1. Introduction
Autism Spectrum Disorder (ASD) is a heterogeneous neurodevelopmental condition defined by persistent deficits in social communication and interaction, alongside restricted interests and repetitive behaviors [
1]. Affecting approximately 1–2% of the global population [
2,
3], ASD imposes a substantial individual, familial, and societal burden [
4]. Beyond its core diagnostic features, many individuals exhibit sensory processing abnormalities [
5], impaired cognitive flexibility [
6], and difficulties in emotional and behavioral regulation [
7]. These associated features often exacerbate functional impairment and reduce participation in educational, social, and community life.
The neurobiological underpinnings of ASD are multifaceted [
8], involving genetic, epigenetic, and environmental factors that converge on atypical brain development [
9]. Converging evidence from neuroimaging and electrophysiological studies has highlighted the role aberrant neural oscillations [
10] and altered functional connectivity across large-scale networks [
11]. Such dysregulation affects the temporal coordination of neural activity both within and between cortical and subcortical structures [
12,
13], thereby compromising the neural basis of perception, cognition, socio-emotional behaviors, and adaptive functioning [
14]. Specifically, dysregulated oscillatory activity has been linked to impaired sensory integration [
15,
16,
17], diminished socio-communicative responsiveness [
14], and increased behavioral rigidity domains that are often resistant to conventional therapeutic strategies [
15,
18].
Current treatment paradigms for ASD primarily rely on behavioral and educational interventions [
19], sometimes supplemented by symptom-targeted pharmacotherapy [
20]. While these approaches can yield measurable improvements, their impact is often partial and they do not directly address the neurophysiological mechanisms of the disorder. This has spurred interest in novel interventions aimed at modulating brain network dynamics, with the goal of promoting adaptive plasticity and improving functional outcomes. Among these, non-invasive neurobiological modulation techniques have emerged as promising adjunctive options, capable of influencing endogenous neural activity without the risks associated with invasive procedures [
21,
22]. However, most existing non-invasive neuromodulation methods require individualized parameter adjustment, may show variable reproducibility, and are not always feasible for intensive treatment cycles in pediatric populations.
Radio Electric Asymmetric Conveyer (REAC) technology was developed to interact with the body’s endogenous bioelectric activity in a targeted and asymmetrically conveyed manner, promoting functional optimization of neural circuits [
23,
24]. Its therapeutic protocols are pre-set and operator-independent. By acting only where bioelectrical alterations are present, REAC interventions avoid unwanted interference with normal function, while facilitating the progressive restoration of optimal neural activity. Previous REAC applications in neurodevelopmental and neuropsychiatric contexts particularly through Neuro Postural Optimization (NPO), Neuro Psycho Physical Optimization (NPPO), and Neuro Psycho Physical Optimization—Cervico-Brachial (NPPO-CB) protocols have demonstrated improvements in cognitive performance [
25], behavioral adaptation, and emotional regulation [
26,
27,
28,
29]. In ASD populations, these protocols have been associated with enhanced adaptive functioning and reduced behavioral dysregulation [
21,
30,
31].
Building on this foundation, the REAC brain wave optimization (BWO) Neurodevelopment—Autism (BWO ND-A) protocol was specifically conceived to address electrophysiological patterns frequently altered in ASD. It employs a standardized sequence of endogenous modulation steps targeting neural oscillations implicated in sensory integration, socio-emotional processing, and attentional control. By restoring network coherence and improving cross-frequency coupling, BWO ND-A seeks to enhance information processing and promote adaptive behavioral and cognitive responses.
Given its specific neurofunctional targeting and the encouraging evidence from previous REAC applications in ASD [
21,
30,
31], the BWO ND-A protocol preceded by a single NPO session to prime central nervous system modulatory capacity [
23] represents a promising therapeutic option with potential impact beyond the clinical setting. By addressing core and associated symptoms through standardized, reproducible neurobiological modulation, this approach may offer tangible benefits for individuals with ASD and their families, complementing existing behavioral and educational interventions. The present study provides real-world evidence of its multidomain efficacy, supporting its integration not only in clinical practice but also in broader social and community-based initiatives aimed at improving quality of life and social participation in ASD.
2. Materials and Methods
2.1. Overview of REAC Technology and BWO ND-A Protocol
REAC technology (Radio Electric Asymmetric Conveyer) neurobiological modulation platform designed to interact with endogenous bioelectric activity [
23,
24,
32]. Treatments are delivered through Asymmetric Conveyor Probes (ACPs), which asymmetrically convey very low-intensity radio electric fields to the body. All therapeutic protocols are pre-set, operator-independent, and standardized to ensure reproducibility and safety. Unlike other neuromodulation techniques, REAC acts selectively on sites with altered endogenous bioelectrical activity [
33], without affecting areas functioning within normal parameters.
The BWO ND-A protocol is specifically configured to modulate brain oscillatory patterns frequently altered in ASD [
34,
35], with the aim of improving sensory integration [
15,
16,
17], socio-communicative responsiveness, and behavioral regulation [
3]. It consists of a standardized and non-modifiable sequence of modulation steps, developed from prior clinical and neurophysiological research [
35,
36,
37], and is delivered identically across all patients to ensure comparability of outcomes.
Within the REAC neurobiological modulation platform, Brain Wave Optimization (BWO) protocols are preconfigured to modulate specific patterns of brain oscillatory activity. Standard or “base” BWO protocols target a single specific frequency within a fundamental brain rhythm, such as BWO-Delta (deep sleep rhythms), BWO-Theta (drowsiness and meditative states), BWO-Alpha (relaxation and quiet wakefulness), and BWO-Gamma (complex cognitive processing). Each of these base protocols acts on functions that are highly specific to the selected frequency.
Over time, clinical and neurophysiological research revealed that many functional alterations involve not only individual frequency bands but also their dynamic cross-frequency orchestration [
35,
36,
37]. This led to the development of “composite” BWO protocols, each consisting of a specific sequence of modulations drawn from the base protocols, tailored to specific clinical profiles.
The Neuro Psycho Physical Optimization—Cervico-Brachial (NPPO-CB) was the first clinically introduced BWO protocol, historically referred to without the BWO suffix for ease of communication [
38]. It primarily operates within the delta frequency range and is aimed at subcortical neurobiological modulation for emotional adaptation.
From this experience, further composite protocols were developed. The NPPO-CB BWO Neurodevelopment—Autism (BWO ND-A) used in this study combines modulations from multiple base frequency bands to re-orchestrate altered brain rhythms frequently observed in ASD, targeting sensory over/under-responsiveness, socio-communicative impairments, and behavioral inflexibility.
2.2. Study Design and Setting
This retrospective observational pre–post clinical study was conducted at a single clinical center under the coordination of the Rinaldi Fontani Institute & Foundation (Florence, Italy), with treatments delivered in accordance with the same standardized clinical protocols and data collection procedures established by the coordinating institute. The aim was to assess changes in global and domain-specific symptomatology in individuals with Autism Spectrum Disorder (ASD) following the REAC BWO Neurodevelopment—Autism (BWO ND-A) protocol, preceded by a single NPO session. The retrospective design was chosen to capture real-world data from routine clinical practice, allowing the inclusion of all consecutive eligible cases over the observation period. All procedures were performed according to established clinical practice, by experienced personnel trained in REAC methodology.
2.3. Participants
Participants were 39 children with a prior clinical diagnosis of ASD, confirmed by specialist evaluation. This cohort represents the entire population of ASD patients treated in the first seven months of the year at participating center, with no prior history of REAC treatments. Inclusion criteria were: (1) age between 4 and 13 years, (2) confirmed ASD diagnosis, (3) ability to complete the planned treatment cycle, and (4) stable pharmacological and behavioral treatment regimens for at least eight weeks prior to study entry. Exclusion criteria included: (1) acute medical conditions, (2) recent changes in ongoing therapies, and (3) inability to comply with the treatment schedule.
Of the 39 participants, 31 (79.5%) were male and 8 (20.5%) were female, with a mean age of 7.85 ± 2.90 years (range 4–13). Age distribution was as follows: 14 participants (35.9%) were aged 4–6 years, 13 (33.3%) were 7–9 years old, and 12 (30.8%) were 10–13 years old. All completed the treatment cycle without adverse events (
Table 1).
Baseline demographic and clinical data were collected from medical records and verified by the treating clinicians.
2.4. Intervention
All participants first received a single session of the NPO protocol, lasting only a few milliseconds, designed to optimize central nervous system adaptive capacity and prime the response to subsequent REAC treatments [
23,
39].
Following NPO, each underwent the BWO ND-A protocol: 18 sessions of ~8 minutes each, over one to two weeks, delivered 3–4 times per day with at least one hour between sessions (max four per day), according to established REAC safety parameters.
Treatments used a REAC medical device (BENE mod 110, ASMED, Scandicci, Italy) with asymmetric conveyer probe (ACPs) positioned in the cervico-brachial area per standard procedure. The ACP does not deliver current but channels interaction between the device’s radio electric field and endogenous bioelectrical activity. Protocol parameters are fixed and cannot be modified by the operator.
2.5. Outcome Measures
The primary outcome was the change in ATEC total score from pre- to post-intervention [
40]. Secondary outcomes were changes in its four subscales: (1) Speech/Language/Communication, (2) Sociability, (3) Sensory/Cognitive Awareness, and (4) Health/Physical/Behavior [
41]. Assessments occurred within one week before and after the treatment cycle. The ATEC, completed by parents or primary caregivers, has been validated for ASD research [
41,
42] and used in prior REAC studies [
31].
2.6. Statistical Analysis
Analyses used SPSS v.22 (IBM Corp., Armonk, NY, USA). Continuous variables are mean ± SD. Pre–post differences were tested with paired-sample t-tests (p < 0.05, two-tailed). Effect sizes were calculated as Cohen’s dz with 95% CI. No imputation for missing data was necessary as all participants completed the study.
2.7. Ethical Considerations
The study was approved by the Institutional Review Board of the Rinaldi Fontani Institute (Protocol: IRB-RFI-2025-07-1-2). Given its retrospective nature and use of standard clinical practice, additional local IRB approvals were not required. All data were anonymized, and written informed consent for research use was obtained from parents or legal guardians. All procedures were conducted in accordance with the Declaration of Helsinki.
3. Results
All thirty-nine participants completed the treatment cycle, and no adverse events were reported during the study period. Treatment adherence was 100%, with no interruptions or dropouts, and all sessions were performed according to the standardized protocol.
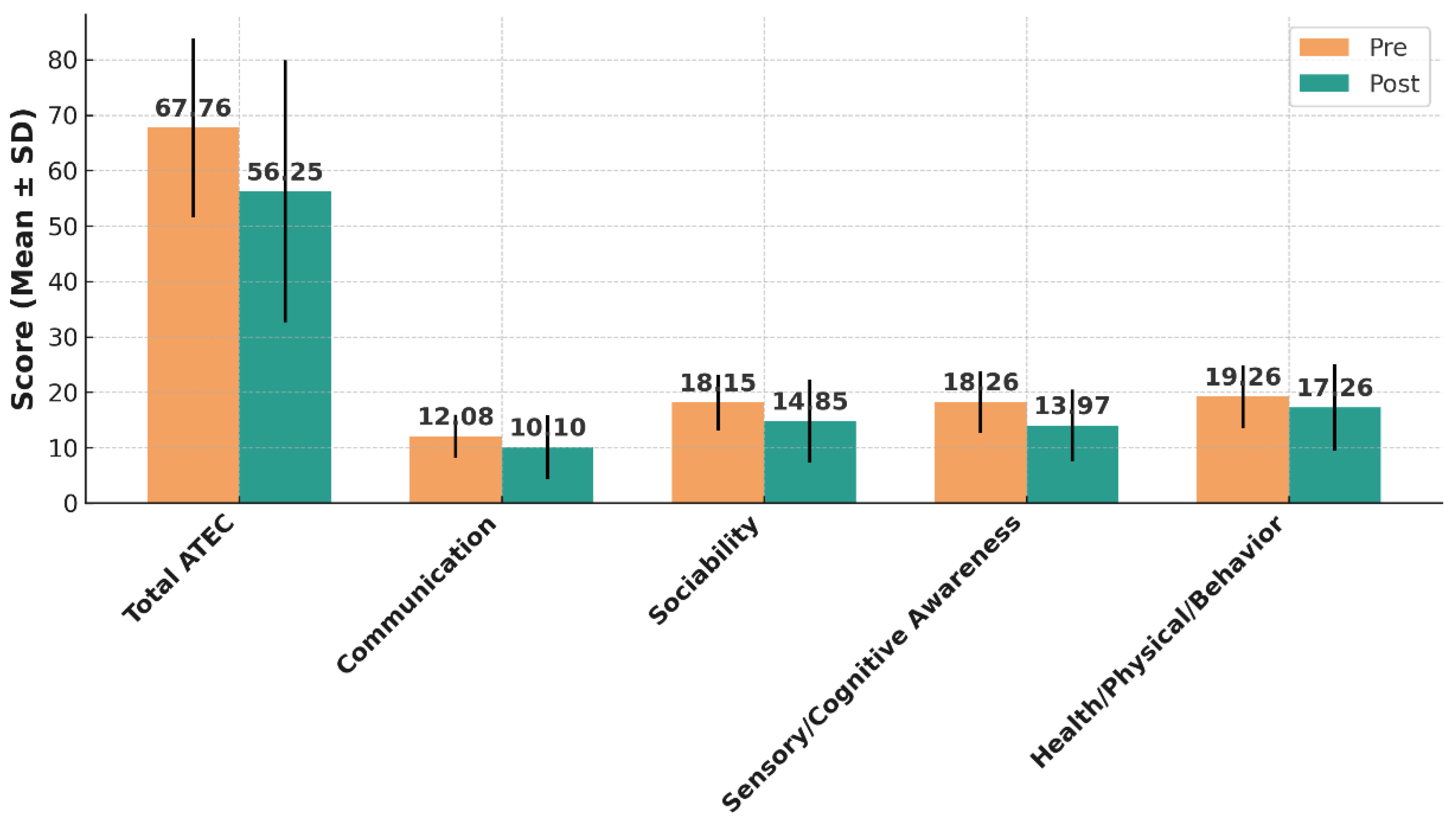
At baseline, the mean total ATEC score for the cohort was 67.76 ± 16.11. Following the intervention, the mean total score decreased to 56.25 ± 23.66, corresponding to a mean reduction of 11.51 ± 14.48 points (95% CI: 6.98 to 16.03; t(38) = 4.90; p < 0.0001). This change represents a large treatment effect, as indicated by a Cohen’s dz value of 0.78 (95% CI: 0.43 to 1.11) (
Table 2 and
Figure 1).
Clinically significant improvements, defined as a reduction of at least eight points in the total ATEC score, were observed in 23 participants (59.0%; 95% CI: 42.1%–74.4%). No clinically relevant change (< 8 points) was recorded in 12 participants (30.8%), and clinically significant worsening (≥ 8-point increase) was observed in 4 participants (10.3%) (
Table 3). The subgroup of participants showing worsening did not report adverse behavioral effects but exhibited fluctuations in caregiver perception of symptom severity.
Improvements were evident across all four ATEC subscales (
Table 2 and
Figure 1). In the Communication domain, mean scores decreased from 12.08 ± 3.86 to 10.10 ± 5.74 (mean change 1.97 ± 5.10; 95% CI: 0.33 to 3.60; p = 0.021; dz = 0.38, 95% CI: 0.06–0.69). In Sociability, baseline scores of 18.15 ± 5.01 declined to 14.85 ± 7.51 (mean change 3.31 ± 6.48; 95% CI: 1.19 to 5.43; p = 0.0032; dz = 0.50, 95% CI: 0.17–0.82). Sensory/Cognitive Awareness scores demonstrated the largest improvement, dropping from 18.26 ± 5.56 to 13.97 ± 6.48 (mean change 4.28 ± 6.23; 95% CI: 2.25 to 6.31; p = 0.00014; dz = 0.68, 95% CI: 0.33–1.02). In the Health/Physical/Behavior domain, mean scores fell from 19.26 ± 5.70 to 17.26 ± 7.75 (mean change 2.00 ± 5.71; 95% CI: 0.14 to 3.86; p = 0.036; dz = 0.35, 95% CI: 0.03–0.66).
3.1. Outcomes by Age Group
Participants aged 4–6 years (n = 14) had the largest mean ATEC reduction (−13.4 points), followed by ages 7–9 years (−11.1) and 10–13 years (−9.6). Although differences between groups were not statistically significant, the trend suggests potentially greater responsiveness in younger children.
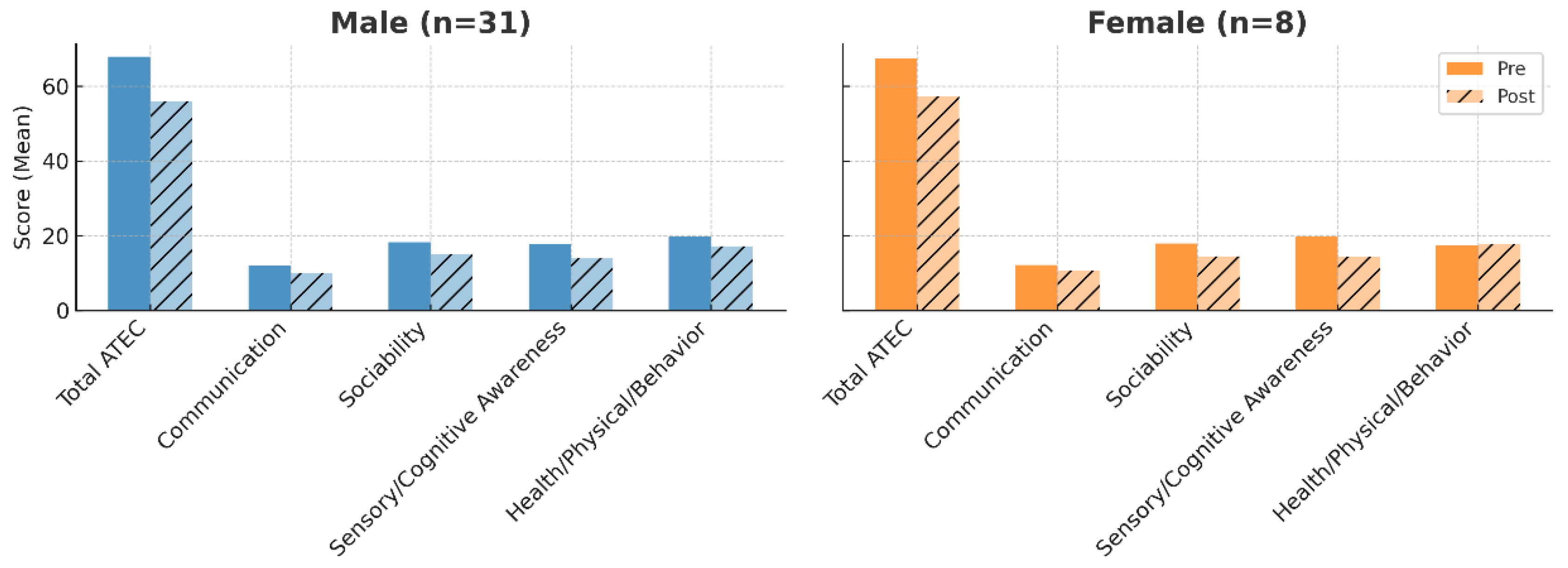
3.2. Outcomes by Sex
Both sexes improved in all outcome measures. In males, the mean total ATEC score decreased from 67.87 to 55.96; in females, from 65.21 to 54.91. Females showed slightly greater relative gains in Sensory/Cognitive Awareness, while males had marginally larger improvements in Sociability (
Figure 2).
3.3. Clinical Observations
Caregivers frequently reported qualitative improvements consistent with the measured outcomes, including greater social engagement, improved responsiveness to verbal cues, and reduced behavioral rigidity. Additional comments described better tolerance of sensory stimuli, fewer tantrums, and more consistent eye contact. Although not formally quantified, these observations support the multidomain benefits detected by standardized assessments.
4. Discussion
This study provides real-world clinical evidence supporting the efficacy of the REAC BWO Neurodevelopment—Autism (BWO ND-A) protocol, preceded by a single Neuro Postural Optimization (NPO) session, in improving core and associated symptoms of ASD. The observed reduction in total ATEC scores [
40,
41], with a large effect size (Cohen’s dz = 0.78, 95% CI: 0.43–1.11) [
43] and a clinically significant improvement in 59% of participants, indicates a substantial impact across multiple functional domains. These changes were achieved in a heterogeneous pediatric cohort aged 4–13 years, with a male-to-female ratio consistent with epidemiological trends in ASD prevalence [
44]. The improvement pattern, with the largest gains in Sensory/Cognitive Awareness and Sociability, closely matches the neurofunctional targets of the BWO ND-A sequence, suggesting a specific rather than diffuse neuromodulatory effect.
From a neurophysiological standpoint, ASD is characterized by atypical oscillatory patterns [
34,
35] and disrupted connectivity in large-scale neural networks [
11], affecting sensory integration, socio-communicative responsiveness, and cognitive flexibility [
3]. The BWO ND-A protocol is specifically designed to target and modulate these electrophysiological patterns through a standardized, operator-independent sequence of endogenous bioelectric modulation steps. By enhancing coherence within frequency bands and restoring balanced cross-frequency coupling, the protocol may facilitate more efficient information flow between cortical and subcortical structures, thereby improving functional outcomes in targeted domains.
The most pronounced improvements in this study were observed in the Sensory/Cognitive Awareness and Sociability subscales, domains closely aligned with the neurofunctional targets of the protocol. Gains in Speech/Language/Communication and Health/Physical/Behavior, although of moderate effect size, further highlight the multidomain benefits of BWO ND-A. Qualitative reports from caregivers—such as increased social engagement, improved responsiveness to verbal cues, reduced behavioral rigidity, and better tolerance of sensory input—were consistent with quantitative findings and provide additional insight into the real-life impact of the intervention.
Previous studies on REAC neurobiological modulation in ASD, particularly those employing NPO and NPPO protocols, have demonstrated improvements in cognitive, behavioral, and emotional regulation domains [
21,
30,
31]. In these earlier protocols, NPO was used to prime the central nervous system’s modulatory capacity before delivering broader neuromodulatory interventions [
23,
39]. The present study follows this same methodological approach but applies a more targeted protocol—BWO ND-A—designed specifically to modulate cortical oscillatory sequences associated with ASD. This targeted approach may help explain why improvements were more concentrated in sensory [
17] and social domains [
36,
45], while still extending to communication [
46] and behavior regulation.
When compared to other non-invasive neuromodulation modalities, such as transcranial direct current stimulation (tDCS) [
47] and repetitive transcranial magnetic stimulation (rTMS) [
48], BWO ND-A offers several advantages: no direct current delivery, operator-independent administration, no need for individualized parameter adjustment, short sessions, and the possibility of multiple daily applications without increased risk. These features make it suitable for pediatric populations, including those less tolerant of conventional neuromodulation.
The consistency of improvements across sexes and age groups supports generalizability, although trends toward greater benefit in younger participants suggest that early intervention may yield enhanced neuroplastic effects consistent with literature on critical developmental periods. This warrants confirmation in larger, stratified samples.
The single-group pre–post design without a control group limits causal inference, as placebo effects or regression to the mean cannot be entirely excluded. Nevertheless, several considerations reduce the likelihood that the observed changes were solely due to placebo. The magnitude of improvements, particularly in Sensory/Cognitive Awareness, is consistent with neurofunctional targets previously documented in REAC protocols [
23,
24,
28,
29,
32] and is unlikely to result from expectancy alone. Moreover, the short duration of the intervention cycle (one to two weeks) makes it improbable that natural developmental progression could account for the observed effects. These findings are also in line with prior REAC studies conducted in independent cohorts and settings, where similar multidomain improvements were consistently observed [
28,
29]. Additionally, the multidomain structure of the ATEC reduces the risk that isolated subjective impressions could significantly influence the total score.
The sample size, while adequate for detecting large effects, remains modest, and the heterogeneity in age and baseline severity may have influenced responsiveness. The absence of objective neurophysiological measures, such as quantitative EEG, limits the mechanistic interpretation of the results, although previous REAC research has demonstrated protocol-specific reorganization of brain network activity [
23,
24]. Future research should therefore aim to replicate these findings in larger, multicenter observational cohorts, ideally with age- and severity-matched comparison groups to strengthen causal inference. Where possible, integrating non-invasive neurophysiological monitoring could provide direct evidence of oscillatory modulation and clarify the neural mechanisms underlying clinical improvements. Long-term follow-up would also be valuable to assess the persistence of benefits and the potential cumulative effects of repeated treatment cycles. Given the safety, tolerability, and reproducibility of the intervention, prospective randomized controlled trials appear both feasible and ethically justifiable.
5. Conclusions
In this real-world observational study, the REAC BWO Neurodevelopment—Autism (BWO ND-A) protocol, preceded by a single NPO session, produced significant multidomain improvements in children with ASD, with a large overall effect size and clinically meaningful changes in the majority of participants. The most notable gains were in sensory/cognitive awareness and sociability domains, which are closely linked to the specific neurofunctional targets of the protocol and suggest a targeted neuromodulatory action rather than a non-specific improvement. Additional benefits were observed in communication and behavioral regulation, further confirming the multidomain relevance of the intervention.
These findings, which are consistent with previous REAC neurobiological modulation studies, strengthen the rationale for integrating BWO ND-A as a safe, non-invasive, and operator-independent adjunct to established therapeutic programs for ASD. The standardization of the protocol ensures reproducibility across different settings, making it adaptable not only to specialized clinical environments but also to broader community and educational contexts where early and intensive interventions are beneficial.
Given the absence of reported adverse events, the short treatment duration, and the possibility of multiple daily sessions, BWO ND-A emerges as a feasible option for large-scale implementation in ASD care pathways. While further controlled prospective studies are necessary to confirm these results and clarify long-term outcomes, the present evidence supports the inclusion of this approach in multidisciplinary management strategies aimed at improving adaptive functioning, social participation, and quality of life for individuals with ASD and their families.
Author Contributions
Conceptualization, A.R., S.R. and V.F.; methodology, S.R. and V.F.; validation, S.R., V.F. and A.R.; formal analysis, A.R.; investigation, A.R. and H.A.B.M. (patient management and clinical assessments); data curation, A.R. and H.A.B.M.; writing—original draft preparation, A.R.; writing—review and editing, S.R., V.F. and H.A.B.M.; visualization, A.R.; supervision, S.R. and V.F.; project administration, S.R., V.F. and H.A.B.M.; clinical coordination, H.A.B.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Rinaldi Fontani Institute (Protocol code: IRB-RFI-2025-07-1-2) on 1 July 2025.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data supporting the findings of this study are contained within the manuscript.
Acknowledgments
The authors thank the International Scientific Society of Neuro Psycho Physical Optimization with REAC Technology for covering the article processing charge for open access publication.
Conflicts of Interest
S.R. and V.F. are co-inventors of patents related to REAC technology, and founders of ASMED Srl, the manufacturer of REAC medical devices. A.R. is the daughter of S.R. and V.F. H.A.B.M. declares no conflict of interest.
References
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr 2020, 9, S55–S65. [Google Scholar] [CrossRef]
- Nobrega, I.S.; Teles, E.S.A.L.; Yokota-Moreno, B.Y.; Sertie, A.L. The Importance of Large-Scale Genomic Studies to Unravel Genetic Risk Factors for Autism. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Talantseva, O.I.; Romanova, R.S.; Shurdova, E.M.; Dolgorukova, T.A.; Sologub, P.S.; Titova, O.S.; Kleeva, D.F.; Grigorenko, E.L. The global prevalence of autism spectrum disorder: A three-level meta-analysis. Front Psychiatry 2023, 14, 1071181. [Google Scholar] [CrossRef] [PubMed]
- Dückert, S.; Gewohn, P.; König, H.; Schöttle, D.; Konnopka, A.; Rahlff, P.; Vogeley, K.; Schulz, H.; David, N.; Peth, J. Multidimensional Burden on Family Caregivers of Adults with Autism Spectrum Disorder: a Scoping Review. Review Journal of Autism and Developmental Disorders 2023. [Google Scholar] [CrossRef]
- Cheung, P.P.P.; Lau, B.W.M. Neurobiology of sensory processing in autism spectrum disorder. Prog Mol Biol Transl Sci 2020, 173, 161–181. [Google Scholar] [CrossRef]
- Lage, C.; Smith, E.S.; Lawson, R.P. A meta-analysis of cognitive flexibility in autism spectrum disorder. Neurosci Biobehav Rev 2024, 157, 105511. [Google Scholar] [CrossRef]
- Restoy, D.; Oriol-Escude, M.; Alonzo-Castillo, T.; Magan-Maganto, M.; Canal-Bedia, R.; Diez-Villoria, E.; Gisbert-Gustemps, L.; Setien-Ramos, I.; Martinez-Ramirez, M.; Ramos-Quiroga, J.A.; et al. Emotion regulation and emotion dysregulation in children and adolescents with Autism Spectrum Disorder: A meta-analysis of evaluation and intervention studies. Clin Psychol Rev 2024, 109, 102410. [Google Scholar] [CrossRef]
- Holanda, M.V.F.; Paiva, E.D.S.; de Souza, L.N.; Paiva, K.M.; Oliveira, R.F.; Tavares, E.A.F.; Morais, P.; de Andrade, A.M.; Knackfuss, M.I.; do Nascimento, E.G.C.; et al. Neurobiological basis of autism spectrum disorder: mini review. Front Psychol 2025, 16, 1558081. [Google Scholar] [CrossRef]
- La Monica, I.; Di Iorio, M.R.; Sica, A.; Rufino, F.; Sotira, C.; Pastore, L.; Lombardo, B. Autism Spectrum Disorder: Genetic Mechanisms and Inheritance Patterns. Genes (Basel) 2025, 16. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, A.S.; van den Honert, R.N.; Huppert, T.J.; Wallace, G.L.; Martin, A. Aberrant Oscillatory Synchrony Is Biased Toward Specific Frequencies and Processing Domains in the Autistic Brain. Biol Psychiatry Cogn Neurosci Neuroimaging 2017, 2, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Zhang, H.; Du, L.; Deng, Z. Abnormalities of brain dynamics based on large-scale cortical network modeling in autism spectrum disorder. Neural Netw 2025, 189, 107561. [Google Scholar] [CrossRef]
- Maximo, J.O.; Kana, R.K. Aberrant “deep connectivity” in autism: A cortico-subcortical functional connectivity magnetic resonance imaging study. Autism Res 2019, 12, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiao, Y.; Chen, R.; Wang, X.H.; Han, Y. Aberrant dynamic and static functional connectivity of the striatum across specific low-frequency bands in patients with autism spectrum disorder. Psychiatry Res Neuroimaging 2023, 336, 111749. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Burraco, A.; Murphy, E. The Oscillopathic Nature of Language Deficits in Autism: From Genes to Language Evolution. Front Hum Neurosci 2016, 10, 120. [Google Scholar] [CrossRef]
- Suprunowicz, M.; Bogucka, J.; Szczerbinska, N.; Modzelewski, S.; Oracz, A.J.; Konarzewska, B.; Waszkiewicz, N. Neuroplasticity-Based Approaches to Sensory Processing Alterations in Autism Spectrum Disorder. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Seymour, R.A.; Rippon, G.; Gooding-Williams, G.; Schoffelen, J.M.; Kessler, K. Dysregulated oscillatory connectivity in the visual system in autism spectrum disorder. Brain 2019, 142, 3294–3305. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.M.; Wallace, M.T. Dysfunction of sensory oscillations in Autism Spectrum Disorder. Neurosci Biobehav Rev 2016, 68, 848–861. [Google Scholar] [CrossRef]
- Ilic, N.; Sarajlija, A. Neuroglial Dysregulation in Autism Spectrum Disorder: Pathogenetic Insights, Genetic Threads, and Therapeutic Horizons. Neuroglia 2025, 6, 11. [Google Scholar] [CrossRef]
- Qin, L.; Wang, H.; Ning, W.; Cui, M.; Wang, Q. New advances in the diagnosis and treatment of autism spectrum disorders. Eur J Med Res 2024, 29, 322. [Google Scholar] [CrossRef]
- Aishworiya, R.; Valica, T.; Hagerman, R.; Restrepo, B. An Update on Psychopharmacological Treatment of Autism Spectrum Disorder. Neurotherapeutics 2022, 19, 248–262. [Google Scholar] [CrossRef]
- Rinaldi, A.; Maioli, M.; Marins Martins, M.C.; de Castro, P.C.F.; de Oliveira Silva, N.A.P.; de Mattos, J.A.V.; Fontani, V.; Rinaldi, S. REAC Non-invasive Neurobiological Stimulation for Mitigating the Impact of Internalizing Disorders in Autism Spectrum Disorder. Advances in Neurodevelopmental Disorders 2021, 5, 446–456. [Google Scholar] [CrossRef]
- Andre Nogueira, J.A.; Souza Bulle Oliveira, A.; Pereira Motta, M.; Vieira de Souza Moscardi, A.A.; Manchim Favaro, V.; Munhoz Teixeira, C.; Orasmo Simcsik, A.; Patrizi, M.C.; Conde, M.S.; Rinaldi, A.; et al. Neurobiological modulation with REAC technology: enhancing pain, depression, anxiety, stress, and quality of life in post-polio syndrome subjects. Sci Rep 2024, 14, 17222. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Mura, M.; Castagna, A.; Fontani, V. Long-lasting changes in brain activation induced by a single REAC technology pulse in Wi-Fi bands. Randomized double-blind fMRI qualitative study. Sci Rep 2014, 4, 5668. [Google Scholar] [CrossRef]
- Rinaldi, S.; Oliveira, A.S.; Modestto, V.; Rinaldi, A.; Fontani, V. Functional Brain Reorganization After Radio Electric Asymmetric Conveyer (REAC) Brain Wave Optimization Gamma (BWO-G) Neuromodulation in Individuals With Chronic Stress Exposure: A Retrospective Case Series With Multimodal Evaluation. Cureus 2025, 17, e90951. [Google Scholar] [CrossRef] [PubMed]
- Modesto, V.; Rinaldi, A.; Fontani, V.; Rinaldi, S. Non-invasive Gamma Brain Wave Optimization (BWO-G) for Cognitive and Emotional Recovery in an Adolescent: A Case Study on Radio Electric Asymmetric Conveyer (REAC) Neuro Psycho Physical Optimization (NPPO) BWO-G Treatment. Cureus 2024, 16, e72819. [Google Scholar] [CrossRef]
- Pinheiro Barcessat, A.R.; Nolli Bittencourt, M.; Goes Goncalves, R.; Goncalves de Oliveira Cruz, A.V.; Coelho Pereira, J.A.; Bechelli, F.A.; Rinaldi, A. REAC Neuromodulation Treatments in Depression, Anxiety and Stress. A Comparative Retrospective Study. Psychol Res Behav Manag 2020, 13, 1247–1256. [Google Scholar] [CrossRef]
- Goncalves de Oliveira Cruz, A.V.; Goes Goncalves, R.; Nunes, L.; Douglas Quaresma de Oliveira, J.; Lima Monteiro, E.S.; Soares Eneias, I.; Guilherme Lima, T.C.; Duarte Ferreira, L.; Souza Neri, E.; da Cunha Pena, J.L.; et al. Neuro Postural Optimization Neuromodulation Treatment of Radio Electric Asymmetric Conveyer Technology on Stress and Quality of Life in Institutionalized Children in a Capital City of the Brazilian Amazon. Cureus 2022, 14, e26550. [Google Scholar] [CrossRef]
- Pereira Motta, M.; Oliveira, A.S.B.; Andre Nogueira, J.A.; Vieira de Souza Moscardi, A.A.; Munhoz Teixeira, C.; Manchim Favaro, V.; Simcsik, A.O.; Conde, S.; Patrizi, M.C.; Rinaldi, C.; et al. Improving Strength and Fatigue Resistance in Post-Polio Syndrome Individuals with REAC Neurobiological Treatments. J Pers Med 2023, 13, 1536. [Google Scholar] [CrossRef]
- Rinaldi, C.; Landre, C.B.; Volpe, M.I.; Goncalves, R.G.; Nunes, L.D.S.; Darienso, D.; Cruz, A.V.; Oliveira, J.D.; Rinaldi, S.; Fontani, V.; et al. Improving Functional Capacity and Quality of Life in Parkinson’s Disease Patients through REAC Neuromodulation Treatments for Mood and Behavioral Disorders. J Pers Med 2023, 13, 937. [Google Scholar] [CrossRef]
- Rinaldi, A.; Marins Martins, M.C.; De Almeida Martins Oliveira, A.C.; Rinaldi, S.; Fontani, V. Improving Functional Abilities in Children and Adolescents with Autism Spectrum Disorder Using Non-Invasive REAC Neuro Psycho Physical Optimization Treatments: A PEDI-CAT Study. J Pers Med 2023, 13, 792. [Google Scholar] [CrossRef]
- Rinaldi, A.; Martins, M.C.M.; Maioli, M.; Rinaldi, S.; Fontani, V. REAC Noninvasive Neurobiological Stimulation in Autism Spectrum Disorder for Alleviating Stress Impact. Adv Neurodev Disord 2023, 7, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Zippo, A.G.; Rinaldi, S.; Pellegata, G.; Caramenti, G.C.; Valente, M.; Fontani, V.; Biella, G.E. Electrophysiological effects of non-invasive Radio Electric Asymmetric Conveyor (REAC) on thalamocortical neural activities and perturbed experimental conditions. Sci Rep 2015, 5, 18200. [Google Scholar] [CrossRef] [PubMed]
- Fontani, V.; Cruciani, S.; Santaniello, S.; Rinaldi, S.; Maioli, M. Impact of REAC Regenerative Endogenous Bioelectrical Cell Reprogramming on MCF7 Breast Cancer Cells. J Pers Med 2023, 13, 1019. [Google Scholar] [CrossRef]
- Maslennikova, A.V.; Portnova, G.V.; Martynova, O.V. Brain oscillatory patterns of affective prosody perception in children with autism spectrum disorder. Research in Autism Spectrum Disorders 2022, 96, 101993. [Google Scholar] [CrossRef]
- Larrain-Valenzuela, J.; Zamorano, F.; Soto-Icaza, P.; Carrasco, X.; Herrera, C.; Daiber, F.; Aboitiz, F.; Billeke, P. Theta and Alpha Oscillation Impairments in Autistic Spectrum Disorder Reflect Working Memory Deficit. Sci Rep 2017, 7, 14328. [Google Scholar] [CrossRef]
- Soto-Icaza, P.; Soto-Fernandez, P.; Kausel, L.; Marquez-Rodriguez, V.; Carvajal-Paredes, P.; Martinez-Molina, M.P.; Figueroa-Vargas, A.; Billeke, P. Oscillatory activity underlying cognitive performance in children and adolescents with autism: a systematic review. Front Hum Neurosci 2024, 18, 1320761. [Google Scholar] [CrossRef] [PubMed]
- Kessler, K.; Seymour, R.A.; Rippon, G. Brain oscillations and connectivity in autism spectrum disorders (ASD): new approaches to methodology, measurement and modelling. Neurosci Biobehav Rev 2016, 71, 601–620. [Google Scholar] [CrossRef]
- Pinheiro Barcessat, A.R.; Nolli Bittencourt, M.; Duarte Ferreira, L.; de Souza Neri, E.; Coelho Pereira, J.A.; Bechelli, F.; Rinaldi, A. REAC Cervicobrachial Neuromodulation Treatment of Depression, Anxiety, and Stress During the COVID-19 Pandemic. Psychol Res Behav Manag 2020, 13, 929–937. [Google Scholar] [CrossRef]
- Fontani, V.; Rinaldi, A.; Rinaldi, C.; Araldi, L.; Azzara, A.; Carta, A.M.; Casale, N.; Castagna, A.; Del Medico, M.; Di Stasio, M.; et al. Long-Lasting Efficacy of Radio Electric Asymmetric Conveyer Neuromodulation Treatment on Functional Dysmetria, an Adaptive Motor Behavior. Cureus 2022, 14, e25768. [Google Scholar] [CrossRef]
- Mahapatra, S.; Vyshedsky, D.; Martinez, S.; Kannel, B.; Braverman, J.; Edelson, S.M.; Vyshedskiy, A. Autism Treatment Evaluation Checklist (ATEC) Norms: A “Growth Chart” for ATEC Score Changes as a Function of Age. Children (Basel) 2018, 5. [Google Scholar] [CrossRef]
- Mahapatra, S.; Khokhlovich, E.; Martinez, S.; Kannel, B.; Edelson, S.M.; Vyshedskiy, A. Longitudinal Epidemiological Study of Autism Subgroups Using Autism Treatment Evaluation Checklist (ATEC) Score. J Autism Dev Disord 2020, 50, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Netson, R.; Schmiedel Fucks, A.; Schmiedel Sanches Santos, A.; Poloni, L.E.P.; Nacano, N.N.; Fucks, E.; Radi, K.; Strong, W.E.; Carnaval, A.A.; Russo, M.; et al. A Comparison of Parent Reports, the Mental Synthesis Evaluation Checklist (MSEC) and the Autism Treatment Evaluation Checklist (ATEC), with the Childhood Autism Rating Scale (CARS). Pediatr Rep 2024, 16, 174–189. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J Grad Med Educ 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Posserud, M.B.; Skretting Solberg, B.; Engeland, A.; Haavik, J.; Klungsoyr, K. Male to female ratios in autism spectrum disorders by age, intellectual disability and attention-deficit/hyperactivity disorder. Acta Psychiatr Scand 2021, 144, 635–646. [Google Scholar] [CrossRef]
- Blanco, B.; Lloyd-Fox, S.; Begum-Ali, J.; Pirazzoli, L.; Goodwin, A.; Mason, L.; Pasco, G.; Charman, T.; Jones, E.J.H.; Johnson, M.H.; et al. Cortical responses to social stimuli in infants at elevated likelihood of ASD and/or ADHD: A prospective cross-condition fNIRS study. Cortex 2023, 169, 18–34. [Google Scholar] [CrossRef]
- Jochaut, D.; Lehongre, K.; Saitovitch, A.; Devauchelle, A.D.; Olasagasti, I.; Chabane, N.; Zilbovicius, M.; Giraud, A.L. Atypical coordination of cortical oscillations in response to speech in autism. Front Hum Neurosci 2015, 9, 171. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, S.; Lugo-Marin, J.; Setien-Ramos, I.; Gisbert-Gustemps, L.; Arteaga-Henriquez, G.; Diez-Villoria, E.; Ramos-Quiroga, J.A. Transcranial direct current stimulation in Autism Spectrum Disorder: A systematic review and meta-analysis. Eur Neuropsychopharmacol 2021, 48, 89–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, P.; Wang, Y. Assessing the impact of repetitive transcranial magnetic stimulation on effective connectivity in autism spectrum disorder: An initial exploration using TMS-EEG analysis. Heliyon 2024, 10, e31746. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).