1. Introduction
Actinic keratosis (AK), also known as solar keratosis, is a precancerous skin lesion caused by chronic exposure to ultraviolet (UV) radiation. It is a marker of photoaging and an early stage in the progression that can lead to cutaneous squamous cell carcinoma, making it a major public health issue [
1,
2].
Epidemiologically, its prevalence increases with age and cumulative sun exposure. It is reported in 15 to 25% of people over the age of 60 in Europe and can reach more than 60% in sunny regions such as Australia [
1]. Risk factors include chronic sun exposure, advanced age, male gender, immunosuppression, and certain outdoor occupations [
2].
Although it is more common in fair skin types (I–II), HA can also occur in darker skin types, particularly in cases of advanced baldness, which exposes the scalp directly to UV rays [
1].
Clinically, HA manifests as erythematous-squamous macules or papules that are rough to the touch and located on sun-exposed areas. The diagnosis is primarily clinical, but can be confirmed by biopsy, which reveals hyperkeratosis, parakeratosis, keratinocyte atypia limited to the epidermis, and solar elastosis of the dermis [
3,
4].
Treatment is based on several modalities: cryotherapy, dynamic phototherapy, laser, curettage, or topical treatments such as 5-fluorouracil, imiquimod, or ingenol mebutate [
5]. Although these options are effective, they are often perceived as aggressive, and recurrences are frequent [
6]. In addition, systemic approaches are also being studied with a view to preventing skin cancer, in particular the use of certain vitamins (vitamin D) and oral molecules such as cyanamide [
1].
Furthermore, in up to 85% of cases of treated AK, recurrence or the appearance of new lesions can be observed after a one-year follow-up, making AK a chronic and recurring disease [
7]. In this context, retinoids appear to be an interesting dermocosmetic alternative. Their role in regulating keratinocyte proliferation and differentiation is well documented [
8]. However, their topical use suffers from several limitations: chemical instability, poor skin penetration, and sometimes reduced tolerance [
9].
We recently published results concerning the use of retinol combined with a polylysine vector (22.5kDa) in the treatment of inflammatory skin diseases, particularly psoriasis and eczema [
10]. The encapsulation of vitamins, particularly retinol and ascorbic acid, within a polylysine biovector represents an innovative strategy that ensures molecular protection, enhances stability, improves cutaneous bioavailability, and minimizes irritative potential.
We present the case of a 64-year-old patient with actinic hyperkeratosis of the scalp that had been developing for more than 24 months, treated with a dermocosmetic formulation based on retinol encapsulated in a polylysine biovector, equivalent to 3.2 × 10⁶ IU of vitamin A per gram of polylysine.
2. Clinical Case
Symptoms
A 64-year-old male patient, residing in the south of France, with fair Mediterranean skin (Fitzpatrick skin type IV), with no significant medical history or comorbidities, consulted for a chronic scalp lesion.
- Advanced baldness exposing the vertex to UV rays.
- Lesion evolving for more than 24 months.
- Rough, well-defined erythematous-squamous plaque measuring approximately 1 cm.
- Mild pruritus and cosmetic discomfort.
Biology
A recent biological assessment showed no significant abnormalities:
- Normal blood count (Hb 15.1 g/dL, leukocytes 9.74 G/L, platelets 351 G/L).
- CRP 1.6 mg/L (N < 5).
- Slightly reduced renal function (creatinine 111 µmol/L, GFR 61 mL/min/1.73 m²).
- Normal liver function.
Histology
The skin biopsy confirmed the diagnosis of actinic hyperkeratosis, with hyperkeratosis, parakeratosis, basal and suprabasal keratinocyte atypia, and solar elastosis of the dermis [
3,
4].
Treatment
The patient was treated with a dermocosmetic formulation of retinol encapsulated in a polylysine biovector, equivalent to 3.2 × 10⁶ IU of vitamin A per gram of polylysine, provided by Dermabel Cosmetics France (Myrameha®).
The method of application consisted of using a hazelnut-sized dose, applied twice a day to the lesion, massaging the affected area gently to promote skin penetration.
The patient thus received approximately 1280 IU of vitamin A per day.
Table 1.
Concentrations and administered doses.
Table 1.
Concentrations and administered doses.
| Parameter |
Value |
Unit/Equivalence |
| Retinol concentration |
0.05 % |
≈1600 IU/g of gel |
| Poly-L-lysine concentration |
0.05 % |
0.5 mg/g of gel |
| Retinol / polylysine ratio |
3.2 × 10⁶ |
IU of vitamin A per g of polylysine |
| Applied dosage |
2 × pea-sized amount (~0.4 g) |
i.e., 0.8 g/day of gel |
| Daily retinol dose |
≈ 1280 IU/day |
administered topically |
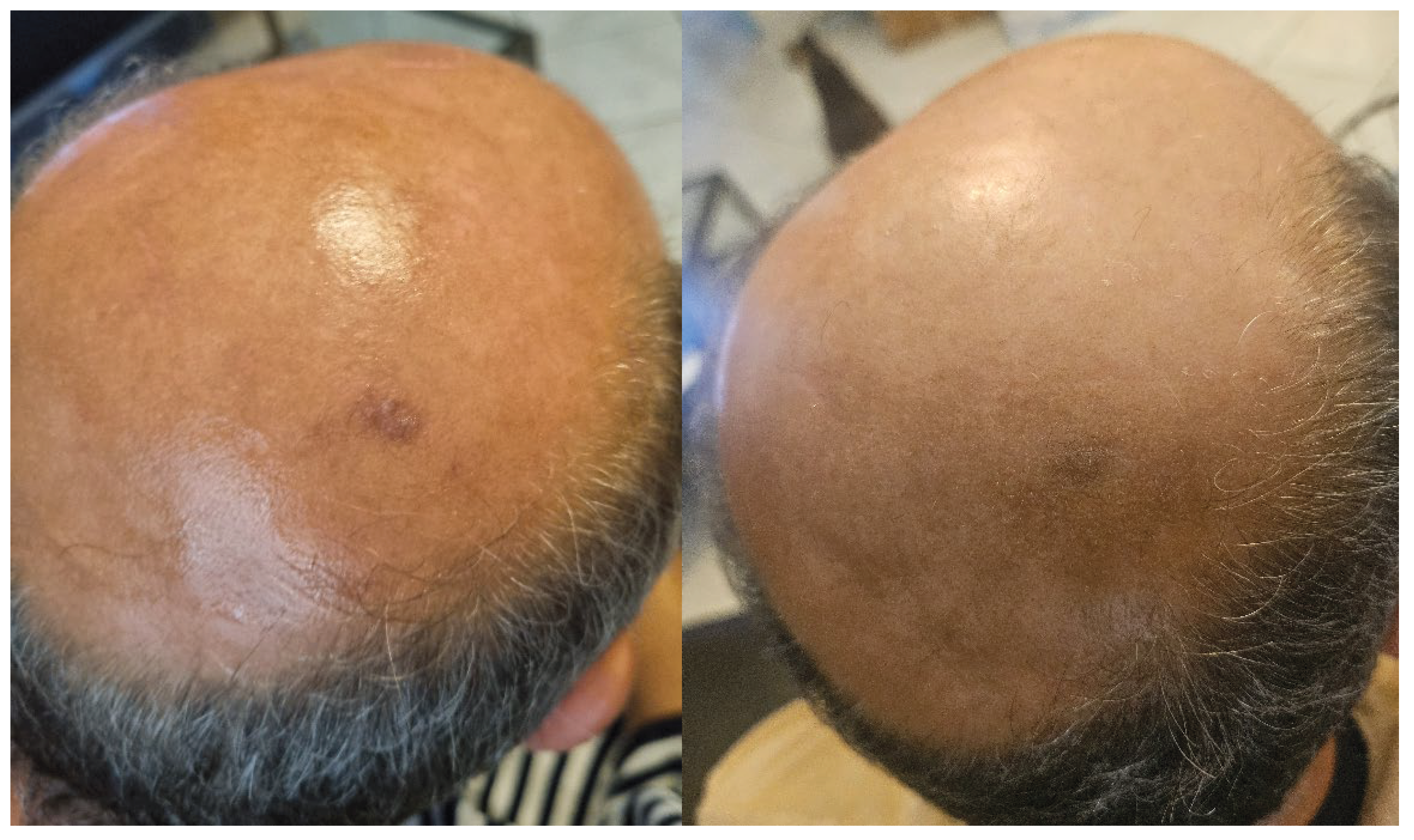
Results
After 21 days of treatment:
- Progressive regression of the lesion.
- Complete disappearance of the hyperkeratotic plaque.
- Limited hair regrowth at the initial site of the lesion.
The treatment was well tolerated, with no irritation or adverse effects reported.
Figure 1.
Clinical progression. Clinical comparison before treatment (left) and after 21 days of treatment (right).
Figure 1.
Clinical progression. Clinical comparison before treatment (left) and after 21 days of treatment (right).
3. Discussion
Actinic keratosis is a common skin condition, the prevalence of which increases with age and cumulative sun exposure. It affects 15 to 25% of people over the age of 60 in Europe and can reach more than 60% in regions with high levels of sunshine such as Australia [
1,
2]. Although it is more common in fair skin types, it can also occur in individuals with skin type IV when there is advanced baldness, removing the natural protection of the scalp.
There are many treatment options available: cryotherapy, dynamic phototherapy, laser, curettage, or topical treatments such as 5-fluorouracil, imiquimod, and ingenol mebutate [
6]. However, each of these options has limitations. Retinoids have been shown to be useful in the prevention and treatment of actinic keratoses and other precancerous lesions [
8]. Nevertheless, systemic retinoids (acitretin, isotretinoin) are limited by their metabolic and hepatic toxicity, their teratogenicity, and frequent adverse effects such as skin xerosis, cheilitis, arthralgia, and, more rarely, neuropsychiatric disorders [
9]. Topical forms (tretinoin, adapalene, tazarotene, retinol) are better tolerated but often cause transient local skin reactions: erythema, irritation, desquamation, or photosensitivity [
9].
Ingenol mebutate had attracted particular interest due to its efficacy and ultra-short treatment regimen. However, its use was limited by intense local reactions that were visually dramatic for patients, as well as by reports of skin cancer after marketing authorization, which led to its withdrawal in Europe [
10]
Furthermore, long-term follow-up data indicate that up to 85% of treated patients experience recurrence or new lesions within one year, underscoring the chronic and relapsing nature of AK [
11]. In this context, it is essential to explore new management strategies, both preventive and curative in the long term. Dermocosmetics are a promising avenue, particularly when based on innovative encapsulation systems. Our recent work has highlighted the benefits of retinol and vitamin C encapsulated in poly-L-lysine biovectors, which improve stability, skin penetration, and clinical tolerance [
12,
13]. The case presented here illustrates the potential benefits of such an approach in the management of actinic hyperkeratosis.
4. Conclusions
This case illustrates the potential benefits of an innovative dermocosmetic formulation combining retinol and polylysine biovector in the treatment of actinic hyperkeratosis. The favorable outcome observed suggests that this approach could represent a complementary alternative to conventional treatments. However, this is a single observation that should be interpreted with caution. Larger clinical studies will be needed to confirm these preliminary results.
Conflict of interest statement
The author declares that he has no conflict of interest
Informed Consent Statement
Written informed consent was obtained from the patient for the publication of this case report and the accompanying clinical images. The patient was informed about the objectives of this work, the anonymous handling of personal data, and consented to the use of his clinical information for scientific and publication purposes.
References
- George CD, Lee T, Hollestein LM, Asgari MM, Nijsten T. Global epidemiology of actinic keratosis in the general population: a systematic review and meta-analysis. British Journal of Dermatology. 2024 Apr;190(4):465-76. [CrossRef]
- Thamm JR, et al. Epidemiology and risk factors of actinic keratosis. DPCJ. 2024. [PubMed]
- Conforti C, et al. Clinical and dermoscopic diagnosis of actinic keratosis. J Clin Med. 2024. PMC11566822. [CrossRef]
- Calderone DC, Fenske NA. The clinical spectrum of actinic elastosis. Journal of the American Academy of Dermatology. 1995 Jun 1;32(6):1016-24. [CrossRef]
- Dianzani C, Conforti C, Giuffrida R, Corneli P, di Meo N, Farinazzo E, Moret A, Magaton Rizzi G, Zalaudek I. Current therapies for actinic keratosis. International Journal of Dermatology. 2020 Jun;59(6):677-84. [CrossRef]
- Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. Journal of the American Academy of Dermatology. 2013 Jan 1;68(1):S39-48. [CrossRef]
- Steeb T, Wessely A, Petzold A, Brinker TJ, Schmitz L, Schöffski O, Berking C, Heppt MV. Long-term recurrence rates of actinic keratosis: A systematic review and pooled analysis of randomized controlled trials. J Am Acad Dermatol.2022 May 1;86(5):1116-9. [CrossRef]
- Ianhez M, Fleury LF, Miot HA, Bagatin E. Retinoids for prevention and treatment of actinic keratosis. Anais brasileiros de dermatologia. 2013 Aug;88(4):585-93. [CrossRef]
- Beckenbach L, Baron JM, Merk HF, Löffler H, Amann PM. Retinoid treatment of skin diseases. Eur J Dermatol. 2015;25(5):384–91. [CrossRef]
- Heron CE, Feldman SR. Ingenol mebutate and the treatment of actinic keratosis. J Drugs Dermatol. 2021 Jan 1;20(1):102-4. [CrossRef]
- Noels EC, Lugtenberg M, van Egmond S, et al. Insight into the management of actinic keratosis: Br J Dermatol. 2019;181(1):96–104. [CrossRef]
- Nassar B, Belhadj-Tahar H, Jin W, Yang G. A Prospective Open-Label Study of Tolerance and Effectiveness of Sequential Dermocosmetic Treatments Combining Poly-L-Lysine Biovectors With Vitamins A and C. Health Sci Rep. 2025 Apr;8(4):e70676. [CrossRef]
- Belhadj-Tahar, H. Impact of Poly-L-Lysine Dendrimers on the Stability and Effectiveness of Vitamin C in Dermocosmetic Use: Research Article. J Biochem Physiol 7. 2024; 3:2.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).