Submitted:
10 September 2025
Posted:
23 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Outcomes
2.2. Biomarkers
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Biomarker Description and Validation
3.3. Cognition, Depression and Anxiety
4. Discussion
4.1. Cognitive Impairment in PD
4.2. Depression and Anxiety in PD
4.3. Strengths and Limitations
5. Conclusion
Funding
Data Availability Statement
Conflicts of Interest
References
- Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers, 1: 2017;3, 2017. [CrossRef]
- Cong S, Xiang C, Zhang S, Zhang T, Wang H, Cong S. Prevalence and clinical aspects of depression in Parkinson's disease: A systematic review and meta-analysis of 129 studies. Neurosci Biobehav Rev, 1047. [CrossRef]
- Blundell EK, Grover LE, Stott J, Schrag A. The experience of Anxiety for people with Parkinson’s disease. npj Parkinson's Disease, 7: 2023;9(1), 2023. [CrossRef]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord, 8: 2008;23(6), 2008. [CrossRef]
- Ravina B, Camicioli R, Como PG, et al. The impact of depressive symptoms in early Parkinson disease. Neurology, 3: 2007;69(4), 2007. [CrossRef]
- Watson GS, Leverenz JB. Profile of cognitive impairment in Parkinson's disease. Brain Pathol, 20 May. [CrossRef]
- Factors impacting on quality of life in Parkinson's disease: results from an international survey. Mov Disord. [CrossRef]
- Luo Y, Qiao L, Li M, Wen X, Zhang W, Li X. Global, regional, national epidemiology and trends of Parkinson's disease from 1990 to 2021: findings from the Global Burden of Disease Study 2021. Front Aging Neurosci, 1498. [CrossRef]
- Chikatimalla R, Dasaradhan T, Koneti J, Cherukuri SP, Kalluru R, Gadde S. Depression in Parkinson's Disease: A Narrative Review. Cureus, 2775. [CrossRef]
- Hatcher-Martin JM, McKay JL, Pybus AF, et al. Cerebrospinal fluid biomarkers in Parkinson's disease with freezing of gait: an exploratory analysis. NPJ Parkinsons Dis, 1: 2021;7(1), 2021. [CrossRef]
- Irwin DJ, Lee VM, Trojanowski JQ. Parkinson's disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. [CrossRef]
- Liampas I, Kyriakoulopoulou P, Siokas V, et al. Apolipoprotein E Gene in α-Synucleinopathies: A Narrative Review. Int J Mol Sci, 2024. [CrossRef]
- Ronzitti G, Bucci G, Emanuele M, et al. Exogenous α-synuclein decreases raft partitioning of Cav2.2 channels inducing dopamine release. J Neurosci, 1: 2014;34(32), 2014. [CrossRef]
- Putzier I, Kullmann PH, Horn JP, Levitan ES. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J Neurosci, 1: 2009;29(49), 2009. [CrossRef]
- Wong YC, Luk K, Purtell K, et al. Neuronal vulnerability in Parkinson disease: Should the focus be on axons and synaptic terminals? Mov Disord, 1406. [CrossRef]
- Calabresi P, Mechelli A, Natale G, Volpicelli-Daley L, Di Lazzaro G, Ghiglieri V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death & Disease, 1: 2023;14(3), 2023. [CrossRef]
- Fan TS, Liu SC, Wu RM. Alpha-Synuclein and Cognitive Decline in Parkinson Disease. Life (Basel), 2021. [CrossRef]
- Miquel-Rio L, Sarriés-Serrano U, Pavia-Collado R, Meana JJ, Bortolozzi A. The Role of α-Synuclein in the Regulation of Serotonin System: Physiological and Pathological Features. Biomedicines, 2023. [CrossRef]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A, 19 May 1858. [CrossRef]
- Li HL, Wang HH, Liu SJ, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer's neurodegeneration. Proc Natl Acad Sci U S A, 3: 2007;104(9), 2007. [CrossRef]
- Stokin GB, Lillo C, Falzone TL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science, 1: 2005;307(5713), 2005. [CrossRef]
- Iqbal K, Grundke-Iqbal I, Zaidi T, et al. Defective brain microtubule assembly in Alzheimer's disease. Lancet, 4: 1986;2(8504), 1986. [CrossRef]
- Medeiros R, Baglietto-Vargas D, LaFerla FM. The role of tau in Alzheimer's disease and related disorders. CNS Neurosci Ther. [CrossRef]
- Dean J, Keshavan M. The neurobiology of depression: An integrated view. Asian J Psychiatr. [CrossRef]
- Liu C, Cholerton B, Shi M, et al. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson's disease. Parkinsonism Relat Disord. [CrossRef]
- Kwon EH, Tennagels S, Gold R, Gerwert K, Beyer L, Tönges L. Update on CSF Biomarkers in Parkinson's Disease. Biomolecules, 2022. [CrossRef]
- The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. [CrossRef]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. [CrossRef]
- Shin C, Park MH, Lee SH, et al. Usefulness of the 15-item geriatric depression scale (GDS-15) for classifying minor and major depressive disorders among community-dwelling elders. J Affect Disord, 3: 2019;259, 2019. [CrossRef]
- Oei TP, Evans L, Crook GM. Utility and validity of the STAI with anxiety disorder patients. Br J Clin Psychol. [CrossRef]
- Shaw LM, Waligorska T, Fields L, et al. Derivation of cutoffs for the Elecsys(®) amyloid β (1-42) assay in Alzheimer's disease. Alzheimers Dement (Amst). [CrossRef]
- Bittner T, Zetterberg H, Teunissen CE, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement, 20 May. [CrossRef]
- Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement, 1470. [CrossRef]
- Lim EW, Aarsland D, Ffytche D, et al. Amyloid-β and Parkinson's disease. J Neurol, 2605. [CrossRef]
- Goldman JG, Williams-Gray C, Barker RA, Duda JE, Galvin JE. The spectrum of cognitive impairment in Lewy body diseases. Mov Disord, 6: 2014;29(5), 2014. [CrossRef]
- Irizarry MC, Growdon W, Gomez-Isla T, et al. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. [CrossRef]
- Sugeno N, Takeda A, Hasegawa T, et al. Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J Biol Chem, 2: 2008;283(34), 2008. [CrossRef]
- Power JH, Barnes OL, Chegini F. Lewy Bodies and the Mechanisms of Neuronal Cell Death in Parkinson's Disease and Dementia with Lewy Bodies. Brain Pathol. [CrossRef]
- Klucken J, Poehler AM, Ebrahimi-Fakhari D, et al. Alpha-synuclein aggregation involves a bafilomycin A 1-sensitive autophagy pathway. Autophagy, 1 May. [CrossRef]
- Olanow CW, Perl DP, DeMartino GN, McNaught KS. Lewy-body formation is an aggresome-related process: a hypothesis. Lancet Neurol. [CrossRef]
- Cooper AA, Gitler AD, Cashikar A, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science, 3: 2006;313(5785), 2006. [CrossRef]
- Chin KS, Yassi N, Churilov L, Masters CL, Watson R. Prevalence and clinical associations of tau in Lewy body dementias: A systematic review and meta-analysis. Parkinsonism Relat Disord. [CrossRef]
- Gomperts, SN. Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia. Continuum (Minneap Minn), 4: Dementia). [CrossRef]
- Førland MG, Tysnes OB, Aarsland D, et al. The value of cerebrospinal fluid α-synuclein and the tau/α-synuclein ratio for diagnosis of neurodegenerative disorders with Lewy pathology. Eur J Neurol. [CrossRef]
- Fayyad M, Salim S, Majbour N, et al. Parkinson's disease biomarkers based on α-synuclein. J Neurochem. [CrossRef]
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci, 26 May 7281. [CrossRef]
- Palmio J, Suhonen J, Keränen T, Hulkkonen J, Peltola J, Pirttilä T. Cerebrospinal fluid tau as a marker of neuronal damage after epileptic seizure. Seizure. [CrossRef]
- Fischer DL, Menard M, Abdelaziz OZ, et al. Distinct subcellular localization of tau and alpha-synuclein in lewy body disease. Acta Neuropathologica Communications, 1: 2025;13(1), 2025. [CrossRef]
- Compta Y, Parkkinen L, O'Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain, 1: 5), 20 May 1493. [CrossRef]
- Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. [CrossRef]
- Vasconcelos B, Stancu IC, Buist A, et al. Heterotypic seeding of Tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol. [CrossRef]
- Williams T, Sorrentino Z, Weinrich M, Giasson BI, Chakrabarty P. Differential cross-seeding properties of tau and α-synuclein in mouse models of tauopathy and synucleinopathy. Brain Commun. [CrossRef]
- Colloby SJ, McAleese KE, Walker L, et al. Patterns of tau, amyloid and synuclein pathology in ageing, Alzheimer's disease and synucleinopathies. Brain, 13 May 1562. [CrossRef]
- Sahoo TA, Chand J, Kandy AT, Antony S, Subramanian G. Unravelling the Proteinopathic Engagement of α-Synuclein, Tau, and Amyloid Beta in Parkinson's Disease: Mitochondrial Collapse as a Pivotal Driver of Neurodegeneration. Neurochem Res, 1: 2025;50(3), 2025. [CrossRef]
- Ray S, Agarwal P. Depression and Anxiety in Parkinson Disease. Clin Geriatr Med. [CrossRef]
- Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Recognition and treatment of depression in Parkinson's disease. J Geriatr Psychiatry Neurol. [CrossRef]
- Patriquin MA, Mathew SJ. The Neurobiological Mechanisms of Generalized Anxiety Disorder and Chronic Stress. Chronic Stress (Thousand Oaks), 2017. [CrossRef]
- Leonard, BE. Stress, norepinephrine and depression. J Psychiatry Neurosci, S: Suppl(Suppl).
- van Dooren FE, Schram MT, Schalkwijk CG, et al. Associations of low grade inflammation and endothelial dysfunction with depression - The Maastricht Study. Brain Behav Immun. [CrossRef]
- Watt DF, Panksepp J. Depression: An evolutionarily conserved mechanism to terminate separation distress? A review of aminergic, peptidergic, and neural network perspectives. Neuropsychoanalysis. [CrossRef]
- Caudal D, Alvarsson A, Björklund A, Svenningsson P. Depressive-like phenotype induced by AAV-mediated overexpression of human α-synuclein in midbrain dopaminergic neurons. Experimental Neurology, 2: 2015;273, 2015. [CrossRef]
- Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. [CrossRef]
- Fregni F, Santos CM, Myczkowski ML, et al. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. Aug 2004;75(8):1171-4. [CrossRef]
- Twait EL, Wu JH, Kamarioti M, et al. Association of amyloid-beta with depression or depressive symptoms in older adults without dementia: a systematic review and meta-analysis. Transl Psychiatry, 2: 2024;14(1), 2024. [CrossRef]
- Rotter A, Lenz B, Pitsch R, Richter-Schmidinger T, Kornhuber J, Rhein C. Alpha-Synuclein RNA Expression is Increased in Major Depression. Int J Mol Sci, 2019. [CrossRef]
- Du T, Li G, Luo H, Pan Y, Xu Q, Ma K. Hippocampal alpha-synuclein mediates depressive-like behaviors. Brain Behav Immun. [CrossRef]
- Mollenhauer B, Caspell-Garcia CJ, Coffey CS, et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson's disease. Mov Disord, 1354. [CrossRef]
| MoCA test | |||
| ≥ 26 (n = 304) | < 26 (n = 134) | P-Value | |
| Age | 59.09 (±9.01) | 64.28 (±8.96) | <0.001 |
| Sex (Male) | 179 (58.9%) | 79 (59.0%) | 1 |
| White | 301 (99.0%) | 124 (92.5%) | <0.001 |
| Hispanic/Latino | 19 (6.2%) | 8 (6.0%) | 1 |
| Asian | 2 (0.7%) | 4 (3.0%) | 0.14 |
| Black | 0 | 3 (2.2%) | 0.05 |
| Education Years | 16.21 (±3.34) | 14.81 (±4.02) | <0.001 |
| MDS UPDRS Part III | 19.64 (±9.54) | 19.58 (±9.83) | 0.96 |
| Total STAI | 47.12 (±5.02) | 46.66 (±6.32) | 0.45 |
| Total GDS | 5.39 (±1.55) | 5.85 (±1.68) | 0.008 |
| MoCA Test | 28.01 (±1.37) | 22.66 (±3.09) | <0.001 |
| LRRK2 | 6 (5.6%) | 8 (13.8%) | 0.13 |
| Data are presented as mean (± standard deviation) or frequency (valid %). P-values refer to t-tests (age and education) or chi-squared tests (sex and ethnicity). LRKK2: LRKK2 gene positive Parkinson’s Disease. MDS UPDRS: Movement Disorders Society Unified Parkinson's Disease Rating Scale. MoCA: Montreal Cognitive Assessment. STAI: State-Trait Anxiety Inventory. | |||
| Depression Screening | |||
| Positive (GDS ≥ 5) (n = 349) |
Negative (GDS < 5) (n = 96) |
P-Value | |
| Age | 60.4 (±9.5) | 62.0 (±8.2) | 0.09 |
| Sex (Male) | 200 (57.3%) | 63 (65.6%) | 0.18 |
| Ethnicity | |||
| White | 336 (96.3%) | 95 (99.0%) | 0.32 |
| Hispanic/Latino | 24 (6.9%) | 4 (4.2%) | 0.47 |
| Asian | 5 (1.4%) | 1 (1.0%) | 1 |
| Black | 4 (1.1%) | 0 | 0.66 |
| Other | 8 (2.3%) | 0 | 0.53 |
| MDS UPDRS Part III | 19.5 (±9.9) | 20.1 (±8.9) | 0.59 |
| STAI | 47.5 (±5.2) | 45.1 (±5.9) | <0.001 |
| MoCA Test | 26.2 (±3.4) | 26.9 (±2.2) | 0.01 |
| LRRK2 | 12 (9.0%) | 2 (6.1%) | 0.84 |
| Education (Years) | 15.6 (±3.7) | 16.7 (±3.1) | 0.003 |
| Data are presented as mean (± standard deviation) or frequency (valid %). P-values refer to t-tests (age and education) or chi-squared tests (sex and ethnicity). GDS: Geriatric Depression Scale. LRKK2: LRKK2 gene positive Parkinson’s Disease. MDS UPDRS: Movement Disorders Society Unified Parkinson's Disease Rating Scale. MoCA: Montreal Cognitive Assessment. STAI: State-Trait Anxiety Inventory. | |||
| Anxiety screening | |||
| Positive (STAI > 40) (n = 395) |
Negative (STAI ≤ 40) (n = 50) |
P-Value | |
| Age | 60.6 (±9.3) | 61.8 (±8.6) | 0.35 |
| Sex (Male) | 231 (58.5%) | 32 (64.0%) | 0.55 |
| Ethnicity | |||
| White | 382 (96.7%) | 49 (98.0%) | 0.95 |
| Hispanic/Latino | 25 (6.3%) | 3 (6.0%) | 1 |
| Asian | 5 (1.3%) | 1 (2.0%) | 1 |
| Black | 4 (1.0%) | 0 | 1 |
| Other | 8 (2.1%) | 0 | 0.93 |
| Education Years | 15.9 (±3.7) | 15.2 (±2.7) | 0.09 |
| MDS UPDRS Part III | 19.4 (±9.7) | 21.9 (±9.3) | 0.08 |
| MoCA Test | 26.5 (±3.1) | 25.1 (±3.8) | 0.02 |
| LRRK2 | 12 (8.5%) | 2 (8.3%) | 1 |
| Data are presented as mean (± standard deviation) or frequency (valid %). P-values refer to t-tests (age and education) or chi-squared tests (sex and ethnicity). LRKK2: LRKK2 gene positive Parkinson’s Disease. MDS UPDRS: Movement Disorders Society Unified Parkinson's Disease Rating Scale. MoCA: Montreal Cognitive Assessment. STAI: State-Trait Anxiety Inventory. | |||
|
Healthy subjects (n = 108) |
Parkinson’s Disease (n = 300) |
P-Value | |
| Alpha-synuclein | 119.7 (±51.6) | 100.62 (±47.0) | < 0.01 |
| pTau | 17.9 (±9.3) | 14.1 (±5.0) | <0.001 |
| tTau | 195.0 (±83.7) | 163.0 (±55.5) | <0.001 |
| Aβ-42 | 1031.3 (±527.8) | 923.0 (±398.3) | 0.05 |
| Aβ-42 < 1100 | 71 (65.7%) | 217 (72.3%) | 0.244 |
| pTau/Aβ-42 > 0.022 | 14 (14.3%) | 19 (6.9%) | 0.05 |
| tTau/Aβ-42 > 0.26 | 16 (15.2%) | 21 (7.2%) | 0.03 |
| Data are presented as mean (± standard deviation) or frequency (valid %). P-values refer to t-tests. | |||
|
Overall (n = 300) |
MoCA ≥ 26 (n = 213) |
MoCA < 26 (n = 87) |
P-Value | |
| Alpha-synuclein | 100.6 (±47.0) | 98.7 (±42.3) | 106.2 (±58.8) | 0.46 |
| Alpha-synuclein > p75 | 27 (17.2%) | 20 (17.1%) | 7 (17.5%) | 1 |
| pTau | 14.09 (±4.97) | 13.64 (±4.49) | 15.1 (±5.8) | 0.02 |
| tTau | 163.0 (±55.4) | 158.1 (±51.9) | 174.2 (±61.6) | 0.01 |
| Aβ-42 | 923.0 (±398.3) | 928.6 (±388.4) | 909.1 (±423.6) | 0.71 |
| Aβ-42 < 1100 | 217 (72.3%) | 152 (71.4%) | 65 (74.7%) | 0.65 |
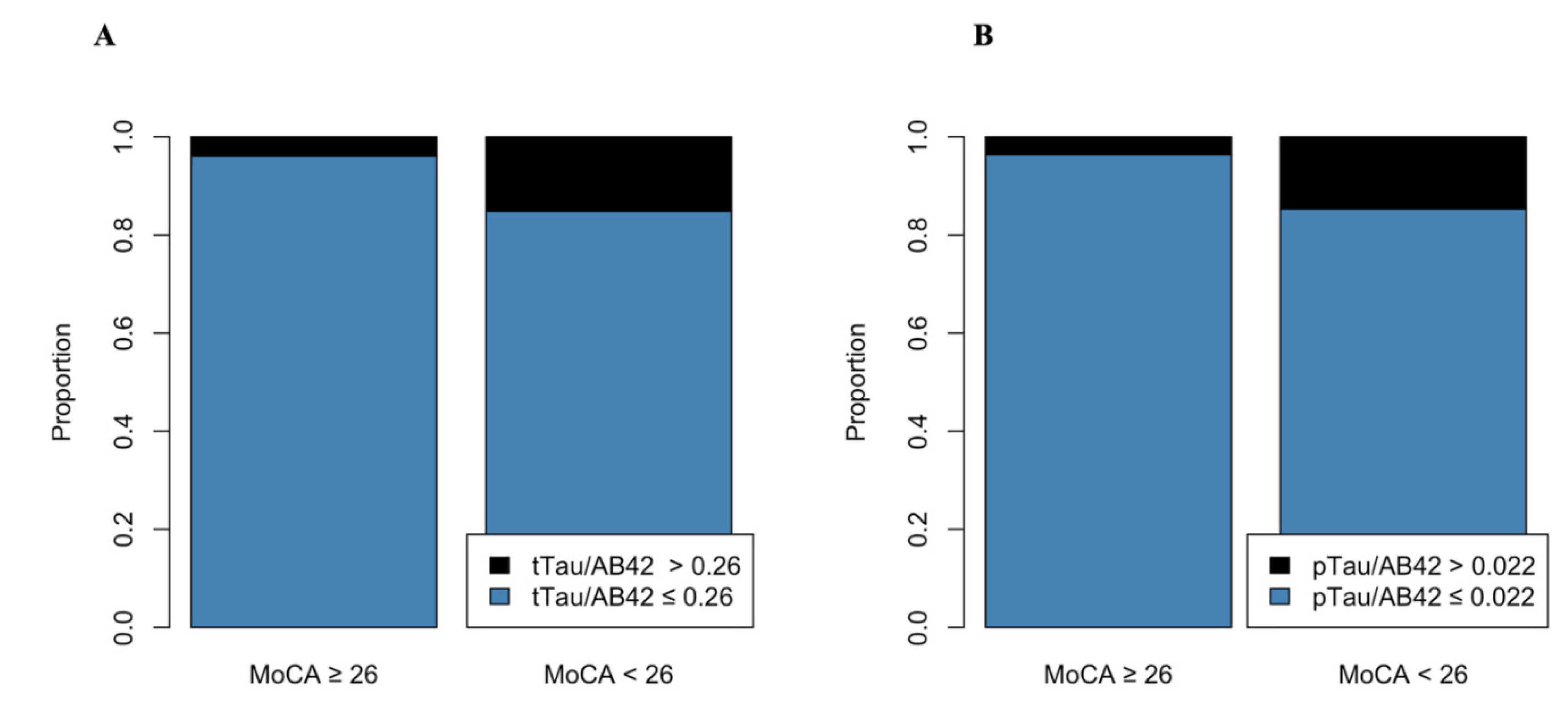
| pTau/Aβ-42 > 0.022 | 19 (6.9%) | 7 (3.6%) | 12 (14.6%) | 0.003 |
| tTau/Aβ-42 > 0.26 | 21 (7.2%) | 8 (3.9%) | 13 (15.1%) | 0.002 |
| Data are reported as mean (± standard deviation) P-values refer to t-tests. | ||||
| Univariable | OR | 95% Confidence Interval | P-value | |
| Alpha-synuclein | 1.003 | 0.996 | 1.011 | 0.38 |
| pTau | 1.06 | 1.01 | 1.11 | 0.01 |
| tTau | 1.005 | 1.001 | 1.009 | 0.01 |
| Aβ-42 | 0.999 | 0.999 | 1.000 | 0.70 |
| Alpha-synuclein > p75 | 1.03 | 0.37 | 2.56 | 0.95 |
| Aβ-42 < 1100 | 1.19 | 0.68 | 2.12 | 0.56 |
| pTau/Aβ-42 > 0.022 | 4.53 | 1.75 | 12.6 | < 0.01 |
| tTau/Aβ-42 > 0.26 | 4.40 | 1.78 | 11.5 | < 0.01 |
| Multivariable* | OR | 95% Confidence Interval | P-value | |
| Alpha-synuclein | 1.00 | 0.99 | 1.01 | 0.69 |
| pTau | 1.02 | 0.98 | 1.07 | 0.31 |
| tTau | 1.00 | 0.99 | 1.01 | 0.36 |
| Aβ-42 | 0.999 | 0.998 | 1.00 | 0.35 |
| Alpha-synuclein > p75 | 0.79 | 0.26 | 2.11 | 0.65 |
| Aβ-42 < 1100 | 1.35 | 0.75 | 2.51 | 0.32 |
| pTau/Aβ-42 > 0.022 | 4.64 | 1.67 | 13.8 | < 0.01 |
| tTau/Aβ-42 > 0.26 | 4.18 | 1.60 | 11.5 | < 0.01 |
| The regressions evaluated the association between biomarkers and cognitive decline (MoCA < 26). *Adjusted for age and education years | ||||
| Overall | GDS ≥ 5 | GDS < 5 | P-Value | |
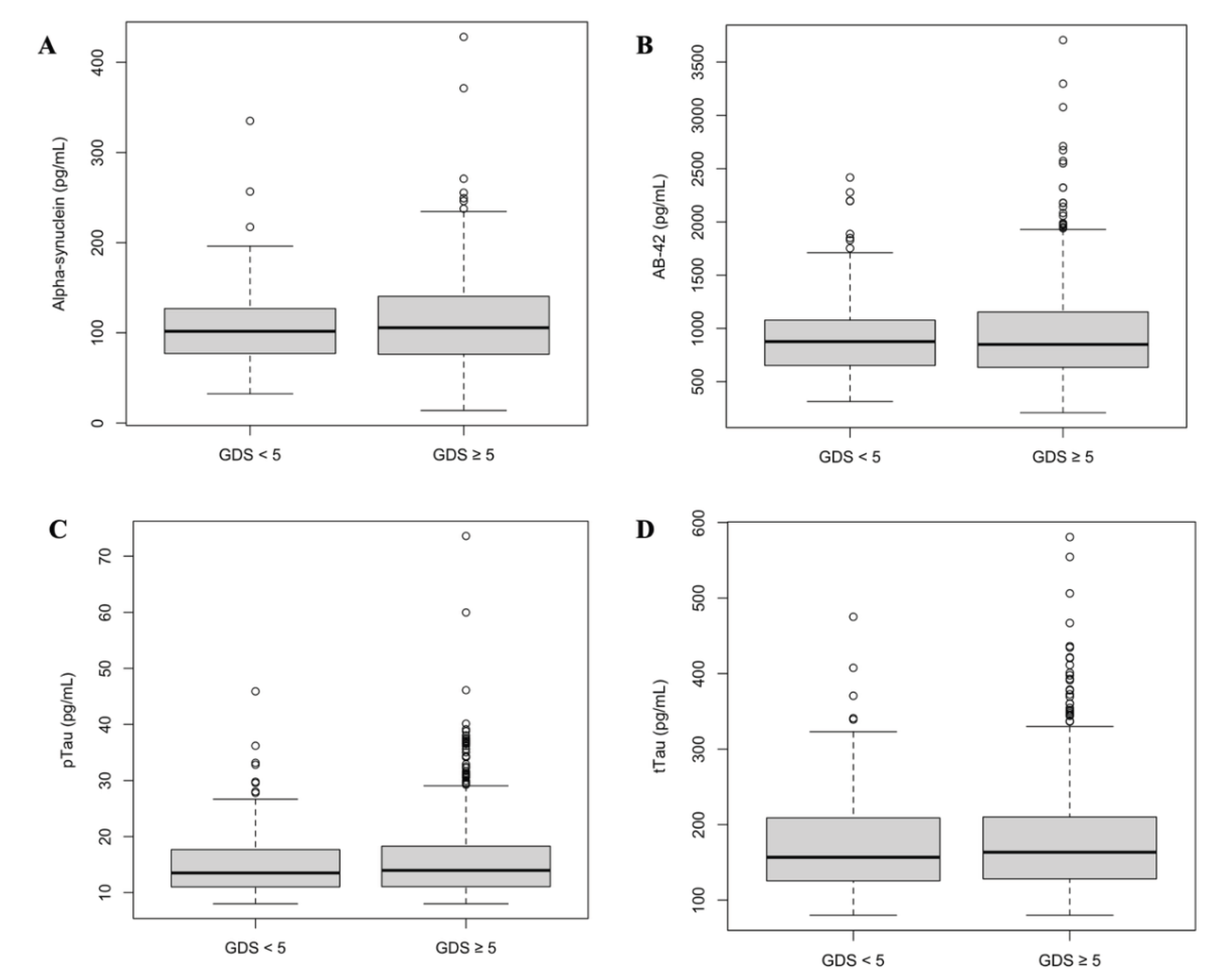
| Alpha-synuclein | 100.62 (±47.0) | 102.9 (±49.6) | 94.5 (±39.2) | 0.27 |
| pTau | 14.09 (±5.0) | 14.0 (±4.8) | 14.3 (±5.5) | 0.62 |
| tTau | 163.0 (±55.5) | 161.1 (±53.8) | 169.5 (±60.5) | 0.25 |
| Aβ-42 | 924.2 (±397.8) | 917.7 (±413.1) | 946.1 (±343.7) | 0.57 |
| Alpha-synuclein > p75 | 27 (17.2%) | 22 (19.3%) | 5 (11.6%) | 0.37 |
| Aβ-42 < 1100 | 217 (72.3%) | 167 (72.3%) | 50 (72.5%) | 0.99 |
| pTau/Aβ-42 > 0.022 | 19 (6.9%) | 16 (7.7%) | 3 (4.5%) | 0.53 |
| tTau/Aβ-42 > 0.26 | 21 (7.2%) | 17 (7.6%) | 4 (5.9%) | 0.83 |
| Overall | GDS ≥ 9 | GDS < 9 | P-Value | |
| Alpha-synuclein | 100.62 (±47.0) | 100.60 (±47.3) | 101.39 (±33.4) | 0.97 |
| pTau | 14.09 (±5.0) | 14.14 (±5.0) | 12.57 (±4.1) | 0.20 |
| tTau | 163.0 (±55.5) | 163.7 (±55.3) | 146.85 (±58.3) | 0.29 |
| Aβ-42 | 924.2 (±397.8) | 930.0 (±400.7) | 770.4 (±286.0) | 0.1 |
| Alpha-synuclein > p75 | 27 (17.2%) | 27 (17.5%) | 0 | 0.98 |
| Aβ-42 < 1100 | 217 (72.3%) | 208 (72.0%) | 9 (81.8%) | 0.71 |
| pTau/Aβ-42 > 0.022 | 19 (6.9%) | 19 (7.2%) | 0 | 0.87 |
| tTau/Aβ-42 > 0.26 | 21 (7.2%) | 21 (7.5%) | 0 | 0.73 |
| Data are reported as mean (± standard deviation) P-values refer to t-tests. | ||||
| Univariable | OR | 95% Confidence Interval | P-value | |
| Alpha-synuclein | 1.004 | 0.996 | 1.013 | 0.32 |
| pTau | 0.987 | 0.942 | 1.037 | 0.59 |
| tTau | 0.997 | 0.993 | 1.002 | 0.22 |
| Aβ-42 | 0.999 | 0.999 | 1.001 | 0.60 |
| Alpha-synuclein > p75 | 1.82 | 0.68 | 5.74 | 0.26 |
| Aβ-42 < 1100 | 0.99 | 0.53 | 1.79 | 0.98 |
| pTau/Aβ-42 > 0.022 | 1.79 | 0.57 | 7.86 | 0.37 |
| tTau/Aβ-42 > 0.26 | 1.31 | 0.47 | 4.69 | 0.63 |
| Multivariable* | OR | 95% Confidence Interval | P-value | |
| Alpha-synuclein | 1.005 | 0.997 | 1.014 | 0.25 |
| pTau | 0.990 | 0.943 | 1.042 | 0.71 |
| tTau | 0.997 | 0.993 | 1.002 | 0.30 |
| Aβ-42 | 0.999 | 0.999 | 1.001 | 0.60 |
| Alpha-synuclein > p75 | 2.03 | 0.75 | 6.54 | 0.19 |
| Aβ-42 < 1100 | 0.98 | 0.53 | 1.78 | 0.95 |
| pTau/Aβ-42 > 0.022 | 2.03 | 0.64 | 9.08 | 0.28 |
| tTau/Aβ-42 > 0.26 | 1.48 | 0.51 | 5.36 | 0.50 |
| The regressions evaluated the association between biomarkers and positive depression screening (GDS ≥ 5). *Adjusted for age and education years | ||||
| . | Anxiety screening | ||
| Positive (STAI > 40) (n = 265) |
Negative (STAI ≤ 40) (n = 35) |
P-value | |
| Alpha-synuclein | 100.7 (±47.7) | 100.3 (±41.6) | 0.97 |
| Alpha-synuclein > p75 | 23 (16.4%) | 4 (23.5%) | 0.69 |
| pTau | 14.1 (±5.03) | 14.2 (±4.6) | 0.87 |
| tTau | 163.1 (±56.6) | 162.6 (±47.3) | 0.95 |
| Aβ-42 | 922.0 (±404.8) | 930.4 (±350.5) | 0.90 |
| Aβ-42 < 1100 | 192 (72.5%) | 25 (71.4%) | 1 |
| pTau/Aβ-42 > 0.022 | 18 (7.5%) | 1 (3.0%) | 0.57 |
| tTau/Aβ-42 > 0.26 | 20 (7.8%) | 1 (2.9%) | 0.48 |
| Data are reported as mean (± standard deviation) P-values refer to t-tests. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





