Submitted:
30 September 2025
Posted:
30 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Sampling Procedure
2.3. Sample Size Estimation
2.4. Data Collection
2.5. Laboratory Methodology
2.6. Statistical Analysis
3. Results
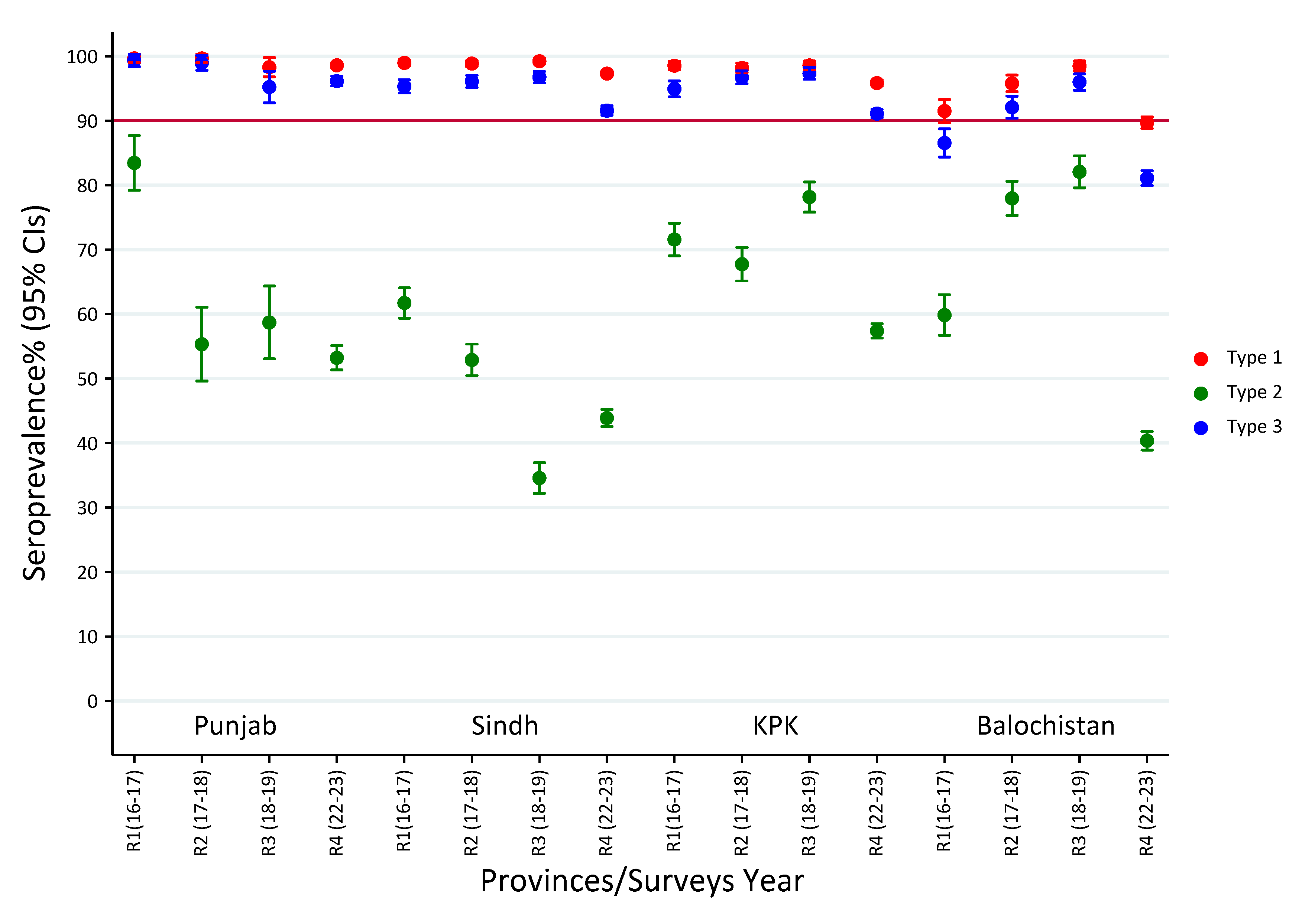
3.1. Seroprevalence of Polio Type-1 Across Provinces and Survey Rounds
3.2. Seroprevalence of Polio Type-2 Across Provinces and Survey Rounds
3.3. Seroprevalence of Polio Type-3 Across Provinces and Survey Rounds
3.4. Seroprevalence of Polio Type-1 Across High-Risk Cities
3.5. Seroprevalence Trends of Polio Type-2 Across High-Risk Cities
3.6. Seroprevalence of Polio Type-3 Across High-Risk Cities

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Assembly. Global eradication of poliomyelitis by the year 2000: resolution of the 41st World Health Assembly. Geneva: WHO; 1988.
- Haqqi A, Zahoor S, Aftab MN, Tipu I, Rehman Y, Ahmed H, et al. COVID-19 in Pakistan: impact on global polio eradication initiative. J Med Virol. 2020, 93, 141–143. [CrossRef]
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol. 2015, 10, 791–808. [CrossRef]
- Pons-Salort M, Molodecky NA, O’Reilly KM, Wadood MZ, Safdar RM, Etsano A, et al Population immunity against Serotype-2 poliomyelitis leading up to the global withdrawal of the Oral poliovirus vaccine: Spatio-temporal modelling of surveillance data. PLoS Med. 2016, 13, e1002140. [CrossRef]
- Hsu CH, Kader M, Mahamud A, et al. Progress Toward Poliomyelitis Eradication — Pakistan, January 2022–July 2023. MMWR Morb Mortal Wkly Rep. 2023, 72, 1057–1062. [Google Scholar]
- Chard AN, Datta SD, Gumede N, et al. Progress Toward Global Polio Eradication — Worldwide, January 2021–March 2023. MMWR Morb Mortal Wkly Rep. 2023, 72, 533–538. [Google Scholar]
- Akseer N, Rizvi A, Bhatti Z, et al. Association of Polio Eradication Initiative with the Delivery of Essential Maternal and Child Health Interventions in Pakistan: A Population-Based Cross-Sectional Study. Lancet Glob Health. 2022, 10, e1302–e1312. [Google Scholar]
- Ghafoor S, Sheikh N. Eradication and Current Status of Poliomyelitis in Pakistan: Ground Realities. J Immunol Res. 2016, 2016, 6837824. [Google Scholar]
- Raza F, Svensson J, Shehzad S, et al. Endemic Polio in Peshawar, Pakistan: Perceptions, Practices, and Risk Analysis. Health Policy Plan. 2021, 36, 383–394. [Google Scholar]
- Shah M, Khan MK, Shakeel S, et al. Resistance of Polio to Its Eradication in Pakistan. Virol J. 2021, 18, 108. [Google Scholar]
- Mbaeyi C, Alleman MM, Ehrhardt D, et al. Update on Vaccine-Derived Poliovirus Outbreaks — Worldwide, January 2020–June 2021. MMWR Morb Mortal Wkly Rep. 2021, 70, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Macklin GR, O’Reilly KM, Grassly NC, et al. Evolving Epidemiology of Poliovirus Serotype 2 Following Withdrawal of the Type 2 Oral Poliovirus Vaccine. Science. 2020, 368, 401–405. [Google Scholar] [CrossRef]
- Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-Derived Polioviruses. J Infect Dis. 2014, 210 (Suppl. 1), S283–S293.
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol. 2015, 10, 791–808. [Google Scholar] [CrossRef]
- Pons-Salort M, Molodecky NA, O’Reilly KM, Wadood MZ, Safdar RM, Etsano A, et al. Population immunity against Serotype-2 poliomyelitis leading up to the global withdrawal of the Oral poliovirus vaccine: Spatio-temporal modelling of surveillance data. PLoS Med. 2016, 13, e1002140. [Google Scholar] [CrossRef]
- Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine — Worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016, 65, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Sutter RW, Platt L, Mach O, et al. The New Polio Eradication End Game: Rationale and Supporting Evidence. J Infect Dis. 2014, 210 (Suppl. 1), S434–S438. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio Vaccination: Past, Present and Future. Future Microbiol. 2015, 10, 791–808. [Google Scholar] [CrossRef]
- Duintjer Tebbens RJ, Hampton LM, Thompson KM. Implementation of Coordinated Global Serotype 2 Oral Poliovirus Vaccine Cessation: Risks of Potential Non-Synchronous Cessation. BMC Infect Dis. 2016, 16, 237. [Google Scholar]
- Garon J, Seib K, Orenstein WA, Gonzalez AR, Blanc DC, Zaffran M, et al. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines. 2016, 15, 693–708. [CrossRef]
- Taniuchi M, Famulare M, Zaman K, Uddin MJ, Upfill-Brown AM, Ahmed T, et al. Community transmission of type 2 poliovirus after cessation of trivalent oral polio vaccine in Bangladesh: an open-label cluster-randomised trial and modelling study. Lancet Infect Dis. 2017, 17, 1069–1079. [CrossRef]
- Concepción FE, Mark AP, Abhijeet A, Steven GFW, Roland WS, Jay DW, et al. Poliovirus vaccination options for achieving eradication and securing the endgame. Curr Opin Virol. 2013, 3, 309–315. [CrossRef]
- Pakistan Polio Eradication Programme. (2025). Polio cases in provinces. Available at: https://www.endpolio.com.pk/polioin-pakistan/polio-cases-in-provinces.
- Mir F, Quadri F, Mach O, et al. Monovalent Type-1 Oral Poliovirus Vaccine Given at Short Intervals in Pakistan: A Randomised Controlled, Four-Arm, Open-Label, Non-Inferiority Trial. Lancet Infect Dis. 2015, 15, 889–897. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pakistan Polio Update—January-December 2022. WHO Regional Office for the Eastern Mediterranean; 2023.
- Asghar H, Diop OM, Weldegebriel G, et al. Environmental Surveillance for Polioviruses in the Global Polio Eradication Initiative. J Infect Dis. 2014, 210 (Suppl. 1), S294–S303. [Google Scholar] [CrossRef]
- Alam MM, Shaukat S, Sharif S, et al. Detection of Multiple Cocirculating Wild Poliovirus Type 1 Lineages Through Environmental Surveillance: Impact and Progress During 2011-2013 in Pakistan. J Infect Dis.
- Zafar M, Ullah MA, Zahoor MA, et al. COVID-19 and Polio in Pakistan: Challenges, Efforts, and Recommendations. Int J Infect Dis. 2022, 115, 172–179. [Google Scholar]
- Chandir S, Siddiqi DA, Mehmood M, et al. Impact of COVID-19 Pandemic on Polio Vaccination in Pakistan: A Retrospective Cross-Sectional Study. BMJ Open. 2020, 10, e043813. [Google Scholar]
- Zainab SM, Khowaja S, Akhtar I, et al. Polio Vaccination Amidst COVID-19 in Pakistan: What Are the Efforts and Challenges? J Glob Health. 2022, 12, 03023. [Google Scholar]
- Rana MS, Usman M, Salman M, et al. Impact of COVID-19 Pandemic on Polio Surveillance in Pakistan. J Infect. 2021, 82, 414–451. [Google Scholar] [CrossRef]
- Khan MT, Zaheer S, Shafique K. Maternal Education, Empowerment, Economic Status and Child Polio Vaccination Uptake in Pakistan: A Population Based Cross Sectional Study. BMJ Open. 2017, 7, e013853. [Google Scholar] [CrossRef]
- Khan AJ, Akber A, Nawaz M, et al. Transmission Dynamics of Wild Poliovirus Type 1 in a High-Risk District of Pakistan with Continuously High Vaccination Coverage. BMC Infect Dis. 2022, 22, 677. [Google Scholar]
- Naqvi AA, Naqvi SBS, Zehra F, et al. Estimation of the Burden of Children Affected by Poliomyelitis in Pakistan and an Assessment of the Physical Limitations Faced by these Children. J Taibah Univ Med Sci. 2018, 13, 241–246. [Google Scholar]
- Hsu CH, Mahamud A, Morales M, et al. Progress Toward Poliomyelitis Eradication — Afghanistan, January 2021–May 2023. MMWR Morb Mortal Wkly Rep. 2023, 72, 683–688. [Google Scholar]
- Jorba J, Diop OM, Iber J, et al. Update on Vaccine-Derived Poliovirus Outbreaks — Worldwide, January 2018–June 2019. MMWR Morb Mortal Wkly Rep. 2019, 68, 1024–1028. [Google Scholar] [CrossRef]
- Cowger TL, Burns CC, Sharif S, et al. The Role of Supplementary Environmental Surveillance to Complement Acute Flaccid Paralysis Surveillance for Wild Poliovirus in Pakistan – 2011-2013. PLoS One. 2017, 12, e0180608. [Google Scholar]
- Hsu CH, Rehman MS, Ray P, et al. Progress Toward Poliomyelitis Eradication — Pakistan, January 2021–July 2022. MMWR Morb Mortal Wkly Rep. 2022, 71, 1359–1364. [Google Scholar]
- Ahmad SO, Bux R, Yousuf F. Health Care in Pakistan—A Systems Perspective. In: Cockerham WC, ed. The Wiley Blackwell Encyclopedia of Health, Illness, Behavior, and Society. Chichester: John Wiley & Sons.
- Sutter RW, John TJ, Jain H, et al. Immunogenicity of Bivalent Types 1 and 3 Oral Poliovirus Vaccine: A Randomised, Double-Blind, Controlled Trial. Lancet. 2010, 376, 1682–1688. [Google Scholar] [CrossRef]
- Grassly, NC. The Final Stages of the Global Eradication of Poliomyelitis. Philos Trans R Soc Lond B Biol Sci. 2013, 368, 20120140. [Google Scholar] [CrossRef] [PubMed]
- Habib MA, Soofi SB, Sheraz A, et al. Evaluation of Immunogenicity and Safety of the 13-Valent Pneumococcal Conjugate Vaccine in Pakistani Children. Vaccine. 2016, 34, 4739–4744. [Google Scholar]
- Estivariz CF, Pallansch MA, Anand A, et al. Poliovirus Vaccination Options for Achieving Eradication and Securing the Endgame. Curr Opin Virol. 2013, 3, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Hussain I, Mach O, Habib A, et al. Seroprevalence of Anti-Polio Antibodies in Children from Polio High-Risk Areas of Pakistan: A Cross-Sectional Survey 2015-2016. BMJ Open. 2017, 7, e013014. [Google Scholar]
- Grassly NC, Wadood MZ, Safdar RM, et al. Effect of Inactivated Poliovirus Vaccine Campaigns, Pakistan, 2014-2017. Emerg Infect Dis. 2018, 24, 2113–2115. [Google Scholar] [CrossRef]
- Voorman A, Habib MA, Hussian I, Safdar RM, Ahmed JA, Weldon WC, et al. Immunity and field efficacy of type 2-containing polio vaccines after cessation of trivalent oral polio vaccine: a population-based serological study in Pakistan. Vaccine. 2020, 5, 100067. [CrossRef]
- Hussain I, Umer M, Khan A, Sajid M, Ahmed I, Begum K, Iqbal J, Alam MM, Safdar RM, Baig S, Voorman A, Partridge J and Soofi S. Exploring the path to polio eradication: insights from consecutive seroprevalence surveys among Pakistani children. Front. Public Health 2024, 12, 1384410. [CrossRef]
- Weldon WC, Oberste MS, MA P. Standardized methods for detection of poliovirus antibodies. New York: Springer (2016).
- Plotkin, SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC.; 2024.
- Chandir S, Siddiqi DA, Mehmood M, et al. Impact of COVID-19 Pandemic on Polio Vaccination in Pakistan: A Retrospective Cross-Sectional Study. BMJ Open. 2020, 10, e043813. [Google Scholar]
- Fatima M, Khan MA, Ali S, et al. Projection of Spatial Spread of Wild Polio Virus Type 1 in Pakistan. Commun Nonlinear Sci Numer Simul. 2022, 113, 106523. [Google Scholar]
- Bigouette JP, Wilkinson AL, Tallis G, et al. Progress Toward Polio Eradication — Worldwide, January 2019–June 2021. MMWR Morb Mortal Wkly Rep. 2021, 70, 1129–1135. [Google Scholar] [CrossRef]
- O’Reilly KM, Durry E, ul Islam O, et al. The Effect of Mass Immunisation Campaigns and New Oral Poliovirus Vaccines on the Incidence of Poliomyelitis in Pakistan and Afghanistan, 2001–2011: A Retrospective Analysis. Lancet. 2012, 380, 491–498. [Google Scholar] [CrossRef]
- Shaikh NB, Soomro TR, Ghouri A, et al. Key Determinants of Polio Immunity Gaps in Areas of High Risk in Pakistan. J Infect Dis. 2020, 221, 744–750. [Google Scholar]
- Minor, PD. Polio Vaccines and the Eradication of Poliomyelitis. Lancet. 2021, 398, 1731–1740. [Google Scholar] [CrossRef]
- World Health Organization. Certification of the Global Eradication of Wild Poliovirus Type 3. Wkly Epidemiol Rec. 2019, 94, 545–547.
- Kew OM, Cochi SL, Jafari HS, et al. Possible Eradication of Wild Poliovirus Type 3 — Worldwide, 2012. MMWR Morb Mortal Wkly Rep. 2014, 63, 1031–1033. [Google Scholar]
- Rehman AU, Sahito A, Alvi ZA, et al. Geographical Disparities in the Access of Polio Vaccination in Pakistan: A Secondary Analysis. J Prim Care Community Health. 2021, 12, 21501327211027735. [Google Scholar]
- Molodecky NA, Blake IM, O’Reilly KM, et al. Risk Factors and Short-term Projections for Serotype-1 Poliomyelitis Incidence in Pakistan: A Spatiotemporal Analysis. PLoS Med. 2017, 14, e1002323. [Google Scholar]
| Survey rounds | Number of Districts | Clusters covered | Households covered | Number of children surveyed (N) | Male (%) | Vaccination Card (%) | OPV3 Coverage (%) | IPV Coverage (%) | Caregiver Education level (Illiteracy rate- %) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 326 | 4143 | 4146 | 52.0 | 65.3 | 75.3 | 69.2 | 73.3 |
| 2 | 10 | 325 | 4093 | 4094 | 52.2 | 60.3 | 69.0 | 74.0 | 76.6 |
| 3 | 10 | 324 | 3985 | 3987 | 53.0 | 70.8 | 68.8 | 81.9 | 73.2 |
| 4 | 38 | 1109 | 20150 | 20680 | 51.9 | 40.0 | 74.1 | 55.4 | 67.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





