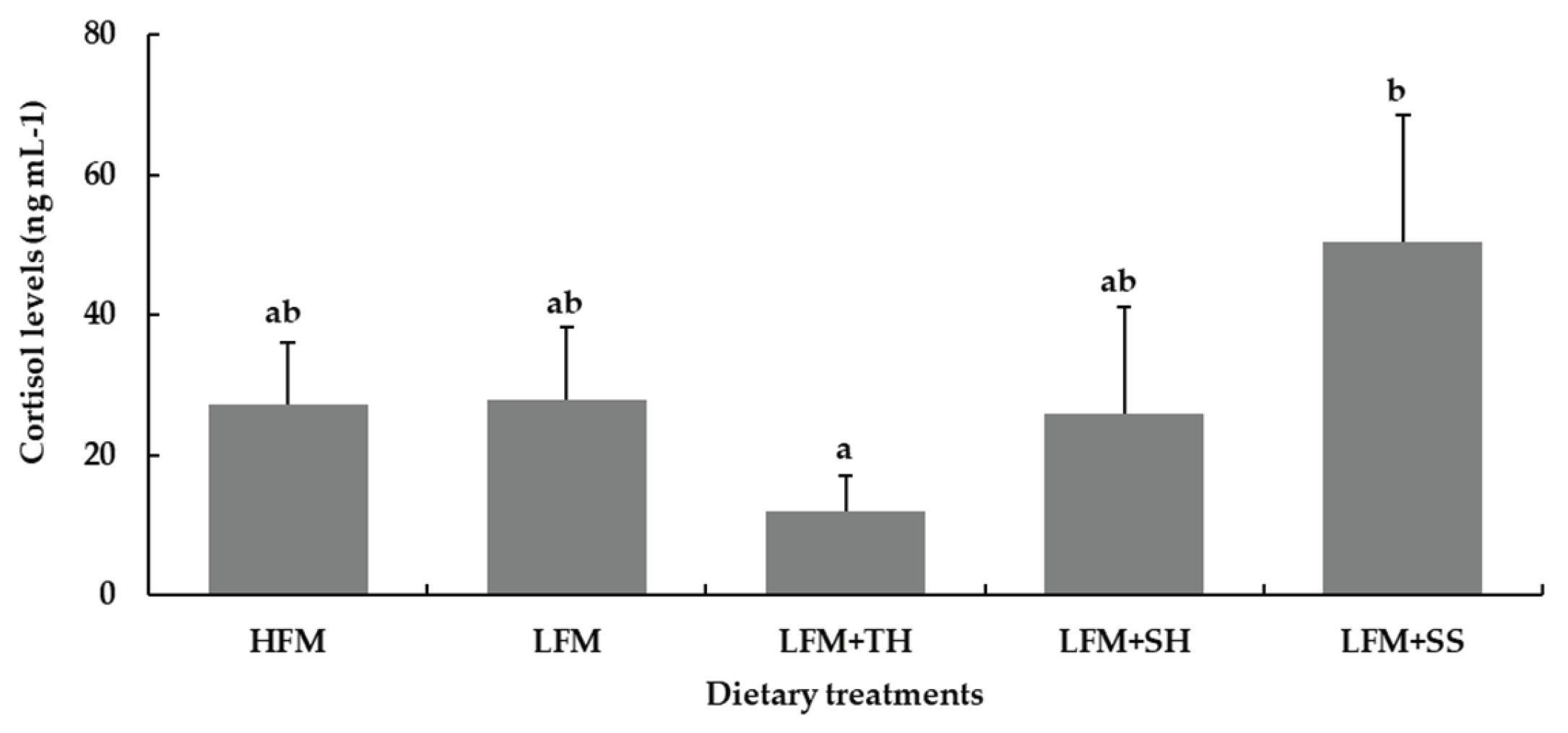
3.7. Ammonia Stress Challenge Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glencross, B. The nutritional management of barramundi, Lates calcarifer -A review. Aquac. Nutr. 2006, 12, 291–309. [Google Scholar] [CrossRef]
- Boonyaratpalin, M.; Williams, K.; Asian sea bass Lates calcarifer In, C.D. Asian sea bass Lates calcarifer. In Nutrient requirements and feeding of finfish for aquaculture; Webster, C.D., Lim, C.E., Eds.; 2009; pp. 40–50. [Google Scholar]
- Siddik, M.A.B.; Islam, M.A.; Hanif, M.A.; Chaklader, M.R.; Kleindienst, R. Barramundi, Lates calcarifer (Bloch, 1790): A new dimension to the fish farming in coastal Bangladesh. J. Aquacult. Res. Dev. 2016, 7, 1–3. [Google Scholar]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquac. 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Tantikitti, C. Feed palatability and the alternative protein sources in shrimp feed. Songklanakarin J. Sci. Technol. 2014, 36, 51–55. [Google Scholar]
- Khosravi, S.; Bui, D.T.H.; Fournier, V.; Kim, K.W.; Lee, K. Supplementation of protein hydrolysates to a low-fishmeal diet improves growth and health status of juvenile olive flounder, (Paralicthys olivaceus). JWAS. 2018, 49, 897–911. [Google Scholar]
- Ma, Z.; Hassan, M.M.; Allais, L.; He, T.; Leterme, S.; Ellis, A.V.; McGraw, B.; Qin, J.G. Replacement of fishmeal with commercial soybean meal and EnzoMeal in juvenile barramundi Lates calcarifer. Aquac. Res. 2018, 49, 3258–3269. [Google Scholar] [CrossRef]
- Tola, S.; Fukada, H.; Masumoto, T. Effects of feeding a fishmeal-free soy protein concentrate-based diet on the growth performance and nutrient utilization of red sea bream (Pagrus major). Aquac. Res. 2016, 50, 1087–1095. [Google Scholar] [CrossRef]
- Tola, S.; Sommit, N.; Seel-audom, M.; Khamtavee, P.; Waiho, K.; Boonmee, T.; Yuangsoi, B.; Munpholsri, N. Effect of dietary tuna hydrolysate supplementation on feed intake, growth performance, feed utilization and health status of Asian seabass (Lates calcarifer) fed a low fish meal soybean meal-based diet. Aquac. Res. 2022, 00, 1–15. [Google Scholar]
- Gunathilaka, B.E.; Khosravi, S.; Herault, M.; Fournier, V.; Lee, C.; Jeong, J.; Lee, K. Evaluation of shrimp or tilapia protein hydrolysate at graded dosages in low fish meal diet for olive flounder (Paralichthys olivaceus). Aquac. Nutr. 2020, 26, 1592–1603. [Google Scholar] [CrossRef]
- Suratip, N.; Charoenwattanasak, S.; Klahan, R.; Herault, M.; Yuangsoi, B. An investigation into the effects of using protein hydrolysate in low fish meal diets on growth performance, feed utilization and health status of snakehead fish (Channa striata) fingerling. Aquac. Rep. 2023, 30, 101623. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh, K.B.; Hemalatha, R.; Tummala, J. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Gamarro, E.; Orawattanamateekul, W.; Sentina, J.; Gopal, S. By-products of tuna processing GLOBEFISH Res. Program. FAO, 112. 2013; pp. 1–48. [Google Scholar]
- Siddik, M.A.B.; Pham, H.D.; Francis, D.S.; Vo, B.V.; Shahjahan, M. Dietary supplementation of fish protein hydrolysate in high plant protein diets modulates growth, liver and kidney health, and immunity of barramundi (Lates calcarifer). Aquac. Nutr. 2021, 27, 86–98. [Google Scholar] [CrossRef]
- Chotikachinda, R.; Tantikitti, T.; Benjakul, S.; Rustad, T.; Kumarnsit, E. Production of protein hydrolysate from skipjack tuna (Katsuwonus pelamis) viscera as feeding attractants for Asia seabass (Lates calcarifer). Aquac. Nutr. 2013, 19, 773–784. [Google Scholar] [CrossRef]
- Egerton, S.; Wan, A.; Murphy, K.; Collins, F.; Ahern, G.; Sugrue, I.; Busca, K.; Egan, F.; Muller, N.; Whooley, J.; McGinnity, P.; Culloty, S.; Ross, R.; Stanton, C. Replacing fishmeal with plant protein in Atlantic salmon (Salmo salar) diets by supplementation with fish protein hydrolysate. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Leduc, A.; Hervy, M.; Rangama, J.; Delépée, R.; Fournier, V.; Henry, J. Shrimp by-product hydrolysate induces intestinal myotropic activity in European seabass (Dicentrarchus labrax). Aquac. 2018, 497, 380–388. [Google Scholar] [CrossRef]
- Bui, H.T.D.; Khosravi, S.; Fournier, V.; Herault, M.; Lee, K. Growth performance, feed utilization, innate immunity, digestibility and disease resistance of juvenile red seabream (Pagrus major) fed diets supplemented with protein hydrolysates. Aquac. 2014, 418–419, 11–16. [Google Scholar] [CrossRef]
- Kim, H.S.; Jung, W.G.; Myung, S.H.; Cho, S.H.; Kim, D.S. Substitution effects of fishmeal with tuna byproduct meal in the diet on growth, body composition, plasma chemistry and amino acid profiles of juvenile olive flounder (Paralichthys olivaceus). Aquac. 2014, 431, 92–98. [Google Scholar] [CrossRef]
- Khosravi, S.; Bui, D.T.H.; Rahimnejad, S.; Herault, M.; Fournier, V.; Kim, S.; Jeong, J.; Lee, K. Dietary supplementation of marine protein hydrolysate in fish-meal base diet for red sea bream (Pagrus major) and olive flounder (Paralicthys olivaceus). Aquac. 2015, 435, 371–376. [Google Scholar] [CrossRef]
- Leal, A.L.G.; Fernandes de Castro, P.F.; de Lima, J.P.V.; de Souza Correia, E.; de Souza Bezerra, R. Use of shrimp protein hydrolysate in Nile tilapia (Oreochromis niloticus, L.) feeds. Aquac. Int. 2010, 18, 635–646. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Howieson, J.; Fotedar, R. Beneficial effects of tuna hydrolysate in poultry by-product meal diets on growth, immune response, intestinal health, and disease resistance to Vibrio harveyi in juvenile barramundi, Lates calcarifer. Fish Shellfish Immunol. 2019, 89, 61–71. [Google Scholar] [CrossRef]
- Blaxhall, P.C.; Daisley, K.W. Routine hematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- England, J.M.; Walford, D.M. Re-assessment of the reliability of haematocrit. Br. J. Hematol. 1972, 23, 247–253. [Google Scholar] [CrossRef]
- Parry, R.M.; Chandau, R.C.; Shahani, R.M. A rapid and sensitive assay of muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.L.; Hsieh, Y.T. Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: Analysis of reactive oxygen species. Dev. Comp. Immunol. 1994, 18, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kewcharoen, W.; Srisapoome, P. Probiotic effects of Bacillus spp. From Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish Shellfish Immunol. 2018, 94, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Clark, G. Tissue preparation and basic staining techniques. In Neuroanatomical Research Techniques; Robertson, R.T., Ed.; Academic Press, 1978; pp. 25–45. [Google Scholar]
- AOAC 2005. Official methods of analysis (18th edition) Association of Official Analytical Chemists International. Maryland, USA.
- Rungruangsak-Torrissen, K.; Moss, R.; Andresen, L.H.; Berg, A.; Waagbø, R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2006, 32, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Rungruangsak-Torrissen, K.; Rustad, A.; Sunde, J.; Eiane, S.A.; Jensen, H.B.; Opstvedt, J.; Nygard, E.; Samuelsen, T.A.; Mundheim, H.; Luzzana, U.; Venturini, G. In vitro digestibility based on fish crude enzyme extract for prediction of feed quality in growth trials. J. Sci. Food Agric. 2002, 82, 644–654. [Google Scholar] [CrossRef]
- Rungruangsak-Torrissen, K. Digestive efficiency, growth and qualities of muscle and oocyte in Atlantic salmon (Salmo salar L.) fed on diets with krill meal as an alternative protein source. J. Food Biochem. 2007, 31, 509–540. [Google Scholar] [CrossRef]
- Austreng, E. Digestibility determination in fish using chromic oxide marking and analysis of contents from different segments of the gastrointestinal tract. Aquac. 1978, 13, 265–272. [Google Scholar] [CrossRef]
- Cho, C.Y.; Slinger, S.J.; Bayley, H.S. Bioenergetics of salmonid fishes: Energy intake, expenditure and productivity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1982, 73, 25–41. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C. Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. J. Exp. Mar. Biol. Ecol. 2001, 259, 109–119. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Arias, M.V.; Papadakis, I.E.; Divanach, P. Evaluation of feed stimulants in diets for Sea Bream (Sparus aurata). Isr. J. Aquacult.Bamidgeh 2009, 61, 315–321. [Google Scholar] [CrossRef]
- Zheng, K.; Liang, M.; Yao, H.; Wang, J.; Chang, Q. Effect of dietary fish protein hydrolysate on growth, feed utilization and IGF-I levels of Japanese flounder (Paralichthys olivaceus). Aquac. Nutr. 2012, 18, 297–303. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids. 2009, 37, 283–293. [Google Scholar] [CrossRef]
- Wei, Y.; Li, B.; Xu, H.; Liang, M. Effects of lysine and leucine in free and different dipeptide forms on growth and amino acid transporters in turbot (Scophthalmus maximus). Fish Physiol. Biochem. 2020, 46, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Liang, M.; Yao, H.; Wang, J.; Chang, Q. Effect of size-fractionated fish protein hydrolysate on growth and feed utilization of turbot (Scophthalmus maximus L.). Aquac. Res. 2013, 44, 895–902. [Google Scholar] [CrossRef]
- Morais, S. The Physiology of Taste in Fish: Potential Implications for Feeding Stimulation and Gut Chemical Sensing. Rev Fish Sci Aquac. 2017, 25, 133–149. [Google Scholar] [CrossRef]
- Kasumyan, A.O.; Døving, K.B. Taste preferences in fisheries. Fish Physiol. Biochem. 2003, 4, 289–347. [Google Scholar]
- Dabrowski, K.; Zhang, Y.; Kwasek, K.; Hliwa, P.; Ostaszewska, T. Effects of protein-, peptide-and free amino acid-based diets in fish nutrition. Aquac. Res. 2010, 41, 668–683. [Google Scholar] [CrossRef]
- Mamauag, R.E.P.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Gao, J.; Nguyen, B.T.; Ragaza, J.A. Soy peptide inclusion levels influence the growth performance, proteolytic enzyme activities, blood biochemical parameters and body composition of Japanese flounder, Paralichthys olivaceus. Aquac. 2011, 321, 252–258. [Google Scholar] [CrossRef]
- Zheng, K.; Xu, T.; Qian, C.; Liang, M.; Wang, X. Effect of low molecular weight fish protein hydrolysate on growth performance and IGF-I expression in Japanese flounder (Paralichthys olivaceus) fed high plant protein diets. Aquac. Nutr. 2014, 20, 372–380. [Google Scholar] [CrossRef]
- Lin, X.; Chen, W.; Lin, S.; Luo, L. Effects of dietary cecropin on growth, non-specific immunity and disease resistance of tilapia (Oreochromis niloticus × O. aureus). Aquac. Res. 2015, 46, 2999–3007. [Google Scholar] [CrossRef]
- Xu, D.; He, G.; Mai, K.; Zhou, H.; Xu, W.; Song, F. Postprandial nutrient sensing and metabolic responses after partial dietary fishmeal replacement by soyabean meal in Turbot (Scophthalmus Maximus L.). Brit. J. Nutr. 2016, 115, 379–388. [Google Scholar] [CrossRef]
- Dai, M.; Li, S.; Fu, C.; Qiu, H.; Chen, N. The potential role of marine protein hydrolysates in elevating nutritive values of diets for largemouth bass, Micropterus Salmoides. Front. Mar. Sci. 2020, 7, 197. [Google Scholar] [CrossRef]
- 49 Bröer, S. Amino Acid Transport Across Mammalian Intestinal and Renal Epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef]
- Verri, T.; Barca, A.; Pisani, P.; Piccinni, B.; Storelli, C.; Romano, A. Di-and tripeptide transport in vertebrates: The contribution of teleost fish models. J. Comp. Physiol. B. 2017, 187, 395–462. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. Control of protein synthesis by amino acid availability. Curr Opin Clin Nutr Metab Care. 2002, 5, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell. 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Kilberg, M.S.; Pan, Y.X.; Chen, H.; Leung-Pineda, V. Nutrition Control of Gene Expression: How Mammalian Cells Respond to Amino Acid Limitation. Annu. Rev. Nutr. 2005, 25, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.W.; Yanke, D.; Mirza, J.; Ballantyne, J.S. Plasma Free Amino Acid Kinetics in Rainbow Trout (Oncorhynchus Mykiss) Using a Bolus Injection of 15N-Labeled. Amino Acids. 2011, 40, 689–696. [Google Scholar] [CrossRef]
- Lansard, M.; Panserat, S.; Plagnes-Juan, E.; Seiliez, I.; Skiba-Cassy, S. Integration of insulin and amino acid signals that regulate hepatic metabolism-related gene expression in Rainbow Trout: Role of TOR. Amino Acids. 2010, 39, 801–810. [Google Scholar] [CrossRef]
- Chen, G.; Feng, L.; Kuang, S.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.; Li, S.; Tang, L.; Zhou, X. Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian Carp (Cyprinus Carpio Var. Jian). Brit. J. Nutr. 2012, 8, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Xu, D.; Mai, K.; Zhou, H.; Xu, W.; He, G. Comparative study on the cellular and systemic nutrient sensing and intermediary metabolism after partial replacement of fishmeal by meat and bone meal in the diet of Turbot (Scophthalmus Maximus L.). PloS. One. 2016, 11, e0165708. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Mu, Y.; Zhang, Y.; Li, J.; Liang, M.; Zheng, K.; Wei, Y. Graded levels of fish protein hydrolysate in high plant diets for turbot (Scophthalmus maximus): Effects on growth performance and lipid accumulation. Aquac. 2016, 454, 140–147. [Google Scholar] [CrossRef]
- Sheng, Z.; Turchini, G.M.; Xu, J.; Fang, Z.; Chen, N.; Xie, R.; Zhang, H.; Li, S. Functional properties of protein hydrolysates on growth, digestive enzyme activities, protein metabolism, and intestinal health of larval largemouth bass (Micropterus salmoides). Front. Immunol. 2022, 13, 913024. [Google Scholar] [CrossRef] [PubMed]
- Cahu, C.; Zambonio-Infante, J.; Takeuchi, T. Nutritional components affecting skeletal development in fish larvae. Aquac. 2003, 227, 245–258. [Google Scholar] [CrossRef]
- Moriyama, S.; Ayson, F.G.; Kawauchi, H. Growth Regulation by Insulin-like Growth Factor-I in Fish. Biosci Biotechnol Biochem. 2000, 64, 1553–1562. [Google Scholar] [CrossRef]
- Gómez-Requeni, P.; Mingarro, M.; Calduch-Giner, J.A.; Médale, F.; Martin, S.A.M.; Houlihan, D.F.; Kaushik, S.; Pérez-Sánchez, J. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquac. 2004, 232, 493–510. [Google Scholar] [CrossRef]
- Men, K.; Ai, Q.; Mai, K.; Xu, W.; Zhang, Y.; Zhou, H. Effects of dietary corn gluten meal on growth, digestion and protein metabolism in relation to IGF-I gene expression of Japanese seabass (Lateolabrax japonicus). Aquac. 2014, 428–429, 303–309. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, C.Y.; Wang, D.D.; Li, X.F.; Xiao, L.; Zhang, X.; You, X.; Shi, Q.; Hu, QJ.; Fang, C.; Lin, H.R.; Zhang, Y. Transcriptome analysis reveals the molecular mechanisms underlying growth superiority in a novel grouper hybrid (Epinephelus fuscoguttatus♀×E. lanceolatus♂). BMC Genetics. 2006, 17, 24. [Google Scholar]
- Fuentes, E.N.; Björnsson, B.T.; Valdés, J.A.; Molina, A. Insulin-like growth factor-I (IGF-I) and its signaling pathways in skeletal muscle are regulated by nutrition and contribute to somatic growth in the fine flounder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1532–1542. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Gallinetti, J.; Harputlugil, E.; Mitchell, J.R. Amino acid sensing in dietary-restriction-mediated longevity: Roles of signal-transducing kinases GCN2 and TOR. Biomolecules. 2013, 499, 1–10. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Howieson, J.; Partridge, G.J.; Fotedar, R.; Gholipourkanani, H. Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi, Lates calcarifer. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Pham, H.C.; Siddik, M.A.B.; Le, H.M.; Ngo, M.V.; Nguyen, M.V.; Francis, D. Effects of Dietary Tuna Viscera Hydrolysate Supplementation on Growth, Intestinal Mucosal Response, and Resistance to Streptococcus iniae Infection in Pompano (Trachinotus blochii). Aquac. Nutr. 2022, 2022, 3645868. [Google Scholar] [CrossRef]
- Tang, H.G.; Wu, T.X.; Zhoa, Z.Y.; Pan, X.D. Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.). J. Zhejiang Univ. Sci. B. 2008, 9, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Wang, J.; Chang, Q.; Mai, K. Effects of different levels of fish protein hydrolysate in the diet on the nonspecific immunity of Japanese sea bass, Lateolabrax japonicus (Cuvieret Valenciennes, 1828). Aquac. Res. 2006, 37, 102–106. [Google Scholar] [CrossRef]
- Murray, A.L.; Pascho, R.J.; Alcorn, S.W.; Fairgrieve, W.T.; Shearer, K.D.; Roley, D. Effects of various feed supplements containing fish protein hydrolysate or fish processing by-products on the innate immune functions of juvenile coho salmon (Oncorhynchus kisutch). Aquac. 2003, 220, 643–653. [Google Scholar] [CrossRef]
- Ambardekar, A.A.; Reigh, R.C.; Williams, M.B. Absorption of amino acids from intact dietary proteins and purified amino acid supplements follows different time-courses in channel catfish (Ictalurus punctatus). Aquac. 2009, 291, 179–187. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Fotedar, R.; Howieson, J.; Siddik, M.A.B.; Foysal, J. The ameliorative effects of various fish protein hydrolysates in poultry by-product meal based diets on muscle quality, serum biochemistry and immunity in juvenile barramundi, Lates calcarifer. Fish Shellfish Immunol. 2000, 104, 567–578. [Google Scholar] [CrossRef]
- Salamat, N.; Ardeshir, R.A.; Movahedinia, A.; Rastgar, S. Liver histophysiological alterations in pelagic and benthic fish as biomarkers for marine environmental assessment. Int. J. Environ. Res. 2007, 11, 251–262. [Google Scholar] [CrossRef]
- Tenji, D.; Micic, B.; Sipos, S.; Miljanovic, B.; Teodorovic, I.; Kaisarevic, S. Fish biomarkers from a different perspective: Evidence of adaptive strategy of Abramis brama (L.) to chemical stress. Environ. Sci. Eur. 2020, 32, 47. [Google Scholar] [CrossRef]
- Shukla, G. A review on liver enzymes as useful biomarkers to evaluate the effects of pesticides on freshwater fish. WJBPHS. 2024, 19, 171–176. [Google Scholar]
- Kotzamanis, Y.P.; Gisbert, E.; Gatesoupe, F.J.; Zambonino Infante, J.; Cahu, C. Effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European seabass (Dicentrarchus labrax) larvae. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 205–214. [Google Scholar] [CrossRef]
- Caspary, W.F. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 1992, 55, 299S–308S. [Google Scholar] [CrossRef]
- Baeverfjord, G.; Krogdahl, Å. Development regression of soybean meal induced enteritis in Atlantic salmon Salmo salar L, distal intestine: A comparison with the intestines of fasted fish, J. Fish Dis. 1996, 19, 375–387. [Google Scholar] [CrossRef]
- Escaffre, A.-M.; Kaushik, S.; Mambrini, M. Morphometric evaluation of changes in the digestive tract of rainbow trout (Oncorhynchus mykiss) due to fish meal replacement with soy protein concentrate. Aquac. 2007, 273, 127–138. [Google Scholar] [CrossRef]
- Zhang, C.; Rahimnejad, S.; Wang, Y.; Lu, K.; Song, K.; Wang, L.; Ma, K. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquac. 2018, 483, 173–182. [Google Scholar] [CrossRef]
- Domeneghini, C.; Arrighi, S.; Radaelli, G.; Bosi, G.; Veggetti, A. Histochemical analysis of glycoconjugate secretion in the alimentary canal of Anguilla anguilla L. Acta Histochemica. Acta Histochemica. 2005, 106, 477–487. [Google Scholar] [CrossRef]
- Srichanun, M.; Tantikitti, C.; Kortner, T.M.; Krogdahl, A.; Chotikachinda, R. Effects of different protein hydrolysate products and levels on growth, survival rate and digestive capacity in Asian seabass (Lates calcarifer Bloch) larvae. Aquac. 2014, 428–429, 195–202. [Google Scholar] [CrossRef]
- Hevrøy, E.M.; Espe, M.; Waagbø, R.; Sandnes, K.; Ruud, M.; Hemre, G.I. Nutrient utilization in Atlantic salmon (Salmo salar L.) fed increased levels of fish protein hydrolysate during a period of fast growth. Aquac. Nutr. 2005, 11, 301–313. [Google Scholar] [CrossRef]
- Saidi, S.; Saoudi, M.; Ben, A.R. Valorisation of Tuna Processing Waste Biomass: Isolation, Purification and Characterisation of Four Novel Antioxidant Peptides from Tuna by-Product Hydrolysate. Environ. Sci. Pollut. Res. 2018, 25, 17383–17392. [Google Scholar] [CrossRef]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. CBP. 1998, 120C, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 21–268. [Google Scholar] [CrossRef]
| Ingredients | |||||||
|---|---|---|---|---|---|---|---|
| TH | SH | SS | |||||
| Chemical composition (% dry matter) | |||||||
| Dry matter | 35.5 | 95.6 | 45.1 | ||||
| Crude protein | 62.1 | 71.2 | 68.7 | ||||
| Crude lipid | 8.2 | 9.2 | 19.1 | ||||
| Crude ash | 20.2 | 12.3 | 6.7 | ||||
| Energy (MJ kg-1) | 17.8 | 21.8 | 24.2 | ||||
| Soluble protein | >90 | >90 | n.d.* | ||||
| Molecular weight (Dalton, % wet basis) | |||||||
| <500 | 64 | 79 | 82 | ||||
| 500-1000 | 5 | 10 | 7 | ||||
| 1000-5000 | 15 | 10 | 9 | ||||
| 5000-10000 | 8 | 1 | 1 | ||||
| >10000 | 8 | 0 | 0 | ||||
| Essential amino acids (% wet basis) | |||||||
| Arginine | 1.14 | 4.19 | 1.97 | ||||
| Histidine | 0.85 | 1.03 | 0.46 | ||||
| Isoleucine | 0.59 | 2.70 | 1.20 | ||||
| Leucine | 1.09 | 4.04 | 2.31 | ||||
| Lysine | 1.16 | 4.30 | 2.40 | ||||
| Methionine | 0.40 | 1.30 | 0.95 | ||||
| Phenylalanine | 0.59 | 2.90 | 1.17 | ||||
| Threonine | 0.72 | 2.50 | 1.17 | ||||
| Tryptophan | 0.15 | 0.80 | 0.37 | ||||
| Valine | 0.77 | 3.20 | 1.51 | ||||
| Total essential amino acids | 7.46 | 26.96 | 13.51 | ||||
| Non-essential amino acids (% wet basis) | |||||||
| Alanine | 1.34 | 4.30 | 1.85 | ||||
| Aspartic acid | 1.37 | 5.70 | 2.86 | ||||
| Glutamic acid | 2.12 | 9.00 | 4.31 | ||||
| Glycine | 1.91 | 4.90 | 1.85 | ||||
| Proline | 1.09 | 2.30 | 1.05 | ||||
| Serine | 0.74 | 2.40 | 1.85 | ||||
| Tyrosine | 0.47 | 2.30 | 0.28 | ||||
| Total essential amino acids | 7.46 | 26.96 | 13.51 | ||||
| Total non-essential amino acids | 9.04 | 30.90 | 14.05 | ||||
| Total amino acids | 16.50 | 59.08 | 27.56 | ||||
| Ingredients (% crude basis) |
Dietary treatment | ||||
|---|---|---|---|---|---|
| HFM | LFM | LFM+TH | LFM+SH | LFM+SS | |
| Fish meal, 65%CP | 25.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Soybean meal, 44%CP | 23.00 | 48.46 | 42.88 | 45.03 | 42.60 |
| Poultry by-product meal | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Wheat gluten | 5.06 | 5.06 | 5.06 | 5.06 | 5.06 |
| Wheat flour | 28.29 | 14.13 | 15.01 | 15.56 | 15.69 |
| Tuna crude oil | 4.50 | 5.80 | 5.50 | 5.80 | 5.40 |
| Choline chloride | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mono calcium phosphate | 0.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| DL-Methionine | 0.00 | 0.35 | 0.35 | 0.35 | 0.25 |
| L-Lysine | 0.10 | 0.20 | 0.20 | 0.20 | 0.00 |
| Salt, NaCl | 0.20 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin premix1 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Mineral premix2 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Antioxidants | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Antimicrobial agents | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Tuna hydrolysate | 5.00 | ||||
| Shrimp hydrolysate | 2.00 | ||||
| Salmon silage | 5.00 | ||||
| Analyzed chemical composition (% dry matter)3 | |||||
| Dry matter | 92.50 | 89.52 | 90.69 | 89.60 | 89.39 |
| Crude protein | 48.84 | 48.51 | 48.43 | 48.09 | 48.18 |
| Crude lipid | 11.89 | 11.95 | 11.91 | 11.83 | 11.97 |
| Ash | 10.18 | 9.75 | 10.34 | 10.67 | 10.18 |
| Crude fiber | 2.34 | 2.52 | 1.64 | 1.62 | 2.61 |
| Nitrogen-free extract | 26.75 | 27.26 | 27.68 | 27.79 | 27.06 |
| Gross energy (MJ kg-1) | 19.92 | 19.62 | 19.70 | 19.61 | 19.36 |
| Parameters | Dietary treatments | ||||
|---|---|---|---|---|---|
| HFM | LFM | LFM+TH | LFM+SH | LFM+SS | |
| Peptides profile (Dalton, % wet basis)1 | |||||
| <500 | 48.8 | 49.1 | 50.7 | 40.4 | 49.1 |
| 500-1000 | 3.9 | 2.7 | 3.4 | 3.0 | 3.8 |
| 1000-5000 | 7.0 | 5.6 | 6.6 | 7.2 | 6.6 |
| 5000-10000 | 3.9 | 4.0 | 4.1 | 5.1 | 3.8 |
| 10000-20000 | 9.1 | 9.4 | 5.6 | 11.2 | 9.8 |
| >20000 | 27.4 | 29.1 | 25.6 | 33.2 | 36.7 |
| Essential amino acids (% dry matter)2 | |||||
| Arginine | 2.60 | 2.70 | 2.48 | 2.72 | 2.52 |
| Histidine | 0.96 | 1.03 | 0.99 | 1.16 | 1.04 |
| Isoleucine | 1.70 | 1.67 | 1.57 | 1.64 | 1.68 |
| Leucine | 2.83 | 2.91 | 2.67 | 2.83 | 2.87 |
| Lysine | 2.65 | 2.72 | 2.59 | 2.81 | 2.73 |
| Methionine | 1.01 | 1.04 | 1.01 | 1.10 | 1.09 |
| Phenylalanine | 1.81 | 1.82 | 2.03 | 2.01 | 1.83 |
| Threonine | 1.26 | 1.33 | 1.22 | 1.33 | 1.32 |
| Tryptophan | 0.50 | 0.55 | 0.48 | 0.50 | 0.47 |
| Valine | 2.24 | 2.33 | 2.21 | 2.35 | 2.35 |
| Non-essential amino acids (% dry matter)2 | |||||
| Alanine | 1.80 | 1.79 | 1.65 | 2.03 | 2.05 |
| Aspartic acid | 3.39 | 3.56 | 3.20 | 3.53 | 3.16 |
| Cystine + Cysteine | 0.75 | 0.69 | 0.68 | 0.69 | 0.69 |
| Glutamic acid | 6.60 | 7.14 | 7.51 | 7.42 | 7.01 |
| Glycine | 2.24 | 2.19 | 2.14 | 2.29 | 2.31 |
| Proline | 2.39 | 2.35 | 2.45 | 2.68 | 2.59 |
| Taurine | 0.16 | 0.14 | 0.20 | 0.20 | 0.28 |
| Tyrosine | 1.36 | 1.38 | 1.50 | 1.66 | 1.55 |
| Serine | 1.77 | 1.98 | 1.73 | 1.94 | 1.83 |
| Total essential amino acids | 17.57 | 18.10 | 17.23 | 18.47 | 17.91 |
| Total non-essential amino acids | 20.48 | 21.22 | 21.06 | 22.44 | 21.47 |
| Sum-total amino acids | 38.05 | 39.32 | 38.29 | 40.90 | 39.38 |
| Parameters | Dietary treatments | |||||
|---|---|---|---|---|---|---|
| HFM | LFM | LFM+TH | LFM+SH | LFM+SS | P-value | |
| Initial body weight (g fish-1) | 2.61±0.01 | 2.62±0.01 | 2.62±0.01 | 2.62±0.01 | 2.62±0.01 | 0.067 |
| Final body weight (g fish-1) | 47.96±0.83a | 40.30±1.27b | 48.05±0.94a | 38.38±1.48c | 41.34±0.35b | <0.001 |
| Percent weight gain (%) | 1736.3±32.6a | 1436.4±47.2b | 1737.1±40.9a | 1367.5±52.7c | 1476.2±13.0b | <0.001 |
| Average daily gain (g day-1) | 0.81±0.01a | 0.67±0.02b | 0.81±0.02a | 0.64±0.03c | 0.69±0.01b | <0.001 |
| Specific growth rate (% day-1) | 5.20±0.03a | 4.88±0.06b | 5.20±0.04a | 4.80±0.06c | 4.92±0.01b | <0.001 |
| Feed intake (g fish-1) | 51.57±2.04a | 49.14±2.07b | 50.49±0.98ab | 46.65±1.98c | 52.40±1.05a | <0.001 |
| Feed conversion ratio | 1.16±0.05a | 1.42±0.94c | 1.14±0.01a | 1.34±0.03b | 1.38±0.04bc | <0.001 |
| Protein efficiency ratio | 1.80±0.05b | 1.58±0.06cd | 1.86±0.02a | 1.59±0.02c | 1.53±0.04d | <0.001 |
| Nitrogen retention (%) | 27.26±1.07a | 25.65±1.06b | 28.03±0.53a | 27.51±1.11a | 23.38±0.46c | <0.001 |
| Lipid retention (%) | 33.71±1.32b | 30.50±1.26c | 35.29±0.67a | 29.81±1.20c | 30.55±0.61c | <0.001 |
| Intraperitoneal fat (%) | 1.74±0.39 | 1.48±0.40 | 1.81±0.33 | 1.81±0.23 | 1.75±0.29 | 0.505 |
| Hepatosomatic index (%) | 2.38±0.26 | 1.95±0.46 | 2.09±0.34 | 2.43±0.34 | 1.92±0.34 | 0.090 |
| Viscerosomatic index (%) | 8.55±0.62 | 8.41±0.97 | 8.18±0.42 | 8.75±0.46 | 8.08±1.00 | 0.615 |
| Survival rate (%) | 96.8±3.0a | 90.0±2.7b | 96.0±2.4a | 96.8±3.0a | 96.8±2.3a | 0.023 |
| Parameters | Dietary treatments | |||||
|---|---|---|---|---|---|---|
| HFM | LFM | LFM+TH | LFM+SH | LFM+SS | P-value | |
| Red blood cells (×109 cells mL-1) | 1.72±0.08 | 1.63±0.15 | 1.66±0.08 | 1.67±0.16 | 1.64±0.15 | 0.803 |
| White blood cells (×107 cells mL-1) | 0.87±0.16 | 0.88±0.19 | 0.90±0.20 | 0.83±0.16 | 0.90±0.12 | 0.946 |
| Hematocrit (%) | 39.5±0.5 | 38.3±1.68 | 38.8±2.99 | 38.8±2.41 | 38.1±2.27 | 0.861 |
| Hemoglobin (g dL-1) | 11.73±1.21 | 10.88±1.14 | 11.22±0.78 | 11.69±0.68 | 10.62±1.63 | 0.470 |
| MCHC (g dL-1)1 | 29.78±3.01 | 28.61±2.58 | 28.92±1.37 | 29.95±0.76 | 27.95±3.55 | 0.691 |
| NBT (absorbance 540 nm)2 | 1.134±0.290 | 1.087±0.477 | 1.296±0.367 | 1.103±0.327 | 0.867±0.452 | 0.555 |
| SOD (%inhibition)3 | 66.39±4.48 | 59.29±7.32 | 65.26±4.82 | 53.56±16.48 | 60.85±13.78 | 0.350 |
| CAT (unit mL-1)4 | 33.46±8.20 | 36.80±10.32 | 34.01±14.36 | 39.48±10.04 | 45.43±9.68 | 0.414 |
| Parameters | Dietary treatments | |||||
|---|---|---|---|---|---|---|
| HFM | LFM | LFM+TH | LFM+SH | LFM+SS | P-value | |
| Cholesterol (mg dL-1) | 194.0±11.7 | 194.9±19.3 | 203.6±24.3 | 201.5±19.2 | 182.2±12.0 | 0.390 |
| Triglyceride (mg dL-1) | 265.8±55.5 | 230.9±59.1 | 228.7±27.1 | 203.0±68.2 | 194.4±46.2 | 0.274 |
| Glucose (mg dL-1) | 16.6±7.3 | 23.7±9.4 | 25.5±14.9 | 18.9±14.9 | 23.8±9.0 | 0.714 |
| Albumin (g dL-1) | 1.41±0.30 | 1.69±0.20 | 1.70±0.37 | 1.62±0.50 | 1.73±0.34 | 0.623 |
| Total protein (g dL-1) | 4.30±0.32 | 4.42±0.23 | 4.55±0.41 | 4.67±0.18 | 4.68±0.34 | 0.157 |
| Creatinine (mg dL-1) | 0.26±0.02 | 0.28±0.03 | 0.27±0.03 | 0.27±0.04 | 0.26±0.02 | 0.184 |
| Aspartate transaminase (U L-1) | 127.3±52.2 | 127.3±45.9 | 113.0±35.7 | 122.0±14.4 | 80.2±12.1 | 0.873 |
| Alkaline phosphatase (U L-1) | 63.8±6.7 | 60.0±2.3 | 60.2±12.5 | 59.0±6.9 | 64.8±6.8 | 0.701 |
| Parameters | Dietary treatments | |||||
|---|---|---|---|---|---|---|
| HFM | LFM | LFM+TH | LFM+SH | LFM+SS | P-value | |
| Proximate composition (%)1 | ||||||
| Dry matter | 26.23±0.09 | 26.53±0.12 | 26.67±1.20 | 26.00±0.00 | 26.17±0.38 | 0.759 |
| Crude protein | 48.29±0.14 | 47.54±0.08 | 48.28±0.98 | 47.60±0.09 | 47.33±0.95 | 0.158 |
| Crude lipid | 12.00±0.30 | 11.72±0.43 | 12.05±0.97 | 11.57±0.13 | 11.85±0.16 | 0.823 |
| Crude Ash | 12.56±0.75 | 12.18±0.08 | 12.69±0.79 | 12.24±0.00 | 12.61±0.79 | 0.900 |
| Parameters | Dietary treatments | |||||
|---|---|---|---|---|---|---|
| HFM | LFM | LFM+TH | LFM+SH | LFM+SS | P-value | |
| ADCd1 | 73.1±0.3a | 68.8±0.8b | 73.5±1.0a | 69.4±1.2b | 70.4±3.7ab | 0.035 |
| ADCp2 | 90.3±0.6a | 88.7±0.3b | 90.7±0.6a | 88.3±0.1b | 88.0±1.1b | 0.001 |
| ADCl3 | 83.8±1.5 | 79.6±2.7 | 82.5±2.9 | 78.1±0.2 | 77.6±3.9 | 0.066 |
| Parameters | Dietary treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| HFM | LFM | LFM+TH | LFM+SH | LFM+SS | P-value | |||
| Hematological parameters | ||||||||
| Red blood cells (×109 cells mL-1) | 1.38±0.12 | 1.20±0.31 | 1.23±0.22 | 1.36±0.62 | 1.17±0.06 | 0.668 | ||
| White blood cells (×107 cells mL-1) | 0.92±0.18 | 0.81±0.11 | 0.77±0.14 | 0.73±0.24 | 0.79±0.31 | 0.246 | ||
| Hematocrit (%) | 36.77±1.42 | 36.42±4.88 | 37.00±4.09 | 35.75±6.61 | 36.81±3.88 | 0.778 | ||
| Hemoglobin (g dL-1) | 9.94±0.45 | 9.80±1.29 | 9.58±2.29 | 9.88±2.74 | 9.79±1.14 | 0.707 | ||
| MCHC (g dL-1)1 | 26.83±1.41 | 26.85±1.82 | 25.72±3.85 | 27.40±3.20 | 26.80±3.15 | 0.226 | ||
| Non-specific immunity parameters | ||||||||
| NBT (absorbance 540 nm)2 | 0.892±0.440 | 0.974±0.489 | 1.130±0.191 | 0.661±0.085 | 0.834±0.073 | 0.937 | ||
| SOD (%inhibition)3 | 91.88±19.62 | 74.50±31.83 | 91.37±12.83 | 76.14±8.95 | 86.43±12.22 | 0.593 | ||
| CAT (unit mL-1)4 | 33.96±4.79 | 25.72±12.83 | 37.82±8.72 | 28.55±10.66 | 43.00±9.79 | 0.943 | ||
| Total immunoglobulin (µg dL-1) | 140.00±13.00 | 171.41±51.68 | 146.48±16.26 | 149.71±30.79 | 155.33±46.77 | 0.318 | ||
| Lysozyme activity (unit mg protein-1) |
48.59±29.43 |
33.81±23.66 |
38.41±22.01 |
48.18±15.92 |
39.96±9.83 |
0.439 |
||
| Blood plasma metabolic markers parameters | ||||||||
| Cholesterol (mg dL-1) | 162.2±10.3 | 171.2±6.2 | 164.8±12.8 | 170.2±22.8 | 157.7±17.0 | 0.912 | ||
| Triglyceride (mg dL-1) | 200.3±65.7 | 244.7±19.3 | 207.5±31.4 | 238.7±70.5 | 202.5±41.5 | 0.929 | ||
| Glucose (mg dL-1) | 15.2±11.3 | 14.5±4.1 | 17.5±3.6 | 10.3±1.8 | 9.2±4.2 | 0.997 | ||
| Albumin (mg dL-1) | 1.13±0.31 | 1.07±0.06 | 0.95±0.25 | 1.40±0.20 | 1.17±0.21 | 0.963 | ||
| Total protein (mg dL-1) | 3.98±0.33 | 3.93±0.13 | 3.62±0.35 | 4.25±0.40 | 3.95±0.39 | 0.999 | ||
| Creatinine (mg dL-1) | 0.23±0.06 | 0.23±0.03 | 0.22±0.03 | 0.23±0.06 | 0.25±0.05 | 0.483 | ||
| Aspartate transaminase (U L-1) | 72.3±10.6 | 79.3±16.1 | 75.0±23.5 | 82.3±20.9 | 81.8±14.0 | 0.845 | ||
| Alkaline phosphatase (U L-1) | 94.0±5.3 | 120.3±29.2 | 112.8±54.4 | 121.8±40.1 | 144.7±37.5 | 0.888 | ||
| Blood plasma electrolytes parameters | ||||||||
| Sodium (Na+) (mmol L-1) | 169.7±6.0 | 184.2±25.0 | 163.8±13.7 | 168.0±10.6 | 159.8±21.6 | 0.500 | ||
| Chloride (Cl-) (mmol L-1) | 140.8±8.5 | 132.2±15.1 | 133.7±18.7 | 132.3±15.0 | 126.5±16.6 | 0.843 | ||
| Potassium (K+) (mmol L-1) | 5.1±0.9 | 5.1±1.5 | 5.1±0.3 | 5.0±0.7 | 7.6±2.5 | 0.185 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





