1. Introduction
According to possessed data, TB affects 10 million people per year (range 8.9 to 11.0 million people) and is one of the major infectious diseases that accounts for the largest number of deaths in the world. TB is estimated to cause 1.2 million deaths in HIV-negative people (range 1.1 to 1.3 million) and another 208,000 deaths in HIV-positive people (range 177,000 to 242,000). Of the approximately 10 million new cases, about 70% are diagnosed and treated and recorded in reporting systems, data from which are transmitted to the World Health Organization (WHO). Accordingly, national TB control programs have notified WHO of 7.1 million TB cases. Of the 7.1 million TB patients diagnosed in 2019., 5.9 million (84%) had pulmonary TB [
1].
TB strains that are resistant to TB drugs are more difficult to treat than drug-sensitive strains and pose a serious threat to patients, health professionals and health services. In addition, the increase in the number of cases of DR-TB (drug-resistant tuberculosis) is jeopardizing global progress in achieving the goals set out in the WHO TB Strategy [
2].
DR-TB remains a public health problem today, with a heavy burden on patients, local communities and health systems. According to recent estimates, in 2018 approximately 500,000. people in the world first fell ill with MDR / RR (rifampicin-resistant)-TB, with less than 40% of cases reported from the total estimated number, and only 32% of patients began drug therapy with second-line drugs [
2].
Current treatment regimens for MDR/RR-TB patients are far from perfect. Compared with the treatment of drug-sensitive forms of TB, these regimens require a longer course of treatment, more tablets and the use of more toxic drugs; in addition, patients may experience serious adverse reactions and may experience a deterioration in treatment results. Despite the increase in the proportion of successful treatment outcomes, almost 15% of patients die from MDR/RU-TB worldwide today [
2].
A high-level meeting in New-York was held on 22 September 2023 at the 78th session of the UN General Assembly (United Nations). The Republic of Uzbekistan and Poland were co-coordinators of the Meeting [
3].
STUDY OBJECTIVE: The aim of the study was to investigate the effectiveness of different treatment regimens for MDR/XDR TB (multi-drug-resistant/extensively drug-resistant tuberculosis).
2. Materials and Methods:
We observed 163 patients with M/XDR-TB (multi/extensively drug-resistant tuberculosis) aged 18 to 82 years. Males – 91 (55,8%), females – 72 (44,2%). Age: 18-20 years old – 7, 21-30 years old – 29, 31-40 years old – 50, 41-50 years old – 29, 51-60 years old – 19, over 61 years old – 29. As much as 64.4% of patients (105 people) were at the most able-bodied age from 20 to 50 years old. Number of women of childbearing age (15-49 years): 52 (31.9%). Students — 5 (3.0%), college students — 1 (0.6%), from places of deprivation of liberty or patients previously imprisoned (“prison tuberculosis”) — 14 (8.6%), migrants (who were abroad for work) — 38 (23.3%). Of the number of patients treated, the number of patients with disabilities due to tuberculosis: 18 patients with disabilities of the 2nd group (11.0%), 2 of the 1st group (1.2%), which totals to 20 patients with disabilities (12.3%) out of 163 treated.
Among the treated, the number of patients with the firstly diagnosed active tuberculosis: 75 (46.0%), previously treated - 88 (54.0%).
According to the forms of the disease (diagnosis): fibrocavernous TB - 47, cavernous - 5, infiltrative TB - 75, disseminated TB - 13 (5 of them miliary tuberculosis), tuberculoma - 10, cirrhotic TB - 4, focal TB - 4, pleuritis - 5.
According to the structure of the disease: with destruction and bacterial excretion: with destruction - 158 (96.9%), with bacteria excretion - 158 (96.9%), neglected/untreated processes - 142 (87.1%).
To determine the resistance, both molecular genetic tests (G/XDR-Xpert, HAIN MTBDR plus / sl) and phenotypic tests of drugs of the first, second and backup series were used, and genome sequencing was carried out in some patients. After determining resistance according to genotypic tests, treatment for DR-TB was started, later the treatment was adjusted according to phenotypic tests for drug sensitivity (TDS).
Resistance to anti-TB medications (MDR, XDR): XDR TB– у 82 patients (50,3%), MDR TB – in 81(49,7%). Of patients with XDR TB: pre-XDR TB with resistance to fluoroquinolones - 68 (41.7%), XDR TB - 14 (8.6%). In these 14 patients, along with resistance to fluoroquinolones, resistance to bedaquiline, linezolid, in some cases, and clofazimin, delamanid was established, in 2 patients - to pretomanidus.
Associated diseases were observed in 85 patients out of 163 treated (52.1%). Diabetes mellitus - in 30 (18.4%), hepatitis B and C - in 9 patients (5.5%): hepatitis B - in 3 (1.8%), hepatitis C - in 6 (3.7%); in total with hepatitis 9 (5.5%); HIV / AIDS - in 12 (7.4%); Coronary heart disease (CHD) and hypertension (GB) - in 16 (9.8%). This is due to the fact that patients older than 60 years are increasing; COPD - chronic obstructive pulmonary disease - in 4 (2.4%); mental illness in 4 (2.4%); anemia - in 3 (1.8%). In addition, secondary syphilis in 3 (1.8%), neonatal pemphigus and duodenal ulcer, goiter and gastroptosis in 1 (0.6%). Most of the concomitant diseases were diabetes mellitus (30) and HIV (12). The number of HIV/TB has increased. Sometimes several concomitant pathologies were observed (diabetes + CHD and Hypertension, hepatitis B or C, diabetes + CHD and hypertension, HIV + hepatitis C, etc.
A blood test for HIV infection was conducted in all 163 treated patients (100%), of which IFA positive - in 2 (1.2%), in which HIV infection was verified by immunoblotting. A total of 10 patients were previously registered with an infectious disease specialist with HIV infection. Thus, TB/HIV is established in 12 (7.3%) patients.
3. Results:
New alternative treatment regimen with bedaquiline in patients with hepatitis was used in 12 patients (7.4%). In this case, for patients with initially increased transaminases bedaquiline was prescribed 200 mg daily for 28 days, then 200 mg 3 times a week, or 100 mg daily.
New short-term treatment regimens for MDR-TB: m-STTR (modified short-term treatment regimen) — 9 (11) monthly treatment (BdqLfxLzdCfzCs) — bedaquiline, levofloxacin, linezolid, clophazimine, cycloserine, was used in 14 patients (8.5%); Alternative variant of m-STTR — 9 months treatment with delamanide (BdqLfxLzdCfzDlm) - bedaquiline, levofloxacin, linezolid, clophazimine, delamanide, was used in 2 patients (1.2%); 6-month treatment with BPaLM (26 weeks) using a new anti-tuberculosis drug pretomanide (bedaquiline, pretomanide, linezolid, moxifloxacin) - was used in 14 patients (8.5%). In 53 (32.5%) patients with MDR-TB (with large common forms of pulmonary tuberculosis), standard long-term treatment regimens of BdqLfxLzdCfzCs were carried out.
New short-term treatment regimens for XDR-TB: 6-month BPaL treatment (bedaquiline, pretomanide, linezolid) was used in 2 (1.2%) patients; BPaLC (bedaquiline, pretomanide, linezolid, clophasimin) was used in 7 patients (4.3%). A total of 59 (35.2) patients with pre-BDR TB with fluoroquinolones resistance and XDR TB were given standard long-term treatment regimens BdqLfxLzdCfzDlm. In the remaining 14 patients (8.6%) with resistance to reserve drugs (bedaquilin, linezolid, clophasimin, delamanid, in some cases to pretomanid) individual treatment regimens with moxifloxacin 800 mg, prothionamide, ethambutol, pyrazinamide, amikacin (if there is sensitivity), imipenema / cylastatin with amoxiclavoma were carried out.
During the course of treatment, side effects on chemotherapy were observed: nausea and vomiting were observed in 125 patients (76.7%) (mainly the first 2-3 weeks of treatment); headaches, dizziness - in 23 (14.1%), mainly with cycloserine; hepatotoxicity - in 16 (9.8%), mainly in patients receiving bedaquiline, pretomanide, clophazimine, pyrazinamide in treatment regimens; prolongation of the Qt interval - in 14 (8.6%), mainly in patients receiving bedaquiline, moxifloxacin, delamanid, clophasimin in treatment regimens; anemia - in 6 (3.7%); skin rash - in 5 (3.1%), in 1 case - Stevens-Johnson syndrome; arthritis, arthralgia - in 4 (2.4%) on levofloxacin and pyrazinamide; peripheral neuropathy - in 3 (1.8%), mainly on linezolid and cycloserine, which was confirmed by determining vibration on 128 Hertz tuning fork; ophthalmotoxicity - in 2 (1.2%) in the form of reduced visual acuity and impaired color differentiation mainly on linezolid and ethambutol; lactoacidosis -in 2 (1.2%) for linezolid and bedaquiline, confirmed by an increase in LDH (lactate dehydrogenase) in the blood, pancreatitis - in 1 (0.6%) for bedaquiline, confirmed by an increase in amylase in the blood; convulsive syndrome in 1 (0.6%) for cycloserine; depression - in 1 (0.6%) for cycloserine; psychosis - in 1 for cycloserine; hypokalemia - in 1 (0.6%), mainly in patients taking imipenem/cylastatin and amicacin, hearing loss - in 1 (0.6 per cent) per amikacin.
Operative treatment was carried out in 17 (10.4%) patients, 21 operations were performed (lobectomy - 4, combined resections (section + segment) - 1, combined segmental resections - 1, segmental resections - 9, pleurectomy and decortication - 2, posterior 4-rib thoracoplasty - 2, thoracocentesis and drainage - 2).
Effectiveness of treatment of patients, i.e. resorption, compaction or closure of decay cavities and abacillation among the firstly diagnosed and previously treated patients (firstly diagnosed 75 (of which 3 patients - 4.0% - died) and previously treated 88 (of which 5 patients - 5.7% - died):
- -
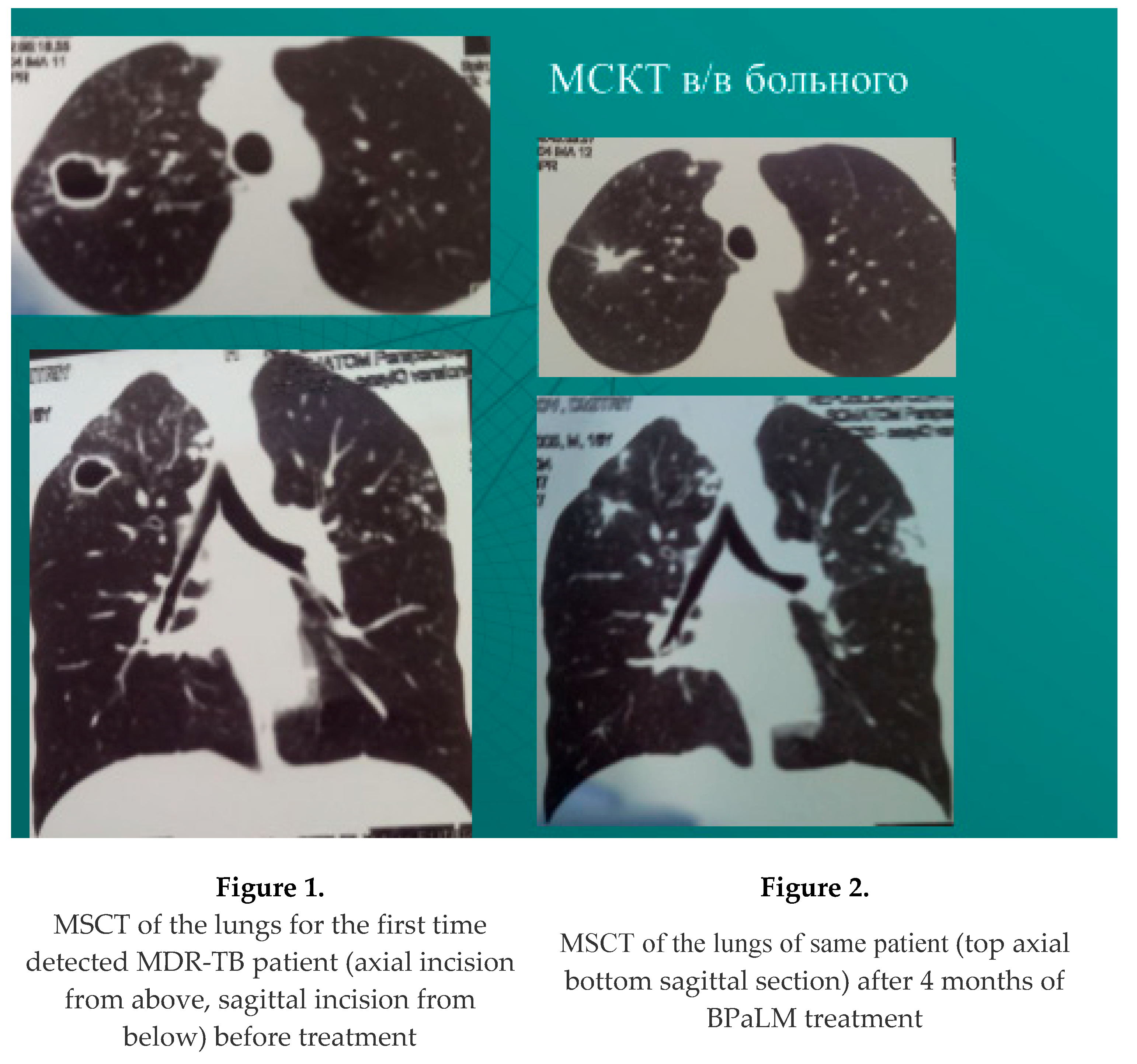
resorption, compaction or closure of decay cavities among the first identified patients - in 59 of 72 discharged - 81.9%, of which 9 patients underwent surgical treatment (in 5 XDR TB, in 4 MDR TB). Abacillation occurred in 63 of 72 discharged - 87.5%; Example: Figure 1, Figure 2.
- -
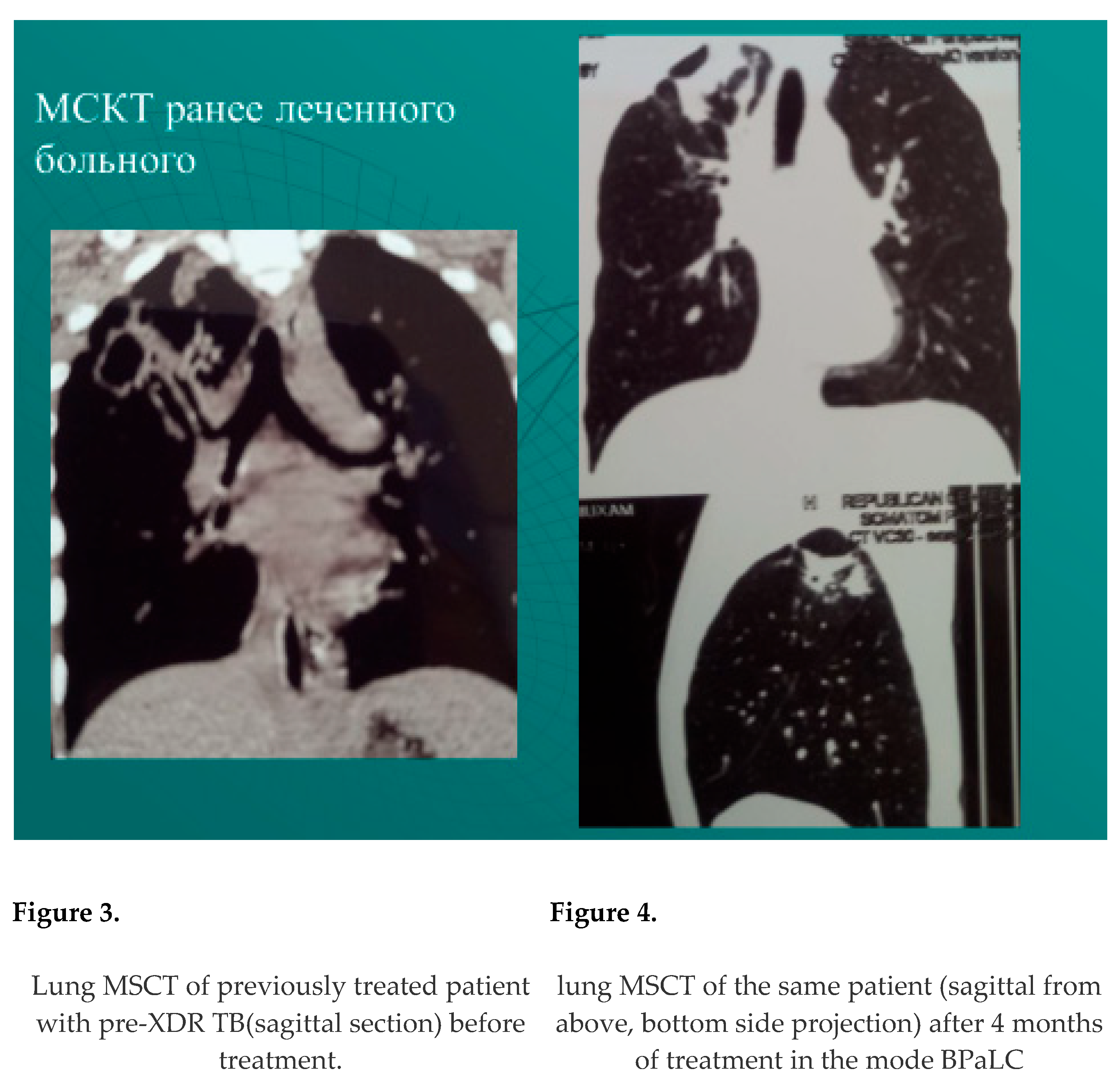
resorption, compaction or closure of decay cavities among previously treated patients - in 46 patients out of 83 discharged - 55.4%. Abacillation occurred - 63 of 83 discharged - 75.9%. Example: Figure 3, Figure 4.
4. Discussions:
In total, resorption, compaction, closure of decay cavities was achieved in 105 patients out of 155 discharged - 67.7%, in 105 out of 163 treated - 64.4%). Abacillation occurred in 127 patients out of 155 discharged - 81.9%, 127 out of 163 treated - 77.9%. Abacillation, although higher, does not yet reach the WHO target indicators (over 85%). This is due to the fact that the number of patients with resistance to reserve drugs (bedaquiline, linezolid, clofazimin, delamanid, pretomanid) increased - in 14, as well as the premature departure of 42 patients from the hospital (13 of them were discharged for violations of the hospital regime, 29 according to the statement due to family circumstances).
Effectiveness of treatment of patients according to regimes:
In 81 patients with MDR TB (49.7%), 3 treatment regimens were carried out: 1-subgroup 14 patients (8.6%) 6-month BPaLM regimen, 2-subgroup also 14 patients (8.6%) with 9(11)-month m-STTR regimen (of which 12 were taking BdqLfxLzdCfzCs - bedaquiline, levofloxacin, linezolid, clofazimine, cycloserine, 2 alternative treatment option for m-STTR with delanide BdqLzdCfzCsDlm - bedaquiline, linezolid, clofazimin, cycloserine, delamanid. 3-subgroup 53 patients who are prescribed a long-term standard treatment regimen BdqLfxLzdCfzCs.
In patients with 6-month BPaLM treatment regimens for MDR-TB, out of 14 (8.6%) - in 4 patients who underwent surgical treatment after 4 months of preliminary treatment, mycobacteria of tuberculosis (MTB) was microscopically detected in the operating material. In this regard, in these 4 operated patients, the treatment regimen was extended after 26 weeks of treatment to 9 months in the form of BPaL (without moxifloxacin), in 1 patient with a BPaLM regimen who received only therapeutic treatment, at the 4th month, microscopically, MTB was positive, also extended after 6 months (26 weeks) of treatment to 9 months in the form of BPaL (without moxifloxacin). In all 14 patients (100%) with BPaLM regimen, abacyllation confirmed by seeding occurred. Closure of decay cavities in 13 of 14 (92.8%).
In patients with m-STTR (14 - 8.6%), also in all 14, abacillation confirmed by seeding occurred (100%), closure of decay cavities in 12 of 14 (85.7%).
Abacillation occurred in patients with MDR-TB in the standard treatment mode (53-32.5%) - in 37 of 53 (69.8%), closure of decay cavities in 25 of 53 (47.2%).
Abacillation in patients with MDR TB 80.2% (in 65 of 81), 81.3% of 80 discharged. Closure of decay cavities — 65.4% (in 53 of 81) treated, 66.3% of 80 discharged.
82 patients with XDR TB (50.3%) are also divided into 3 subgroups: 1-subgroup 9 (5.5%) patients with pre-XDR carried out 6 monthly BPaL (2 patients), BPaLC (7 patients) regimens, 2-subgroup 59 (36.2%) patients with pre-XDR TB with resistance to fluoroquinolones standard treatment regimen for XDR TB BdqLzdCfzCsDlm (bedaquiline, linezolid, clofazimine, cycloserine, delamanid), 3-subgroup 14 (8.6%) patients individualized treatment regimens from-for resistance to reserve drugs (bedaquilin, linezolid, clofazimin, delamanid, in 4 cases and pretomanid).
In 9 patients (5.5%) with pre-BDR TB with fluoroquinolones resistance who underwent short-term 6-month BPaL treatment regimens, BPaLC abacillation occurred in all 9 patients (100%). Of the 9 in 5 who were operated on, MTB was detected in the operating material, treatment was extended to 9 months. (up to 39 weeks). Closure of the decay cavities was observed in all 9 patients (100%), taking into account the operated patients, in which the decay cavities were eliminated with the help of operations.
In patients with pre-XDR TB with resistance to fluoroquinolones (68 - 41.7%), 59 (36.2%) carried out standard treatment regimens BdqLzdCfzCsDlm (bedaquiline, linezolid, clofazimin, cycloserine, delamanide). In 48 patients (81.3%) out of 59, abacillation was observed. Closure of decay cavities was observed in 41 of 59 (69.5%).
In 14 (8.6%) patients with XDR-TB (resistance along with fluoroquinolones, bedaquiline, linezolid, sometimes clofazimin and delanmaid, in 2 cases and pretomanid). Abacillation was observed in 5 patients with XDR TB out of 14 (35.7%), out of 12 discharged (41.6%), Closure of decay cavities in 2 patients with XDR TB out of 14 (14.3%) treated, out of 12 discharged (16.6%).
Abacillation occurred in patients with XDR-TB 76.8% (in 63 of 82) treated, 84% of 75 discharged. Closure of decay cavities 63.4% (in 52 of 82) treated, in 69.3% of 75 discharged.
8 patients died (4.9%): 7 - men, 1 - woman. 1 with MDR TB, 7 with XDR TB. Age in 3 patients from 20 to 50 years (at the most able-bodied age) of 8 deaths. Cause of death: 7 - from increasing respiratory failure and increasing pulmonary heart failure; 1 - from pulmonary embolism. There was no discrepancy in the diagnosis of the deceased due to death (in the clinic and according to the results of the autopsy).).. A high degree of mortality (4.9%) is due to the fact that patients with involuntary processes arrived.
5. Conclusions:
1. Abacillation in patients with resistant forms of tuberculosis was 77.9% (in 127 of 163) treated. Closure of decay cavities was observed 64.4% in 105 of 163 treated.
2. In patients with MDR pulmonary tuberculosis, the abacillation was 80.5% (in 65 out of 81) treated, closure of decay cavities - 65.4% (53 out of 81).
3. Abacillation occurred in patients with XDR-TB 76.8% (in 63 of 82) treated, closure of decay cavities 63.4% (in 52 of 82).
4. In patients with short-term treatment regimens with pretomanid in MDR TB, abacillation was 100% (in all 14 patients), closure of decay cavities of 85.7% (in 12 of 14).
5. In patients with short-term regimens of m-STTR with MDR TB, abacillation confirmed by sowing 100% (in all 14 patients), closure of decay cavities of 85.7% (in 12 of 14).
6. In patients with standard long-term treatment regimens for MDR TB, abacillation occurred in 69.8% of treated patients (in 37 out of 53), closure of decay cavities 47.2% (in 25 out of 53).
7. In patients with XDR TB, short-term treatment regimens with pretomanid in 9 patients, abatsillation and closure of decay cavities came 100% (in all 9 patients).
8. In patients with pre-XDR TB with fluoroquinolones resistance, 81.3% (48 out of 59 patients) were in long-term mode with a standard treatment regimen, abacillation was observed, cavities of decay were closed in 69.5% (41 out of 59 patients).
9. Abacillation was observed in 5 patients with XDR-TB out of 14 (35.7%), closure of decay cavities in 2 patients out of 14 (14.3%) treated.
10. Hospital mortality was 4.9% (8 out of 163) of treated patients with resistant forms of tuberculosis (1 with MDR TB, 7 with XDR TB).
Author Contributions
Conceptualization, Sunnatilla Abulkasimov; Methodology, Sunnatilla Abulkasimov, Nodira Rustamova and Janibek Pulatov; Software, Yulduzxon Abulkasimova; Formal analysis, Sunnatilla Abulkasimov and Yulduzxon Abulkasimova; Investigation, Sunnatilla Abulkasimov and Nargiza Parpieva; Resources, Nargiza Parpieva; Data curation, Nargiza Parpieva and Janibek Pulatov; Writing – original draft, Sunnatilla Abulkasimov; Writing – review & editing, Sunnatilla Abulkasimov; Supervision, Sunnatilla Abulkasimov; Project administration, Nodira Rustamova, Yulduzxon Abulkasimova and Janibek Pulatov.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the ERC of the MOH of Uzbekistan (protocol code No. 1/16-1480, dated February 27, 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-susceptible tuberculosis treatment: web annexes [Proposed Russian translation]. Geneva: World Health Organization; 2022 (https://apps.who.int/iris/handle/10665/353398).
- WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. Geneva: World Health Organization; 2022».
- 78th session of the UN General Assembly of 22 September 2023 https://news.un.org/ru/tags/78-ya-sessiya-genassamblei-oon.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).