Submitted:
02 October 2025
Posted:
06 October 2025
You are already at the latest version
Abstract
Aim of the work: This review aims to systematically integrate the mechanism of action of Astragalus membranaceus and its active components on central nervous system(CNS) diseases, with a focus on exploring its therapeutic potential, and introducing related health food products that use it as an adjunct treatment for CNS diseases.Materials and methods: Searches were conducted through the PubMed and Web of Science databases using the keywords "Astragalus membranaceus", "Astragali Radix", "Astragaloside IV (AS-IV)", "Astragalus membranaceus polysaccharides (APS)" and "CNS diseases". Reports on the effects of Astragalus membranaceus and its components on CNS diseases were identified and reviewed.Results: This review provides a comprehensive summary of the latest research advancements concerning Astragalus membranaceus across several pivotal domains, including its extensive historical usage, active constituents, pharmacological properties, potential therapeutic applications for CNS disorders, safety profile, contemporary formulations, and significant findings in the realm of health food applications. The medicinal history of Astragalus membranaceus membranaceus is both long-standing and profound, with usage spanning over 2,000 years. Its active components, such as AS-IV and APS, have demonstrated considerable therapeutic efficacy in the treatment of CNS diseases. The pharmacological effects of Astragalus membranaceus membranaceus are diverse, encompassing anti-neuroinflammatory, anti-oxidative stress, anti-apoptotic activities, modulation of autophagy, anti-ferroptotic effects, and protection of the blood-brain barrier. Furthermore, the practical applications of Astragalus membranaceus extend beyond the medical field, encompassing modern pharmaceutical preparations and health food products, among other areas. Despite the promising potential of Astragalus membranaceus in the treatment of CNS diseases, numerous challenges persist in the comprehensive investigation of its pharmacological mechanisms and the standardization of its quality. Nonetheless, Astragalus membranaceus occupies an essential and significant role in the management of CNS disorders and is anticipated to have an increasingly prominent impact in related fields in the future.Conclusion: Astragalus membranaceus has emerged as a promising pharmacological agent in the global health sector, attributed to its notable efficacy in addressing CNS disorders. Combining traditional knowledge with innovative research and based on the concept of "homology of medicine and food", the application of Astragalus membranaceus as an auxiliary therapy in the field of health food fully leverages its neuroprotective and anti-inflammatory properties, which is an important direction for future research.
Keywords:
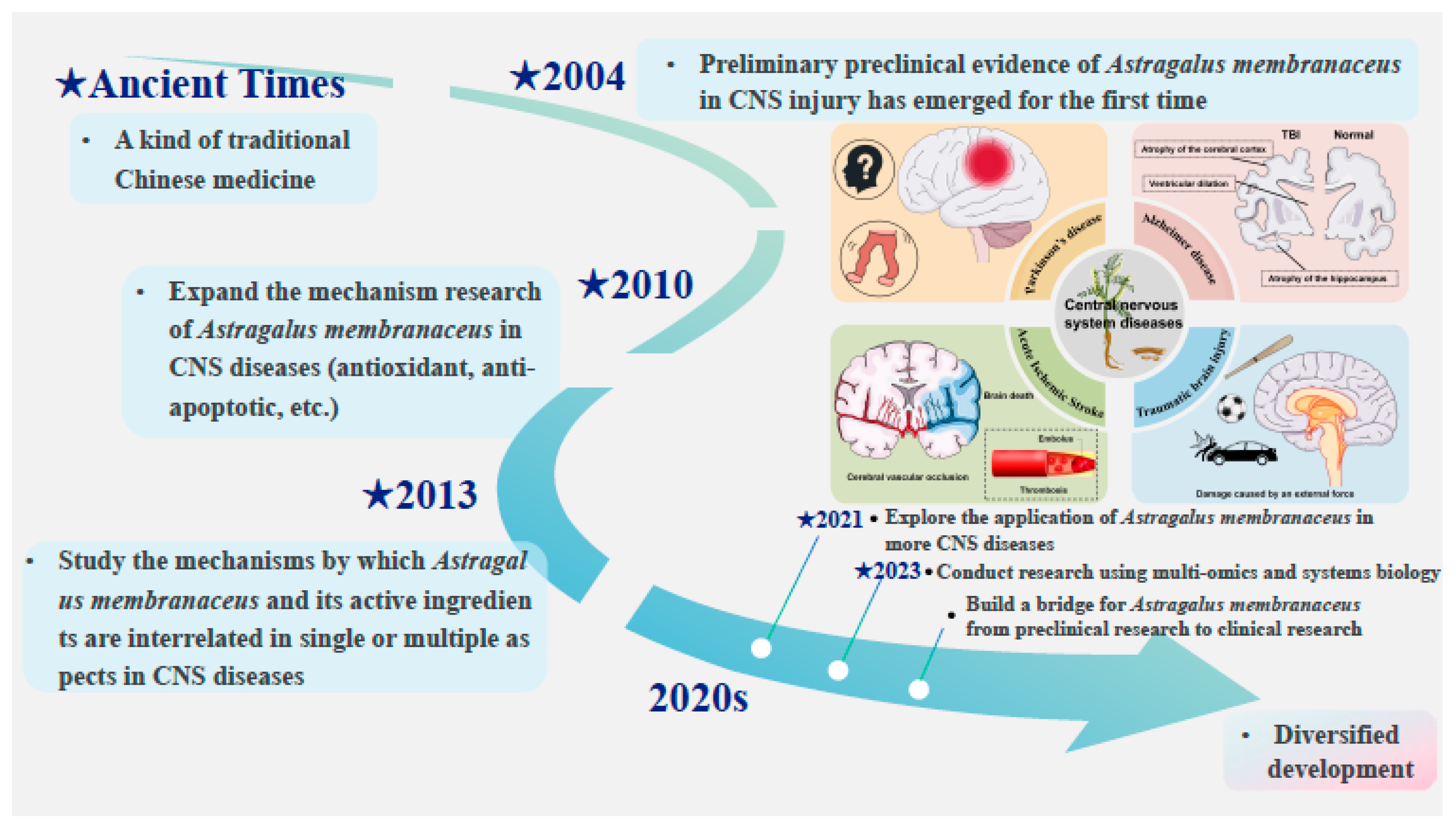
1. Introduction
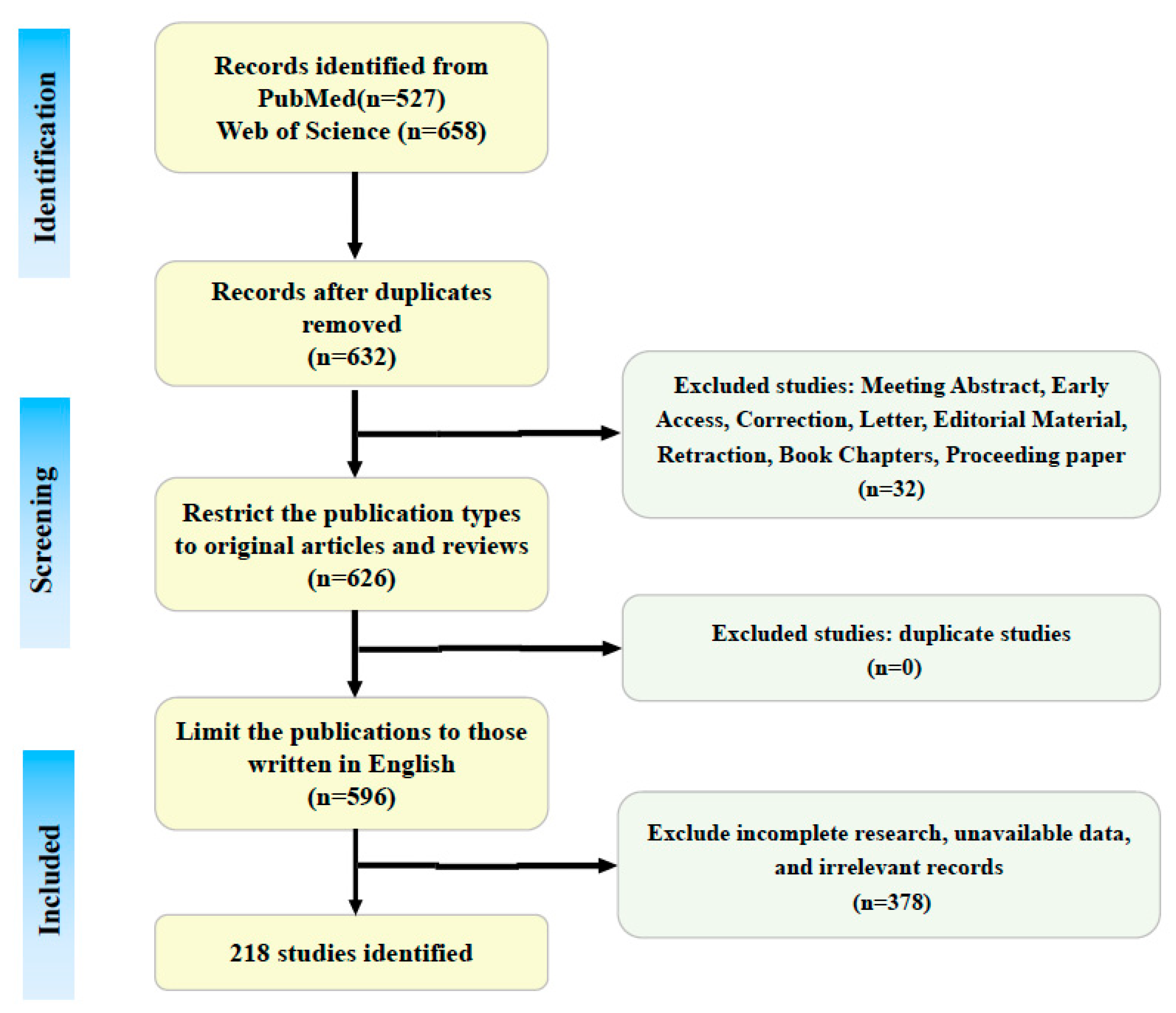
2. Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Results
3. Impact of Astragalus Membranaceus and Its Active Components on the Cns
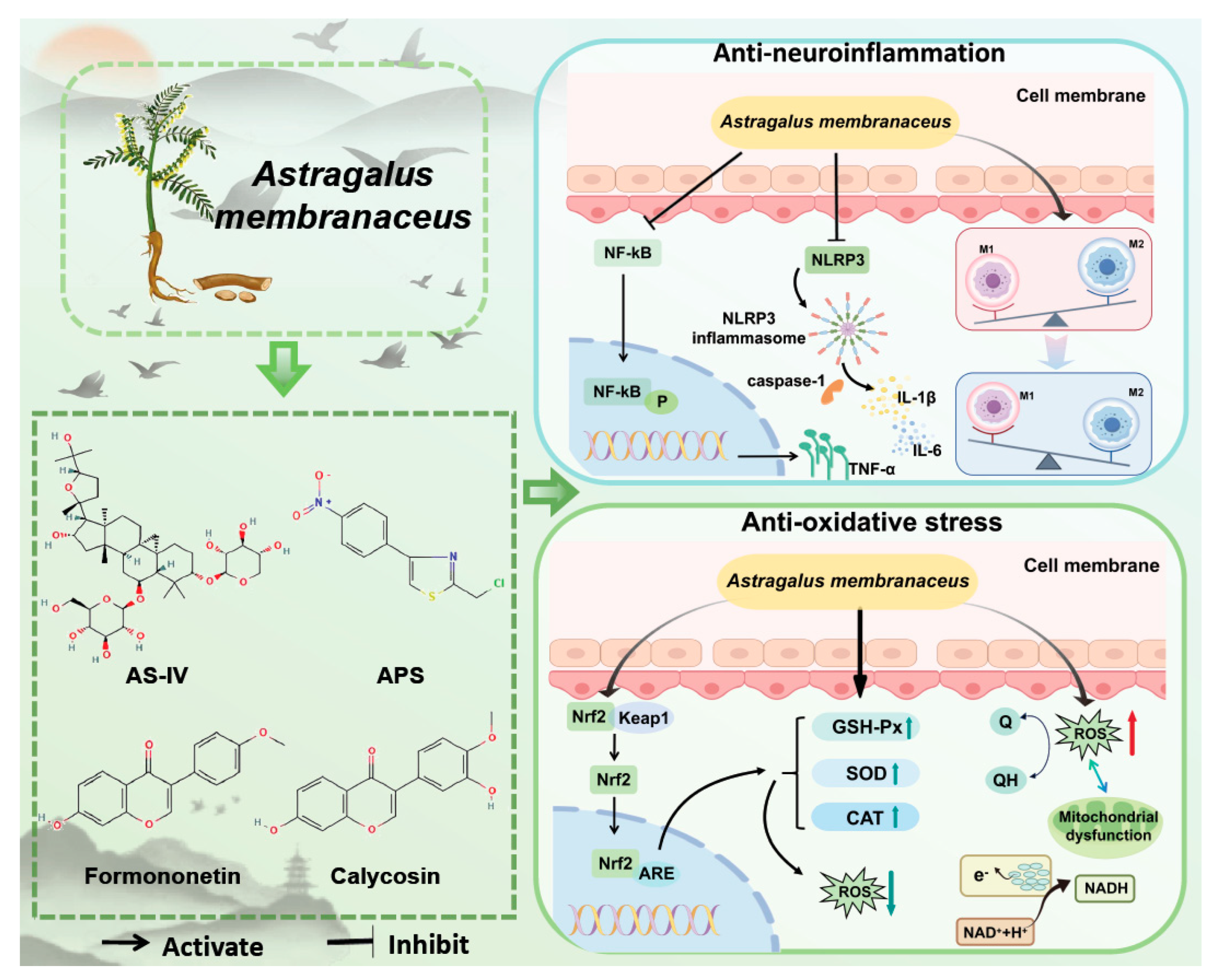
3.1. Anti-Neuroinflammation
3.1.1. Regulatory Effects of Astragalus Membranaceus Components (as-Iv, Aps) on Microglia/Macrophages
3.1.2. Inhibitory Effect of as-Iv on Nlrp3 Inflammatory Body and Related Damage
3.1.3. Regulatory Effect of Astragalus Membranaceus on Astrocytes
3.1.4. Regulatory Effect of Astragalus Membranaceus on Neutrophils
3.1.5. Regulatory Effects of Astragalus Membranaceus Through Multiple Inflammatory Signaling Pathways
3.2. Anti-Oxidative Stress
3.2.1. Astragalus Membranaceus Regulates the Expression Levels of Antioxidant Enzymes
3.2.2. The Regulation of Mitochondrial Function by Astragalus Membranaceus
3.2.3. Nrf2 Plays a Core Role in the Antioxidant Stress Resistance of Astragalus Membranaceus
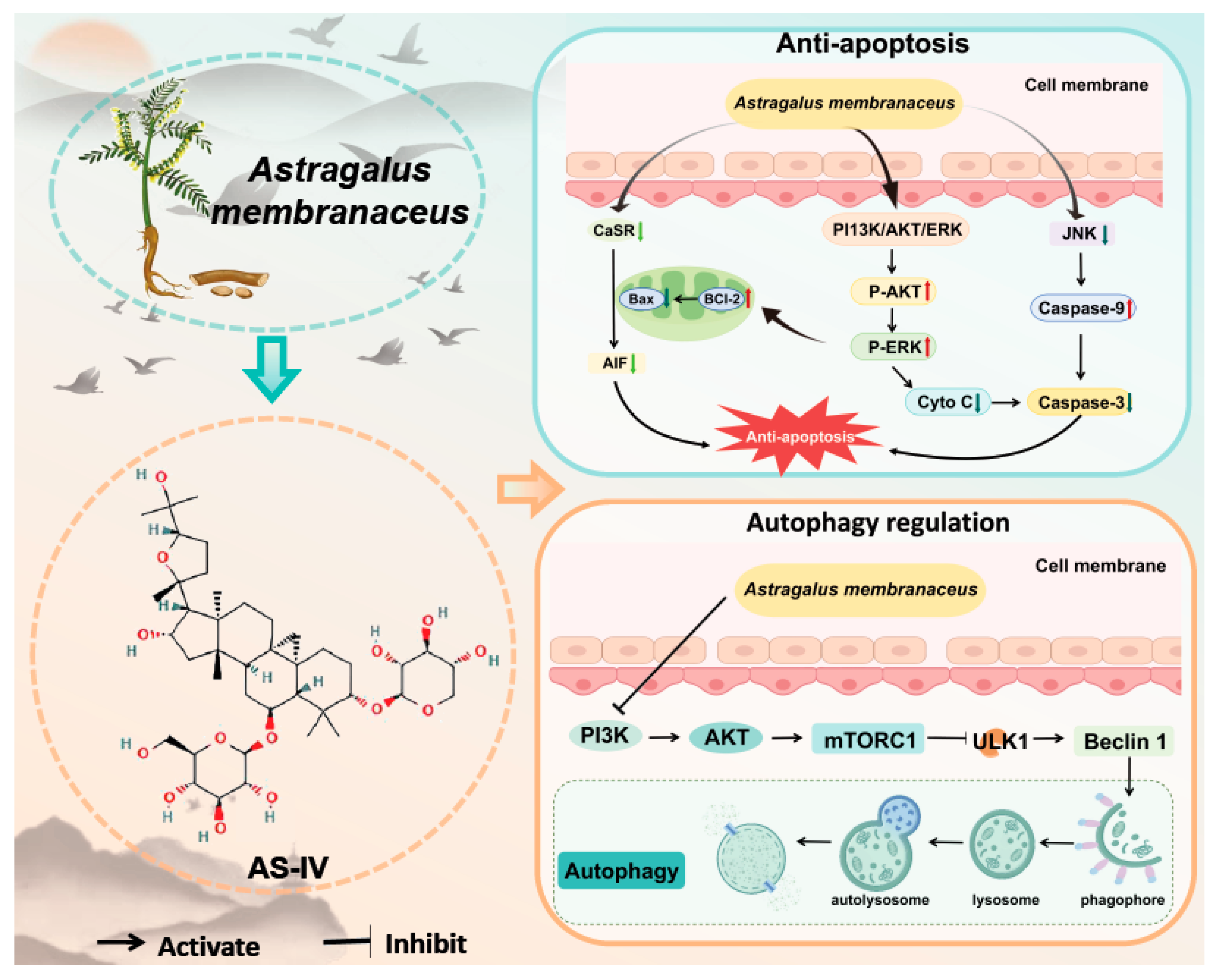
3.3. Anti-Apoptosis
3.3.1. Astragalus Membranaceus Inhibits Jnk Phosphorylation
3.3.2. Astragalus Membranaceus Regulates the Expression of Casr
3.3.3. Astragalus Membranaceus Inhibits Apoptosis Through Other Pathways
3.4. Autophagy Regulation
Astragalus Membranaceus Regulates Autophagy via Ampk and Mtor
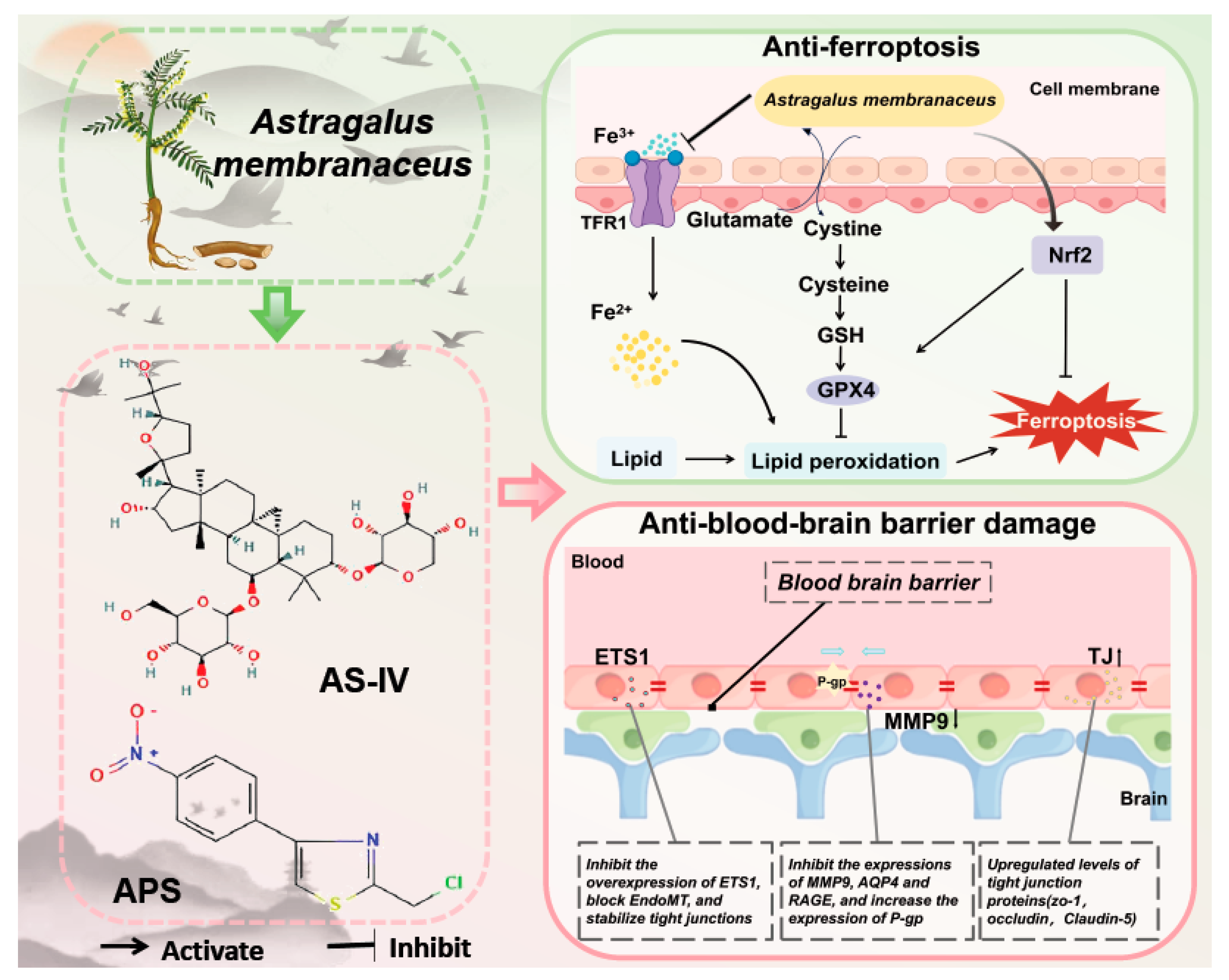
3.5. Anti-Ferroptosis
3.5.1. The Regulation of Gpx4 by Astragalus Membranaceus
3.5.2. Astragalus Membranous Relies on Nadph to Function
3.6. Anti-Blood-Brain Barrier Damage
3.6.1. The Regulation of Tight Junction Proteins by Active Ingredients of Astragalus Membranaceus
3.6.2. The Regulation of Ets1 by Astragalus Membranaceus
3.6.3. Astragalus Membranaceus Combats Bbb Through Other Means
4. Pharmacognosy of Astragalus Membranaceus
4.1. Safety
4.2. Application of Astragalus Membranaceus in the Field of Food and Medicine
4.2.1. Application of Astragalus Membranaceus in Formulated Preparations
4.2.2. Application of Astragalus Membranaceus in Health Foods
Liquid Products
Semi-Liquid Products
Solid Products
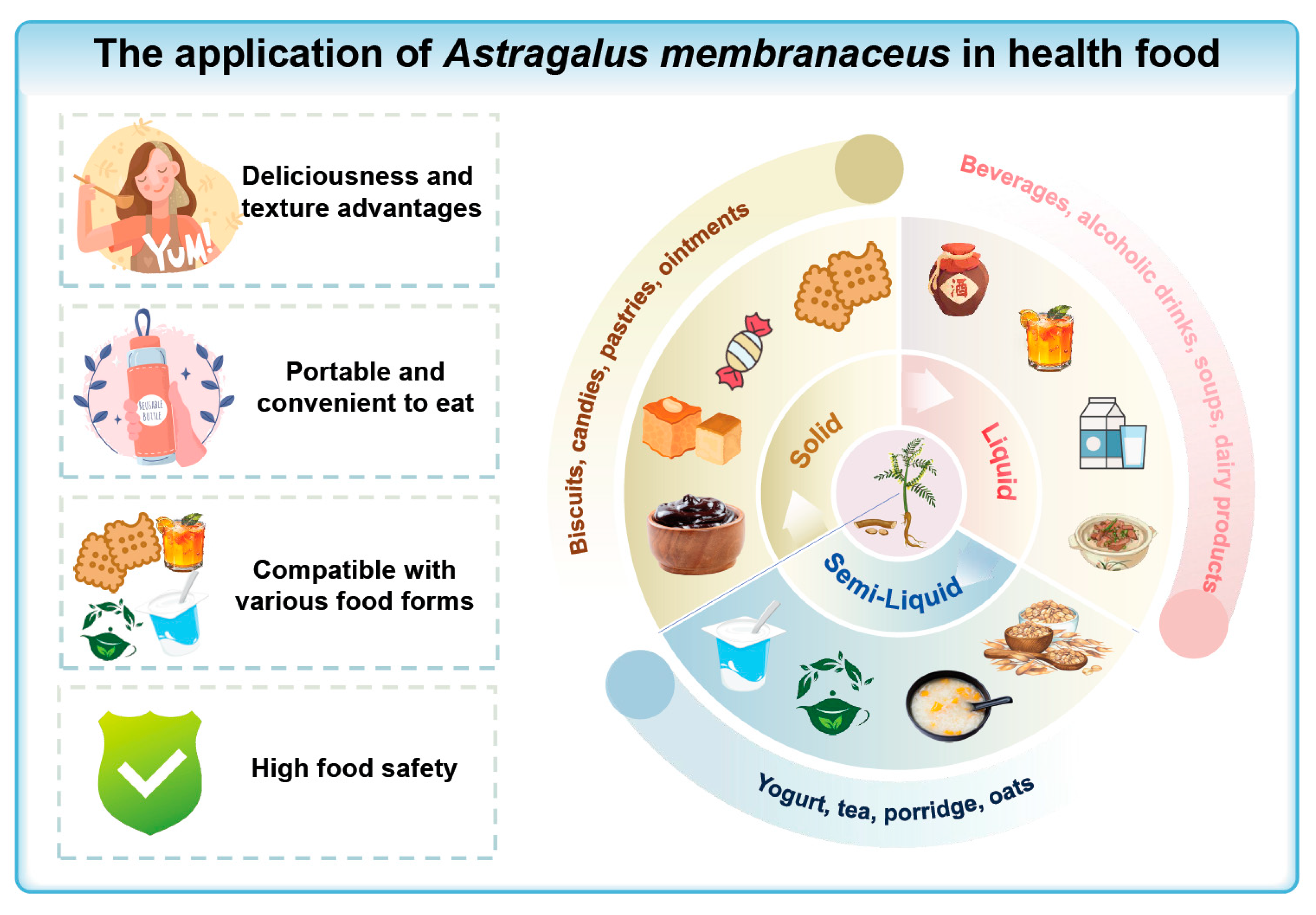
5. Summary
5.1. Neuroprotective Potential and Mechanisms of Astragalus Membranaceus Membranaceus in Cns Disorders
5.2. The Limitations Faced in the Clinical Application and Promotion of Astragalus Membranaceus
- (1)
- The pathways and targets still need to be clarified. Existing studies focus on a single signaling axis (e.g., PI3K/AKT, Nrf2), and there is a lack of systematic analysis of the whole target network of Astragalus membranaceus in neuron-glia-vascular unit using a multi-omics integration strategy, which makes it difficult to translate the synergistic advantage of "multi-component-multi-target" into quantifiable biomarkers and precise intervention programs.
- (2)
- The drug combination does not work. The role of the drug combination is unclear. When Astragalus membranaceus is used in combination with other drugs, it may affect the efficacy or increase the risk of adverse reactions, which still needs to be proved by clinical studies.
- (3)
- Lack of clinical evidence. At present, most of the domestic and international studies on Astragalus membranaceus treatment of CNS diseases are cellular and animal experiments, lacking large-sample, multicenter, randomized controlled clinical data; in addition, the dose-exposure relationship of Astragalus membranaceus in various types of animal models and the effect of the route of administration on the bioavailability are still lacking systematic elucidation; whether long-term use of the drug induces tolerance, or leads to dry mouth, constipation, and other adverse effects, has not been seen in a definitive conclusion.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TCM | traditional Chinese medicine |
| CNS | Central Nervous System |
| PD | Parkinson's disease |
| AS-IV | Astragaloside IV |
| APS | Astragalus membranaceus polysaccharides |
| CAG | Cycloastragenol |
| SCI | spinal cord injury |
| AD | Alzheimer's disease |
| TBI | traumatic brain injury |
| CI | cerebral infarction |
| NF-κB | Nuclear factor-κB |
| NLRP3 | NOD-like receptor thermal protein domain associated protein 3 |
| PPARγ | peroxisome proliferator-activated receptor γ |
| RRS | repeated restraint stress |
| LPS | Lipopolysaccharides |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| SOD | superoxide dismutase |
| GSH-Px | glutathione peroxidase |
| NOX2/4 | NADPH oxidase 2/4 |
| T-AOC | total antioxidant capacity |
| AIS | Acute Ischemic Stroke |
| CA | Calycosin |
| PTZ | pentylenetetrazole |
| Rg1 | Panax ginseng's |
| FMN | Formononetin |
| HGWD | Huangqi Guizhi WuWu Tang |
| JNK | c-Jun N-terminal kinase |
| p-ERK | phosphorylated extracellular signal-regulated kinase |
| CIRI | cerebral ischemia-reperfusion injury |
| CaSR | calcium-sensitive receptor |
| AMPK | Adenosine activated protein kinase |
| I/R | ischemia/reperfusion |
| OGD/R | oxygen-glucose deprivation/reoxygenation |
| TON | traumatic optic neuropathy |
| GPX4 | Glutathione peroxidase 4 |
| TFA | Total flavonoids |
| SAH | subarachnoid hemorrhage |
| NOX4 | NADPH oxidase 4 |
| LPO | Peroxidation |
| BBB | blood-brain barrier |
| MS | multiple sclerosis |
| SAE | sepsis-associated encephalopathy |
| P-gp | P-glycoprotein |
| RAGE | receptor for advanced glycation end-products |
| ISOI | Isoastragaloside I |
References
- Abd Elrahim Abd Elkader, H., Essawy, A. E., & Al-Shami, A. S. (2022). Astragalus species: Phytochemistry, biological actions and molecular mechanisms underlying their potential neuroprotective effects on neurological diseases. Phytochemistry, 202, 113293. [CrossRef]
- Aldarmaa, J., Liu, Z., Long, J., Mo, X., Ma, J., & Liu, J. (2010). Anti-convulsant Effect and Mechanism of Astragalus mongholicus Extract In Vitro and In Vivo: Protection Against Oxidative Damage and Mitochondrial Dysfunction. Neurochem Res, 35(1), 33-41. [CrossRef]
- Alharbi, K. M., Alshehri, S. A., Almarwani, W. A., Aljohani, K. K., Albalawi, A. Z., Alatawi, A. S., ... Al-Gayyar, M. (2024). Effects of Cycloastragenol on Alzheimer's Disease in Rats by Reducing Oxidative Stress, Inflammation, and Apoptosis. Curr Alzheimer Res, 21(2), 141-154. [CrossRef]
- Alves, F., Lane, D., Nguyen, T., Bush, A. I., & Ayton, S. (2025). In defence of ferroptosis. Signal Transduct Target Ther, 10(1), 2. [CrossRef]
- Araya, L. E., Soni, I. V., Hardy, J. A., & Julien, O. (2021). Deorphanizing Caspase-3 and Caspase-9 Substrates In and Out of Apoptosis with Deep Substrate Profiling. ACS Chem Biol, 16(11), 2280-2296. [CrossRef]
- Bahar, E., Kim, J., & Yoon, H. (2017). Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, iNOS/NF-κB and HO-1/Nrf2 Pathways. International Journal of Molecular Sciences, 18(9), 1989. [CrossRef]
- Berry, C., Ley, E. J., Tillou, A., Cryer, G., Margulies, D. R., & Salim, A. (2009). The Effect of Gender on Patients With Moderate to Severe Head Injuries. Journal of Trauma: Injury, Infection & Critical Care, 67(5), 950-953. [CrossRef]
- Biala, A. K., Dhingra, R., & Kirshenbaum, L. A. (2015). Mitochondrial dynamics: Orchestrating the journey to advanced age. J Mol Cell Cardiol, 83, 37-43. [CrossRef]
- Cai, J., Pan, R., Jia, X., Li, Y., Hou, Z., Huang, R. Y., ... Huang, Y. (2014). The combination of astragalus membranaceus and ligustrazine ameliorates micro-haemorrhage by maintaining blood-brain barrier integrity in cerebrally ischaemic rats. J Ethnopharmacol, 158 Pt A, 301-309. [CrossRef]
- Cao, M., Guo, Z., Wang, J., Ma, H., Qin, X., Hu, Y., & Lan, R. (2025). Astragalin alleviates lipopolysaccharide-induced depressive-like behavior in mice by preserving blood-brain barrier integrity and suppressing neuroinflammation. Free Radic Biol Med, 232, 340-352. [CrossRef]
- Castillo Ferrer, C., Berthenet, K., & Ichim, G. (2021). Apoptosis – Fueling the oncogenic fire. The FEBS Journal, 288(15), 4445-4463. [CrossRef]
- Chen, G., Jiang, N., Zheng, J., Hu, H., Yang, H., Lin, A., ... Liu, H. (2023). Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus. Int J Biol Macromol, 241, 124386. [CrossRef]
- Cherry, J. D., Olschowka, J. A., & O'Banion, M. K. (2014). Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation, 11, 98. [CrossRef]
- Coll, R. C., Schroder, K., & Pelegrin, P. (2022). NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci, 43(8), 653-668. [CrossRef]
- Dai, X., Liu, Y., Meng, F., Li, Q., Wu, F., Yuan, J., ... Chang, Y. (2023). Amplification of oxidative damage using near-infrared II-mediated photothermal/thermocatalytic effects for periodontitis treatment. Acta Biomater, 171, 519-531. [CrossRef]
- Debnath, J., Gammoh, N., & Ryan, K. M. (2023). Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol, 24(8), 560-575. [CrossRef]
- Deleyto-Seldas, N., & Efeyan, A. (2021). The mTOR–Autophagy Axis and the Control of Metabolism. Front Cell Dev Biol, 9, 655731. [CrossRef]
- Deng, Z., Zhou, X., Lu, J., & Yue, Z. (2021). Autophagy deficiency in neurodevelopmental disorders. Cell & Bioscience, 11(1), 214. [CrossRef]
- Ding, C., Wu, Y., Chen, X., Chen, Y., Wu, Z., Lin, Z., ... Chen, F. (2022). Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990–2019. Front Public Health, 10, 952161. [CrossRef]
- Du SJ, Zhang, Y., Zhao, Y. M., Dong, Y. J., Tang, J. L., Zhou, X. H., & Gao, W. J. (2021). Astragaloside IV attenuates cerebral ischemia-reperfusion injury in rats through the inhibition of calcium-sensing receptor-mediated apoptosis. Int J Mol Med, 47(1), 302-314. [CrossRef]
- Engler-Chiurazzi, E. B., Brown, C. M., Povroznik, J. M., & Simpkins, J. W. (2017). Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Prog Neurobiol, 157, 188-211. [CrossRef]
- Feng, W. D., Liu, D. N., Shang, Y. F., Zhang, W. F., Xu, S., Feng, D. H., & Wang, Y. H. (2025). Neuroimmune modulators derived from natural products: Mechanisms and potential therapies. Pharmacol Ther, 269, 108830. [CrossRef]
- Gao, P., Shi, H., Jin, X., Guo, S., Zhou, X., & Gao, W. (2024). Mechanism of astragaloside IV regulating NLRP3 through LOC102555978 to attenuate cerebral ischemia reperfusion induced microglia pyroptosis. Int Immunopharmacol, 131, 111862. [CrossRef]
- Gao, Z., Wang, G., Chen, Y., Yuan, W., Cai, J., Feng, A., ... Wu, X. (2024). Total flavonoids of Astragalus membranaceus protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice by inhibiting ferroptosis through SLC7A11/GPX-4 signaling pathway. Food Sci Human Wellness, 13(1), 414-420. [CrossRef]
- Gong, A. G. W., Duan, R., Wang, H. Y., Kong, X. P., Dong, T. T. X., Tsim, K. W. K., & Chan, K. (2018). Evaluation of the Pharmaceutical Properties and Value of Astragali Radix. Medicines, 5(2), 46. [CrossRef]
- Gu, D., Lu, P., Zhang, K., Wang, X., Sun, M., Chen, G., & Wang, Q. (2015). EGFR mediates astragaloside IV-induced Nrf2 activation to protect cortical neurons against in vitro ischemia/reperfusion damages. Biochem Biophys Res Commun, 457(3), 391-397. [CrossRef]
- Gupta, I., Ganguly, S., Rozanas, C. R., Stuehr, D. J., & Panda, K. (2016). Cigarette Smoke Induced Emphysema and Pulmonary Hypertension Are Triggered by Tobacco Smoke Oxidants: Attenuation by Vitamin C. Free Radic Biol Med. [CrossRef]
- Hadian, K., & Stockwell, B. R. (2020). SnapShot: Ferroptosis. Cell, 181(5), 1188. [CrossRef]
- Haftcheshmeh, S. M., Abedi, M., Mashayekhi, K., Mousavi, M. J., Navashenaq, J. G., Mohammadi, A., & Momtazi-Borojeni, A. A. (2022). Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF-kappaB, JAK/STAT, and MAPK signaling pathways. Phytother Res, 36(3), 1216-1230. [CrossRef]
- Hagemann, N., Mohamud Yusuf, A., Martiny, C., Zhang, X., Kleinschnitz, C., Gunzer, M., ... Hermann, D. M. (2020). Homozygous Smpd1 deficiency aggravates brain ischemia/ reperfusion injury by mechanisms involving polymorphonuclear neutrophils, whereas heterozygous Smpd1 deficiency protects against mild focal cerebral ischemia. Basic Res Cardiol, 115(6), 64. [CrossRef]
- Hao, H., Yang, J., & Zhu, J. (2023). Astragaloside IV ameliorates cerebral ischemic damage by restraining adenosine monophosphate-activated protein kinase/mTOR-triggered autophagic process and apoptotic activity in neurons. Mater Express, 13(7), 1265-1273. [CrossRef]
- He, Y. X., Du M, Shi, H. L., Huang, F., Liu, H. S., Wu, H., ... Wang, Z. T. (2014). Astragalosides from Radix Astragali benefits experimental autoimmune encephalomyelitis in C57BL /6 mice at multiple levels. BMC Complement Altern Med, 14, 313. [CrossRef]
- He, Y., Du M, Gao, Y., Liu, H., Wang, H., Wu, X., & Wang, Z. (2013). Astragaloside IV attenuates experimental autoimmune encephalomyelitis of mice by counteracting oxidative stress at multiple levels. PLoS One, 8(10), e76495. [CrossRef]
- Hoffman, B., & Liebermann, D. A. (1998). The proto-oncogene c-myc and apoptosis. Oncogene, 17(25), 3351-3357. [CrossRef]
- Hou, B., Liu, R., Wu, Y., & Huang, S. (2020a). Astragaloside IV Reduces Cerebral Ischemia/Reperfusion-Induced Blood-Brain Barrier Permeability in Rats by Inhibiting ER Stress-Mediated Apoptosis. Evid Based Complement Alternat Med, 2020(1), 9087873. [CrossRef]
- Hou, B., Liu, R., Wu, Y., & Huang, S. (2020b). Astragaloside IV Reduces Cerebral Ischemia/Reperfusion-Induced Blood-Brain Barrier Permeability in Rats by Inhibiting ER Stress-Mediated Apoptosis. Evid Based Complement Alternat Med, 2020(1), 9087873. [CrossRef]
- Hou, Y., Yan, Z., Wan, H., Yang, J., Ding, Z., & He, Y. (2024). A Combination of Astragaloside IV and Hydroxysafflor Yellow A Attenuates Cerebral Ischemia-Reperfusion Injury via NF-κB/NLRP3/Caspase-1/GSDMD Pathway. Brain Sci, 14(8), 781. [CrossRef]
- Huang, M. Y., & Yu, G. R. (2021). Cycloastragenol inhibits Abeta(1-42)-induced blood-brain barrier disruption and enhances soluble Abeta efflux in vitro. J Asian Nat Prod Res, 23(6), 556-569. [CrossRef]
- Huang, X. P., Qiu, Y. Y., Wang, B., Ding, H., Tang, Y. H., Zeng, R., & Deng, C. Q. (2014). Effects of Astragaloside IV combined with the active components of Panax notoginseng on oxidative stress injury and nuclear factor-erythroid 2-related factor 2/heme oxygenase-1 signaling pathway after cerebral ischemia-reperfusion in mice. Pharmacogn Mag, 10(40), 402-409. [CrossRef]
- Jang, M., Park, R., Kim, H., Namkoong, S., Jo, D., Huh, Y. H., ... Park, J. (2018). AMPK contributes to autophagosome maturation and lysosomal fusion. Sci Rep, 8(1), 12637. [CrossRef]
- Jia, X., Xie, L., Liu, Y., Liu, T., Yang, P., Hu, J., ... Chen, C. (2022). Astragalus polysaccharide (APS) exerts protective effect against acute ischemic stroke (AIS) through enhancing M2 micoglia polarization by regulating adenosine triphosphate (ATP)/ purinergic receptor (P2X7R) axis. Bioengineered, 13(2), 4468-4480. [CrossRef]
- Jiang, C., Yan, Y., Long, T., Xu, J., Chang, C., Kang, M., ... Qiu, J. (2025). Ferroptosis: a potential therapeutic target in cardio-cerebrovascular diseases. Mol Cell Biochem, 480(7), 4379-4399. [CrossRef]
- Jomova, K., Raptova, R., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K., & Valko, M. (2023). Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol, 97(10), 2499-2574. [CrossRef]
- Kapil, L., Kumar, V., Kaur, S., Sharma, D., Singh, C., & Singh, A. (2024). Role of Autophagy and Mitophagy in Neurodegenerative Disorders. CNS & neurological disorders drug targets, 23(3), 367. [CrossRef]
- Kodi, T., Sankhe, R., Gopinathan, A., Nandakumar, K., & Kishore, A. (2024). New Insights on NLRP3 Inflammasome: Mechanisms of Activation, Inhibition, and Epigenetic Regulation. J Neuroimmune Pharmacol, 19(1), 7. [CrossRef]
- Kong, H., Xu, T., Wang, S., Zhang, Z., Li, M., Qu, S., ... Cong, Z. (2024). The molecular mechanism of polysaccharides in combating major depressive disorder: A comprehensive review. Int J Biol Macromol, 259(Pt 2), 129067. [CrossRef]
- Lei, P., Walker, T., & Ayton, S. (2025). Neuroferroptosis in health and diseases. Nat Rev Neurosci. [CrossRef]
- Li, H. L., Shao, L. H., Chen, X., Wang, M., Qin, Q. J., Yang, Y. L., ... Tian, Y. H. (2024). Anti-inflammatory and DNA Repair Effects of Astragaloside IV on PC12 Cells Damaged by Lipopolysaccharide. Curr Med Sci, 44(4), 854-863. [CrossRef]
- Li, H., Jin, J., Yang, C., Wang, P., Huang, F., Wu, H., ... Wu, X. (2018). Isoastragaloside I suppresses LPS-induced tight junction disruption and monocyte adhesion on bEnd.3 cells via an activating Nrf2 antioxidant defense system. RSC Adv.
- Li, H., Wang, P., Huang, F., Jin, J., Wu, H., Zhang, B., ... Wu, X. (2018). Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol Appl Pharmacol, 340, 58-66. [CrossRef]
- Li, L., Gan, H., Jin, H., Fang, Y., Yang, Y., Zhang, J., ... Chu, L. (2021). Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARγ pathway after cerebral ischemia/reperfusion injury in rats. Int Immunopharmacol, 92, 107335. [CrossRef]
- Li, M., Han, B., Zhao, H., Xu, C., Xu, D., Sieniawska, E., ... Kai, G. (2022a). Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine, 98, 153918. [CrossRef]
- Li, M., Han, B., Zhao, H., Xu, C., Xu, D., Sieniawska, E., ... Kai, G. (2022b). Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine, 98, 153918. [CrossRef]
- Li, M., Huan, Y., Jiang, T., He, Y., & Gao, Z. (2024). Rehabilitation training enhanced the therapeutic effect of calycosin on neurological function recovery of rats following spinal cord injury. J Chem Neuroanat, 136, 102384. [CrossRef]
- Li, M., Huan, Y., Jiang, T., He, Y., & Gao, Z. (2024). Rehabilitation training enhanced the therapeutic effect of calycosin on neurological function recovery of rats following spinal cord injury. J Chem Neuroanat, 136, 102384. [CrossRef]
- Li, M., Ma, R. N., Li, L. H., Qu, Y. Z., & Gao, G. D. (2013). Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. Eur J Pharmacol, 715(1-3), 189-195. [CrossRef]
- Li, M., Qu, Y. Z., Zhao, Z. W., Wu, S. X., Liu, Y. Y., Wei, X. Y., ... Gao, G. D. (2012). Astragaloside IV protects against focal cerebral ischemia/reperfusion injury correlating to suppression of neutrophils adhesion-related molecules. Neurochem Int, 60(5), 458-465. [CrossRef]
- Li, W., Shao, C., Huang, P., Yu, D., Yang, J., Wan, H., & He, Y. (2023). Optimization, characterization of Astragalus polysaccharides, and evaluation of anti-inflammation effect in primary cultured astrocytes via HMGB1/RAGE/NF-κB/NLRP3 signal pathway. Ind Crops Prod, 197, 116594. [CrossRef]
- Lin, J., Pan, X., Huang, C., Gu, M., Chen, X., Zheng, X., ... Wang, X. (2020). Dual regulation of microglia and neurons by Astragaloside IV-mediated mTORC1 suppression promotes functional recovery after acute spinal cord injury. J Cell Mol Med, 24(1), 671-685. [CrossRef]
- Liu, H. X., Li, Y. C., Su, R. B., Liu, C. X., & Wen, S. Y. (2025). Astragalus injection inhibits the growth of osteosarcoma by activating cytotoxic T lymphocyte and targeting CTSL. J Ethnopharmacol, 345, 119607. [CrossRef]
- Liu, J., Han, X., Zhang, T., Tian, K., Li, Z., & Luo, F. (2023). Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: from mechanism to therapy. J Hematol Oncol, 16(1), 116. [CrossRef]
- Liu, K., Wan, G., Jiang, R., Zou, L., Wan, D., Zhu, H., & Feng, S. (2022). Astragalus injection ameliorates lipopolysaccharide-induced cognitive decline via relieving acute neuroinflammation and BBB damage and upregulating the BDNF-CREB pathway in mice. Pharm Biol, 60(1), 825-839. [CrossRef]
- Liu, L., Zhang, K., Sandoval, H., Yamamoto, S., Jaiswal, M., Sanz, E., ... Bellen, H. J. (2015a). Glial Lipid Droplets and ROS Induced by Mitochondrial Defects Promote Neurodegeneration. Cell, 160(1-2), 177-190. [CrossRef]
- Liu, L., Zhang, K., Sandoval, H., Yamamoto, S., Jaiswal, M., Sanz, E., ... Bellen, H. J. (2015b). Glial Lipid Droplets and ROS Induced by Mitochondrial Defects Promote Neurodegeneration. Cell, 160(1-2), 177-190. [CrossRef]
- Liu, X., Ma, J., Ding, G., Gong, Q., Wang, Y., Yu, H., & Cheng, X. (2021). Microglia Polarization from M1 toward M2 Phenotype Is Promoted byAstragalus Polysaccharides Mediated through Inhibition of miR-155 in Experimental Autoimmune Encephalomyelitis. Oxid Med Cell Longev, 2021(1), 5753452. [CrossRef]
- Liu, Z., Zhou, Z., Ai, P., Zhang, C., Chen, J., & Wang, Y. (2022). Astragaloside IV attenuates ferroptosis after subarachnoid hemorrhage via Nrf2/HO-1 signaling pathway. Front Pharmacol, 13, 924826. [CrossRef]
- Lizama, B. N., & Chu, C. T. (2021). Neuronal autophagy and mitophagy in Parkinson's disease. Mol Aspects Med, 82, 100972. [CrossRef]
- Lu, Q., Ma, J., Zhao, Y., Ding, G., Wang, Y., Qiao, X., & Cheng, X. (2024). Disruption of blood-brain barrier and endothelial-to-mesenchymal transition are attenuated by Astragalus polysaccharides mediated through upregulation of ETS1 expression in experimental autoimmune encephalomyelitis. Biomed Pharmacother, 180, 117521. [CrossRef]
- Lv, Z., Shen, J., Gao, X., Ruan, Y., Ling, J., Sun, R., ... Cao, P. (2021). Herbal formula Huangqi Guizhi Wuwu decoction attenuates paclitaxel-related neurotoxicity via inhibition of inflammation and oxidative stress. Chin Med, 16(1), 76. [CrossRef]
- Ma, T., Du J, Zhang, Y., Wang, Y., Wang, B., & Zhang, T. (2022). GPX4-independent ferroptosis-a new strategy in disease's therapy. Cell Death Discov, 8(1), 434. [CrossRef]
- Mai, L., Liu, J., Wu, H., Wang, H., Lin, Z., Rao, S., ... Chen, B. (2025). Enhanced inhibition of neuronal ferroptosis and regulation of microglial polarization with multifunctional traditional Chinese medicine active ingredients-based selenium nanoparticles for treating spinal cord injury. Mater Today Bio, 32, 101758. [CrossRef]
- Mao, H., Zhao, X., & Sun, S. (2025). NF- κ Bininflammation and cancer. Cell Mol Immunol.
- Patabendige, A., & Janigro, D. (2023). The role of the blood–brain barrier during neurological disease and infection. Biochem Soc Trans, 51(2), 613-626. [CrossRef]
- Rathod, S. S., Agrawal, Y. O., Nakhate, K. T., Meeran, M. F. N., Ojha, S., & Goyal, S. N. (2023). Neuroinflammation in the Central Nervous System: Exploring the Evolving Influence of Endocannabinoid System. Biomedicines, 11(10), 2642. [CrossRef]
- Reshi, L., Wu, H., Wu, J., Wang, H., & Hong, J. (2016). GSIV serine/threonine kinase can induce apoptotic cell death via p53 and pro-apoptotic gene Bax upregulation in fish cells. Apoptosis, 21(4), 443-458. [CrossRef]
- Ruan, J., Shi, Z., Cao, X., Dang, Z., Zhang, Q., Zhang, W., ... Wang, T. (2024). Research Progress on Anti-Inflammatory Effects and Related Mechanisms of Astragalin. Int J Mol Sci, 25(8). [CrossRef]
- Senapati, P. K., Mahapatra, K. K., Singh, A., & Bhutia, S. K. (2025). mTOR inhibitors in targeting autophagy and autophagy-associated signaling for cancer cell death and therapy. Biochimica et Biophysica Acta, 1880(3), 189342. [CrossRef]
- Shao, A., Guo, S., Tu, S., Ammar, A., Tang, J., Hong, Y., ... Zhang, J. (2014). Astragaloside IV Alleviates Early Brain Injury Following Experimental Subarachnoid Hemorrhage in Rats. Int J Med Sci, 11(10), 1073-1081. [CrossRef]
- Shi, Y., Shi, X., Zhao, M., Ma, S., & Zhang, Y. (2024). Pharmacological potential of Astragali Radix for the treatment of kidney diseases. Phytomedicine, 123, 155196. [CrossRef]
- Silva-Islas, C. A., & Maldonado, P. D. (2018). Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol Res, 134, 92-99. [CrossRef]
- Sirnonian, N. A., & Coyle, J. T. (1996). OXIDATIVE STRESS IN NEURODEGENERATIVE DISEASES. ANNUAL REVIEWS.
- Soliman, D. H., & Nafie, M. S. (2023). Design, synthesis, and docking studies of novel pyrazole-based scaffolds and their evaluation as VEGFR2 inhibitors in the treatment of prostate cancer. RSC Adv. [CrossRef]
- Song, M., Ruan, J., Zhang, R., Deng, J., Ma, Z., & Ma, S. (2018). AstragalosidelVameliorates neuroinflammationinduced depressive-like behaviors in mice via thePPARV/NF-kB/NLRP3 inflammasome axis. Acta Pharmacol Sin.
- Song, R., Guo, Y., Fu, Y., Ren, H., Wang, H., Yan, H., & Ge, Y. (2023). Trends of mitochondrial changes in AD: a bibliometric study. Front Aging Neurosci, 15, 1136400. [CrossRef]
- Souza, D. G., Almeida, R. F., Souza, D. O., & Zimmer, E. R. (2019). The astrocyte biochemistry. Semin Cell Dev Biol, 95, 142-150. [CrossRef]
- Sun, J., Chen, X. L., Zheng, J. Y., Zhou, J. W., & Ma, Z. L. (2016). Astragaloside IV protects new born rats from anesthesia-induced apoptosis in the developing brain. Exp Ther Med, 12(3), 1829-1835. [CrossRef]
- Sun, Q., Jia, N., Wang, W., Jin, H., Xu, J., & Hu, H. (2014). Protective effects of astragaloside IV against amyloid beta1-42 neurotoxicity by inhibiting the mitochondrial permeability transition pore opening. PLoS One, 9(6), e98866. [CrossRef]
- Sun, W., Chao, G., Wu, Q., Xia, Y., Shang, M., Wei, Q., ... Liao, L. (2025). Astragaloside IV improves the survival rates of retinal ganglion cells in traumatic optic neuropathy by regulating autophagy mediated by the AMPK-MTOR-ULK signaling pathway. Mol Vis, 31, 99-112.
- Tak, P. P., & Firestein, G. S. (2001). NF-κB: a key role in inflammatory diseases. The Journal of clinical investigation, 107(1), 7-11. [CrossRef]
- Tang, Y., Zhang, Y., Chen, C., Cao, Y., Wang, Q., & Tang, C. (2025). Gut microbiota: A new window for the prevention and treatment of neuropsychiatric disease. J Cent Nerv Syst Dis, 17, 1585117614. [CrossRef]
- Teleanu, D. M., Niculescu, A., Lungu, I. I., Radu, C. I., Vladâcenco, O., Roza, E., ... Teleanu, R. I. (2022). An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. International Journal of Molecular Sciences, 23(11), 5938. [CrossRef]
- Thakur, A., Wang, X., Siedlak, S. L., Perry, G., Smith, M. A., & Zhu, X. (2007). c-Jun phosphorylation in Alzheimer disease. J Neurosci Res, 85(8), 1668-1673. [CrossRef]
- Tujula, I., Hyvärinen, T., Lotila, J., Rogal, J., Voulgaris, D., Sukki, L., ... Hagman, S. (2025). Modeling neuroinflammatory interactions between microglia and astrocytes in a human iPSC-based coculture platform. Cell Commun Signal, 23(1), 298. [CrossRef]
- Wang, M., Li, M., Jiang, Y., Wang, S., Yang, X., Naseem, A., ... Liu, Y. (2025). Saponins fromAstragalus membranaceus (Fisch.) Bge Alleviated Neuronal Ferroptosis in Alzheimer’s Disease by Regulating the NOX4/Nrf2 Signaling Pathway. J Agric Food Chem, 73(13), 7725-7740. [CrossRef]
- Wang, S., Li, H., Yuan, M., Fan, H., & Cai, Z. (2022). Role of AMPK in autophagy. Front Physiol, 13, 1015500. [CrossRef]
- Wang, S., Yang, Y., Lin, J., Zhang, W., Yang, C., Zhang, R., ... Ma, Y. (2025). Astragalin actives autophagy and inhibits apoptosis of astrocytes in AD mice via down-regulating Fas/Fasl-VDAC1 pathway. Free Radic Biol Med, 232, 72-85. [CrossRef]
- Wang, T., Sun, Q., Yang, J., Wang, G., Zhao, F., Chen, Y., & Jin, Y. (2021). Reactive astrocytes induced by 2-chloroethanol modulate microglia polarization through IL-1β, TNF-α, and iNOS upregulation. Food Chem Toxicol, 157, 112550. [CrossRef]
- Wang, X., Xu, W., Chen, H., Li, W., Li, W., & Zhu, G. (2020). Astragaloside IV prevents Aβ 1-42 oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ / BDNF signaling pathway. Brain Res, 1747. [CrossRef]
- Weng, W., Lin, B., Zheng, J., Sun, Y., Li, Z., Chen, X., ... Pan, X. (2025). Novel application of cycloastragenol target microglia for the treatment of Alzheimer's disease: Evidence from single-cell analysis, network pharmacology and experimental assessment. Phytomedicine, 139, 156502. [CrossRef]
- Wijdicks, E. F. (2016). Hepatic Encephalopathy. N Engl J Med, 375(17), 1660-1670. [CrossRef]
- Wu, Y. Y., Wu, W. Y., Gong, H. L., Li, W. Z., & Yin, Y. Y. (2014). Astragalosides attenuate learning and memory impairment in rats following ischemia-reperfusion injury. Mol Med Rep, 9(4), 1319-1324. [CrossRef]
- Xia, M., Xie, X., Ding, J., Du, R., & Hu, G. (2020). Astragaloside IV inhibits astrocyte senescence: implication in Parkinson’s disease. J Neuroinflammation, 17(1), 105. [CrossRef]
- Xiao, Y., Cheng, Y., Liu, W., Liu, K., Wang, Y., Xu, F., ... Yang, Y. (2023). Effects of neutrophil fate on inflammation. Inflamm Res, 72(12), 2237-2248. [CrossRef]
- Xie, J., Chen, Z., Pan, Y., Luo, D., Su, Z., Chen, H., ... Lei, G. (2016). Evaluation of safety of modified-Danggui Buxue Tang in rodents:immunological, toxicity and hormonal aspects. J Ethnopharmacol, 183, 59-70. [CrossRef]
- Xu, H., & Hu, F. (2022). The role of autophagy and mitophagy in cancers. Arch Physiol Biochem, 128(2), 281-289. [CrossRef]
- Xu, N., Kan, P., Yao, X., Yang, P., Wang, J., Xiang, L., & Zhu, Y. (2018). Astragaloside IV reversed the autophagy and oxidative stress induced by the intestinal microbiota of AIS in mice. J Microbiol, 56(11), 838-846. [CrossRef]
- Xu, X. F., Shi, M. M., Luo, M. Y., Liu, D. D., Guo, D. M., Ling, C., ... Cao, W. Y. (2022). Targeting PERK mediated endoplasmic reticulum stress attenuates neuroinflammation and alleviates lipopolysaccharide-induced depressive-like behavior in male mice. Int Immunopharmacol, 111, 109092. [CrossRef]
- Yang, C. Z., Wang, S. H., Zhang, R. H., Lin, J. H., Tian, Y. H., Yang, Y. Q., ... Ma, Y. X. (2023). Neuroprotective effect of astragalin via activating PI3K/Akt-mTOR-mediated autophagy on APP/PS1 mice. Cell Death Discov, 9(1), 15. [CrossRef]
- Yang, L., Huang, H., Fan, Y., Xu, L., Jin, X., Xiao, B., ... Chai, Z. (2025). Astragaloside IV ameliorates Parkinson's disease by inhibiting TLR4/NF-κB-dependent neuroinflammation. Int Immunopharmacol, 160, 114972. [CrossRef]
- Yang, Y., Wang, H., Kouadir, M., Song, H., & Shi, F. (2019). Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis, 10(2), 128. [CrossRef]
- Yao, J., Peng, T., Shao, C., Liu, Y., Lin, H., & Liu, Y. (2024). The Antioxidant Action of Astragali radix: Its Active Components and Molecular Basis. Molecules, 29(8), 1691. [CrossRef]
- Yin, F., Zhou, H., Fang, Y., Li, C., He, Y., Yu, L., ... Yang, J. (2020). Astragaloside IV alleviates ischemia reperfusion-induced apoptosis by inhibiting the activation of key factors in death receptor pathway and mitochondrial pathway. J Ethnopharmacol, 248. [CrossRef]
- Yin, M., Liu, Z., Wang, J., & Gao, W. (2023). Buyang Huanwu decoction alleviates oxidative injury of cerebral ischemia-reperfusion through PKCε/Nrf2 signaling pathway. J Ethnopharmacol, 303, 115953. [CrossRef]
- Yu, J., Guo, M., Li, Y., Zhang, H., Chai, Z., Wang, Q., ... Cungen, M. (2019). Astragaloside IV protects neurons from microglia-mediated cell damage through promoting microglia polarization. Folia Neuropathol, 57(2), 170-181. [CrossRef]
- Yu, J., Mu, B., Guo, M., Liu, C., Meng, T., Yan, Y., ... Ma, C. (2023). Astragaloside IV inhibits experimental autoimmune encephalomyelitis by modulating the polarization of both microglia/macrophages and astrocytes. Folia Neuropathol, 61(3), 273-290. [CrossRef]
- Yu, S. Y., Ouyang, H. T., Yang, J. Y., Huang, X. L., Yang, T., Duan, J. P., ... Qiong, P. (2007). Subchronic toxicity studies of Radix Astragali extract in rats and dogs. J Ethnopharmacol, 110(2), 352-355. [CrossRef]
- Yunna, C., Mengru, H., Lei, W., & Weidong, C. (2020). Macrophage M1/M2 polarization. Eur J Pharmacol, 877, 173090. [CrossRef]
- Zhang, C., Shi, Z., Xu, Q., He, J., Chen, L., Lu, Z., ... Cui, G. (2023). Astragaloside IV alleviates stroke-triggered early brain injury by modulating neuroinflammation and ferroptosis via the Nrf2/HO-1 signaling pathway. Acta Cir Bras, 38, e380723. [CrossRef]
- Zhang, Y., Zhang, Y., Jin, X., Zhou, X., Dong, X., Yu, W., & Gao, W. (2019). The Role of Astragaloside IV against Cerebral Ischemia/Reperfusion Injury: Suppression of Apoptosis via Promotion of P62-LC3-Autophagy. Molecules, 24(9), 1838. [CrossRef]
- Zhang, Z., Wu, L., Wang, J., Yang, J., Zhang, J., Zhang, J., ... Gao, G. (2012). Astragaloside IV prevents MPP+-induced SH-SY5Y cell death via the inhibition of Bax-mediated pathways and ROS production. Mol Cell Biochem, 364(1-2), 209-216. [CrossRef]
- Zhao, J., Zhao, G., Lang, J., Sun, B., Feng, S., Li, D., & Sun, G. (2024). Astragaloside IV ameliorated neuroinflammation and improved neurological functions in mice exposed to traumatic brain injury by modulating the PERK-eIF2α-ATF4 signaling pathway. J Investig Med, 72(7), 747-762. [CrossRef]
- Zhao, X., Fang, K., Liu, X., Yao, R., Wang, M., Li, F., ... Luo, Z. (2023). QSER1 preserves the suppressive status of the pro-apoptotic genes to prevent apoptosis. Cell Death Differ, 30(3), 779-793. [CrossRef]
- Zhao, X., Lu, M., Yuan, D., Xu, D., Yao, P., Ji, W., ... Ma, Q. (2019). Mitochondrial Dysfunction in Neural Injury. Front Neurosci, 13, 30. [CrossRef]
- Zheng, Y., Ren, W., Zhang, L., Zhang, Y., Liu, D., & Liu, Y. (2020). A Review of the Pharmacological Action of Astragalus Polysaccharide. Front Pharmacol, 11, 349. [CrossRef]
- Zhong, Q., & Zhu, F. (2022). Trends in Prevalence Cases and Disability-Adjusted Life-Years of Parkinson’s Disease: Findings from the Global Burden of Disease Study 2019. Neuroepidemiology, 56(4), 261-270. [CrossRef]
- Zhu, X., Chen, Y., Du Y, Wan, Q., Xu, Y., & Wu, J. (2018). Astragaloside IV attenuates penicillin-induced epilepsy via inhibiting activation of the MAPK signaling pathway. Mol Med Rep, 17(1), 643-647. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




