1. Introduction
The global incidence of dengue has markedly increased over the last decades. With an estimated 390 million infections annually, the global incidence of dengue escalated between 1990 and 2021 from 26.45 million to 58.96 million cases, accompanied by an increase in related deaths from 14,315 to 29,075, and an endemic occurrence of dengue in over 100 countries [
1]. Consequently, the risk for infection has increased significantly for travellers. Record numbers of imported cases have been reported in various European countries and elsewhere in 2024. In addition, large numbers of travellers with dengue were treated in the endemic destination countries.
Dengue presents unique challenges for immunization due to the need for balanced protection against all four serotypes. Primary infection with one serotype typically results in lifelong immunity to that serotype but only transient protection against the others. Subsequent infections with a different serotype tend to increase the risk of severe dengue due to antibody-dependent enhancement [
2]. This immunological complexity has made vaccine development particularly difficult.
The first dengue vaccine to be licensed, CYD-TDV (Dengvaxia
®, Sanofi Pasteur), is a live attenuated tetravalent vaccine. It has shown variable efficacy depending on prior dengue exposure, age, and serotype. Importantly, CYD-TDV has been associated with an increased risk of severe disease in dengue-naïve individuals, leading to a World Health Organization (WHO) recommendation that it be used only in individuals with confirmed prior dengue infection [
3]. Consequently, its use in travellers—who are often dengue-naïve—has been limited and controversial. This vaccine is currently being withdrawn from usage.
More recently, another vaccine has been developed. TAK-003 (QDENGA
®, Takeda) is another live attenuated tetravalent vaccine that has demonstrated favourable efficacy and safety profiles in both seropositive and seronegative individuals [
4]. In 2022, it received regulatory approval in the European Union for use in individuals aged 4 and older, regardless of prior exposure. The vaccine became available in Germany in February 2023.
For travellers, dengue vaccination offers a potentially valuable preventive tool, particularly for those undertaking long-term or repeated travel to endemic areas. However, implementation requires careful consideration of pre-travel counselling, timing of vaccine administration, and regional epidemiology. The availability of a first new-generation vaccine that is safe and effective in seronegative individuals has the potential to significantly change dengue prevention strategies in the travel medicine setting.
3. Results
TAK003 (Qdenga®) received market authorization by the European Medicines Agency (EMA) in November 2022 and was first distributed in February 2023. Germany was the first country to receive vaccine and at February 9th, 2023, the Berlin Centre for Travel & Tropical Medicine was the first institution where travellers were vaccinated. During the first two years of using TAK003, 266,519 pre-travel visits occurred at our 10 sites. In this population, 56,459 (21.2%) doses of TAK003 were administered. Our of these, 30,077 (53.3%) of recipients were female, 25,495 (45.2%) male, and 887 (1.6%) classified themselves as undefined sex. The age range of vaccinees was 4-86 years, with an average of 38.3 years (median 35 years). When asked to being contacted four weeks later for a follow-up, 30,306 (53.7%) of vaccinees agreed. Out of these, 11,827 (39.0%) submitted anonymous information after receiving a letter that asked them to access our study web site. These data were further evaluated. Among these, 6,856 (58%) subjects were female, 4,938 (41.8%) male, and 18 (0.2%) of undefined sex. The age was given in clusters and ranged from 4-70+ years with a median of 35 years. A previous dengue infection was reported by 565 (4.8%) subjects. The first dose of TAK003 was received by 9,268 (78.4%) subjects, while the second dose was given to 2,521 (21.4%, no answer given by 32), since a large proportion of vaccinees received their second dose after data sampling for the study was already closed.
Travel destinations included 181 countries, the 3 most frequently stated countries were Thailand (n=1,485), Indonesia (n=1,180), and India (n=695). Travel duration varied from 1 week to 21 years (latter given by an emigrant) with a median of 3 weeks.
Before completing the survey, 6,309 (53.3%) subjects had already travelled and were thus potentially exposed to dengue infection. Among these, 46 (0.7%) stated that they were diagnosed with dengue during their journey. All these dengue episodes were classified as mild by subjects, no complications or hospitalisations were reported.
Local adverse events were reported by 5,623 (47.5%), systemic AEs by 4,891 (41.4%) subjects (
Table 1). Using the possibility to give open replies, 1,294 (10.9%) subjects reported “other” symptoms, with headache (n=457, 3.9%) being the most frequent in this group. There was no difference between sexes: men reported local adverse events in 48.7% (n=2.406), while woman did so in 46.9% (n=3.217) (p>0.05).
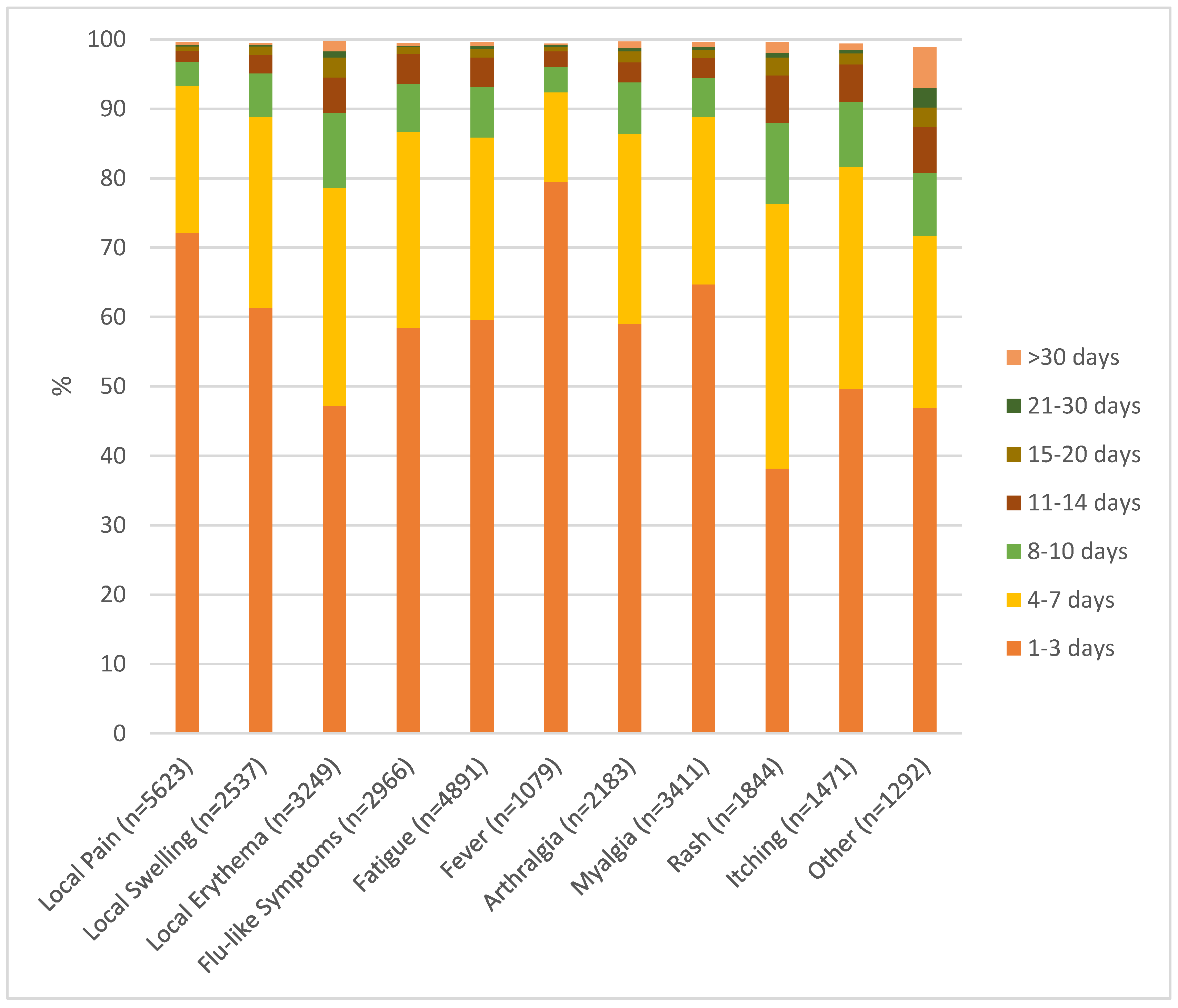
Most systemic adverse events appeared in the second week after vaccination (mean onset 9.3 days, median 8 days) and were brief. A majority of adverse events were reported with a duration of 1-3 days (
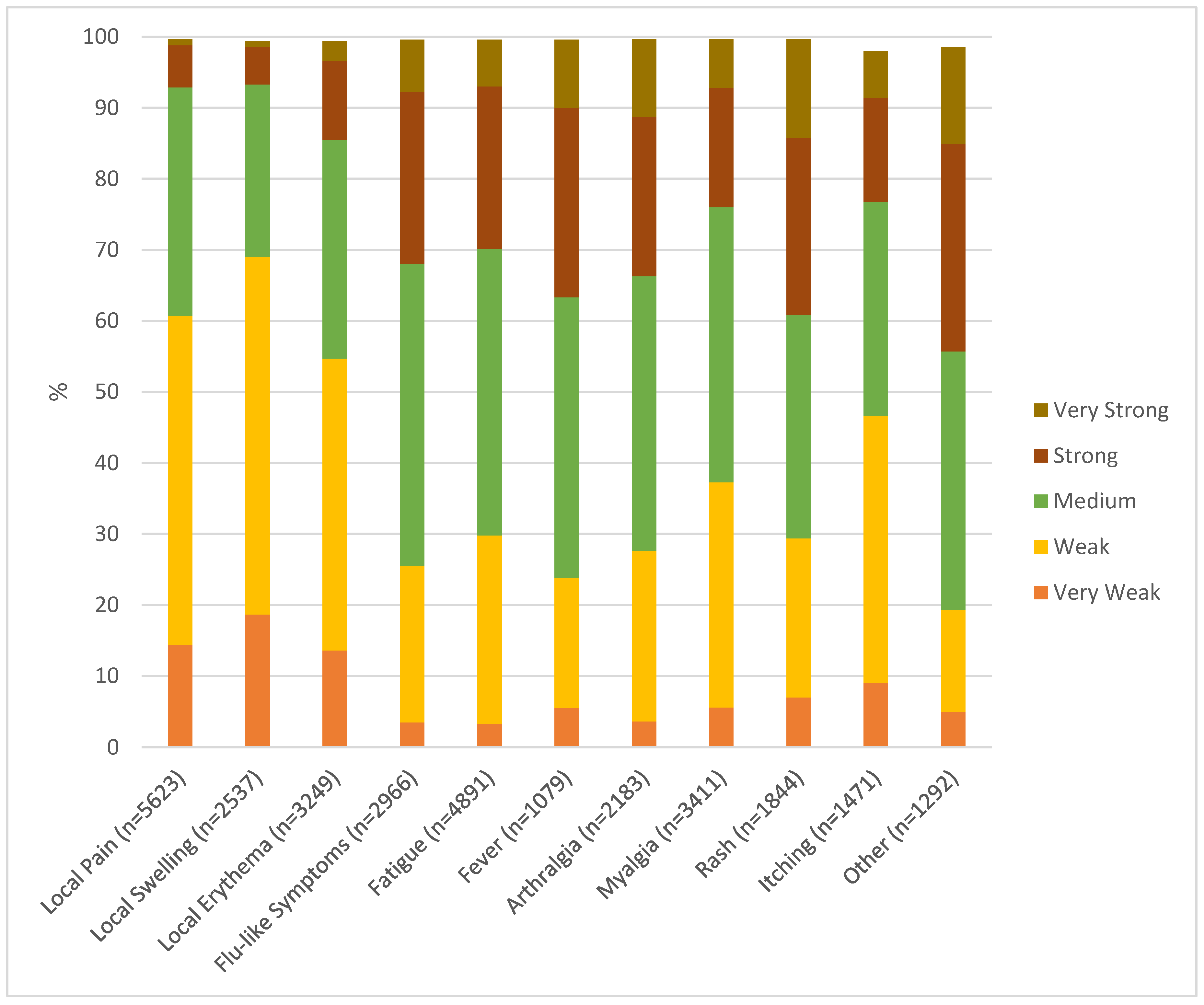
Figure 1). These included local pain 72.2%, local swelling 61.3%, local erythema 47.2%, flu-like symptoms 58.4%, fatigue 59.6%, fever 79.5%, arthralgia 59.0%, and myalgia 64.7%. Slightly longer lasting (up to 7 days) were the majority of exanthemas (76.3%), itching (81.6%), and “others” (71.7%). In terms of severity, most events were classified as very weak, weak, or medium (
Figure 2). However, an important part of systemic side effects were classified as strong or very strong: flu-like symptoms in 24.2% and 7.4%, respectively, fatigue in 22.9% and 6.6%, fever in 26.7% and 9.6%, arthralgia in 22.4% and 11.0%, myalgia in 16.8% and 6.9%, exanthema in 25.0% and 13.9%, itching in 14.6% and 6.6%, “other”, here primarily headache, in 29.2% and 13.6%.
Five subjects reported side effects that could potentially classify as severe adverse events:
A relapse of a thyroiditis (Hashimoto’s disease) was reported by a female travellers to Panama aged 30-40 years with a planned travel duration of 2 weeks. She had no prior dengue, received her first dengue vaccination and was co-vaccinated against yellow fever. She stated that she had Hashimoto’s Disease for 20 years. With fever after the vaccination, hormonal levels were checked and showed hyperthyroidism for a few days before becoming normal.
The first manifestation of hyperthyroidism was reported by a man in the age group 50-60 years who planned to travel to Colombia, had no prior dengue and was not co-vaccinated and developed hyperthyroidism 6 weeks after being vaccinated.
Myocarditis was reported by a 30-40 year old man who intended to travel to Cambodia for 12 weeks. He had no prior dengue, received his first dengue vaccination and was co-vaccinated against Japanese encephalitis. He reported local pain, erythema and arthralgia (all medium, 1-3 days), flu-like symptoms, fatigue, and fever (all strong, 8-10 days), and headache (very strong, >30 days). He stated that a myocarditis was confirmed by MRI 14 days after vaccination which resolved over 4 weeks.,
Sterile meningitis was reported by a woman in the age group 40-50 years who had no prior dengue and received her second dengue vaccination without co-vaccinations for a planned journey to Uganda. About 6 weeks after the vaccination, she developed an aseptic meningitis/encephalitis and was briefly hospitalized. Symptoms resolved over 4 weeks.
Thrombosis at both legs was reported by a woman in the age group 30-40 years who planned to travel to Barbados. She had no prior dengue and no co-vaccination was given. 16 days after vaccination and still pre-flight, thrombosis at both lower legs was diagnosed. She was hospitalised and treatment was started, the issue was resolved.
Dengue infection prior to vaccination was reported by 564 subjects. Compared to the 11,183 immunologically naïve vaccinees, significant differences were seen with local pain (238 (42.2%) in prior infection vs. 5,366 (48.0%) in naïve, p=0.007) and local erythema (114 (20.2%) vs. 3,126 (28.0%), p<0.0001) while there were no differences in other adverse events.
When comparing local and systemic adverse events after first (n=9,237) and second (n=2,523) vaccination, all were more frequent after the first dose, most significantly so: local pain (4,616 (50.0%) after 1st vs. 997 (39.5%) after 2nd, p<0.0001), flu-like symptoms (2,699 (29.2%) vs. 263 (10.4%), p<0.0001), fatigue (4,290 (46.3%) vs. 594 (23.5%), p<0.0001), fever (1,015 (11.0%) vs. 62 (2.5%), p<0.0001), arthralgia (1,995 (21.6%) vs. 183 (7.3%), p<0.0001), myalgia (2,960 (32.1%) vs. 445 (17.6%), p<0.0001), exanthema (1,705 (18.5%) vs. 137 (5.4%), p<0.0001), itching (1,222 (13.2%) vs. 246 (9.8%), p<0.0001), other (1,165 (12.6%) vs. 124 (4.9%), p<0.0001).
4,416 subjects (37.3%) received only TAK003, while between one and four co-vaccinations were given in a clear majority of 7,363 subjects (62.3%), 48 (0.4%) subjects gave no answer. When comparing subjects with any co-vaccination with those who received none, local pain, flu-like symptoms, fatigue, fever, arthralgia, myalgia, and rash were significantly more frequent in the former, while local swelling and erythema, generalised itching and other adverse events were not (
Table 1). The five most frequent co-vaccinations were rabies (n=2,598), typhoid (n=1,841), Japanese encephalitis (n=1,542), yellow fever (n=1,272), and hepatitis A (n=681), followed by almost all vaccines licensed for adult use in Germany (
Table 2). The only vaccines that were not combined with TAK003 were routine childhood vaccines and chikungunya, which was not licensed at that time.
When analysing all adverse events and age groups, reports after the second vaccination were less frequent in all groups (
Table 3). Most frequent reports were noted for the first vaccination in the age groups 20-30 and 30-40 years, with a clear tendency to decrease for all types of events in older age groups. Fever was reported in 15.5% of those 20-30 years, as compared to 4.5% in the group above 70 years. Similarly, rash was reported by most frequently in the 20-30 years group (24.6%), whereas only 3.2% of those above 70 years reported it.
4. Discussion
To our knowledge, this is the largest follow-up study on TAK003 vaccinees in a primarily adult population, and the largest study in travellers. The study results mirror the real-life situation of vaccinating travellers in a professional travel clinic setting. These tend to present on short notice before travel (in our clinics on average 13 days prior to departure), they are a largely adult population with a high proportion of subjects above 60 years of age, and they frequently need several vaccinations before departure which will be given at the same time. All these specifics were not mirrored in the pivotal studies that led to licensure of TAK003 [
4]. These were performed in endemic areas, and therefore the study population consisted of children and adolescents. Data on adults were limited with vaccine licensure, and no data were available for subjects above 60 years.
An active surveillance study that recruits from a network with documented patients visits provides a good foundation for denominator data. Prior published work on reactogenicity of TAK003 in travellers had to rely on anonymous reporting without knowledge of the underlying numbers of vaccinations [
6]. In our setting, we were able to assess the total number of travellers that were counselled during the study period (n=266,519), the exact number for patients who received a dengue vaccination (n=56,459), the number of subjects who agreed to being contacted 4 weeks after vaccination (n=30,306), and the number of those who eventually replied to the anonymous questionnaire (n=11,827). This loss to follow-up provides a clear signal of the reporting bias that is inherent to real-world studies, and even more so in passive surveillance. A large proportion of vaccinees did not report on the reactogenicity of the vaccines they received, particularly if they experienced no or only limited effects. A common statement of subjects returning to our travel clinics for the second vaccination was that they saw no reason to complete the online questionnaire when side effects were absent or mild. As consequence of this, the percentage of local and systemic side effects reported in this survey is most likely at least double the real percentage in all vaccinees. This aligns with the results of the pivotal studies where reactogenicity was markedly lower than in our setting [
4,
7].
Most vaccinees were immunologically naïve (95.2%) and received their first vaccination before travel (78.4%). The second dose of TAK003 was typically given after the journey to provide long-term protection, only 21.4% received both doses before departure. A strength of this study is the extended time interval between vaccination and sending the request to report, which was four weeks. Unlike in previous studies [
6], this period covered the journey in 53.3% of subjects and provided a robust data set of dengue naïve travellers who were potentially exposed in endemic areas after receiving the first vaccination. Previously, concerns have been raised about potential complications of dengue infections in vaccinated subjects due to antibody dependent enhancement [
8], leading to very restrictive recommendations in many European countries . In this study, dengue infection during the journey was reported by 47 subjects (0.7% out of those who travelled). All episodes were classified as mild, no complications or signs of antibody dependent enhancement were reported. This provides additional evidence on the lack of an ADE risk upon single dose vaccination with TAK003 in a dengue naïve population prior to exposure.
Local and systemic adverse events were frequently reported in our population (47.5% and 41.4% respectively,
Figure 1). Unlike in previous published work [
6], there was no difference between men and women. The percentage of reports was higher than in the pivotal studies [
4,
7]. but similar to a previous study in travellers [
6]. With the exception of exanthema, reactions were comparable to previous studies. Exanthema is a systemic reaction that had only rarely been described in pivotal studies [
4,
7], and not at all in the only prior study in travellers [
6], but occurred in 15.6% of our vaccinees. In clinical practice, this macular rash out to be an important symptom since it initially caused confusion in the affected vaccinees. Like all systemic adverse events, it typically occurred in the second week after vaccination. Subjects did not connect it to the previous vaccination and tended to interpret it as an allergic reaction. This led to consultation with health care providers and in some cases presentations at emergency rooms with the risk of receiving antiallergic treatment. Once the symptom had been identified as a common occurrence, vaccination counselling was adapted accordingly. The discrepancy with the pivotal studies [
4,
7]. may be attributed to differences in the study populations. Our study cohort comprised older, more light skinned and predominantly dengue-naïve vaccinees. As for the previously published work in travellers [
6], subjects in this study were asked to report one week after vaccination which may have led to an under-reporting of symptoms that occur later. The longer follow-up interval of 4 weeks after vaccination in our study allowed for these events to be captured.
In general, reported adverse events were short lived with a duration of 1-3 days (
Figure 2). This aligns with previously published work [
4,
6,
7]. Only exanthema and connected symptoms like itching tended to last slightly longer (duration of up to 7 days).
Most subjects who reported systemic side effects classified them as weak or medium (Figure 3). However, 21.2% to 38.9% of subjects who experienced systemic reactions classified their symptoms as strong or very strong. This is a higher rate than in pivotal studies [
4,
7]. and might be explained by differences in the study populations. Five subjects reported side effects that might be classified as severe adverse events. Two of these resulted in hospitalization, all resolved without sequelae. All subjects in this group were below 60 years, medical history and prior medication are largely unknown. As subjects self-reported adverse events through an anonymous data base, there is no possibility of verifying reports or following them up. This is clearly a weakness of this study design which intended to make reporting as easy as possible. It is quite possible that reports on strong or very strong symptoms or even potential severe adverse events might have been re-assessed upon physician evaluation.
Prior dengue infection was reported in a subset of our 564 vaccinees, as compared to 11,183 immunologically naïve travellers who received the vaccination. Comparing side effects in these two groups, there was no difference in systemic effects, while local reactions were significantly more frequent in naïve persons. However, since dengue infections are frequently asymptomatic, differentiating between dengue-naïve individuals and those with a prior dengue infection is difficult and self-reporting may be misleading.
When comparing the effects of the first and second vaccine dose, all local and systemic side effects decreased with administration of the second dose, most of them significantly. This is in line with previous studies [
4,
6,
7]. Most prior clinical investigations included vaccinees up to 60 years, whereas this survey included participants aged 4–86 years (
Table 3), thus adding safety data for older travellers who are considered at higher risk from dengue infection [
9]. Due to the absence of individuals over the age of 60 in pivotal studies, a comparison could not be made. Our findings show peaks for all adverse events after the first and second dose for the age group 20-40 years with a decline with further age. This kinetic was not shown in pivotal studies since they included only young subjects [
4,
7], but an indication of this trend was already shown in a previous study done in German travellers [
6]. This result does not justify a contraindication for the vaccine in elderly travellers [
3], particularly in view of the fact that older patients have a more severe course of dengue.
Upon licensure, data on co-vaccination with yellow fever and hepatitis A vaccines were published [
10,
11], demonstrating proof of principle for life attenuated and inactivated co-vaccination. The actual situation in a travel clinic with combination of up to 4 vaccines in one session could only be assessed and followed up in a real-world situation. Co-administration is a common vaccination practice due to time and cost savings. It has also been shown to be well tolerated [
12]. In our data set, 62.3% of vaccinees received TAK003 in combination with at least one other vaccine (
Table 1). Some adverse events increased significantly with co-vaccination. This is in line with previous studies done on this topic [
12,
13], which showed similar increases in co-vaccinations. Previous work has also shown that an increase of local and systemic side effects with co-vaccination does not lead to increased incapacitation or reduced routine activities [
12]. Due to the anonymous nature of reporting, this detail was not followed up in our study. TAK003 was most frequently co-administered in combination with rabies, typhoid fever, Japanese encephalitis, or yellow fever vaccine (
Table 2). Specific symptoms tended to increase with specific vaccine combinations, e.g., myalgia was more common after co-vaccination with TdapP (40.7% vs. 23.9%). The only prior study done on TAK003 in travellers showed no differences in the incidence of adverse events but results may have been impaired by a comparatively small number of study participants [
6]. However, it has to be acknowledged that the analysis of adverse events related to specific combinations of vaccines is difficult due to lack of a control group not receiving TAK-003. This makes it difficult to assess whether observed reactions were primarily due to the combination of vaccines or specific to TAK003.
This study was based on a single voluntary survey conducted 4 weeks after vaccination. We are confident that it identified early and late-onset adverse events. To make reporting as easy as possible for subjects, access to a website was offered for rapid anonymous reporting. These data cannot be directly related to individual subjects and are impossible to verify. Respondent bias, always an issue when collecting real world data, was made transparent in this study since denominator data were available. Participants with adverse events were more likely to answer the questionnaire, thus creating a bias towards higher reactogenicity. Also, the categorization of severity was a subjective perception of subjects which probably led to a bias towards strong and very strong side effects. Furthermore, the self-reporting of fever may result in limited accuracy when compared to phase 3 trials. It is challenging to attribute reported adverse events to the vaccine and to compare them against potential baseline reactions in an unvaccinated control group since a placebo group is missing. Overall, the results of this study demonstrate that adverse events are frequently reported following the administration of TAK003, yet most cases are not severe and do not pose a risk to travellers. Also, there was no signal of safety issues in vaccinated travellers during their journey to endemic areas.