Submitted:
28 September 2025
Posted:
29 September 2025
You are already at the latest version
Abstract
Keywords:
Introduction
2. Experimental
2.1. Reagents and Instrumentation
2.2. Synthesis of Actived Biochar
2.3. Determination of Iodine and Methylene Blue Numbers
2.4. Electrochemical Properties of Biochar
3. Results and Discussion
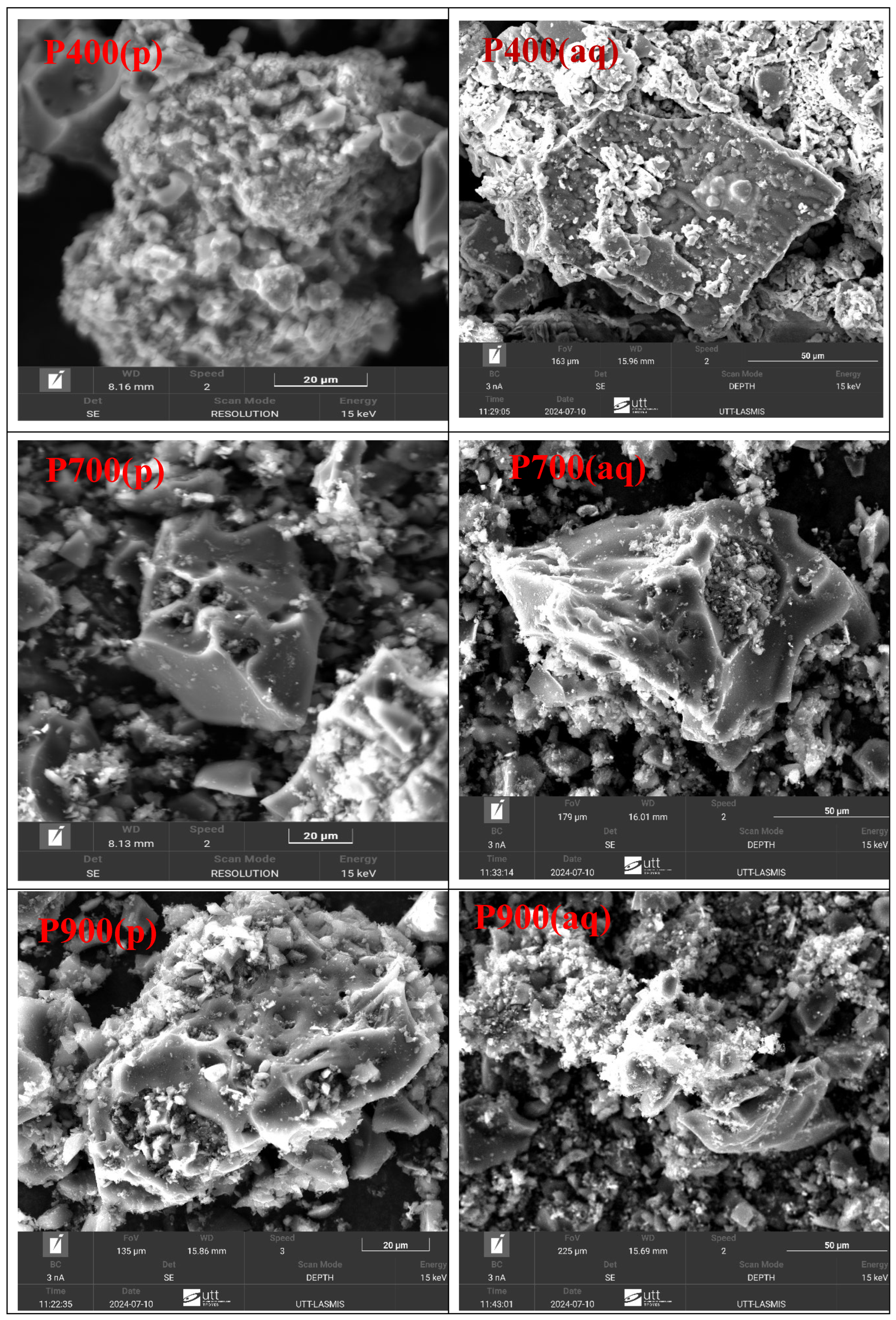
3.1. Morphology of the Biochar Powder Particles
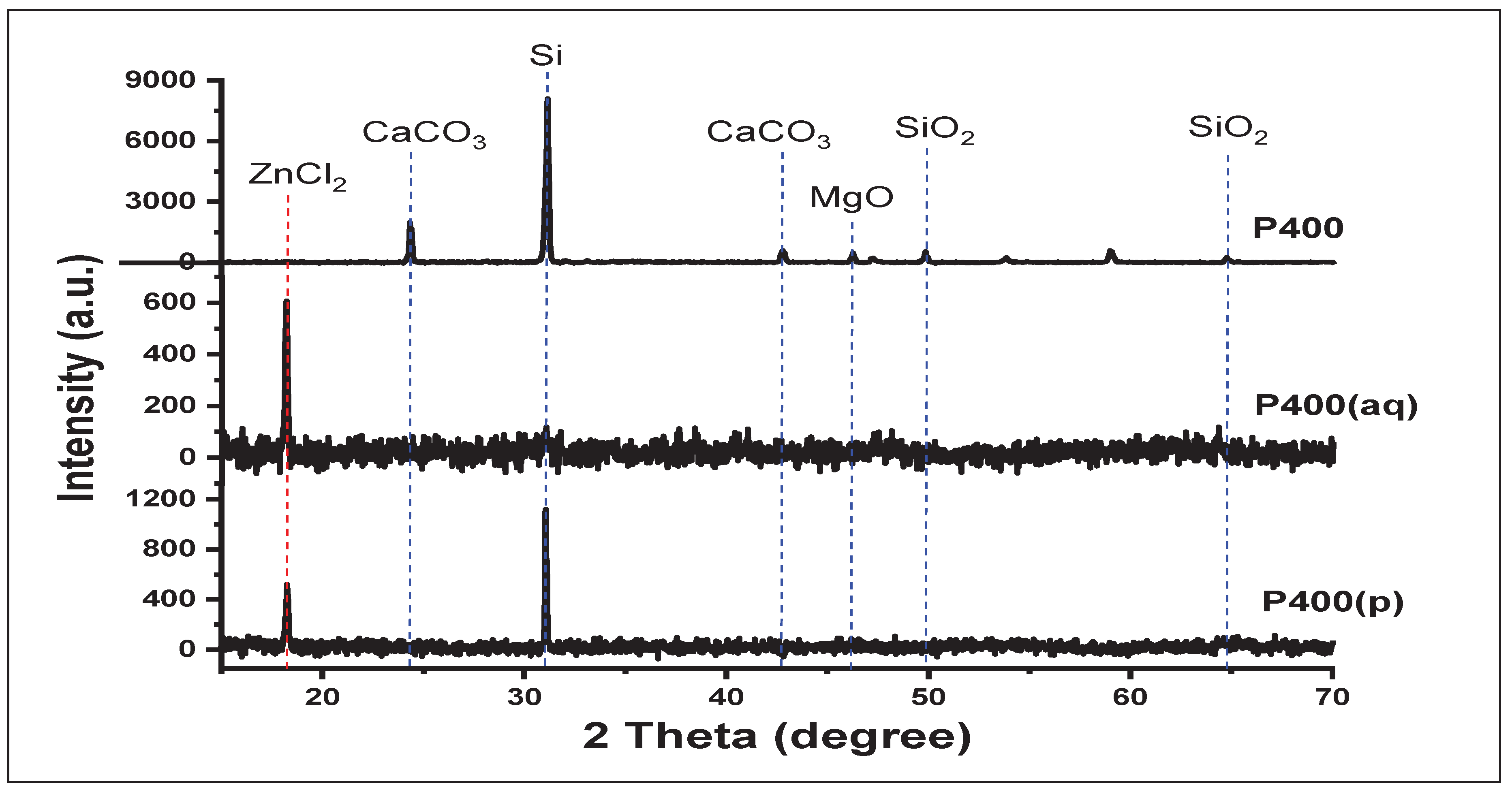
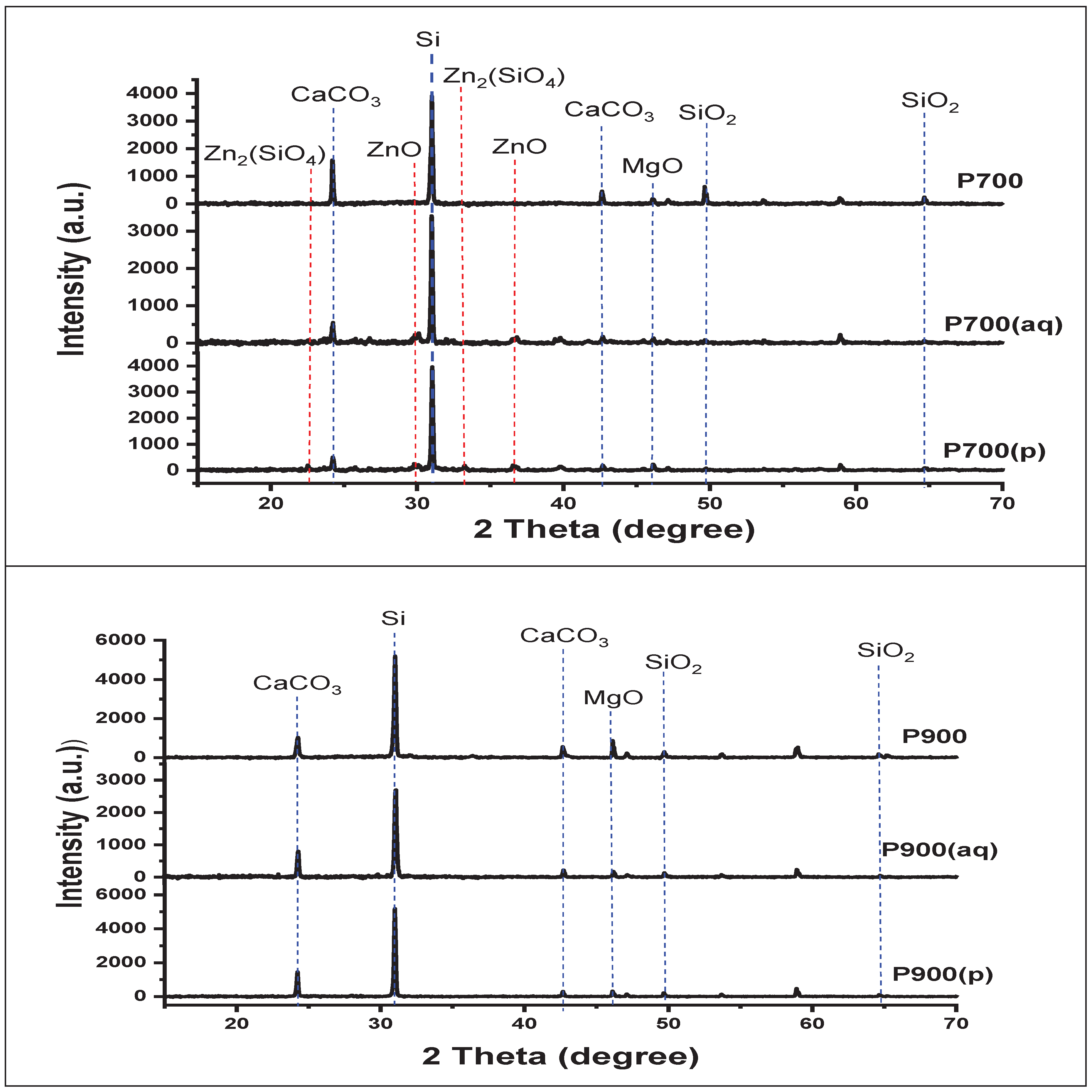
3.2. XDR Analysis
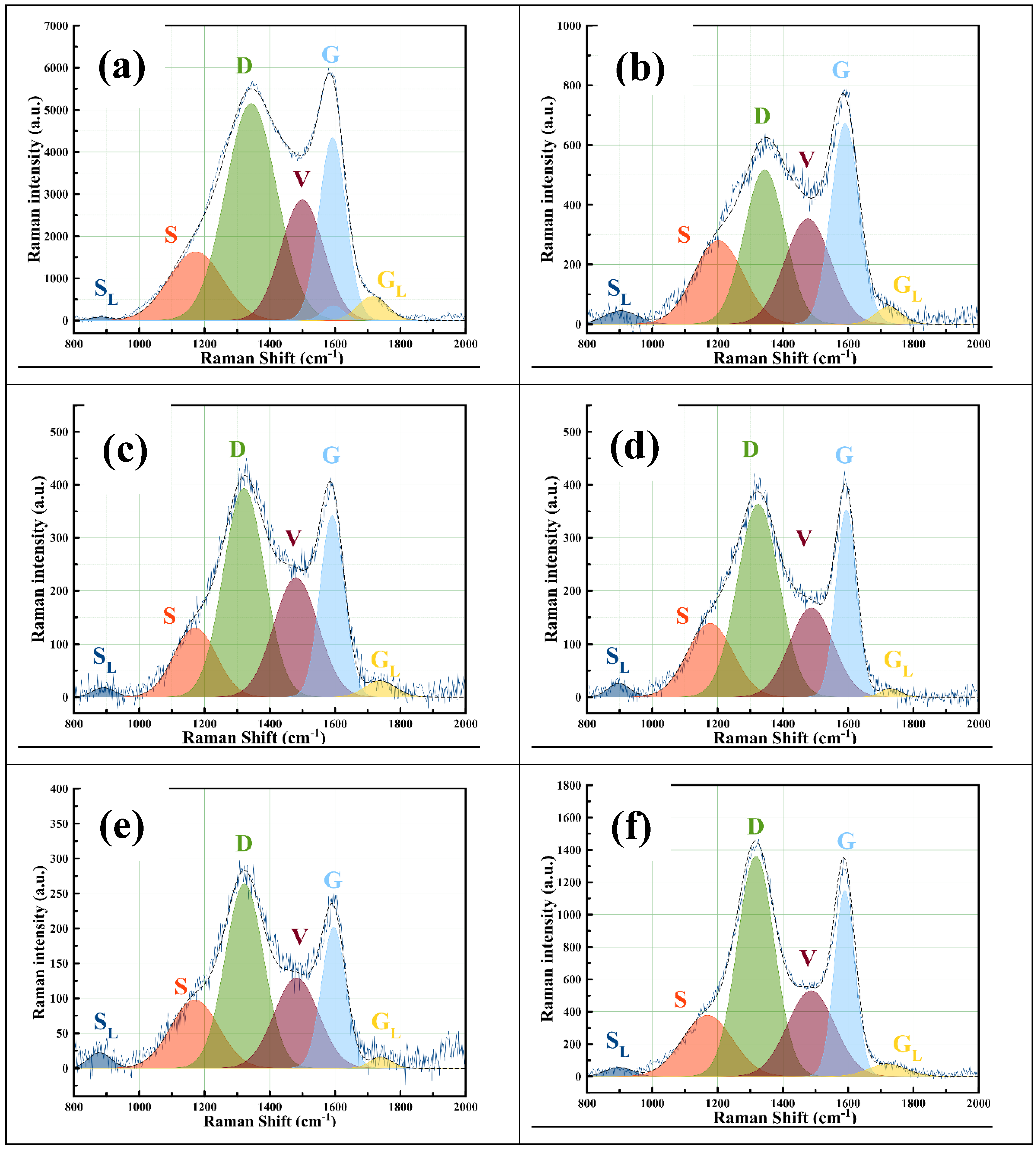
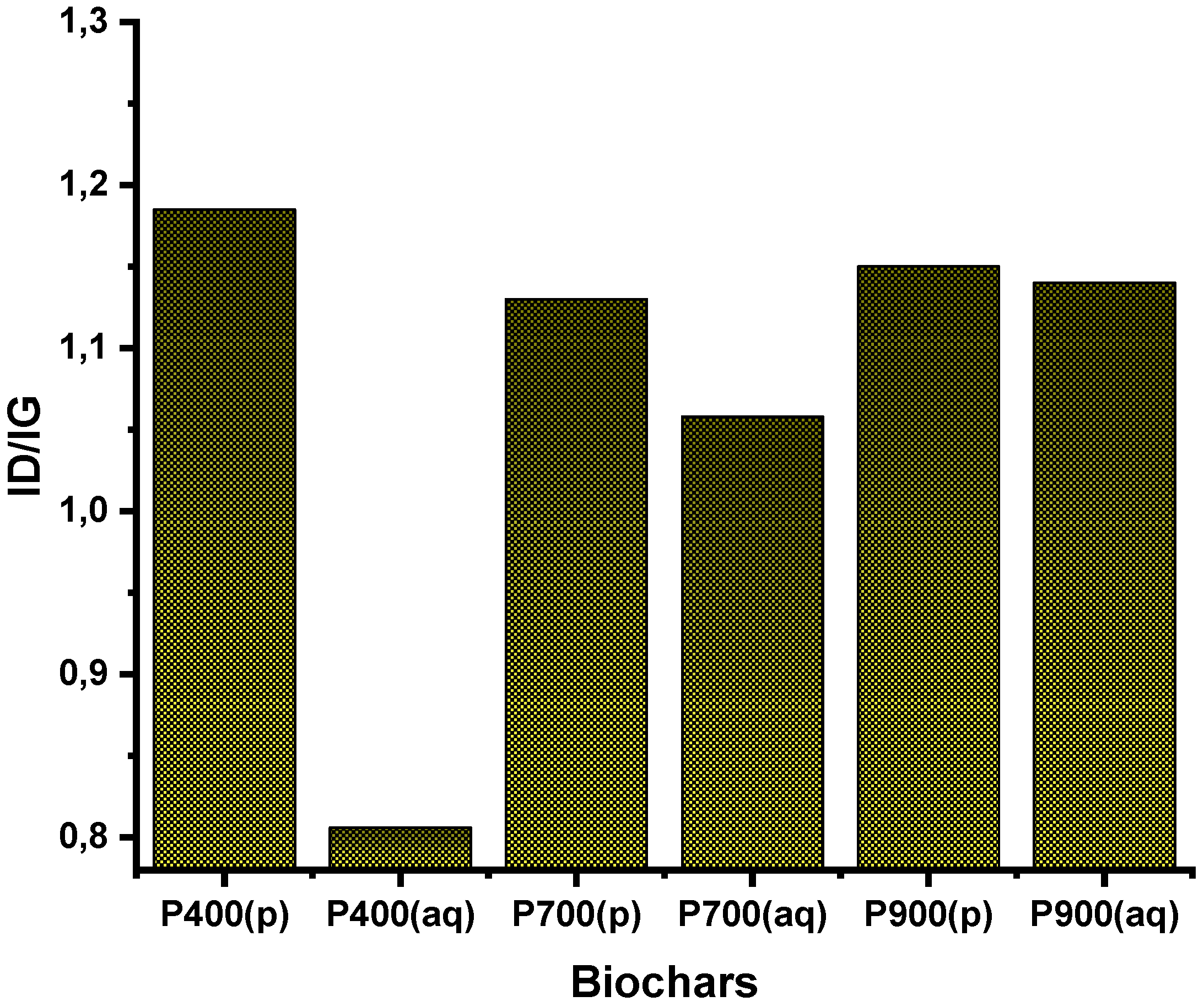
3.3. Raman Spectroscopy
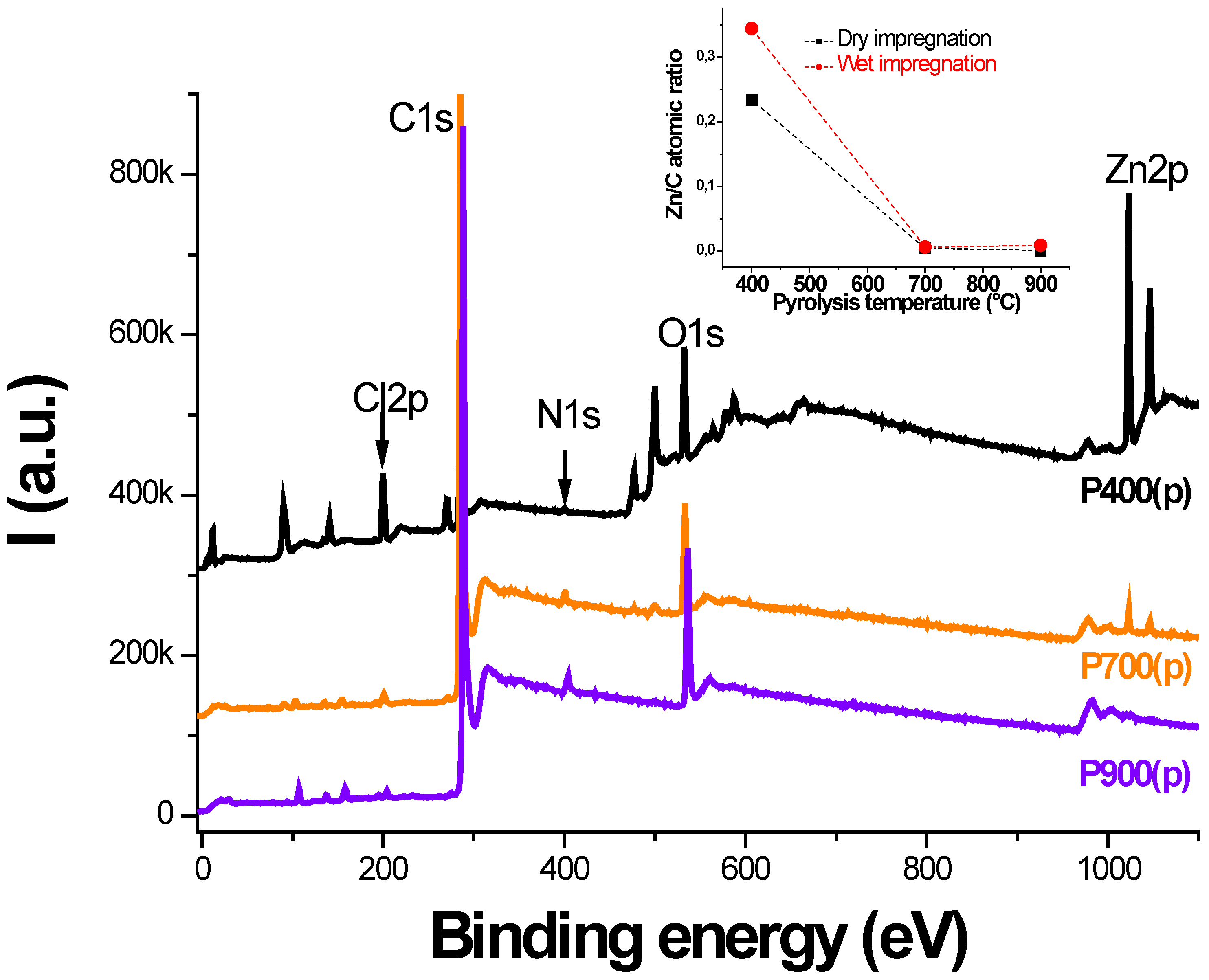
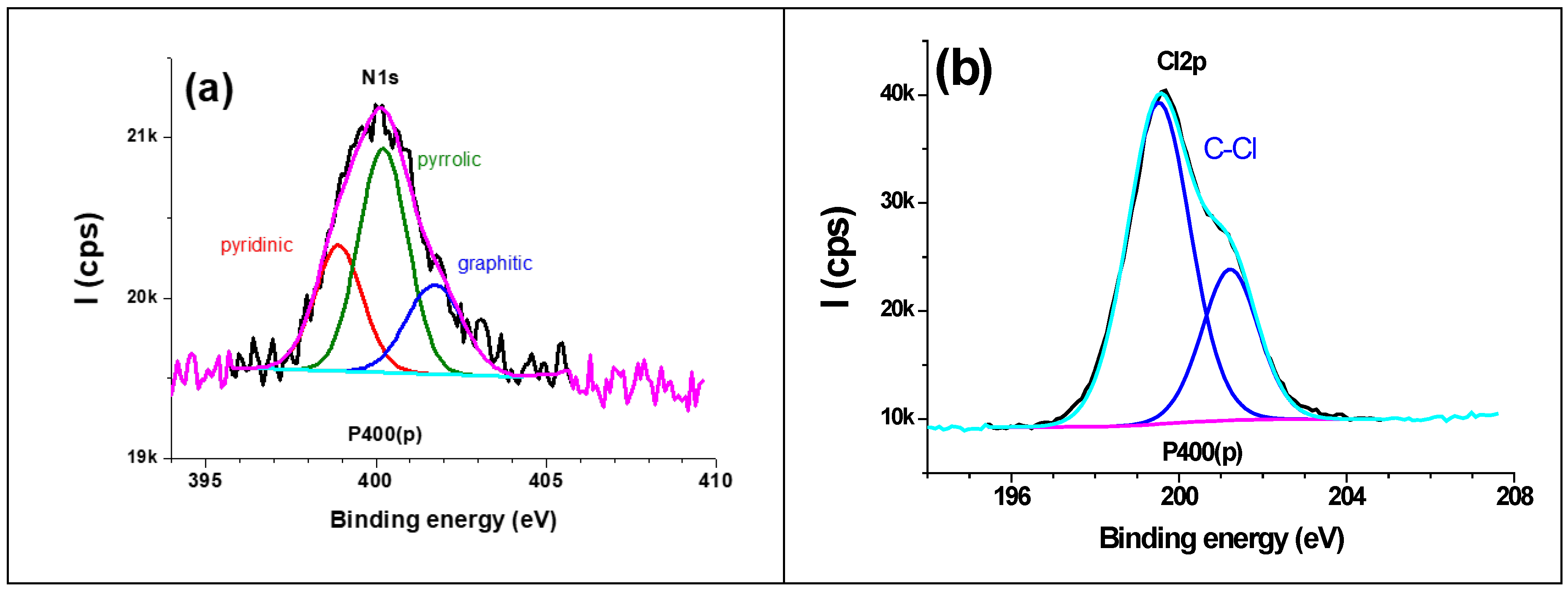
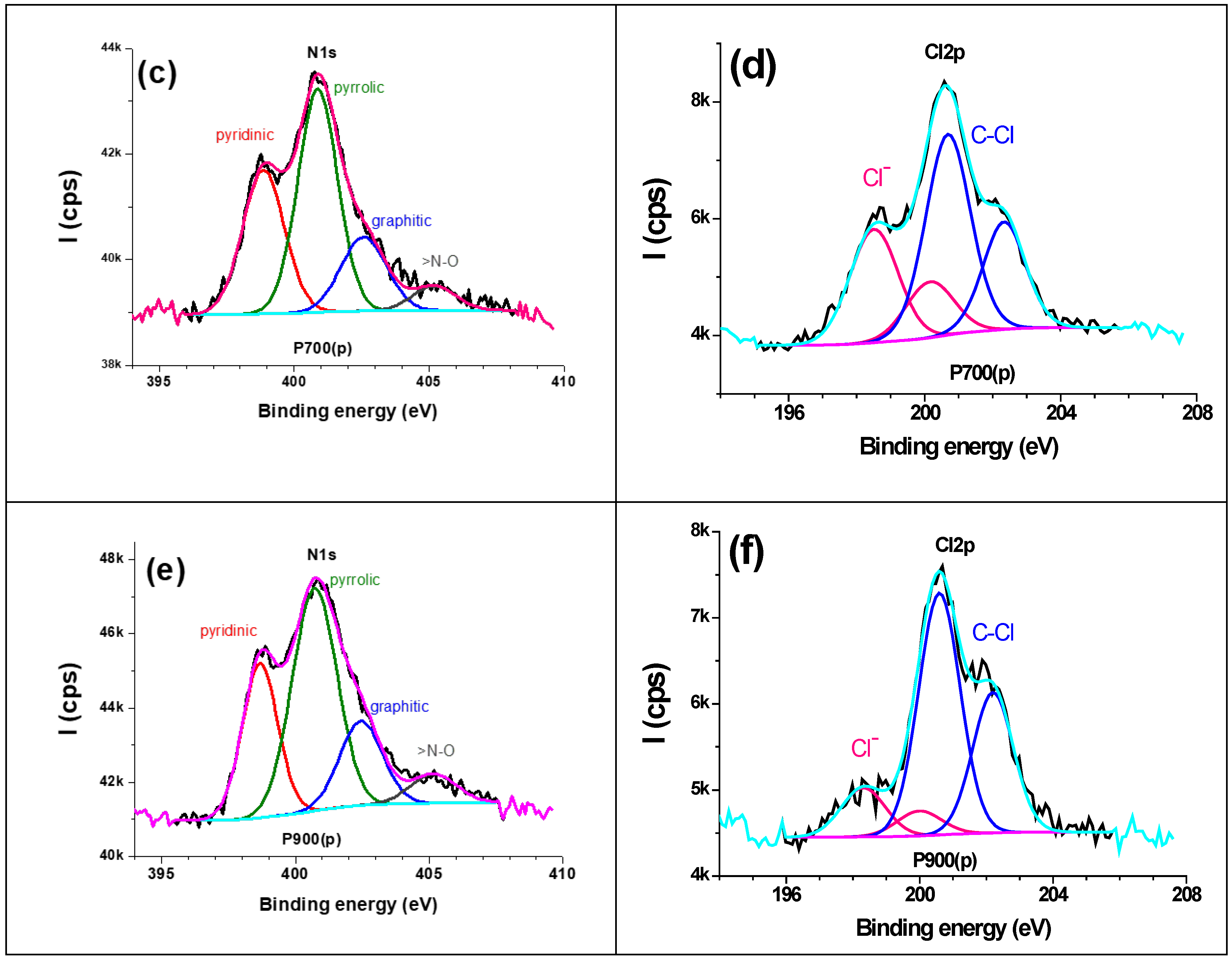
3.4. XPS
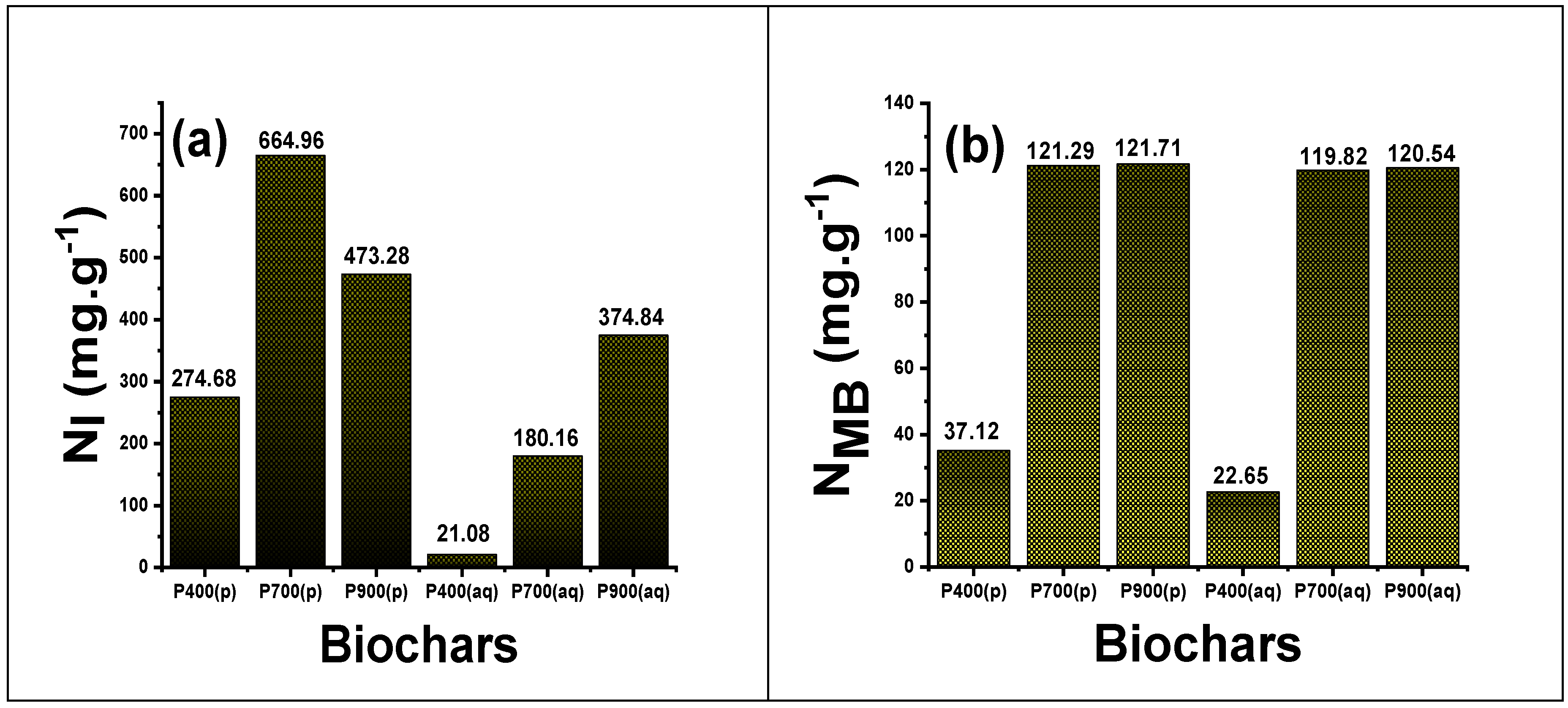
3.5. Determination of MB and Iodine Indices
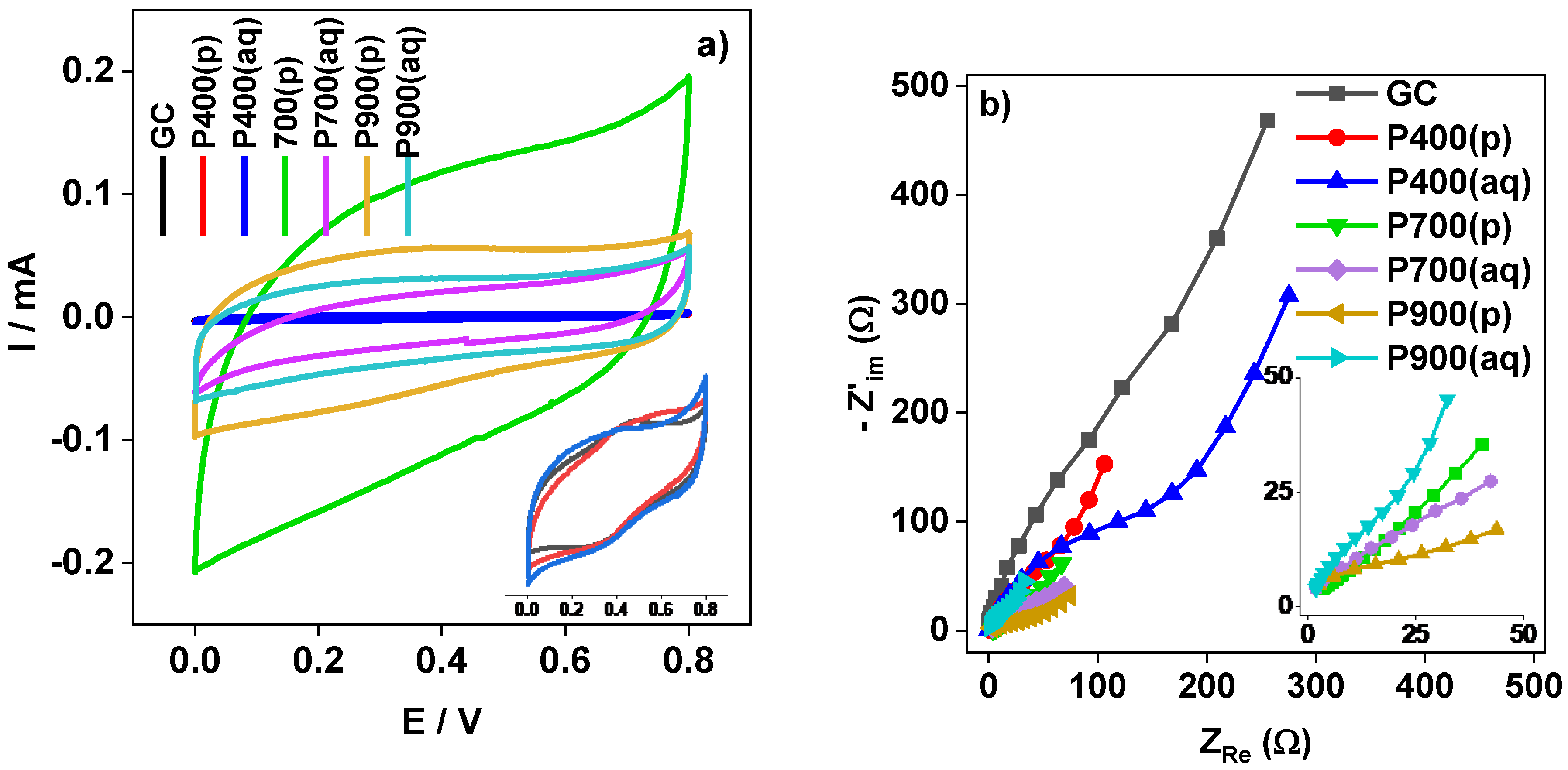
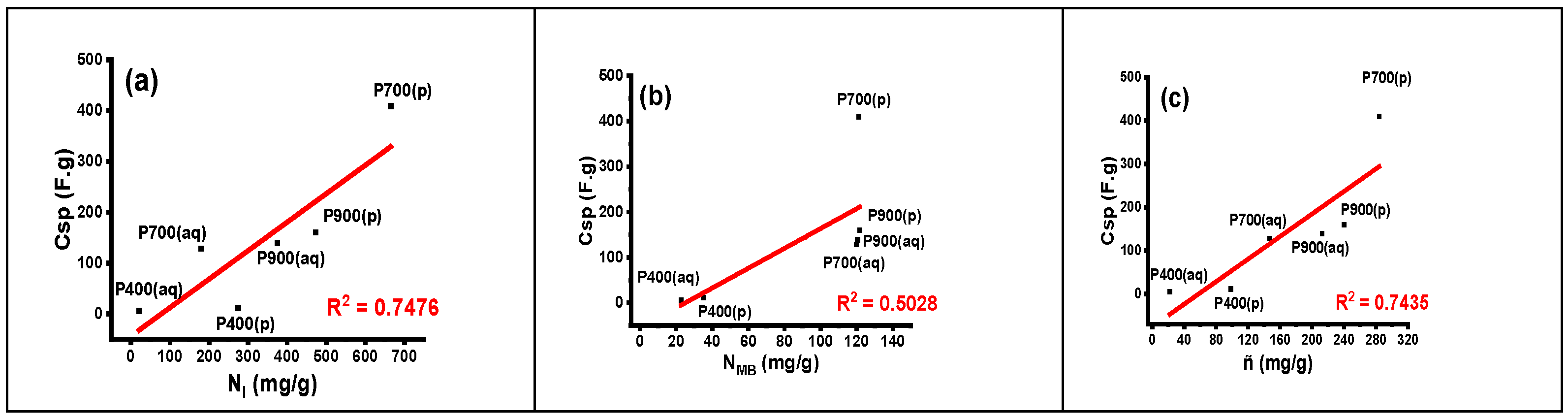
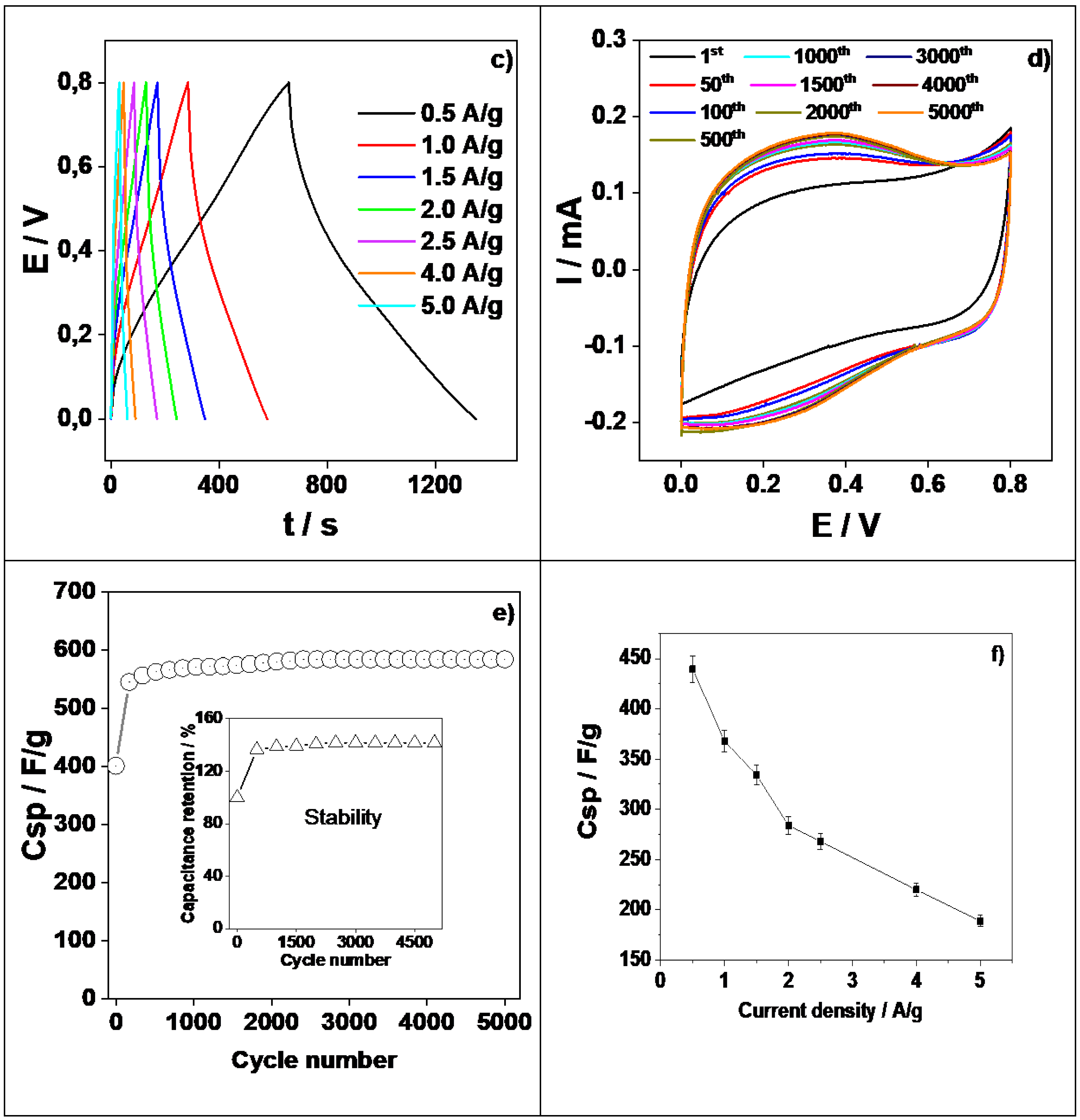
3.6. Electrochemical Characterization
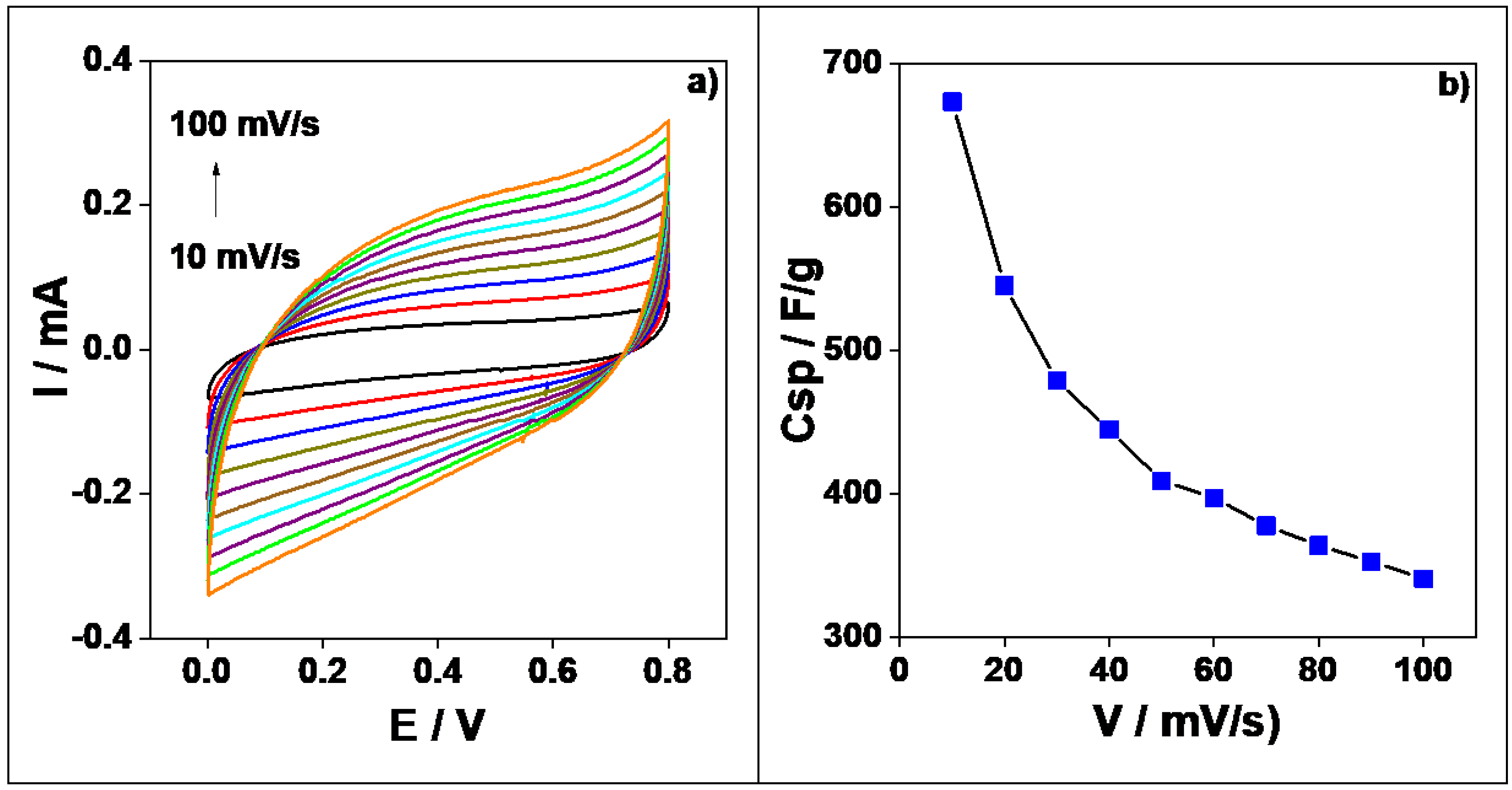
3.7. Supercapacitor Studies
Conclusion
CRediT Authorship Contribution Statement
Data Availability
Declaration of Competing Interest
References
- S. Jellali, P. S. Jellali, P. Dutournié, M. Jeguirim, Materials and clean processes for sustainable energy and environmental applications: Foreword, 26, 3-5, (2023). [CrossRef]
- A.A. Kebede, T. A.A. Kebede, T. Kalogiannis, J. V. Mierlo, M. Berecibar, A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration, Renewable Sustainable Energy Rev., 159, 112213, (2022). [CrossRef]
- M. E. Şahin, Frede Blaabjerg, A. Sangwongwanich, A Comprehensive Review on Supercapacitor Applications and Developments, Energies, 15, 674, (2022).
- S. Sharma, P. S. Sharma, P. Chand, Supercapacitor and electrochemical techniques: A brief review, Results Chem., 5, 100885, (2023). [CrossRef]
- M. A. Dar, S. R. M. A. Dar, S. R. Majid, M. Satgunam, C. Siva, S. Ansari, P. Arularasan, S. R. Ahamed, Advancements in Supercapacitor electrodes and perspectives for future energy storage technologies, I. J. Hydrogen Energy, 70, 10-28, (2024). [CrossRef]
- Z. Zhai, L. Z. Zhai, L. Zhang, T. Du, B. Ren, Y. Xu, S. Wang, J. Miao, Z. Liu, A review of carbon materials for supercapacitors, Mater. Des, 221, 111017, (2022).
- W. Chen, X. W. Chen, X. Shi, Y. Peng, Y. Wang, J. Li, Y. Wang, C. Gong, C. Dong, S. Gao, L. Xu, Z. Fang, H. Yang, Biomass pyrolysis for N-doped biochar: Relationship among preparation process, N-doped biochar properties, and supercapacitors, Fuel, 404, 136372, (2026). [CrossRef]
- F. Mahmood, M. F. Mahmood, M. Ali, M. Khan, C. F. M. Mbeugang, Y. M. Isa, A. Kozlov, M. Penzik, X. Xie, H. Yang, S. Zhang, B. Li, A review of biochar production and its employment in synthesizing carbon-based materials for supercapacitors, Ind. Crops Prod., 227, 120830, (2025). [CrossRef]
- C. A. B. Diop, M. C. A. B. Diop, M. Lo, Y. Snoussi, S. Gam-Derouich, M. E. Garah, M. Jouini, D. Gningue-Sall, M. M. Chehimi, Functional hydrochar/biochar through thermochemical conversion of millet Bran from Senegal: physicochemical, morphological and electrochemical properties, emergent mater., 8, 2663–2678, (2025). [CrossRef]
- M. Ahmad, A. U. M. Ahmad, A. U. Rajapaksha, J. E. Lim, M. Zhang, N. Bolan, D. Mohan, M. Vithanage, S. S. Lee and Y. S. Ok, « Biochar as a sorbent for contaminant management in soil and water: A review », Chemosphere vol. 99, pp. 19–33, (2014). [CrossRef]
- D. Wang, C. D. Wang, C. Li, S. J. Parikh and K. M. Scow, «Impact of biochar on water retention of two agricultural soils – A multi-scale analysis», Geoderma, vol. 340, pp.185-191, (2019). [CrossRef]
- A. Kumar, K. A. Kumar, K. Sharma and A. R. Dixit, «Carbon nanotube- and graphene-reinforced multiphase polymeric composites: review on their properties and applications», J. Mater., vol. 55, pp. 2682-2724, (2020). [CrossRef]
- M. Bassyouni, A. E. M. Bassyouni, A. E. Mansi, A. Elgabry, B. A. Ibrahim, O. A. Kassem and R. Alhebeshy, Utilization of carbon nanotubes in removal of heavy metals from wastewater: a review of the CNTs’ potential and current challenges, Appl. Phys. A: Mater. Sci. Process. Vol. 126, pp. 1-33, (2020). [CrossRef]
- X. Li, J. X. Li, J. Zhang, B. Liu, Z. Su, A critical review on the application and recent developments of post-modified biochar in supercapacitors, J. Cleaner Prod., 310, 127428, (2021). [CrossRef]
- Uppugalla, S. , Pothu, R., Boddula, R. et al. Nitrogen and sulfur co-doped activated carbon nanosheets for high-performance coin cell supercapacitor device with outstanding cycle stability. emergent mater. 6, 1167–1176 (2023). [CrossRef]
- Sandeep, A. , Ravindra, A.V. Peanut shell-derived porous carbons activated with iron and zinc chlorides as electrode materials with improved electrochemical performance for supercapacitors. emergent mater. 8, 235–250 (2025). [CrossRef]
- Gęca, M. , Khalil, A. M., Tang, M., Bhakta, A. K., Snoussi, Y., Nowicki, P., Wiśniewska, M., Chehimi, M. M. (2023). Surface treatment of biochar—methods, surface analysis and potential applications: a comprehensive review. Surfaces, 6(2), 179-213. [CrossRef]
- N. Hagemann, K. N. Hagemann, K. Spokas, H.-P. Schmidt, R. Kägi, M. A. Böhler and T. D. Bucheli, Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s ABCs, vol. 182, pp. 1-19, (2018).
- K. Velusamy, J. B. K. Velusamy, J. B. Isabel, S. Periyasamy, A. Thiruvenkadam, H. Ravikumar, S. K. Gupta, E. A. Lopez-Maldonado, Role of biochar as a greener catalyst in biofuel production: Production, activation, and potential utilization – A review, J. Taiwan Inst. Chem. Eng., 1-18, 105732, (2024). [CrossRef]
- X. Yuan, Y. X. Yuan, Y. Cao, J. Li, A. K. Patel, C.-D. Dong, X. Jin, C. Gu, A. C.K. Yip, D. C.W. Tsang, Y. S. Ok, Recent advancements and challenges in emerging applications of biochar-based catalysts, Biotechnol. Adv., 67, 108181, (2023). [CrossRef]
- X. Zhou, Y. X. Zhou, Y. Zhu, Q. Niu, G. Zeng, C. Lai, S. Liu, D. Huang, L. Qin, X. Liu, B. Li, H. Yi, Y. Fu, L. Li, M. Zhang, C. Zhou, J. Liu, New notion of biochar: A review on the mechanism of biochar applications in advannced oxidation processes, Chem. Eng. J. 416, 129027, (2021). [CrossRef]
- H. I. Abdu, S. H. I. Abdu, S. Adnan, T. Aboudou, Y. Guo, L. N. nian, L. Xiaowei, L. Ziyu, K. Eid, Hierarchical porous carbon biochar nanotubes encapsulated metal nanocrystals with a strong metal-carbon interaction for high-performance supercapacitors, Ultrason. Sonochem., 107476, (2025). [CrossRef]
- R. M. A. P. Lima, G. S. R. M. A. P. Lima, G. S. dos Reis, M. Thyrel, J. J. Alcaraz-Espinoza, S. H. Larsson, H. P. de Oliveira, Facile Synthesis of Sustainable Biomass-Derived Porous Biochars as Promising Electrode Materials for High-Performance Supercapacitor Applications. Nanomaterials, 2022; 12, 866. [Google Scholar] [CrossRef]
- J. Sun, L. J. Sun, L. E, C. Ma, S. Luo, Z. Wu, W. Li, S. Liu, Effects of the Pore Structure of Commercial Activated Carbon on the Electrochemical Performance of Supercapacitors, K. Zhang, J. Energy Storage, 45, 103457, (2022). [CrossRef]
- T. Jamnongkan, N. T. Jamnongkan, N. Intaramongkol, N. Kanjanaphong, K. Ponjaroen, W. Sriwiset, R. Mongkholrattanasit, P. Wongwachirakorn, K.-Y. A. Lin, C.-F. Huang, Study of the Enhancements of Porous Structures of Activated Carbons Produced from Durian Husk Wastes, Sustainability, 14, 5896, (2022). [CrossRef]
- Porosity Analysis of Acacia catechu Seed-derived Carbon Materials Activated with Sodium Hydroxide and Potassium Hydroxide: Insights from Methylene Blue and Iodine Number Methods, P. K. Porosity Analysis of Acacia catechu Seed-derived Carbon Materials Activated with Sodium Hydroxide and Potassium Hydroxide: Insights from Methylene Blue and Iodine Number Methods, P. K. Mishra, S. Aryal, H. B. Oli, T. Shrestha, D. Jha, R. L. (Swagat) Shrestha, D. Pr. Bhattarai, J. Nepal Chem. Soc., 45, 57-65, (2025). [CrossRef]
- C. A. Nunes, M. C. C. A. Nunes, M. C. Guerreiro, Estimation of surface area and pore volume of activated carbons by methylene blue and iodine numbers, Quim. Nova, 34, 3, 472-476, (2011). [CrossRef]
- C. Du, B. C. Du, B. Liu, J. Hu, H. Li, Determination of iodine number of activated carbon by the method of ultraviolet–visible spectroscopy. Mater. Lett., 285, 129137, (2021). [CrossRef]
- M. Lo, M. M. Lo, M. Tang, D. Faye, V. Vaiyapuri, A. Jayaram, N. Mani, M. Jouini, M. M. Chehimi, Silver-modified sugarcane bagasse biochar-based electrode materials for the electrochemical detection of mercury ions in aqueous media, Electrochimica Acta, 540, 147214, (2025). [CrossRef]
- S. Umino and J. Newman, Diffusion of sulfuric acid in concentrated solutions, J. Electrochem. Soc. 140, 2217-2221, (1993). [CrossRef]
- D. Faye, M. D. Faye, M. Lo, D. Seye, C.A.B. Diop, M.G. Diop, A. Ngom, M. Dieng, A.K. Bhakta, D.G. Sall, M.M. Chehimi, A. Koné, Silver Nanoparticles Supported by Carbon Nanotubes Functionalized with 1, 2, 3-Benzenetricarboxylic Acid: Spectroscopic Analysis and Electrochemical Capacitance, J. Inorg Organomet Polym Mater. 1-14 (2025). [CrossRef]
- D. Thomas, N. B. D. Thomas, N. B. Fernandez, M. D. Mullassery and R. Surya, Iron oxide loaded biochar/polyaniline nanocomposite: Synthesis, characterization and electrochemical analysis, Inorg. Chem. Com., 119, 1-10, (2020). [CrossRef]
- B. Li, C. B. Li, C. Li, D. Li, L. Zhang, S Zhang, Z. Cui, D. Wang, Y. Tang, X. Hu, Activation of pine needles with zinc chloride: Evolution of functionalities and structures of activated carbon versus increasing temperature, Fuel Process. Technol., 252, 107987, (2023). [CrossRef]
- L. Yan, Y. L. Yan, Y. Liu, Y. Zhang, S. Liu, C. Wang, W. Chen, C. Liu, Z. Chen, Y. Zhang, ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline, Bioresource Technology, 297, 122381, (2020). [CrossRef]
- J. Zhao, D. J. Zhao, D. Zhou, J. Zhang, F. Li, G. Chu, M. Wu, B. Pan, C. E.W. Steinber, The contrasting role of minerals in biochars in bisphenol A and sulfamethoxazole sorption, J. Pre-proof, 264, 128490, (2021). [CrossRef]
- D. Xia, F. D. Xia, F. Tan, C. Zhang, X. Jiang, Z. Chen, H. Li, Y. Zheng, Q. Li, Y. Wanga, ZnCl2-activated biochar from biogas residue facilitates aqueous As(III) removal, Appl. Surf. Sci., 377, 361-369, (2016). [CrossRef]
- A.S. Yusuff, M. A. A.S. Yusuff, M. A. Lala, K. A. Thompson-Yusuff, E. O. Babatunde, ZnCl2-modified eucalyptus bark biochar as adsorbent: preparation, characterization and its application in adsorption of Cr(VI) from aqueous solutions, South Afr. J. Chem. Eng., 42, 138–145, (2022). [CrossRef]
- S. Minaei, K. Z. S. Minaei, K. Z. Benis, K. N. McPhedran, J. Soltan, Evaluation of a ZnCl2-modified biochar derived from activated sludge biomass for adsorption of sulfamethoxazole, Chem. Eng. Res. Des., 190, 407–420, (2023). [CrossRef]
- S. Wei, Q. S. Wei, Q. Qin, Z. Liu, Thermal behavior analysis and reaction mechanism in the preparation of activated carbon by ZnCl2 activation of bamboo fibers, J. Anal. Appl. Pyrolysis, 179, 106500, (2024). [CrossRef]
- M. W. Smith, I. M. W. Smith, I. Dallmeyer, T. J. Johnson, C. S. Brauer, J.-S. McEwen, J. F. Espinal, M. Garcia-Perez, Structural analysis of char by Raman spectroscopy: Improving band assignments through computational calculations from first principles, Carbon, 100, 678-692, (2016). [CrossRef]
- M. Ayiania, E. M. Ayiania, E. Weiss-Hortala, M. Smith, J.-S. McEwen and M. Garcia-Perez, Microstructural analysis of nitrogen-doped char by Raman spectroscopy: Raman shift analysis from first principles, Carbon, vol. 167, pp. 559-574, 2020. [CrossRef]
- C. Wan, H. C. Wan, H. Li, L. Zhao, Z. Li, C. Zhang, X. Tan, X. Liu, Mechanism of removal and degradation characteristics of dicamba by biochar prepared from Fe-modified sludge, J. Environ. Manage., 299, 113602, (2021). [CrossRef]
- Leng, L. , Xu, S., Liu, R., Yu, T., Zhuo, X., Leng, S.,... & Huang, H. Nitrogen containing functional groups of biochar: An overview. Bioresource Technol., 298, 122286 (2020). [CrossRef]
- Jansen, R. J. J. , & Van Bekkum, H.. XPS of nitrogen-containing functional groups on activated carbon. Carbon, 33, 1021-1027 (1995). [CrossRef]
- Q. Shi, X. Q. Shi, X. Zhang, B. Shen, K. Ren, Y. Wang, J. Luo, Enhanced elemental mercury removal via chlorine-based hierarchically porous biochar with CaCO3 as template, Chem. Eng. J., 406, 126828, (2021). [CrossRef]
- Z. Song, T. F. Z. Song, T. F. Donald, W. Kirk, C. Q. Jia, Electrochemical Performance of Pre-Modified Birch Biochar Monolith Supercapacitors by Ferric Chloride and Ferric Citrate, Batteries, 11, 47, (2025).
- Igor, V. Esarev, D. V. Agafonov, Y. V. Surovikin, S. N. Nesov, A. V. Lavrenov, On the causes of non-linearity of galvanostatic charge curves of electrical double layer capacitors, Electrochim. Acta, 390, 138896, (2021). [CrossRef]
- M. Omidvar, S. M. Omidvar, S. Dalvand, A. Asghari, N. Yazdanfar, HY Sadat, N. Mohammadi, Fabrication of an efficient supercapacitor based on defective mesoporous carbon as electrode material utilizing Reactive Blue 15 as novel redox mediator for natural aqueous electrolyte, Fuel, 347, 128472, (2023). [CrossRef]
- B. A. Hemdan, D. A. B. A. Hemdan, D. A. Jadhav, A. Dutta, P. Goswami, Facilitating the electrochemical characterization and biofilm enrichment through anode modification in microbial fuel cells, J. Water Process Eng., 54, 104065, (2023). [CrossRef]
- Y. Lia, D. Y. Lia, D. Zhang, Y. Zhang, J. He, Y. Wang, K. Wang, Y. Xu, H. Li, Yi Wang, Biomass-derived microporous carbon with large micropore size for high-performance supercapacitors, J. Power Sources, 448, 227396, (2020). [CrossRef]
- H. Noh, Y. H. Noh, Y. Park, A. Bhadouria, B. M. Tackett, Effects of electrochemical active surface area of Cu on electrochemical CO reduction in acidic electrolyte using Cu nanoparticles on surfactant-treated carbon, J. Catalysis, 437, 115662, (2024). [CrossRef]
- Z. Ouyang, Y. Z. Ouyang, Y. Lei, Y. Chen, Z. Zhang, Z. Jiang, J. Hu, Y. Lin, Nanoscale Res. Lett. 2019; 14, 1–9. [Google Scholar]
- S.A. Mousavianfard, A. S.A. Mousavianfard, A. Molaei, M. Manouchehri, A. Foroozandeh, A. Shahmohammadi, S. Dalvand, J. Energy Storage. 109, 115232 (2025).
- Hsia, B. , Kim, M. S., Carraro, C., & Maboudian, R. Cycling characteristics of high energy density, electrochemically activated porous-carbon supercapacitor electrodes in aqueous electrolytes. Journal of Materials Chemistry A, 1(35), 10518-10523 (2013). [CrossRef]
- T. Zhu, F. T. Zhu, F. Tian, J. Wu, H. Li, D. Chen, R. Qi, Y. Niu, F. Zhang, Z. Yang, Synthesis of various nanostructured N-doped Cu7S4 materials for aqueous supercapacitors, Electrochim. Acta, 511, 145396, (2025). [CrossRef]
- Wang, Z. , Pan, S., Wang, B., Qi, J., Tang, L., & Liu, L. Asymmetric supercapacitors based on Co3O4@MnO2@PPy porous pattern core-shell structure cathode materials. J. Electrochem. Sci. Technol. 12, 346-357 (2021). [CrossRef]
- Ye, Z. , Wang, F., Jia, C., Mu, K., Yu, M., Lv, Y., & Shao, Z. Nitrogen and oxygen-codoped carbon nanospheres for excellent specific capacitance and cyclic stability supercapacitor electrodes. Chemical Engineering Journal, 330, 1166-1173 (2017). [CrossRef]
- W. Dong, M. W. Dong, M. Xie, S. Zhao, Q. Qin, F. Huang, Materials design and preparation for high energy density and high power density electrochemical supercapacitors, Mater. Sci. Eng., R., 152, 100713, (2023). [CrossRef]
- Z. Song, W. Z. Song, W. Liu, P. Xiao, Z. Zhao, G. Liu, J. Qiu, Nano-iron oxide (Fe2O3)/three-dimensional graphene aerogel composite as supercapacitor electrode materials with extremely wide working potential window, Mater. Lett., 145, 44-47, (2015). [CrossRef]
- F.-Y. Zeng, Z.-Y. F.-Y. Zeng, Z.-Y. Sui, S. Liu, H.-P. Liang, H.-H. Zhan, B.-H. Han, Nitrogen-doped carbon aerogels with high surface area for supercapacitors and gas adsorption, Mater. Today Commun., 16, 1-7, (2018). [CrossRef]
- G. A. Tafete, A. G. A. Tafete, A. Uysal, N. G. Habtu, M. K. Abera, T. A. Yemata, K. S. Duba, S. Kinayyigit, Hydrothermally synthesized nitrogen-doped hydrochar from sawdust biomass for supercapacitor electrodes, Int. J. Electrochem. Sci., 19, 100827 (2024). [CrossRef]
- S. Rawat, T. S. Rawat, T. Boobalan, B. B. Krishna, M. Sathish, S. Hotha, T. Bhaskar, Biochar for Supercapacitor Application: A Comparative Study, 17, e202200982, (2022). [CrossRef]
- Z. Ma, H. Z. Ma, H. Su, Y. Li, K. Yang, L. Dang, F. Li, B. Xue, Preparation of porous biochar and its application in supercapacitors, New J. Chem., 46, 21788-21797, (2022). [CrossRef]
- S. Nirmaladevi, R. S. Nirmaladevi, R. Boopathiraja, S. K. Kandasamy, S. Sathishkumar, M. Parthibavarman, Wood based biochar supported MnO2 nanorods for high energy asymmetric supercapacitor applications, Surf. Interfaces, 27, 101548, (2021). [CrossRef]
- C.-F. Xue, Y. C.-F. Xue, Y. Lin, W. Zhao, T. Wu, Y-Y. Wei, X.-H. Li, W.-J. Yan, X.-G. Hao, Green preparation of high active biochar with tetra-heteroatom self-doped surface for aqueous electrochemical supercapacitor with boosted energy density, J. Storage Mater., 90, 111872, (2024). [CrossRef]
- J. Cheng, Y. J. Cheng, Y. Lu, Y. Sun, S. Deng, H. Yang, M. Zhang, C. Wang, J. Yan, Impact of Activation Conditions on the Electrochemical Performance of Rice Straw Biochar for Supercapacitor Electrodes, Molecules, 30, 632, (2025). [CrossRef]
| Electrode materiels | Electrolyte | Current density (A/g) | Csp (F/g) | Ref |
|---|---|---|---|---|
| Fe2O3/GA | 0.5 M Na2SO4 | 1 | 81.3 | [59] |
| NCA | 1 M H2SO4 | 0.1 | NCA-800: 166 NCA-900: 136 |
[60] |
| HC2 | 2 M KOH | 0.5 | 80 | [61] |
| Biochar litchi seed | 1 M H2SO4 | 1 | 190 | [62] |
| Biochar apricot shell | 3 M KOH | 0.5 | 216 | [63] |
| BC@MnO2 | 1 M Na2SO4 | 0.5 | 512 | [64] |
| SGB-700 | 1 M H2SO4 | 0.5 | 638 | [65] |
| RSBC | 6 M KOH | 0.2 | RSBC: 197.2 RSBC-2: 296 |
[66] |
| P700(p) | 0.5 M H2SO4 | 0.5 | 440 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





