1. Introduction
Conversion of a failed medial unicompartmental knee arthroplasty (UKA) to total knee arthroplasty (TKA) presents several technical challenges, such as implant removal, management of bone defects, restoration of alignment, and soft-tissue balancing. These cases often necessitate revision-type components with stems or augments, even in the absence of loosening or infection [
1,
2,
3,
4].
Advances in robotic-assisted TKA, including CT-based three-dimensional planning, real-time balance assessment, and precise bone preparation, offer new opportunities for a bone-preserving strategy in this setting [
5,
6,
7]. When applied within a functional alignment framework, 3D planning enables resections on healthy bone, optimizing fixation conditions and potentially allowing the use of cementless primary implants without augments or stems- an outcome less likely with conventional mechanical alignment.
The present technical note describes a step-by-step workflow for robotic-assisted conversion of medial UKA to TKA using standard condylar-stabilized components without stems or augments. The approach was inspired by the work of Marchand’s group and refined through institutional experience in high-volume robotic-assisted TKA, leading us to adopt a functional alignment philosophy based on native bone morphology and physiological ligament balance [
8]. As highlighted by Shatrov et al., this alignment strategy may reduce the need for augments or ligament releases in appropriately selected cases [
9]. Nevertheless, its successful application requires a thorough understanding of robotic workflow and an appreciation of the learning curve associated with UKA-to-TKA conversion [
6,
10,
11].
To our knowledge, no previous technical note has detailed a robotic-assisted UKA-to-TKA conversion workflow that combines pre-explantation registration, functional alignment, and exclusive use of cementless primary components without stems or augments.
2. Surgical Technique
This protocol was developed to enable a bone-preserving UKA-to-TKA conversion workflow using advanced robotic assistance. The strategy emphasizes functional alignment, precise 3D preoperative planning, and dynamic intraoperative adjustment based on real-time kinematic and soft-tissue assessment, as described by recent reference techniques [
8,
9].
2.1. Pre-Operative Planning
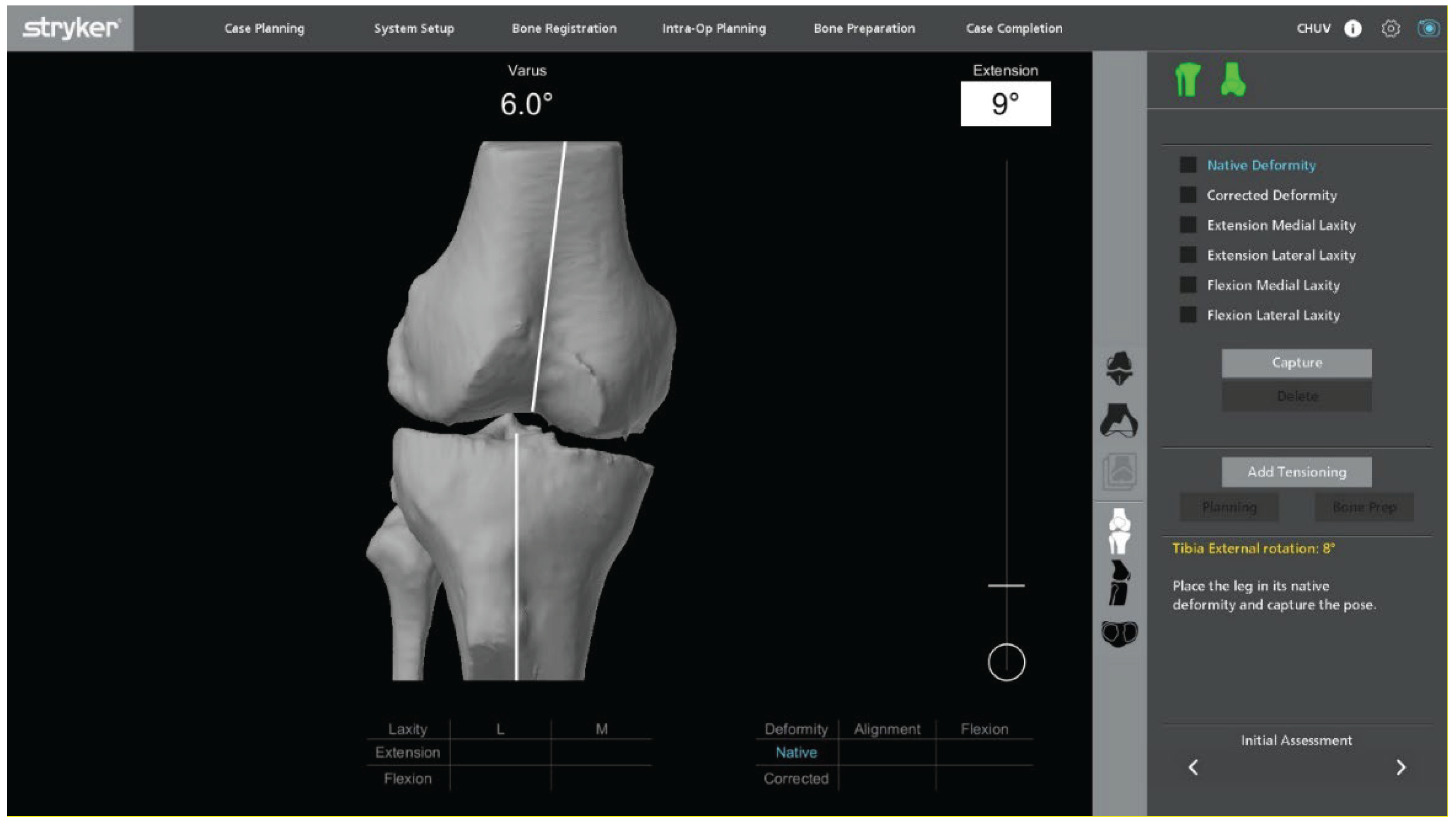
Pre-operative planning begins with the acquisition of a standard protocol CT scan, imported into the Mako robotic software (Stryker, Mahwah, NJ, USA). No dedicated metal–artifact–reduction protocol is applied during CT acquisition. Artifact mitigation is performed during image processing, and residual artifacts from the in-situ UKA are explicitly considered during planning. Three-dimensional reconstruction allows precise assessment of bone stock, implant position, and soft-tissue envelope. The surgical objective is to restore functional alignment based on native bone morphology and physiological ligament tension. The plan is refined to maximize bone preservation and minimize the need for stems or augments. Target parameters include restoring the hip–knee–ankle angle within 175–180°, maintaining joint line height within ±2 mm, and achieving 1–2 mm of controlled laxity in terminal extension and 90° of flexion while avoiding mid-flexion instability.
2.2. Patient Positioning and Approach
The patient is positioned supine, without a tourniquet. A medial parapatellar approach is used, re-entering the prior incision. The patella is retracted laterally without eversion. Ensure stable fixation of the limb to allow smooth robotic arm tracking and full range of motion during intraoperative assessment.
2.3. Pins Placement and Landmark Registration
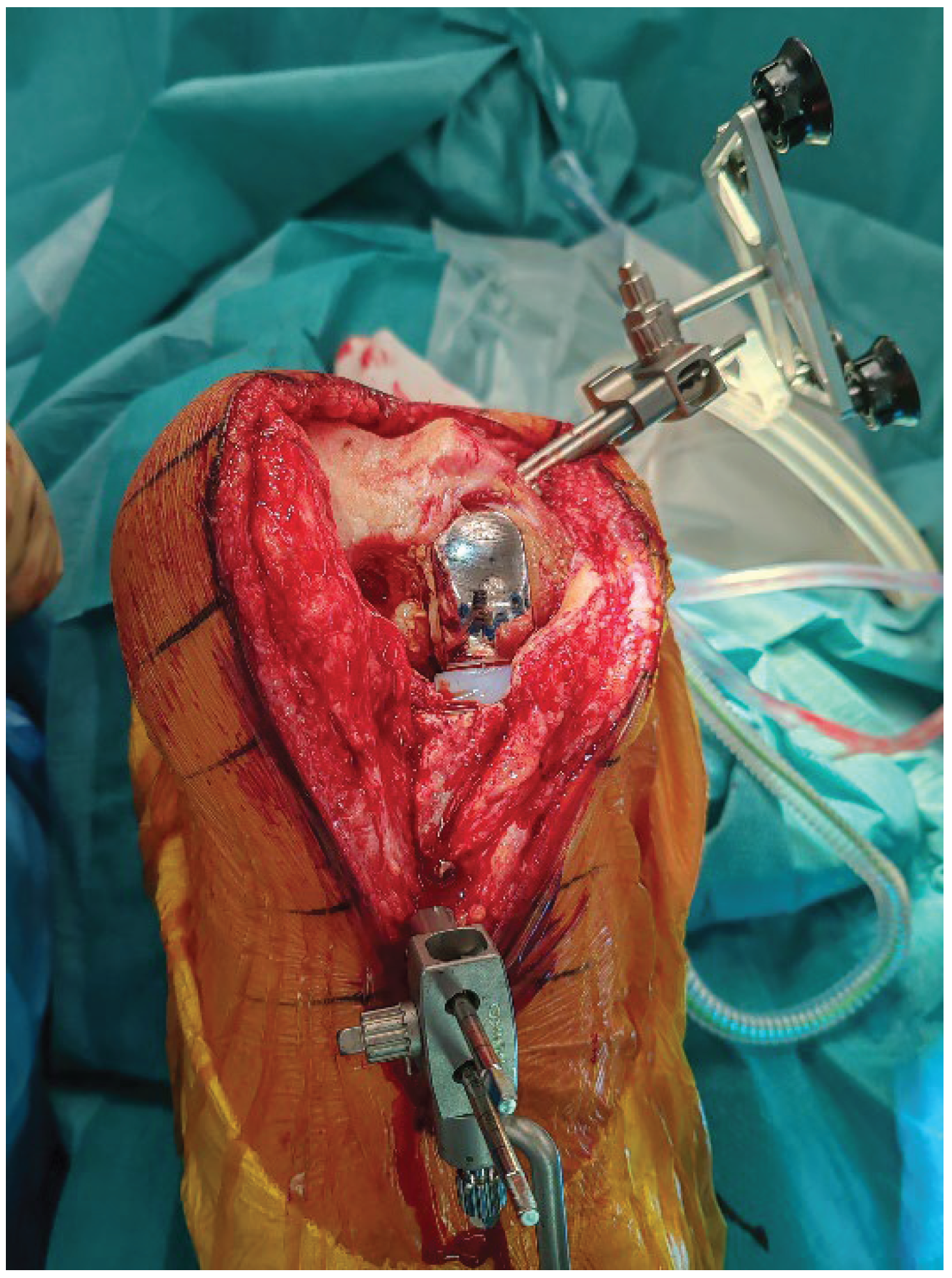
Array pins for the robotic system are inserted according to protocol (4 mm femur, 3 mm tibia) through the same medial parapatellar incision to minimize soft-tissue trauma (
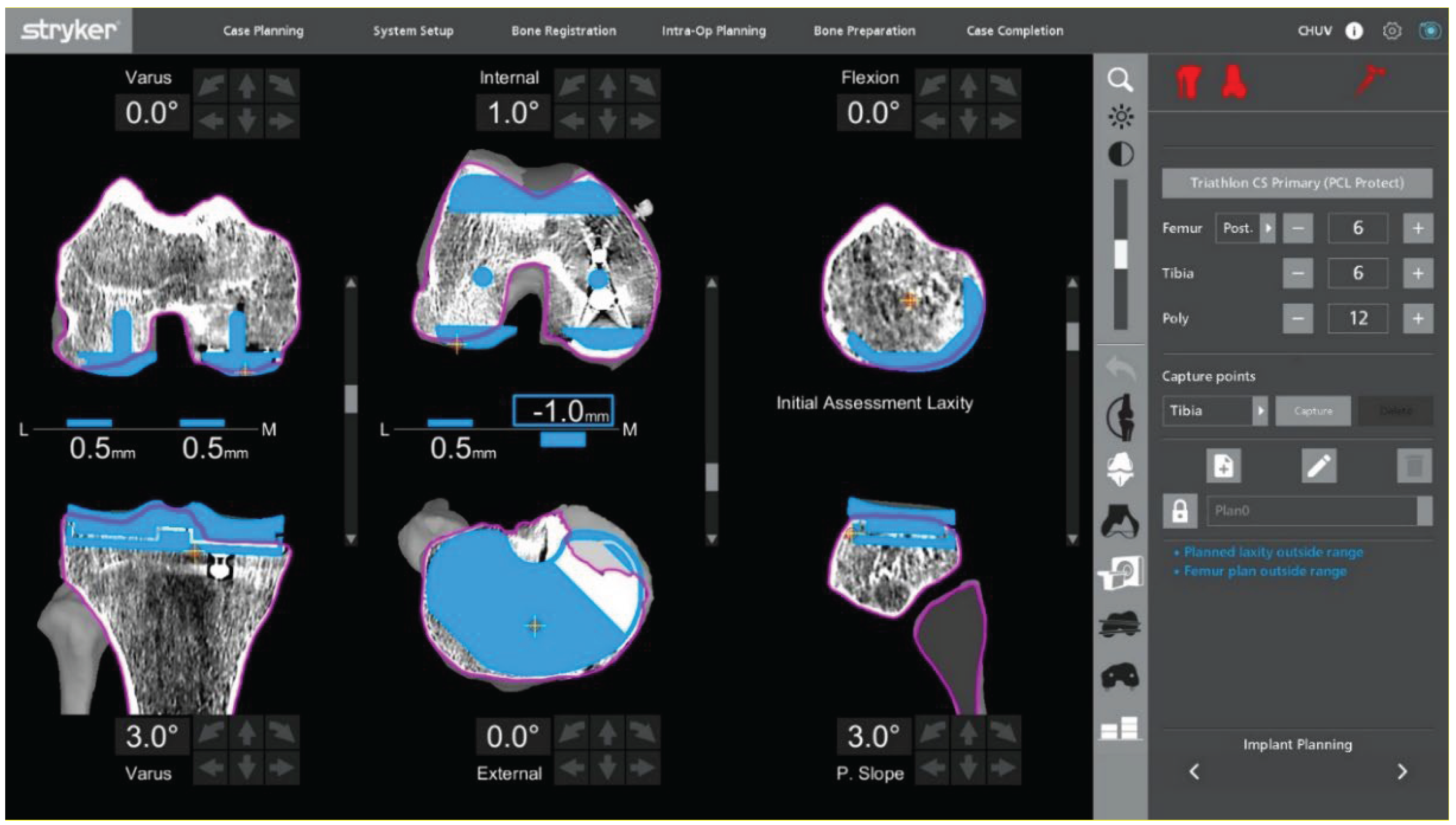
Figure 1). Before explantation, registration is performed onto the sagittal central axis of in-situ UKA components to limit the impact of CT metal artifacts (
Figure 2). On the femoral component, the sagittalcentral axis is drawn with a dermographic pen to create a clear sagittal reference. Landmark acquisition is then performed directly along this marked midline, which serves as a surrogate of the native medial condyle morphology and improves the accuracy of the registration. The polyethylene insert is temporarily removed to access reference surfaces and reinserted immediately afterward to allow dynamic balance assessment. Pin stability is verified prior to proceeding, because any motion can compromise tracking accuracy throughout the procedure. Registration accuracy is confirmed with checkpoint validation. Osteophytes are left in place during registration and removed only afterward to avoid altering reference points.
2.4. Intraoperative Kinematic and Ligament Balance Assessment
With the UKA components in place, an initial intraoperative kinematic assessment is performed (
Figure 3). The polyethylene insert is reinserted to enable a dynamic evaluation of ligament balance in both flexion and extension, providing essential data for potential plan adjustments. After registration and verification of reference points, this assessment is repeated through the full range of motion, allowing real-time adaptation to refine the 3D plan.
Varus–valgus stress tests are applied at multiple flexion angles to assess ligament balance. The goal is to achieve physiological, symmetric gaps in both extension and flexion while maintaining optimal soft-tissue tension and avoiding mid-flexion instability.
Within a functional-alignment strategy, balance is restored by adjusting implant positioning and resection targets rather than by performing ligament or soft-tissue releases. This kinematic, bone-preserving approach maintains the native joint line and creates optimal conditions for accurate, conservative implantation.
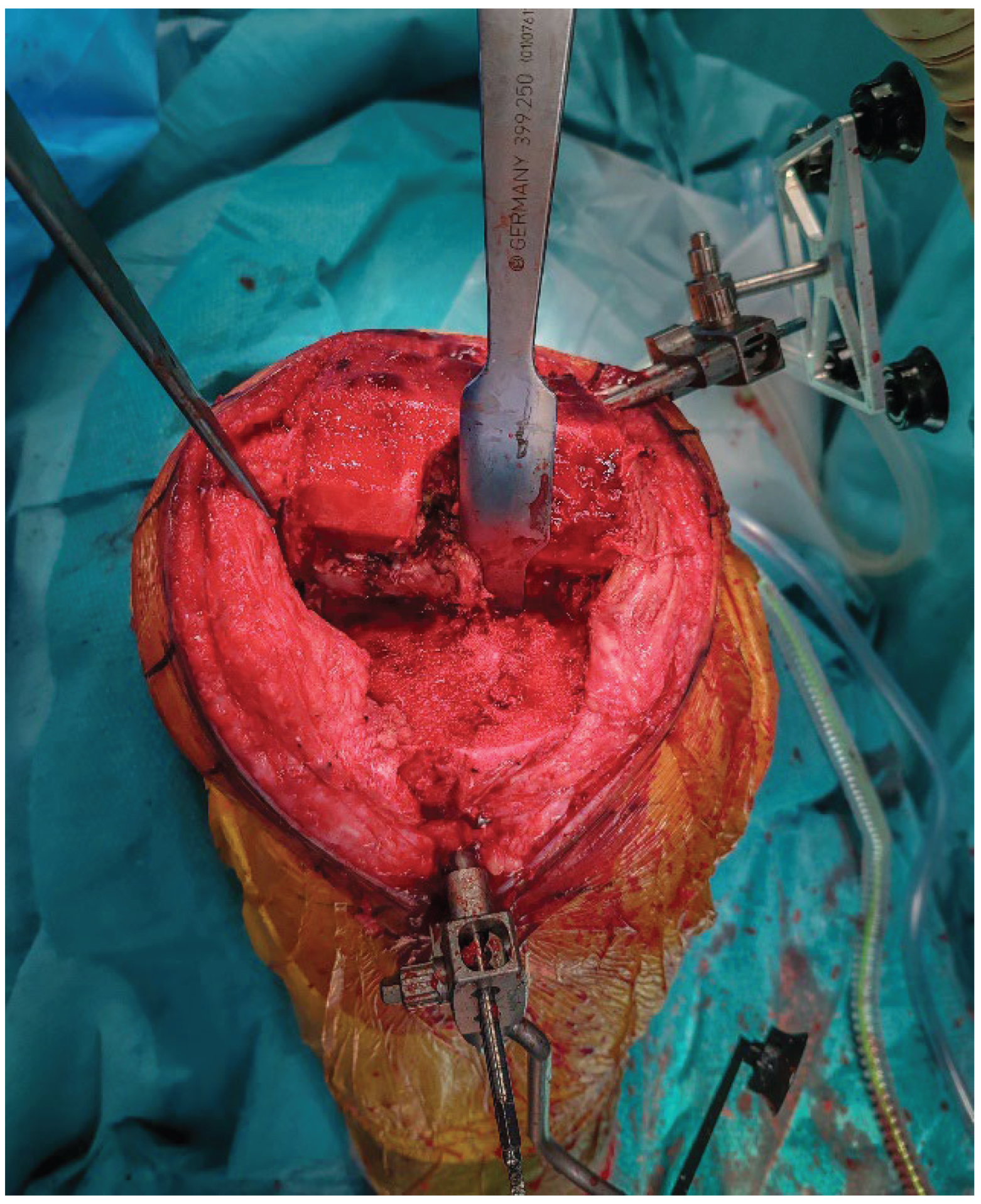
2.5. Implant Removal
The femoral and tibial components are removed using thin osteotomes and an oscillating saw, preserving as much bone as possible (
Figure 4). Careful sequential release of the bone–cement interface, combined with minimal lever forces, helps reduce the risk of iatrogenic bone defects.
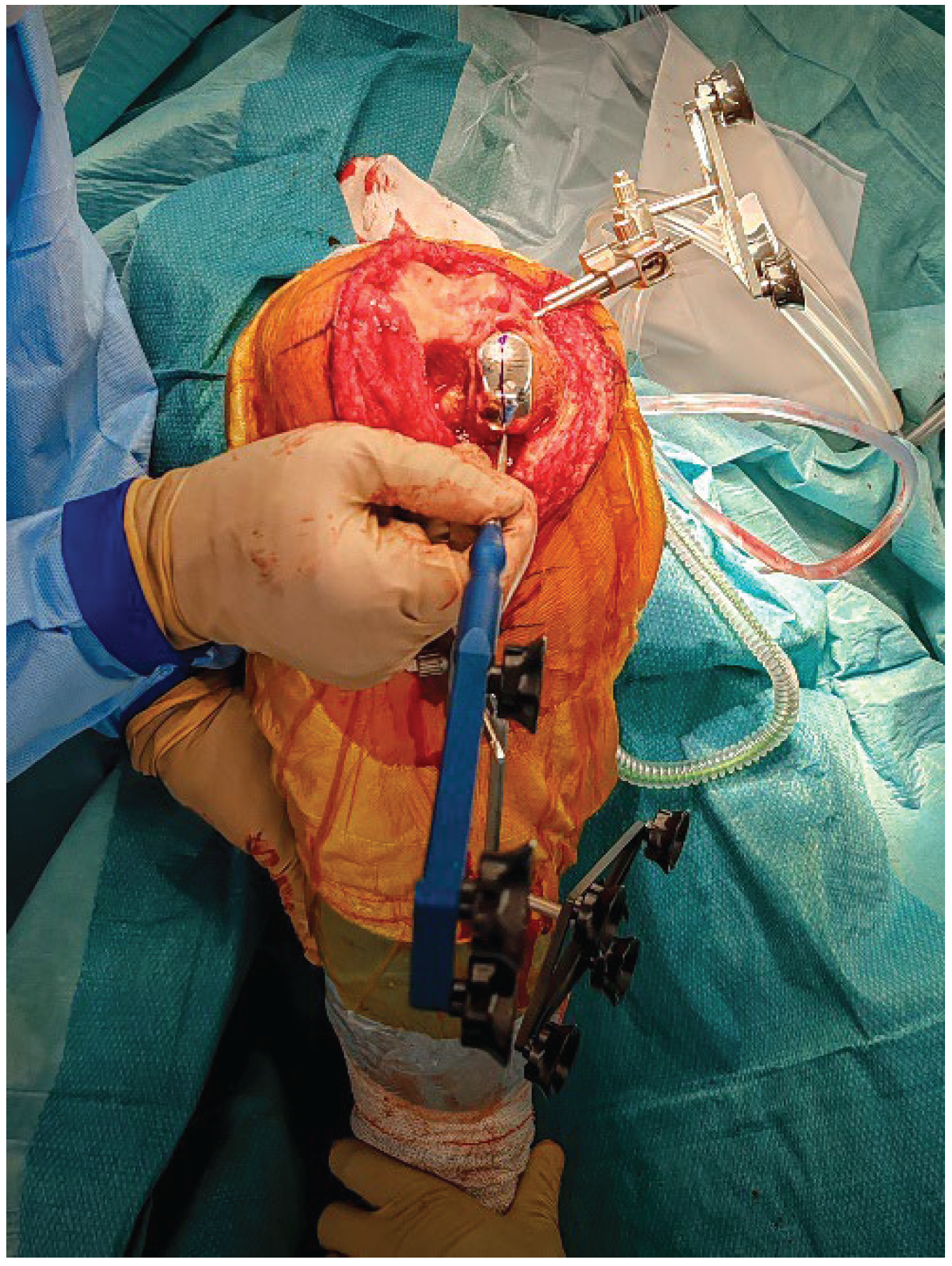
2.6. Robotic Planning and Bone Resection
After confirming ligament balance, the preoperative plan is adjusted if necessary to optimize bone preservation and soft-tissue tension. Robotic-guided resections are performed according to the final plan (
Figure 5).
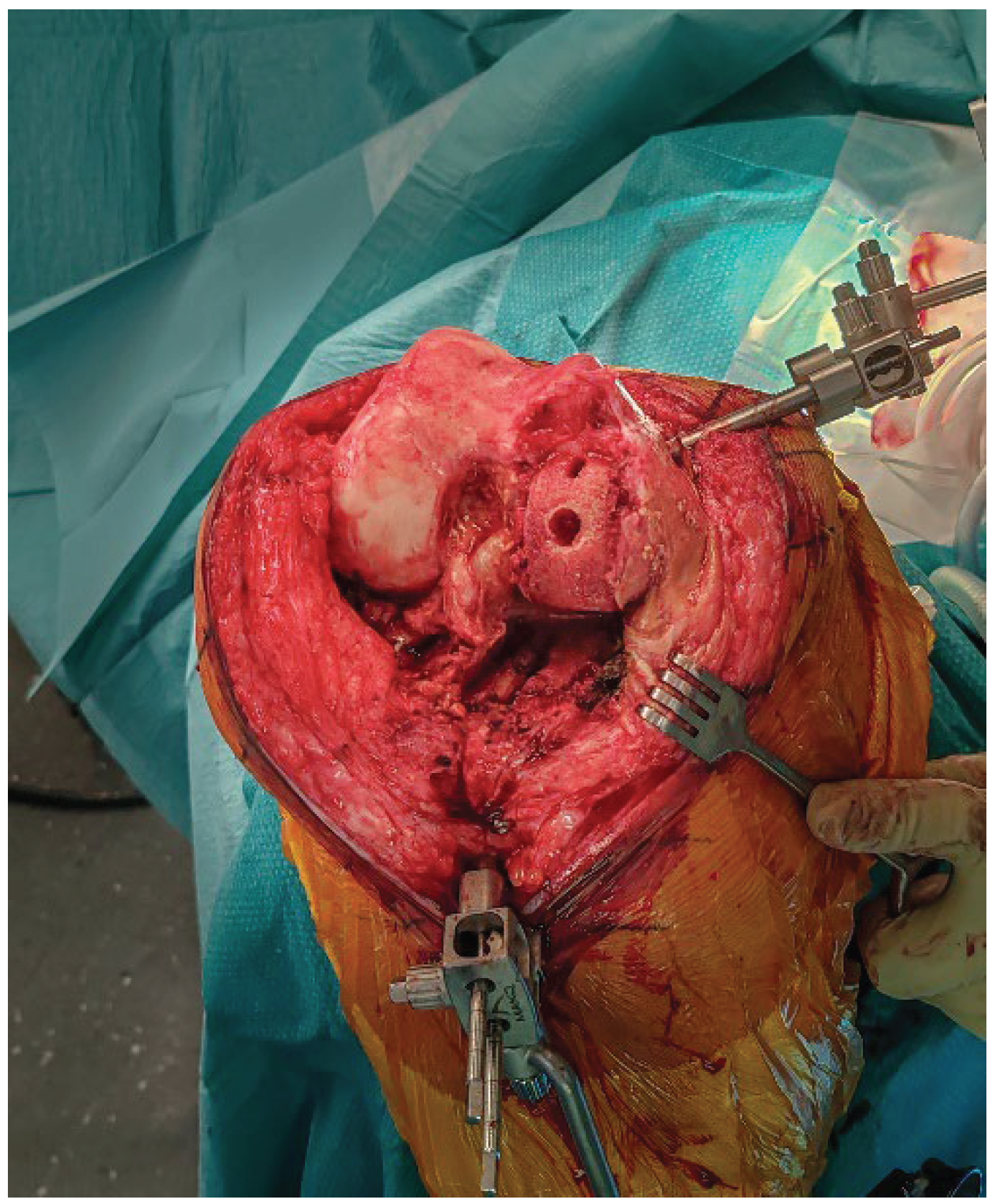
2.7. Bone Defect Management
Following explantation, any residual metaphyseal defect, most commonly located at the site of the former UKA tibial keel, is managed using autologous cancellous bone graft harvested from the femoral resections. The graft is then impacted with a tamp and mallet to recreate a stable and supportive bed for the tibial baseplate (
Figure 6).
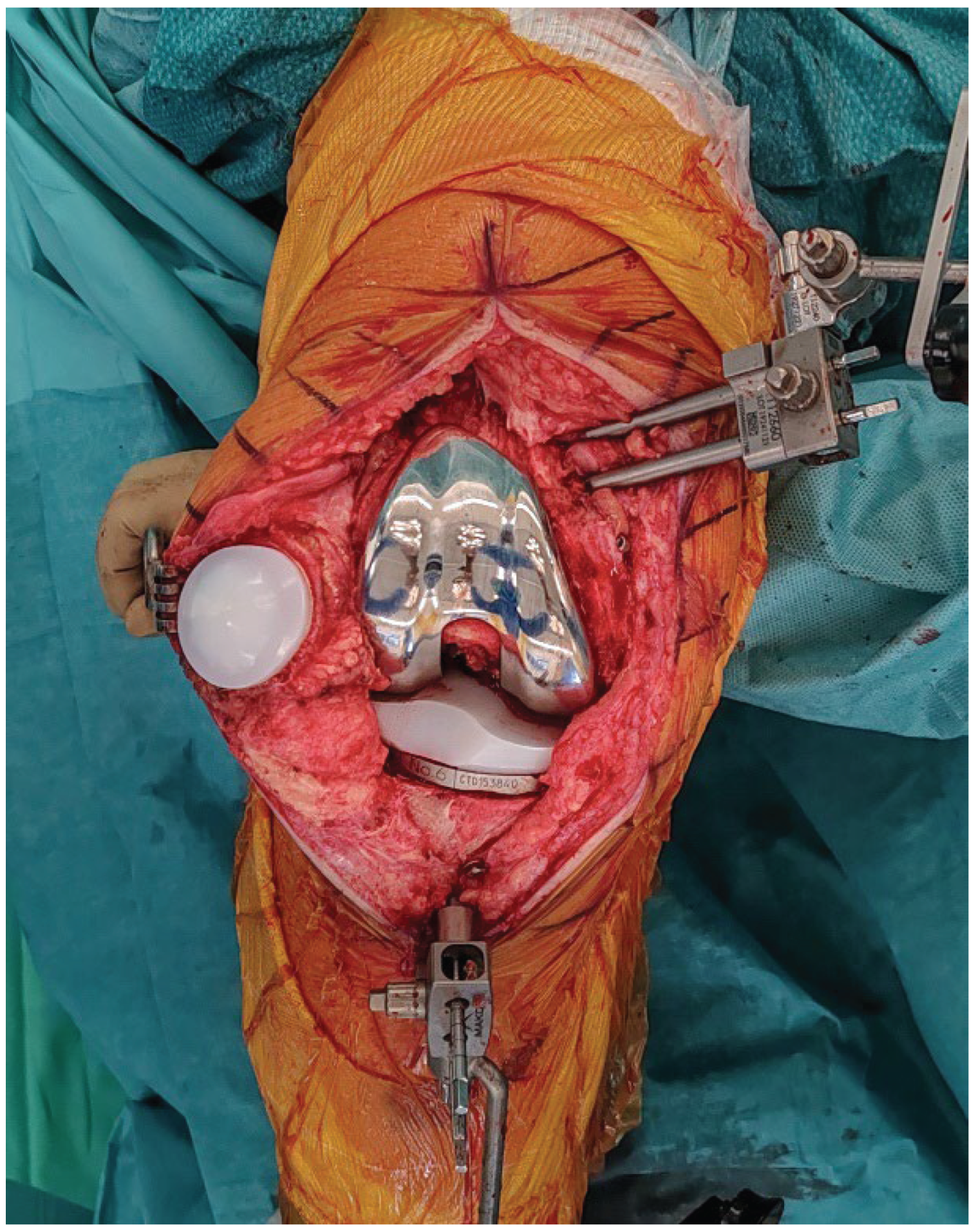
2.8. Final Implantation
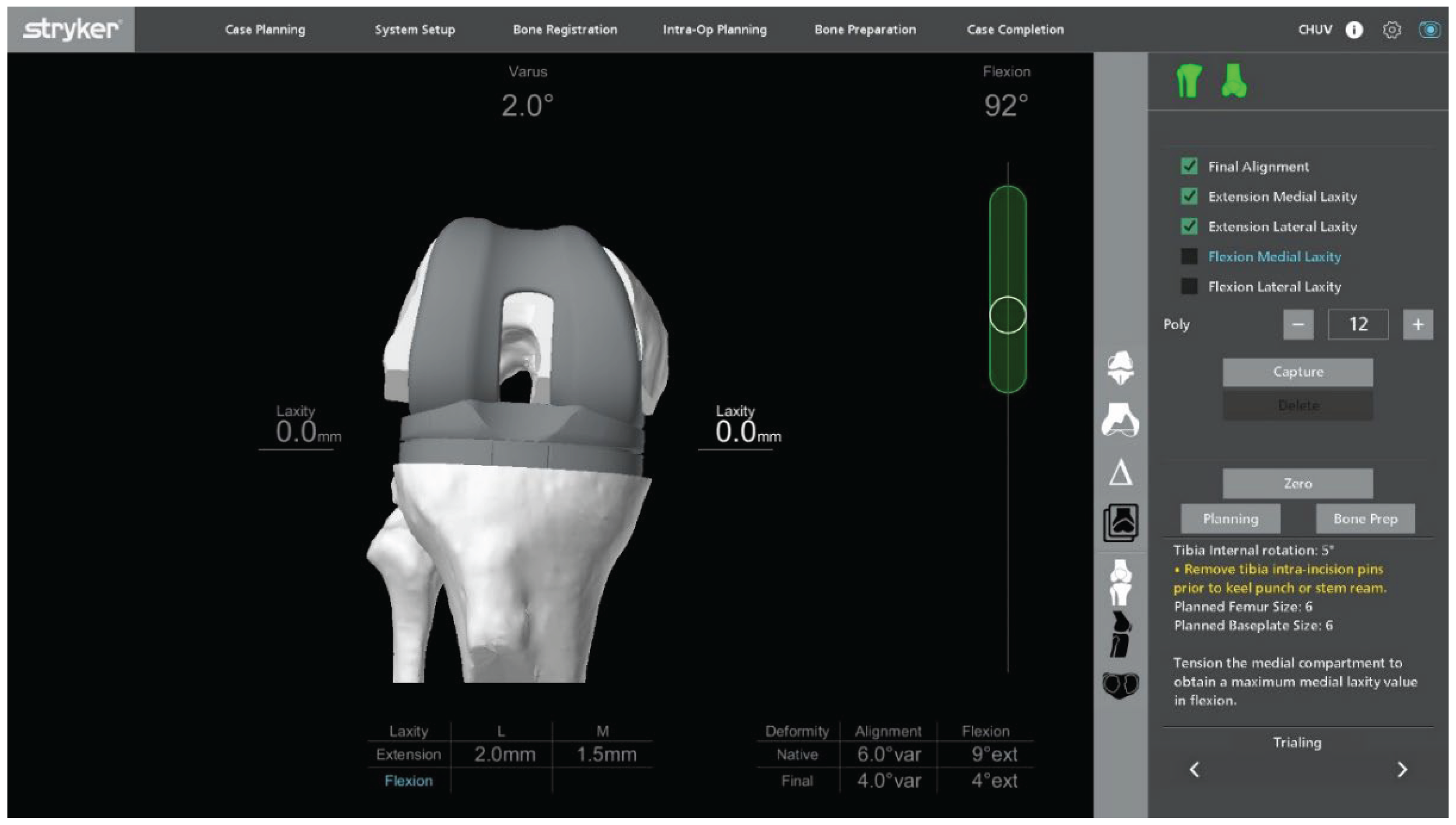
Trial components are inserted to confirm flexion–extension gap balance and overall stability (
Figure 7). Once satisfactory alignment and soft-tissue tension are achieved, definitive cementless condylar-stabilizing components are implanted (
Figure 8). Stems, augments, or ligament releases are typically not required. The patella is systematically resurfaced to decrease the risk of future anterior knee pain and revision surgery. Ensure that the final implant position matches the 3D planning and that all the implant press-fit interfaces are fully seated before closure.
2.9. Irrigation and Closure
The joint is irrigated with saline. Check for hemostasis. The capsule is closed in a watertight fashion, followed by the subcutaneous tissues and skin, using staples. A hydrocolloid dressing is applied for wound protection.
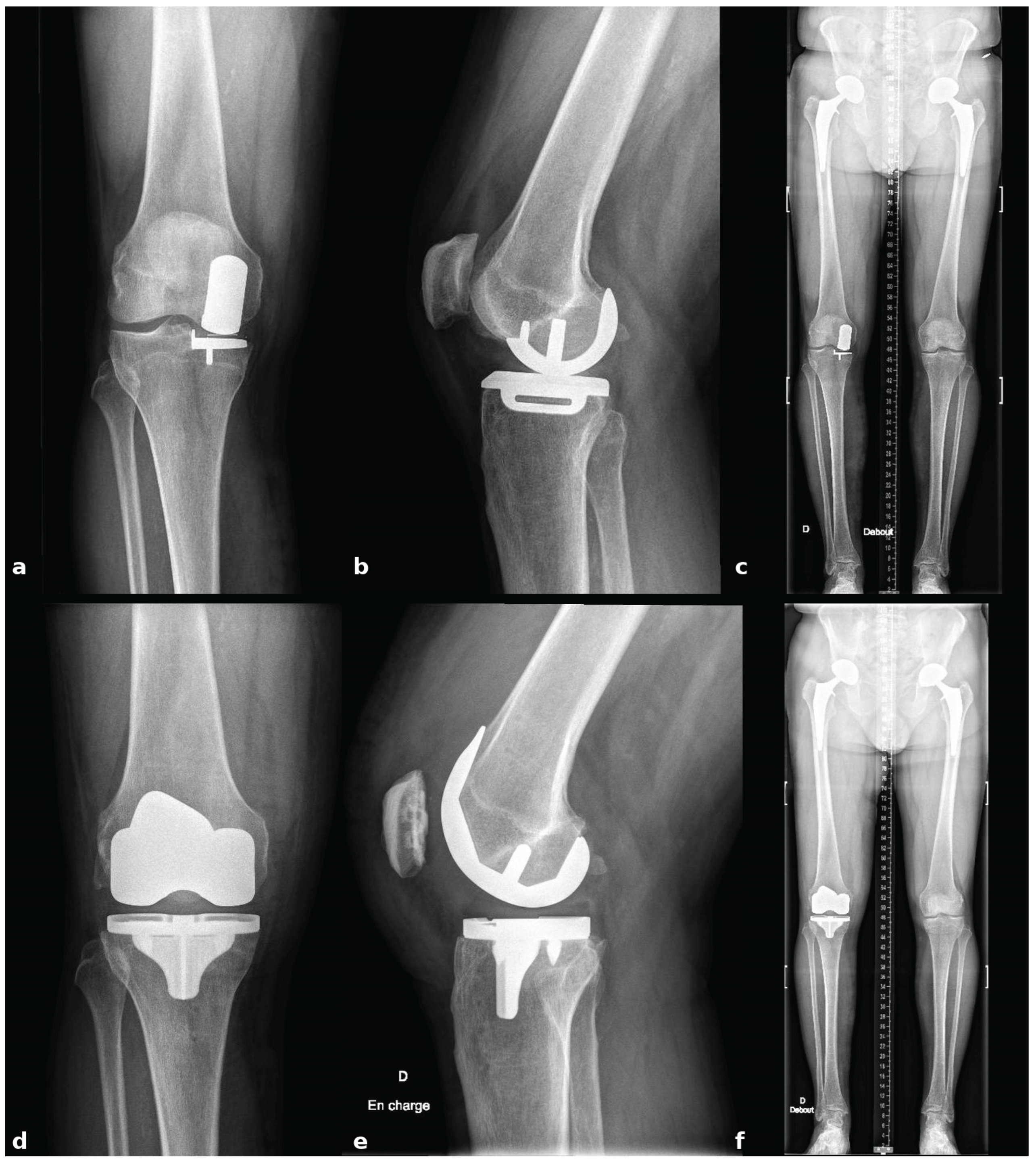
ostoperatively, patients follow a standardized rehabilitation program that encourages immediate mobilization and full weight-bearing. Deep venous thrombosis prophylaxis and early quadriceps activation exercises are initiated on the day of surgery. Postoperative radiographs are obtained to confirm accurate component positioning and restoration of limb alignment (
Figure 9).
3. Results
The described workflow enables stable registration despite metallic artifacts from the in-situ UKA components and allows bone-sparing implant removal. Functional-alignment planning combined with robotic guidance achieves symmetric extension and flexion gaps without ligament or soft-tissue releases. Localized metaphyseal defects at the former tibial keel site are managed with impacted autologous cancellous graft harvested from femoral resections. Final implant placement matched the preoperative plan, and postoperative radiographs confirmed restoration of limb alignment and appropriate component positioning.
4. Discussion
Conversion of UKA to TKA remains technically demanding, especially when the aim is to use standard primary implants. These cases frequently share the complexity, risks, and perioperative management considerations of revision procedures due to pre-existing bone resections, retained implants and altered joint mechanics, often related to malpositioning or wear. Even in the absence of infection, wear-induced osteolysis or loosening, stems and augments could be required [
2,
3,
4].
Robotic assistance offers distinct advantages in this particular context. The combination of CT-based 3D planning, real-time ligament balance assessment, and precise bone resections supports an individualized and bone-preserving strategy. In contrast to conventional mechanical alignment, which may position cuts away from healthy bone and thereby necessitate stems or augments, functional alignment aims to restore native bone morphology and the surrounding soft-tissue envelope. This maximizes the available bone stock for fixation and supports the use of cementless implants while minimizing the need for additional augmentation [
5,
6,
7,
9].
Magruder et al. demonstrated that performing pre-explantation registration directly on the UKA components is technically feasible and allows for maintaining registration accuracy despite CT metal artifacts [
8]. In our workflow, this concept is combined with a functional alignment strategy to preserve bone stock, maintain soft-tissue balance, and achieve stable fixation on healthy bone without the use of stems or augments. By acquiring landmarks along the sagittal central axis of the implants before removal, accurate mapping of both femoral and tibial anatomy can be achieved despite artifact interference. Successful execution of this technique requires proficiency with robotic workflows, and the learning curve for UKA-to-TKA conversion should be taken into account [
6,
10,
11].
Management of limited bone defects with autologous cancellous bone grafting is incorporated to restore a stable platform for tibial baseplate fixation without stems or augments. This combination of robotic pre-explantation registration, functional alignment planning, and bone-preserving defect management offers a reproducible pathway for accurate reconstruction in selected UKA-to-TKA conversions [
5,
6,
8,
9].
Although this report focuses on the technical workflow, the combination of functional alignment and cementless fixation may translate into improved implant longevity and reduced need for future revision, particularly in younger or more active patients, and further comparative studies are warranted to determine whether this bone-sparing robotic approach influences long-term functional outcomes, implant survival, and revision rates compared with conventional conversion techniques.
While robotic-assisted UKA-to-TKA conversions have been described, few reports have outlined such a detailed, stepwise workflow combining these elements to allow the exclusive use of standard cementless components [
5,
6,
7,
8,
10,
11]. This protocol may offer a reliable and reproducible option for carefully selected patients.
This technical note is limited by its descriptive nature and absence of long-term clinical outcome data. The workflow may not be applicable in cases with extensive bone loss, ligament insufficiency, infection, or compromised bone stock, where revision-type implants remain necessary. In addition, the technique requires proficiency with robotic workflows and may involve a learning curve for surgeons unfamiliar with these. Larger prospective studies are needed to validate reproducibility, assess functional outcomes, and determine implant survival compared with conventional conversion techniques.
5. Conclusions
This technical note outlines a reproducible, bone-preserving, robot-assisted workflow for the conversion of medial UKA to TKA using standard cementless condylar-stabilizing components, without the need for stems or augments. The procedure integrates 3D CT-based planning, pre-explantation registration on in situ implants, and dynamic soft-tissue assessment to enable functional alignment and secure fixation on healthy bone. By targeting functional soft-tissue balance and maximizing bone preservation, this strategy reduces the need for additional implants and may offer reliable outcomes in carefully selected patients. As with any advanced robotic technique, appropriate training and familiarity with robotic workflows are essential for safe and effective implementation. Future studies will help clarify the indications and further validate the safety and effectiveness bone-sparing approach in UKA-to-TKA conversion.
Author Contributions
All authors read and approved the final version submitted for publication; JM: Conceptualization, Methodology, Investigation, Writing – Original Draft, Writing – Review & Editing; AA: Conceptualization, Methodology, Writing – Review & Editing; TR: Review & Editing; JW: Conceptualization, Methodology, Writing – Review & Editing, Supervision
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
No datasets were generated or analyzed. De-identified operative images underlying the figures are available from the corresponding author upon reasonable request, subject to institutional and patient-privacy restrictions.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest related to the work reported. Outside the submitted work, J.W. serves as a consultant for Stryker and Enovis, and receives royalties from Dedienne Santé. The technique described was developed independently by the authors. No author received funding, support, materials, or honoraria from Stryker (or any other company) for this study.
Abbreviations
The following abbreviations are used in this manuscript:
UKA
TKA |
Unicompartmental Knee Arthroplasty
Total Knee Arthroplasty |
| CS |
Condylar-Stabilizing |
References
- Niinimäki T, Eskelinen A, Mäkelä K, Ohtonen P, Puhto AP, Remes V. Unicompartmental knee arthroplasty survivorship is lower than TKA survivorship: a 27-year Finnish registry study. Clin Orthop Relat Res. 2014;472(5):1496–1501. [CrossRef]
- Lunebourg A, Parratte S, Ollivier M, Abdel MP, Argenson JN. Are revisions of unicompartmental knee arthroplasties more like a primary or revision TKA? J Arthroplasty. 2015;30(12):1985–1989. [CrossRef]
- Leta TH, Lygre SHL, Skredderstuen A, et al. Outcomes of unicompartmental knee arthroplasty after aseptic revision to total knee arthroplasty: a comparative study of 768 TKAs and 578 UKAs revised to TKAs from the Norwegian arthroplasty register (1994 to 2011). J Bone Joint Surg Am. 2016;98(6):431–440. [CrossRef]
- Lee JK, Kim HJ, Park JO, Yang JH. Inferior outcome of revision of unicompartmental knee arthroplasty to total knee arthroplasty compared with primary total knee arthroplasty: systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2018;26(11):3403–3418. [CrossRef]
- Tuecking LR, Savov P, Windhagen H, Jennings S, Nathwani D, Ettinger M. Imageless robotic-assisted revision arthroplasty from UKA to TKA: surgical technique and case-control study compared with primary robotic TKA. Orthopä. 2021;50(12):1018–1025. [CrossRef]
- Yun AG, Qutami M, Chen CM, Pasko KBD. Management of failed UKA to TKA: conventional versus robotic-assisted conversion technique. Knee Surg Relat Res. 2020;32:38. [CrossRef]
- Lachance AD, Edelstein A, Stilwell M, Lutton J. No difference in range of motion, components, or complications following conversion of robotic-assisted total knee arthroplasty compared to manual TKA after undergoing manual or robotic-assisted unicompartmental knee arthroplasty. Arthroplast Today. 2023;24:101269. [CrossRef]
- Magruder ML, McClure T, Marchand K, Mont MA, Marchand RC. Robotic-arm-assisted conversion of unicompartmental knee arthroplasty to total knee arthroplasty. J Orthop. 2024;52:119–123. [CrossRef]
- Shatrov J, Battelier C, Sappey-Marinier E, Gunst S, Servien E & Lustig S. Functional Alignment Philosophy in Total Knee Arthroplasty– Rationale and technique for the varus morphotype using a CT based robotic platform and individualized planning. SICOT-J, 2022; 8, 11.
- Lee HJ, Park YB, Song MK, Kwak YH, Kim SH. Comparison of the outcomes of navigation-assisted revision of unicompartmental knee arthroplasty to total knee arthroplasty versus navigation-assisted primary TKA. Int Orthop. 2019;43(2):315–322. [CrossRef]
- Ngim HJ, Van Bavel D, De Steiger R, Tang AWW. Robotic-assisted revision total knee arthroplasty: a novel surgical technique. Arthroplasty. 2023;5(1):5. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).