Submitted:
01 October 2025
Posted:
02 October 2025
You are already at the latest version
Abstract
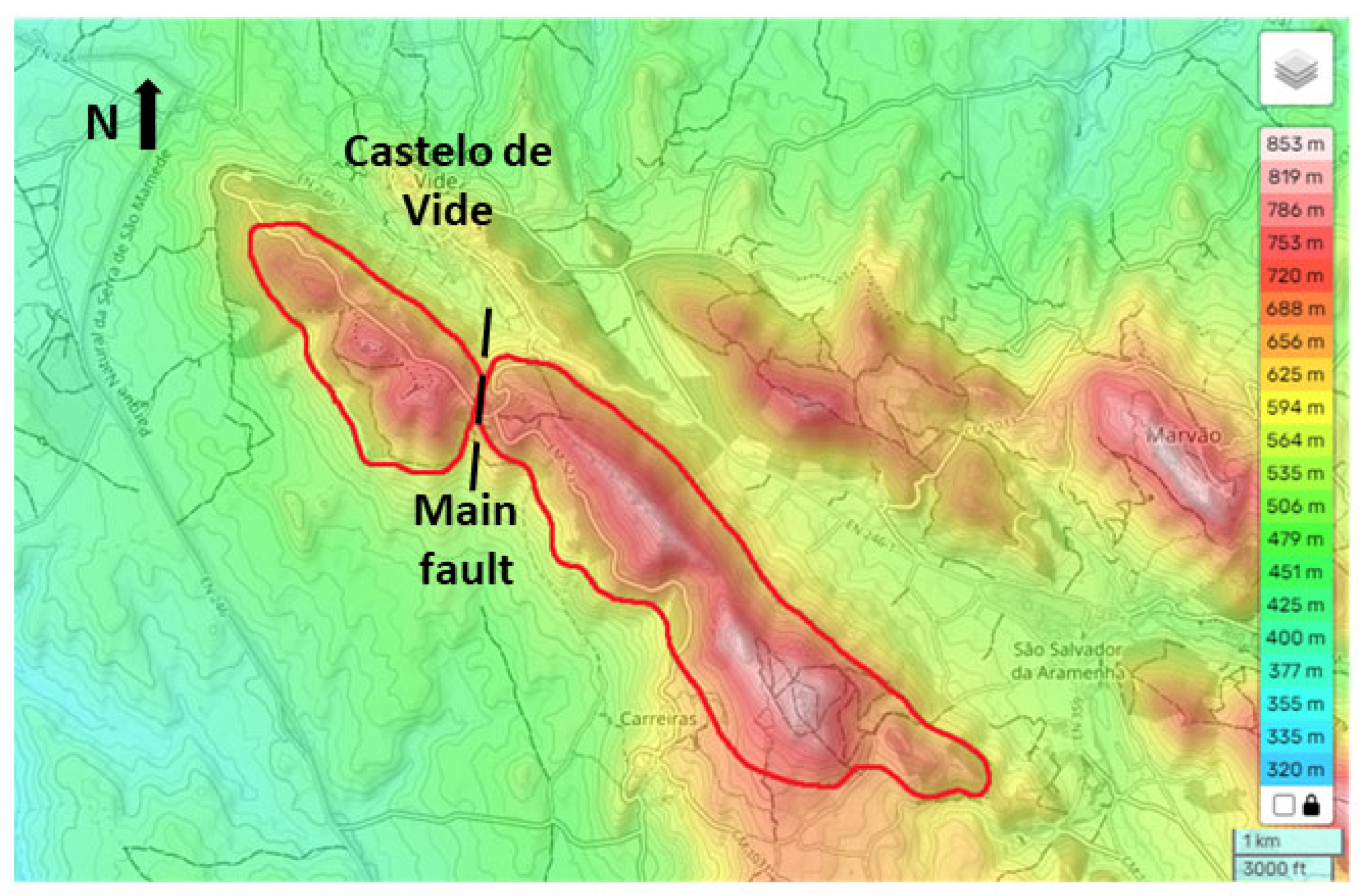
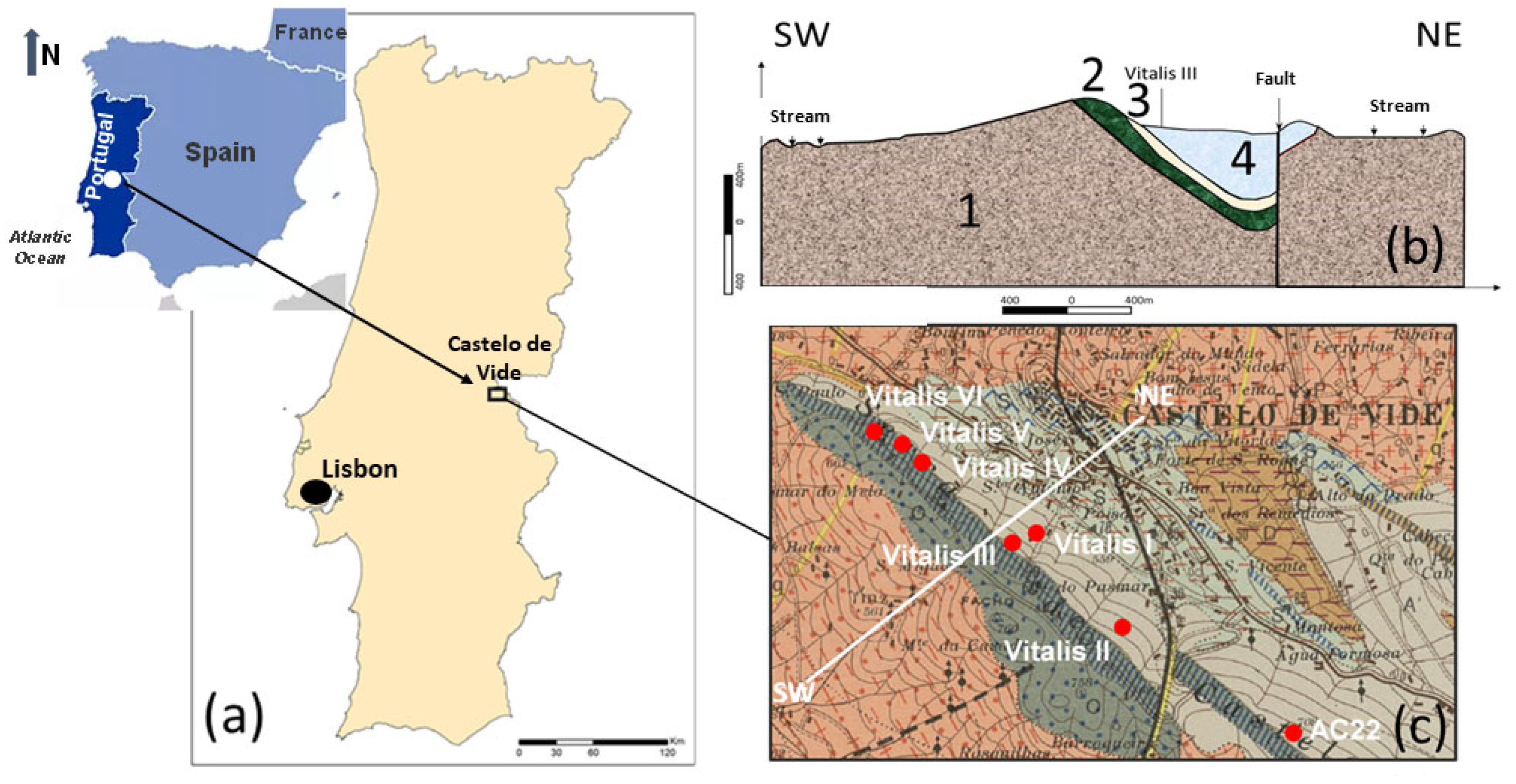
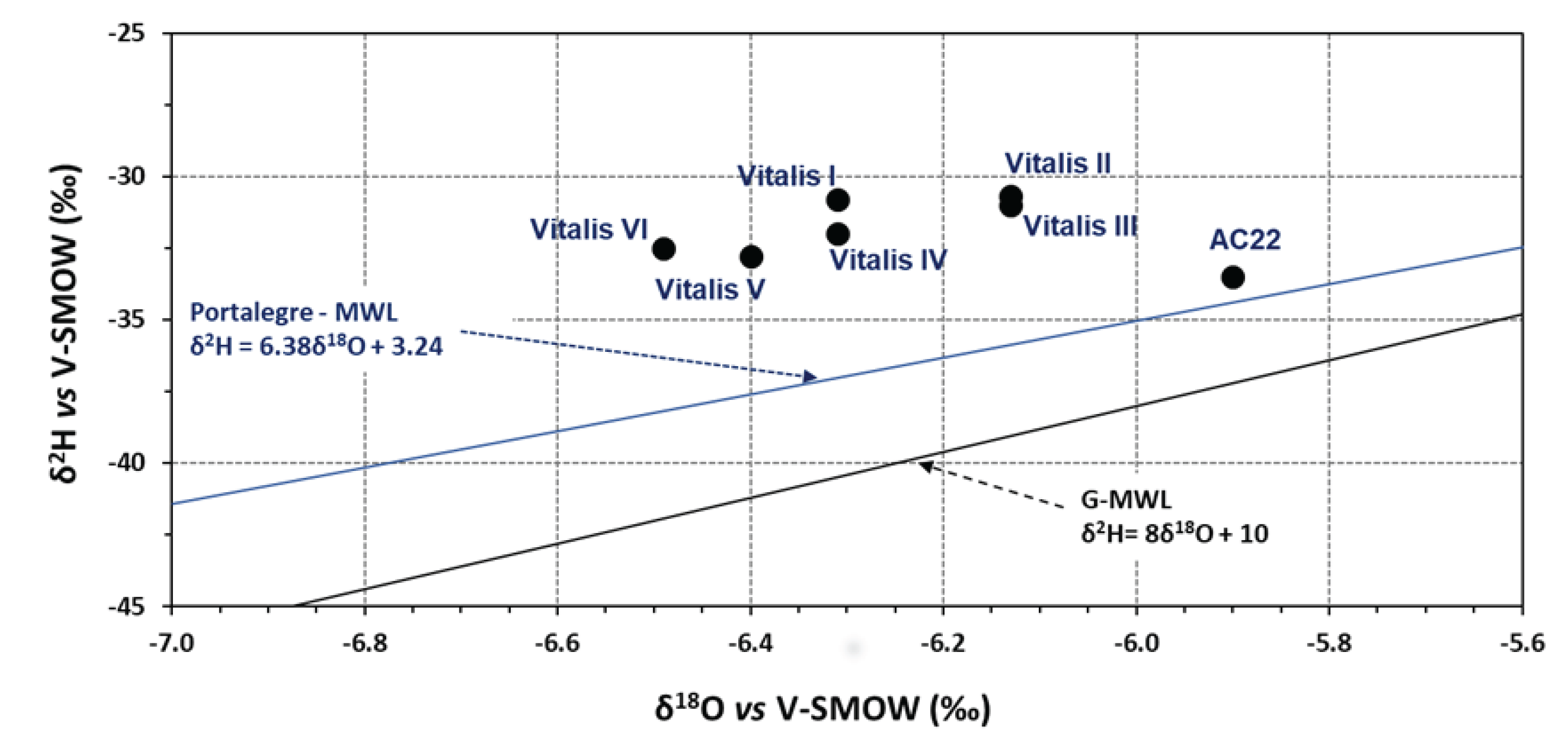
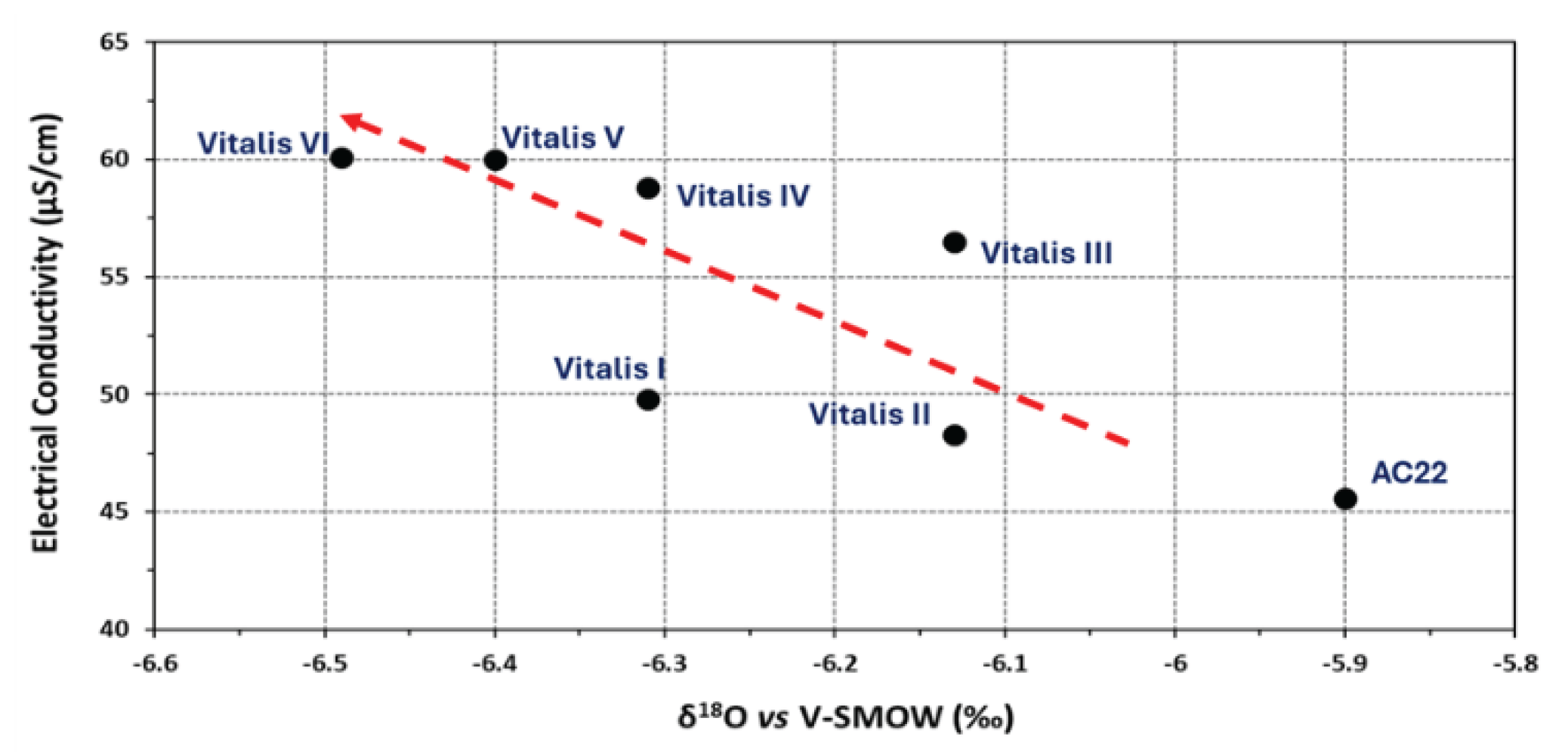
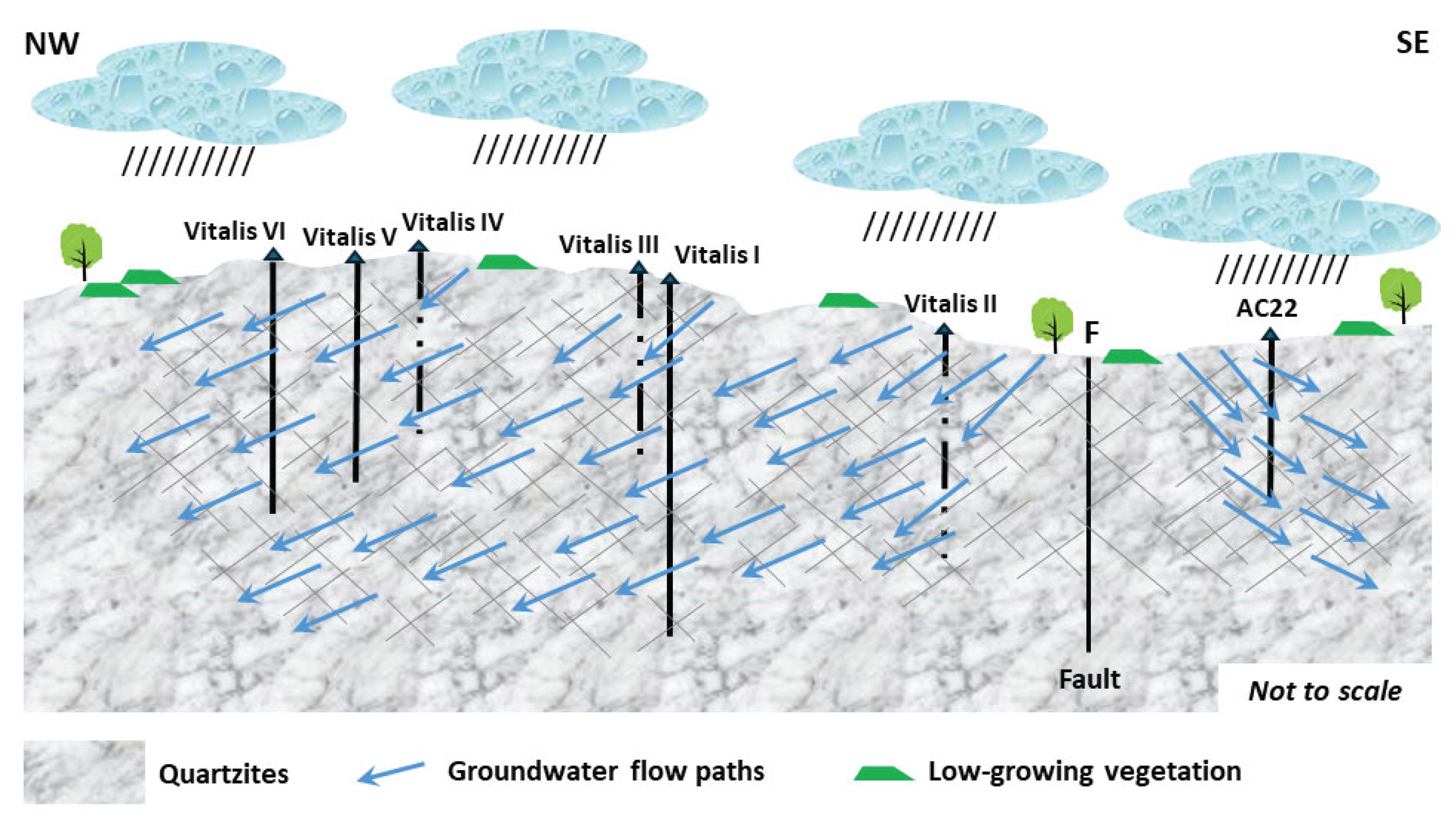
In this paper, the conceptual hydrogeological circulation model of natural mineral waters from Ribeirinho and Fazenda do Arco hydromineral concession (Castelo de Vide) is updated. These waters are exploited by the Super Bock Group, as bottled waters, and are commercially labelled as Água Vitalis. The physico-chemical data (2004 – 2024) of these waters were processed regarding their joint interpretation with recent isotopic (δ2H and δ18O) data. The study region is dominated by the Castelo de Vide syncline that develops along the southern limit of the Central Iberian Zone. These natural mineral waters have low electrical conductivity (EC) mean values (42.80 < ECmean < 54.45 μS/cm) and a slightly acidic pH (5.14 < pHmean < 5.46), making them hyposaline waters. The recharge area of this aquifer system coincides fundamentally with the outcrops of Lower Ordovician quartzites. The updated conceptual circulation model presented in this work is essentially based on the chloride-sodium signatures of these waters, explained by the preferential recharge of meteoric waters (δ2H and δ18O) and low water-rock interaction temperature. Such isotopic results seem to indicate the non-existence of a flow continuity between the two blocks (NW and SE) of the quartzite ridges, separated by a fault with a local orientation approximately N-S, as indicated by the most enriched isotopic values of the waters from borehole AC22 (δ18O = -5.90 o/oo vs. V-SMOW) located in the SE block, compared to the average isotopic value of the waters from the other boreholes (Vitalis I, II, III, IV, V and VI) located in the NW block (δ18Omean = -6.30 o/oo vs. V-SMOW).
Keywords:
1. Introduction
2. Geomorphological, Climatological, Geological and Hydrogeological Framework
3. Materials and Methods
4. Results and Discussion
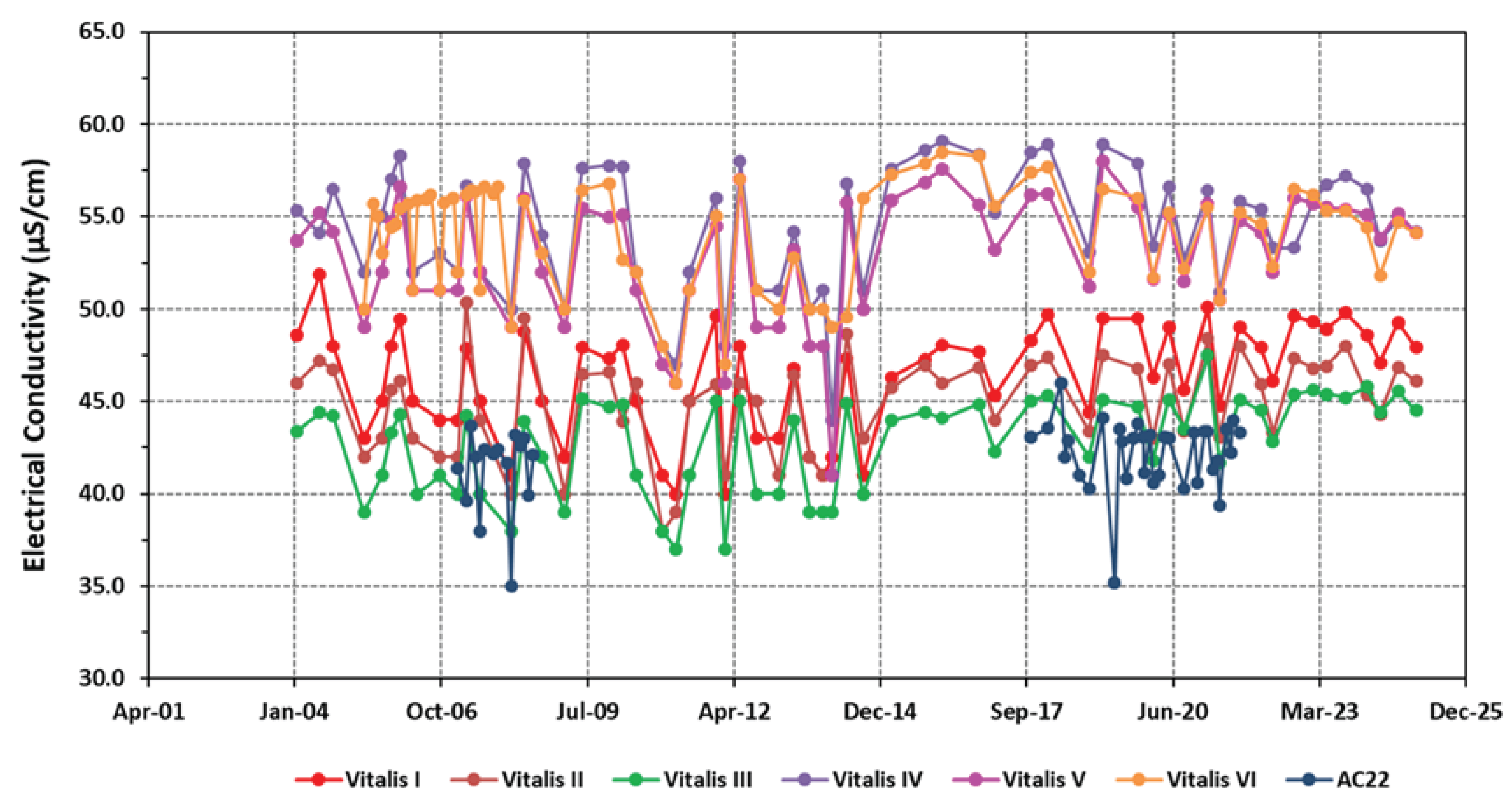
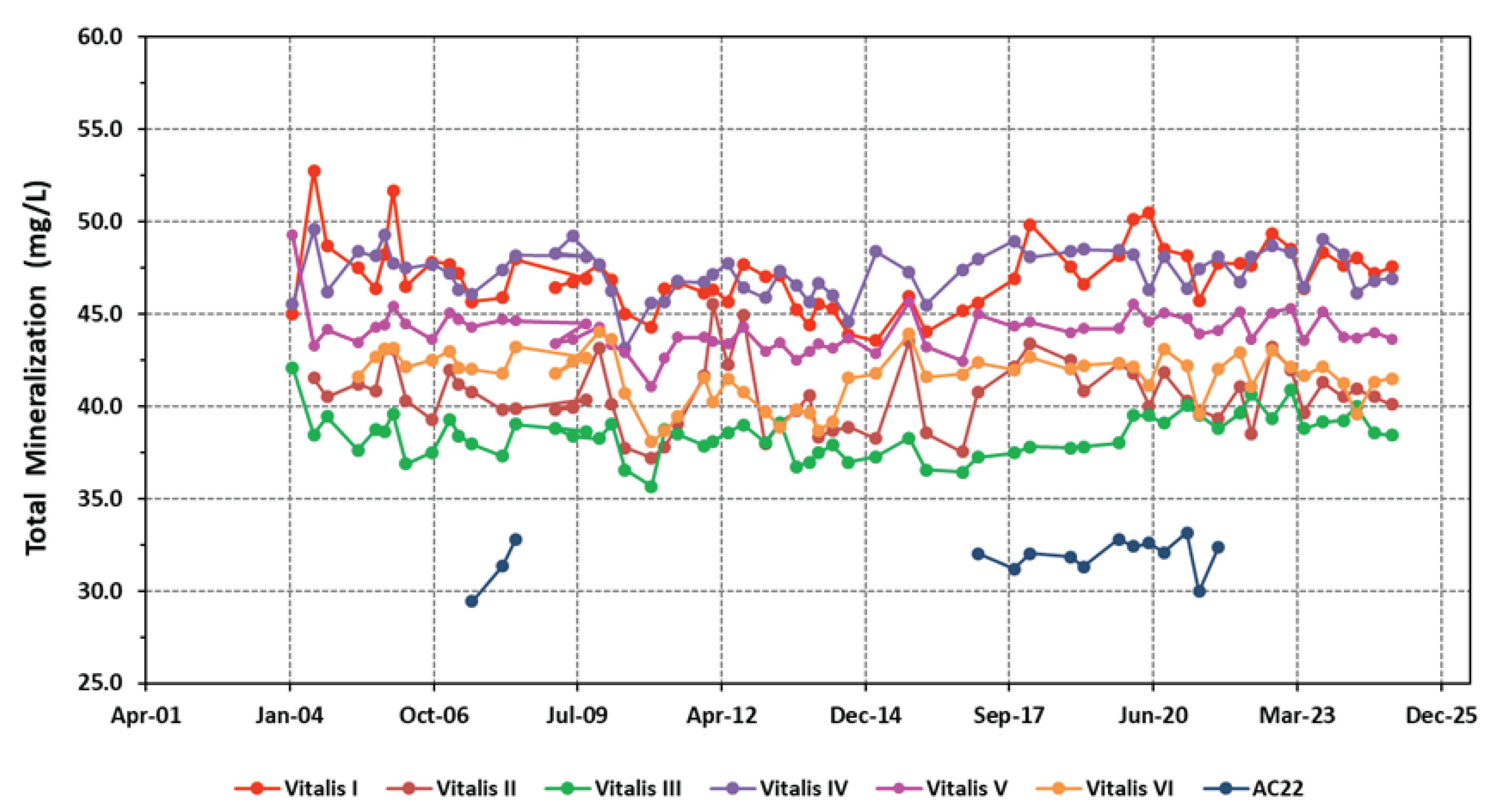
4.1. Physico-Chemical Signatures of the Waters
4.2. Isotopic (δ2H and δ18O) Signatures of the Waters
5. Updating of the Conceptual Hydrogeological Model
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moore, J.E. Field hydrogeology: A guide for site investigations and report preparation; Lewis Publishers: New York, NY, USA, 2002. [Google Scholar]
- Brassington, F.C. A proposed conceptual model for the genesis of the Derbyshire thermal springs. Q. J. Eng. Geol. Hydrogeol 2007, 40, 35–46. [Google Scholar] [CrossRef]
- Marques, J.M.; Carreira, P.M.; Caçador, P.; Antunes da Silva, M. Update of the Interpretive Conceptual Model of Ladeira de Envendos Hyposaline Hydromineral System (Central Portugal): A Contribution to Its Sustainable Use. Sustainability 2024, 16, 5179. [Google Scholar] [CrossRef]
- Vieira da Silva, A.M.; Condesso Melo, M.T.; Marques da Silva, M.A. Modelo conceptual e caracterização hidrogeológica preliminar do sistema aquífero da Serra do Buçaco. In Actas das Jornadas Luso-Espanholas sobre As Águas Subterrâneas no Noroeste da Península Ibérica, La Coruña, Espanha, 3-6 Julho de 2000 [in Portuguese].
- Simões Cortez, A. Águas Minerais Naturais e de Nascente da Região Centro, 1st ed.; Mare Liberum, Aveiro, Portugal, 2012; pp. 483–485 [in Portuguese].
- Feio, M.; Almeida, G. A Serra de S. Mamede. Finisterra 1980, 15(29), 30–52. (In Portuguese) [Google Scholar] [CrossRef]
- Monteiro, J.P.; Silva, M.L. Aspectos da Hidrogeologia e Qualidade das Águas Associadas à formação Carbonatada de Escusa (Castelo de Vide). Revista de Geologia Económica Aplicada e do Ambiente (GEOLIS), Secção de Geologia Económica e Aplicada da Faculdade de Ciências da Universidade de Lisboa 1992, 6, 19–32. [Google Scholar]
- Monteiro, J.P. Hidrogeologia da Formação Carbonatada de Escusa (Castelo de Vide), Dissertação de Mestrado, Faculdade de Ciências da Universidade de Lisboa, Departamento de Geologia, Lisboa, 1993. (In Portuguese)
- Monteiro, J.P.; Silva, M.L.; Carreira, P.M.; Soares, A.M. Aplicação de Métodos Geoquímicos Isotópicos à Interpretação da Hidrodinâmica do Aquífero Carbonatado da Serra de S. Mamede (Castelo de Vide). In VII Congresso de Espanha de Geoquímica, Ed. Cedex, 1997, pp. 544–551 [in Portuguese].
- Fernandes, A.P.; Perdigão, J.C.; Carvalho, H.F.; Peres, A.M. Notícia Explicativa da Folha 28 D - Castelo de Vide, Carta Geológica de Portugal na Escala 1/50000. Direcção-Geral de Minas e Serviços Geológicos, Lisboa, 1973 [in Portuguese].
- Morais Almeida, A.; Dias, D.; Almeida, M.; Marques, J.M.; Antunes da Silva, M. Contribuição para o desenvolvimento do modelo conceptual de circulação da Água Mineral Natural de Castelo de Vide. In Livro de Actas do 12º Seminário sobre Águas Subterrâneas, Coimbra, 7 e 8 de março de 2019, pp. 18–21 [in Portuguese].
- IAEA. Training Course Series 35 Laser spectroscopic analysis of liquid water samples for stable hydrogen and oxygen isotopes. Performance testing and procedures for installing and operating the LGR DT-100 liquid water stable analyser, 1st ed.; International Atomic Energy Agency: Vienna, Austria, 2009; pp. 1–27. [Google Scholar]
- IAEA. Procedure and technique critique for tritium enrichment by electrolysis at IAEA laboratory. Technical Procedure nº19, 1st ed.; International Atomic Energy Agency: Vienna, Austria, 1976; pp. 1–42. [Google Scholar]
- Freeze, A.R.; Cherry, J.A. Groundwater; Englewood Cliffs: Prentice-Hall, NJ, USA, 1979; pp. 82–134. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, groundwater and pollution, 1st ed.; Balkema: Rotterdam, The Netherlands, 1993; pp. 1–168. [Google Scholar]
- Lourenço, C.; Ribeiro, L. Classificação das águas minerais naturais e de nascente de Portugal segundo as suas características físico-químicas. In 7º Congresso da Água, LNEC, Lisboa, 2004 [in Portuguese].
- Directive 2009/54/EC. Available online: http://data.europa.eu/eli/dir/2009/54/oj (accessed on 15 November 2023).
- Todd, E.C.T.; Sherman, D.M.S.; Purton, J.A.P. Surface Oxidation of Pyrite under Ambient Atmospheric and Aqueous (pH = 2 to 10) Conditions: Electronic Structure and Mineralogy from X-Ray Absorption Spectroscopy. Geochim. Cosmochim. Acta 2003, 67, 881–893. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Publishers: New York, NY, USA, 1997. [Google Scholar]
- Geyh, M. Environmental isotopes in hydrological cycle. Principles and applications. IHP-V, Technical Documents in Hydrology, No. 39, vol. IV.
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133(3465), 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Carreira, P.; Nunes, D.; Valério, P.; Araújo, M.F. A 15-year record of seasonal variation in the isotopic composition. J. Radioanal. Nucl. Chem. 2009, 281, 153–156. [Google Scholar] [CrossRef]

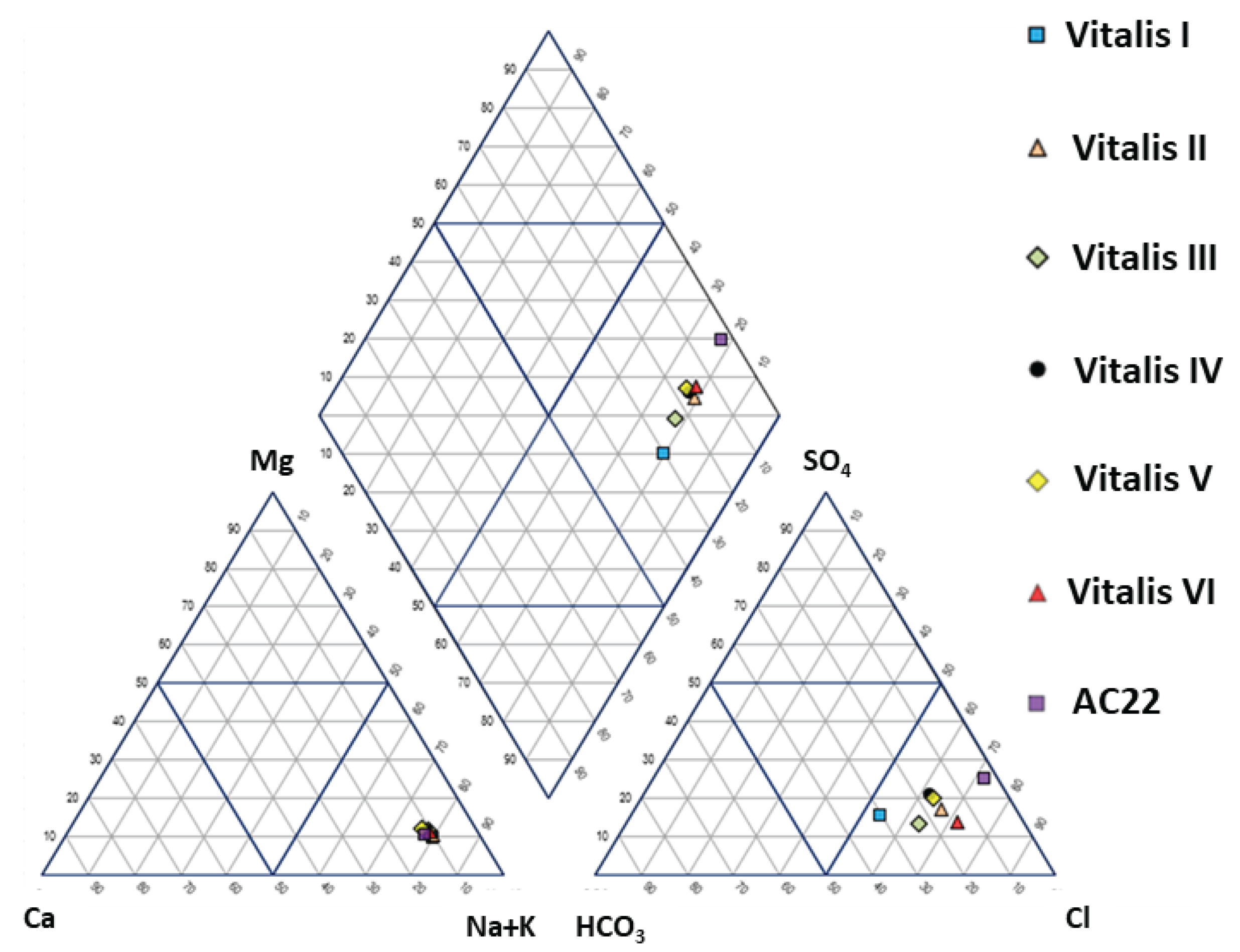
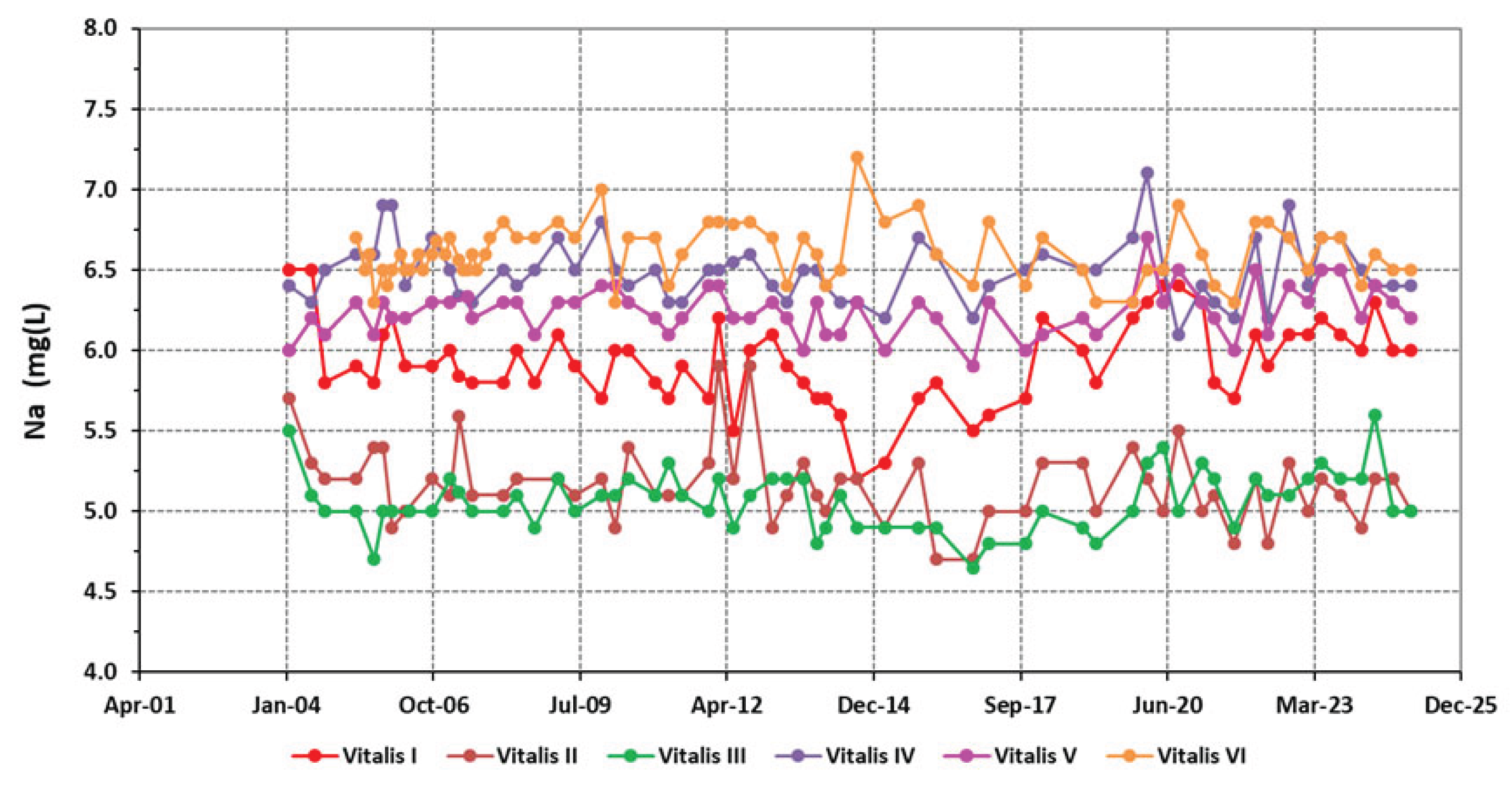
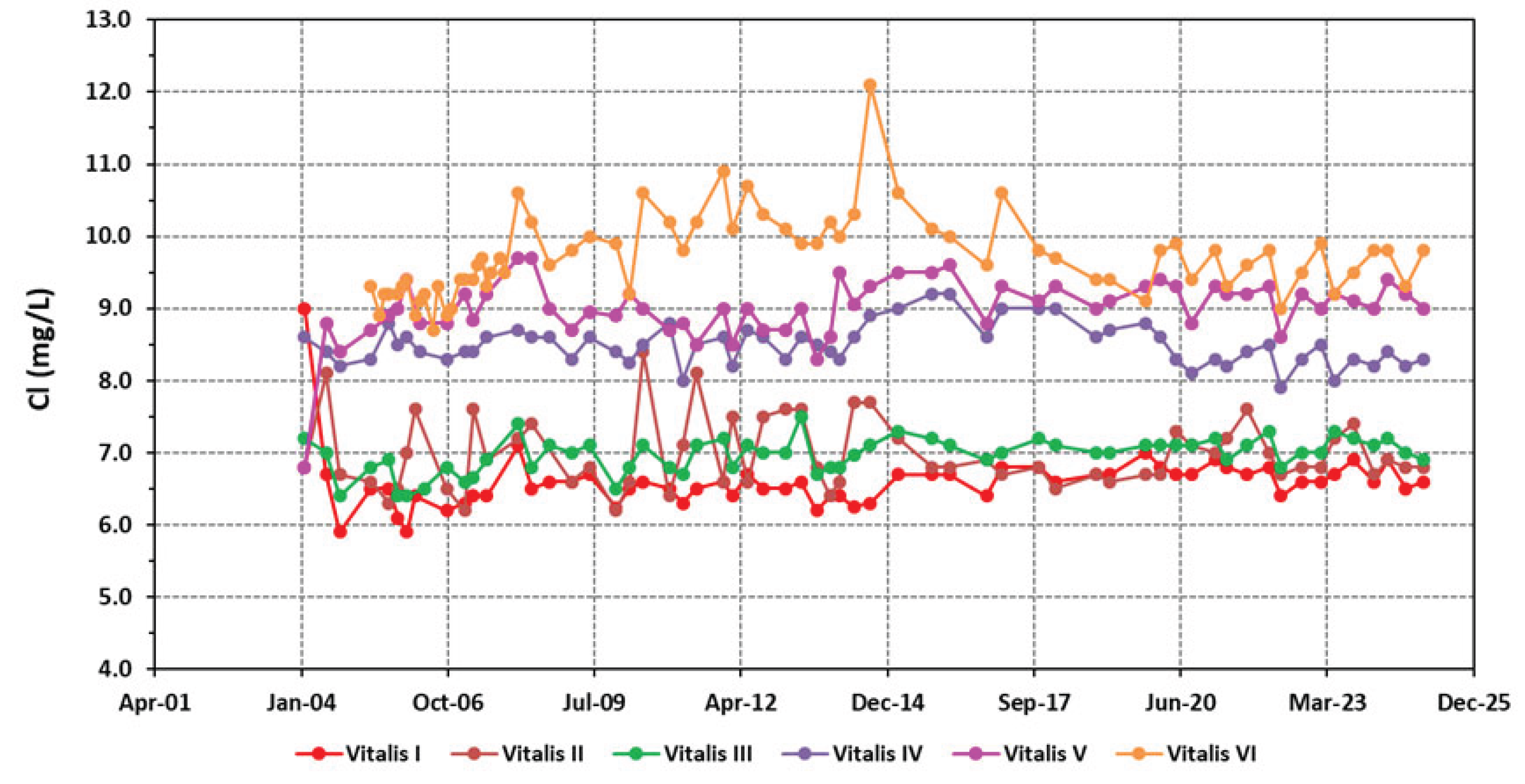
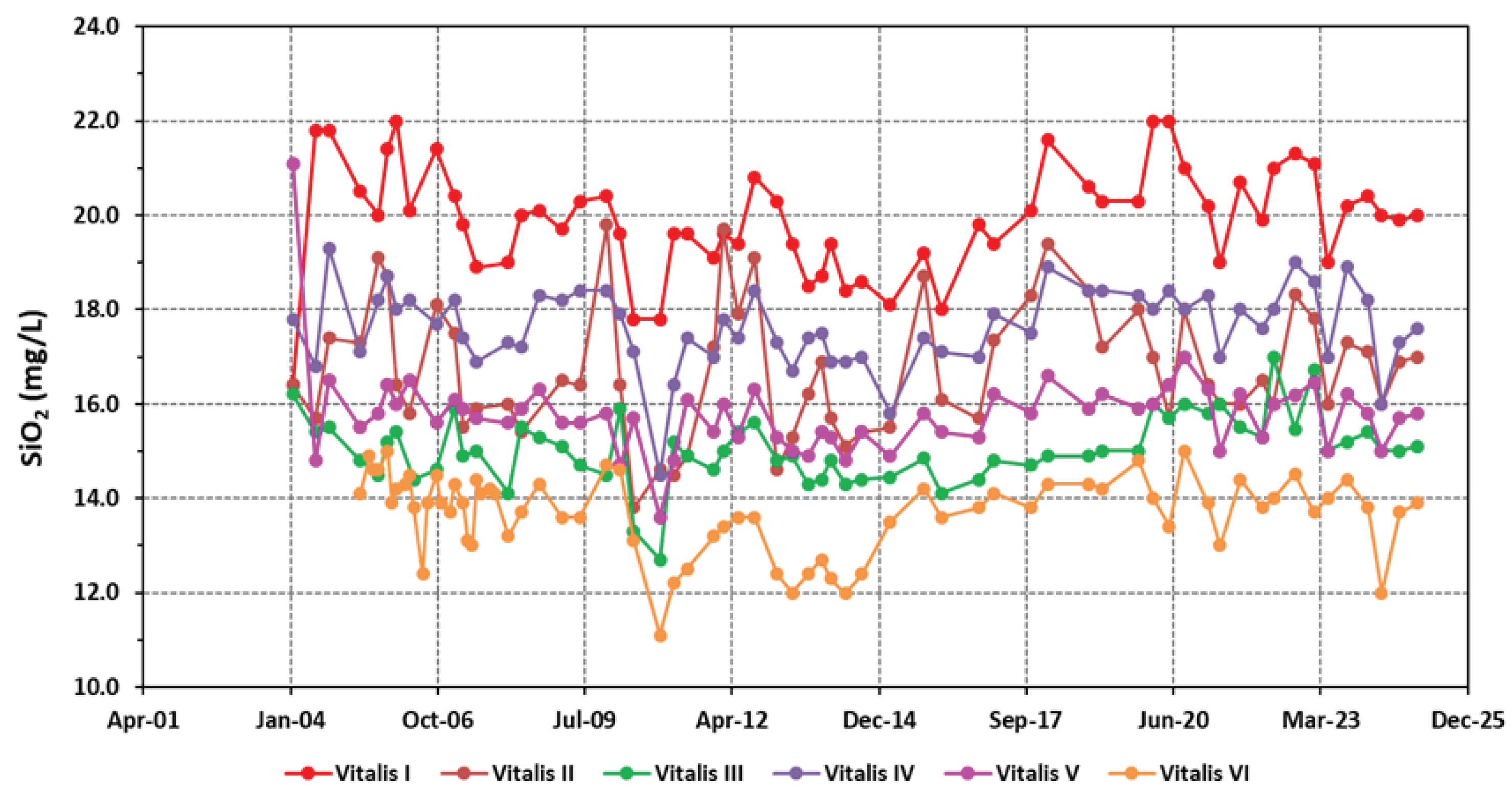
| Borehole | Vitalis I | Vitalis II | Vitalis III | Vitalis VI | Vitalis V | Vitalis VI |
|---|---|---|---|---|---|---|
| HsIL (m) | 0 | 13 | 20.4 | 32.5 | 47.6 | 32.2 |
| EHdL (m) | 16 | 35 | 44 | 54 | 61 | 60 |
|
Vitalis I (n=60) |
Vitalis II (n=59) |
Vitalis III (n=60) |
Vitalis IV (n=61) |
Vitalis V (n=60) |
Vitalis VI (n=72) |
|
| pH | 5.46±0.08 | 5.17±0.11 | 5.26±0.07 | 5.22±0.07 | 5.18±0.08 | 5.14±0.09 |
| EC | 46.34±2.95 | 44.90±2.69 | 42.80±2.59 | 54.45±3.36 | 52.97±3.41 | 54.02±3.03 |
| HCO3- | 6.40±0.63 | 3.05±0.30 | 4.35±0.24 | 4.07±0.30 | 4.08±0.37 | 3.40±0.44 |
| Cl- | 6.58±0.40 | 6.97±0.49 | 6.96±0.25 | 8.50±0.28 | 9.00±0.43 | 9.69±0.58 |
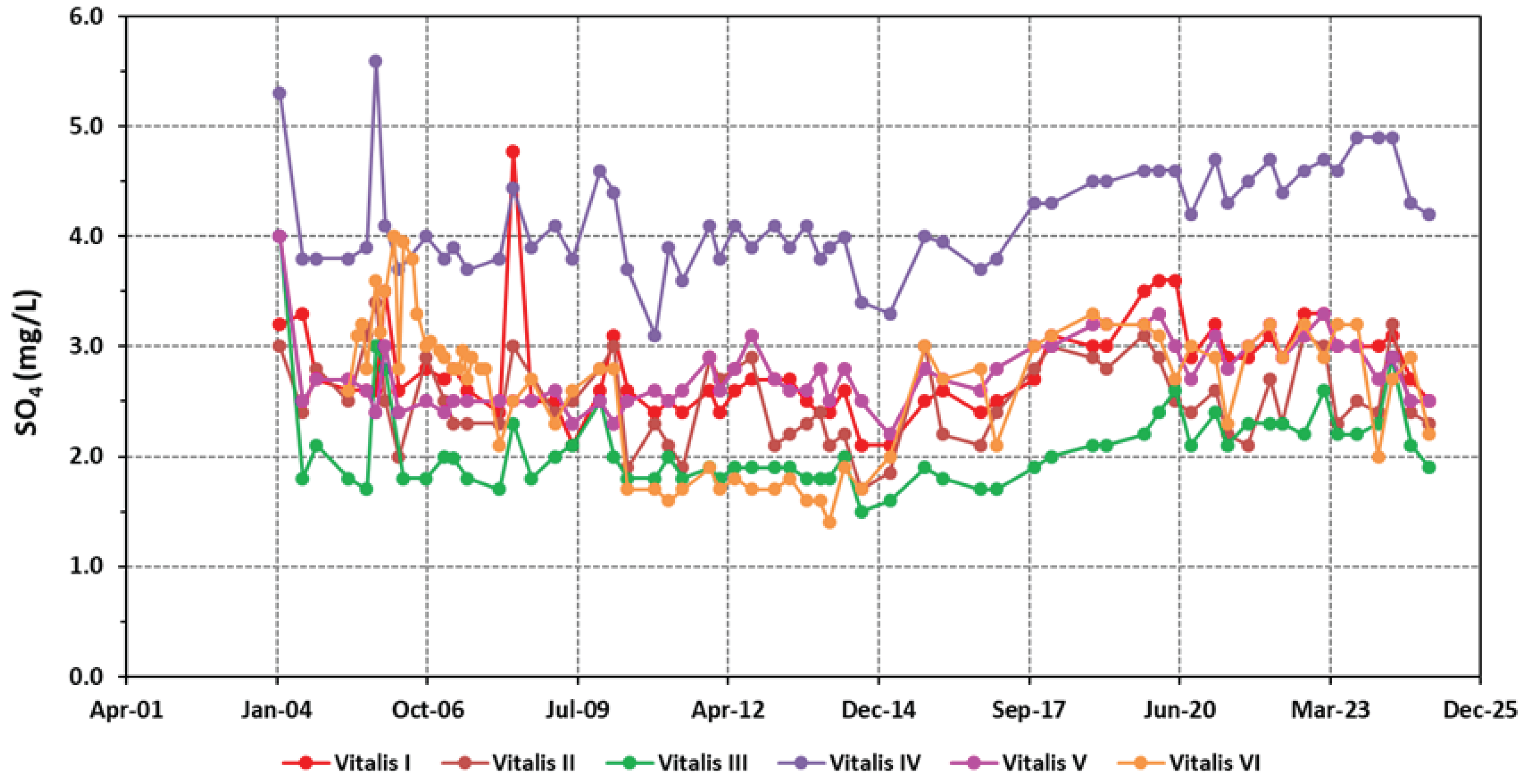
| SO42- | 2.79±0.44 | 2.53±0.39 | 2.09±0.41 | 4.16±0.49 | 2.74±0.33 | 2.67±0.63 |
| Na+ | 5.93±0.27 | 5.17±0.24 | 5.06±0.18 | 6.50±0.19 | 6.24±0.15 | 6.60±0.18 |
| K+ | 2.07±0.08 | 2.06±0.12 | 1.91±0.08 | 2.35±0.10 | 2.06±0.10 | 1.85±0.13 |
| Ca2+ | 0.81±0.06 | 0.72±0.07 | 0.71±0.06 | 0.97±0.07 | 0.99±0.09 | 0.89±0.07 |
| Mg2+ | 0.45±0.03 | 0.45±0.07 | 0.50±0.03 | 0.62±0.03 | 0.63±0.03 | 0.59±0.03 |
| SiO2 | 19.92±1.15 | 16.79±1.41 | 15.04±0.71 | 17.66±0.83 | 15.79±0.91 | 13.72±0.84 |
| Sampling site | October 2023 Field work campaign |
||
|---|---|---|---|
| δ18O | δ2H | d | |
| Vitalis I | -6.31 | -30.8 | 19.68 |
| Vitalis II | -6.13 | -30.7 | 18.34 |
| Vitalis III | -6.13 | -31 | 18.04 |
| Vitalis IV | -6.31 | -32 | 18.48 |
| Vitalis V | -6.4 | -32.8 | 18.4 |
| Vitalis VI | -6.49 | -32.5 | 19.42 |
| AC22 | -5.9 | -33.5 | 13.7 |
| Sampling site | pH |
EC (µS/cm) |
T (oC) |
Eh (mV) |
|---|---|---|---|---|
| Vitalis I | 4.82 | 49.8 | 16.1 | 96.2 |
| Vitalis II | 5.44 | 48.3 | 16.2 | 61.9 |
| Vitalis III | 4.76 | 56.5 | 15.8 | 99.4 |
| Vitalis IV | 4.69 | 58.8 | 16.2 | 103.5 |
| Vitalis V | 4.41 | 60.0 | 16.7 | 119.3 |
| Vitalis VI | 4.84 | 60.1 | 16.4 | 95.0 |
| AC22 | 4.37 | 45.6 | 15.5 | 120.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





