Key Points
Strong evidence shows MDMA-AT can produce large, lasting reductions in PTSD symptoms.
Blinding remains problematic because MDMA’s psychoactive effects are obvious to participants.
TAML model explains how trauma memories are stored, reactivated, and reinforced in brain circuits.
MDMA creates a “therapeutic window” by lowering fear signals and enhancing emotional regulation.
Different trauma types respond differently: acute trauma may resolve quickly, while complex trauma needs extended care.
Introduction of BMPP, a new clinical trial design uses role-based masking to reduce bias while supporting effective therapy.
1. Introduction
MDMA-AT is a highly effective treatment for PTSD (Slomski, 2021). Multiple phase II and III trials have demonstrated large, durable reductions in PTSD symptoms (Christie et al., 2022; Jardim et al., 2021; Mitchell et al., 2021; Mitchell et al., 2023; Mithoefer et al., 2011; Mithoefer et al., 2018; Oehen et al., 2013; Ot'alora et al., 2018; Wang et al., 2021b), and the therapy is already being adopted in Australia, Israel, United States, Canada, and Switzerland (Avanceña et al., 2022; Brinzei et al., 2023; Karp Barnir et al., 2025; Mocanu et al., 2022; Oehen and Gasser, 2022). The effect sizes are substantial, the functional outcomes are clear, and patients are receiving the treatment now. The critical questions are no longer if MDMA is effective, but “why it is effective?”, “for whom it is most effective?”, and “how clinical trials can be structured to capture these truths without methodological artefact?”.
Against this backdrop, the U.S. Food and Drug Administration’s (FDA) recent rejection of MDMA on the grounds of inadequate blinding exposes a deep flaw in regulatory logic (Roseman, 2025). It was the FDA itself that initiated a plan in 2002 to develop MDMA as a treatment for PTSD (Doblin, 2002). However, as early as 1965, placebo-blinding for psychedelic drug trials was discussed by researchers as methodologically problematic (Metzner et al., 1965), By the early 2000s, leading researchers such as Professor Roland Griffiths had already warned that conventional double-blind methods were inadequate for studying psychedelics in humans (Griffiths et al., 2006; Griffiths et al., 2016), precisely because their subjective psychoactive effects inevitably compromise masking (Aday et al., 2022; Butler et al., 2022; Hovmand et al., 2023). Instead of engaging collaboratively to adapt methodology, professional and regulatory bodies continued to demand trials using conventional placebo-blinding, knowing it was inadequate (Doblin et al., 2019). Decades later, after two full phase III trials and an extension study were completed under these very conditions, the same regulators dismissed the findings based on the expected methodological weakness (Wilkinson and Sanacora, 2025). This is not methodological rigor, but bureaucratic bad faith, to demand the use of methods known to be flawed, and then reject the data for using them, represents a failure of both governance and public duty. The hypocrisy is underscored by the FDA’s own 2023 draft guidance for psychedelic trials (FDA, 2023), which explicitly acknowledged that placebo-controlled designs are inherently problematic because psychoactive effects compromise blinding (Harris, 2023), yet such placebo-controlled phase III trials remain a prerequisite for regulatory approval (Doblin et al., 2019). To both mandate and condemn the same design is not scientific oversight; it is institutional inconsistency that delays effective treatment. Researchers and regulators must work collaboratively to resolve the blinding methodology issue, rather than perpetuate a cycle of demand and dismissal (Schenberg, 2025).
This paper deliberately shifts the focus back to science. Its aim is fourfold. First, it introduces TAML, a conceptual model of trauma encoding, retrieval, and reconsolidation that isolates the core circuits most relevant to PTSD pathology (Brinzei, 2025b). Second, it clarifies what is meant when we say that MDMA “enhances fear extinction” (Feduccia and Mithoefer, 2018), that it “decreases amygdala activity” (Johansen and Krebs, 2009), and that MDMA opens a “therapeutic window” (Huestis et al., 2025; O’Brien and Nutt, 2025). These phrases are too often repeated without mechanistic clarity. MDMA disrupts functional connectivity (FC) between corticolimbic and self-referential systems (Singleton et al., 2022), particularly in the brain regions of the amygdala, anterior cingulate cortex (ACC), hippocampus, insula, dorsolateral prefrontal cortex (DLPFC), and default mode network (DMN) (Walpola et al., 2017). During this disruption, threat signalling loses its intrusive dominance, and introspective networks become more available. It is in this repeatable state that reconsolidation can occur. Put simply, MDMA is effective because it reliably creates a condition of safety and access that does not exist in ordinary brain states. If this therapeutic window is not carefully supported, the patient cannot fully benefit from the medicine.
Third, by integrating the TAML model with MDMA’s neurofunctional effects, I advance a phenotype-specific hypothesis: that acute, discrete traumatic events may be resolved with the standard one-to-three-session MDMA current treatment model, while complex or developmental PTSD, shaped over years of neurodevelopmental adaptation, likely requires longer, scaffolded therapeutic approaches.
Finally, this paper offers a methodological response to the very critique regulators have raised. BMPP employs a role-segregated, quadruple-masked design with epistemic blinding of participants to therapeutic hypotheses (Brinzei, 2025a). This framework directly mitigates expectancy effects while preserving therapeutic fidelity, providing trial architecture that psychedelic medicine has long needed.
The evidence that MDMA is an effective treatment for PTSD is overwhelming. To continue stalling on placebo comparisons and antiquated blinding standards is not scientific caution, it is an abdication of responsibility. The task now is precision, to map mechanisms, stratify phenotypes, and design fit-for-purpose trials that advance health outcomes rather than delay them.
2. A Functional Model of Dysregulated Memory Recall in Trauma
2.1. Mapping the Trauma-Affective .Memory Loop
The following section presents TAML, a conceptual model that helps clarify how trauma-related memories may become emotionally charged, persistently reactivated, and difficult to integrate (
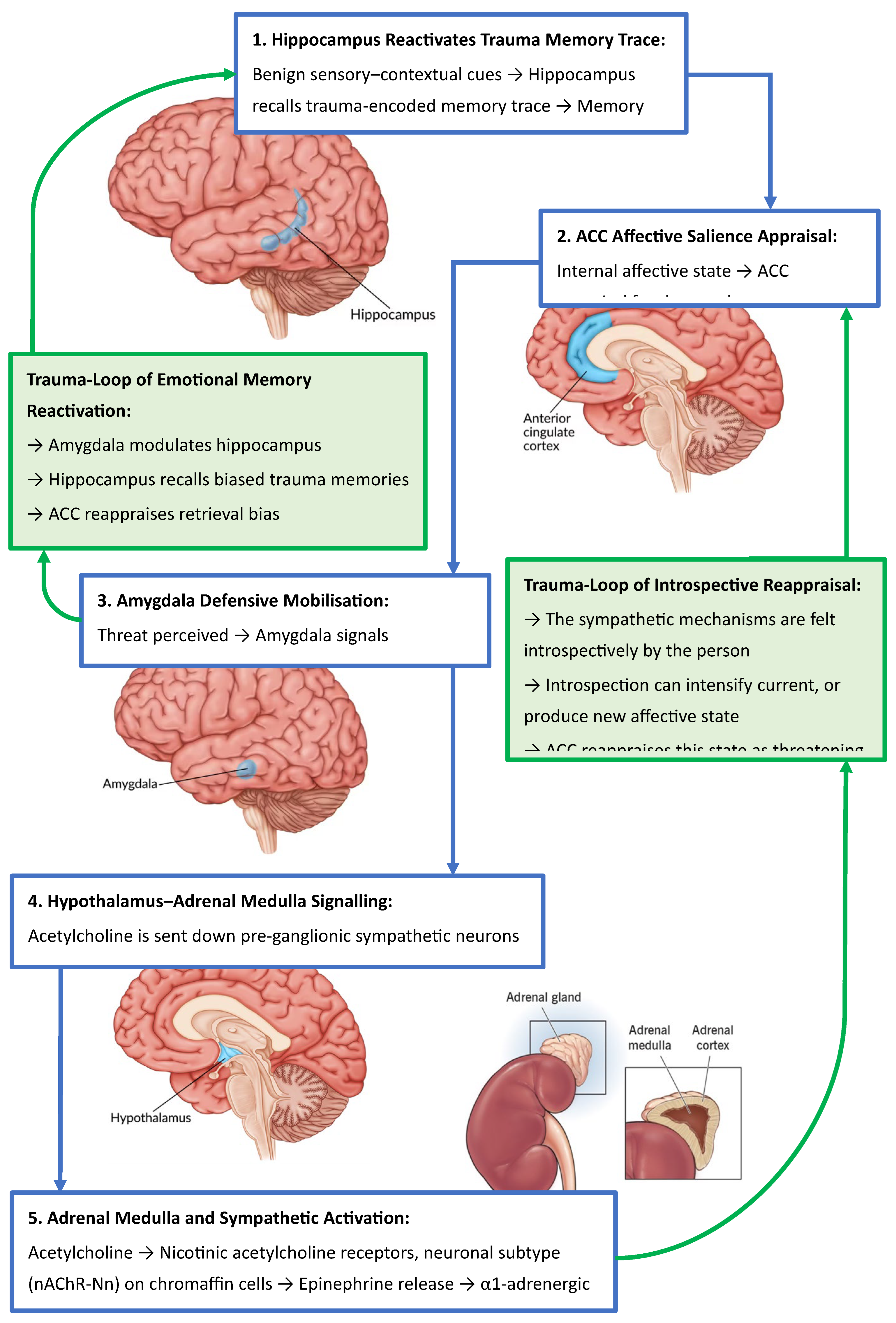
Figure 2). TAML is a heuristic tool intended to simplify the neurobiological process of trauma memory processing, its encoding, retrieval, and reconsolidation. Trauma memory processing involves numerous brain regions, neuromodulators, and systemic interactions that extend beyond the core loop described in TAML (Morris et al., 2025). Nonetheless, by isolating a core circuit involving the hippocampus, ACC, amygdala, and hypothalamus, then following the downstream pathway to the adrenal medulla, the TAML model offers a practical framework for understanding some of the core dysfunctions underlying trauma recall and emotional dysregulation.
Trauma encoding refers to the process by which the nervous system stores salient aspects of an overwhelming or threatening experience (Maddox et al., 2019). This includes sensory-perceptual information, spatial and temporal context, emotional states, autonomic responses, and unprocessed affect that may remain dormant but reactive.
Trauma retrieval and reconsolidation describes the process through which a stored traumatic memory is ‘reactivated’, often in response to environmental cues, the memory once recalled becomes temporarily labile, to be restabilised in memory by updating, modifying, or disrupting the trauma (Chiamulera et al., 2014). If the affective tone is not updated in a safe context, the memory may reconsolidate with its original emotional charge intact or worsen, with greater negative affective valence, perpetuating maladaptive responses. This is the loop aspect of TAML.
As we explore the neuropsychological mechanisms of MDMA, the TAML framework provides a conceptual scaffold for mapping how these mechanisms may interact with TAMLs, enabling the formulation of targeted hypotheses about MDMA’s differential efficacy across specific phenotypes of PTSD.
2.2. From Context to Crisis: Memory Retrieval and Affective Appraisal
The ACC is a key brain region involved in emotion regulation, attention, and the integration of affective experiences into retrieval of autobiographical memory (Martinelli et al., 2013). It acts as a bridge between feeling and remembering (García-Cabezas and Barbas, 2017), determining the emotional relevance of an experience linked via memory circuits from the hippocampus to the ACC (Wang et al., 2021a), then sending information from the ACC to the amygdala (Rolls, 2019). In the context of trauma, this function can become maladaptive, potentially leading to the development of PTSD (Kredlow et al., 2022; Thomaes et al., 2013).
2.3. Trauma as an Adaptive Response of the Amygdala
Trauma is not solely the consequence of external events but is fundamentally rooted in the affective state experienced by the nervous system at the time of the event, states such as helplessness, terror, betrayal, or abandonment (van der Kolk, 2000). The amygdala, a limbic structure central to the detection of emotional salience, particularly threat, encodes these affective experiences as dangerous (Kredlow et al., 2022). It subsequently adapts by sensitising the individual’s nervous system to similar internal states, activating the sympathetic nervous system (SNS) as a protective response when those affective patterns re-emerge.
Notably, the amygdala–ACC circuit does not encode episodic detail but rather affective valence (Kensinger et al., 2011). While much of the literature refers to “emotional information” in the context of memory encoding, this typically denotes external stimuli that elicit emotional responses (Murty et al., 2010). In the current trauma-framework, the focus is on internal affective states; physiological-emotional configurations that the amygdala–ACC circuit uses to guide salience detection and threat reactivity (Šimić et al., 2021). Its function in this context is not to recall the specific content of a past event, but to detect and respond to reoccurring internal affective states associated with prior threat (Liberzon and Abelson, 2016). In essence, it operates on a heuristic akin to: “this internal state once co-occurred with danger, if it arises again, initiate defensive mechanisms”.
Rather than reflecting a dysfunction or adaptation, this represents the amygdala’s core functional role in affective pattern recognition and threat detection (Fox et al., 2015). In PTSD, this mechanism becomes sensitised, triggering disproportionate or misaligned threat responses in the absence of actual danger (Kredlow et al., 2022), a pattern described in the TAML model. TAML frames this process as a kind of feedback loop, where reactivation of a prior affective state amplifies the emotional memory network and maintains the sense of threat even when no external danger is present. This framework also provides a foundation for linking TAML to the current understanding of MDMA’s neurobiological mechanisms and how they modulate trauma-related processes; processes that will be explored later in this paper.
2.4. The Role of the ACC in Triggering the Adaptive Response of the Amygdala
As outlined in TAML, the ACC compares present affective states to past traumatic imprints. When they resemble, it reactivates the amygdala and sympathetic cascade, perpetuating PTSD responses (Etkin et al., 2011; Pitts et al., 2022). This neural mechanism exemplifies how trauma-related affective conditioning can perpetuate dysregulated physiological and psychological responses in the absence of actual threat.
2.5. Affective Fusion: When Emotion Becomes Identity
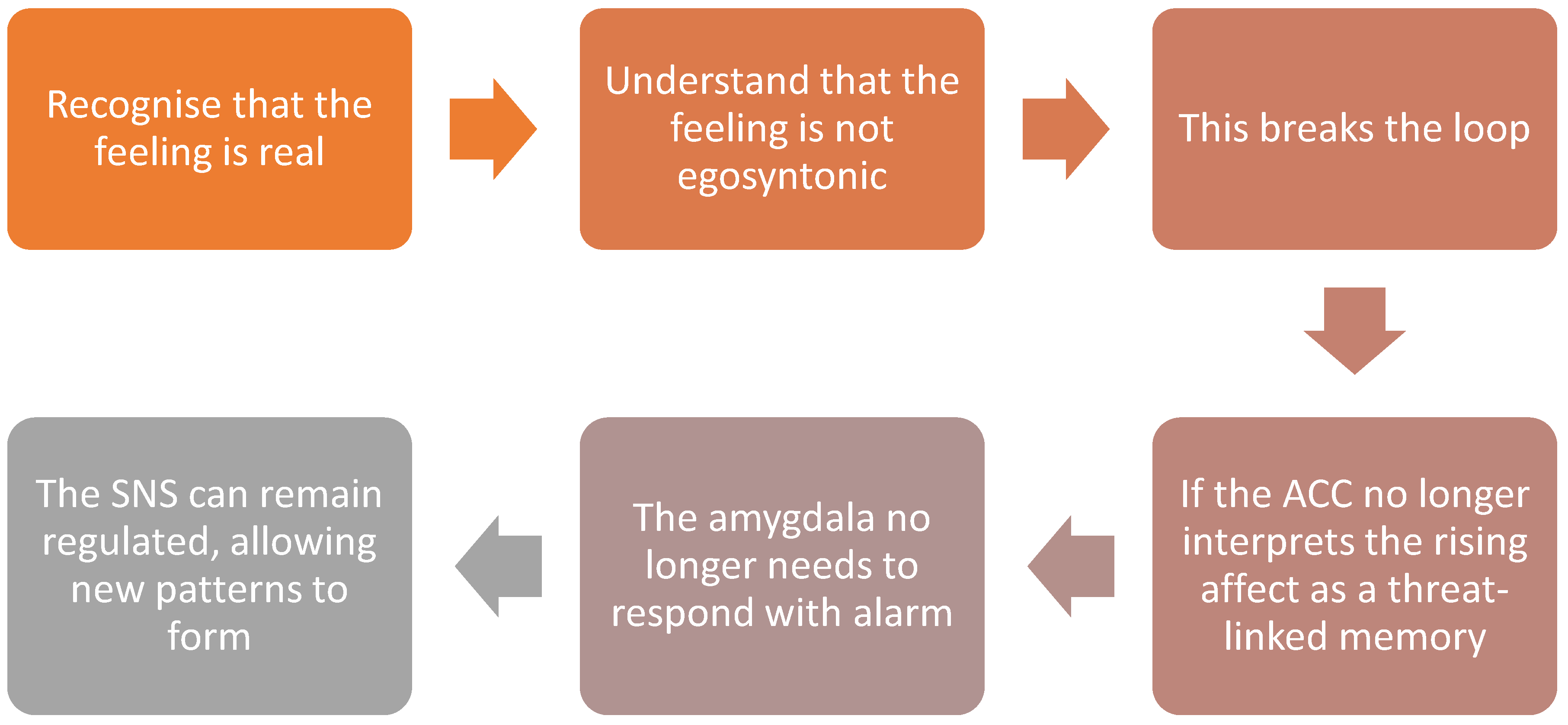
Beyond threat appraisal, the ACC contributes to self-referential meaning, explaining why trauma-related states can fuse with identity (Sharot et al., 2007). Functional Magnetic Resonance Imaging (fMRI) studies have particularly found that the rostral substructure of the ACC (rACC) is engaged during negative self-referential processing (Wagner et al., 2013). Over time, these affective imprints may shift from transient emotional states to egosyntonic identity structures. That is, the individual no longer merely experiences fear, but comes to identify with it: “I am unsafe”, “I am broken”, “I am unworthy”. This fusion of affect and identity forms a psychological trap, in which emotionally conditioned neural patterns are mistaken for intrinsic selfhood, thereby perpetuating dysregulation and obstructing integration or healing.
Figure 1.
Dis-identification: interrupting trauma-driven affective loops by recognising emotions as states rather than identity.
Figure 1.
Dis-identification: interrupting trauma-driven affective loops by recognising emotions as states rather than identity.
Figure 2.
Diagrammatic representation of TAML illustrating the conceptual model of the neurobiological mechanisms by which benign environmental cues trigger PTSD episode activation.
Figure 2.
Diagrammatic representation of TAML illustrating the conceptual model of the neurobiological mechanisms by which benign environmental cues trigger PTSD episode activation.
2.6. Dis-identification and the Deconstruction of Affective Imprints
Psychological healing begins with the capacity to bring conscious awareness to the affective signature that the ACC has associated with a traumatic memory (Lanius et al., 2011). This signature is not usually recalled as a discrete memory but as an implicit affective tone, a felt state of fear, shame, or helplessness, that dominates perception and behaviour when left unexamined (van der Kolk, 2006). Over time, such affective tones can become egosyntonic, fusing with identity. Dis-identification interrupts this fusion, shifting perspective from “this is me” to “this is a state I am experiencing”, enabling neural and psychological restructuring (Barber et al., 2025). This distinction is crucial, when the self is equated with trauma, every recurrence of affective arousal reinforces the trauma schema. By stepping back into observation, the individual introduces psychological distance between the arising affect and their core identity.
This decoupling allows both neural and psychological restructuring (Mueser et al., 2015). On the neural level, repeated observation of affect without immediate defensive mobilisation weakens the overlearned FC between the ACC, amygdala, and DNM, reducing the likelihood of automatic hyperarousal. On the psychological level, dis-identification opens the possibility of new self-narratives, where traumatic states are reframed as experiences rather than identities (Fisher, 2017). This process is not about denying the trauma but about reclaiming agency in the face of it, recognising that while the affective imprint was once a survival response, it no longer needs to define the self in the present.
3. Neuropsychopharmacology of MDMA
3.1. Affective Circuitry Modulation in MDMA-Assisted Therapy
This section does not revisit the well-known pharmacodynamics of MDMA on serotonin, dopamine, and oxytocin systems. Instead, it highlights a less discussed but crucial element: how MDMA changes network-level activity in the corticolimbic system, especially amygdala–ACC FC that underpins trauma-related affective memory, threat detection, and emotional appraisal (Zhang et al., 2025).
3.2. Corticolimbic Decoupling and Salience Recalibration
Neuroimaging confirms the amygdala–ACC threat loop described in TAML: amygdala hyperactivity paired with biased ACC appraisal (Kredlow et al., 2022; Young et al., 2018).
MDMA interrupts this loop. Imaging studies show decreased amygdala hyperactivity alongside increased ACC engagement (Carhart-Harris et al., 2015; Singleton et al., 2022). Importantly, these are related but separable mechanisms:
Decreasing amygdala hyperactivity: MDMA directly dampens the amygdala’s excitability and weakens its bottom-up output to the hypothalamus (Sottile and Vida, 2022), reducing the body’s automatic fear-driven reactions.
Enhancing fear extinction: By modulating amygdala–ACC FC, MDMA raises the threshold for the ACC to classify a stimulus as threatening (Doss et al., 2018; Luoma and Lear, 2021; Walpola et al., 2017). This recalibration allows traumatic memories to be revisited without triggering the defensive sympathetic cascade, creating the conditions for extinction learning.
Together, this corticolimbic decoupling reduces the flow of exaggerated fear signals and recalibrates affective salience. The individual can engage with traumatic material in a more regulated state, without being overwhelmed (Bedi et al., 2009).
In sum, “decreasing amygdala hyperactivity” and “enhancing fear extinction” describe distinct but synergistic effects. One suppresses the raw intensity of fear signals; the other alters how those signals are interpreted and integrated. This dual action, combined with the loosening of DMN dominance, defines MDMA’s therapeutic window, a temporary brain state where trauma can be processed safely and constructively to create new self-referential introspection.
3.3. The Therapeutic Window: Self-Referential Network Remapping and Affective Decoupling
Beyond fear extinction, MDMA alters DMN activity, especially in circuits tied to self-referential processing and autobiographical meaning-making (Gattuso et al., 2023; Schmid and Bershad, 2024). Under unresolved trauma, affective states such as shame or fear are embedded within the ACC–DMN–insula network as enduring identity structures: “I am unsafe”, “I am broken”. These are not simply thoughts but embodied self-states, reinforced by neural circuitry.
MDMA facilitates a transient decoupling of affect from self-schema (van der Kolk et al., 2024). With the amygdala–ACC circuit less likely to misclassify interoceptive affect as threat, defensive amygdala intrusion recedes. In this quieter state, the insula’s interoceptive signals can rise to conscious awareness (Walpola et al., 2017). This represents a period of insula upregulation, when interoceptive input carries more weight than fear-conditioned affect.
This is the therapeutic window: a brain state where emotional and affective states can be observed rather than fused with, enabling metacognitive awareness. Within this altered context, traumatic memories can be re-encountered without defensive mobilisation. Instead, they are re-encoded against a backdrop of coherence, empathy, and agency. Over repeated exposures, the traumatic imprint shifts from a danger-laden signal to a survivorship narrative, supporting integration.
3.4. Role of the Prefrontal Cortex in Supporting Integration
During this process, the prefrontal cortex (PFC) supports integration by providing reflective and rational scaffolding for the raw interoceptive signals emerging from the insula, particularly via the insula–DLPFC circuit (Walpola et al., 2017). While not the primary driver of therapeutic change, the PFC works in concert with ACC recalibration and insula upregulation, enabling the individual to link felt bodily states with higher-order meaning. This network synergy allows emotional experiences to be observed without fusion, reinterpreted within a safe context without amygdala intrusion, and then woven into a coherent self-narrative. The potency of MDMA-AT lies in the integrative process of insula-driven anchoring of new affective states, concurrent with amygdala–ACC circuit recalibration to reduce maladaptive threat appraisal, while the PFC helps organise these shifts into a survivable and adaptive identity structure. In this way, cognition does not override emotion but instead supports the consolidation of new learning, ensuring that re-encoded traumatic memories can be stably integrated into a broader narrative of recovery.
4. The Brinzei MDMA-PTSD Protocol
4.1. Formulating the Hypothesis
The central neurobiological hypothesis of this protocol is that MDMA-AT is most effective when trauma is encoded as an acute affective imprint, typically arising from single-event or time-bound exposures. In such cases, the dysregulated circuitry, principally the amygdala–ACC circuit, can often be recalibrated through one to three well-structured MDMA sessions.
Under MDMA, amygdala hyperactivity is dampened, reducing fear signals, while the ACC recalibrates salience appraisal, and the insula brings interoceptive signals to awareness without being hijacked by defensive responses (Walpola et al., 2017). The PFC then provides reflective scaffolding, enabling traumatic memories to be re-encoded as survivable experiences within a coherent self-narrative. For acute trauma phenotypes, this network-level modulation can rapidly reorganise the trauma loop, producing durable resolution.
By contrast, complex or developmental PTSD arises from years of chronic threat, relational neglect, or environmental adversity (Sar, 2011). Here, trauma is not a discrete imprint but has shaped self-referential networks, embedding dysregulation into the DMN, ACC, and insula over neurodevelopmental time (Akiki et al., 2017; Thomason and Marusak, 2017). In these cases, MDMA’s neuromechanisms while still catalytic in generating windows of metacognitive clarity, embodied safety, and affective recontextualisation are unlikely to produce full reorganisation within one to three sessions. Instead, repeated, scaffolded interventions are required, with MDMA acting as a facilitator of long-term neural and psychological integration.
This distinction between acute and complex trauma is thus neuroarchitectural. It reflects differences in how memories are encoded and embedded across corticolimbic and self-referential systems. This neuroarchitectural distinction should guide both therapeutic design and clinical expectations.
4.2. Differentiating Trauma Exposure: Neurobiological Implications for MDMA-Assisted Therapy
4.2.1. Neurophenomenological Stratification of Trauma Exposure
To refine the clinical use of MDMA-AT, trauma must be differentiated not only by symptoms but by the underlying encoding architecture. The following formulations represent a working model to guide precision-targeted interventions, acknowledging the need for empirical validation.
4.2.2. Direct Exposure and Affective Traceability
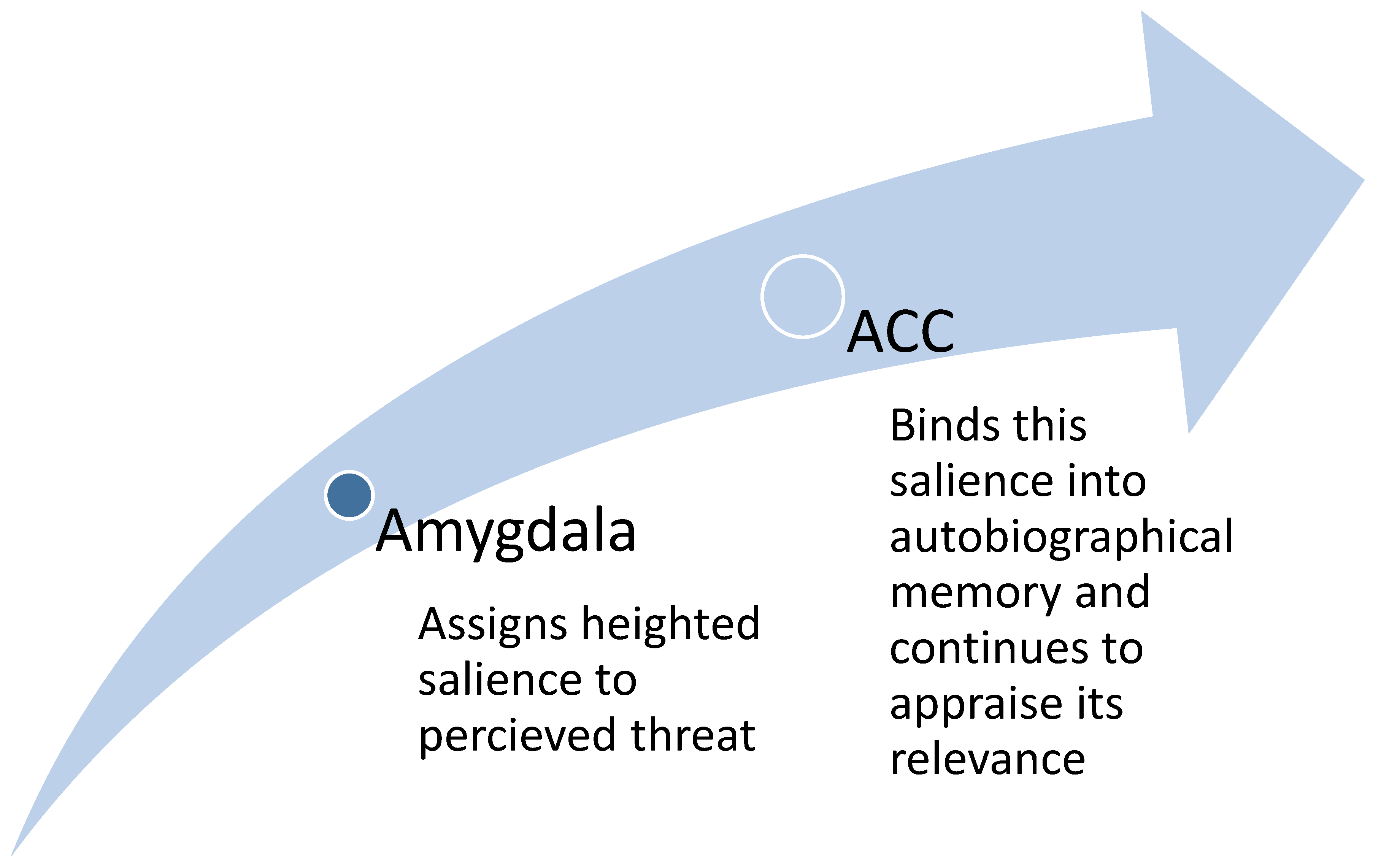
Acute, direct traumas such as combat, assault, or catastrophic injury tend to create sharply defined imprints within the corticolimbic system (Edmondson, 2014), particularly in the amygdala–ACC circuit. In these cases, the amygdala assigns intense salience to the threat (Fox et al., 2015), while the ACC integrates this salience into autobiographical memory and continues to appraise its relevance over time (García-Cabezas and Barbas, 2017). This combination produces a clear trauma trace that can be readily reactivated and is strongly linked to TAML.
Figure 3.
Amygdala–ACC circuit in PTSD: dysregulated connectivity drives salience misclassification and defensive mobilisation.
Figure 3.
Amygdala–ACC circuit in PTSD: dysregulated connectivity drives salience misclassification and defensive mobilisation.
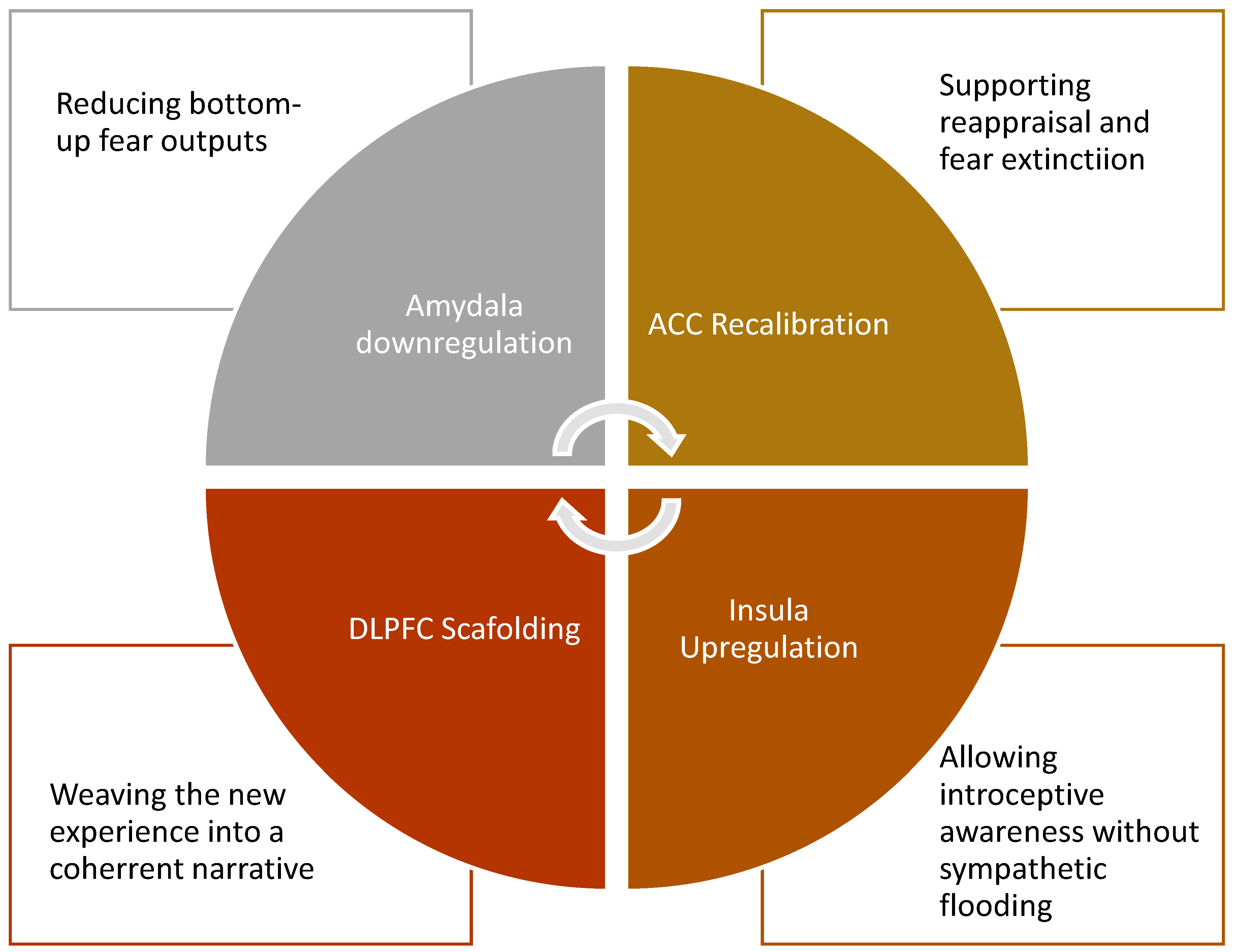
MDMA facilitates resolution of these imprints through several complementary mechanisms. It downregulates the amygdala, dampening bottom-up fear signals, and recalibrates the ACC, allowing more accurate reappraisal and extinction of threat associations (Zhang et al., 2025). At the same time, it upregulates the insula, enabling interoceptive states to rise into awareness without sympathetic flooding, while the PFC provides reflective scaffolding, weaving these revised affective states into a coherent and survivable narrative (Walpola et al., 2017). Together, these network-level effects enable affective decoupling, in which the traumatic memory can be revisited without overwhelming physiological responses, permitting reconsolidation within a safer and more adaptive emotional frame (Luoma and Lear, 2021). Acute trauma phenotypes therefore align closely with MDMA’s neuromechanisms and are often responsive to limited-session interventions.
Figure 4.
MDMA’s network-level effects: amygdala downregulation, ACC recalibration, insula upregulation, and PFC scaffolding enable safe trauma reconsolidation.
Figure 4.
MDMA’s network-level effects: amygdala downregulation, ACC recalibration, insula upregulation, and PFC scaffolding enable safe trauma reconsolidation.
4.2.3. Indirect Exposure and Affective Enmeshment
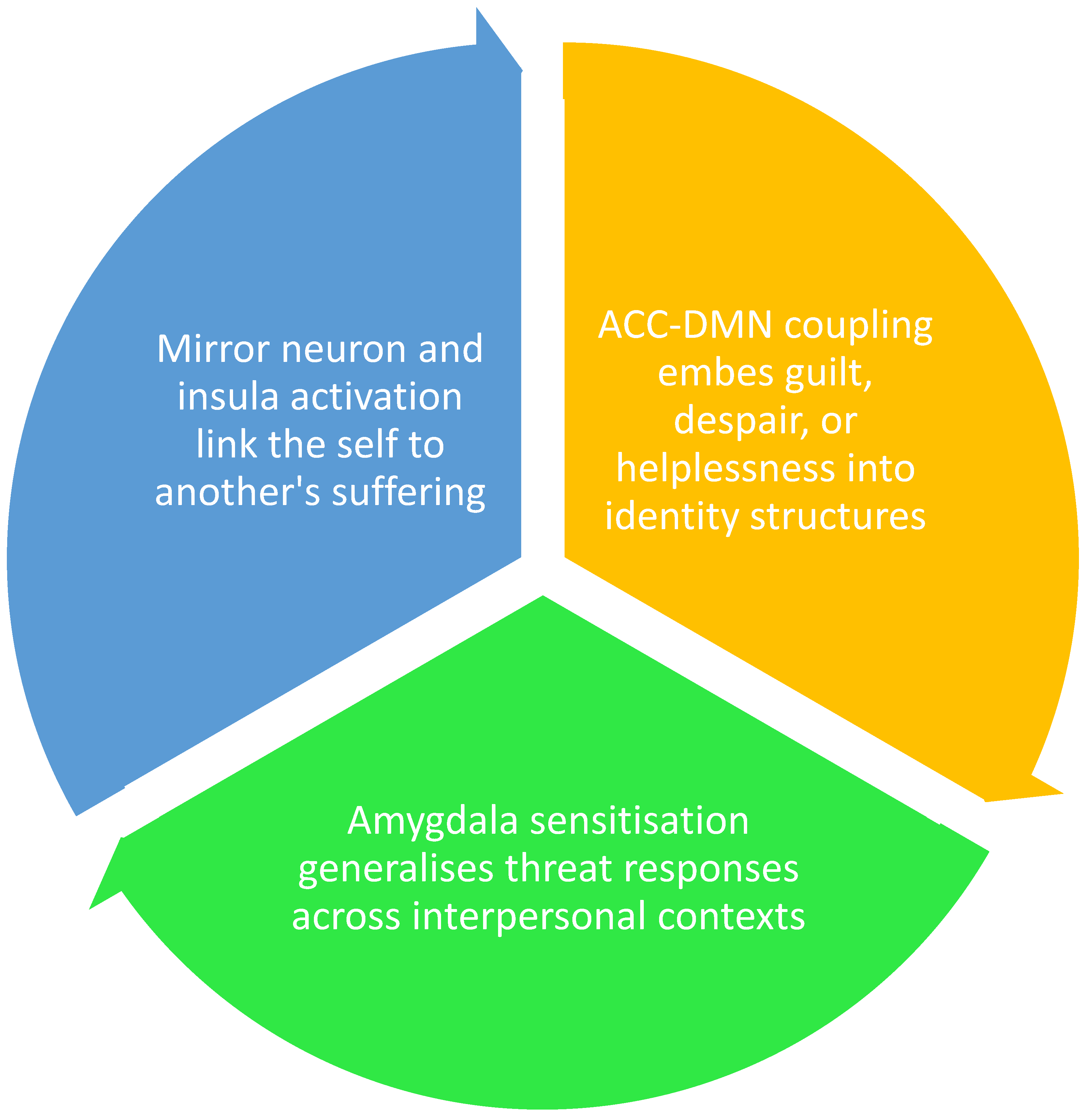
Not all trauma comes from a single, sudden event (Birkeland et al., 2022). For many people, trauma builds up gradually through relationships, caregiving, neglect, or long-term exposure to someone else’s suffering (Kiser et al., 2008). This is called relational, vicarious, or developmental trauma, referred to as enmeshment trauma (Baroncelli et al., 2025). Unlike direct trauma, which creates a sharp ‘imprint’ in the amygdala–ACC loop, this type of trauma spreads across broader brain systems that deal with identity, empathy, and self-concept.
Enmeshed trauma develops when the brain’s empathy networks, particularly when the mirror neurons and insula cause another person’s suffering to be felt almost as if it were one’s own (Schott, 2015). Over time, this repeated resonance blurs the boundaries between self and other. The ACC and DMN then consolidate these emotions into self-identity, embedding guilt, helplessness, or shame so deeply that they stop being temporary states and instead become enduring self-concepts (Ford and Courtois, 2021). Meanwhile, the amygdala, which typically responds to immediate threat, becomes hypersensitised, misinterpreting interpersonal conflict or reminders of others’ pain as signals of danger. These interacting processes generate TAML that perpetually reactivates distress. Unlike direct trauma, where the loop is tied to a specific memory trace, enmeshed trauma binds the loop to relationships and identity itself, making it both more diffuse and more resistant to resolution.
Figure 5.
Enmeshment trauma: mirror neuron and insula resonance blur self–other boundaries, consolidated by ACC and DMN into identity structures.
Figure 5.
Enmeshment trauma: mirror neuron and insula resonance blur self–other boundaries, consolidated by ACC and DMN into identity structures.
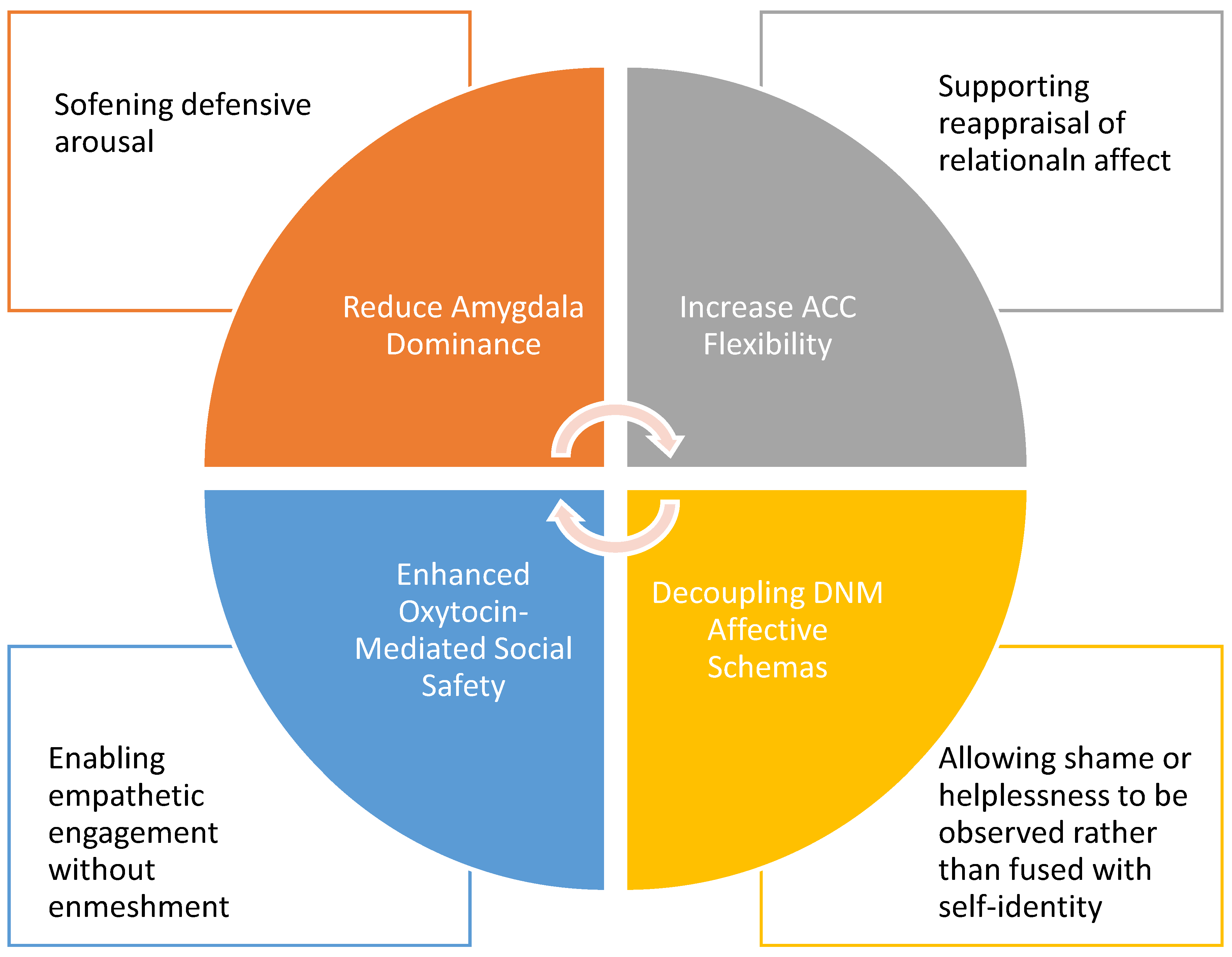
MDMA interrupts the cycle of enmeshed trauma by modulating the very brain networks that sustain it (Zhang et al., 2025). First, it reduces amygdala dominance, quieting the exaggerated fear responses that usually arise in relational contexts (Singleton et al., 2022). This calming effect prevents the nervous system from defaulting to defensive arousal when interpersonal cues are encountered. At the same time, MDMA increases the flexibility of the ACC, allowing it to reappraise emotional signals more accurately, distinguishing sadness or frustration from immediate threat. The loosening of DMN patterns further enables negative self-beliefs such as “I am unworthy” or “I am broken” to be decoupled from identity (Barber et al., 2025), shifting them from fixed truths into transient experiences that can be observed with psychological distance. Finally, MDMA enhances oxytocin-mediated social safety (Dumont et al., 2009), fostering a sense of trust and empathic connection that allows engagement with others without becoming emotionally enmeshed in their pain (Borissova et al., 2021). In terms of TAML, these combined effects soften the amygdala–ACC–DMN feedback cycle, creating temporary windows of safety and clarity in which entrenched relational patterns can be reprocessed (Albert and Back, 2025). Although such windows rarely dismantle enmeshed trauma in a single session, their repeated occurrence through structured therapy gradually rewires identity-level dysregulation and supports long-term integration.
Figure 6.
MDMA modulation of enmeshment trauma: quiets amygdala hyperreactivity, recalibrates ACC appraisal, loosens DMN-based self-fusion, and enhances oxytocin-mediated social safety.
Figure 6.
MDMA modulation of enmeshment trauma: quiets amygdala hyperreactivity, recalibrates ACC appraisal, loosens DMN-based self-fusion, and enhances oxytocin-mediated social safety.
These mechanisms create temporary conditions for insight and recontextualisation (Bedi et al., 2009), but because the trauma is relationally embedded and not affectively autonomous, resolution is less immediate. Here, MDMA functions as a recurrent catalyst within a longer therapeutic arc, gradually restructuring identity-level dysregulation.
4.2.4. Clinical Implications
This proposed stratification, between direct affective traceability and relational enmeshment, has implications for treatment planning. While individuals with acute, direct trauma may benefit significantly from short-course MDMA interventions, those with developmental, relational, or cumulative trauma may require multi-modal, phased therapeutic models. It must be emphasised that this distinction is based on a neurophenomenological hypothesis, not a validated biomarker system.
Table 1.
Hypothesised associations between trauma exposure and MDMA responsiveness.
Table 1.
Hypothesised associations between trauma exposure and MDMA responsiveness.
| Trauma Type |
PTSD Phenotype |
Nature of Exposure |
Affective Encoding Profile |
MDMA Therapeutic Potential |
| Acute direct trauma |
Classic PTSD |
First-hand, time-bound |
Amygdala–ACC circuit; discrete imprint |
High; rapid decoupling and re-encoding |
| Chronic direct trauma |
Complex PTSD |
Sustained, developmental |
Multi-network dysregulation (ACC, DMN) |
Moderate; requires extended integration |
| Witnessed traumaa |
Secondary PTSD |
Vicarious, empathic |
Partial co-activation, less personal |
Moderate; depends on identity fusion |
| Witnessed traumab |
Relational PTSD |
Deeply personal, empathic |
Affective enmeshment, insula/ACC load |
Variable; depends on healing in the other |
| Repeated exposurec |
Occupational PTSD |
Chronic, cumulative |
Layered micro-trauma, moral injury |
Moderate; may require phased intervention |
5. Methodological Foundations of Masking Design
5.1. Clinical Rationale for Expectancy Control
The masking strategy employed in BMPP is designed to preserve internal validity by mitigating expectancy effects and minimising sources of expectancy bias. In studies involving MDMA-AT, the FDA have made clear that expectancy from breaking-blind is not merely a nuisance variable but a core methodological concern (FDA, 2023). Participant expectations, therapeutic framing, and assessor interpretation can each influence outcome measures, particularly when these outcomes are subjective or phenomenologically rich (Harris, 2023).
Masking in this context is designed to control for expectancy effects across relevant trial stakeholders, preserving the methodological conditions necessary for reliable causal inference. By limiting access to information about the specific therapeutic hypotheses or predicted response patterns, the proposed protocol maintains a controlled interpretive environment that reduces the influence of demand characteristics, placebo effects, and observer-expectancy bias.
5.2. Role-Based Masking Justification
To minimise expectancy and observer bias, BMPP employs a role-specific masking framework. All participants receive the same intervention, but they are stratified by trauma phenotype. Participants are masked to the protocol’s therapeutic hypothesis and to their own phenotype classification to reduce demand characteristics and placebo effects. Care providers and outcome assessors are masked to trauma phenotype to ensure unbiased support and outcome measurement. Therapists, who must tailor treatment to the participant’s trauma profile, are unmasked but restricted from outcome assessment and analysis. Investigators remain masked to participant phenotype while retaining knowledge of the study’s mechanistic rationale. This structure results in a quadruple-masked design.
Table 2.
Role-masking protocol matrix.
Table 2.
Role-masking protocol matrix.
| Role |
Masked To |
Rationale |
| Participants |
Phenotype classification;
Therapeutic hypothesis |
Reduces expectancy effects and demand characteristics |
| Care Providers |
Phenotype classification |
Prevents bias in supportive care delivery |
| Outcome Assessors |
Phenotype classification |
Ensures objectivity in clinical outcome assessment |
| Therapists |
Not blinded |
Must know phenotype and rationale to deliver targeted psychotherapy |
| Investigators |
Phenotype classification |
Preserves analytic objectivity while maintaining scientific oversight |
5.3. Masking Integrity: Role Conflict Management
To preserve the methodological integrity of the trial, BMPP enforces strict role separation to minimise the risk of unintentional unmasking and mitigate expectancy-related bias. Because access to specific information, such as trauma phenotype or therapeutic rationale, can compromise masking, individuals in roles that require such access (therapists) must be clearly segregated from those responsible for outcome assessment, recruitment, or data analysis. Therapists must understand the participant’s trauma presentation to deliver tailored psychotherapy and are therefore incompatible with blinded functions (Yadav et al., 2025). Recruiters, if involved in study screening or consent processes, may inadvertently acquire knowledge of participant phenotype or trial rationale; as such, they are excluded from therapeutic, analytical, or assessment duties and functionally isolated from outcome data. Investigators, who design and oversee the study, are masked to trauma phenotype to prevent analytical bias and ensure objective interpretation. They may access treatment assignment only when required for safety monitoring or compliance but are restricted from therapeutic or participant-facing roles. This deliberate separation of responsibilities is designed to prevent role conflicts from undermining the masking strategy and to ensure that any observed therapeutic effects reflect genuine treatment impact rather than bias introduced through role contamination or expectancy effects.
Table 3.
Rationale for role masking.
Table 3.
Rationale for role masking.
| Role |
Excluded Function |
Access Restrictions |
| Therapist |
Cannot act as investigator, recruiter, care provider, or outcome assessor |
Access to trauma phenotype and rationale; excluded from assessment and analysis |
| Recruiter |
Cannot act as therapist, care provider, outcome assessor, or data-analysing |
Blinded to phenotype where possible; firewalled if also acting in investigator role |
| Investigator |
Cannot act as therapist, recruiter, care provider, or outcome assessor |
Masked to phenotype; may access treatment data for monitoring; no direct participant contact |
6. Discussion
The evidence increasingly supports MDMA-assisted therapy (MDMA-AT) as a potent intervention for PTSD, though its efficacy may be contingent upon the nature and neurobiological imprint of the trauma (Slomski, 2021). PTSD is a heterogeneous disorder: some individuals develop symptoms in response to discrete, time-bound events, while others suffer from chronic, relational, or developmental exposures that permeate identity and relational schemas (Yehuda, 2004). This variability may help explain differential treatment outcomes.
Conventional pharmacological strategies for PTSD have often focused on adrenergic antagonism, particularly through the use of α1-adrenergic receptor (α1) inverse agonists such as prazosin (Minipress®) (Green, 2014), or α1 antagonists such as doxazosin (Cardura®), and terazosin (Hytrin®) (Nirmalani-Gandhy et al., 2015; Smith and Koola, 2016). These agents blunt the sympathetic cascade at its terminal effector level, blocking norepinephrine and epinephrine activity on central and peripheral α1 receptors, thereby reducing symptoms such as hypervigilance, nightmares, and autonomic arousal (Taylor and Raskind, 2002). While sometimes effective, this approach operates at the bottom level of the sympathetic cascade, targeting the expression of symptoms rather than the upstream affective circuitry in which the traumatic memory is encoded (Arnsten et al., 2015). In other words, α1 antagonists provide a form of symptomatic palliation, they reduce the physiological manifestations of trauma-related hyperarousal but do little to alter the maladaptive circuits that sustain PTSD. By contrast, MDMA allows a therapeutic window where traumatic memories to be re-experienced in a safe neurochemical context and re-encoded with reduced fear salience (Bedi et al., 2009; Kredlow et al., 2022; Walpola et al., 2017). Importantly, MDMA does not forgo adrenergic modulation altogether, by dampening amygdala–hypothalamic threat signalling upstream (Sottile and Vida, 2022), the sympathetic cascade is never fully mobilised, meaning that α1 receptors are not driven by excessive epinephrine in the first place. This represents, in effect, a form of functional α1 antagonism that MDMA provides, the benefit of adrenergic quieting without the need for direct receptor blockade, while simultaneously enabling higher-order processes that conventional antagonists cannot achieve. Thus, MDMA combines the symptomatic relief traditionally sought through α1 blockade with the deeper neurobiological effects of network recalibration, including amygdala–ACC–insula FC disruption, DMN modulation, and oxytocin-mediated pro-social signalling (Vaslavski et al., 2025). In this sense, α1 antagonists reflect a earlier-generation approach that addresses symptoms alone, whereas MDMA represents a paradigm shift whereby both muting the sympathetic cascade and fostering durable integration at the level of trauma-informed memory and identity. A related class of conventional interventions involves α2-adrenergic receptor (α2) agonists such as clonidine (Catapres®), guanfacine (Tenex®, Intuniv®), and dexmedetomidine (Precedex®). α2 agonists pursue the same principle from the opposite end of the cascade, reducing adrenergic output presynaptically (Burek et al., 2021; Cheung, 2025; Yu et al., 2023). Like α1 antagonists, they mute sympathetic overdrive and provide relief of insomnia, hyperarousal, and startle responses. Yet, they too leave untouched the amygdala–ACC–insula circuitry where trauma is encoded. α1 antagonists and α2 agonists represent earlier-generation approaches: they quiet adrenergic tone but cannot open the therapeutic window required for trauma reconsolidation (Arluk et al., 2022; Belkin and Schwartz, 2015). Moreover, chronic suppression of noradrenergic tone can be sedating and cognitively dulling, limiting tolerability for some patients (Recchia et al., 2023). By contrast, MDMA simultaneously dampens adrenergic signalling upstream (preventing α1 drive in the first place), modulates corticolimbic connectivity, and enhances interoceptive and pro-social networks. MDMA also works downstream, agonising α2 itself (Bexis and Docherty, 2005; Sessa et al., 2019), working at all levels as an ‘entourage effect’, further explaining MDMA’s superiority over conventional adrenergic agents.
Neurobiologically, MDMA attenuates hyperactive amygdala signalling while enhancing ACC regulation and insula-mediated interoception (Walpola et al., 2017; Singleton et al., 2022; Carhart-Harris et al., 2014). This recalibration is particularly suited to trauma encoded as acute affective imprints, where hyperarousal, hypervigilance, and intrusive re-experiencing are dominant. In these cases, MDMA facilitates affective decoupling and re-encoding of the traumatic memory in a regulated state, often within just a few sessions.
However, in complex or relational PTSD, trauma is not a single memory trace but a developmental architecture (Birkeland et al., 2022; Kiser et al., 2008). Here, TAML highlights how affective imprints shift from transient defensive states into enduring self-referential structures: “I am unsafe” or “I am unworthy”. For such individuals, MDMA may assist in reducing fear responses and fostering self-compassion, but resolution also requires extended therapeutic work, relational repair, grief integration, and existential processing of powerlessness (Mahoney and Markel, 2016). This distinction underscores that MDMA is not a universal cure, but a catalyst best matched to certain PTSD phenotypes (Arluk et al., 2022).
Emerging neuroimaging findings support this stratification. For example, adolescents exposed to the 2013 Boston Marathon bombing with heightened amygdala reactivity and reduced hippocampal engagement during regulation tasks were more likely to develop PTSD (Busso et al., 2014). Such pre-existing neural vulnerabilities map onto the TAML model, suggesting that individual neurobiological profiles may predict therapeutic responsiveness. Personalising treatment according to trauma type and neural phenotype therefore represents the next frontier in MDMA-AT.
7. Conclusions
BMPP advances the field by framing MDMA-AT not as a uniform intervention, but as a precision tool most effective when trauma is self-contained and affectively traceable. When the traumatic imprint resides primarily in heightened sympathetic responses, MDMA can rapidly recalibrate defensive circuits, allowing memories to be re-encoded without fear dominance.
By contrast, when trauma is relationally entangled or developmentally embedded, MDMA may provide essential affective safety but requires integration within longer-term psychotherapeutic frameworks. Healing in these cases depends not only on neurobiological decoupling but also on reconstructing meaning, repairing relationships, and processing existential loss.
Thus, the efficacy of MDMA hinges on the ownership and localisation of the traumatic wound, whether it lies in the individual’s own nervous system or in unresolved relational fields. By distinguishing these pathways, BMPP offers a roadmap for tailoring psychedelic therapy to the complexity of trauma. As future trials integrate symptom profiling with neuroimaging and the TAML framework, MDMA-AT may evolve into a truly personalised medicine, transforming PTSD treatment and restoring agency to those most burdened by trauma.
BMPP represents a significant step forward in the tailored application of MDMA therapy for PTSD. By distinguishing between different types of trauma exposure and understanding the underlying neural mechanisms, we can better identify those who will benefit most from this promising treatment. This personalised approach not only maximises therapeutic outcomes but also paves the way for future innovations in the field of psychedelic medicine. As research continues, this methodology holds the potential to transform the landscape of PTSD treatment, offering hope and healing to countless individuals.
Funding
The author received no financial support for the research, authorship, and/or publication of this article.
Acknowledgements
The author gratefully acknowledges Flint Rehab (
www.flintrehab.com) for the use of brain images that supported the development of TAML.
Declaration of conflicting interests
The author declares a financial interest in consulting services provided to psychiatrists seeking Authorised Prescriber (AP) approval for the use of MDMA in clinical practice. This work has the potential to generate financial benefit for the author through such consulting activities.
References
- Aday JS, Heifets BD, Pratscher SD, et al. (2022) Great Expectations: recommendations for improving the methodological rigor of psychedelic clinical trials. Psychopharmacology 239(6): 1989-2010. [CrossRef]
- Akiki TJ, Averill CL and Abdallah CG (2017) A Network-Based Neurobiological Model of PTSD: Evidence From Structural and Functional Neuroimaging Studies. Curr Psychiatry Rep 19(11): 81. [CrossRef]
- Albert AE and Back AL (2025) Psychoanalytically informed MDMA-assisted therapy for pathological narcissism: a novel theoretical approach. Front Psychiatry 16: 1529427.
- Arluk S, Matar MA, Carmi L, et al. (2022) MDMA treatment paired with a trauma-cue promotes adaptive stress responses in a translational model of PTSD in rats. Transl Psychiatry 12(1): 181. [CrossRef]
- Arnsten AFT, Raskind MA, Taylor FB, et al. (2015) The effects of stress exposure on prefrontal cortex: Translating basic research into successful treatments for post-traumatic stress disorder. Neurobiology of Stress 1: 89-99. [CrossRef]
- Avanceña ALV, Kahn JG and Marseille E (2022) The Costs and Health Benefits of Expanded Access to MDMA-assisted Therapy for Chronic and Severe PTSD in the USA: A Modeling Study. Clinical Drug Investigation 42(3): 243-252. [CrossRef]
- Barber M, Evans S, Marks R, et al. (2025) “I am not pain, I have pain”: A pilot study examining iRest yoga nidra as a mind-body intervention for persistent pain. Complementary Therapies in Clinical Practice 59: 101955. [CrossRef]
- Baroncelli CMC, Lodder P, van der Lee M, et al. (2025) The role of enmeshment and undeveloped self, subjugation and self-sacrifice in childhood trauma and attachment related problems: The relationship with self-concept clarity. Acta Psychologica 254: 104839. [CrossRef]
- Bedi G, Phan KL, Angstadt M, et al. (2009) Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 207(1): 73-83. [CrossRef]
- Belkin MR and Schwartz TL (2015) Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context 4: 212286.
- Bexis S and Docherty JR (2005) Role of alpha2A-adrenoceptors in the effects of MDMA on body temperature in the mouse. Br J Pharmacol 146(1): 1-6.
- Birkeland MS, Skar AS and Jensen TK (2022) Understanding the relationships between trauma type and individual posttraumatic stress symptoms: a cross-sectional study of a clinical sample of children and adolescents. J Child Psychol Psychiatry 63(12): 1496-1504. [CrossRef]
- Borissova A, Ferguson B, Wall MB, et al. (2021) Acute effects of MDMA on trust, cooperative behaviour and empathy: A double-blind, placebo-controlled experiment. Journal of Psychopharmacology 35(5): 547-555. [CrossRef]
- Brinzei O, Donley CN and Dixon Ritchie G (2023) From prohibited to prescribed: The rescheduling of MDMA and psilocybin in Australia. Drug Science, Policy and Law 9: 20503245231198472. [CrossRef]
- Brinzei OV (2025a) The Brinzei MDMA-PTSD Protocol. Available at: https://brinzei.solutions/blogs/neuroscience/brinzei-mdma-ptsd-protocol.
- Brinzei OV (2025b) Trauma-Effective Memory Loop. Available at: https://brinzei.solutions/blogs/neuroscience/trauma-effective-memory-loop. [CrossRef]
- Burek GA, Waite MR, Heslin K, et al. (2021) Low-dose clonidine in veterans with Posttraumatic stress disorder. Journal of Psychiatric Research 137: 480-485. [CrossRef]
- Busso DS, McLaughlin KA and Sheridan MA (2014) Media exposure and sympathetic nervous system reactivity predict PTSD symptoms after the Boston marathon bombings. Depress Anxiety 31(7): 551-558. [CrossRef]
- Butler M, Jelen L and Rucker J (2022) Expectancy in placebo-controlled trials of psychedelics: if so, so what? Psychopharmacology 239(10): 3047-3055. [CrossRef]
- Carhart-Harris RL, Murphy K, Leech R, et al. (2015) The Effects of Acutely Administered 3,4-Methylenedioxymethamphetamine on Spontaneous Brain Function in Healthy Volunteers Measured with Arterial Spin Labeling and Blood Oxygen Level-Dependent Resting State Functional Connectivity. Biol Psychiatry 78(8): 554-562. [CrossRef]
- Carhart-Harris RL, Wall MB, Erritzoe D, et al. (2014) The effect of acutely administered MDMA on subjective and BOLD-fMRI responses to favourite and worst autobiographical memories. Int J Neuropsychopharmacol 17(4): 527-540. [CrossRef]
- Cheung LK (2025) Guanfacine as an Adjunct Treatment for Complex Post-Traumatic Stress Disorder: A Case Report. J Korean Acad Child Adolesc Psychiatry 36(2): 78-82. [CrossRef]
- Chiamulera C, Hinnenthal I, Auber A, et al. (2014) Reconsolidation of maladaptive memories as a therapeutic target: pre-clinical data and clinical approaches. Front Psychiatry 5: 107. [CrossRef]
- Christie D, Yazar-Klosinski B, Nosova E, et al. (2022) MDMA-assisted therapy is associated with a reduction in chronic pain among people with post-traumatic stress disorder. Front Psychiatry 13: 939302. [CrossRef]
- Doblin R (2002) A Clinical Plan for MDMA (Ecstasy) in the Treatment of Posttraumatic Stress Disorder (PTSD): Partnering with the FDA. Journal of Psychoactive Drugs 34(2): 185-194. [CrossRef]
- Doblin RE, Christiansen M, Jerome L, et al. (2019) The Past and Future of Psychedelic Science: An Introduction to This Issue. Journal of Psychoactive Drugs 51(2): 93-97. [CrossRef]
- Doss MK, Weafer J, Gallo DA, et al. (2018) MDMA Impairs Both the Encoding and Retrieval of Emotional Recollections. Neuropsychopharmacology 43(4): 791-800. [CrossRef]
- Dumont GJ, Sweep FC, van der Steen R, et al. (2009) Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci 4(4): 359-366. [CrossRef]
- Edmondson D (2014) An Enduring Somatic Threat Model of Posttraumatic Stress Disorder Due to Acute Life-Threatening Medical Events. Soc Personal Psychol Compass 8(3): 118-134. [CrossRef]
- Etkin A, Egner T and Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15(2): 85-93. [CrossRef]
- Feduccia AA and Mithoefer MC (2018) MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Prog Neuropsychopharmacol Biol Psychiatry 84(Pt A): 221-228. [CrossRef]
- Fisher J (2017) Trauma-informed stabilisation treatment: A new approach to treating unsafe behaviour. Australian Clinical Psychologist 3(1): 1744.
- Food and Drugs Administration (FDA) (2023) Psychedelic Drugs: Considerations for Clinical Investigations. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/psychedelic-drugs-considerations-clinical-investigations.
- Ford JD and Courtois CA (2021) Complex PTSD and borderline personality disorder. Borderline Personality Disorder and Emotion Dysregulation 8(1): 16.
- Fox AS, Oler JA, Tromp do PM, et al. (2015) Extending the amygdala in theories of threat processing. Trends Neurosci 38(5): 319-329. [CrossRef]
- García-Cabezas M and Barbas H (2017) Anterior Cingulate Pathways May Affect Emotions Through Orbitofrontal Cortex. Cereb Cortex 27(10): 4891-4910. [CrossRef]
- Gattuso JJ, Perkins D, Ruffell S, et al. (2023) Default Mode Network Modulation by Psychedelics: A Systematic Review. Int J Neuropsychopharmacol 26(3): 155-188. [CrossRef]
- Green B (2014) Prazosin in the treatment of PTSD. J Psychiatr Pract 20(4): 253-259.
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 30(12): 1181-1197. [CrossRef]
- Griffiths RR, Richards WA, McCann U, et al. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187(3): 268-283; discussion 284-292. [CrossRef]
- Harris E (2023) FDA Proposes First Guidance for Researchers Studying Psychedelics. JAMA 330(4): 307-307. [CrossRef]
- Hovmand OR, Poulsen ED, Arnfred S, et al. (2023) Risk of bias in randomized clinical trials on psychedelic medicine: A systematic review. Journal of Psychopharmacology 37(7): 649-659. [CrossRef]
- Huestis MA, Smith WB, Leonowens C, et al. (2025) MDMA pharmacokinetics: A population and physiologically based pharmacokinetics model-informed analysis. CPT: Pharmacometrics & Systems Pharmacology 14(2): 376-388. [CrossRef]
- Jardim AV, Jardim DV, Chaves BR, et al. (2021) 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for victims of sexual abuse with severe post-traumatic stress disorder: an open label pilot study in Brazil. Braz J Psychiatry 43(2): 181-185. [CrossRef]
- Johansen P and Krebs T (2009) How could MDMA (ecstasy) help anxiety disorders? A neurobiological rationale. Journal of Psychopharmacology 23(4): 389-391. [CrossRef]
- Karp Barnir E, Rubinstein Z, Abend R, et al. (2025) Peri-traumatic consumption of classic psychedelics is associated with lower anxiety and post-traumatic responses 3 weeks after exposure. J Psychopharmacol 39(9): 1031-1036. [CrossRef]
- Kensinger EA, Addis DR and Atapattu RK (2011) Amygdala activity at encoding corresponds with memory vividness and with memory for select episodic details. Neuropsychologia 49(4): 663-673. [CrossRef]
- Kiser LJ, Nurse W, Lucksted A, et al. (2008) Understanding the Impact of Trauma on Family Life From the Viewpoint of Female Caregivers Living in Urban Poverty. Traumatology (Tallahass Fla) 14(3): 77-90. [CrossRef]
- Kredlow MA, Fenster RJ, Laurent ES, et al. (2022) Prefrontal cortex, amygdala, and threat processing: implications for PTSD. Neuropsychopharmacology 47(1): 247-259. [CrossRef]
- Lanius RA, Bluhm RL and Frewen PA (2011) How understanding the neurobiology of complex post-traumatic stress disorder can inform clinical practice: a social cognitive and affective neuroscience approach. Acta Psychiatrica Scandinavica 124(5): 331-348. [CrossRef]
- Liberzon I and Abelson JL (2016) Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 92(1): 14-30. [CrossRef]
- Luoma J and Lear MK (2021) MDMA-Assisted Therapy as a Means to Alter Affective, Cognitive, Behavioral, and Neurological Systems Underlying Social Dysfunction in Social Anxiety Disorder. Front Psychiatry 12: 733893.
- Maddox SA, Hartmann J, Ross RA, et al. (2019) Deconstructing the Gestalt: Mechanisms of Fear, Threat, and Trauma Memory Encoding. Neuron 102(1): 60-74. [CrossRef]
- Mahoney D and Markel B (2016) An Integrative Approach to Conceptualizing and Treating Complex Trauma. Psychoanalytic Social Work 23(1): 1-22. [CrossRef]
- Martinelli P, Sperduti M, Devauchelle AD, et al. (2013) Age-related changes in the functional network underlying specific and general autobiographical memory retrieval: a pivotal role for the anterior cingulate cortex. PLoS One 8(12): e82385. [CrossRef]
- Metzner R, Litwin G and Weil G (1965) The relation of expectation and mood to psilocybin reactions: a questionnaire study. Psychedelic Rev 5: 3-39.
- Mitchell JM, Bogenschutz M, Lilienstein A, et al. (2021) MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med 27(6): 1025-1033. [CrossRef]
- Mitchell JM, Ot'alora GM, van der Kolk B, et al. (2023) MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial. Nat Med 29(10): 2473-2480.
- Mithoefer MC, Mithoefer AT, Feduccia AA, et al. (2018) 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 5(6): 486-497. [CrossRef]
- Mithoefer MC, Wagner MT, Mithoefer AT, et al. (2011) The safety and efficacy of {+/-}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol 25(4): 439-452. [CrossRef]
- Mocanu V, Mackay L, Christie D, et al. (2022) Safety considerations in the evolving legal landscape of psychedelic-assisted psychotherapy. Substance Abuse Treatment, Prevention, and Policy 17(1): 37. [CrossRef]
- Morris KR, Jaeb M, Dunsmoor JE, et al. (2025) Decoding threat neurocircuitry representations during traumatic memory recall in PTSD. Neuropsychopharmacology 50(3): 568-575. [CrossRef]
- Mueser KT, Gottlieb JD, Xie H, et al. (2015) Evaluation of cognitive restructuring for post-traumatic stress disorder in people with severe mental illness. Br J Psychiatry 206(6): 501-508. [CrossRef]
- Murty VP, Ritchey M, Adcock RA, et al. (2010) fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia 48(12): 3459-3469. [CrossRef]
- Nirmalani-Gandhy A, Sanchez D and Catalano G (2015) Terazosin for the treatment of trauma-related nightmares: a report of 4 cases. Clin Neuropharmacol 38(3): 109-111.
- O’Brien S and Nutt D (2025) MDMA-assisted therapy: challenges, clinical trials, and the future of MDMA in treating behavioral disorders. CNS spectrums 30(1): e15.
- Oehen P and Gasser P (2022) Using a MDMA- and LSD-Group Therapy Model in Clinical Practice in Switzerland and Highlighting the Treatment of Trauma-Related Disorders. Front Psychiatry 13: 863552. [CrossRef]
- Oehen P, Traber R, Widmer V, et al. (2013) A randomized, controlled pilot study of MDMA (+/- 3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD). J Psychopharmacol 27(1): 40-52. [CrossRef]
- Ot'alora GM, Grigsby J, Poulter B, et al. (2018) 3,4-Methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized phase 2 controlled trial. J Psychopharmacol 32(12): 1295-1307. [CrossRef]
- Pitts BL, Eisenberg ML, Bailey HR, et al. (2022) PTSD is associated with impaired event processing and memory for everyday events. Cognitive Research: Principles and Implications 7(1): 35. [CrossRef]
- Recchia A, Tonti MP, Mirabella L, et al. (2023) The Pharmacological Class Alpha 2 Agonists for Stress Control in Patients with Respiratory Failure: The Main Actor in the Different Acts. Stresses 3(1): 1-10. [CrossRef]
- Rolls ET (2019) The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct 224(9): 3001-3018. [CrossRef]
- Roseman L (2025) A reflection on paradigmatic tensions within the FDA advisory committee for MDMA-assisted therapy. Journal of Psychopharmacology 39(4): 313-315. [CrossRef]
- Sar V (2011) Developmental trauma, complex PTSD, and the current proposal of DSM-5. Eur J Psychotraumatol 2. [CrossRef]
- Schenberg EE (2025) From Efficacy to Effectiveness: Evaluating Psychedelic Randomized Controlled Trials for Trustworthy Evidence-Based Policy and Practice. Pharmacology Research & Perspectives 13(2): e70097. [CrossRef]
- Schmid Y and Bershad AK (2024) Altered States and Social Bonds: Effects of MDMA and Serotonergic Psychedelics on Social Behavior as a Mechanism Underlying Substance-Assisted Therapy. Biol Psychiatry Cogn Neurosci Neuroimaging 9(5): 490-499. [CrossRef]
- Schott GD (2015) Pictures of pain: their contribution to the neuroscience of empathy. Brain 138(Pt 3): 812-820. [CrossRef]
- Sessa B, Higbed L and Nutt D (2019) A Review of 3,4-methylenedioxymethamphetamine (MDMA)-Assisted Psychotherapy. Front Psychiatry 10: 138. [CrossRef]
- Sharot T, Riccardi AM, Raio CM, et al. (2007) Neural mechanisms mediating optimism bias. Nature 450(7166): 102-105. [CrossRef]
- Šimić G, Tkalčić M, Vukić V, et al. (2021) Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 11(6). [CrossRef]
- Singleton SP, Wang JB, Mithoefer M, et al. (2022) Altered brain activity and functional connectivity after MDMA-assisted therapy for post-traumatic stress disorder. Front Psychiatry 13: 947622. [CrossRef]
- Slomski A (2021) MDMA-Assisted Therapy Highly Effective for PTSD. JAMA 326(4): 299-299. [CrossRef]
- Smith C and Koola MM (2016) Evidence for Using Doxazosin in the Treatment of Posttraumatic Stress Disorder. Psychiatr Ann 46(9): 553-555. [CrossRef]
- Sottile RJ and Vida T (2022) A proposed mechanism for the MDMA-mediated extinction of traumatic memories in PTSD patients treated with MDMA-assisted therapy. Front Psychiatry 13: 991753.
- Taylor F and Raskind MA (2002) The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol 22(1): 82-85. [CrossRef]
- Thomaes K, Dorrepaal E, Draijer N, et al. (2013) Increased anterior cingulate cortex and hippocampus activation in Complex PTSD during encoding of negative words. Soc Cogn Affect Neurosci 8(2): 190-200. [CrossRef]
- Thomason ME and Marusak HA (2017) Toward understanding the impact of trauma on the early developing human brain. Neuroscience 342: 55-67. [CrossRef]
- van der Kolk B (2000) Posttraumatic stress disorder and the nature of trauma. Dialogues in Clinical Neuroscience 2(1): 7-22.
- van der Kolk BA (2006) Clinical Implications of Neuroscience Research in PTSD. Annals of the New York Academy of Sciences 1071(1): 277-293.
- van der Kolk BA, Wang JB, Yehuda R, et al. (2024) Effects of MDMA-assisted therapy for PTSD on self-experience. PLoS One 19(1): e0295926.
- Vaslavski A, Gross AH, Israel S, et al. (2025) The effect of MDMA administration on oxytocin concentration levels: systematic review and a multilevel meta-analysis in humans. Neuroscience & Biobehavioral Reviews 177: 106324. [CrossRef]
- Wagner G, Koch K, Schachtzabel C, et al. (2013) Self-referential processing influences functional activation during cognitive control: an fMRI study. Soc Cogn Affect Neurosci 8(7): 828-837. [CrossRef]
- Walpola IC, Nest T, Roseman L, et al. (2017) Altered insula connectivity under MDMA. Neuropsychopharmacology 42(11): 2152-2162. [CrossRef]
- Wang J, John Y and Barbas H (2021a) Pathways for Contextual Memory: The Primate Hippocampal Pathway to Anterior Cingulate Cortex. Cereb Cortex 31(3): 1807-1826. [CrossRef]
- Wang JB, Lin J, Bedrosian L, et al. (2021b) Scaling Up: Multisite Open-Label Clinical Trials of MDMA-Assisted Therapy for Severe Posttraumatic Stress Disorder. Journal of Humanistic Psychology. [CrossRef]
- Wilkinson ST and Sanacora G (2025) Issues in Clinical Trial Design—Lessons From the FDA’s Rejection of MDMA. JAMA Psychiatry 82(6): 545-546.
- Yadav G, McNamara S and Gunturu S (2025) Trauma-Informed Therapy. StatPearls. Treasure Island (FL).
- Yehuda R (2004) Understanding Heterogeneous Effects of Trauma Exposure: Relevance to Postmortem Studies of PTSD. Psychiatry 67(4): 391-397. [CrossRef]
- Young DA, Chao L, Neylan TC, et al. (2018) Association among anterior cingulate cortex volume, psychophysiological response, and PTSD diagnosis in a Veteran sample. Neurobiol Learn Mem 155: 189-196. [CrossRef]
- Yu Y, Li Y, Han D, et al. (2023) Effect of Dexmedetomidine on Posttraumatic Stress Disorder in Patients Undergoing Emergency Trauma Surgery: A Randomized Clinical Trial. JAMA Network Open 6(6): e2318611-e2318611.
- Zhang X, Hack LM, Bertrand C, et al. (2025) Negative Affect Circuit Subtypes and Neural, Behavioral, and Affective Responses to MDMA: A Randomized Clinical Trial. JAMA Network Open 8(4): e257803-e257803.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).