Submitted:
13 September 2025
Posted:
16 September 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Case Definition and Inclusion Criteria
Data Collection
Outcomes
Statistical Analysis
Ethical considerations
Results
Clinical Manifestations
Laboratory Findings
Discussion
Limitations
Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Reno, E.; Quan, N.G.; Franco-Paredes, C.; Chastain, D.B.; Chauhan, L.; Rodriguez-Morales, A.J.; Henao-Martínez, A.F. Prevention of yellow fever in travellers: an update. Lancet Infect Dis 2020, 20, e129–e137. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Sah, R.; Silva-Ramos, C.R.; Pava-Garzón, D.M. Challenges in Emerging and Reemerging Arboviral Diseases: The Examples of Oropouche and Yellow Fever. Pathogens 2025, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- Forero-Delgadillo, A.J.; Morales-Olivera, J.A.; Celis-Guzmán, J.F.; Zapata-Díaz, O.E.; González-Varona, G.A.; Acevedo-Bedoya, C.A.; Salazar-Fernández, R.; Ordoñez, J.O.; Robayo-Amortegui, H.; Quintero-Altare, A. , et al. Colombian consensus on the care of critically ill patients with suspected or confirmed severe yellow fever. Lancet Reg Health Am 2025, 48, 101144. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J.; Alhazmi, A.H.; Katime, A.; Hameed, A.A.; Morales, A.; Lepetic, A.C.; Risquez, A.; Forero-Delgadillo, A.J.; Holguin, A.; Faccini-Martínez Á, A. , et al. Yellow fever in South America - A plea for action and call for prevention also in travelers from SLAMVI, ESGITM, EVASG, ALEIMC, GEPI-SEIMC, SEMEVI, and CMTZMV-ACIN. Travel Med Infect Dis 2025, 67, 102871. [Google Scholar] [CrossRef]
- Angerami, R.N.; Socorro Souza Chaves, T.D.; Rodríguez-Morales, A.J. Yellow fever outbreaks in South America: Current epidemiology, legacies of the recent past and perspectives for the near future. New Microbes New Infect 2025, 65, 101580. [Google Scholar] [CrossRef] [PubMed]
- Chaves, T.; Orduna, T.; Lepetic, A.; Macchi, A.; Verbanaz, S.; Risquez, A.; Perret, C.; Echazarreta, S.; Rodríguez-Morales, A.J.; Lloveras, S.C. Yellow fever in Brazil: Epidemiological aspects and implications for travelers. Travel Med Infect Dis 2018, 23, 1–3. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Bonilla-Aldana, D.K.; Suárez, J.A.; Franco-Paredes, C.; Forero-Peña, D.A.; Mattar, S.; Villamil-Gómez, W.E.; Ruíz-Sáenz, J.; Cardona-Ospina, J.A.; Figuera, M.E. , et al. Yellow fever reemergence in Venezuela - Implications for international travelers and Latin American countries during the COVID-19 pandemic. Travel Med Infect Dis 2021, 44, 102192. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.A.; Rodriguez-Morales, A.J. Challenges of the current yellow fever outbreak in Colombia. Lancet 2025, 405, 2273. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Bonilla-Aldana, J.L.; Castellanos, J.E.; Rodriguez-Morales, A.J. Importance of Epizootic Surveillance in the Epidemiology of Yellow Fever in South America. Current Tropical Medicine Reports 2025, 12, 16. [Google Scholar] [CrossRef]
- Sanchez-Rojas, I.C.; Bonilla-Aldana, D.K.; Solarte-Jimenez, C.L.; Bonilla-Aldana, J.L.; Belisario-Tovar, M.; Ortega-Gómez, S.; Zambrano-Quenan, V.M.; Perafan-Gomez, J.C.; Gomez-Ocampo, C.H.; Delgado-Cajigas, M.; et al. Fatal yellow fever among captive non-human primates in southern Colombia, 2025. Frontiers in Veterinary Science 2025, 12, 1655474. [Google Scholar] [CrossRef]
- Brandão-de-Resende, C.; Cunha, L.H.M.; Oliveira, S.L.; Pereira, L.S.; Oliveira, J.G.F.; Santos, T.A.; Vasconcelos-Santos, D.V. Characterization of Retinopathy Among Patients With Yellow Fever During 2 Outbreaks in Southeastern Brazil. JAMA Ophthalmol 2019, 137, 996–1002. [Google Scholar] [CrossRef]
- Ho, Y.L.; Joelsons, D.; Leite, G.F.C.; Malbouisson, L.M.S.; Song, A.T.W.; Perondi, B.; Andrade, L.C.; Pinto, L.F.; D'Albuquerque, L.A.C.; Segurado, A.A.C. Severe yellow fever in Brazil: clinical characteristics and management. J Travel Med 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.; de Rezende, I.M.; Pereira, L.S.; Dutra, M.R.T.; Fradico, J.R.B.; Macedo, R.; Marçal, M.C.; Fonte Boa, L.S.C.; Bragato, A.M.C.; Faria, F.A.A. , et al. Risk factors associated with in-hospital mortality during yellow fever outbreak in Brazil. Front Med (Lausanne) 2025, 12, 1505005. [Google Scholar] [CrossRef] [PubMed]
- Baronti, C.; Goitia, N.J.; Cook, S.; Roca, Y.; Revollo, J.; Flores, J.V.; de Lamballerie, X. Molecular epidemiology of yellow fever in Bolivia from 1999 to 2008. Vector Borne Zoonotic Dis 2011, 11, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E. [Yellow Fever National Service of Ecuador]. Bol Oficina Sanit Panam 1949, 28, 1099–1106. [Google Scholar] [PubMed]
- Sanchez-Rojas, I.C.; Solarte-Jimenez, C.L.; Chamorro-Velazco, E.C.; Diaz-Llerena, G.E.; Arevalo, C.D.; Cuasquer-Posos, O.L.; Bonilla-Aldana, J.L.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Yellow fever in Putumayo, Colombia, 2024. New Microbes New Infect 2025, 64, 101572. [Google Scholar] [CrossRef]
- Smith, A. Rise and Progress of Yellow Fever in Peru. Edinb Med Surg J 1855, 82, 165–204. [Google Scholar]
- Cabezas, C. Yellow fever in Peru and the Americas and the latent risk of reurbanuzation: an avoidable threat. Rev Peru Med Exp Salud Publica 2025, 42, 113–114. [Google Scholar] [CrossRef]
- Garcia-Bereguiain, M.A.; Cisneros, S.S.; Narváez, A.; Orlando, S.A.; Rodríguez-Pazmiño Á, S. Yellow fever outbreak in Ecuador and travel vaccination policies. J Travel Med 2025. [Google Scholar] [CrossRef]
- Paupy, C.; Le Goff, G.; Brengues, C.; Guerra, M.; Revollo, J.; Barja Simon, Z.; Hervé, J.P.; Fontenille, D. Genetic structure and phylogeography of Aedes aegypti, the dengue and yellow-fever mosquito vector in Bolivia. Infect Genet Evol 2012, 12, 1260–1269. [Google Scholar] [CrossRef]
- Rifakis, P.M.; Benitez, J.A.; De-la-Paz-Pineda, J.; Rodriguez-Morales, A.J. Epizootics of yellow fever in Venezuela (2004-2005): an emerging zoonotic disease. Ann N Y Acad Sci 2006, 1081, 57–60. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Torres-Hernández, D.; Guevara, M.E.; Chang-Cojulun, A.; Brea-Del Castillo, J.; Rios-Blanco, R.; Mérida-Barrios, M.I.; Palmieri, M.; Avila-Agüero, M.L. Yellow fever in children and adolescents amid the South American Outbreak, 2024/2025. New Microbes and New Infections 2025, 101635. [Google Scholar] [CrossRef]
- Baba, M.M.; Ikusemoran, M. Is the absence or intermittent YF vaccination the major contributor to its persistent outbreaks in eastern Africa? Biochem Biophys Res Commun 2017, 492, 548–557. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Cianchi, V.; Torelli, A.; Montomoli, E. Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects. Vaccines (Basel) 2022, 10, 372. [Google Scholar] [CrossRef]
- Paniz-Mondolfi, A.E.; Rodriguez-Morales, A.J.; Blohm, G.; Marquez, M.; Villamil-Gomez, W.E. ChikDenMaZika Syndrome: the challenge of diagnosing arboviral infections in the midst of concurrent epidemics. Ann Clin Microbiol Antimicrob 2016, 15, 42. [Google Scholar] [CrossRef]
- Srivastava, S.; Sah, R.; Babu, M.R.; Sharma, D.; Sharma, D.; Kumar, S.; Sridhar, S.B.; Wadhwa, T.; Shareef, J.; Rao, G. , et al. The emergence of oropouche fever: A potential new threat? New Microbes New Infect 2025, 65, 101596. [Google Scholar] [CrossRef]
- Giugni, F.R.; Aiello, V.D.; Faria, C.S.; Pour, S.Z.; Cunha, M.D.P.; Giugni, M.V.; Pinesi, H.T.; Ledesma, F.L.; Morais, C.E.; Ho, Y.L. , et al. Understanding yellow fever-associated myocardial injury: an autopsy study. EBioMedicine 2023, 96, 104810. [Google Scholar] [CrossRef]
- Villamil-Gómez, W. Protocolo diagnóstico del síndrome febril con afectación hematológica en áreas geográficas de riesgo endémico de infecciones tropicales. Medicine - Programa de Formación Médica Continuada Acreditado 2022, 13, 3445–3454. [Google Scholar] [CrossRef]
- Carrillo-Hernández, M.Y.; Ruiz-Saenz, J.; Villamizar, L.J.; Gómez-Rangel, S.Y.; Martínez-Gutierrez, M. Co-circulation and simultaneous co-infection of dengue, chikungunya, and zika viruses in patients with febrile syndrome at the Colombian-Venezuelan border. BMC Infect Dis 2018, 18, 61. [Google Scholar] [CrossRef]
- Castellanos, J.E.; Jaimes, N.; Coronel-Ruiza, C.; Rojas, J.P.; Mejía, L.F.; Villarreal, V.H.; Maya, L.E.; Claros, L.M.; Orjuela, C.; Calvo, E. , et al. Dengue-chikungunya coinfection outbreak in children from Cali, Colombia in 2018-2019. Int J Infect Dis 2021, 102, 97–102. [Google Scholar] [CrossRef]
- Villamil-Gómez, W.E.; Rodríguez-Morales, A.J.; Uribe-García, A.M.; González-Arismendy, E.; Castellanos, J.E.; Calvo, E.P.; Álvarez-Mon, M.; Musso, D. Zika, dengue, and chikungunya co-infection in a pregnant woman from Colombia. Int J Infect Dis 2016, 51, 135–138. [Google Scholar] [CrossRef]
- Cardona-Ospina, J.A.; Jiménez-Canizales, C.E.; Vásquez-Serna, H.; Garzón-Ramírez, J.A.; Alarcón-Robayo, J.F.; Cerón-Pineda, J.A.; Rodríguez-Morales, A.J. Fatal Dengue, Chikungunya and Leptospirosis: The Importance of Assessing Co-infections in Febrile Patients in Tropical Areas. Trop Med Infect Dis 2018, 3, 123. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Paniz-Mondolfi, A.E. Venezuela's failure in malaria control. Lancet 2014, 384, 663–664. [Google Scholar] [CrossRef]
- Ortiz-Martínez, Y.; Cabeza-Ruiz, L.D.; Henao-Martínez, A.F.; Rodriguez-Morales, A.J. Clinical challenges of managing advanced AIDS in the tropics: Histoplasmosis, COVID-19, and shigellosis coinfections. New Microbes New Infect 2022, 49-50, 101015. [Google Scholar] [CrossRef]
- Bani Hani, H.; Ibrahim, S.; Esmail, M.; Waleed, S.; Gouher, S. Dengue Fever Complicated by Pneumonia in Pregnancy: A Case Report. Cureus 2024, 16, e73608. [Google Scholar] [CrossRef]
- Frassetto, F.P.; Rosemberg, S. Neuropathology of yellow fever autopsy cases. Tropical Diseases, Travel Medicine and Vaccines 2023, 9, 1. [Google Scholar] [CrossRef]
- Schnyder, J.L.; Bache, B.E.; Welkers, M.R.A.; Spijker, R.; Schaumburg, F.; Goorhuis, A.; Grobusch, M.P.; de Jong, H.K. Yellow fever breakthrough infections after yellow fever vaccination: a systematic review and meta-analysis. Lancet Microbe 2024, 5, 100937. [Google Scholar] [CrossRef]
- Wilder-Smith, A. Yellow Fever in Travelers. Curr Infect Dis Rep 2019, 21, 42. [Google Scholar] [CrossRef]
- de Ávila, R.E.; José Fernandes, H.; Barbosa, G.M.; Araújo, A.L.; Gomes, T.C.C.; Barros, T.G.; Moreira, R.L.F.; Silva, G.L.C.; de Oliveira, N.R. Clinical profiles and factors associated with mortality in adults with yellow fever admitted to an intensive care unit in Minas Gerais, Brazil. Int J Infect Dis 2020, 93, 90–97. [Google Scholar] [CrossRef]
| Variable | n / Median | % / IQR |
|---|---|---|
| Year | ||
| 2025 | 17 | 81 |
| 2023/2024 | 4 | 19 |
| Country | ||
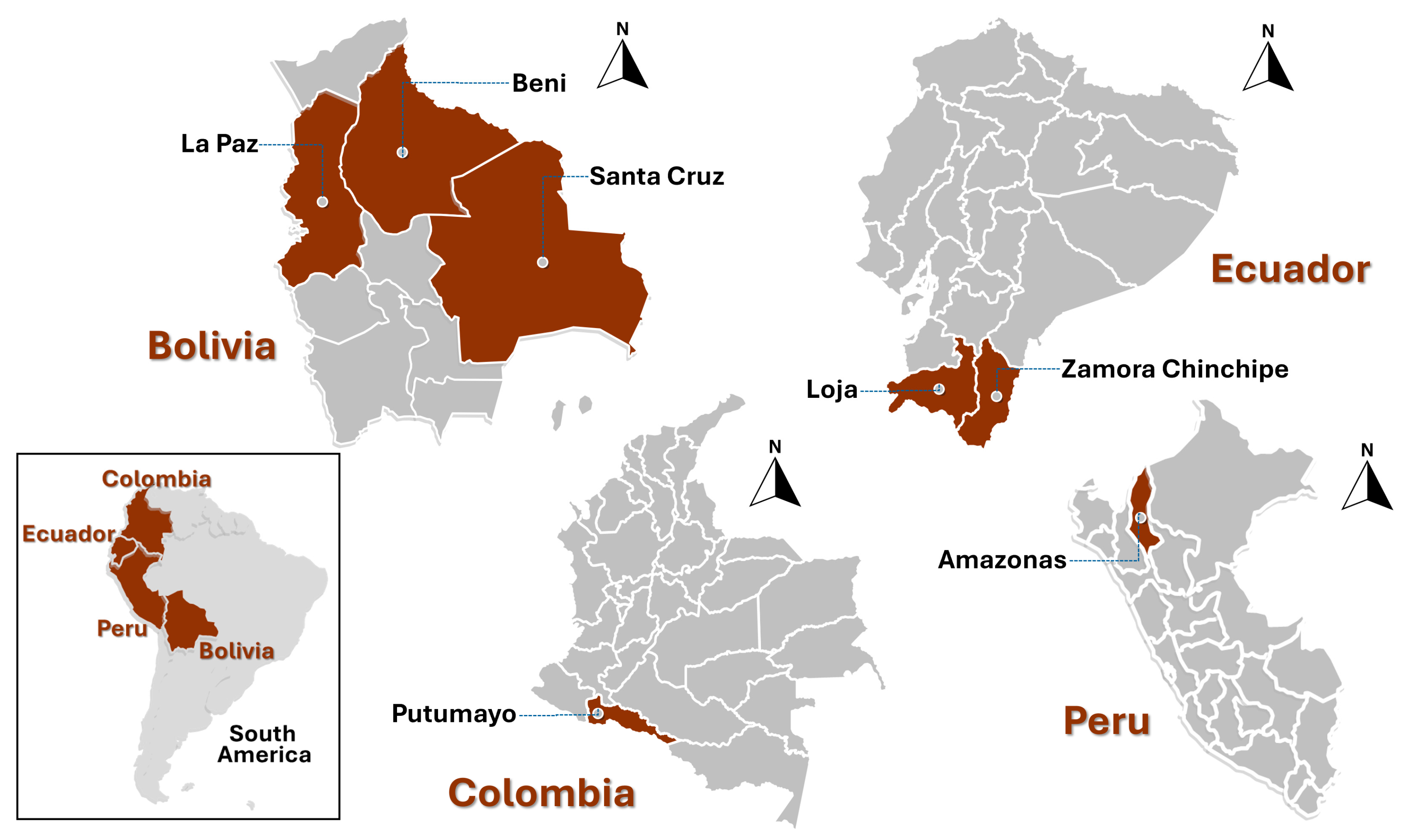
| Bolivia (Santa Cruz, La Paz, and Beni) | 8 | 38 |
| Ecuador (Loja and Zamora Chinchipe) | 5 | 24 |
| Colombia (Putumayo) | 4 | 19 |
| Peru (Amazonas) | 4 | 19 |
| Age (years) | 25 | 16-43 |
| <20 years | 6 | 29 |
| 20-59 years | 13 | 62 |
| ≥60 years | 2 | 9 |
| Sex | ||
| Male | 17 | 81 |
| Female | 4 | 19 |
| Occupation | ||
| Students | 5 | 24 |
| Agricultural worker | 3 | 14 |
| Construction worker | 2 | 10 |
| Mining worker | 1 | 5 |
| Other occupations/unknown | 10 | 48 |
| Vaccination against YFV (status) | ||
| Unknown or none | 18 | 86 |
| Apparently vaccinated | 3 | 14 |
| Days between symptom onset and consultation and hospitalization | 5 | 4-6 |
| Outcome | ||
| Died | 12 | 57 |
| Survived | 9 | 43 |
| Days from symptoms onset to death | 8 | 6-9 |
| Finding | n | % |
|---|---|---|
| Main Initial Clinical Findings (Early Stage) | ||
| Fever | 15 | 71 |
| Arthralgia | 4 | 19 |
| Headache | 4 | 19 |
| Myalgia | 4 | 19 |
| Vomiting | 1 | 5 |
| Severe Findings (Toxic Phase) | ||
| Bleeding | 18 | 86 |
| Jaundice | 13 | 62 |
| Circulatory Collapse | 12 | 57 |
| Hepatic Dysfunction | 11 | 52 |
| Encephalopathy | 8 | 38 |
| Renal Failure | 5 | 24 |
| High Transaminases | 4 | 19 |
| Coagulopathy | 3 | 14 |
| Thrombocytopenia | 2 | 10 |
| Hyperbilirubinemia | 2 | 10 |
| Azotemia | 1 | 5 |
| Coma | 1 | 5 |
| Complications | ||
| Severe Coagulation Disorder | 3 | 14 |
| Pleural Effusion | 2 | 10 |
| Acute Kidney Injury | 2 | 10 |
| Anemia | 2 | 10 |
| Intracranial Hemorrhage | 2 | 10 |
| Multiorgan Dysfunction | 2 | 10 |
| Hypokalemia | 1 | 5 |
| Pancytopenia | 1 | 5 |
| Pneumonia | 1 | 5 |
| Shock | 1 | 5 |
| Bilateral Nephromegaly | 1 | 5 |
| Cerebral Anoxia | 1 | 5 |
| Edema | 1 | 5 |
| ESβL Klebsiella pneumoniae pneumonia | 1 | 5 |
| ESβL Escherichia coli sepsis | 1 | 5 |
| Laboratory Variable | n | Median | IQR | Reference Values (RV) | % <RV | % >RV | |
|---|---|---|---|---|---|---|---|
| pH | 5 | 7.3 | 7.28 | 7.41 | 7.35–7.45 | 60 | 0 |
| HCO3- (mmol/L) | 5 | 19.6 | 13.2 | 26.2 | 21–28 | 60 | 0 |
| Anion Gap (mmol/L) | 5 | 15.2 | 14.9 | 21.9 | 8–16 | 0 | 40 |
| Lactate (mmol/L) | 3 | 13.1 | 12.7 | 13.55 | 0.7–2.5 | 0 | 100 |
| Glucose (mg/dL) | 5 | 49 | 46 | 63 | 70-110 | 80 | 20 |
| Urea (mg/dL) | 3 | 124 | 106.9 | 127.85 | 15–45 | 0 | 100 |
| Creatinine (mg/dL) | 6 | 2.885 | 1.3 | 6.44 | 0.7–1.3 | 0 | 67 |
| Bilirubin total (mg/dL) | 11 | 6.68 | 5.19 | 7.85 | <1.2 | 100 | |
| Bilirubin direct (mg/dL) | 9 | 6.41 | 3.92 | 6.7 | <0.3 | 100 | |
| Bilirubin indirect (mg/dL) | 9 | 1.21 | 0.93 | 1.62 | <1.0 | 33 | |
| AST (U/L) | 11 | 3257 | 1230.5 | 11468.5 | <40 | 100 | |
| ALT (U/L) | 10 | 1570.5 | 726.25 | 4101 | <40 | 100 | |
| Alkaline phosphatase (U/L) | 3 | 154 | 150 | 227.5 | <120 | 67 | |
| GGT (U/L) | 2 | 290.5 | 254.25 | 326.75 | <60 | 100 | |
| Proteins total (g/dL) | 2 | 4.11 | 4.105 | 4.115 | 6.4–8.3 | 100 | 0 |
| Albumin (g/dL) | 4 | 2.465 | 2.1925 | 3.295 | 3.5–5.0 | 75 | 25 |
| Globulin (g/dL) | 2 | 1.95 | 1.925 | 1.975 | 2.3–3.4 | 100 | 0 |
| TP (s) | 12 | 22.8 | 15.125 | 33.45 | 11–14 | 8 | 75 |
| INR | 6 | 1.25 | 1.135 | 2.2425 | 0.9–1.2 | 0 | 50 |
| TTP/TTPA (s) | 11 | 43 | 34.65 | 63.25 | <35 | 27 | |
| Fibrinogen (mg/dL) | 4 | 142 | 119 | 203.5 | 15–20 | 75 | 0 |
| Hemoglobin (g/dL) | 13 | 13.6 | 10.4 | 14.5 | 200–400 | 46 | 8 |
| Hematocrit (%) | 13 | 36 | 25.65 | 41 | ♂13.5–17.5; ♀12–16 | 46 | 0 |
| Leukocytes (x103/µL) | 13 | 5.8 | 3.5 | 7.1 | 35–49 | 38 | 8 |
| Platelets (x103/µL) | 13 | 74 | 62 | 131 | 4–11 | 77 | 8 |
| Procalcitonin (ng/mL) | 4 | 4.96 | 1.5025 | 8.5125 | 150–400 | 25 | 0 |
| CRP (mg/L) | 3 | 5.75 | 4.065 | 50.875 | <0.5 | 33 | |
| LDH (U/L) | 2 | 2580.5 | 1439.75 | 3721.25 | <5 | 100 | |
| CPK total (U/L) | 2 | 797.25 | 694.475 | 900.025 | <250 | 100 | |
| CPK-MB (U/L) | 1 | 10 | 26–162 | ||||
| Troponin I (ng/mL) | 1 | 0.1 | 0–25 | ||||
| D-dimer (µg/mL) | 1 | 3.65 | 0.12–0.60 | ||||
| YFV RT-PCR Ct | 4 | 23.45 | 22.6 | 24.6 | <0.55 | 0 | 0 |
| n | Positive | Negative | %+ | %- | |||
| YFV RT-PCR | 19 | 18 | 1 | 95 | 5 | ||
| IgM for YFY | 7 | 7 | 0 | 100 | 0 | ||
| YFY RT-PCR/IgM | 5 | 4 | 1 | 80 | 0 | ||
| NS1 for DENV | 1 | 0 | 1 | 0 | 100 | ||
| IgM for DENV | 1 | 1 | 0 | 100 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





