Submitted:
13 November 2025
Posted:
14 November 2025
You are already at the latest version
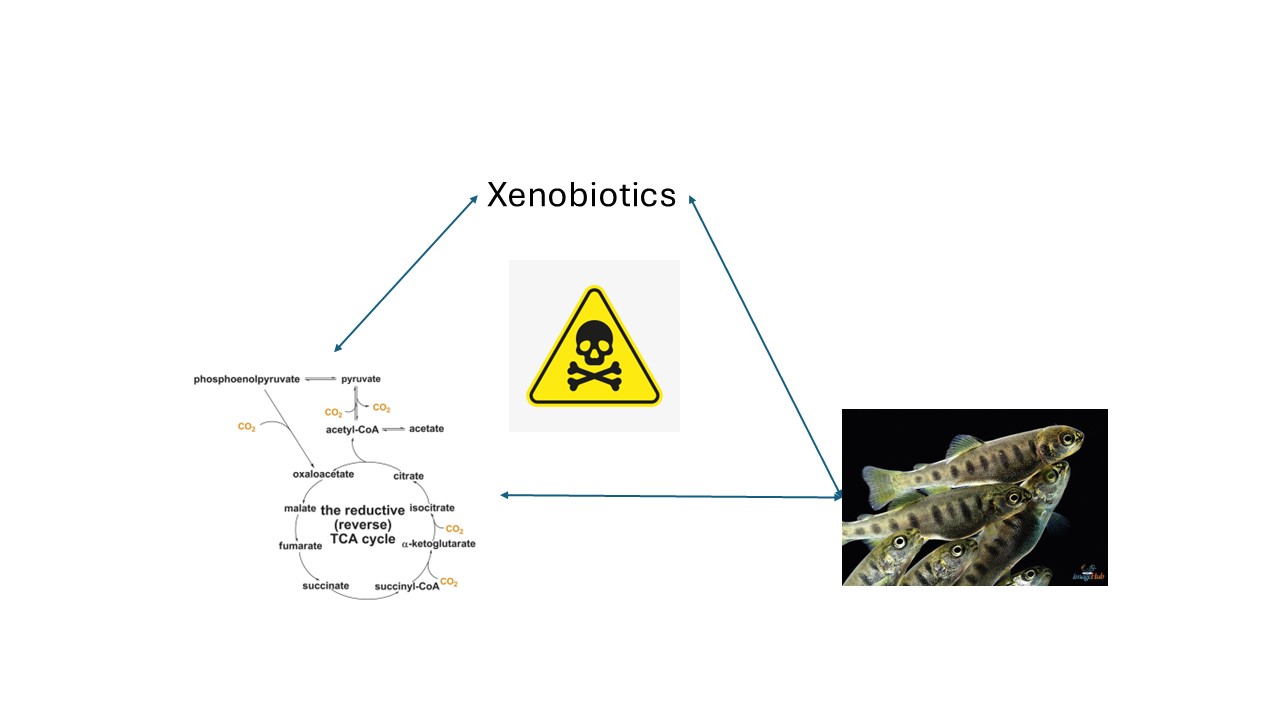
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Water Extraction
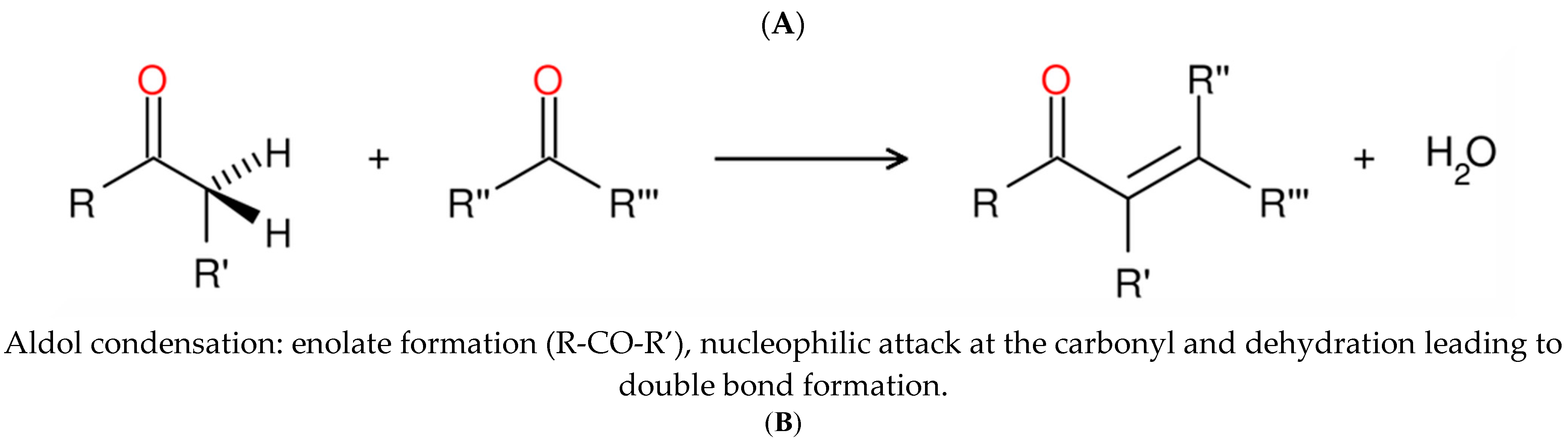
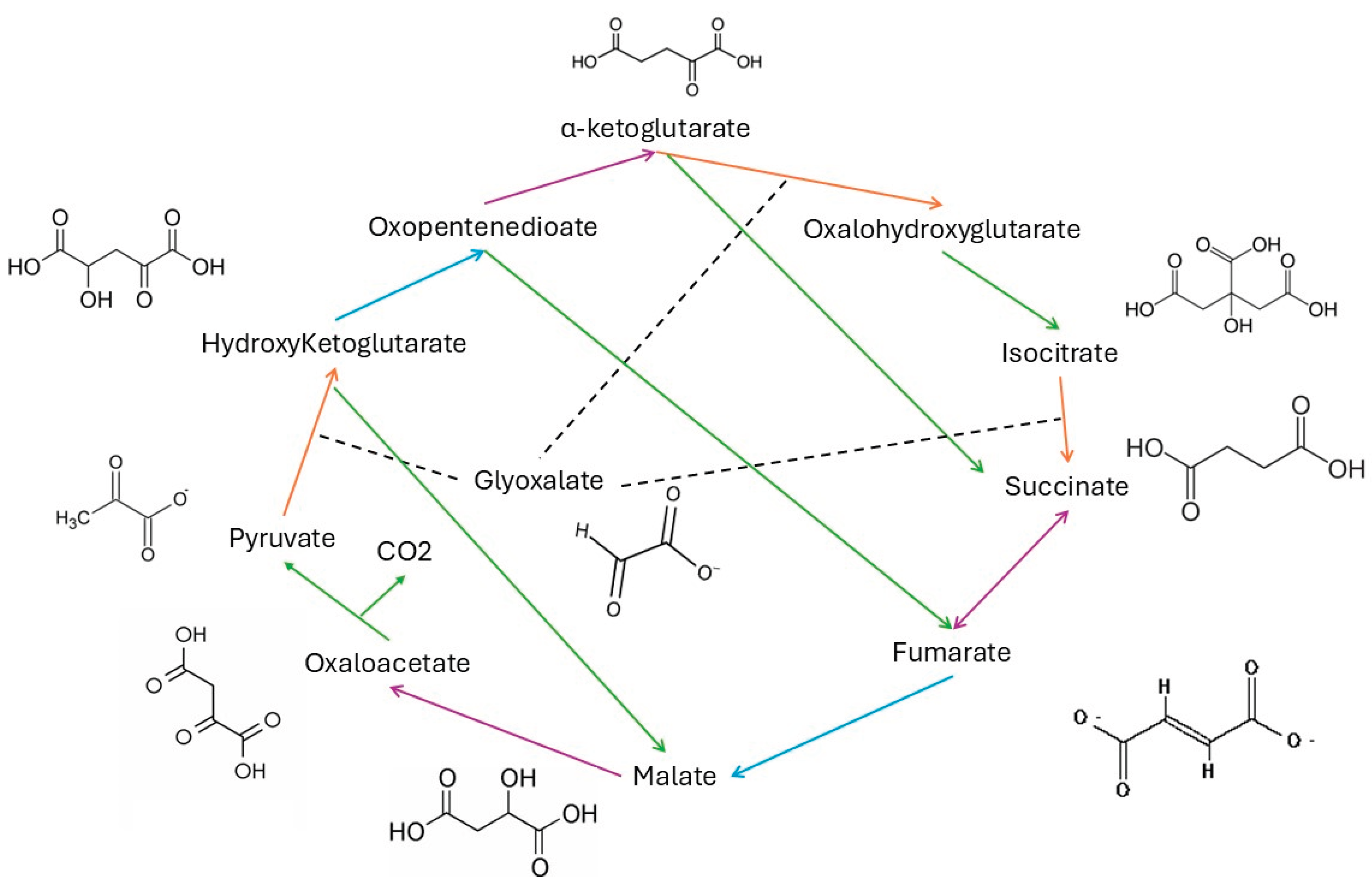
2.2. Pyruvate-Glyoxylate Assay
2.3. Rainbow Trout Acute Lethality Tests
2.4. Data Analysis
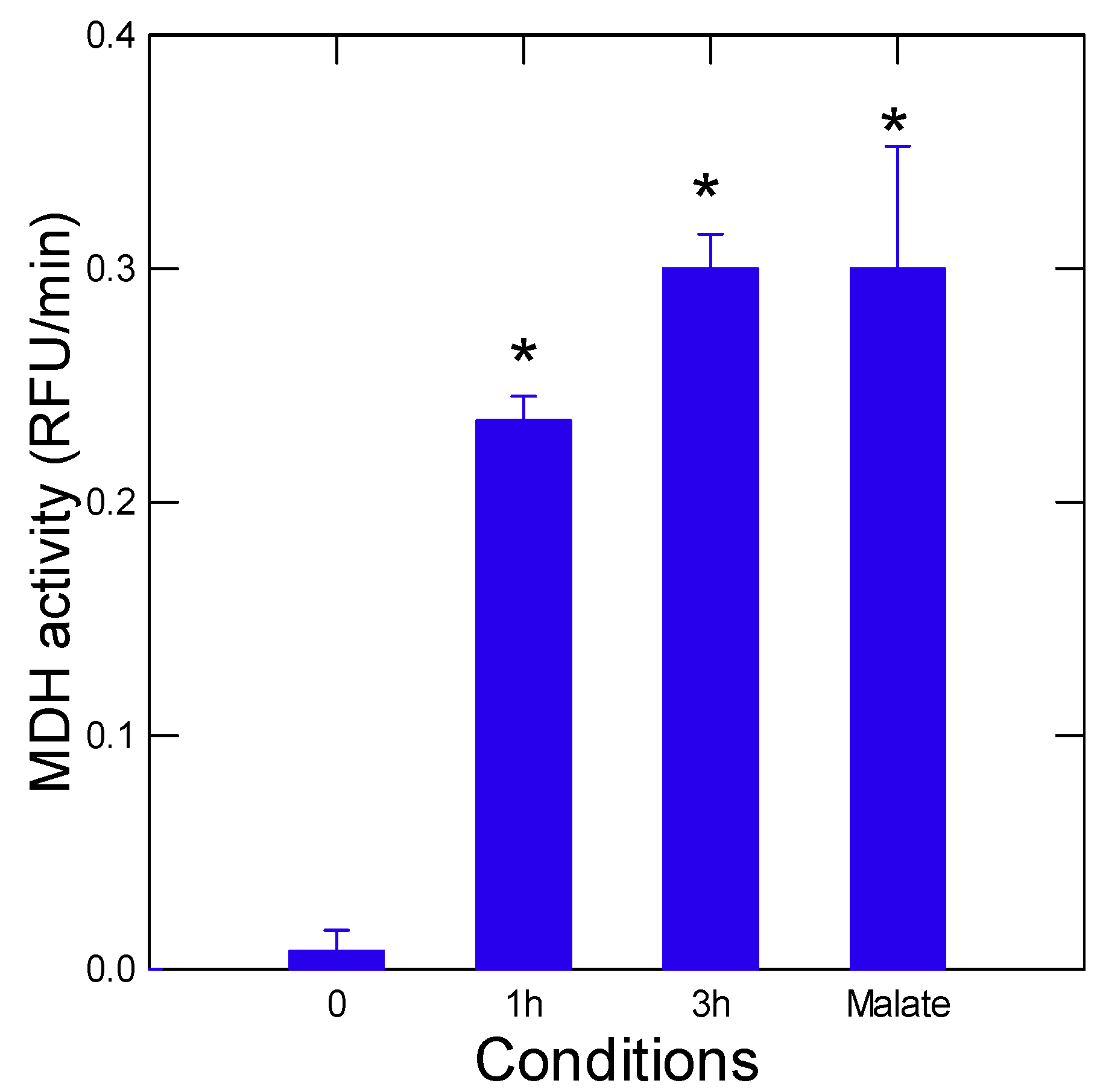
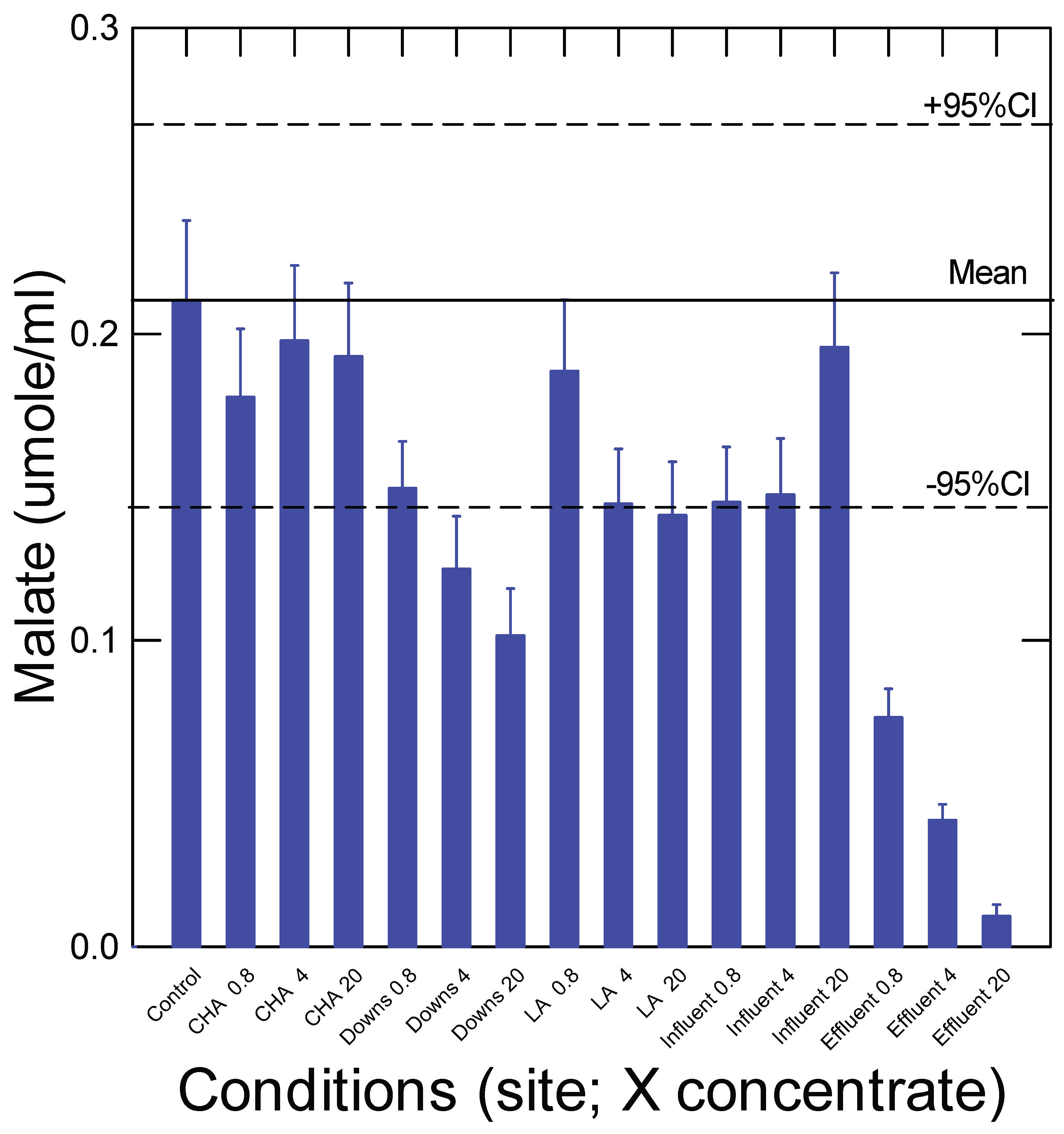
3. Results and Discussion
References
- Ten Brink, B.J.E.; Woudstra, J.H. Towards an effective and rational water management: the aquatic outlook project-integrating water management, monitoring and research. Eur. Water Pollut. Control 1991, 1, 20–27. [Google Scholar]
- Fishery Act. Published by the Minister of Justice 2025, R.S., c. F-14, s. 1, Canada, 111p.
- Costan, G.; Bermingham, N.; Blaise, C.; Ferard, J.F. Potential ecotoxic effects probe (PEEP): A novel index to assess and compare the toxic potential of industrial effluents. Environ. Toxicol. Water Qual. 1993, 8, 115–140. [Google Scholar] [CrossRef]
- Yang, H.; Sun, F.; Liao, H.; Guo, Y.; Wang, J.; Wu, F. Quantitative characteristics and multiple probabilistic risk assessment of small-sized microplastics in the middle and lower reaches of the Hanjiang River, China. Mar. Pollut. Bull. 2025, 220, 118404. [Google Scholar] [CrossRef]
- Farley, G.; Bouchard, P.; Faille, C.; Trottier, S.; Gagné, F. Towards the standardization of Hydra vulgaris bioassay for toxicity assessments of liquid samples. Ecotoxicol. Environ. Saf. 2024, 290, 117560. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; Roubeau Dumont, E.; Chantale, A. The effects of selected metals and rare earth elements on the peroxidase toxicity assay. Adv. Earth Environ. Sci. 2025, 6, 1–8. [Google Scholar]
- Braakman, R.; Smith, E. The compositional and evolutionary logic of metabolism. Phys. Biol. 2012, 10, 011001. [Google Scholar] [CrossRef]
- Nogal, N.; Sanz-Sánchez, M.; Vela-Gallego, S.; Ruiz-Mirazo, K.; de la Escosura, A. The protometabolic nature of prebiotic chemistry. Chem. Soc. Rev. 2023, 52, 7359–7388. [Google Scholar] [CrossRef]
- Tran, Q.P.; Adam, Z.R.; Fahrenbach, A.C. Prebiotic Reaction Networks in Water. Life 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Muchowska, K.B.; Varma, S.J.; Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 2019, 569, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.J.; Muchowska, K.B.; Chatelain, P.; Moran, J. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat. Ecol. Evol. 2018, 2, 1019–1024. [Google Scholar] [CrossRef]
- Emsley, J. The Elements 2019; Oxford University Press: NY, USA; 256p.
- Villamena, F.; Forman, H.J. Molecular basis of oxidative stress: chemistry, toxicology, disease pathogenesis, diagnosis, and therapeutics; John Wiley & Sons, Inc. January 2025 ISBN:9781119790266.
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Environment Canada. Biological test method: acute lethality test using rainbow trout environmental protection series 2007, report EPS 1/RM/9.
- Finney, D.J. Statistical Method in Biological Assay; Hafner Publishing Company: New York, NY, USA, 1964; 668p. [Google Scholar]
- Bhajiwala, H.M.; Patil, H.R.; Gupta, V. Studies of amino acids for inhibition of aldol condensation and dissolution of polymeric product of aldehyde in alkaline media. Appl. Petrochem. Res. 2013, 3, 17–23. [Google Scholar] [CrossRef]
- dos Santos, M.M.; Snyder, S.A. Occurrence of polymer additives 1,3-Diphenylguanidine (DPG), N-(1,3-Dimethylbutyl)-N′-phenyl-1,4-benzenediamine (6PPD), and chlorinated byproducts in drinking water: contribution from plumbing polymer materials. Environ. Sci. Technol. Lett. 2023, 10, 885–890. [Google Scholar] [CrossRef]
- Maxwell, K.L.; Bona, D.; Liu, C.; Arrowsmith, C.H.; Edwards, A.M. Refolding out of guanidine hydrochloride is an effective approach for high-throughput structural studies of small proteins. Protein Sci. 2003, 12, 2073–2080. [Google Scholar] [CrossRef]
- Blanco, F.; Kelly, B.; Sánchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J.; Rozas, I. Non-Covalent Interactions: Complexes of Guanidinium with DNA and RNA Nucleobases. J. Phys. Chem. B 2013, 117, 11608–11616. [Google Scholar] [CrossRef]
- Gasmi, A.; Peana, M.; Arshad, M.; Butnariu, M.; Menzel, A.; Bjørklund, G. Krebs cycle: activators, inhibitors and their roles in the modulation of carcinogenesis. Arch. Toxicol. 2021, 95, 1161–1178. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, B.J.; Wong, A.S.Y.; Semenov, S.N.; Belding, L.; Gmür, S.; Huck, W.T.S.; Whitesides, G.M. Robustness, Entrainment, and Hybridization in Dissipative Molecular Networks, and the Origin of Life. J. Am. Chem. Soc. 2019, 141, 8289–8295. [Google Scholar] [CrossRef]
- Grady, S.R.; Wang, J.K.; Dekker, E.E. Steady-state kinetics and inhibition studies of the aldol condensation reaction catalyzed by bovine liver and Escherichia coli 2-keto-4-hydroxyglutarate aldolase. Biochemistry 1981, 20, 2497–2502. [Google Scholar] [CrossRef]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-Induced Oxidative Stress and Toxicity. J. Toxicol. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Ali, D.; Falodah, F.A.; Almutairi, B.; Alkahtani, S.; Alarifi, S. Assessment of DNA damage and oxidative stress in juvenile Channa punctatus (Bloch) after exposure to multi-walled carbon nanotubes. Environ. Toxicol. 2020, 35, 359–367. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, R.; Hua, Z.; Long, W.; Xiang, Q. Effect of polystyrene nanoplastics on the intestinal histopathology, oxidative stress, and microbiota of Acrossocheilus yunnanensis. Aquat. Toxicol. 2025, 283, 107359. [Google Scholar] [CrossRef]
- Auclair, J.; Quinn, B.; Peyrot, C.; Wilkinson, K.J.; Gagné, F. Detection, biophysical effects, and toxicity of polystyrene nanoparticles to the cnidarian Hydra attenuata. Environ. Sci. Pollut. Res. 2020, 27, 11772–11781. [Google Scholar] [CrossRef] [PubMed]
- Auclair, J.; Roubeau-Dumont, E.; Gagné, F. Lethal and Sublethal Toxicity of Nanosilver and Carbon Nanotube Composites to Hydra vulgaris—A Toxicogenomic Approach. Nanomaterials 2024, 14, 1955. [Google Scholar] [CrossRef]
- André, C.; Smyth, S.-A.; Gagné, F. The peroxidase toxicity assay for the rapid evaluation of municipal effluent quality. Water Emerg. Contam. Nanoplastics 2025, 4. [Google Scholar] [CrossRef]
- Gagné, F.; André, C.; Smyth, S.-A. Screening of municipal effluents with the peroxidase toxicity assay. Discov. Water 2024, 4, 1–11. [Google Scholar] [CrossRef]
- Ichihara, M.; Asakawa, D.; Yamamoto, A.; Sudo, M. Quantitation of guanidine derivatives as representative persistent and mobile organic compounds in water: method development. Anal. Bioanal. Chem. 2023, 415, 1953–1965. [Google Scholar] [CrossRef]
- Brooke, D.N., Nielsen, I.R., Dobson, S., Howe, P.D. Environmental hazard assessment: Di-(2-ethylhexyl) phthalate. TSD 2, 1991. UK Department of the Environment, Garston, United Kingdom.
- Brix, K.V.; Tellis, M.S.; Crémazy, A.; Wood, C.M. Characterization of the effects of binary metal mixtures on short-term uptake of Ag, Cu, and Ni by rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2016, 180, 236–246. [Google Scholar] [CrossRef]
- Delahaut, V.; Rašković, B.; Salvado, M.S.; Bervoets, L.; Blust, R.; De Boeck, G. Toxicity and bioaccumulation of Cadmium, Copper and Zinc in a direct comparison at equitoxic concentrations in common carp (Cyprinus carpio) juveniles. PLOS ONE 2020, 15, e0220485. [Google Scholar] [CrossRef]
- Dubé, M.; Auclair, J.; Hanana, H.; Turcotte, P.; Gagnon, C.; Gagné, F. Gene expression changes and toxicity of selected rare earth elements in rainbow trout juveniles. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2019, 223, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Tan, S.-W.; Wu, W.-L. Stabilization or Oxidation of Nanoscale Zerovalent Iron at Environmentally Relevant Exposure Changes Bioavailability and Toxicity in Medaka Fish. Environ. Sci. Technol. 2012, 46, 8431–8439. [Google Scholar] [CrossRef] [PubMed]
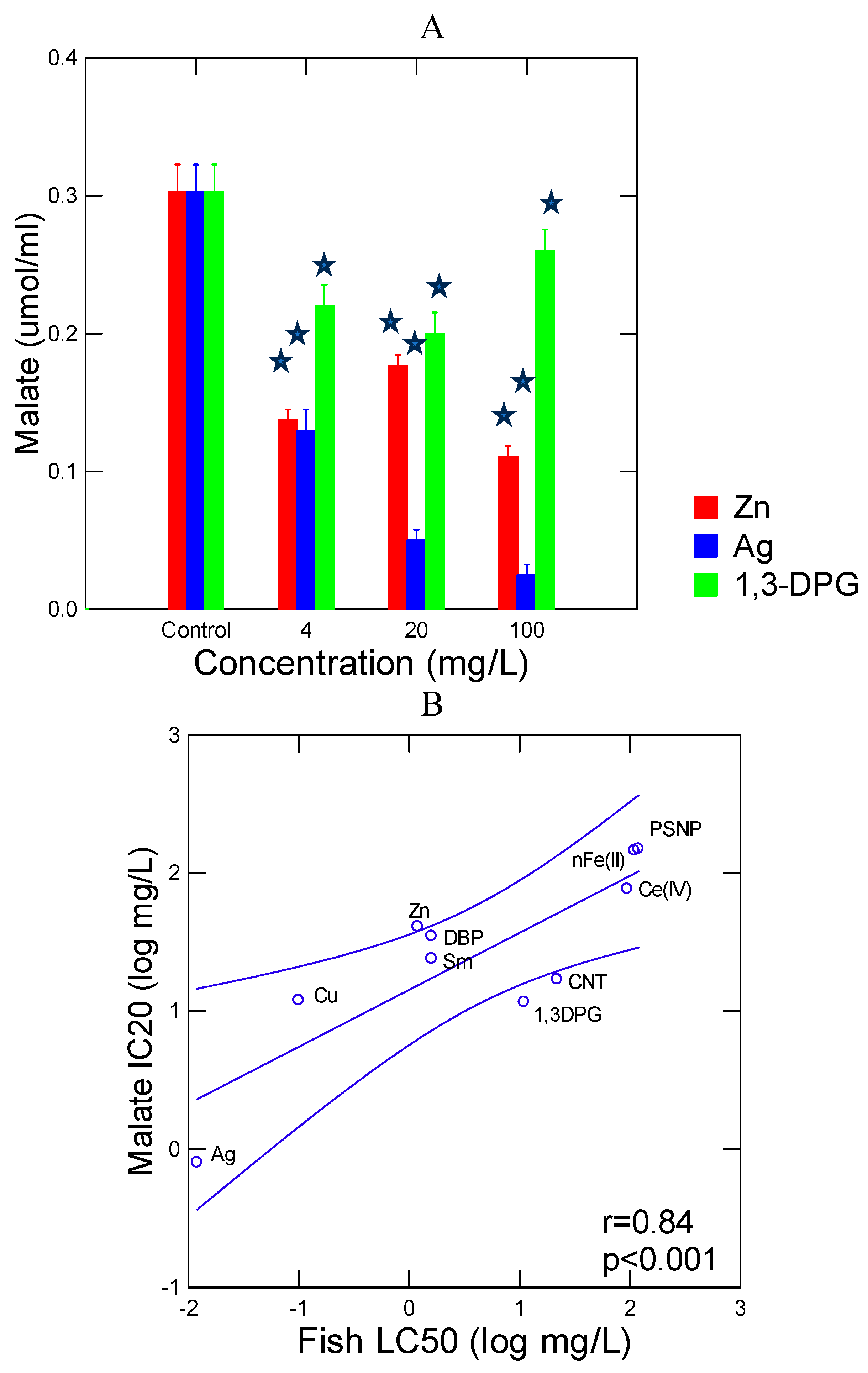
| Compounds | Pyr-Glyox (IC20 mg/l) |
Trout toxicity (LC50 mg/l) |
References |
| Dibutylphthalate (DBP) | 35 | 1.6 | [31] |
| 1,3-Diphenylguanidine (1.3-DPG) | 12 | 4.2 | Registration Dossier - ECHA |
| Copper Cu(II) | 12 | 0.1 | [32] |
| Silver Ag(I) | 1 | 0.02 | [32] |
| Zinc Zn(II) | 41 | 1.6 | [32,33] |
| Samarium Sm(III) | 24 | 2 | [34] |
| Cerium Ce(IV) | 77 | 95 | [34] |
| nFe2O3 | 146 | 100 (Oryzias latipe embryo) |
[35] |
| Polystyrene nanoplastic (PSNP) |
150 | >100 | This lab (section 2.3) |
| Carbon walled nanotubes (CNT) | 17 | 22 (Channa punctatus juvenile) |
[25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





