1. Introduction
Hydrogels—highly hydrated, biocompatible, and tunable three-dimensional polymer networks—have emerged as versatile platforms for DDS. Their ability to encapsulate and release active pharmaceutical ingredients with controlled profiles, while simultaneously reducing undesired systemic exposure, is a key advantage. These properties stem from a delicate balance between their crosslinked structure, water content, and release mechanisms (e.g., diffusion, degradation, swelling), which allows formulations to be tailored to specific therapeutic contexts. "Smart" or stimuli-responsive hydrogels (sensitive to pH, temperature, enzymes, light, or redox conditions) add a further layer of temporal and spatial precision to drug release. Furthermore, hybrid hydrogel-nanotechnology systems integrate nanoparticles or other nanostructures to enhance drug loading, stability, and targeting [
1,
2].
In the management of pain, interest in hydrogels is driven by the goal of enhancing the clinical efficacy of analgesics while minimizing systemic toxicity and exposure variability, particularly for hydrophobic molecules or those with a narrow therapeutic window. The use of natural and synthetic matrices—including injectable or in situ-forming variants—aims to prolong the local residence time of the drug, mitigate the initial "burst release," and promote tissue targeting in settings where systemic administration is suboptimal. Recent literature indicates a growing interest in sustained-release approaches for analgesics (including local anesthetics) and in stimuli-responsive platforms that allow for finer control over release kinetics [
3,
4].
Given the broad technological scope (polymer origin, crosslinking methods, loading modalities, responsiveness) and the variety of relevant outcomes (e.g., targeting precision, reduction of dose-dependent adverse events, improved bioavailability, and delivery of hydrophobic drugs), a scoping review is an appropriate method to systematically map formulations, mechanisms, and applications in the analgesic domain, without being limited to a single class of molecules or setting. Moreover, the emergence of hybrid hydrogel-nanotechnology systems suggests new interdisciplinary research directions—from materials design to clinical pharmacokinetics—that warrant a structured reconnaissance [
5,
6].
Objective. Over the past 20 years, we synthesize the evidence published in peer-reviewed journals on hydrogel formulations used for the delivery of analgesic drugs, with an emphasis on applications and efficacy. In this review, efficacy is operationalized as targeting precision, reduction of systemic toxicity, improved bioavailability, and the capacity to deliver hydrophobic drugs; we also highlight key gaps and advances in hybrid hydrogel-nanotechnology systems.
Beyond drug delivery, hydrogels are widely used as advanced dressings for wounds and burns, where they maintain a moist microenvironment, facilitate re-epithelialization, and can reduce pain (e.g., during dressing changes) through cooling, reduced adherence to the wound bed, and atraumatic removal. A clinical meta-analysis reported reductions in pain scores with hydrogels compared to controls in various types of skin lesions, including second-degree burns and traumatic wounds. Systematic reviews on superficial/partial-thickness burns and recent wound management literature confirm that hydrogels can minimize pain on removal compared to more adherent dressings [
7,
8].
In the domain of tissue hydration and lubrication, hydrogels (e.g., cross-linked hyaluronates in ophthalmic formulations) act as surface lubricants, prolong precorneal retention, and improve signs and symptoms of dry eye, including discomfort/pain from friction. In gynecology, moisturizing and bioadhesive gels based on hydrophilic polymers (hydrogel-like) have been shown in randomized trials to reduce dryness and dyspareunia, thereby modulating pain without delivering active analgesics. While these applications fall outside the primary scope of this review (focused on hydrogels for analgesic release), they are relevant as they demonstrate how the hydrogel matrix itself can contribute to pain modulation through physical mechanisms (lubrication, barrier, moisture, atraumaticity) [
9,
10,
11].
2. Materials and Methods
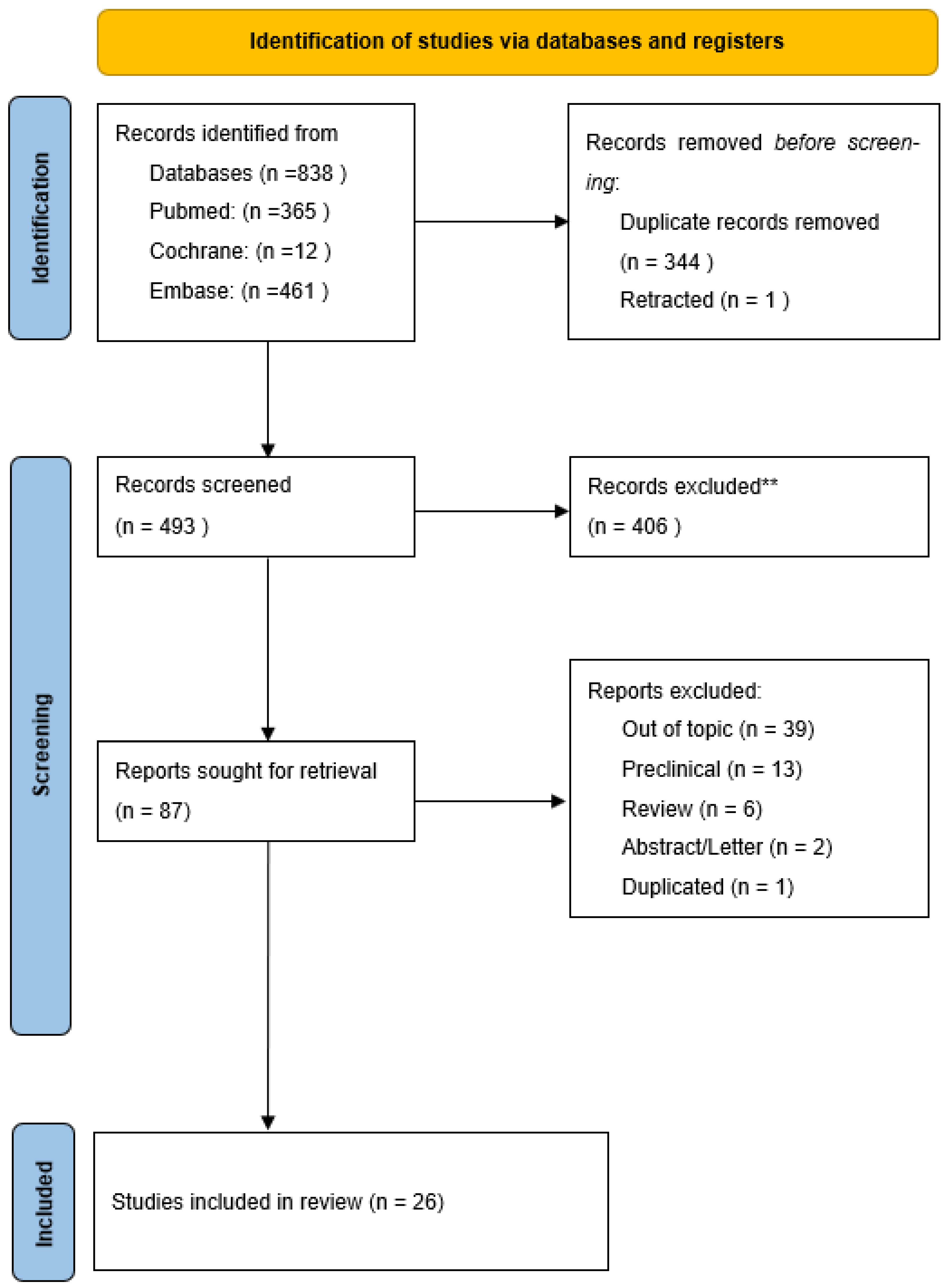
This scoping review was designed and reported in accordance with the PRISMA-ScR (Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews) checklist [
12]. The PRISMA flow diagram, resuming studies inclusion process, is illustrated in
Figure 1.
Only articles published in peer-reviewed journals were included; grey literature was excluded, consistent with the objective of transparently and reproducibly mapping clinical evidence as intended for scoping reviews. PubMed/MEDLINE, Embase, and Cochrane databases were searched with no publication date restrictions. The complete search strings for each database (constructed by combining controlled vocabulary and keywords for hydrogel, drug delivery, and pain) are reported in
Appendix A.
Records retrieved from the databases were aggregated into a single library and deduplicated prior to screening.
Inclusion criteria adopted were: clinical studies only (randomized clinical trials, RCTs, and non-randomized, prospective/retrospective, cohort, case-control, case series/reports) on hydrogel formulations (natural or synthetic, including stimuli-responsive and hybrid systems with nanotechnologies) used as drug-delivery systems for analgesic drugs. Exclusion criteria were: preclinical studies (in vitro/in vivo animal models), formulation-only or proof-of-concept works without clinical data, non-journal articles (proceedings, theses, preprints, websites), and irrelevant studies (non-hydrogel or not related to analgesics).
The selection was performed in two phases: a first phase of title/abstract screening against the eligibility criteria, and a second phase of full-text assessment of potentially relevant articles. Screening was conducted independently by paired reviewers, with discrepancies resolved by consensus; a third reviewer was consulted in cases of persistent disagreement. Reasons for exclusion at the full-text stage were documented and summarized in the PRISMA-ScR flow diagram. For each included study, the following data were extracted: clinical context/pain indication; hydrogel type; analgesic molecule/class; administration route/target; study design, and main results. In line with scoping review recommendations, a formal risk of bias assessment was not planned.
3. Results
The search of PubMed/MEDLINE, Embase, and Cochrane identified a total of 838 records; after deduplication, 493 unique records remained. Title and abstract screening, followed by full-text assessment according to the predefined criteria, led to the inclusion of 87 clinical articles relevant to the hydrogel–pain theme. Within the included set, 26 studies specifically evaluated hydrogel formulations as DDS for analgesics and constitute the primary cohort for the thematic mapping of efficacy and safety. In parallel, the screening identified 38 clinical studies on hydrogel applications involving pain modulation not based on drug delivery (e.g., wound care, hydration/lubrication): this evidence was cataloged separately and does not contribute to the primary DDS-oriented synthesis but is reported for methodological completeness and its potential clinical relevance. The PRISMA-ScR flow diagram summarizes the flow of records through the identification, screening, and inclusion phases, with documentation of reasons for exclusion at the full-text stage.
The screening led to the inclusion of 26 clinical studies evaluating hydrogel formulations as DDS for analgesics (primary cohort). The majority had a randomized design (18/26; 69.2%), followed by case series (3/26; 11.5%), pilot/open label/pharmacokinetic studies (4/26; 15.4%), retrospective studies (2/26; 7.7%). The timeframe of the studies ranged from 1996 to 2024 (median 2021).
By application site/route, the distribution shows a predominant use for topical/transdermal (10/26; 38.5%) and perioperative/incisional/wound (8/26; 30.8%) applications, with smaller contributions from oral/mucosal (2/26; 7.7%), rectal/anal (2/26; 7.7%), intra-articular (2/26; 7.7%), paranasal sinuses (1/26; 3.8%), and regional block (1/26; 3.8%). Regarding the pain context, studies were mainly perioperative (acute post-operative pain) (9/26; 34.6%), with the remaining indications being heterogeneous (chronic ulcers/wounds 4/26; 15.4%, knee osteoarthritis 2/26; 7.7%, and single occurrences in acute oral pain, burns, chronic neck pain, acute sinusitis, anal fissure, neuropathic pain, chronic low back pain, mucositis, pediatric pain, and toe pain).
Regarding drug class, local anesthetics were most prevalent (12/26; 46.2%; e.g., lidocaine, ropivacaine, bupivacaine), followed by opioids (5/26; 19.2%; e.g., morphine, loperamide), intra-articular corticosteroids (2/26; 7.7%; triamcinolone in Hyaluronic Acid, HA-gel), NSAIDs (1/26; 3.8%; loxoprofen), topical neuromodulators (1/26; 3.8%; capsaicin), and Nitric Oxide (NO) donors for anal fissure (1/26; 3.8%). In four studies (15.4%), the nature of the active agent or the analgesic profile was not uniquely classified within the main categories but fell within the analgesic clinical scope.
As for hydrogel technology, various types were observed: thermo-responsive hydrogels (poloxamer/proprietary; 5/26; 19.2%), hyaluronates (2/26; 7.7%, including HA-steroid systems), muco-adhesives or bio-adhesives (2/26; 7.7%), amorphous wound gels (2/26; 7.7%), hydrophilic suppositories (1/26; 3.8%), and cellulosic gels (1/26; 3.8%). In approximately 11/26 studies (42.3%), the description of the material or platform did not allow for a more detailed classification; no included study explicitly reported a hybrid integration with nanotechnologies within the hydrogel matrix.
The clinical outcomes measured were consistent with the review's scope: pain control, consumption of rescue analgesics, time to/duration of the effect, tolerability, and local or systemic adverse events. In some perioperative studies with local anesthetics, clinical pharmacokinetic proxies were reported. Comparators included placebo, standard of care or non-hydrogel formulations; in non-controlled studies, interpretation was based on pre and post outcomes and feasibility.
In parallel, 38 non-DDS clinical studies were identified (excluded from the primary synthesis) in which the hydrogel modulated pain through physical mechanisms (advanced dressings, post photorefractive keratectomy (PRK) bandage contact lenses (BCL), intradiscal implants, tissue hydration/lubrication). In this group, ocular (≈24% of the excluded set), burns and wounds (≈20%), and discogenic/orthopedic pain contexts were frequent; these studies were designed as case series or randomized studies. This evidence is cataloged separately and does not feed into the efficacy mapping of DDS for analgesics but illustrates the broad spectrum of hydrogel use in "drug-free" pain modulation.
In the primary cohort (26 clinical studies on hydrogels used as drug-delivery systems for analgesics), the most frequently reported clinical outcomes were pain intensity (Visual Aanalogue Scale, VAS/ Numerical Rating Scale, NRS), consumption of rescue analgesics, time to pain relief and pain-free period, and tolerability/adverse events. Based on the summaries provided, 22 studies reported a relevant improvement in analgesic outcomes compared to control or baseline (22/26; 84.6%), with a limited number of neutral results (1/26; 3.8%) and a few cases that were not clearly determinable due to insufficient detail (3/26; 11.5%). Safety reports were found in a minority of studies while tolerability was widely described as favorable..
3.1. Drug Classes
Local Anesthetics (12/26; 46.2%). Designs were mostly randomized; predominant routes of administration were perioperative, incisional or wound injection (8 studies), with contributions from topical/transdermal (2), paranasal sinuses (1), and regional block (1). Outcomes included reduction in VAS/NRS, decreased of rescue medication use, and prolonged duration of analgesic effect; 10 of these 12 studies that analyzed local anesthetics in pain management reported great pain solving effect, while 1 study was neutral and 1 not definable.
Opioids and Peripheral Opioid Receptor Modulators (5/26; 19.2%). To administrate these drugs there was a prevalence of topical and transdermal application (3), with evidence also for rectal/anal (1) and oral/mucosal (1) routes. The main endpoints were pain release and need for rescue medication; 4 of 5 studies reported improvement in pain management and 1 was not definable.
Intra-articular Corticosteroids (2/26; 7.7%). Intra-articular (i.e. knee infiltration) in an Osteoarthritis context; both studies described pain relief and better clinical outcomes.
NSAIDs (1/26; 3.8%). Topical/transdermal application guaranteed a good pain control and outcomes.
Topical Neuromodulator (i.e. capsaicin) (1/26; 3.8%). Topical/transdermal application of capsaicin improved pain relief if pain was related to local symptoms.
Other/Not Classified (4/26; 15.4%). Heterogeneous contexts (topical/transdermal, oral/mucosal, perioperative/incisional/wound); 3 studies reported good pain management and 1 was not definable.
3.2. Route and Site of Administration
Hydrogel application was predominantly topical and transdermal (10/26; 38.5%) followed by their use in perioperative, incisional and wound setting (8/26; 30.8%), or by oral and mucosal route (2/26; 7.7%), rectal or anal (2/26; 7.7%) and intra-articular delivery (2/26; 7.7%). The application in frontal sinuses cavity was reported in one case (1/26; 3.8%) such as their usage in regional block anesthesia (1/26; 3.8%). The direction of effect was positive across all site categories (with single neutral or undefinable cases in perioperative/incisional/wound and topical/transdermal).
3.3. Hydrogel Technology
Thermo-responsive (poloxamer/proprietary; 5/26; 19.2%), hyaluronate (2/26), muco/bio-adhesive (2/26), amorphous wound gels (2/26), hydrophilic suppositories (1/26), and cellulosic (1/26) platforms were identified. In 11 studies of the total 26 (42.3%), the description of the matrix did not allow a precise classification (other/not specified). None of the included clinical studies reported hybrid hydrogel–nanotechnology systems in the examined formulation.
In
Table 1 there is a short report that summarizes DDS hydrogels characteristics and settings of usage, while a comprehensive report is presented in
Appendix B.
3.4. Non-DDS Clinical Studies (Pain Modulation Without Drug Release)
In the 38 non-DDS clinical studies (cataloged separately), the hydrogel modulated pain through physical mechanisms (protection, cooling, lubrication, atraumatic barrier and adhesion), without acting as a drug vehicle. The prevalent areas were ocular (≈24%), burns or wounds (≈20%), and musculoskeletal and discal damages; These were mostly case series and randomized studies. A thematic summary follows.
3.4.1. Ocular
Silicone hydrogel BCLs are used to reduce pain and promote re-epithelialization after PRK; comparative studies and clinical trials show benefits in pain and comfort, with differences also related to lens design, material, and curvature. In comparison of silicone-hydrogel BCLs, the authors reported improved pain management and patient’s comfort and epithelial stability; other trials evaluated fitting parameters comparing different silicone-hydrogels, and confirmed the reduction of post-PRK discomfort with good tolerability profiles. This evidence supports the andavantage in using BCLs as a non-pharmacological analgesic intervention in the early stages post-corneal ablation [
38,
39].
3.4.2. Burns and Wounds
In burn patients (especially pediatric), clinical trials have evaluated cooling and moisturizing hydrogel dressings to alleviate pain immediately after first aid and during initial dressing changes. A pediatric RCT showed a reduction in pain scores with a hydrogel compared to plastic film, while protocols and further studies have investigated the early analgesic effect of specific products. Recent reviews emphasize that cooling, water retention, and atraumatic adhesion contribute to pain reduction and better dressing management. Overall, the evidence indicates that hydrogel dressings can be a non-pharmacological analgesic adjuvant in superficial/partial-thickness burns and some painful wounds, despite heterogeneity in settings and outcomes [
40,
41].
3.4.3. Musculoskeletal and Discal
In discogenic pain, the use of intradiscal hydrogel implants/augmentations aims to restore hydration/cushioning of the nucleus pulposus with potential pain benefits. However, the clinical literature highlights safety signals: cases of hydrogel migration into the spinal canal with radicular pain or neurological complications requiring invasive management have been reported. Recent reviews underscore the insufficiency of controlled clinical data and the need for rigorous prospective evaluations before widespread clinical adoption. In this area, therefore, the "non-DDS" use appears promising but still immature, with a pri-mary focus on safety [
42,
43].
3.4.4. Other Contexts (Lubrication/Barrier)
At mucosal or cutaneous sites, bio-adhesive and muco-adhesive hydrogels or hydrophilic sheets and gels have been used as moisturizing and lubricating barriers, reducing pain from friction or dressing removal and improving the overall tolerability of local care. Clinical evidence is heterogeneous and often small-scale but converges on the idea that the hydrogel matrix, even without a drug, can help reduce procedural and incidental pain through physical effects (moisture, cooling, protection, atraumaticity) [
44].
In
Table 2 there is a report that summarize Non-DDS hydrogels characteristic and setting of usage.
4. Discussion
This scoping review clinically maps the use of hydrogels as drug-delivery platforms for analgesics (DDS), revealing a heterogeneous landscape in terms of drug classes, hydrogel types, administration sites and routes, but with a predominantly favorable direction of effect on pain outcomes and the use of rescue analgesics. These findings are consistent with the engineering rationale of hydrogel-based DDS—the ability to modulate release, local residence time, and tissue concentrations—which is well-documented in the literature on design and mechanisms (e.g., mesh size, hydrogel–drug interactions, crosslinking, responsiveness) and associated with clinical benefits when local tissue distribution is a key determinant of the outcome [
45].
In perioperative/incisional contexts, thermo-hydrogels (e.g., poloxamer 407) that gel
in situ have enabled the "single-shot" application of local anesthetics with outcomes non-inferior to continuous catheter techniques and with less procedural complexity, suggesting a possible operational advantage and a limited systemic toxicity profile. In an RCT in minimally invasive thoracic surgery, local injection of ropivacaine in poloxamer 407 was non-inferior to a continuous paravertebral block for postoperative pain control, supporting the use of thermo-hydrogels as a viable alternative to the standard of care in selected settings [
23].
In knee osteoarthritis, HA in a hydrogel form combined with triamcinolone acetonide showed signals of efficacy and tolerability in a randomized feasibility study, with a faster onset of relief compared to hyaluronate alone. While not conclusive, this line of research indicates that viscoelastic gelling matrices can enhance the analgesic response of known active ingredients by modulating their intra-articular availability [
29].
For topical/transdermal and mucosal indications, the use of muco-adhesive and bio-adhesives has improved drug contact time and adherence. The clinical literature on topical opioids in gel form shows heterogeneous results—with positive studies on the reduction of ulcerative pain and negative trials in specific dermatological settings—indicating that matrix composition, concentration, and the pain model strongly influence efficacy. This heterogeneity reinforces the need for pragmatic trials and shared core outcome sets for measures of pain, rescue consumption, and duration of effect [
46].
Our non-DDS subgroup (excluded from the primary synthesis) confirms that the hydrogel matrix can modulate pain even without a drug through physical mechanisms (cooling, barrier, lubrication), as shown by RCTs on hydrogel dressings in pediatric burns and the established practice of BCLs after PRK to reduce pain and promote re-epithelialization. This context is relevant because, in combination with or as an alternative to DDS, hydrogels can offer multimodal strategies for local pain management [
40,
47].
A cross-cutting finding is the scarcity of clinical trials on hybrid hydrogel–nanotechnology systems and stimuli-responsive hydrogels in the analgesic field, despite a robust preclinical pipeline and evidence in other inflammatory areas. The lack of standardization (types of comparators, follow-up times, patient-centered outcomes) and incomplete reporting of safety profiles limit the cumulativeness of the evidence. Future studies should: (i) harmonize clinical endpoints (pain intensity, rescue consumption, time to first rescue), (ii) integrate clinical Pharmacokinetic-Pharmacodynamic proxies (e.g., time to/duration of action for local anesthetics), (iii) compare hydrogel-based DDS with standards of care in adequately powered randomized trials, and (iv) implement safety registries for use in confined or high mechanical risk sites (where the non-DDS literature reports, albeit rarely, complications from migration into enclosed spaces) [
2,
48].
The main strength of this review is the breadth of the clinical mapping of hydrogel-based DDS for analgesics across various settings and administration routes, with classification by drug class and technology. Limitations arise from the heterogeneity of study designs, the variability of the matrices (often not fully characterized in the materials and methods), and the absence, in the mapped analgesic clinical trials, of widespread adoption of frontier hybrid platforms (e.g., liposomes-in-hydrogel, microparticles-in-gel). Bridging this clinical-translational gap—already well-delineated in engineering and drug delivery reviews—will require coordinated efforts between materials science, clinical pharmacology, and trial methodology [
45,
49].
For clinical practice, the data available to date suggest that: (i) in perioperative settings, thermo-hydrogels with local anesthetics can be simplified alternatives to continuous catheter techniques in selected settings; (ii) in knee osteoarthritis, the addition of a corticosteroid to an HA hydrogel may accelerate analgesia compared to HA alone; (iii) at topical/mucosal sites, mucoadhesion is a critical driver, but efficacy is sensitive to the pain model and formulation. These considerations must be verified with head-to-head comparative studies, standardized measures, and adequate follow-up to capture clinically significant differences and rare safety profiles [
23,
29].
5. Conclusions
This scoping review highlights that hydrogel formulations used as DDS for analgesics show, across the different clinical settings mapped, convergent signals of benefit on pain outcomes and rescue medication consumption, with generally favorable tolerability profiles. This is especially true when the therapeutic goal is prolonged local exposure to analgesics with low systemic toxicity (e.g., in perioperative/incisional contexts, and via topical/mucosal routes). The consistency between the engineering rationale (e.g., in situ gelling, muco/bioadhesion, polymer network modulation, stimuli-responsiveness) and the observed clinical endpoints supports the position of hydrogels as one of the most promising platforms for the targeted delivery of analgesics. Established technological trajectories—from thermo-hydrogels to stimuli-responsive systems—indeed converge towards more controlled and translatable release profiles, although they still suffer from formulation and reporting variability that hinders direct comparisons and formal meta-analyses.
A second key finding is that the "non-DDS ecosystem"—hydrogels used without a drug for cooling, barrier, or lubrication purposes—confirms the intrinsic ability of the matrix to modulate pain in a clinically appreciable manner (e.g., after PRK or in the early management of burns/wounds). This supports multimodal strategies where the hydrogel acts as a physical component complementary to the DDS. This landscape, while distinct from the primary scope, reinforces the idea that the material-tissue interface is an integral part of the efficacy perceived by the patient.
However, translational gaps remain: (i) a scarcity of clinical trials on hybrid hydrogel–nanotechnology platforms in analgesia despite encouraging preclinical signals; (ii) heterogeneity of comparators, follow-up, and patient-centered outcomes; and (iii) incomplete documentation of safety (rare local events, material-tissue interactions). The priorities for clinical research are therefore: to standardize endpoints (pain intensity, time to/consumption of rescue medication, duration of effect, QoL), to adopt head-to-head comparisons with the standard of care in adequately powered pragmatic RCTs, and to implement prospective safety registries for "constrained" or high mechanical risk sites. In parallel, the further maturation of responsive systems (pH, temperature, light/ultrasound) and injectable formulations could accelerate broader clinical adoption where local targeting is a critical determinant of the analgesic outcome.
Overall, the mapped clinical data indicates that hydrogels as DDS for analgesics constitute a field that is already useful in practice in specifical settings and a growing platform for more sophisticated applications. Consolidating the evidence with rigorous comparative studies and harmonized reporting will be crucial to move from fragmented evidence to operational recommendations and, in the long term, to safely and effectively integration of "smart" technologies into pain management.
Author Contributions
Conceptualization, S.D.F., A.A. and M.F.; methodology, A.A. and M.F.; software, A.A.; validation, S.D.F., A.A. and M.F.; formal analysis, A.A. and M.F.; investigation, S.D.F., A.A. and M.F.; resources, M.C.P. and M.B.P.; data curation, P.S. and A.A.; writing—original draft preparation, A.A. and S.D.F.; writing—review and editing, M.F.; visualization, A.A.; supervision, M.F.; All authors have read and agreed to the published version of the manuscript..
Funding
This research received no external funding.
Data Availability Statement
Data are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI |
Multidisciplinary Digital Publishing Institute |
| ADL |
Activities of Daily Living |
| AEs |
Adverse Events |
| AMT |
Thermoresponsive hydrogel formulation (AMT-143) |
| APIs |
Active Pharmaceutical Ingredients |
| BCL |
Bandage Contact Lens |
| DDS |
Drug Delivery System(s) |
| EHO |
Experimental Hydrogel with Olea europaea extract (EHO-85) |
| ENT |
Ear, Nose and Throat |
| HA |
Hyaluronic Acid |
| LD |
Lidocaine |
| NO |
Nitric Oxide |
| NRS |
Numerical Rating Scale |
| NSAIDs |
Non-Steroidal Anti-Inflammatory Drugs |
| OA |
Osteoarthritis |
| PF |
Poloxamer Formulation (e.g., PF72 hydrogel) |
| PK |
Pharmacokinetics |
| PRISMA-ScR |
Preferred Reporting Items for Systematic Reviews and Meta-Analyses—Scoping Review extension |
| PRK |
Photorefractive Keratectomy |
| RCT |
Randomized Controlled Trial |
| RCTs |
Randomized Controlled Trials |
| TA |
Triamcinolone Acetonide |
| TLA |
Topical Local Anesthetic |
| VAS |
Visual Analogue Scale |
| |
|
| |
|
| |
|
Appendix A
The search strings for each database are detailed in
Table A1.
Table A1.
Complete search strings for each database.
Table A1.
Complete search strings for each database.
| Database |
Search String |
Results |
| Pubmed |
("hydrogel s"[All Fields] OR "hydrogelating"[All Fields] OR "hydrogelation"[All Fields] OR "hydrogelations"[All Fields] OR "hydrogelator"[All Fields] OR "hydrogelators"[All Fields] OR "hydrogels"[MeSH Terms] OR "hydrogels"[All Fields] OR "hydrogel"[All Fields]) AND ("drug delivery systems"[MeSH Terms] OR ("drug"[All Fields] AND "delivery"[All Fields] AND "systems"[All Fields]) OR "drug delivery systems"[All Fields] OR ("drug"[All Fields] AND "delivery"[All Fields]) OR "drug delivery"[All Fields]) AND ("pain"[MeSH Terms] OR "pain"[All Fields]) |
365 |
| Cochrane |
hydrogels and pain |
12 |
| Embase |
('analgesia'/exp OR 'analgaesia' OR 'analgesia' OR 'pain management' OR 'pain relief' OR 'sequential analgetic analgesia' OR 'surgical analgesia') AND ('hydrogel'/exp OR 'gamma polymerized hydrogel' OR 'hydrogel' OR 'hydrogels') |
461 |
Appendix B
Table A2.
DDS hydrogels characteristics and settings of usage.
Table A2.
DDS hydrogels characteristics and settings of usage.
| Year |
Setting |
Application Site |
Drug |
Hydrogel |
Direction of Effect |
Safety |
Reference |
| 2015 |
Knee osteoarthritis |
Intra-articular (knee) |
Triamcinolone acetonide |
Hyaluronic acid hydrogels |
Improved vs control/baseline |
Unclear/Not stated |
[29] |
| 2012 |
Knee osteoarthritis |
Intra-articular (knee) |
Triamcinolone acetonide |
Hyaluronic acid + triamcinolone hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[30] |
| 2014 |
Purulent frontal sinusitis |
Sinus cavity |
Dioxidine, epsilon-aminocaproic acid, lidocaine |
Sodium alginate hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[13] |
| 2024 |
Post-craniotomy pain |
Scalp incision site |
Lidocaine |
Lidocaine 5% hydrogel plaster |
No difference/Neutral |
Unclear/Not stated |
[14] |
| 2021 |
Localized neuropathic pain |
Transdermal |
Lidocaine |
Topical system 1.8% vs 5% patch |
Improved vs control/baseline |
Unclear/Not stated |
[15] |
| 2009 |
Postherpetic neuralgia |
Topical |
Lidocaine |
Lidocaine medicated plaster (5%) |
Improved vs control/baseline |
Favorable safety/tolerability |
[16] |
| 2022 |
Minimally invasive colorectal surgery |
Surgical wound |
Ropivacaine or Bupivacaine |
Gel infusion kit |
Improved vs control/baseline |
Unclear/Not stated |
[17] |
| 2024 |
Inguinal hernia repair |
Surgical site infiltration |
Ropivacaine |
AMT-143 thermosensitive hydrogel |
Unclear/Not stated |
Unclear/Not stated |
[18] |
| 2024 |
Bimaxillary surgery |
Surgical site (orthognathic surgery) |
Ropivacaine |
PF 72 thermoresponsive hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[19] |
| 2024 |
Ear reconstruction surgery |
Donor site (iliac crest) |
Ropivacaine |
Sodium carboxymethylcellulose hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[20] |
| 2024 |
Postoperative pain relief |
Surgical site (breast augmentation) |
Ropivacaine |
PF72 thermoresponsive hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[21] |
| 2022 |
Postoperative pain management |
Surgical incision site |
Ropivacaine |
PF 72 thermoresponsive hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[22] |
| 2022 |
Thoracoscopic surgery |
Thoracic port site |
Ropivacaine |
Poloxamer 407-based hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[23] |
| 2021 |
Laparoscopic nephrectomy |
Regional block |
Ropivacaine or Bupivacaine |
Unspecified |
Improved vs control/baseline |
Unclear/Not stated |
[24] |
| 2002 |
Anal fissure |
Anal |
Glyceryl trinitrate |
Topical nitrate ointment |
Improved vs control/baseline |
Unclear/Not stated |
[31] |
| 2024 |
Chronic low back pain |
Topical (lower back) |
Loxoprofen |
Loxoprofen sodium hydrogel patch |
Improved vs control/baseline |
Favorable safety/tolerability |
[32] |
| 1996 |
Pediatric pain management |
Rectal |
Morphine |
Hydrophilic hydrogel suppository |
Improved vs control/baseline |
Unclear/Not stated |
[25] |
| 2011 |
Chronic leg ulcers |
Topical (leg ulcer) |
Morphine |
Topical morphine gel |
Improved vs control/baseline |
Unclear/Not stated |
[26] |
| 2009 |
Arterial leg ulcers |
Topical |
Morphine |
Topical morphine hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[27] |
| 2020 |
Oral mucositis pain |
Oral mucosa |
Morphine (topical) |
Mucoadhesive oral hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[28] |
| 2021 |
Painful dermal ulcers |
Topical |
Morphine and Loperamide |
Intrasite gel |
Unclear/Not stated |
Unclear/Not stated |
[50] |
| 2001 |
Aphthous ulcers |
Oral |
2-octyl cyanoacrylate |
Bioadhesive hydrogel device |
Unclear/Not stated |
Unclear/Not stated |
[33] |
| 2013 |
Burn wounds |
Topical |
Aloe vera vs silver sulphadiazine |
Aloe vera gel |
Improved vs control/baseline |
Unclear/Not stated |
[34] |
| 2022 |
Chronic ulcer treatment |
Topical (chronic ulcers) |
Olea europaea leaf extract |
EHO-85 amorphous hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[35] |
| 2024 |
Onychocryptosis |
Topical (toenail) |
Ozoile (ozonized oil) |
Ozoile-based hydrogel |
Improved vs control/baseline |
Unclear/Not stated |
[36] |
| 2012 |
Myofascial neck pain |
Topical (neck) |
Capsaicin 0.1% |
Capsaicin hydrogel patch |
Improved vs control/baseline |
Unclear/Not stated |
[37] |
References
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10. [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv Sci (Weinh) 2024, 11, e2306152. [CrossRef]
- Getachew, M.; Tesfaye, H.; Yihunie, W.; Ayenew, T.; Alemu, S.; Dagnew, E.M.; Biyazin, Y.; Abebe, D.; Degefu, N.; Abebaw, A. Sustained release local anesthetics for pain management: relevance and formulation approaches. Front Pain Res (Lausanne) 2024, 5, 1383461. [CrossRef]
- Chen, Y.; Xu, J.; Li, P.; Shi, L.; Zhang, S.; Guo, Q.; Yang, Y. Advances in the use of local anesthetic extended-release systems in pain management. Drug Deliv 2024, 31, 2296349. [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: current achievements and future directions. Signal Transduct Target Ther 2024, 9, 166. [CrossRef]
- Choi, W.; Kohane, D.S. Hybrid Nanoparticle-Hydrogel Systems for Drug Delivery Depots and Other Biomedical Applications. ACS Nano 2024, 18, 22780-22792. [CrossRef]
- Lee, K.; Lee, S.; Kim, J. Wound Pain Management: The Present and the Future. Journal of Wound Management and Research 2024, 20, 199-211. [CrossRef]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front Bioeng Biotechnol 2019, 7, 342. [CrossRef]
- Yu, Y.; Chow, D.W.Y.; Lau, C.M.L.; Zhou, G.; Back, W.; Xu, J.; Carim, S.; Chau, Y. A bioinspired synthetic soft hydrogel for the treatment of dry eye. Bioeng Transl Med 2021, 6, e10227. [CrossRef]
- Loprinzi, C.L.; Abu-Ghazaleh, S.; Sloan, J.A.; vanHaelst-Pisani, C.; Hammer, A.M.; Rowland, K.M., Jr.; Law, M.; Windschitl, H.E.; Kaur, J.S.; Ellison, N. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol 1997, 15, 969-973. [CrossRef]
- Kim, Y.H.; Park, S.; Lee, M.; Hahn, S.; Jeon, M.J. Effect of a pH-Balanced Vaginal Gel on Dyspareunia and Sexual Function in Breast Cancer Survivors Who Were Premenopausal at Diagnosis: A Randomized Controlled Trial. Obstet Gynecol 2017, 129, 870-876. [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O'Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018, 169, 467-473. [CrossRef]
- Khar’Kova, N.A.; Gerasimenko, M.Y.; Egorova, E.A. The use of hydrogel materials coletex-adl and colegel in the treatment of purulent frontal sinusitis. Sovremennye Tehnologii v Medicine 2014, 6, 176-180, doi:doi:.
- Han, X.; Yang, Y.; Ren, T.; Ji, N.; Luo, F. Efficacy of Preemptive Topical Lidocaine 5% Plaster in the Prevention of Post-Craniotomy Pain, a Randomized Clinical Trial. J Pain Res 2024, 17, 4251-4261. [CrossRef]
- Gudin, J.; Webster, L.R.; Greuber, E.; Vought, K.; Patel, K.; Kuritzky, L. Open-Label Adhesion Performance Studies of a New Lidocaine Topical System 1.8% versus Lidocaine Patches 5% and Lidocaine Medicated Plaster 5% in Healthy Subjects. J Pain Res 2021, 14, 513-526. [CrossRef]
- Hans, G.; Sabatowski, R.; Binder, A.; Boesl, I.; Rogers, P.; Baron, R. Efficacy and tolerability of a 5% lidocaine medicated plaster for the topical treatment of post-herpetic neuralgia: results of a long-term study. Curr Med Res Opin 2009, 25, 1295-1305. [CrossRef]
- Shin, J.K.; Jeong, H.; Lee, W.Y.; Yun, S.H.; Cho, Y.B.; Huh, J.W.; Park, Y.A.; Sim, W.S.; Kim, H.C. Efficacy of a local anesthetic gel infusion kit for pain relief after minimally invasive colorectal surgery: an open-label, randomized clinical trial. Sci Rep 2022, 12, 17429. [CrossRef]
- Nct. Postsurgical Analgesia After Hernia Repair. https://clinicaltrials.gov/ct2/show/NCT06709612 2024.
- Yun, C.W.; Kim, K.H.; Lee, W.; Kim, S.H. Comparative Analysis of Temperature-Responsive Hydrogel (PF 72) for Postoperative Pain After Bimaxillary Surgery: A Retro-spective Study. Aesthetic Plast Surg 2024, 48, 1271-1275. [CrossRef]
- Deng, Y.; Wang, B.; Jiang, H.; Luan, W.; Pan, B.; Li, C. Sodium Carboxymethylcellulose Serves as Sustained-Release Agents for Providing Analgesia at the Donor Site After Costal Cartilage Harvesting in Ear Reconstruction. Aesthetic Plast Surg 2025, 49, 2350-2356. [CrossRef]
- Cho, J.; Kim, K.H.; Lee, W.; Go, J.Y.; Kim, S.H. Effectiveness of a Novel Temperature-Responsive Hydrogel (PF72) for Postoperative Pain Relief in Breast Augmentation. J Clin Med 2023, 13. [CrossRef]
- Choi, B.M.; Hwang, C.S.; Yoon, Y.S.; Park, I.J.; Yoo, M.W.; Kim, B.S. Novel temperature-responsive hydrogel injected to the incision site for postoperative pain relief in laparoscopic abdominal surgery: a single-blind, randomized, pivotal clinical trial. Surg Endosc 2022, 36, 5794-5802. [CrossRef]
- Jeon, J.H.; Seong, Y.W.; Han, J.E.; Cho, S.; Kim, J.H.; Jheon, S.; Kim, K. Randomized Trial of Poloxamer 407-Based Ropivacaine Hydrogel After Thoracoscopic Pulmonary Resection. Ann Thorac Surg 2022, 114, 1189-1196. [CrossRef]
- Dam, M.; Hansen, C.; Poulsen, T.D.; Azawi, N.H.; Laier, G.H.; Wolmarans, M.; Chan, V.; Bendtsen, T.F.; Borglum, J. Transmuscular quadratus lumborum block reduces opioid consumption and prolongs time to first opioid demand after laparoscopic nephrectomy. Reg Anesth Pain Med 2021, 46, 18-24. [CrossRef]
- Lundeberg, S.; Beck, O.; Olsson, G.L.; Boreus, L.O. Rectal administration of morphine in children. Pharmacokinetic evaluation after a single-dose. Acta Anaesthesiol Scand 1996, 40, 445-451. [CrossRef]
- Huptas, L.; Rompoti, N.; Herbig, S.; Korber, A.; Klode, J.; Schadendorf, D.; Dissemond, J. [A new topically applied morphine gel for the pain treatment in patients with chronic leg ulcers: first results of a clinical investigation]. Hautarzt 2011, 62, 280-286. [CrossRef]
- Jansen, M.M.; van der Horst, J.C.; van der Valk, P.G.; Kuks, P.F.; Zylicz, Z.; van Sorge, A.A. Pain-relieving properties of topically applied morphine on arterial leg ulcers: a pilot study. J Wound Care 2009, 18, 306-311. [CrossRef]
- Hood, A.; Scullion, B.; McGuire, H. QP2: Challenges Treating Severe Oral Mucositis Pain in Patients at High Risk for Aspiration. Journal of Pain & Palliative Care Pharmacotherapy 2021, 34, 173-174. [CrossRef]
- Petrella, R.J.; Emans, P.J.; Alleyne, J.; Dellaert, F.; Gill, D.P.; Maroney, M. Safety and performance of Hydros and Hydros-TA for knee osteoarthritis: a prospective, multicenter, randomized, double-blind feasibility trial. BMC Musculoskelet Disord 2015, 16, 57. [CrossRef]
- Petrella, R.J.; Eamans, P.; Alleyne, J.; Maroney, M. A prospective, multi-center, randomized, double-blind feasibility study to evaluate the safety and performance of hydros joint therapy and Hydros-TA joint therapy for management of pain associated with osteoarthritis in the knee. Osteoarthritis and Cartilage 2012, 20, S172-S173. [CrossRef]
- Tankova, L.; Yoncheva, K.; Muhtarov, M.; Kadyan, H.; Draganov, V. Topical mononitrate treatment in patients with anal fissure. Aliment Pharmacol Ther 2002, 16, 101-103. [CrossRef]
- Chen, Y.; Bian, X.; Wang, J.; Yan, F.; Gao, J.; Sun, T. Efficacy and Safety of Loxoprofen Sodium Hydrogel Patch in Patients with Chronic Inflammatory Pain: A 4-Week Real-World Study. J Pain Res 2024, 17, 535-541. [CrossRef]
- Kutcher, M.J.; Ludlow, J.B.; Samuelson, A.D.; Campbell, T.; Pusek, S.N. Evaluation of a bioadhesive device for the management of aphthous ulcers. J Am Dent Assoc 2001, 132, 368-376. [CrossRef]
- Shahzad, M.N.; Ahmed, N. Effectiveness of Aloe Vera gel compared with 1% silver sulphadiazine cream as burn wound dressing in second degree burns. J Pak Med Assoc 2013, 63, 225-230, doi:doi:.
- Verdu-Soriano, J.; de Cristino-Espinar, M.; Luna-Morales, S.; Dios-Guerra, C.; Caballero-Villarraso, J.; Moreno-Moreno, P.; Casado-Diaz, A.; Berenguer-Perez, M.; Guler-Caamano, I.; Laosa-Zafra, O.; et al. Superiority of a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract (EHO-85) for the Treatment of Skin Ulcers: A Randomized, Active-Controlled Clinical Trial. J Clin Med 2022, 11. [CrossRef]
- Francavilla, V.; Secolo, G.; D'Armetta, M.; Toscano, R.; Campo, A.; Catanzaro, V.; Manno, M.; Secolo, I.; Messina, G. Onychocryptosis: a retrospective study of clinical aspects, inflammation treatment and pain management using Ozoile as a hydrogel and cream formulation. Eur J Transl Myol 2024, 34, 165-171. [CrossRef]
- Cho, J.H.; Brodsky, M.; Kim, E.J.; Cho, Y.J.; Kim, K.W.; Fang, J.Y.; Song, M.Y. Efficacy of a 0.1% capsaicin hydrogel patch for myofascial neck pain: a double-blinded randomized trial. Pain Med 2012, 13, 965-970. [CrossRef]
- Taylor, K.R.; Molchan, R.P.; Townley, J.R.; Caldwell, M.C.; Panday, V.A. The effect of silicone hydrogel bandage soft contact lens base curvature on comfort and outcomes after photorefractive keratectomy. Eye Contact Lens 2015, 41, 77-83. [CrossRef]
- Taylor, K.R.; Caldwell, M.C.; Payne, A.M.; Apsey, D.A.; Townley, J.R.; Reilly, C.D.; Panday, V.A. Comparison of 3 silicone hydrogel bandage soft contact lenses for pain control after photorefractive keratectomy. J Cataract Refract Surg 2014, 40, 1798-1804. [CrossRef]
- Holbert, M.D.; Griffin, B.R.; McPhail, S.M.; Ware, R.S.; Foster, K.; Bertoni, D.C.; Kimble, R.M. Effectiveness of a hydrogel dressing as an analgesic adjunct to first aid for the treatment of acute paediatric thermal burn injuries: study protocol for a randomised controlled trial. Trials 2019, 20, 13. [CrossRef]
- Surowiecka, A.; Struzyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management-A Review. Gels 2022, 8. [CrossRef]
- Gopalakrishnan, S.; Far, R.; Veilleux, C.; Swamy, G.; Yang, M.M.H. Delayed percutaneous intradiscal hydrogel herniation causing neurological injury after minor trauma: illustrative case. J Neurosurg Case Lessons 2024, 8. [CrossRef]
- Sacks, G.; DeStefano, V.; Parker, C.; Lebens, R.; Mushlin, H. The Artificial Disc Nucleus and Other Strategies for Replacement of the Nucleus Pulposus: Past, Present and Future Designs for an Emerging Surgical Solution. Engineered Regeneration 2024, 5, 269-281. [CrossRef]
- Nasir, N.A.M.; Saad, A.Z.M.; Bachok, N.S.; Rashid, A.H.A.; Ujang, Z.; Noorsal, K.; Yusof, N.; Hashim, K.; Nor, F.M.; Imran, F.H.; et al. A Prospective Multicenter Randomized Controlled Trial to Evaluate the Efficacy of Chitosan Hydrogel Paste in Comparison to Commercial Hydroactive Gel as a Wound Bed Preparation. Indian J Plast Surg 2023, 56, 44-52. [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat Rev Mater 2016, 1. [CrossRef]
- Topical morphine gel in the treatment of painful leg ulcers, a double-blind, placebo-controlled clinical trial: a pilot study. International Wound Journal 2012, 9. [CrossRef]
- Steigleman, W.A.; Rose-Nussbaumer, J.; Al-Mohtaseb, Z.; Santhiago, M.R.; Lin, C.C.; Pantanelli, S.M.; Kim, S.J.; Schallhorn, J.M. Management of Pain after Photorefractive Keratectomy: A Report by the American Academy of Ophthalmology. Ophthalmology 2023, 130, 87-98. [CrossRef]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio 2022, 14, 100223. [CrossRef]
- Binaymotlagh, R.; Hajareh Haghighi, F.; Chronopoulou, L.; Palocci, C. Liposome-Hydrogel Composites for Controlled Drug Delivery Applications. Gels 2024, 10. [CrossRef]
- Jyothi, B.; Mitragotri, M.V.; Kurugodiyavar, M.D.; Shaikh, S.I.; Korikanthimath, V.V. Morphine Versus Loperamide with Intrasite Gel in the Treatment of Painful Dermal Ulcers: A Randomized, Crossover Study. Pain Physician 2021, 24, E37-E44.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).