1. Introduction
Chronic coronary syndrome (CCS) remains one of the leading causes of morbidity, hospitalizations, and mortality worldwide, representing a major public health challenge [
1]. Beyond its direct effect on survival, CCS is strongly associated with impaired health-related quality of life (HRQoL), driven by recurrent angina, functional limitations, and psychological distress [
2]. Over recent decades, management strategies have evolved from a purely disease-centered approach focused on ischemia and event prevention toward a more patient-centered paradigm that emphasizes symptom control, functional capacity, and well-being as primary therapeutic goals [
3,
4].
Patient-reported outcomes (PROs) have emerged as essential tools in this shift, capturing the patient’s perception of symptoms, treatment efficacy, and overall quality of life. Incorporating PROs into clinical research and practice provides clinicians with valuable insights that complement traditional clinical endpoints, ensuring that therapeutic strategies are aligned with patient needs and expectations [
5].
Among disease-specific instruments developed for cardiovascular disease, the Seattle Angina Questionnaire (SAQ) is the most extensively validated and widely applied tool [
6]. Developed in the 1990s, the SAQ is a 19-item self-administered instrument that evaluates five domains: Physical Limitation, Angina Stability, Angina Frequency, Treatment Satisfaction, and Disease-specific Quality of Life [
7]. Compared with generic HRQoL tools such as the Short Form-36 (SF-36) or the EuroQol-5D (EQ-5D), the SAQ demonstrates superior sensitivity in detecting clinically meaningful changes over time [
8].
Importantly, the SAQ is not only descriptive but also prognostic. Lower baseline scores have been independently associated with increased risks of hospitalization, recurrent cardiovascular events, and mortality [
9]. Furthermore, the SAQ has served as a primary or secondary endpoint in landmark clinical trials, including COURAGE, ISCHEMIA, and EuroCTO, which collectively shaped modern strategies for revascularization in stable ischemic heart disease [
10,
11,
12].
Despite its global adoption and validated translations into more than 30 languages [
13], the SAQ has not yet been systematically translated, culturally adapted, or validated in Bulgaria. This gap is particularly relevant in a country with persistently high cardiovascular mortality rates and limited use of standardized PROs [
14].
The aim of this narrative review is therefore to provide a comprehensive overview of the Seattle Angina Questionnaire, summarize the evidence supporting its clinical utility, and explore the opportunities and challenges of its implementation in the Bulgarian healthcare context.
2. Methods
This narrative review was conducted to summarize the development, validation, and clinical applications of the Seattle Angina Questionnaire (SAQ), with a particular focus on its potential implementation in Bulgarian clinical practice.
A structured literature search was performed in PubMed, Scopus, and Web of Science databases for the period 1995–2025, covering the timeframe from the original validation of the SAQ to the most recent publications. The following keywords and their combinations were applied: “Seattle Angina Questionnaire”, “chronic coronary syndrome”, “chronic stable angina”, “quality of life”, “patient-reported outcomes”, and “health status”.
Eligible publications included randomized controlled trials, observational cohort studies, registries, systematic reviews, and meta-analyses. Articles were included if they:
evaluated the psychometric properties of the SAQ;
reported its use in clinical trials or registries; or
assessed its prognostic value or clinical utility.
Publications not available in English, conference abstracts, and case reports were excluded. Reference lists of included studies and relevant review articles were screened to identify additional sources. The analysis was not limited to international evidence but also considered its implications for potential adaptation and validation of the SAQ in the Bulgarian population.
3. Literature Overview
The Seattle Angina Questionnaire (SAQ) was developed in 1992 by Spertus and colleagues as a disease-specific, patient-reported outcome measure designed to capture the impact of angina pectoris on patients’ daily lives [
2]. Since its introduction, the SAQ has become the most widely accepted and extensively validated instrument for assessing symptoms and health-related quality of life in patients with chronic coronary syndrome (CCS) and coronary artery disease (CAD) [
4,
10,
11]. Its strengths lie in its specificity, reproducibility, and prognostic value, which have been confirmed in multiple populations and healthcare systems.
The SAQ consists of 19 items distributed across five domains: Physical Limitation, Angina Stability, Angina Frequency, Treatment Satisfaction, and Disease-specific Quality of Life. Each domain is scored from 0 to 100, with higher values indicating better status. This multidimensional framework provides a nuanced picture of the patient experience that cannot be achieved by generic instruments such as the Short Form-36 (SF-36) or the EuroQol-5D (EQ-5D) [
12]. Importantly, the SAQ has shown high sensitivity to clinical changes, enabling detection of meaningful improvements after pharmacological therapy, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG) [
3,
7].
Extensive psychometric testing has confirmed the validity and reliability of the SAQ. Internal consistency across domains has consistently exceeded the accepted threshold of Cronbach’s alpha >0.70 [
10]. Test–retest reliability has also been robust, with intraclass correlation coefficients ranging from 0.78 to 0.90 across domains in stable patients, confirming reproducibility when no clinical changes are expected [
13]. Construct validity has been established through correlations with objective measures of ischemia and with physician-assessed Canadian Cardiovascular Society (CCS) angina class [
14].
Beyond descriptive use, the SAQ has demonstrated strong prognostic value. Lower baseline scores in domains such as Angina Frequency and Quality of Life are independently associated with higher risks of hospitalization, recurrent cardiovascular events, and all-cause mortality [
6]. In comparative analyses, SAQ scores provided prognostic information comparable to traditional predictors such as left ventricular ejection fraction and exercise tolerance tests, highlighting its role not only for patient-centered assessment but also for risk stratification [
15,
16].
The SAQ has been translated, culturally adapted, and validated in more than 30 languages, including European, Asian, and Latin American contexts, with consistent confirmation of its psychometric properties [
10]. However, no validated Bulgarian version currently exists, which limits its application in both clinical practice and research.
4. Clinical Applications of the Seattle Angina Questionnaire
4.1. Use in Major Clinical Trials
The SAQ has played a pivotal role in numerous landmark randomized controlled trials (RCTs), providing sensitive and patient-centered endpoints that complement traditional measures such as mortality and myocardial infarction. In the COURAGE trial, the addition of PCI to optimal medical therapy improved angina frequency and quality of life, as measured by the SAQ, despite no difference in survival [
3]. The ISCHEMIA trial confirmed that patients with frequent angina at baseline experienced the most pronounced improvements in SAQ scores with an initial invasive strategy, whereas those with minimal symptoms derived little incremental benefit [
7]. Similarly, the EuroCTO trial demonstrated that successful recanalization of chronic total occlusions led to significant and sustained improvements in angina frequency, physical limitation, and quality of life domains of the SAQ, compared with patients treated with optimal medical therapy alone [
8].
Additional evidence from observational registries has reinforced the prognostic implications of SAQ scores, showing that lower baseline scores predict hospitalizations and mortality independent of traditional risk factors [
6,
15]. A summary of these key findings is presented in
Table 1, which outlines the major clinical studies that have incorporated the SAQ as a primary or secondary endpoint.
4.2. Multidimensional and Reproducible Assessment
The SAQ provides a reproducible and multidimensional evaluation of symptom burden, capturing five distinct but interrelated domains: Physical Limitation, Angina Stability, Angina Frequency, Treatment Satisfaction, and Disease-specific Quality of Life [
2]. Its reproducibility has been confirmed by high test–retest reliability, with intraclass correlation coefficients ranging from 0.78 to 0.90 in clinically stable patients [
13]. This ensures that observed changes in SAQ scores over time are attributable to real clinical changes rather than measurement variability.
Moreover, the multidimensional nature of the SAQ allows for a comprehensive characterization of patient status beyond angina frequency alone. For example, improvements in angina frequency are often paralleled by gains in quality of life and physical function, demonstrating the instrument’s ability to capture holistic benefits of therapy [
10].
4.3. Role in Longitudinal Monitoring and Shared Decision-Making
The SAQ enhances clinical practice by supporting longitudinal monitoring of treatment response. Serial assessments allow clinicians to quantify the trajectory of symptom relief and functional improvement after pharmacological therapy, PCI, or CABG [
3,
7]. Beyond research, the SAQ facilitates shared decision-making, embedding the patient’s perspective into therapeutic planning. For example, patients with persistently low SAQ scores despite guideline-directed medical therapy may be considered for invasive evaluation and revascularization, whereas those with stable or improving scores may be managed conservatively [
4,
10].
In this way, the SAQ bridges the gap between clinical outcomes and patient-centered care, enabling treatment strategies that are both evidence-based and aligned with patient values.
5. Implementation of the Seattle Angina Questionnaire in Bulgaria
5.1. Epidemiological and Clinical Context
Bulgaria continues to face an exceptionally high burden of cardiovascular disease, which remains the leading cause of morbidity and mortality nationwide. According to the European Society of Cardiology Atlas of Cardiology, the country consistently ranks among the European states with the highest age-standardized mortality from ischemic heart disease, and cardiovascular causes account for more than 60% of all annual deaths [
9,
17]. Despite significant progress in acute care and wider access to percutaneous coronary interventions, these figures have remained alarmingly stable over the past decades. Such persistence underscores not only structural weaknesses in the healthcare system but also the absence of systematic patient-centered strategies for managing chronic disease. The prevalence of angina and ischemic heart disease is particularly pronounced, driven both by demographic factors such as rapid population aging and by the high prevalence of modifiable risk factors including hypertension, diabetes, dyslipidemia, and tobacco use [
1,
18].
5.2. Rationale for Implementing the SAQ
In this epidemiological context, the introduction of validated patient-reported outcome measures is not a methodological luxury but rather a pressing necessity. The Seattle Angina Questionnaire (SAQ), by providing a reproducible and multidimensional assessment of symptom burden and quality of life, has the potential to transform the evaluation of patients with chronic coronary syndrome in Bulgaria. In a healthcare environment that continues to rely heavily on mortality and hospitalization data, the SAQ offers a complementary perspective that captures the lived experience of patients. Its integration into clinical practice would allow the systematic documentation of treatment benefits that are otherwise difficult to quantify, thereby aligning therapeutic decision-making with outcomes that matter most to patients [
2,
10,
12]. Furthermore, the prognostic capacity of the SAQ—demonstrated through its ability to predict hospitalizations and mortality independently of traditional risk factors—underscores its utility as a dual-purpose instrument for both clinical care and risk stratification [
6,
15].
5.3. Potential Benefits of SAQ Integration
The potential benefits of adopting the SAQ extend across multiple levels of the healthcare system. At the individual patient level, routine application of the SAQ would enhance the accuracy of symptom assessment, allowing for timely adjustments of medical therapy or revascularization strategies based on patient-reported data rather than solely on physician judgment. At the institutional level, the aggregation of SAQ results across hospitals could serve as a valuable tool for monitoring performance, enabling comparisons of outcomes and quality indicators between centers. This benchmarking process, already introduced in other European healthcare systems, has been shown to foster accountability and stimulate local quality improvement initiatives [19]. At the national level, systematic collection of SAQ data could generate a new dimension of health metrics, complementing conventional indicators such as mortality or readmission rates. Such information could inform health policy, guide resource allocation, and provide a more comprehensive understanding of the societal burden of ischemic heart disease. Moreover, by aligning Bulgaria with international standards where PROs are increasingly required in multicenter registries and clinical trials, SAQ integration would strengthen the country’s research capacity and open opportunities for international collaboration.
5.4. Anticipated Barriers and Challenges
Despite these advantages, the implementation of the SAQ in Bulgaria is likely to face several challenges. The foremost among them is the need for rigorous linguistic translation and cultural adaptation, a process that must ensure both semantic and conceptual equivalence with the original instrument. Equally important is the establishment of the psychometric validity of the Bulgarian version, which requires testing in diverse and representative patient cohorts. Infrastructure also presents a significant obstacle, as electronic health record systems remain fragmented and unevenly distributed across institutions. Integrating PROs such as the SAQ into these digital platforms will require targeted investments in technology and interoperability. Beyond technical considerations, adaptation of clinical workflows poses another hurdle, as clinicians and nursing staff will need adequate training to administer the questionnaire and to interpret its results meaningfully. Finally, long-term sustainability will depend on the political and financial commitment to embed PROs within the framework of national cardiovascular programs, supported by appropriate reimbursement strategies and performance-based incentives.
5.5. Roadmap for Implementation
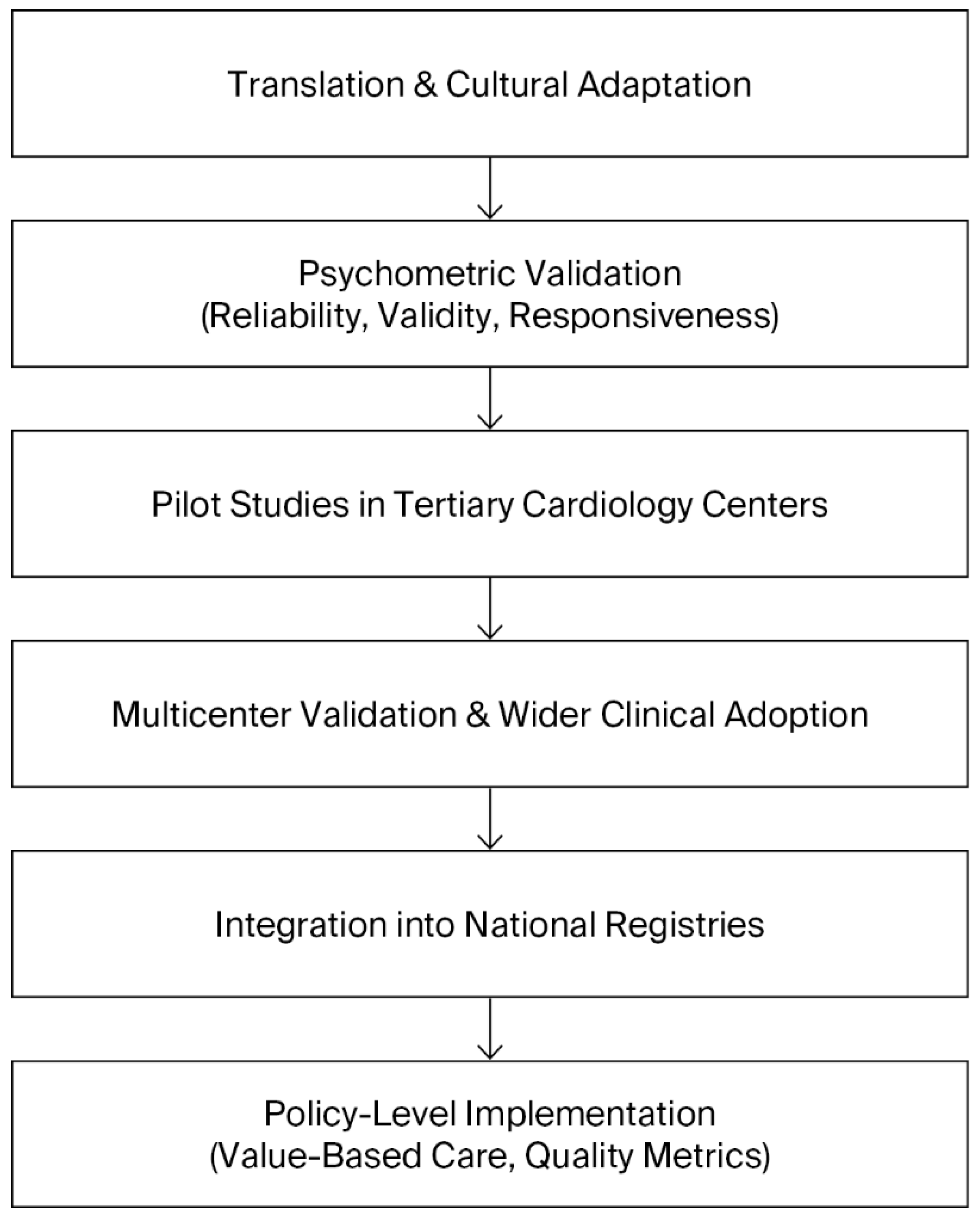
A roadmap for the implementation of the SAQ in Bulgaria should therefore be envisioned as a staged process that balances feasibility with ambition. The initial phase must focus on translation, cultural adaptation, and validation of the instrument, ensuring its reliability and acceptability among Bulgarian patients. Once validated, pilot projects in tertiary cardiology centers should assess the feasibility of routine SAQ use in clinical practice, including its integration into existing workflows and information systems. Insights from these pilots will be crucial for refining the approach before broader implementation. The subsequent phase should aim at systematic integration into electronic health records, with automated scoring and reporting to facilitate clinical use and to minimize administrative burden. Ultimately, the aim should be national adoption of the SAQ as a standardized measure of care quality, formally recognized in clinical guidelines and national cardiovascular registries. This progressive trajectory can be conceptualized as a stepwise roadmap, beginning with translation and validation, followed by pilot implementation, integration into clinical workflows, and culminating in national adoption. The stages of this roadmap are summarized in
Figure 1, which illustrates the sequential strategy for implementing the SAQ in Bulgaria. In this way, the introduction of the SAQ could serve as a catalyst for a more patient-centered, evidence-based, and transparent cardiovascular care system, aligned with contemporary international practice.
6. Conclusions
Over the past three decades, the Seattle Angina Questionnaire (SAQ) has been extensively validated, incorporated into landmark clinical trials such as COURAGE, ISCHEMIA, and EuroCTO, and consistently demonstrated to possess prognostic value that complements traditional clinical predictors [
2,
3,
6,
7,
8,
10,
15]. Its multidimensional structure and reproducibility have established the SAQ as the gold standard for patient-reported outcomes in chronic coronary syndrome and coronary artery disease [
4,
10].
Incorporating the SAQ into routine practice enhances symptom assessment, supports shared decision-making, and provides clinicians with a sensitive and standardized tool to monitor therapeutic effectiveness over time [
4,
10,
12]. These characteristics underscore its potential to improve both clinical care and health system performance by capturing the patient’s perspective in a systematic and comparable manner.
For Bulgaria, where cardiovascular mortality remains among the highest in Europe and the prevalence of ischemic heart disease is compounded by an unfavorable risk factor profile [
1,
9,
17,
18], the integration of the SAQ into clinical practice is both timely and necessary. Its adoption could elevate the quality of care, align national standards with international guidelines, and strengthen the role of patient-centered outcomes in clinical decision-making and policy development. Ultimately, the introduction of the SAQ represents a crucial step towards a more modern, evidence-based, and patient-focused cardiovascular care system in Bulgaria.
Author Contributions
Conceptualization, V.D. and G.G.; Methodology, V.D. and P.N.; Formal analysis, V.D.; Investigation, V.D., G.G., and P.N.; Resources, G.G. and P.N.; Data curation, V.D.; Writing—original draft preparation, V.D.; Writing - review and editing, G.G. and P.N.; Visualization, V.D.; Supervision, G.G.; Project administration, V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external fundings.
Acknowledgments
Authors would like to thank colleagues from the Department of Interventional cardiology, UMHAT “Sveti Georgi” – Plovdiv, for their support and valuable discussions during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
CAD – Coronary Artery Disease
CABG – Coronary Artery Bypass Grafting
CCS – Chronic Coronary Syndrome
CTO – Chronic Total Occlusion
EHR – Electronic Health Record
ESC – European Society of Cardiology
HRQoL – Health-Related Quality of Life
LFU – Lost to Follow-Up
MI – Myocardial Infarction
NSI – National Statistical Institute
OMT – Optimal Medical Therapy
PCI – Percutaneous Coronary Intervention
PRO – Patient-Reported Outcome
QoL – Quality of Life
RCT – Randomized Controlled Trial
SAQ – Seattle Angina Questionnaire
References
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. [CrossRef]
- Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. [CrossRef]
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI vs. medical therapy on quality of life in patients with stable coronary disease: the COURAGE Trial. N Engl J Med. 2008;359(7):677–687.
- Arnold SV, Spertus JA, Jones PG, et al. Interpreting the Seattle Angina Questionnaire as an outcome measure in clinical trials and clinical care: a state-of-the-art review. Am Heart J. 2021;236:56–66.
- Spertus JA, Jones PG. Development and validation of a short version of the Seattle Angina Questionnaire (SAQ-7).Circ Cardiovasc Qual Outcomes. 2014;7(5):640–647. [CrossRef]
- Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106(1):43–49. [CrossRef]
- Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease (ISCHEMIA). N Engl J Med. 2020;382:1395–1407.
- Werner GS, Martin-Yuste V, Hildick-Smith D, et al. EuroCTO randomized trial: Revascularization vs. optimal medical therapy for CTO. Eur Heart J. 2018;39(26):2484–2493.
- National Statistical Institute of Bulgaria. Health Statistics Report. Sofia; 2022.
- Patel KK, Arnold SV, Chan PS, et al. Validation of the Seattle Angina Questionnaire in women with ischemic heart disease. Am J Cardiol. 2018;122(10):1797–1802. [CrossRef]
- Arnold SV, Kosiborod M, Li Y, et al. Comparison of the Seattle Angina Questionnaire with generic health status instruments in CAD. Am Heart J. 2010;160(5):956–963.
- Lawal OA, Rabi DM, Ghali WA, Quan H. Psychometric evaluation of a Canadian version of the Seattle Angina Questionnaire. Health Qual Life Outcomes. 2020;18:389. [CrossRef]
- Chan PS, Jones PG, Arnold SA, et al. Development and validation of a short version of the SAQ (SAQ-7). Circ Cardiovasc Qual Outcomes. 2014;7(5):640–647.
- Arnold SV, Spertus JA, Jones PG, Xiao L, Cohen DJ. Impact of dyspnea on HRQoL in stable angina: results from the PREMIER registry. Am Heart J. 2009;157(6):1042–1049.
- Mills EJ, O’Regan C, Wu P, et al. Revascularization versus medical therapy for stable CAD: systematic review and meta-analysis of RCTs. Eur Heart J. 2011;32(23):3089–3097.
- Townsend N, Kazakiewicz D, Wright FL, et al. Atlas of Cardiovascular Disease in Europe 2022. Eur Heart J. 2022;43(4):382–405.
- Dimitrov P, et al. Cardiovascular risk factors and ischemic heart disease prevalence in Bulgaria: national survey results. Eur J Prev Cardiol. 2021;28(Suppl 1):i21–i29.
- Groene O, Kringos DS, Sunol R, et al. Patient experience measures and health system performance in Europe. Eur J Public Health. 2019;29(Suppl 4):12–17.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).