Submitted:
11 September 2025
Posted:
12 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
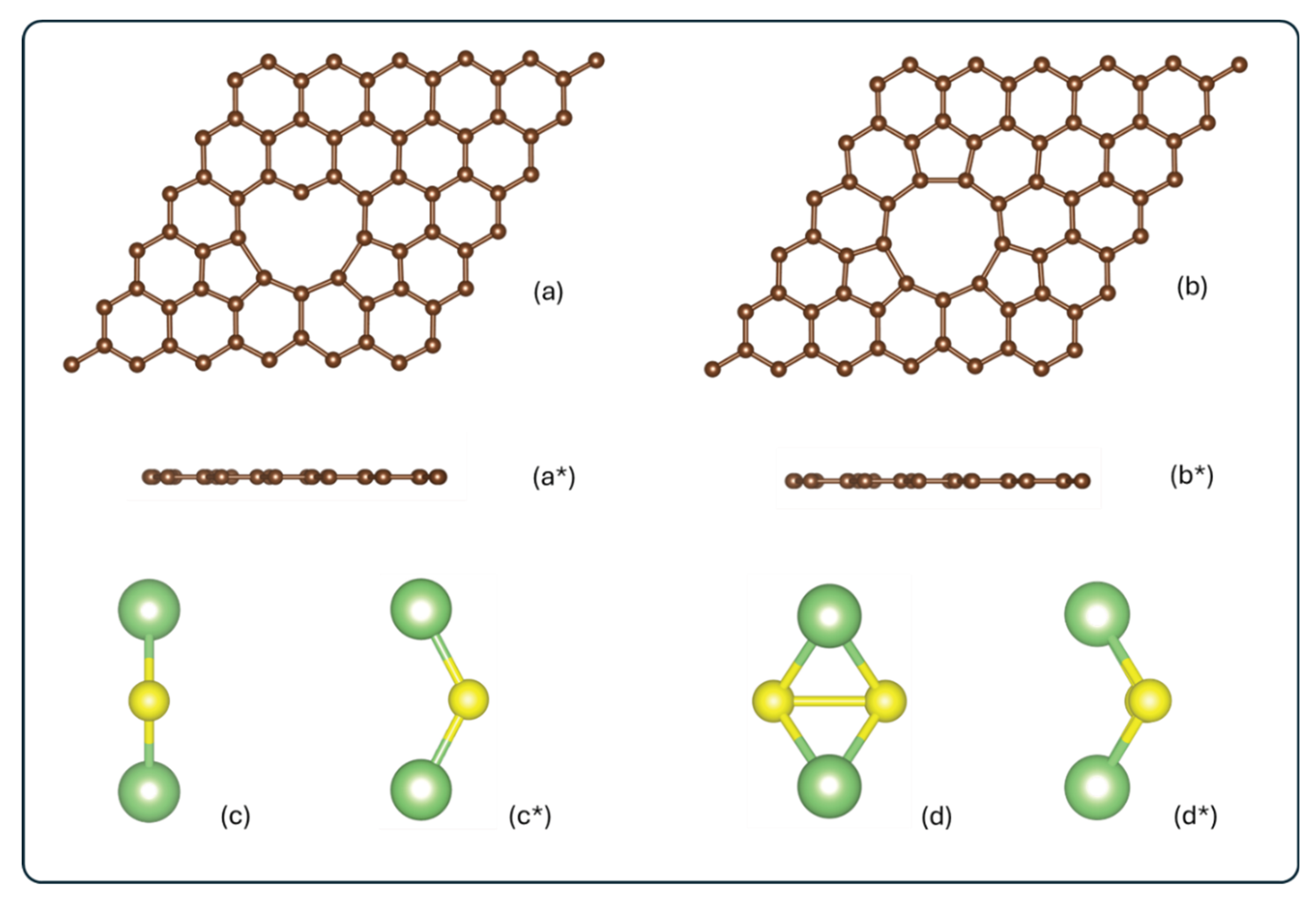
2.1. Methodology
2.2. Model
3. Results and Discussion
3.1. Adsorption Energies, Stability, and Geometry
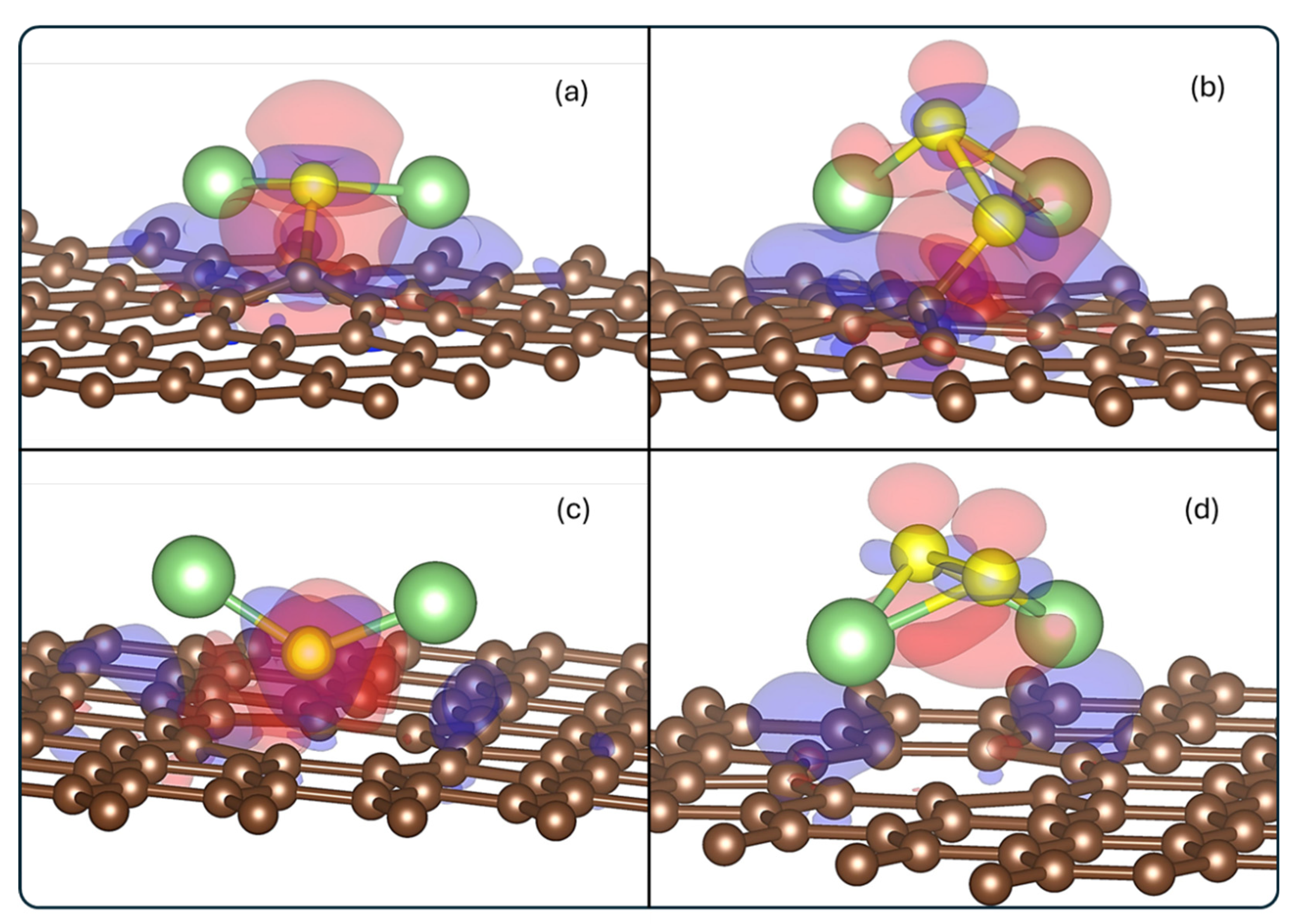
3.2. Electronic Structure
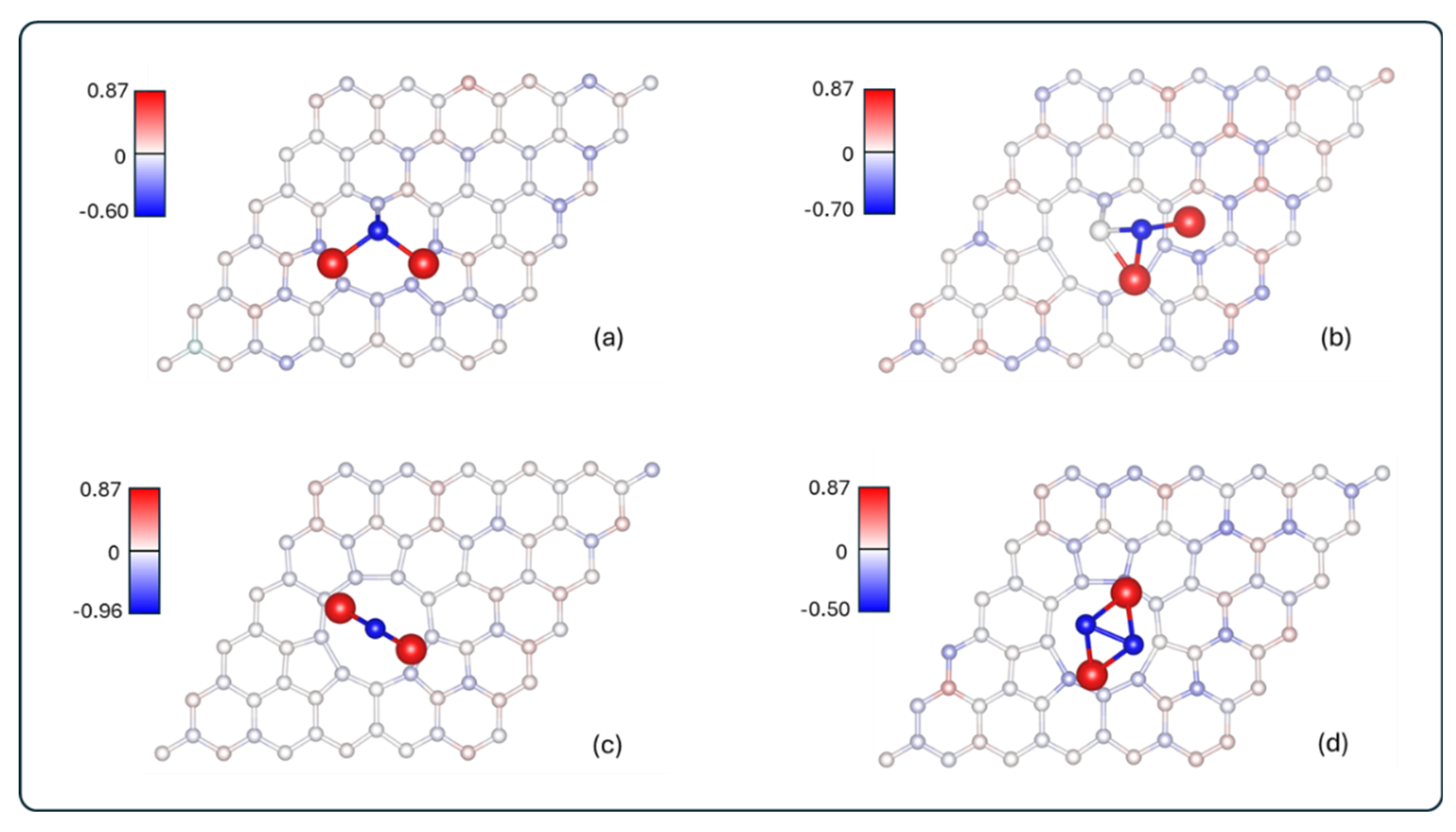
3.2.1. Charge Transfer Analysis
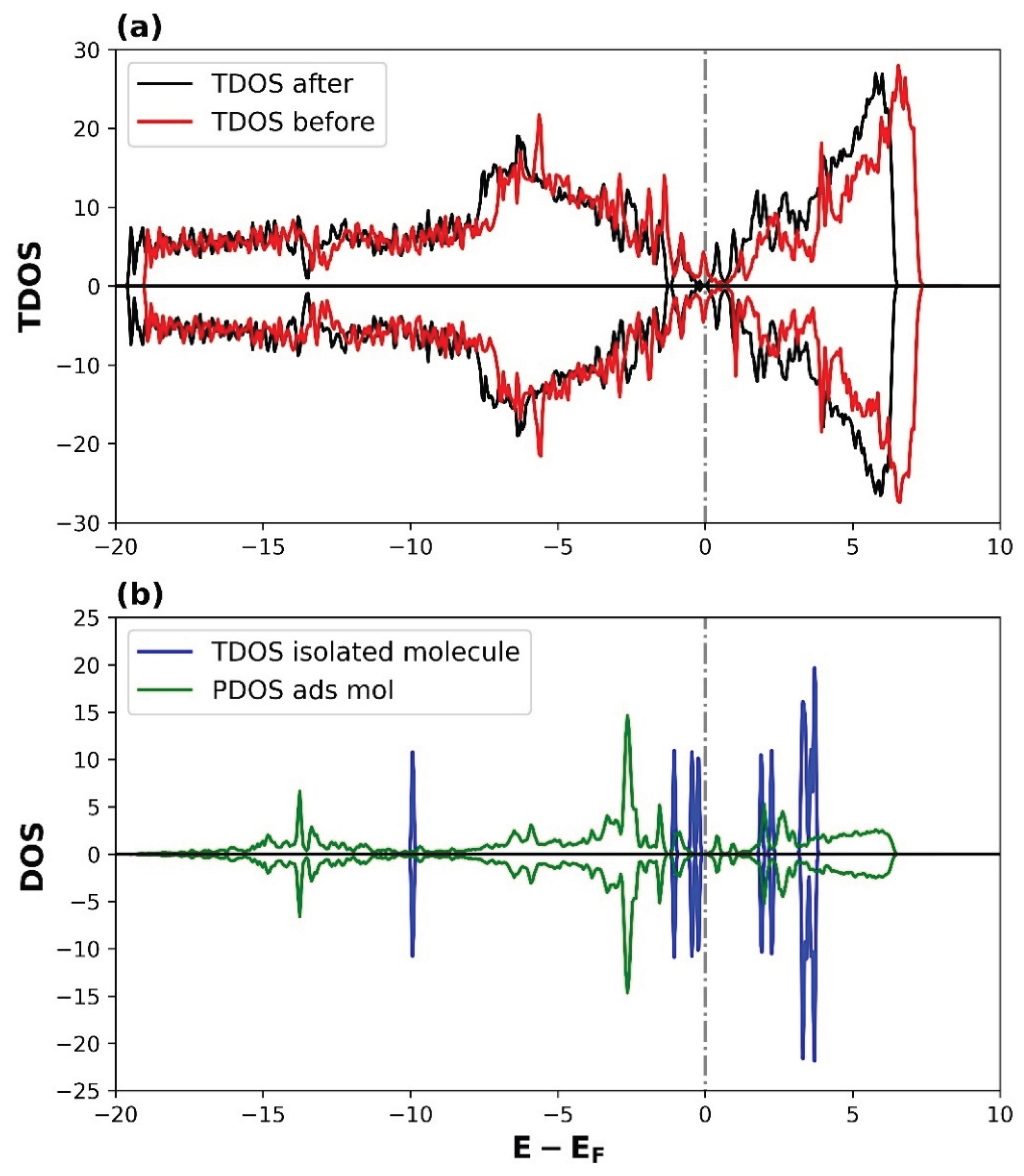
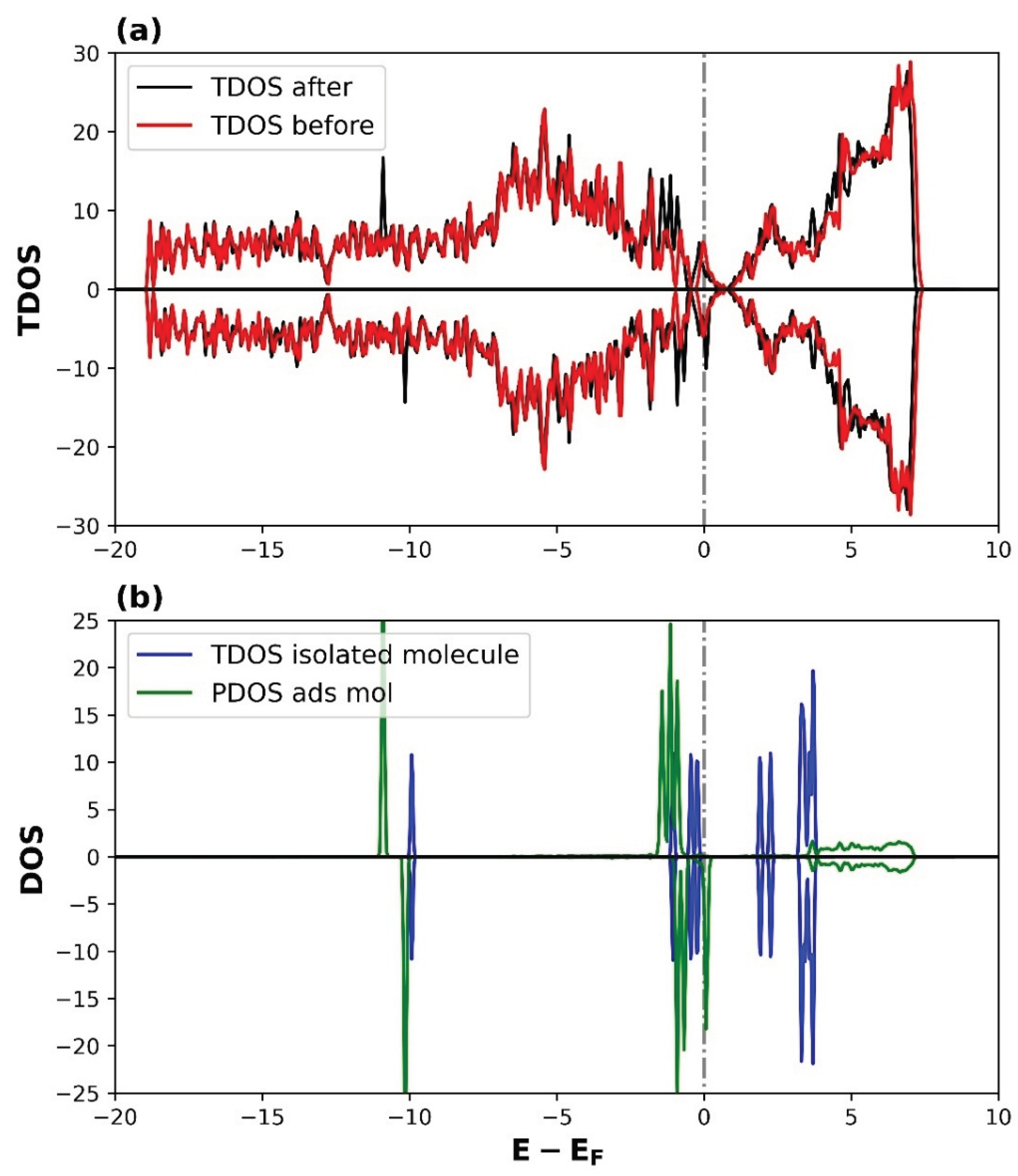
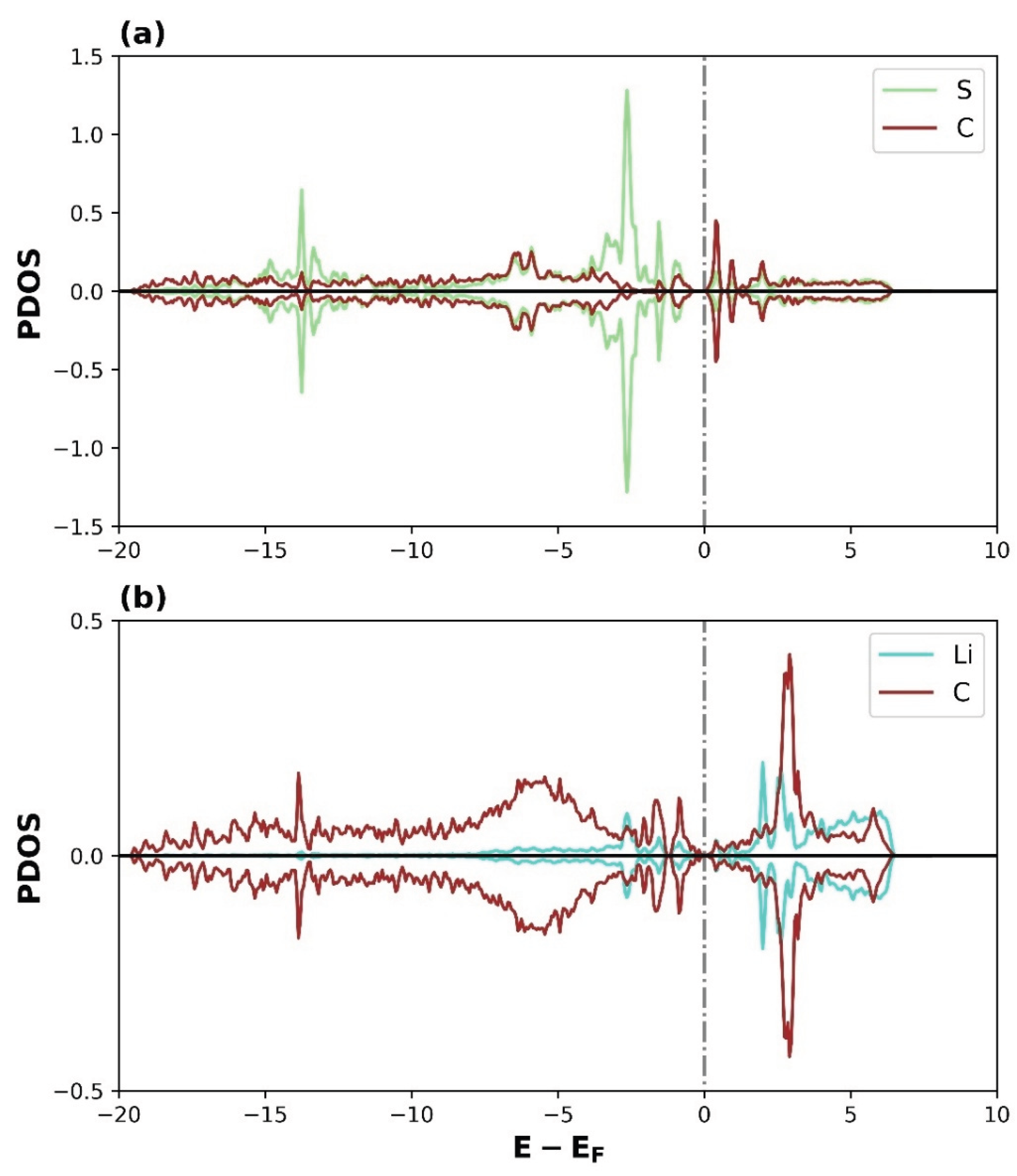
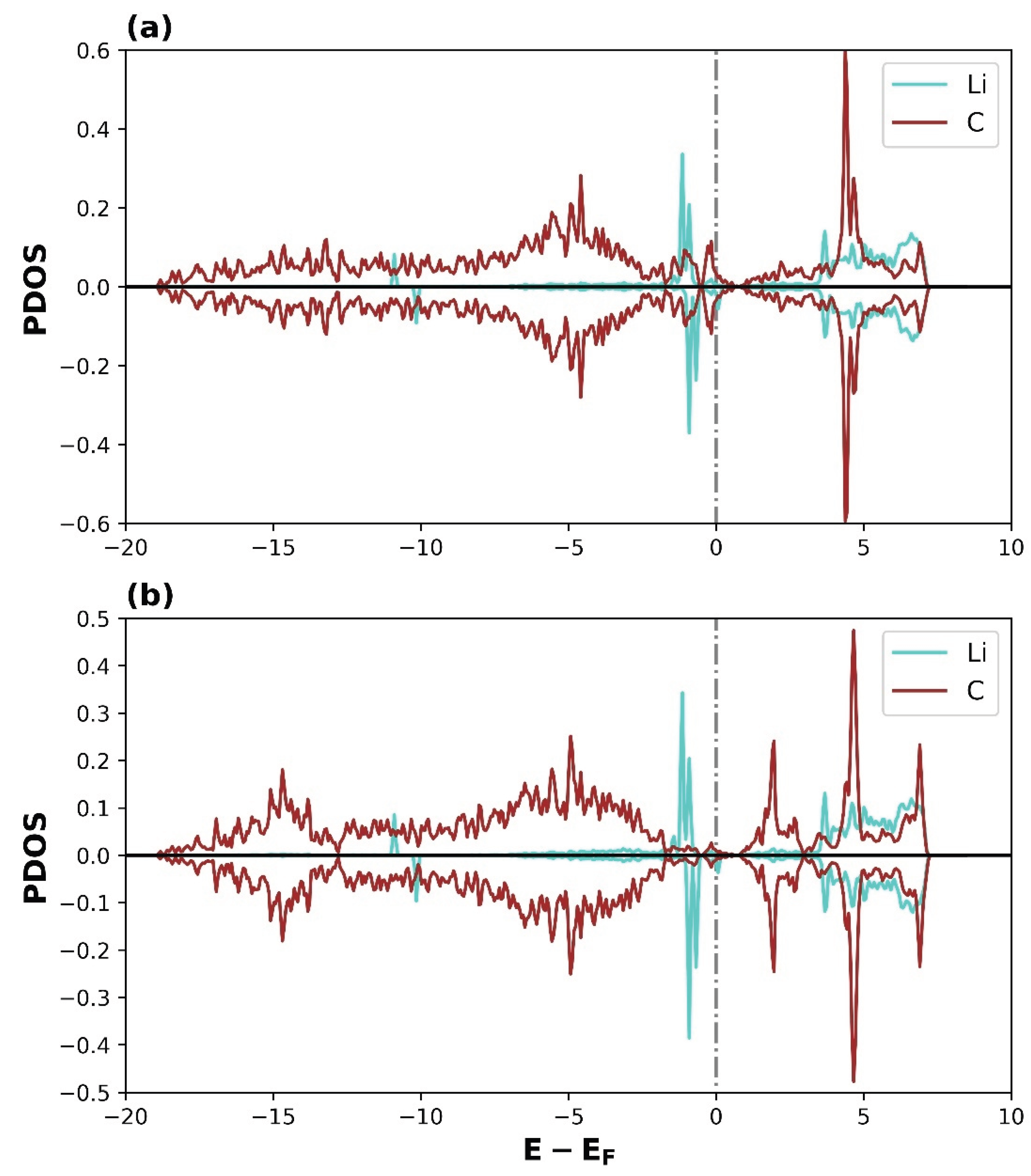
3.2.2. Density of States
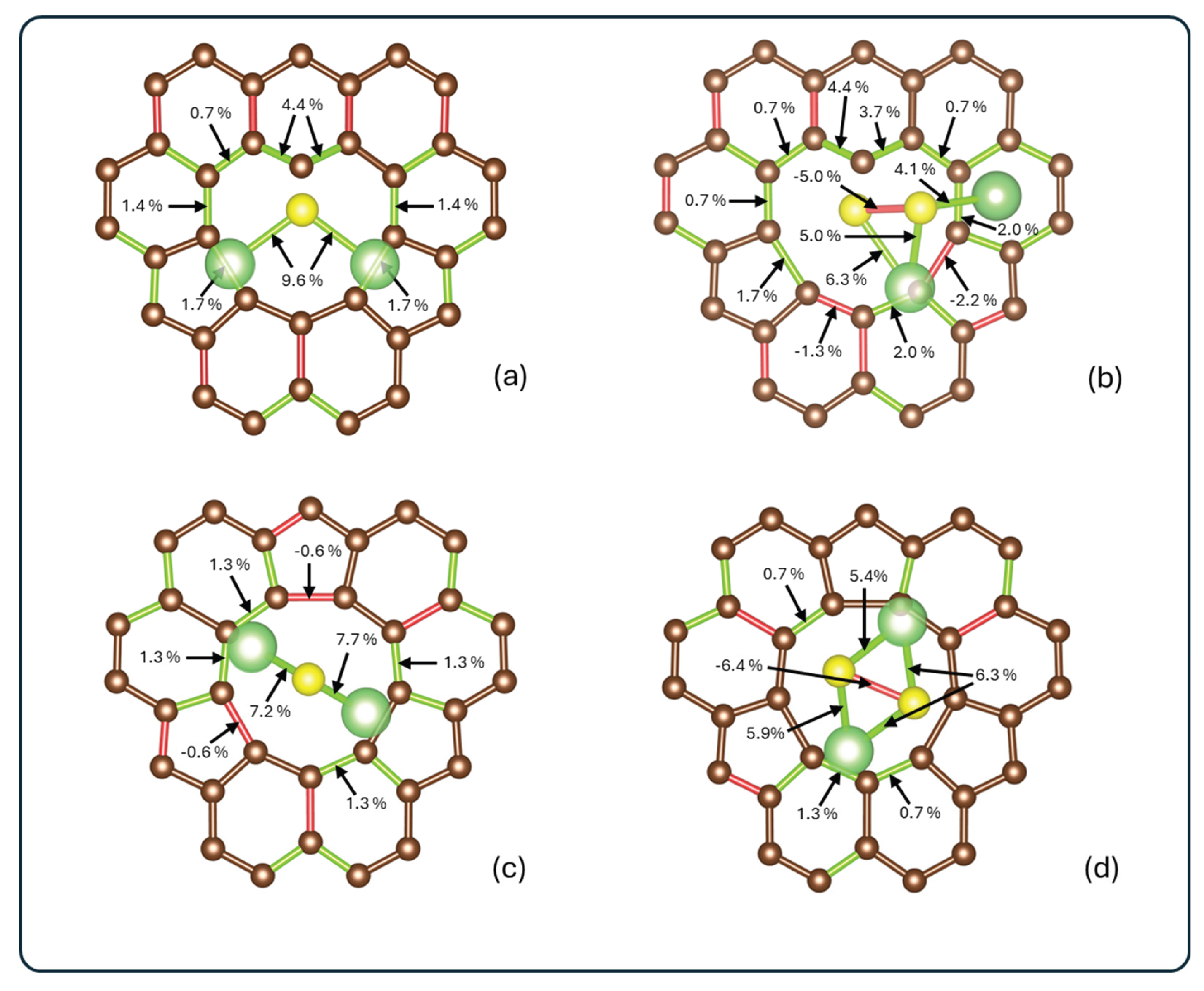
3.3. Bond Order and Interaction Strength
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| ANPCyT | Agencia Nacional de Promoción de Científica y Tecnológica |
| CONICET | Consejo Nacional de Investigaciones Científicas y Técnicas |
References
- Ji, X.; Nazar, L.F. Advances in Li–S Batteries. J Mater Chem 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S Batteries with High Energy Storage. Nat Mater 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc Chem Res 2013, 46, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, J.; Fan, Z.; Chen, S.; Lin, Q.; Lu, X.; Dou, H.; Kumar Nanjundan, A.; Yushin, G.; Zhang, X.; Yamauchi, Y. Solid-State Lithium–Sulfur Batteries: Advances, Challenges and Perspectives. Materials Today 2020, 40, 114–131. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.; Peng, H.; Yuan, H.; Wei, J.; Huang, J. Lithium–Sulfur Batteries under Lean Electrolyte Conditions: Challenges and Opportunities. Angewandte Chemie International Edition 2020, 59, 12636–12652. [Google Scholar] [CrossRef]
- Hou, L.-P.; Zhang, X.-Q.; Li, B.-Q.; Zhang, Q. Challenges and Promises of Lithium Metal Anode by Soluble Polysulfides in Practical Lithium–Sulfur Batteries. Materials Today 2021, 45, 62–76. [Google Scholar] [CrossRef]
- Deshmukh, A.; Thripuranthaka, M.; Chaturvedi, V.; Das, A.K.; Shelke, V.; Shelke, M.V. A Review on Recent Advancements in Solid State Lithium–Sulfur Batteries: Fundamentals, Challenges, and Perspectives. Progress in Energy 2022, 4. [Google Scholar] [CrossRef]
- Raza, H.; Bai, S.; Cheng, J.; Majumder, S.; Zhu, H.; Liu, Q.; Zheng, G.; Li, X.; Chen, G. Li-S Batteries: Challenges, Achievements and Opportunities. Electrochemical Energy Reviews 2023, 6. [Google Scholar] [CrossRef]
- Lv, Z.-C.; Wang, P.-F.; Wang, J.-C.; Tian, S.-H.; Yi, T.-F. Key Challenges, Recent Advances and Future Perspectives of Rechargeable Lithium-Sulfur Batteries. Journal of Industrial and Engineering Chemistry 2023, 124, 68–88. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, T.; Wang, P.; Yuan, M.; Li, Q.; Feng, S. Challenges and Solutions for Low-Temperature Lithium–Sulfur Batteries: A Review. Materials 2023, 16. [Google Scholar] [CrossRef]
- Shao, Q.; Zhu, S.; Chen, J. A Review on Lithium-Sulfur Batteries: Challenge, Development, and Perspective. Nano Res 2023, 16, 8097–8138. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, F.; Ma, Y.; Luo, S.; Li, Y.; Hu, A.; He, M.; Li, F.; Chen, D.; Chen, W.; Lei, T.; Hu, Y. Challenges and Advances on Low-Temperature Rechargeable Lithium-Sulfur Batteries. Nano Res 2023, 16, 8082–8096. [Google Scholar] [CrossRef]
- Shi, H.; Sun, W.; Cao, J.; Han, S.; Lu, G.; Ghazi, Z.A.; Zhu, X.; Lan, H.; Lv, W. Challenges and Solutions for Lithium–Sulfur Batteries with Lean Electrolyte. Adv Funct Mater 2023, 33. [Google Scholar] [CrossRef]
- Pathak, A.D.; Cha, E.; Choi, W. Towards the Commercialization of Li-S Battery: From Lab to Industry. Energy Storage Mater 2024, 72, 103711. [Google Scholar] [CrossRef]
- Qian, W.; Guo, Y.; Zuo, W.; Wu, X.; Zhang, L. Toward Practical Lithium–Sulfur Batteries. Mater Chem Front 2024, 8, 2556–2577. [Google Scholar] [CrossRef]
- Sawangphruk, M. New Materials for Lithium–Sulfur Batteries: Challenges and Future Directions. Chemical Communications 2025, 61, 7770–7794. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, H.; Kumar, G.; Sharma, S.; Aneja, R.; Sharma, A.K.; Kumar, R.; Kumar, P. Progress and Challenges in Electrochemical Energy Storage Devices: Fabrication, Electrode Material, and Economic Aspects. Chemical Engineering Journal 2023, 468, 143706. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-Wrapped Sulfur Particles as a Rechargeable Lithium–Sulfur Battery Cathode Material with High Capacity and Cycling Stability. Nano Lett 2011, 11, 2644–2647. [Google Scholar] [CrossRef]
- Zhou, G.; Pei, S.; Li, L.; Wang, D.; Wang, S.; Huang, K.; Yin, L.; Li, F.; Cheng, H. A Graphene–Pure-Sulfur Sandwich Structure for Ultrafast, Long-Life Lithium–Sulfur Batteries. Advanced Materials 2014, 26, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ding, B.; Pan, J.; Nie, P.; Shen, L.; Zhang, X. High-Performance Lithium–Sulfur Batteries: Advances and Challenges. Small 2015, 11, 4321–4347. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Lee, K.T.; Nazar, L.F. A Highly Ordered Nanostructured Carbon–Sulphur Cathode for Lithium–Sulphur Batteries. Nat Mater 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing High-Energy Lithium–Sulfur Batteries. Chem Soc Rev 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qu, C.; Wen, J.; Wang, C.; Ma, X.; Yang, Y.; Huang, G.; Sun, H.; Xu, S. Progress of Transition Metal Sulfides Used as Lithium-Ion Battery Anodes. Mater Chem Front 2023, 7, 2779–2808. [Google Scholar] [CrossRef]
- Liang, X.; Garsuch, A.; Nazar, L.F. Sulfur Cathodes Based on Conductive MXene Nanosheets for High-Performance Lithium–Sulfur Batteries. Angewandte Chemie 2015, 127, 3979–3983. [Google Scholar] [CrossRef]
- Suzanowicz, A.; Mei, C.; Mandal, B. Approaches to Combat the Polysulfide Shuttle Phenomenon in Li–S Battery Technology. Batteries 2022, 8. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Jia, S.; Zhao, Q.; Zheng, Q.; Ma, Y.; Ma, T.; Li, X. Recent Advances in Inhibiting Shuttle Effect of Polysulfide in Lithium-Sulfur Batteries. J Energy Storage 2023, 72, 108372. [Google Scholar] [CrossRef]
- Rao, X.-Y.; Xiang, S.-F.; Zhou, J.; Zhang, Z.; Xu, X.-Y.; Xu, Y.-Y.; Zhou, X.-C.; Pan, Z.-D.; Tan, S.-C.; Dong, S.-X.; Wang, Z.-L.; Wu, Y.-T.; Zhou, Y.-L.; Liu, X.; Zhang, Y.; Jiang, S. Recent Progress and Strategies of Cathodes toward Polysulfides Shuttle Restriction for Lithium-Sulfur Batteries. Rare Metals 2024, 43, 4132–4161. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. New Approaches for High Energy Density Lithium–Sulfur Battery Cathodes. Acc Chem Res 2013, 46, 1135–1143. [Google Scholar] [CrossRef]
- Pan, H.; Cheng, Z.; He, P.; Zhou, H. A Review of Solid-State Lithium–Sulfur Battery: Ion Transport and Polysulfide Chemistry. Energy & Fuels 2020, 34, 11942–11961. [Google Scholar] [CrossRef]
- Yu, M.; Li, R.; Wu, M.; Shi, G. Graphene Materials for Lithium–Sulfur Batteries. Energy Storage Mater 2015, 1, 51–73. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Padmanaban, S.; Suneel, R.M.; Milton, K.; Subair, N.; Pandey, A.; Khanna, M.; Srivastava, D.; Mathew, R.M.; Selvaraj, S.K.; Banavoth, M.; Sonar, P.; Badoni, B.; Rao, N.S.; Kumar, S.G.; Ray, A.K.; Kumar, A. Review—Contemporary Progresses in Carbon-Based Electrode Material in Li-S Batteries. J Electrochem Soc 2022, 169. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Zhai, X.; Wang, F.; Ren, X.; Xiong, Y.; Akiyoshi, O.; Pan, K.; Ren, F.; Wei, S. Graphene-Based Interlayer for High-Performance Lithium–Sulfur Batteries: A Review. Mater Des 2021, 211, 110171. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, R.; Tang, R.; Cheng, X.; He, Z.; Meng, T. Insight into the Polysulfides Conversion Kinetics and Its Activation Energy Relationship on Ultrafine V8C7 in Li-S Batteries. Chemical Engineering Journal 2024, 500, 156970. [Google Scholar] [CrossRef]
- Rao, D.; Wang, Y.; Zhang, L.; Yao, S.; Qian, X.; Xi, X.; Xiao, K.; Deng, K.; Shen, X.; Lu, R. Mechanism of Polysulfide Immobilization on Defective Graphene Sheets with N-Substitution. Carbon N Y 2016, 110, 207–214. [Google Scholar] [CrossRef]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen-Doped Mesoporous Carbon Promoted Chemical Adsorption of Sulfur and Fabrication of High-Areal-Capacity Sulfur Cathode with Exceptional Cycling Stability for Lithium-Sulfur Batteries. Adv Funct Mater 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Ding, Y.; Zhang, W.; Yu, G. In Situ Reactive Synthesis of Polypyrrole-MnO 2 Coaxial Nanotubes as Sulfur Hosts for High-Performance Lithium–Sulfur Battery. Nano Lett 2016, 16, 7276–7281. [Google Scholar] [CrossRef]
- Zhang, S.; Ueno, K.; Dokko, K.; Watanabe, M. Recent Advances in Electrolytes for Lithium–Sulfur Batteries. Adv Energy Mater 2015, 5. [Google Scholar] [CrossRef]
- Li, J.; Qu, Y.; Chen, C.; Zhang, X.; Shao, M. Theoretical Investigation on Lithium Polysulfide Adsorption and Conversion for High-Performance Li–S Batteries. Nanoscale 2021, 13, 15–35. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Sun, X. Towards High-Performance Solid-State Li–S Batteries: From Fundamental Understanding to Engineering Design. Chem Soc Rev 2020, 49, 2140–2195. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, Y.; He, J.; Li, X. Advanced Computational Methods in Lithium–Sulfur Batteries. Adv Funct Mater 2024, 34. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Wang, Z.; Yan, J.; Liu, J.; Wu, Y. Review on the First-Principles Calculation in Lithium-Sulfur Battery. Progress in Chemistry 2023, 35, 375–389. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef]
- Ambrusi, R.E.; Luna, C.R.; Juan, A.; Pronsato, M.E. DFT Study of Rh-Decorated Pristine, B-Doped and Vacancy Defected Graphene for Hydrogen Adsorption. RSC Adv 2016, 6, 83926–83941. [Google Scholar] [CrossRef]
- Ambrusi, R.E.; Orazi, V.; Morelli, A.; Marchetti, J.M.; Juan, A. Theoretical Study of a Ti4 Cluster Interacting with B-Doped and Non-Doped Multivacancy Graphene. Physica B Condens Matter 2024, 683, 415875. [Google Scholar] [CrossRef]
- Ambrusi, R.E.; Orazi, V.; Marchetti, J.M.; Pronsato, M.E. Ni Clusters Embedded in Multivacancy Graphene Substrates. Journal of Physics and Chemistry of Solids 2020, 138, 109258. [Google Scholar] [CrossRef]
- Orazi, V.; Ambrusi, R.E.; Marchetti, J.M.; Pronsato, M.E. DFT Study of the Hydrogen Adsorption and Storage on Ni4 Cluster Embedded in Multivacancy Graphene. Int J Hydrogen Energy 2020, 45, 30805–30817. [Google Scholar] [CrossRef]
- Ambrusi, R.E.; Orazi, V.; Marchetti, J.M.; Juan, A.; Pronsato, M.E. Hydrogen Storage by Spillover on Ni4 Cluster Embedded in Three Vacancy Graphene. A DFT and Dynamics Study. Journal of Physics and Chemistry of Solids 2022, 167, 110706. [Google Scholar] [CrossRef]
- Faccio, R.; Fernández-Werner, L.; Pardo, H.; Goyenola, C.; Ventura, O.N.; Mombrú, A.W. Electronic and Structural Distortions in Graphene Induced by Carbon Vacancies and Boron Doping. Journal of Physical Chemistry C 2010, 114, 18961–18971. [Google Scholar] [CrossRef]
- Faccio, R.; Mombrú, A.W. Magnetism in Multivacancy Graphene Systems. Journal of Physics Condensed Matter 2012, 24. [Google Scholar] [CrossRef]
- Faccio, R.; Mombrú, A.W. Influence of the Structural Configuration on the Stability and Magnetism in Multivacancy Graphene Systems. Comput Mater Sci 2015, 97, 193–200. [Google Scholar] [CrossRef]
- Mombrú, D.; Faccio, R.; Mombrú, A.W. First Row Transition Metal Atoms Embedded in Multivacancies in a Rippled Graphene System. Appl Surf Sci 2018, 435, 102–107. [Google Scholar] [CrossRef]
- Mombrú, D.; Faccio, R.; Mombrú, A.W. Sulfur Doping in Multivacancy Graphene Systems. Appl Surf Sci 2018, 459, 336–344. [Google Scholar] [CrossRef]
- Rao, D.; Yang, H.; Shen, X.; Yan, X.; Qiao, G. Immobilisation of Sulphur on Cathodes of Lithium–Sulphur Batteries via B-Doped Atomic-Layer Carbon Materials. Physical Chemistry Chemical Physics 2019, 21, 10895–10901. [Google Scholar] [CrossRef]
- Jyothirmai, M.V.; Ravva, M.K. Changes in Structure and Stability of Lithium Polysulfides Encapsulated in Carbon Nanotubes: A DFT Study. J Mol Liq 2022, 359, 119287. [Google Scholar] [CrossRef]
- Yi, Z.; Su, F.; Huo, L.; Cui, G.; Zhang, C.; Han, P.; Dong, N.; Chen, C. New Insights into Li2S2/Li2S Adsorption on the Graphene Bearing Single Vacancy: A DFT Study. Appl Surf Sci 2020, 503, 144446. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys Rev B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput Mater Sci 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys Rev B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys Rev Lett 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector Augmented-+rave Method. Phys Rev B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J Chem Phys 2010, 132. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys Rev B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules; Oxford University PressOxford, 1990. [CrossRef]
- Manz, T.A.; Limas, N.G. Chargemol program for performing DDEC analysis, Version 3.4.4, ddec.sourceforge.net.
- Limas, N.G.; Manz, T.A. Introducing DDEC6 Atomic Population Analysis: Part 2. Computed Results for a Wide Range of Periodic and Nonperiodic Materials. RSC Adv 2016, 6, 45727–45747. [Google Scholar] [CrossRef]
- Manz, T.A.; Limas, N.G. Introducing DDEC6 Atomic Population Analysis: Part 1. Charge Partitioning Theory and Methodology. RSC Adv 2016, 6, 47771–47801. [Google Scholar] [CrossRef]

| System | Eads (eV) | μ(μB) |
| Li2S@3V-graphene | -3.84 | 0 |
| Li2S2@3V-graphene | -2.83 | 0 |
| Li2S@4V-graphene | -1.63 | 0.878 |
| Li2S2@4V-graphene | -1.51 | 0.944 |
| System | Carbon ring charge (e) | Graphene charge (e) | Adsorbate charge (e) |
| Li2S@3V-graphene | -0.80 | -1.01 | 1.13 |
| Li2S2@3V-graphene | -0.63 | -0.92 | 1.04 |
| Li2S@4V-graphene | -0.71 | -0.64 | 0.77 |
| Li2S2@4V-graphene | -0.60 | -0.63 | 0.75 |
| System | Bond | Before | After | % Δ |
| Li2S@3V-graphene | Li1–C5 | - | 0.084 | - |
| Li1–C4 | - | 0.091 | - | |
| Li2–C8 | - | 0.093 | - | |
| Li2–C7 | - | 0.084 | - | |
| S–C1 | - | 1.244 | - | |
| Li1–S | 0.440 | 0.205 | -53% | |
| Li2–S | 0.440 | 0.208 | -53% | |
| C1–C10 | 1.472 | 1.160 | -21% | |
| C1–C2 | 1.471 | 1.162 | -21% | |
| Li2S2@3V-graphene | S2–C1 | - | 1.077 | - |
| Li2–C3 | - | 0.070 | - | |
| Li1–C5 | - | 0.113 | - | |
| S2–Li1 | 0.223 | 0.118 | -47% | |
| S2–Li2 | 0.223 | 0.003 | -98% | |
| S1–Li1 | 0.223 | 0.202 | -9% | |
| S1–S2 | 1.546 | 1.402 | -9% | |
| C1–C10 | 1.472 | 1.221 | -17% | |
| C1–C2 | 1.471 | 1.224 | -17% | |
| C5–C4 | 0.695 | 0.770 | 11% | |
| C5–C6 | 1.240 | 1.180 | -5% | |
| C3–C4 | 1.219 | 1.169 | -4% |
| System | Bond | Before | After | % Δ |
| Li2S@4V-graphene | Li1–C5 | - | 0.082 | - |
| Li1–C4 | - | 0.082 | - | |
| Li2–C9 | - | 0.091 | - | |
| Li1–S | 0.440 | 0.309 | -31% | |
| Li2–S | 0.440 | 0.316 | -28% | |
| C5–C4 | 0.797 | 0.837 | 5% | |
| Li2S2@4V-graphene | Li1–C7 | - | 0.078 | - |
| Li1–C6 | - | 0.068 | - | |
| Li2–C2 | - | 0.092 | - | |
| S1–Li1 | 0.223 | 0.150 | -33% | |
| S1–Li2 | 0.223 | 0.152 | -32% | |
| S2–Li2 | 0.223 | 0.161 | -28% | |
| S2–Li1 | 0.223 | 0.158 | -29% | |
| S1–S2 | 1.546 | 1.664 | 8% | |
| C2–C1 | 0.797 | 0.835 | 5% | |
| C7–C8 | 0.797 | 0.831 | 4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





