1. Introduction
Inflammatory diseases of the dental pulp develop as a complication of dental caries, which can be reversible or irreversible. Irreversible pulpitis is treated through pulpectomy, resulting in the loss of vitality of the dental pulp, disrupting the biological functions of the affected tooth, and making it significantly more brittle and susceptible to fractures [
1,
2].
Research in recent years on the regeneration of dental pulp has demonstrated its significant potential for preserving vitality in young individuals, as well as in adults [
1,
2]. With the introduction of calcium–silicate cements and biomaterials from autologous blood, the possibilities for preserving the pulp’s vitality through direct pulp capping and pulpotomy are expanding. The literature shows significant differences in the success of treatment following vital pulp therapy. According to Bjorndal et al., the success rates for direct pulp capping and partial pulpotomy are 31.8% and 34.5%, respectively [
3]. According to Ricucci et al., the success rate after direct pulp capping is 73.2%. At the same time, for partial and complete pulpotomy, it is 94.6% and 77.8%, respectively [
4]. According to Alovisi M et al., the success rate of direct pulp capping is 23.8% [
5]. Possible reasons for the observed disparity are not only the differences in study design but also in the accurate diagnosis of reversible pulpitis, as confirmed by Asgary S et al. [
6].
The diagnosis of inflammatory diseases of the dental pulp is based on the patient’s subjective complaints, paraclinical methods, and objective examination [
6].
The disclosure of symptoms by the patient is known as anamnesis. The pain characteristics include whether the pain is spontaneous or provoked, its duration, whether it occurs at night or during the day, whether it is continuous, where there is pain upon suction, as well as other factors. This information is subjective and, if the treatment method is singular—such as pulpectomy—this is not a problem. Nevertheless, to preserve the vitality of the dental pulp through direct pulp capping and pulpotomy, it is necessary to precisely determine the phase of the reversible inflammatory process within the dental pulp. Initial changes in the dental pulp indicate the possibility of direct pulp capping [
6].
The paraclinical methods for diagnosis include EPT (electro pulp test), thermal tests, pulse oximetry, X-ray examination, Doppler diagnosis, and marker tests. Electro pulp test enables the determination of the condition of the nerve fibres in the dental pulp. However, the vitality of the dental pulp can be preserved even with partially destroyed nerve fibres. In other words, the obtained values do not always correspond to the phase of inflammation of the dental pulp. Furthermore, there are significant variations in the values provided by the available equipment [
7,
8].
Pulse oximetry provides information about the condition of the blood vessels in the dental pulp and is a reliable method for assessing this condition. However, the devices are difficult to use, due to the contact of the equipment with the teeth [
9].
X-ray diagnostics, particularly through two-dimensional segment imaging, are not entirely accurate for diagnosis, due to their inherent limitations. Through CBCT X-ray examination, we can monitor the integrity of the dental pulp and the deposition of tertiary dentin. Hence, these methods are suitable for tracking the results of vital pulp therapy [
10].
There are also other paraclinical methods, such as thermal methods, Doppler diagnostics, and the application of markers, but they are more research-oriented or still experimental. These methods have limited application in the diagnostic process but are suitable for monitoring and comparing the obtained clinical results [
8,
11,
12].
The objective examination includes the study of the hard dental tissues, especially the dentin and pulp communication. Through it, the criteria for assessing the condition of the dental pulp are objectified, which is essential when selecting the treatment method of direct pulp capping. The inspection of the dentin and pulp communication, along with the results from other examination methods, allows us to decide between direct pulp capping and pulpectomy (partial or complete) [
13,
14]. Calcium silicate cements and autologous blood biomaterial are available as materials for direct pulp capping [
15,
16].
This study aims to monitor the results of treating reversible pulpitis using direct pulp capping with biomaterial of autologous blood A-PRF+ after 12 months.
2. Materials and Methods
In this study, 29 patients were treated. In accordance with the Helsinki Declaration II, all subjects provided informed written consent for participation. The Medical University of Sofia Research Ethics Committee approved this project (approval number: 14/12.07.2024)—a map for scientific developments and projects anticipating scientific research involving human participation.
Twenty-nine patients with a diagnosis of reversible pulpitis were treated. The treated teeth were twenty molars, eight premolars and one incisor. The operator collected anamnestic data and examined the teeth with the EPT (Scorpion, Optica Laser, Sofia, Bulgaria). Twenty-three patients with intact vestibular and lingual surfaces of the examined teeth were examined by pulse oximetry (Contec CMS 8000D, Qinhuangdao, China). An examination of the hard dental tissues was performed. Moreover, the examination of the pulp communication was performed under a microscope. Most of the infected dentin was removed using round burs and when nearing the dental pulp, chemomechanical preparation was performed using BRIX 3000(Argentina) and hand curettes(Medecy). Pulp wound haemostasis was achieved and cavity disinfection was performed using ozone gas for 18 s, a crucial step in our treatment process, using an ozone generator (Prozone, TIP TOP TIPS Sarl, Rolle, Switzerland).
2.1. Measuring the Size of the Pulp Communication
Measuring pulp communication is a complex process, primarily due to the irregular shape and size of the pulp wound. The measurement is carried out using a specialized probe, which is carefully maneuvered along the longest surface of the wound. This meticulous process allows for accurate measurement. Patients are then categorized into three groups based on the size of their pulp wound: 1. Size < 1 mm; 2. Size < 1.5 mm; 3. Size < 2.5 mm.
2.2. Pulp Communication Colour
The colour of the exposed pulp is determined as pink, light red, dark red, livid, or black.
2.3. Bleeding Time
The duration of bleeding after the communication wound is identified is important. Ozone gas and sterile cotton swabs were used to stop the bleeding.
2.4. Level of Pulp Communication Relative to the Surrounding Dentin and Its Color
The level of the dental pulp relative to the surrounding dentin is below, on or above the dentin. The color of the surrounding dentin is also recorded—whitish or slightly yellow, the presence of red or dark areas.
2.5. Method of Treatment
The chosen treatment method was direct pulp capping with biomaterial autologous blood A-PRF+ (biomaterial AB).
2.6. Protocol for the Biomaterial AB A-PRF+ and Its Placement on the Pulp Wound
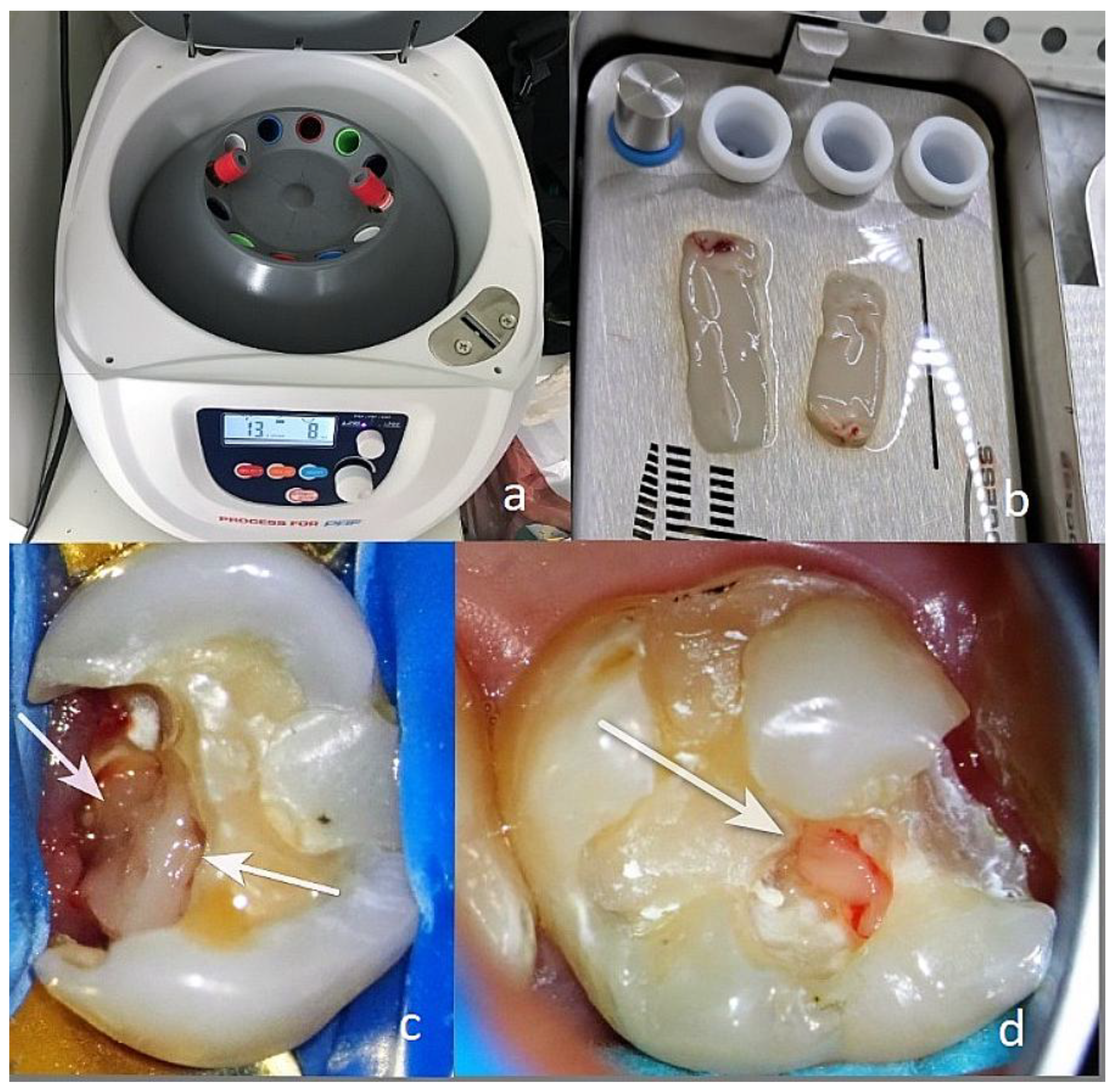
Venous blood (10 mL) was obtained from the patient via venipuncture and centrifuged at 1300 rpm for 14 min (Duo Centrifuge/Process for PRF, Nice, France). After completing the cycle, the test tubes were placed on a rack and opened for 4–5 min. The gelled part was removed, and the red blood cells were separated without cutting. The obtained gel was placed on a grid in a special box and pressed to obtain an A-PRF+ membrane. After 5–6 min, the membrane was ready. For direct pulp coverage, a portion of the membrane located next to the layer of red blood cells, known as the buffy coat, was taken [
15,
16,
17]. A biomaterial AB A-PRF+ from the patient’s blood was prepared and placed over the pulp communication according to the protocol described above. BIODENTINE
TM (Septodont, Saint-Maur-des-Fosses, France) was placed on top of it. The cavity was closed with glass ionomer cement (GIC; Fuji LC II, GC Int. Corp., Tokyo, Japan).
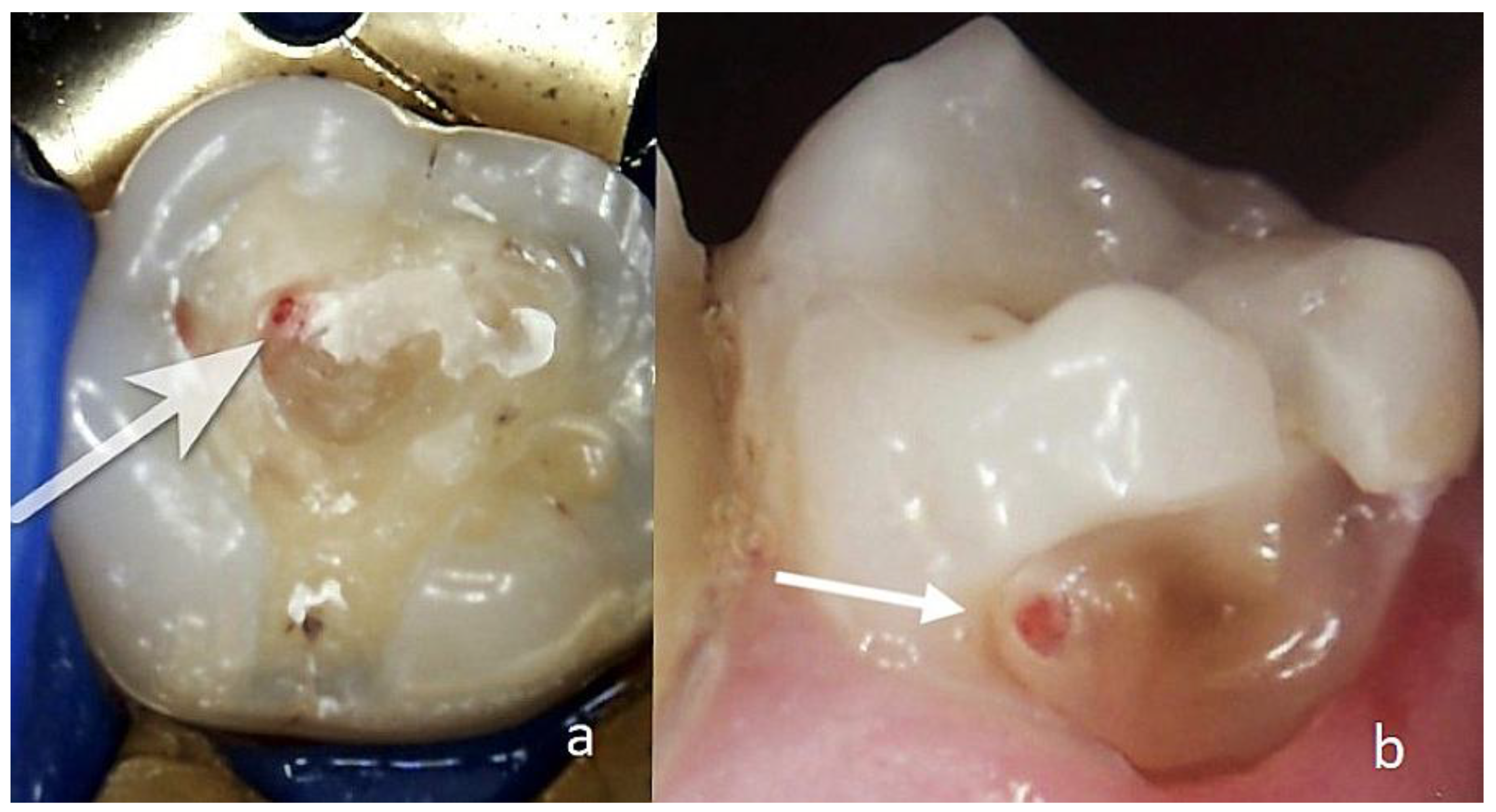
Figure 1.
(a) Centrifuge with test tubes; (b) the prepared biomaterial AB A-PRF+; (c) and (d) biomaterial AB A-PRF+ placed on the exposed dental pulp(white arrows).
Figure 1.
(a) Centrifuge with test tubes; (b) the prepared biomaterial AB A-PRF+; (c) and (d) biomaterial AB A-PRF+ placed on the exposed dental pulp(white arrows).
Follow-up examinations were performed at 1, 3, 6 months clinically with EPT. Patients with clinically pronounced pain symptoms were treated by pulpectomy within 7 days after the start of treatment.
After normalisation of the EPT data in 13 patients with a communication size < 1 after the third month, the temporary restoration was removed, reaching the formed cavity, where the A-PRF+ membrane was. The place of pulp communication was examined for the formation of reparative dentin. If such was established, the final restoration was placed with glass ionomer cement (GIC; Fuji LC II, GC Int. Corp, Tokyo, Japan) and composite resin Daimond (Kulzer, Germany). The crucial CBST examination was performed before the restoration was placed. In the remaining patients with a communication size of <1.5 and <2.5, the treatment was completed at the sixth month. The treated tooth was monitored until the 12th month.
A comprehensive analysis of cases treated with direct pulp capping using biomaterial AB A-PRF+ was conducted, examining the type of dental communication, the nature of the pulp wound, and the surrounding dentin.
The results were statistically processed.
3. Results
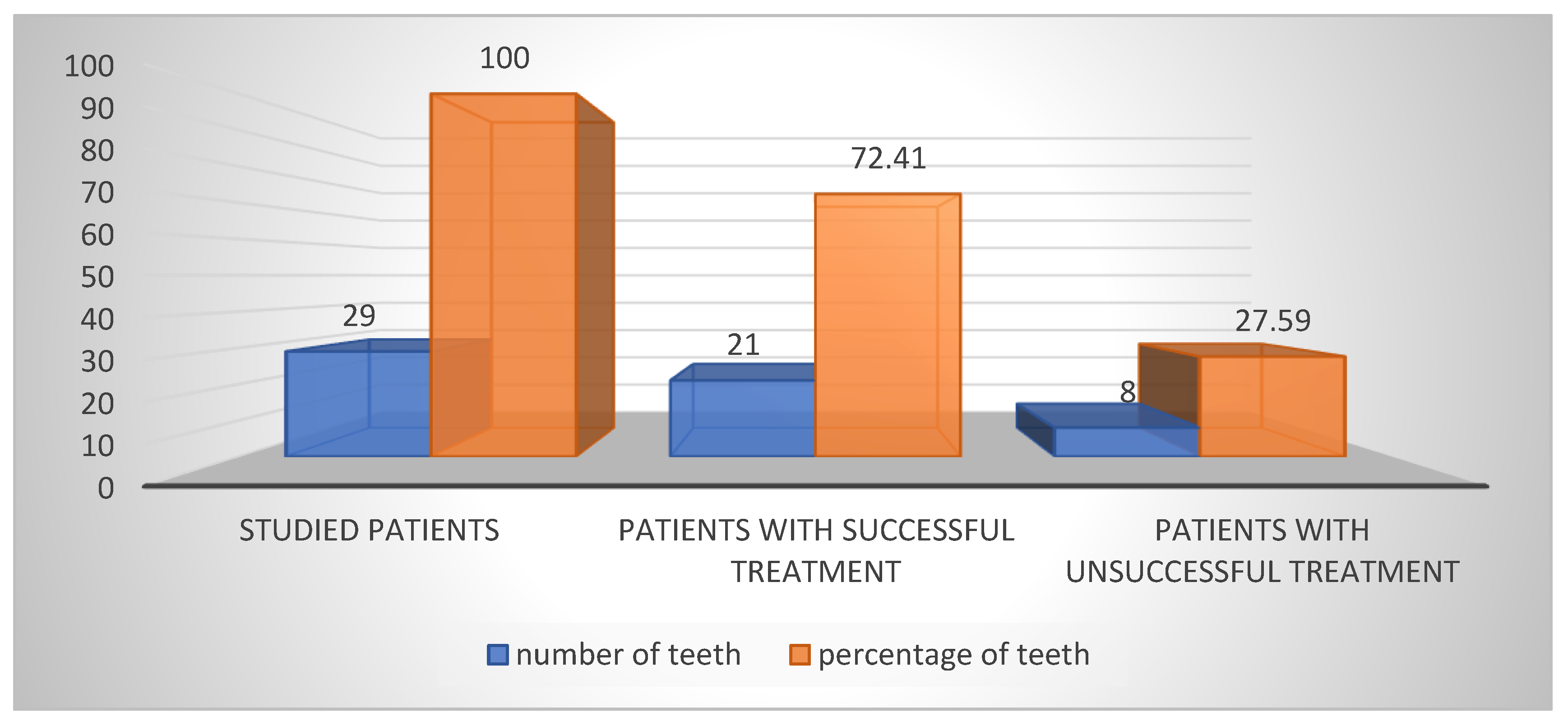
Out of the 29 patients who underwent direct pulp capping with biomaterial AB A-PRF+, 21 experienced successful treatment and maintained vitality, resulting in a 72.41% success rate. The remaining eight patients, constituting 27.59%, required further intervention. In six cases where patients reported severe pain, immediate pulpectomy was necessary within seven days to alleviate their discomfort. In the other two cases, despite the absence of pain, pulpectomy was performed after the third month due to deteriorating EPT test and pulse oximetry data (
Figure 2).
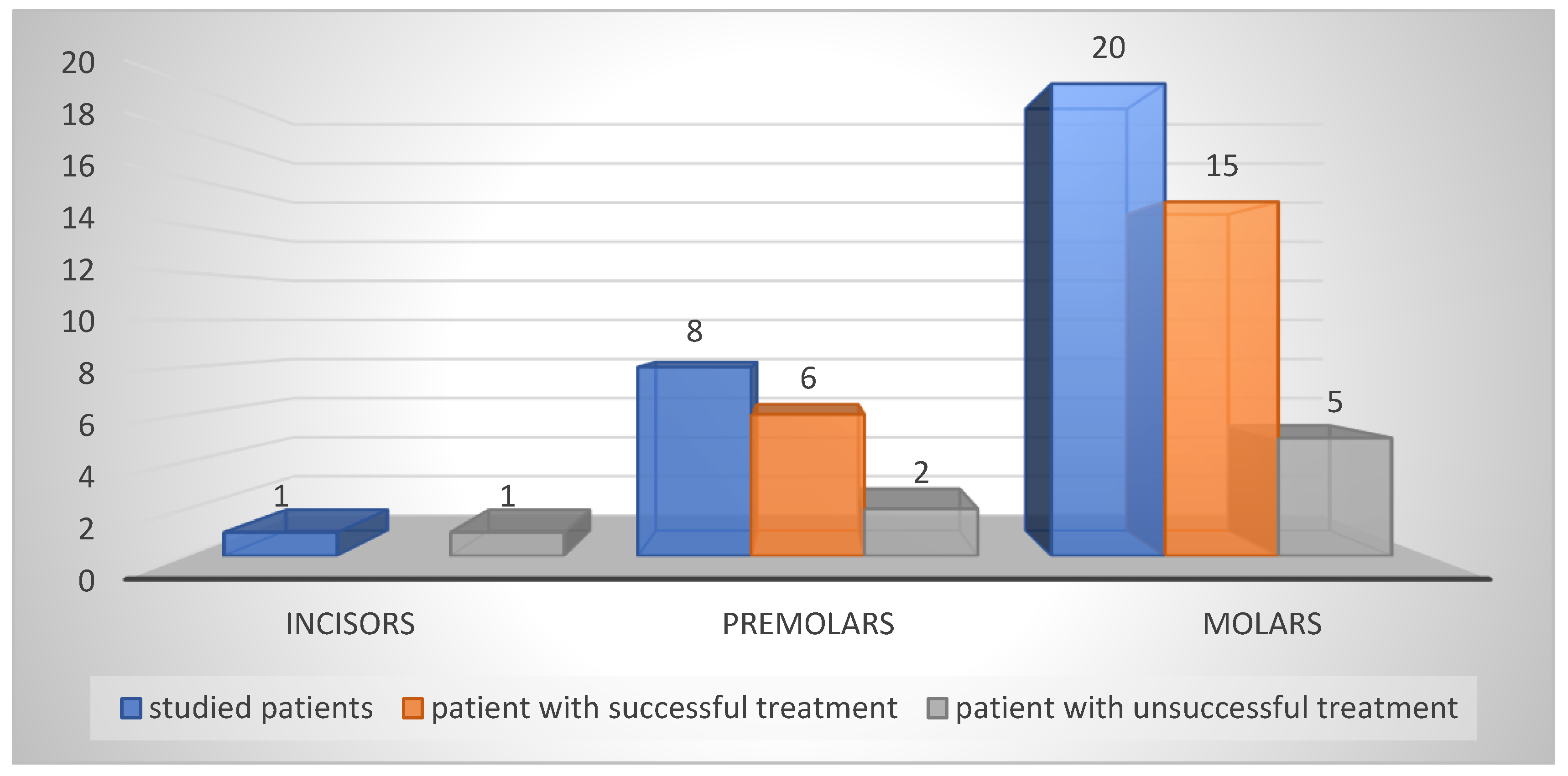
The treated teeth—by type of incisors, premolars and molars are shown in
Figure 3.
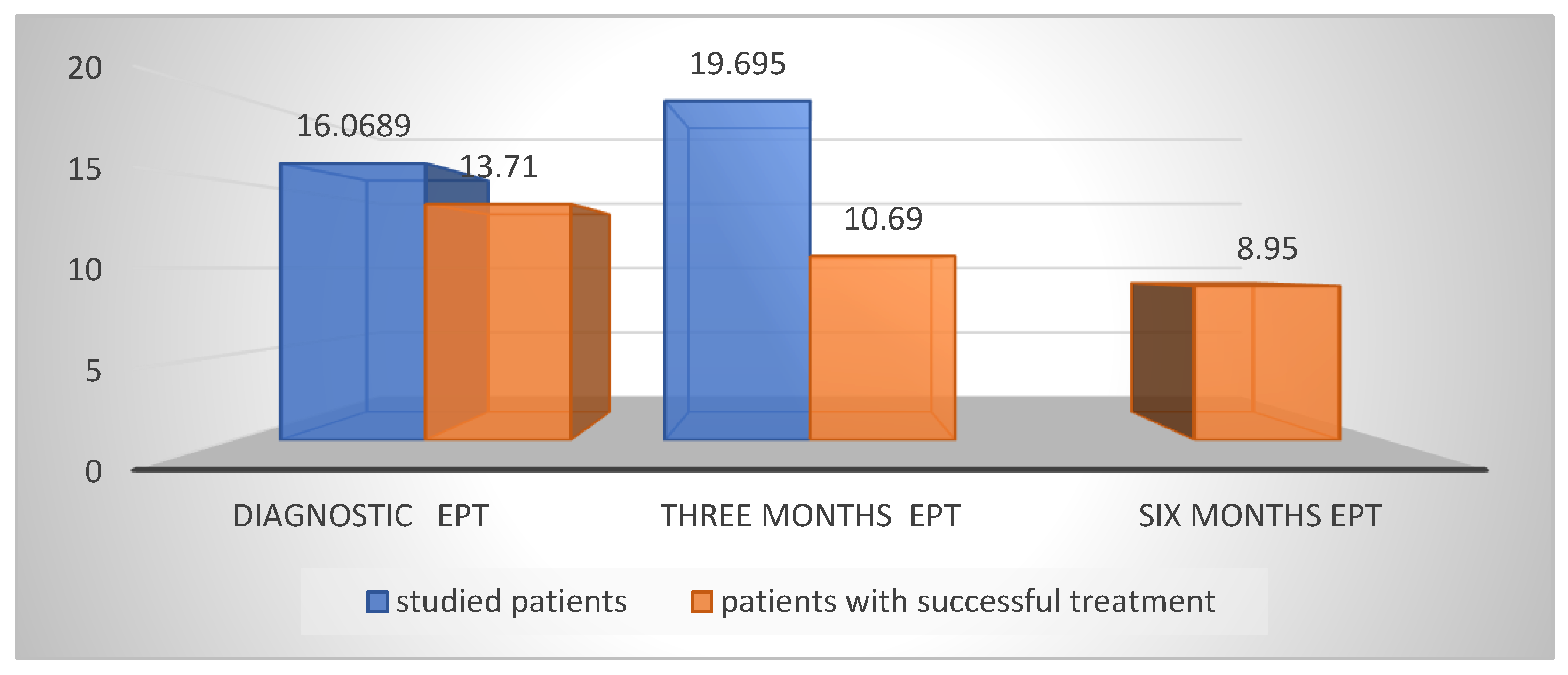
The initial data from the Mean EPT values were on average 16.058 µA; at the third month, the EPT was on average 17.218 µA. The mean increase in EPT values at the third month is likely due to the inclusion of data from both unsuccessful cases in which pulpectomy was performed. Excluding these values, the EPT at the third month was 10.69 µA (
Figure 4).
3.1. Analysis of the Type of Pulp Wound in the Diagnostic Process—Size, Bleeding, Type of Pulp Tissue, and Type of Surrounding Dentin
3.1.1. Size of the Pulp Exposure and Bleeding
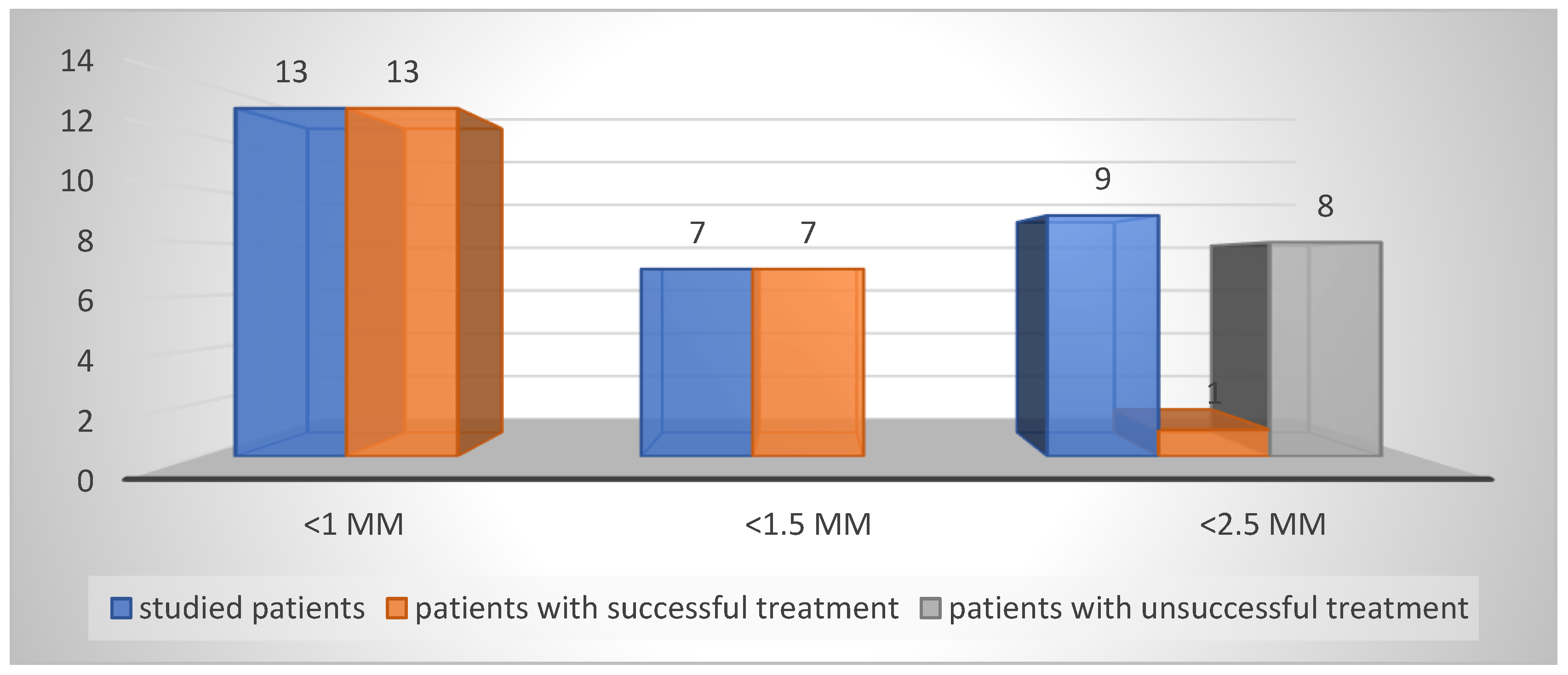
The results of determining the size of the dental pulp communication are presented in
Table 1 and
Figure 5. The results show that failures in the treatment of reversible pulpitis with direct pulp capping and biomaterial AB are almost entirely in patients with a pulp exposure size < 2.5 mm.
3.1.2. Characteristics of the Pulp Wound
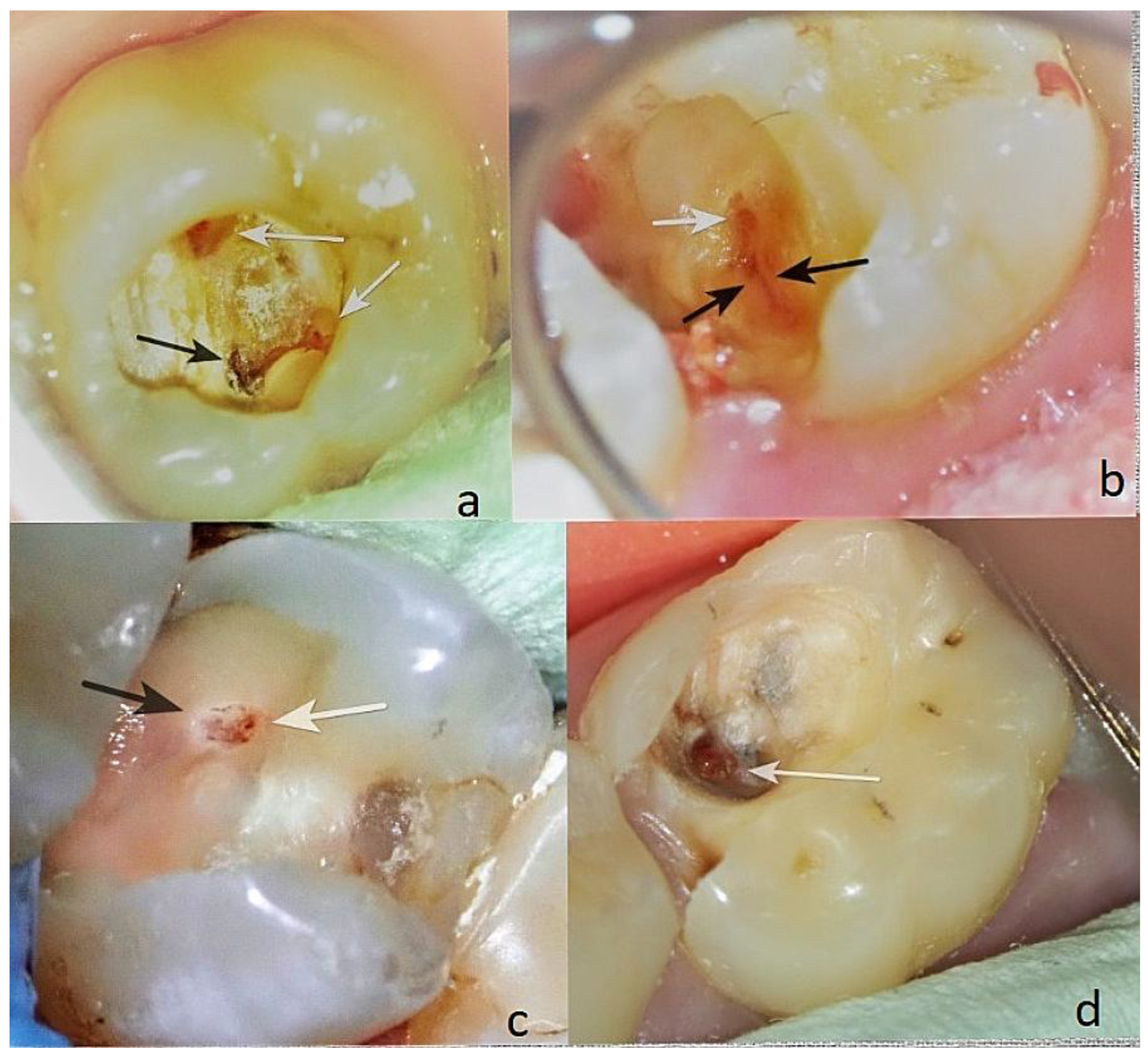
Colour. It is crucial to meticulously assess the colour of the exposed pulp—pink, light red, dark red, livid (
Figure 6(d)), or black (
Figure 6(a)). The most favourable range is from pink to red (
Figure 7), underscoring the importance of this assessment.
The pulp tissue may be at the level, below the level (
Figure 6a,c), or above the level of the surrounding dentin (
Figure 6(d)). It’s important that the pulp communication is at the level of the surrounding dentin (
Figure 7), as this facilitates cleaning and minimizes the risk of infection.
The colour of the surrounding dentin is also important. The intense redness of the dentin, as seen in
Figure 6(b),(c), is a sign of hyperaemia of the dental pulp, and such cases are not suitable for direct pulp capping.
As shown in
Figure 6(d), the dentin is dark in some areas. The pulp communication itself, measuring <1.5 mm, is dark red and is covered with a yellowish tissue fluid, likely indicative of inflammation. Dentin pieces can be seen deep within the pulp tissue. This is unfavourable, as the dentin in the pulp wound is probably infected and will adversely affect the healing process, making this case unsuitable for direct pulp capping.
The consistency of the surface of the dental pulp is most favourable when it is homogeneous and smooth or slightly grainy (
Figure 7).
We present two cases of successful treatment of reversible pulpitis through direct pulp capping with the biomaterial AB A-PRF+.
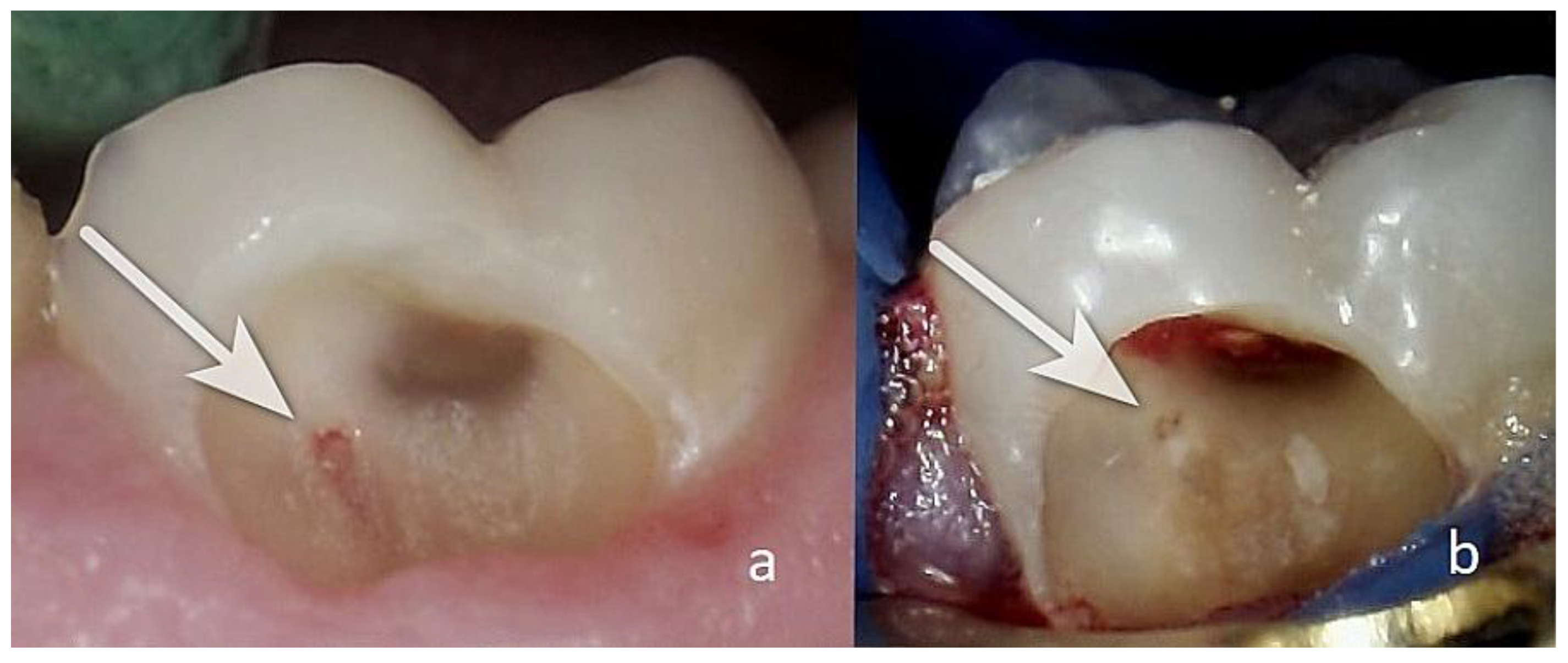
3.1.3. Clinical Case N16
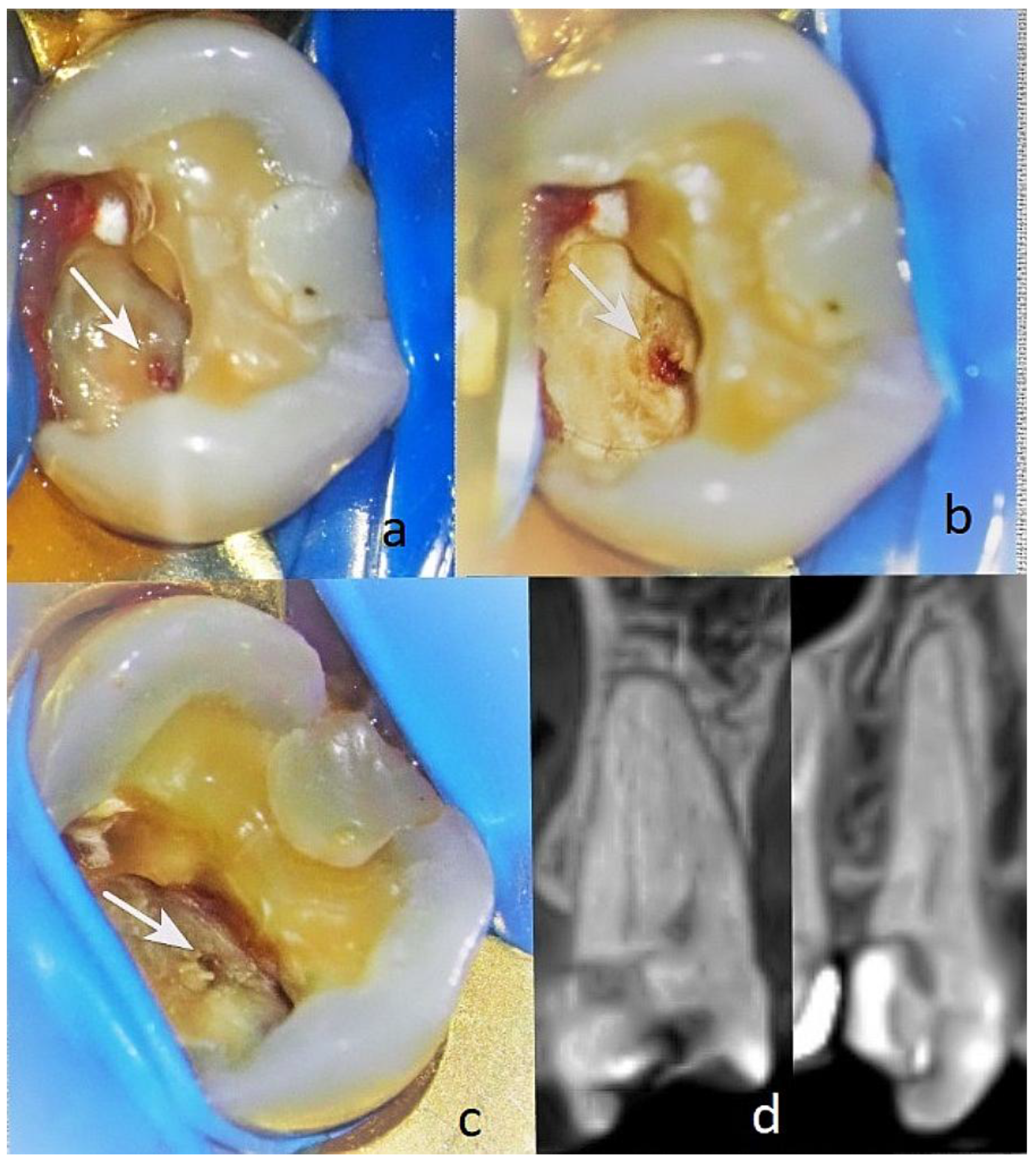
Patient AB, 65 years old. Diagnosis: reversible pulpitis of tooth 14. The patient reported symptoms of hot and cold sensitivity for one month. She identified the causative tooth and reported that, after removing the irritant, the pain subsided within 2–3 s. She also reported a spontaneous pain episode in the evening. Upon examination, a large filling was found distally and a small one mesially. During probing distally, a gap between the filling and the tooth tissue was established distally in the area of the gums with EPT -19µA at the cervical area buccally. The pulse oximetry was 87%. Through microscopic examination, the pulp communication was found to measure about <1.5 mm, and the tissue colour was pink and at the level of the surrounding dentin (
Figure 8a). Pulp wound haemostasis and cavity disinfection with ozone gas was performed for 18 s using an ozone generator (i.e.
Figure 8(b).
Control EPT testing was conducted at one, three, and six months with EPT values of 18 µA, 14 µA, and 10 µA, and the pulse oximetry measurement was 83%. In the sixth month, the Biodentine was removed, and reparative dentin deposition was found in the area of communication (
Figure 8(c)). The formed dentinal bridge was visible in the CBCT examination (
Figure 8(d)). The GIC was also removed, and a final restoration was placed.
3.1.4. Clinical Case N4
A 27-year-old female patient (RL) presented for treatment of tooth 37 with a diagnosis of reversible pulpitis (EPT 17 μA). The caries were located on the entire buccal surface, including the cervical area, as well as on the occlusal and medial surfaces of the dental crown. Upon cleaning the infected dentin from the buccal side, a communication with the dental pulp was found, approximately <1 mm in size with very slight bleeding (
Figure 9(a)). No pulse oximetry examination was performed, as preserving the buccal wall is required.
The communication was located close to the buccal vestibular pulp horn. The pulp lesion was pale pink to reddish and was on the same level as the surrounding dentin. The dentin above the pulp lesion was healthy; however, below it, there were remnants of leathery dentin.
Under anaesthesia, haemostasis of the pulp wound and disinfection of the cavity with ozone gas was performed for 18 s. The protocol for preparing the biocompatible material AB was as described above, as well as for the pulp communication. The cavity was sealed with glass ionomer cement (Fuji LC II, Japan Int. Corp.). The patient complained of spontaneous pain for the next two days, with intervals of a few minutes, without the need for medication. After 3 months, the treated tooth was asymptomatic. The paraclinical findings of the EPT were 6 μA (
Figure 9(b)). A final filling was placed. The tooth was monitored up to 12 months and showed clinically preserved vitality.
4. Discussion
Direct pulp capping is a procedure in which the communication with the dental pulp is covered with a material placed directly over the site of communication. Key factors for the success of this treatment are as follows: 1. accurate diagnosis to determine the stage of changes in the dental pulp and the type of communication with it; 2. the removal of the infected dentin with a chemicomechanical preparation; 3. treatment of the dentin wound to reduce or eliminate the number of microorganisms, using chlorhexidine, ozone gas, etc.; 4. choosing an appropriate pulp capping material; 5. preventing microleakage by sealing the cavity.
In the treatment of the presented cases, the diagnosis of the phase of the inflammatory process in the dental pulp was refined through observation under magnification of the exposed dental pulp and the surrounding dentin. The use of magnifying glasses or a dental microscope is necessary to accurately determine the condition of the dental pulp [
18].
The cases unsuitable for direct pulp capping were discussed in terms of the type of dental communication, highlighting the surrounding dentin as an important element of the diagnostic process. Our results partially coincide with those of Ricucci et al. and Bansal et al. [
18,
19].
Most authors recommend vital pulp therapy when the size of the communication is up to 1 mm. There are also recommendations to perform direct pulp capping when the size of the pulp wound is <1 mm. Authors such as Duncan and Bjørndal et al. note that further research is needed in this area (1, 2, 3). Results of our research show successful treatment in cases with pulp communication size of <1 mm to <1.5 mm. Unsuccessful treatment with direct pulp capping is observed in cases with pulp communication size of <2.5 mm.
The method used to remove the infected dentin and preserve the affected dentin is essential for treatment through direct pulp capping. In this case, we applied a chemomechanical preparation to the tissues close to the dental pulp. Our results coincide with those presented by Duncan et al. [
2,
3].
Determining the duration of bleeding from the pulp tissue is essential. Probing with a sharp instrument in the area of the suspected communication is not recommended, as pressure with the instrument, aside from causing severe pain, could damage the pulp tissue and may create a torn contused wound, which would significantly hinder the healing process. Bleeding up to 2–3 min after cleaning with a disinfectant such as chlorhexidine, 0.5–1% sodium hypochlorite or applying sterile gauze on the dentin wound without pressure is considered favourable [
1,
2,
4]. In this investigation we used ozone for bleeding control.
The plain X-ray examinations have limitations due to the two dimensional nature of the image in the visualisation of pulp communication. This method can provides information about calcifications in the dental pulp, partial or complete obliteration of the root canals and possible periodontal bone loss. According to Ricucci et al., a reduction in the volume of the dental pulp and root canals is observed with prolonged exposure to microorganisms in the dental pulp, and treatment through direct pulp capping is not promising [
4,
18].
The ozone reduces and can eliminate microorganisms in the dentin, thereby preventing their penetration into the dental pulp and subsequent inflammation. Therefore, antibacterial treatment of tissues using ozone is essential. Its effectiveness in stimulating regenerative processes in the dental pulp has been proven. Moreover, the ozone enhances the strength of dentin and stimulates the release of growth factors from pre-dentin [
20].
The introduction of biomaterials from autologous blood gives new opportunities for treatment of reversible pulpitis through direct pulp capping. The biomaterial AB A-PRF+ has been widely studied and has proven qualities for affecting dental pulp, as it stimulates its proliferation and the formation of reparative dentin [
21]. In this study, we used part of the buffy coat, as it contains the highest number of platelets and leukocytes and has a high concentration of growth factors. Recent publications have proven that the biomaterial AB also has antibacterial properties [
22].
5. Conclusions
The analysis of the pulp communication and dentin is essential for diagnosing reversible pulpitis. Examination under magnification is a powerful tool that allows for an objective assessment of the changes that have occurred in the tooth.
We found a 72.41% success rate for direct pulp capping with biomaterial AB, and this were the cases with pulp comunication up to <1.5 mm. A uniform pink or slightly reddish colour of the pulp is considered as favorable.
A pulp comunications between 1.5 mm and 2.5 mm were related with unsuccessful outcome using the same procedure. Red colouration and darkening areas of the surrounding dentin are unfavourable. The location of the pulp wound below the dentin level, and the presence of dentin fragments inside the pulp wound for direct pulp capping are also unfavourable.
According to these results the determination of pulp communication type and the condition of the surrounding dentin are crucial for the successful outcome after direct pulp capping with biomaterial AB A-PRF+.
Author Contributions
Conceptualisation, J.K.; methodology, J.K.; investigation, J.K. and D.Y.; resources, J.K.; data curation, J.K.; writing—original draft preparation, J.K. and D.Y.; writing—review and editing, J.K., D.Y.; visualisation, J.K.; supervision, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by Grant no. ZIU-MP-14/09.08.2024, BG-RRP-2.004-0004-C01. “Strategic Research and Innovation Program for the Development of MU-Sofia” funded by the European Union—Next GenerationEU.
Institutional Review Board Statement
The use of extracted teeth was approved by the Medical University–Sofia Research Ethics Committee (approval number: 14/12.07.2024). Map for scientific developments and projects anticipating scientific research involving human participation.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Biomaterial AB |
biomaterial from autologous blood
|
| EPT
|
electropulp test
|
| A-PRF+ |
advanced platelets rich fibrin+ |
| CBST |
Computer tomography |
References
- Duncan, H. F. Present status and future directions-Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55 (Suppl. 3), 497–511. [CrossRef]
- Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [CrossRef]
- Bjørndal, L.; Reit, C.; Bruun, G.; Markvart, M.; Kjaeldgaard, M.; Näsman, P.; Thordrup, M.; Dige, I.; Nyvad, B.; Fransson, H.; et al. Treatment of deep caries lesions in adults: Randomized clinical trials comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur. J. Oral Sci. 2010, 118, 290–297. [CrossRef]
- Ricucci, D.; Russo, J.; Rutberg, M.; Burleson, J.A.; Spångberg, L.S. A prospective cohort study of endodontic treatments of 1,369 root canals: Results after 5 years. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 825–842. [CrossRef]
- Alovisi, M.; Baldi, A.; Comba, A.; Gamerro, R.; Paolone, G.; Mandurino, M.; Dioguardi, M.; Roggia, A.; Scotti, N. Long-Term Evaluation of Pulp Vitality Preservation in Direct and Indirect Pulp Capping: A Retrospective Clinical Study. J. Clin. Med. 2024, 13, 3962. [CrossRef]
- Asgary, S.; Nosrat, A. Vital Pulp Therapy: Evidence-Based Techniques and Outcomes. Iran. Endod. J. 2025, 20, e2. [CrossRef]
- Sui, H.; Lv, Y.; Xiao, M.; Zhou, L.; Qiao, F.; Zheng, J.; Sun, C.; Fu, J.; Chen, Y.; Liu, Y.; et al. Relationship between the difference in electric pulp test values and the diagnostic type of pulpitis. BMC Oral Health 2021, 21, 339. [CrossRef]
- Castillo-Silva, B.E.; Alegría-Torres, J.A.; Martínez-Castañón, G.A.; Medina-Solís, C.E.; Zavala-Alonso, N.V.; Niño-Martínez, N.; Aguirre-López, E.C.; Patiño-Marín, N. Diagnostic accuracy of three placement sites for the cold test in subjects amongst different age groups. BMC Oral Health 2019, 19, 189. [CrossRef]
- Kosturkov, D. Pulse Oximetry in Dental Medicine. Ph.D. Dissertation, Medical University, Sofia, Bulgaria, 2019; 201p.
- Yovchev, D., Kirilova, J. Diagnostic imaging of chronic apical lesions. Medinform 2022, 9, 1597–1603.
- Ballal, N.V.; Duncan, H.F.; Wiedemeier, D.B.; Rai, N.; Jalan, P.; Bhat, V.; Belle, V.S.; Zehnder, M. 4-Year Pulp Survival in a Randomized Trial on Direct Pulp Capping. J. Endod. 2024, 50, 4–9. [CrossRef]
- Elfezary, M.T.; Waly, A.S.; Mohamed, E.H. Feasibility of a rapid C-reactive protein chairside point-of-care test for detecting inflammation in exposed dental pulp: A pilot exploratory study. BDJ Open 2025, 11, 51. [CrossRef]
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Chua, P.; Elamin, A.D.; Clarke, M.; El-Karim, I.A. Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 556–571. [CrossRef]
- Donnermeyer, D.; Dammaschke, T.; Lipski, M.; Schäfer, E. Effectiveness of diagnosing pulpitis: A systematic review. Int. Endod. J. 2023, 56 (Suppl. 3), 296–325. [CrossRef]
- Hirani, P.; Sarangi, S.; Chandak, M.; Patel, A.; Ikhar, A.; Naladkar, K. Innovative Pulp Preservation: The Use of Platelet-Rich Fibrin (PRF) and Mineral Trioxide Aggregate (MTA) in Treating Dental Pulp Exposure. Cureus 2024, 16, e63740. [CrossRef]
- Kirilova, J.N.; Kosturkov, D. Direct Pulp Capping with Advanced Platelet-Rich Fibrin: A Report of Two Cases. Medicina 2023, 59, 225. [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [CrossRef]
- Ricucci, D.; Siqueira, J.F.; Jr Li, Y.; Tay, F.R. Vital pulp therapy: Histopathology and histobacteriology-based guidelines to treat teeth with deep caries and pulp exposure. J. Dent. 2019, 86, 41–52. [CrossRef]
- Bansal, S.; Singla, M.; Kaur, H.; Gupta, S. Comparative evaluation of various direct pulp capping materials using different blood derivatives on disease-free tooth: A histopathological assessment case series. J. Conserv. Dent. Endod. 2025, 28, 950–954. [CrossRef]
- Veneri, F.; Filippini, T.; Consolo, U.; Vinceti, M.; Generali, L. Ozone therapy in dentistry: An overview of the biological mechanisms involved (Review). Biomed. Rep. 2024, 21, 115. [CrossRef]
- Shobana, S.; Kavitha, M.; Srinivasan, N. Efficacy of Platelet Rich Plasma and Platelet Rich Fibrin for Direct Pulp Capping in Adult Patients with Carious Pulp Exposure—A Randomised Controlled Trial. Eur. Endod. J. 2022, 7, 114–121. [CrossRef]
- Pinto, N.; Yu, J.; Koirala, S.; Mourão, C.F.; Andrade, C.; Rescigno, E.; Zamora, Y.; Pinto, D.; Quirynen, M. L-PRF in extra-oral wound care. Periodontology 2000 2025, 97, 342–362. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).