Submitted:
24 September 2025
Posted:
25 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- 1.1.
- Relevance and Applications of Mesoporous Materials
2. Structural Characterization and Comparison Between Materials
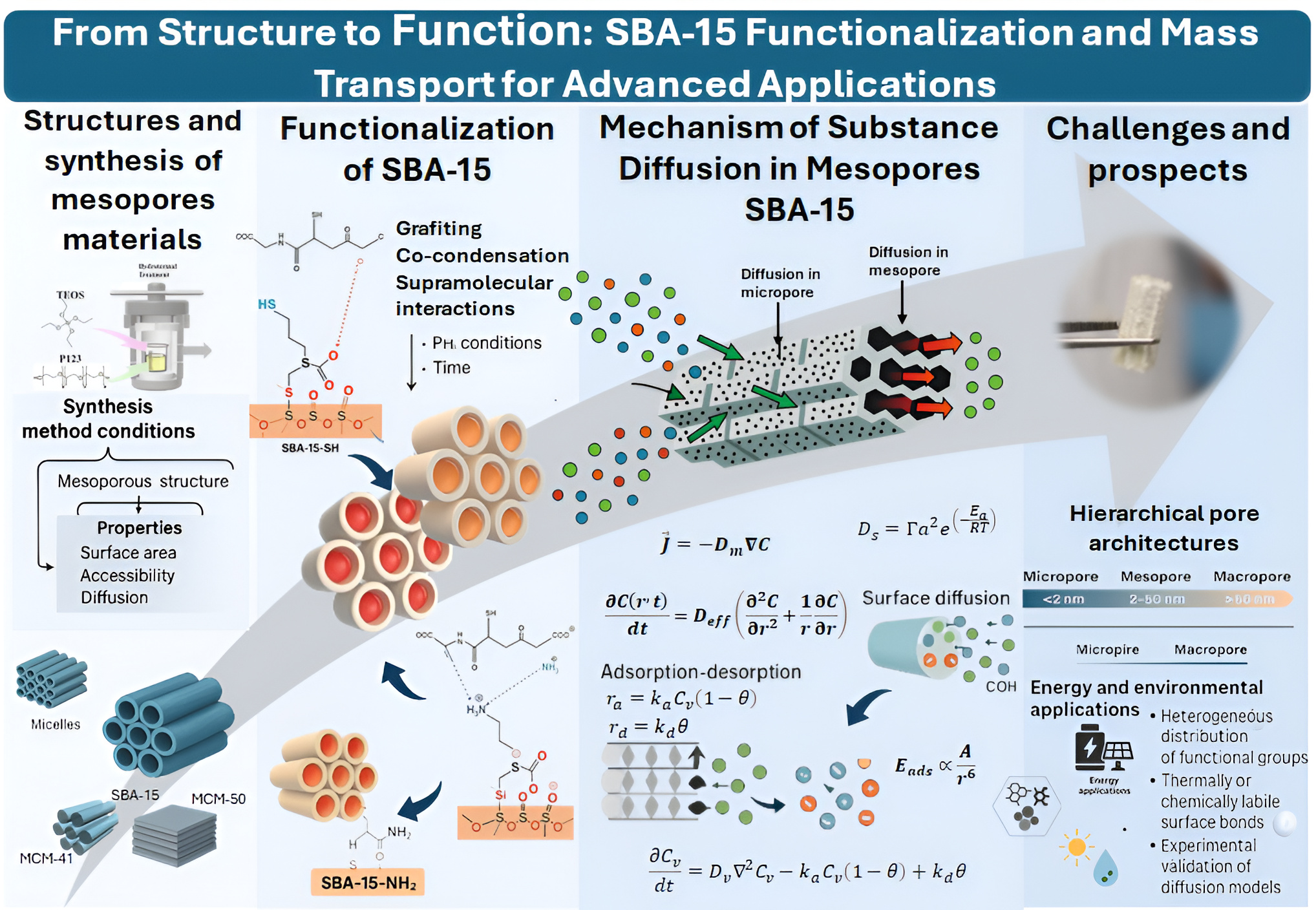
2.1. Structure and Synthesis of SBA-15
2.2. Synthesis Methods and Their Effect on Diffusive Properties
3. Functionalization of Mesoporous Materials: Strategies, Properties
4. Diffusion Mechanism of Substances in Mesopores: General Model Applied to SBA-15
4.1. Modeling Diffusion and Transport Mechanisms
4.1.1. Molecular Diffusion (Fickian)
4.1.2. Knudsen Diffusion
4.1.3. Combined Diffusion: Bosanquet Model
4.1.4. Surface Diffusion
4.1.5. Multicomponent Diffusion: Maxwell–Stefan Model
4.1.6. Adsorption–Desorption: Kinetic Mechanism
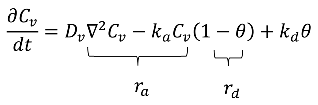
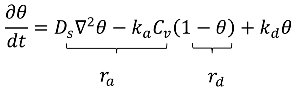
5. Challenges and Future Perspectives
6. Conclusions
7. Further Steps
Abbreviations
| Symbol | Description | Units |
| Distance between active sites on the surface | m | |
| Attractive interaction constant between molecules or between a molecule and the surface of the material | J⋅m6 | |
| Solute concentration in general (may vary across spatial domains) | mol/m³ | |
| Concentration at a radial distance r and time t | mol/m³ | |
| Solute concentration in the gas phase inside the porous channel | mol/m³ | |
| Initial solute concentration | mol/m³ | |
| Pore diameter | m | |
| Effective diffusion coefficient in the porous medium | m²/s | |
| Diffusion coefficient in the channel volume (combined molecular and Knudsen) | m²/s | |
| Molecular diffusion coefficient in the free phase (free motion between molecular collisions) | m²/s | |
| Knudsen diffusion coefficient (dominated by collisions with pore walls) | m²/s | |
| Surface diffusion coefficient (adsorbate migrating along the pore wall) | m²/s | |
| Cross-diffusivity coefficient between species i and j | m²/s | |
| Frictional diffusivity coefficient between species i and the porous solid matrix | m²/s | |
| Overall effective diffusion coefficient in hierarchical materials | m²/s | |
| Activation energy for surface migration (~5–30 kJ/mol for Van der Waals) | kJ/mol | |
| Adsorption energy | kJ/mol | |
| Total molar flux vector | mol/(m²·s) | |
| Adsorption rate constant | 1/s | |
| Desorption rate constant | 1/s | |
| Interfacial spacing between porous domains (used in hierarchical transport) | m | |
| Distance between two particles or between an adsorbed molecule and the material surface | m | |
| Molar mass of the solute | kg/mol | |
| Net charge or transport factor of species i | adimensional | |
| Radial distance from the pore axis | m | |
| Universal gas constant (8.314) | J/mol·K | |
| Absolute temperature | K | |
| Average molecular velocity of the solute | m/s | |
| Interfacial permeability coefficient in hierarchical transport models | m/s | |
| Mean free path of the molecule | m | |
| Porosity of the medium | dimensionless | |
| Tortuosity of the diffusive path | dimensionless | |
| Hopping frequency of adsorbate between sites (~10¹² s⁻¹) | 1/s | |
| Chemical potential of i | dimensionless | |
| Surface coverage fraction (occupied sites over total available) | dimensionless | |
| Mole fraction of species j | dimensionless | |
| Laplacian operator (gradient of the gradient) | 1/m² | |
| Gradient of concentration field | mol/m⁴ | |
| Gradient of chemical potential of species i | J/(mol·m) | |
| Partial derivative related to time | s⁻¹ | |
| Second partial derivative related to the radial coordinate | 1/m² |
References
- D. H. Everett, “Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry,” Pure and Applied Chemistry, vol. 31, no. 4, pp. 577–638, May 1972. [CrossRef]
- C. T. Kresge, M. E. Leonowizc, W. J. Roth, J. C. Vartuli, and J. S. Beck, “Ordered mesoporous molecular sieves synthesised by a liquid-crystal template mechanism,” vol. 359, pp. 710–712, Oct. 1992. [CrossRef]
- Y. Wan and D. Zhao, “On the controllable soft-templating approach to mesoporous silicates,” Jul. 2007. [CrossRef]
- T. Asefa and V. Dubovoy, “Ordered Mesoporous/Nanoporous Inorganic Materials via Self-Assembly,” in Comprehensive Supramolecular Chemistry II, vol. 9, Elsevier Inc., 2017, pp. 158–192. [CrossRef]
- M. Shakeri, Z. K. Shal, and P. Van Der Voort, “An overview of the challenges and progress of synthesis, characterization and applications of plugged sba-15 materials for heterogeneous catalysis,” Sep. 01, 2021, MDPI. [CrossRef]
- S. Yuan, M. Wang, J. Liu, and B. Guo, “Recent advances of SBA-15-based composites as the heterogeneous catalysts in water decontamination: A mini-review,” Jan. 15, 2020, Academic Press. [CrossRef]
- R. Janus, M. Wądrzyk, M. Lewandowski, P. Natkański, P. Łątka, and P. Kuśtrowski, “Understanding porous structure of SBA-15 upon pseudomorphic transformation into MCM-41: Non-direct investigation by carbon replication,” Journal of Industrial and Engineering Chemistry, vol. 92, pp. 131–144, Dec. 2020. [CrossRef]
- J. Asensio, M. I. Beltrán, N. Juáresz-Serrano, and D. Berenguer, “Study of the Decomposition of N-Nitrosonornicotine (NNN)under Inert and Oxidative Atmospheres: Effect of the Additionof SBA-15 and MCM-41,” Applied Sciences, vol. 12, no. 19, p. 9426, Sep. 2022. [CrossRef]
- N. Juárez-Serrano, J. Asensio, I. Blasco, Beltrán Maribel, and A. Marcilla, “Effect of Time, Temperature and Stirring Rate Used in the FirstStep of the Synthesis of SBA-15 on Its Application as Reductorof Tars in Tobacco Smoke,” Catalysis, vol. 11, no. 3, p. 375, Mar. 2021. [CrossRef]
- A. Sayari and Y. Belmabkhout, “Stabilization of amine-containing CO2 adsorbents: Dramatic effect of water vapor,” J Am Chem Soc, vol. 132, no. 18, pp. 6312–6314, May 2010. [CrossRef]
- M. Vallet-Regí, M. Colilla, I. Izquierdo-Barba, and M. Manzano, “Mesoporous silica nanoparticles for drug delivery: Current insights,” 2018, MDPI AG. [CrossRef]
- Z. Ezzeddine, I. Batonneau-Gener, G. Ghssein, and Y. Pouilloux, “Recent Advances in Heavy Metal Adsorption via Organically Modified Mesoporous Silica: A Review,” Mar. 01, 2025, Multidisciplinary Digital Publishing Institute (MDPI). [CrossRef]
- P. N. E. Diagboya and E. D. Dikio, “Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment,” Aug. 01, 2018, Elsevier B.V. [CrossRef]
- A. Galarneau, H. Cambon, F. Di Renzo, and F. Fajula, “True microporosity and surface area of mesoporous SBA-15 silicas as a function of synthesis temperature,” Langmuir, vol. 17, no. 26, pp. 8328–8335, Dec. 2001. [CrossRef]
- F. Tang, L. Li, and D. Chen, “Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery,” Advanced Materials, vol. 24, no. 12, pp. 1504–1534, Mar. 2012. [CrossRef]
- L. Wang and R. T. Yang, “Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density,” Journal of Physical Chemistry C, vol. 115, no. 43, pp. 21264–21272, Nov. 2011. [CrossRef]
- K. Lan and D. Zhao, “Functional Ordered Mesoporous Materials: Present and Future,” Apr. 27, 2022, American Chemical Society. [CrossRef]
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka, and G. D. Stucky, “Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures,” 1998.
- N. Juárez-Serrano, D. Berenguer, I. Martínez-Castellanos, I. Blasco, M. Beltrán, and A. Marcilla, “Effect of reaction time and hydrothermal treatment time on the textural properties of sba-15 synthesized using sodium silicate as a silica source and its efficiency for reducing tobacco smoke toxicity,” Catalysts, vol. 11, no. 7, Jul. 2021. [CrossRef]
- N. Rahmat, A. Z. Abdullah, and A. R. Mohamed, “A review: Mesoporous Santa Barbara amorphous-15, types, synthesis and its applications towards biorefinery production,” 2010, Science Publications. [CrossRef]
- M. Pérez-Page et al., “Template-based syntheses for shape controlled nanostructures,” Aug. 01, 2016, Elsevier B.V. [CrossRef]
- A. Tewodros, Mark J. MacLachlan, Neil Coombs, and Geoffrey A. Ozin, “Periodic mesoporous organosilicas with organic groups inside the channel wall,” vol. 402, pp. 867–871, Dec. 1999. [CrossRef]
- T. Asefa and V. Dubovoy, “Ordered Mesoporous/Nanoporous Inorganic Materials via Self-Assembly,” in Comprehensive Supramolecular Chemistry II, vol. 9, Elsevier Inc., 2017, pp. 158–192. [CrossRef]
- D. Zhao et al., “Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores,” Science (1979), vol. 279, no. 5350, p. 548, Jan. 1998. [CrossRef]
- E. Serra, R. M. Blanco, and I. Díaz, “Síntesis y caracterización de materiales mesoporosos ordenados y su aplicación como soportes en la inmovilización de lipasa,” An. Quím, vol. 104, no. 2, pp. 97–103, 2008, [Online]. Available: www.rseq.org.
- W. Han, Y. Jia, G. Xiong, and W. Yang, “Template-free sol-gel synthesis of mesoporous materials with ZSM-5 structure walls,” in Studies in Surface Science and Catalysis, vol. 165, Elsevier Inc., 2007, pp. 515–518. [CrossRef]
- Y. G. Asfaha, A. K. Tekile, and F. Zewge, “Hybrid process of electrocoagulation and electrooxidation system for wastewater treatment: A review,” Oct. 01, 2021, Elsevier Ltd. [CrossRef]
- J. Zhao and W. Yan, “Microwave-assisted Inorganic Syntheses,” in Modern Inorganic Synthetic Chemistry, Elsevier, 2011, pp. 173–195. [CrossRef]
- C. P. Avanzadas and P. V. Vigón, “Síntesis de materiales mesoporosos compuestos sílice/cabono y su empleo como plataforma para la fabricación de materiales,” 2013.
- J. García Martínez, “Sólidos ordenados desde la nano a la macroestructura,” An. Quim, 2006, [Online]. Available: www.rseq.org.
- J. H. Williams, Q. Zheng, M. D. Mantle, A. J. Sederman, and L. F. Gladden, “Probing the Diffusion Mechanism of n-Alkanes in Mesoporous Confinement Using Pulsed Field Gradient NMR,” Journal of Physical Chemistry C, vol. 127, no. 31, pp. 15326–15335, Aug. 2023. [CrossRef]
- P. Kumar and V. V. Guliants, “Periodic mesoporous organic-inorganic hybrid materials: Applications in membrane separations and adsorption,” Jul. 2010. [CrossRef]
- L. Zheng, Y. Yang, Y. Zhang, T. Zhu, and X. Wang, “Functionalization of SBA-15 mesoporous silica with bis-schiff base for the selective removal of Pb(II) from water,” J Solid State Chem, vol. 301, Sep. 2021. [CrossRef]
- D. Li et al., “β-Cyclodextrin functionalized SBA-15 via amide linkage as a super adsorbent for rapid removal of methyl blue,” J Colloid Interface Sci, vol. 583, pp. 100–112, Feb. 2021. [CrossRef]
- L. Xiang Chuin, S. Kamaruzaman, S. Mangala Praveena, and N. Yahaya, “Recent applications of β-cyclodextrin in selective adsorption of Pesticides, heavy metals, and organic pollutants from water Samples: Mini review,” Microchemical Journal, p. 111583, Nov. 2024. [CrossRef]
- F. das C. M. da Silva, M. J. dos S. Costa, L. K. R. da Silva, A. M. Batista, and G. E. da Luz, “Functionalization methods of SBA-15 mesoporous molecular sieve: a brief overview,” Jun. 01, 2019, Springer Nature. [CrossRef]
- D. Shimon et al., “15N Solid State NMR Spectroscopic Study of Surface Amine Groups for Carbon Capture: 3-Aminopropylsilyl Grafted to SBA-15 Mesoporous Silica,” Environ Sci Technol, vol. 52, no. 3, pp. 1488–1495, Feb. 2018. [CrossRef]
- R. Ojeda-López, I. J. Pérez-Hermosillo, J. Marcos Esparza-Schulz, A. Cervantes-Uribe, and A. Domínguez-Ortiz, “SBA-15 materials: calcination temperature influence on textural properties and total silanol ratio,” Adsorption, vol. 21, no. 8, pp. 659–669, Nov. 2015. [CrossRef]
- Z. Zhang, J. Yin, H. J. Heeres, and I. Melián-Cabrera, “Thermal detemplation of SBA-15 mesophases. Effect of the activation protocol on the framework contraction,” Microporous and Mesoporous Materials, vol. 176, pp. 103–111, 2013. [CrossRef]
- Á. Szegedi, K. Lázár, H. Solt, and M. Popova, “Peculiar redox properties of SBA-15 supported copper ferrite catalysts promoting total oxidation of a model volatile organic air pollutant,” Surfaces and Interfaces, vol. 56, Jan. 2025. [CrossRef]
- A. Gouveia Gil, Z. Wu, D. Chadwick, and K. Li, “Ni/SBA-15 Catalysts for combined steam methane reforming and water gas shift - Prepared for use in catalytic membrane reactors,” Appl Catal A Gen, vol. 506, pp. 188–196, Oct. 2015. [CrossRef]
- Ł. Laskowski et al., “Mesoporous silica SBA-15 functionalized by nickel-phosphonic units: Raman and magnetic analysis,” Microporous and Mesoporous Materials, vol. 200, pp. 253–259, Jul. 2014. [CrossRef]
- H. Wu, Y. Xiao, Y. Guo, S. Miao, Q. Chen, and Z. Chen, “Functionalization of SBA-15 mesoporous materials with 2-acetylthiophene for adsorption of Cr(III) ions,” Microporous and Mesoporous Materials, vol. 292, Jan. 2020. [CrossRef]
- X. Xia, Y. Jin, H. Zhao, G. Wang, and D. Huang, “Optimization and Experiment of Hot Air Drying Process of Cyperus esculentus Seeds,” Agriculture (Switzerland), vol. 13, no. 3, Mar. 2023. [CrossRef]
- Y. Wang and R. T. Yang, “Template Removal from SBA-15 by Ionic Liquid for Amine Grafting: Applications to CO2Capture and Natural Gas Desulfurization,” ACS Sustain Chem Eng, vol. 8, no. 22, pp. 8295–8304, Jun. 2020. [CrossRef]
- F. Hoffmann, M. Cornelius, J. Morell, and M. Fröba, “Silica-based mesoporous organic-inorganic hybrid materials,” Angewandte Chemie - International Edition, vol. 45, no. 20, pp. 3216–3251, May 2006. [CrossRef]
- Y. Yin et al., “Modification of as Synthesized SBA-15 with Pt nanoparticles: Nanoconfinement Effects Give a Boost for Hydrogen Storage at Room Temperature,” Sci Rep, vol. 7, no. 1, Dec. 2017. [CrossRef]
- J. Zhu, T. Wang, X. Xu, P. Xiao, and J. Li, “Pt nanoparticles supported on SBA-15: Synthesis, characterization and applications in heterogeneous catalysis,” Feb. 07, 2013. [CrossRef]
- A. Taguchi and F. Schüth, “Ordered mesoporous materials in catalysis,” in Microporous and Mesoporous Materials, vol. 77, no. 1, 2005, pp. 1–45. [CrossRef]
- Ł. Laskowski et al., “Mesoporous silica SBA-15 functionalized by nickel-phosphonic units: Raman and magnetic analysis,” Microporous and Mesoporous Materials, vol. 200, pp. 253–259, Jul. 2014. [CrossRef]
- F. das C. M. da Silva, M. J. dos S. Costa, L. K. R. da Silva, A. M. Batista, and G. E. da Luz, “Functionalization methods of SBA-15 mesoporous molecular sieve: a brief overview,” Jun. 01, 2019, Springer Nature. [CrossRef]
- J. Kärger and D. M. Ruthven, “Diffusion in nanoporous materials: Fundamental principles, insights and challenges,” 2016, Royal Society of Chemistry. [CrossRef]
- W. Li, J. Liu, and D. Zhao, “Mesoporous materials for energy conversion and storage devices,” May 04, 2016, Nature Publishing Group. [CrossRef]
- J. Kärger and R. Valiullin, “Mass transfer in mesoporous materials: The benefit of microscopic diffusion measurement,” Chem Soc Rev, vol. 42, no. 9, pp. 4172–4197, Apr. 2013. [CrossRef]
- S. Whitaker, The Method of Volume Averaging, vol. 13. in Theory and Applications of Transport in Porous Media, vol. 13. Dordrecht: Springer Netherlands, 1999. [CrossRef]
- R. B. Bird, S. W. Earl, and E. N. Lightfoot, Fenómenos de Transporte, 2nd ed. 2010. [Online]. Available: https://books.google.com/books/about/Transport_Phenomena.html?id=L5FnNlIaGfcC.
- J. Kärger, D. Freude, and J. Haase, “Diffusion in nanoporous materials: Novel insights by combining MAS and PFG NMR,” Sep. 01, 2018, MDPI AG. [CrossRef]
- A. Zhokh, “Size-controlled non-Fickian diffusion in a combined micro- and mesoporous material,” Chem Phys, vol. 520, pp. 27–31, Apr. 2019. [CrossRef]
- D. Do. Duong, Adsorption analysis: equilibria and kinetics, vol. 1. Imperial College Press, 1998.
- V. T. Hoang, Q. Huang, M. Eić, T. O. Do, and S. Kaliaguine, “Structure and diffusion characterization of SBA-15 materials,” Langmuir, vol. 21, no. 5, pp. 2051–2057, Mar. 2005. [CrossRef]
- H. Rusinque and G. Brenner, “Mass transport in porous media at the micro- and nanoscale: A novel method to model hindered diffusion,” Microporous and Mesoporous Materials, vol. 280, pp. 157–165, May 2019. [CrossRef]
- J. Kärger and D. M. Ruthven, “Diffusion in nanoporous materials: Fundamental principles, insights and challenges,” 2016, Royal Society of Chemistry. [CrossRef]
- S. Whitaker, The Method of Volume Averaging, vol. 13. in Theory and Applications of Transport in Porous Media, vol. 13. Dordrecht: Springer Netherlands, 1999. [CrossRef]
- A. Vignes, “Diffusion in Binary Solutions Variation of Diffusion Cocficient with Compositionsion,” Ind. Eng. Chem. Fundam, vol. 5, no. 2, pp. 189–199, 1966. [CrossRef]
- G. Guevara-Carrion, T. Janzen, Y. M. Muñoz-Muñoz, and J. Vrabec, “Mutual diffusion of binary liquid mixtures containing methanol, ethanol, acetone, benzene, cyclohexane, toluene, and carbon tetrachloride,” Journal of Chemical Physics, vol. 144, no. 12, Mar. 2016. [CrossRef]
- J. N. Israelachvili, “Intermolecular and Surface Forces: Third Edition,” in Intermolecular and Surface Forces: Third Edition, 3thd ed., Elsevier Inc., 2011, pp. 1–676. [CrossRef]
- Javier. García-Martínez and Elena. Serrano-Torregrosa, Chemistry Education : Best Practices, Opportunities and Trends. Wiley, 2015.
- A. Larki, S. J. Saghanezhad, and M. Ghomi, “Recent advances of functionalized SBA-15 in the separation/preconcentration of various analytes: A review,” Oct. 01, 2021, Elsevier Inc. [CrossRef]
- N. Linares, A. M. Silvestre-Albero, E. Serrano, J. Silvestre-Albero, and J. García-Martínez, “Mesoporous materials for clean energy technologies,” Nov. 21, 2014, Royal Society of Chemistry. [CrossRef]
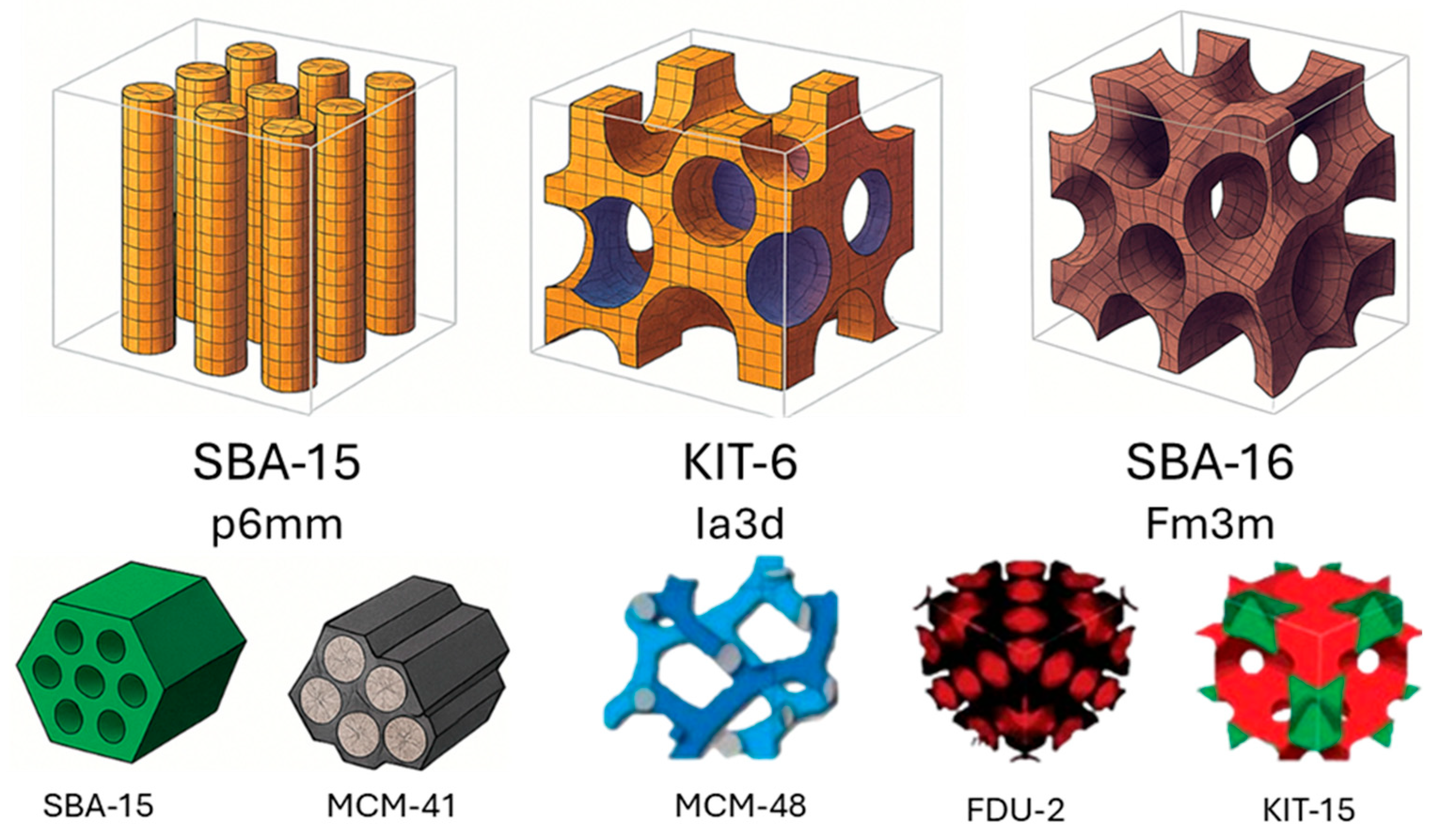
| Mesoporous Material | Structure | Pore size (nm) | Surface Area (m²/g) | Typical Synthesis method | Ref. |
|---|---|---|---|---|---|
| MCM-41 | Hexagonal p6mm |
2–10 | 800–1000 | CTAB-assisted sol-gel | [23,24] |
| SBA-15 | Hexagonal p6mm | 5–30 | 500–1000 | P123-templated sol-gel | [20,24] |
| KIT-6 | Cubic Ia3d |
6–12 | 400–900 | P123/butanol cosurfactant system | [26,27] |
| FDU-12 | Cubic Fm3m |
5–15 | 600–900 | Modified triblock copolymers | [26,28] |
| MSU-X | Disordered | 2–12 | 500–700 | Nonionic self-assembly | [27] |
| TUD-1 | Interconnected | 3–10 | 600–850 | Surfactant-assisted sol-gel | [27] |
| MCM-48 | Cubic Ia3d |
2–4 | 800–1100 | CTAB-templated sol-gel | [23,24] |
| FSM-16 | Lamellar | 2–3 | 900–1000 | Surfactant intercalation | [23] |
| Synthesis method | Conditions | Pore size (nm) | Deff (m²/s) | Key Advantages | Ref. |
|---|---|---|---|---|---|
| Conventional sol-gel | HCl, 35–40 °C, 24–72 h | 6–8 | ~1.0 × 10⁻⁸ | High ordering, easy scalability | [18] |
| Hydrothermal | 100–130 °C, 24–48 h | 6–10 | 1.2 × 10⁻⁸ | Enhanced crystallinity, structural stability | [7,26] |
| Microwave-assisted | 2.45 GHz, 80–100 °C, 1–2 h | 5–8 | 3.5 × 10⁻⁷ | Rapid synthesis, uniform particle size control | [3,28] |
| Sonochemical | Ultrasonic frequency, low T | 5–9 | 1.5 × 10⁻⁷ | Improved homogeneity, morphological dispersion | [21] |
| Solvothermal | Organic solvents, high pressure | 5–7 | ~1.8 × 10⁻⁸ | Precise shape/crystallinity/particle size control | [23] |
| Dual-template (P123/CTAB) | Mixed surfactants | 4–12 (bimodal) | ~1.0 × 10⁻⁸ | Hierarchical meso-macroporous structures | [9] |
| Post-synthesis grafting | Grafting con –NH₂, tioles, etc. | 4–7 | 2.8 × 10⁻⁷ | Tailored surfaces for selective adsorption/catalysis | [10,16] |
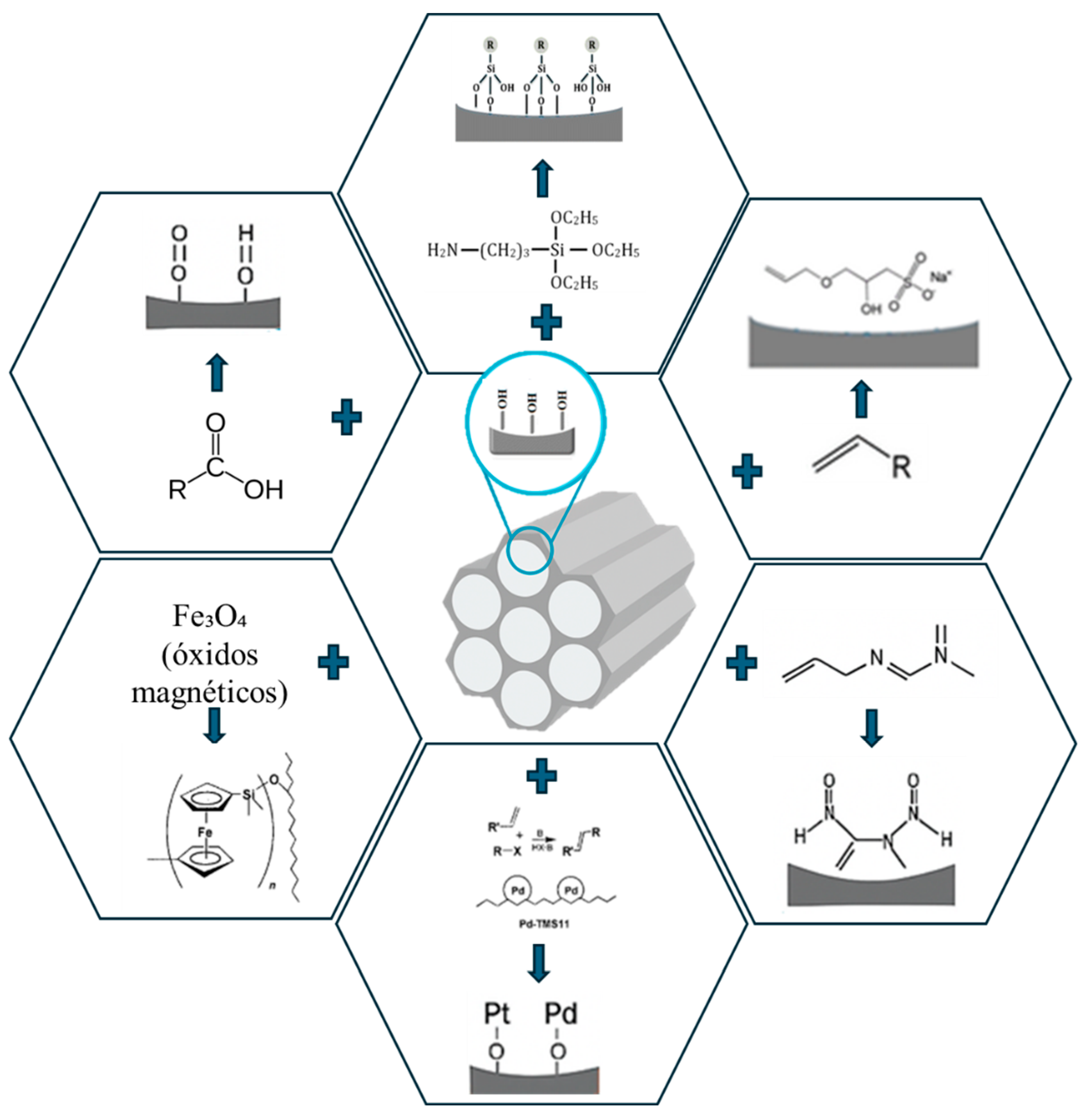
| Base Material | Functional Group / Modifier | Incorporation Method | Conditions | Application | Ref. |
|---|---|---|---|---|---|
| SBA-15 | –NH₂ (aminopropyl, APTES) |
Grafting | EtOH, 60–80 °C, 12–24 h | CO₂ capture, drug immobilization, VOCs | [10,37,45,46] |
| SBA-15 | Bis-Schiff base | 3-step anchoring (silanization + condensation) | Organic solvent, RT–80 °C | Selective removal of Pb(II) and other metals | [12,33] |
| SBA-15 | –COOH (carboxylic acid) | Post-synthesis oxidation | HNO₃, 50–80 °C, 6–12 h | Adsorption of dyes, metals | [15,43] |
| SBA-15 | –SH (tiol) | Co-condensation or grafting | pH acid, 25–50 °C | Adsorption of noble metals (Au, Ag, Pt) | [15,43] |
| SBA-15 | Fe₃O₄ (magnetic oxides) | Coprecipitation in mesostructure | 60–90 °C, pH 8–9 | Magnetic separation, reuse | [15,39] |
| SBA-15 | Pt/Pd o Ni, Cu | Impregnation + reduction | 200–300 °C, H₂ o Ar | Heterogeneous catalysis (hydrogenation, oxidation) | [33], [40,42,47,48] |
| SBA-15 | Organic groups (alkyls, phenyls) | Grafting or co-condensation | RT–120 °C, organic solvent | Hydrophobicity tuning, drug anchoring | [11,49] |
| SBA-15 | β- Cyclodextrin (β-CD) |
Modification by supramolecular anchoring | RT–50 °C, aqueous solvent | Adsorption of organic contaminants | [34,35] |
| SBA-15 | Azobenzene | Photoactive modification | RT–60°C Organic solvent UV (365 nm) or visible (>450 nm) light |
Photoactivated diffusion control, sensors | [3,38] |
| MCM-41 | –COOH (carboxylic acid | Post-synthesis oxidation | APS, HNO₃, 50–80 °C | Adsorption of dyes | [43] |
| KIT-6 | –SH (tiol) | Co-condensation | pH acid, 25–50 °C | Adsorption of heavy metals (Hg²⁺, Cd²⁺) | [15] |
| CMK-3 | –SO₃H (sulfonic acid) | Reflux with H₂SO₄ | 80–120 °C, 6–12 h | Esterification, acid catalysis | [32] |
| MOF-5 | –NH₂, –COOH | Reflux with H₂SO₄ | Solvothermal tempering | Selective adsorption, sensors, catalysis | [4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





