1. Introduction
It is one of the important measures that ironmaking enterprises reduce cost and increase economic efficiency by utilization of cheap bentonite and high alumina ore in blast furnace smelting process. However, excessive Al2O3 in the slag can deteriorate viscous behavior of the slag, permeability of the blast furnace cohesive zone, and make it difficult to separate slag and iron. B2O3 in the blast furnace slag can improve its viscous behaviorError! Reference source not found.. Adding the appropriate amount of CaO and MgO to the slag will reduce the degree of slag polymerization and improve viscous behavior of the slag. However, excessive CaO and MgO will increase the slag amount and energy consumption. It is not conducive to energy conservation and emission reduction. Therefore, it is necessary to study the effect of CaO/SiO2 and MgO/Al2O3 on the viscous flow behaviors of low boron-bearing high alumina blast furnace slag.Error! Reference source not found.
For recent years, some researches about the viscous flow behaviors of boron-containing slag had been reported. Li et al. [
4] studied the roles of MgO and Al
2O
3 in the viscous and structural behavior of CaO-MgO-Al
2O
3-SiO
2-10 mass pct FeO with C/S = 1.4 slag. They found the MgO can prompt the depolymerization of the silicate network structure and reduce the viscosity of slag. The Al
2O
3 content to greater than 10 mass pct has the opposite effect on viscosity, as a result of the polymerization of the silicate network structure. Liang et al. [
5] studied the effects of CaO/SiO
2 and MgO on the metallurgical properties of CaO-SiO
2-MgO-Al
2O
3-TiO
2 blast furnace slag system. The results showed when CaO/SiO
2 increased from 1.10 to 1.30, the polymerization degree of viscous units in the slag decreased, the
η and
Eη decreased, the
TBr increased; when the mass fraction of MgO increases from 6.0% to 12.0%,
η decreased, the
TBr and
Eη decreased first and then increased. Gao et al. [
6] studied the effects of basicity and MgO content on the viscosity of SiO
2-CaO-MgO-9wt%Al
2O
3 slags with basicity from 0.4 to 1.0 and MgO content from 13wt% to 19wt%. They found the viscosity is strongly dependent on the combined action of basic oxide components in the slag. In their study, increasing the basicity is found to be more effective than increasing the MgO content in decreasing the viscosity of the slag. At higher temperatures, the increase of basicity or MgO content does not appreciably decrease the viscosity of the slag, as it does at lower temperatures. Huang et al. [
7] studied the influence of B
2O
3 on the viscosity and degree of polymerization of SiO
2-30wt%Al
2O
3-B
2O
3-12 wt%Na
2O-CaO slag system. The results show that the degree of polymerization of slag decreases, the viscosity and break point temperature of slag decrease with the increase of B
2O
3. The above studies mainly focus on the influence of MgO、Al
2O
3、basicity and B
2O
3 et al. as single factors on the structure and viscosity in different types of slag systems. The effect of CaO/SiO
2 and MgO/Al
2O
3 on the viscosity behavior of low boron-bearing high alumina slag system has not been studied in detail.
In this paper, we verify the effect of CaO/SiO2 and MgO/Al2O3 on the viscous behavior of low boron-bearing high alumina slag. The viscosity, the break temperature and the activation energy of the viscous flow of the slag are considered in detail. Firstly, a series of experiments were conducted to measure the viscosity of the slag. Subsequently, the effect mechanisms of CaO/SiO2 and MgO/Al2O3 on low boron-bearing high alumina slag viscous behavior were expounded using the XRD and FTIR. Then the Factsage was adopted to demonstrate the liquidus temperature of the slags. Finally, the boron-bearing high alumina blast furnace slag with better performance was obtained, which provides guidance for the actual industrial production of the ironmaking company.
2. Experimental
2.1. Raw Material
Based on the on-site BF slag compositions, the slag samples for experiments were synthesized with the analytical reagent oxides of CaO, SiO
2, MgO and Al
2O
3. The basicity of the BF slag is 1.25, the Al
2O
3 is 17.00 %, the B
2O
3 is 0.47 %, and the MgO
/Al
2O
3 is 0.47, which belongs to the boron-bearing high alumina blast furnace slag. The chemical compositions of slag samples are listed in
Table 1. The experimental slag samples were synthesized by adding the analytical-grade oxides with the site blast furnace slag as the reference slag. In order to improve the accuracy of the experiment, the furnace slag and the analytical-grade oxides were roasted, mixed evenly and put into the molybdenum crucible, then pre-melted under the Argon atmosphere to stabilize the temperature and homogenize the compositions. The pre-melted slag samples were used for the determination of viscous flow behaviors
Error! Reference source not found.. The experimental scheme shown in
Table 2. In the series-1, keeping the MgO 7.98%、the Al
2O
3 17.00% and B
2O
3 3.83% in the slag and increase the CaO/SiO
2 from 1.10 to 1.30. In the series-2, keeping the CaO/SiO
2 radio 1.25 in the slag and increase the MgO/Al
2O
3 from 0.40 to 0.65.
2.2. Experimental Procedure
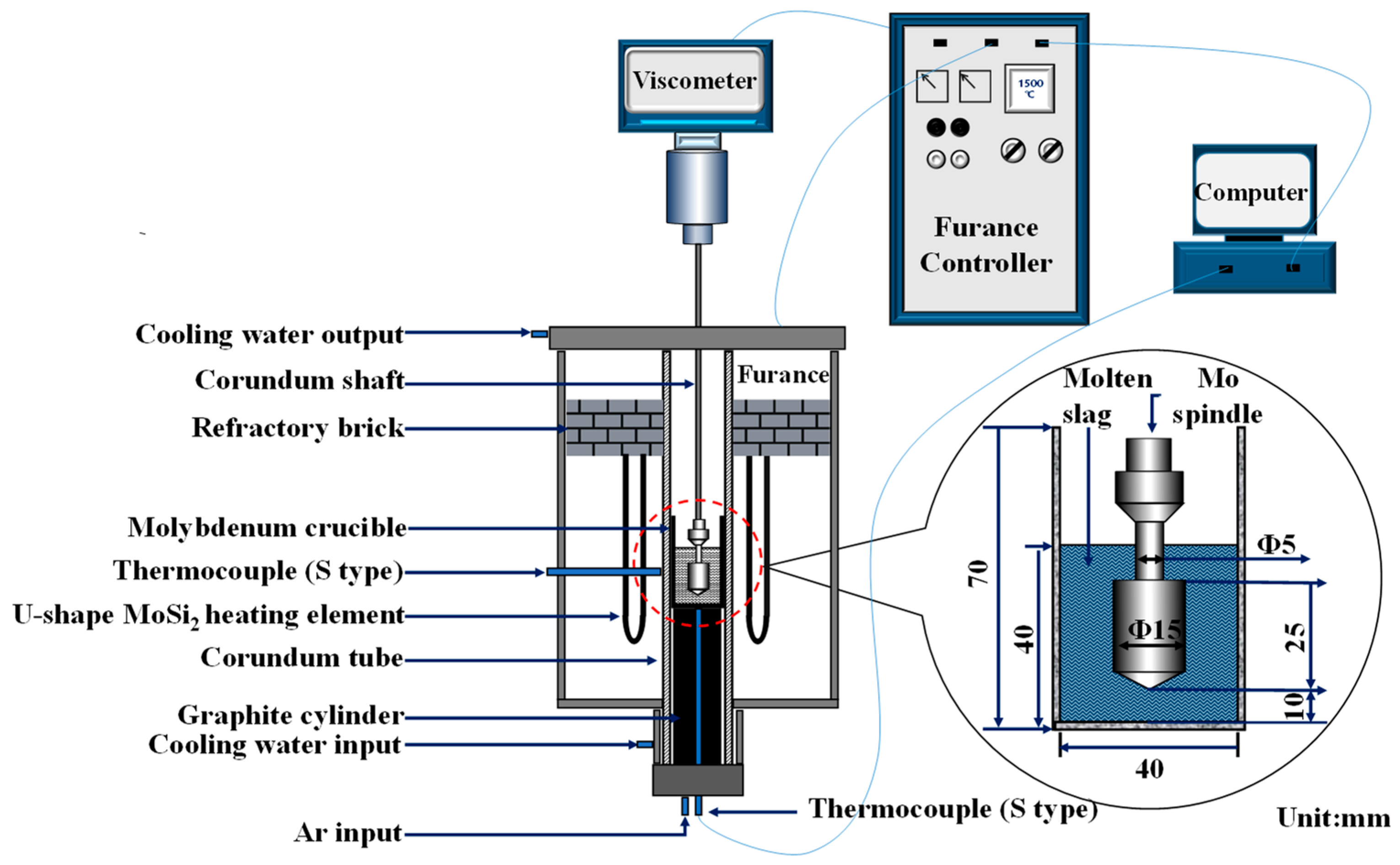
The viscosity-temperature (
η-T) curves of slag were acquired on the RTW-10 melt property tester by the rotating cylinder method.
Figure 1 shown the schematic diagram of the experimental device, which consisted of a heating system, a rotating system, a measuring system, a control system and an atmosphere system.
The crucible containing the experimental slag sample was placed on the graphite base and heated to 1500 °C with the furnace temperature, and then the temperature was held for 30 min. During the constant temperature, the molybdenum probe was used to stir the slag to homogenize the chemical compositions. When the temperature stabilized, the viscosity was measured at a speed of 200r/min. In the process of viscosity measurement, Argon gas was injected into the furnace tube at the flow rate of 1.5 L/min to maintain an inert atmosphere. The measurement results were recorded by the control system. When the viscosity reached about 3.5 Pa·s, the measurements were ended. The experiment was repeated twice. The quenched experimental slags and the natural cooling slags were crushed and grinded to facilitate the analysis by FTIR and XRD.
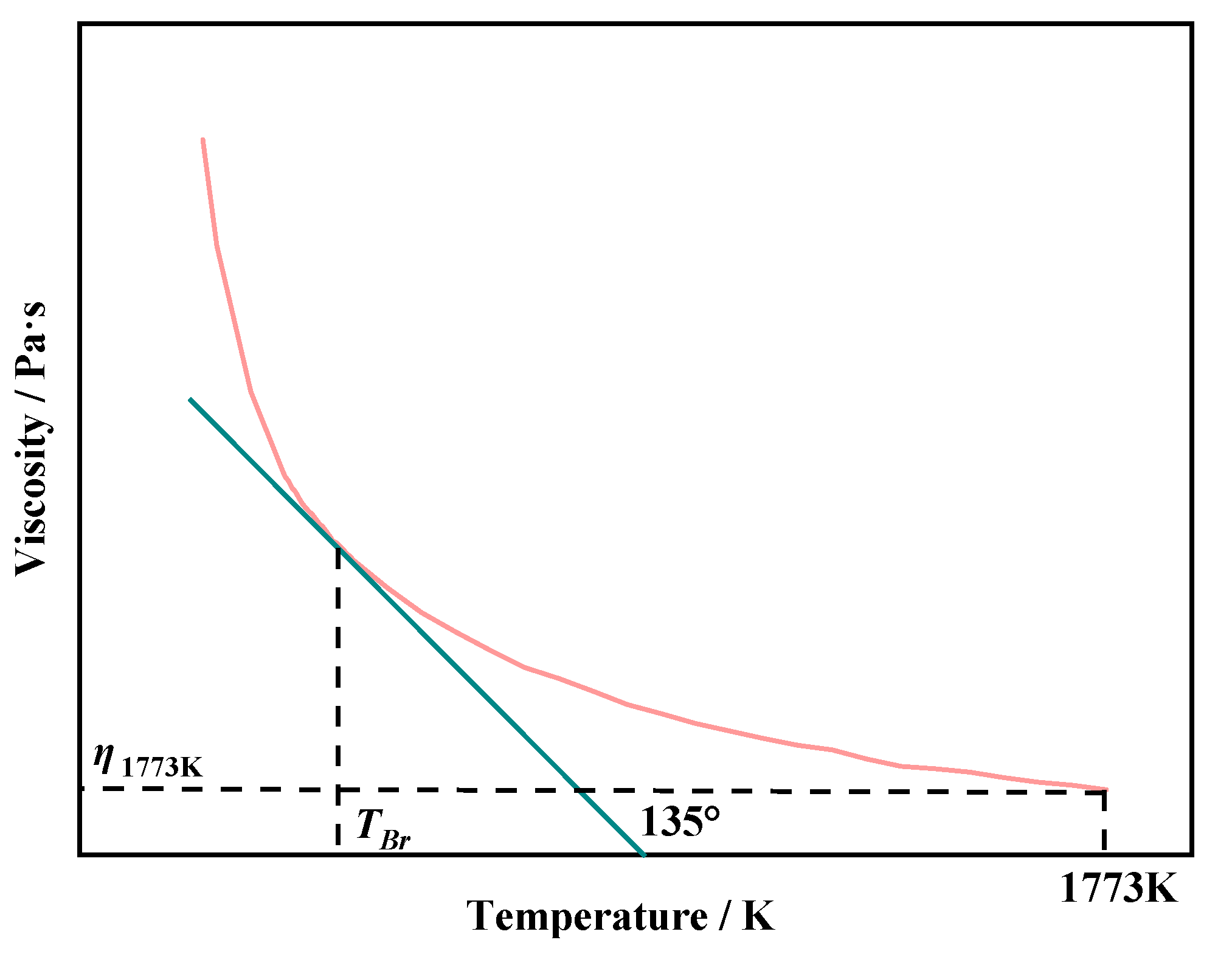
This article defines the viscosity of slag at 1773 K as high-temperature viscosity (
η1773K). Making 135° straight line is tangent to the viscosity-temperature curve(
η-T). The temperature corresponding to the tangent point is defined as the
TBr of the slag, as shown in
Figure 2.
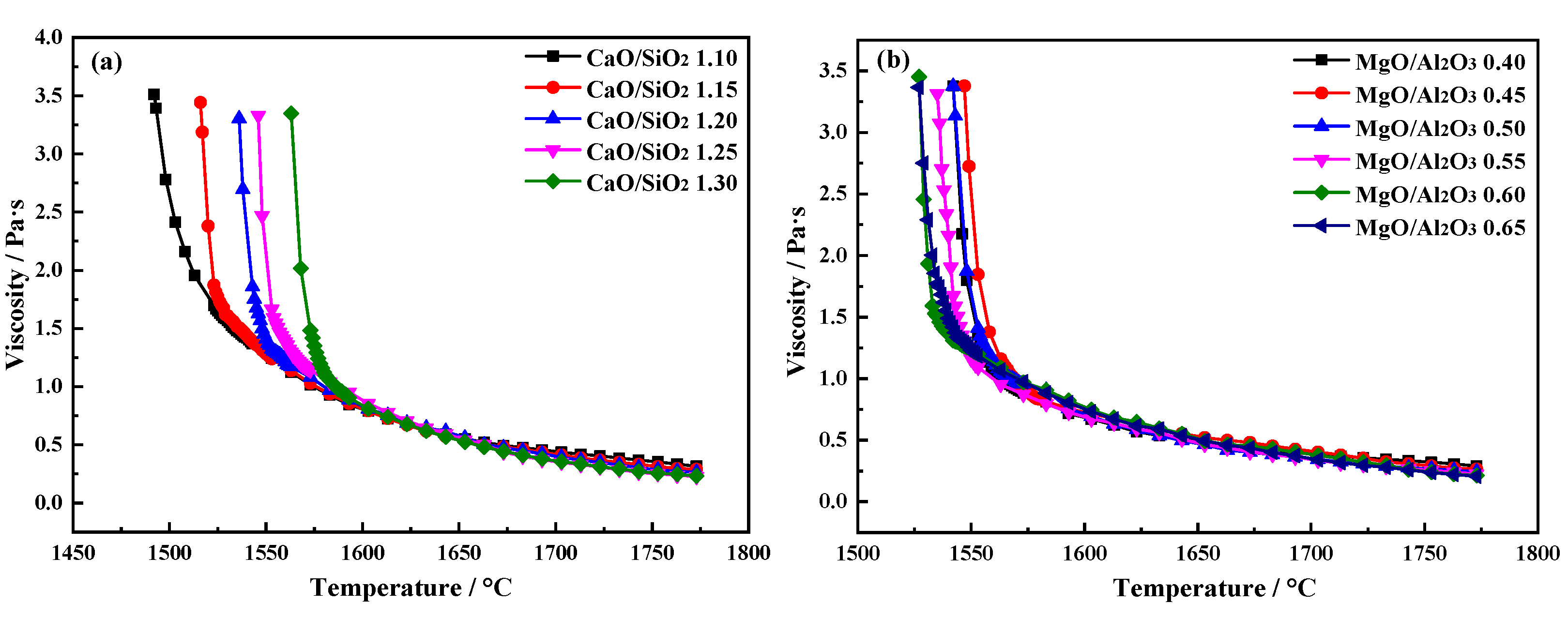
3. Results and Discussion
Different CaO/SiO
2 and MgO/Al
2O
3 slag were obtained through experiments. The
η-T curve is shown in
Figure 3. The viscosity increases with decreasing of temperature and there is a clear turning point on every
η-T curve.
3.1. Effects of CaO/SiO2 and MgO/Al2O3 on the Viscous Behaviors of the Slag
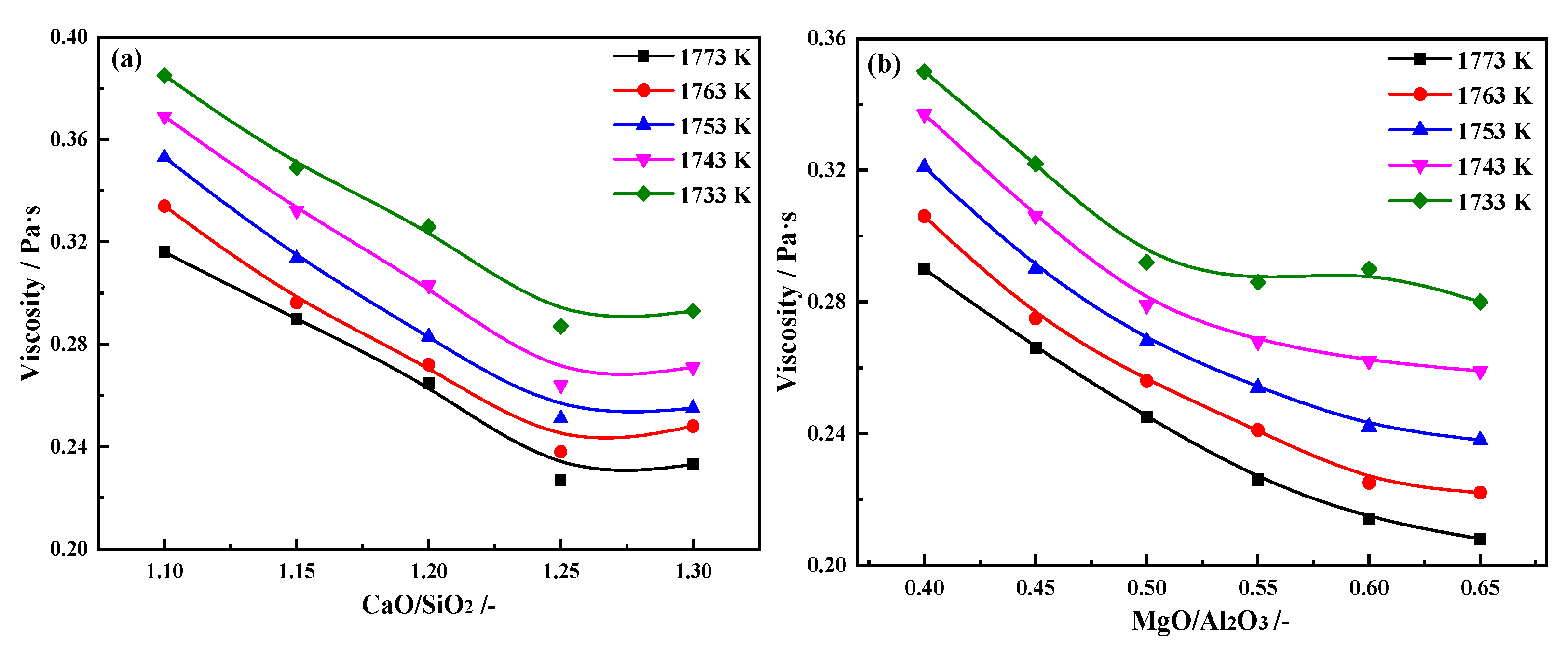
3.1.1. Effects of CaO/SiO2 on the Viscous Behaviors of the Slag
The effects of different CaO/SiO
2 on slag viscosity are shown in
Figure 4(a). When the temperature increases from 1733 to 1773 K, with the increasing of CaO/SiO
2 from 1.10 to 1.30, the
η of the slag decreases significantly first and then slows down. When CaO/SiO
2 is 1.25, the minimum of
η1773K is 0.227 Pa·s.
The viscosity of slag is mainly affected by the internal network structure. The Si
xO
yz- and Al
xO
yz- tetrahedral structures are the main structural units of the slag. The complex network structure inside the slag can be depolymerized to reduce viscosity. As CaO/SiO
2 increases in the slag, the viscosity will decrease. The reason is that the free oxygen ion O
2- dissociated from basic oxide CaO can interact with bridging oxygen O in the network structure of aluminosilicate to form non-bridging oxygen O
- [
9,
10,
11], resulting in the aluminate silicate network structure being depolymerized into smaller network units. By the viscosity module of FactSage prediction, the theoretical viscosity at 1773 K are 0.314, 0.296, 0.281, 0.268, and 0.256 Pa·s respectively. The trend of change is the same as that of the experiment, providing theoretical support for the experimental results.
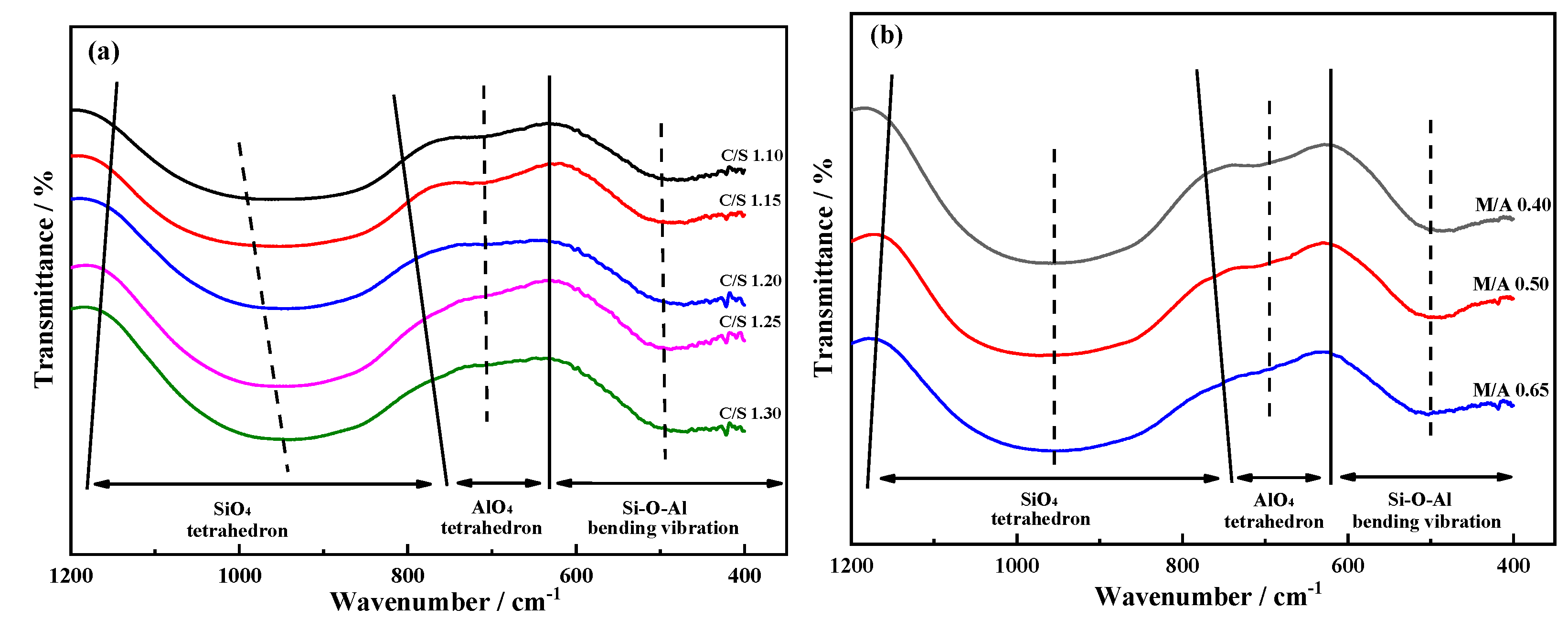
In order to elucidate the relationship between slag viscosity and internal structure, FTIR is employed to analyse the slag of different CaO/SiO
2. The FTIR of silicate aluminate slag is generally divided into three regions, and the wave number range is 400-600 cm
-1, 600-800 cm
-1 and 800-1200 cm
-1, which respectively corresponds to T-O-T (T represents Si or Al) bending vibration, [AlO
4]
5- tetrahedral asymmetric tensile vibration and [SiO
4]
4- tetrahedral symmetric tensile vibration [
12,
13,
14]. The FTIR analysis results of different CaO/SiO
2 are shown in
Figure 5(a). As CaO/SiO
2 increases from 1.10 to 1.30, the transmitted wave valley of the [SiO
4]
4- tetrahedral symmetric stretching vibration band shifts towards lower wave numbers, and the bandwidth widens, indicating the simplification of the aluminosilicate network structure. Gaussian deconvolution was performed on Si-O axisymmetric vibration bands with CaO/SiO
2 ratios of 1.10 and 1.30, and the corresponding areas of each peak are used to characterize the corresponding amount of Q
i (i=0~3). Q
0, Q
1, Q
2, and Q
3 represent the structures of SiO
44-, Si
2O
76-, Si
2O
64-, and Si
2O
52-[
15,
16,
17]. The smaller the value of i, the simpler the slag structure and the higher the degree of polymerization. In brief, when CaO/SiO
2 increases from 1.10 to 1.30, the silicate and aluminate network structure in the slag are depolymerized, resulting in the reduction of the
η in the slag.
3.1.2. Effects of MgO/Al2O3 on the Viscous Behaviors of the Slag
The effect of different MgO/Al
2O
3 on slag viscosity are shown in
Figure 4(b). When the temperature increases from 1733 to 1773 K, with the increasing of CaO/SiO
2 from 0.40 to 0.65, the
η of the slag decreases significantly first and then slows down. When MgO/Al
2O
3 is 0.55, the
η1773K is 0.226 Pa·s.
The effect mechanism of MgO/Al2O3 on slag viscosity is similar to the CaO/SiO2. Both MgO and CaO are basic oxide, and the dissociated O2- ions promote the depolymerization of complex structures. By the viscosity module of FactSage prediction, when MgO/Al2O3 increases from 0.40 to 0.65, the theoretical viscosity at 1773 K are 0.281, 0.272, 0.263, 0.254, 0.247 and 0.239 Pa·s respectively. The trend of change is the same as that of the experiment, providing theoretical support for the experimental results.
In order to elucidate the relationship between slag viscosity and internal structure, FTIR is employed to analyse the slag of different MgO/Al
2O
3. The FTIR analysis results of different MgO/Al
2O
3 slag are shown in
Figure 5(b). When MgO/Al
2O
3 increases from 0.40 to 0.65, the depth of [SiO
4]
4- tetrahedral symmetric tensile vibration becomes shallower and the bandwidth becomes wider, indicating an increase in the distance between Si-O bonds and the disintegration of the slag silicate network structure into smaller network units; The depth of [AlO
4]
5- tetrahedron asymmetric tensile vibration band gradually becomes shallow, and finally almost disappears, indicating the aluminate network structure in the slag is depolymerized; The groove depth of the Si-O-Al bending vibration band is slightly weakened, indicating a decrease in the number of Si-O-Al structures used to connect [AlO
4]
5- and [SiO
4]
4- tetrahedra [
18,
19,
20]. In conclusion, the silicon Aluminate network structure in the slag is depolymerized, resulting in the reduction of the
η in the slag.
3.2. Effects of CaO/SiO2 and MgO/Al2O3 on the Break Point Temperature of Slag
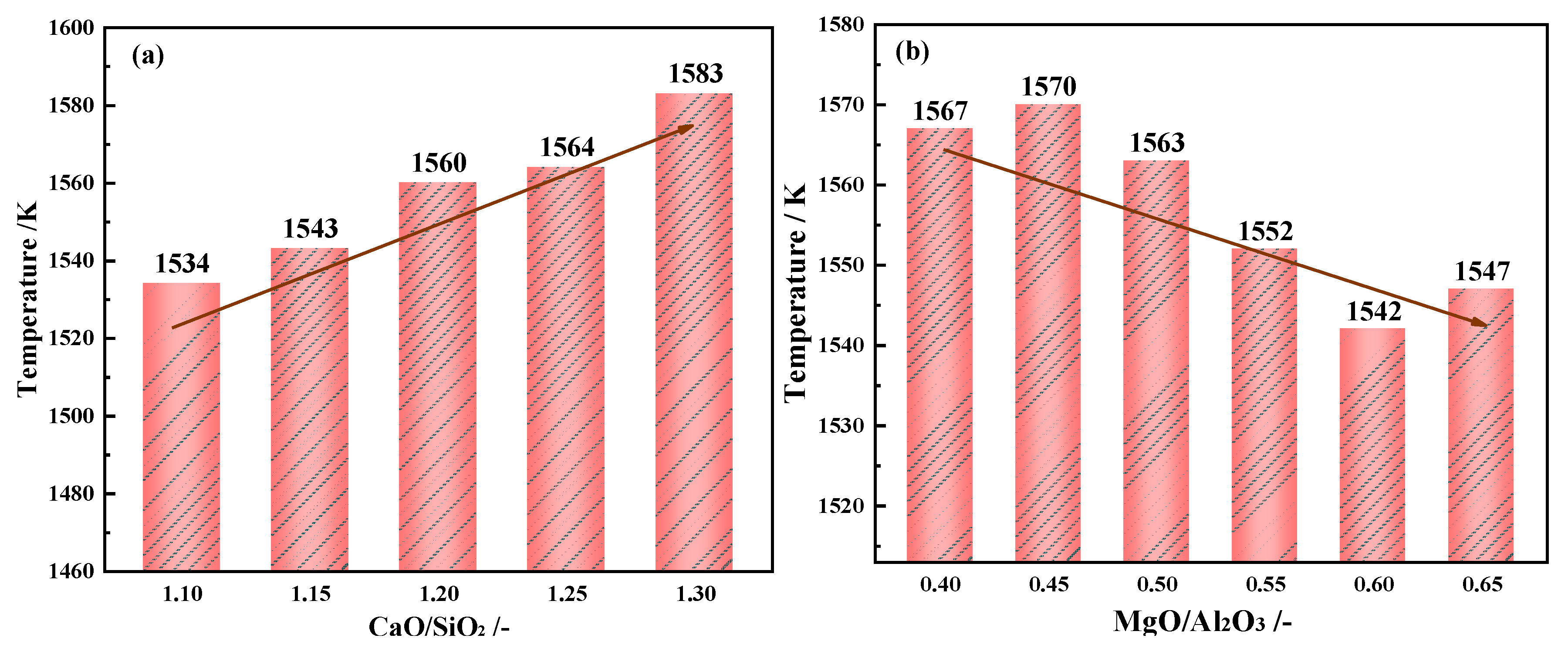
3.2.1. Effects of CaO/SiO2 on the Break Point Temperature of Slag
The effect of different CaO/SiO
2 on the break point temperature is shown in
Figure 8(a). When CaO/SiO
2 increases from 1.10 to 1.30, the
TBr shows an uptrend, increasing from 1534 K to 1583 K.
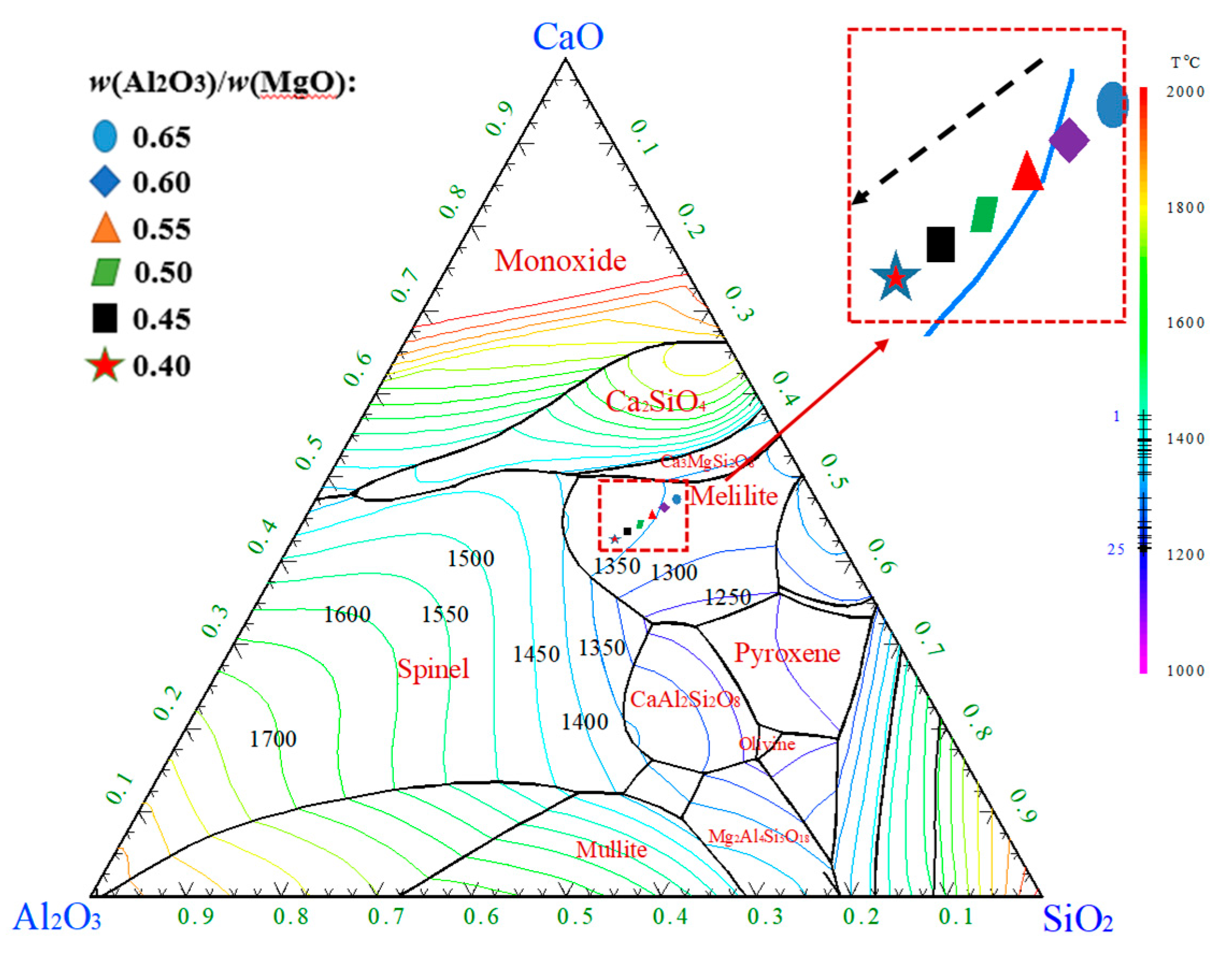
The phase diagram of the five-component slag system CaO-SiO
2-MgO-17.00%Al
2O
3-3.83%B
2O
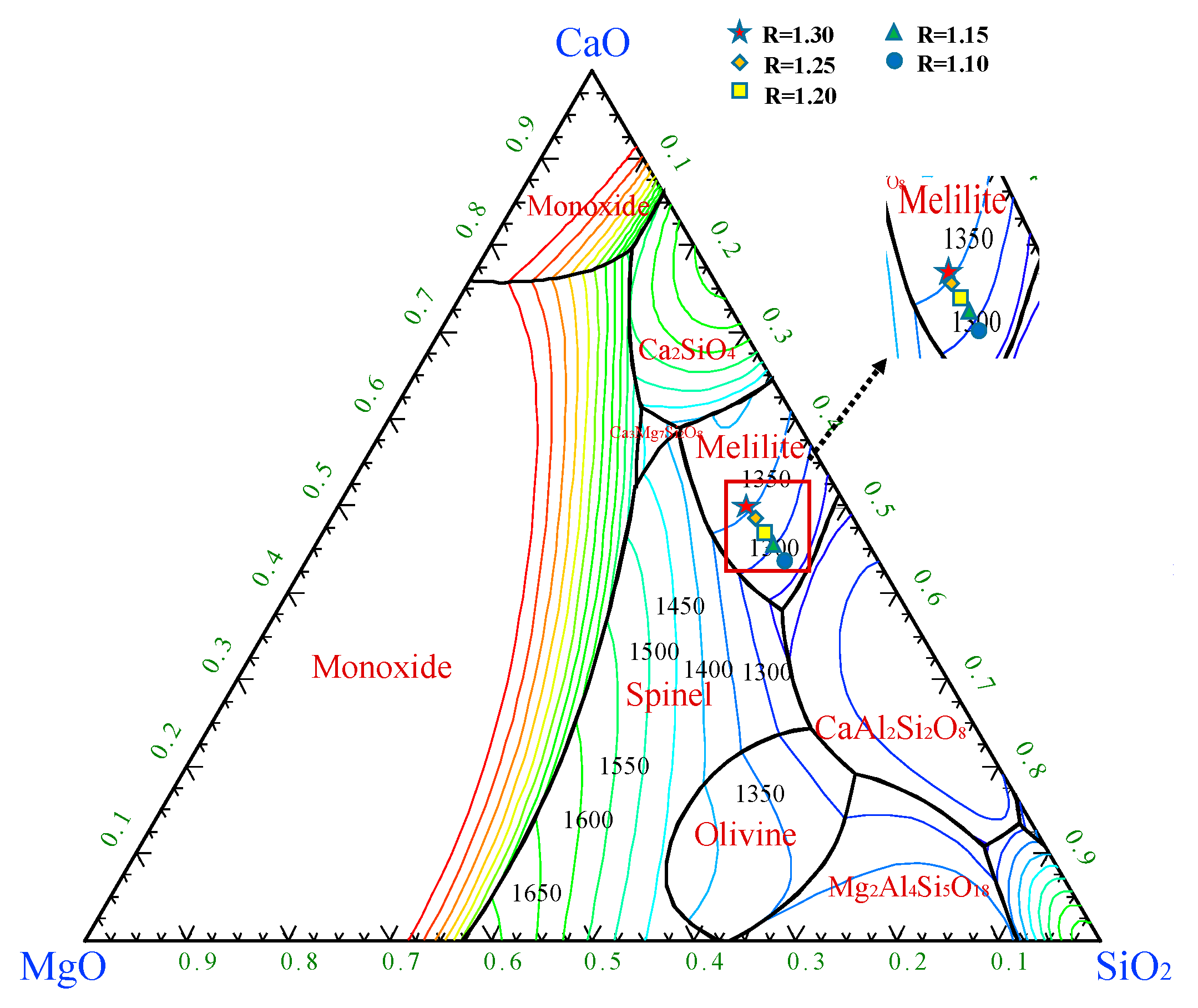
3 as plotted by the phase diagram module in the FactSage is shown in
Figure 9. The different CaO/SiO
2 components are located in the crystalline region of the pyrochlore. With the increasing of CaO/SiO
2, the liquid temperature of the slag increases and the ability to crystallize at high temperatures becomes stronger, leading to an increase in
TBr. The liquid temperatures were 1613.92, 1623.05, 1631.00, 1637.96 and 1643.76 K respectively. The liquidus temperature of slag increased. Thus, the crystallization capacity of slag is enhanced and the
TBr also increases. These results are agreement with the trend of the measurements of
TBr.
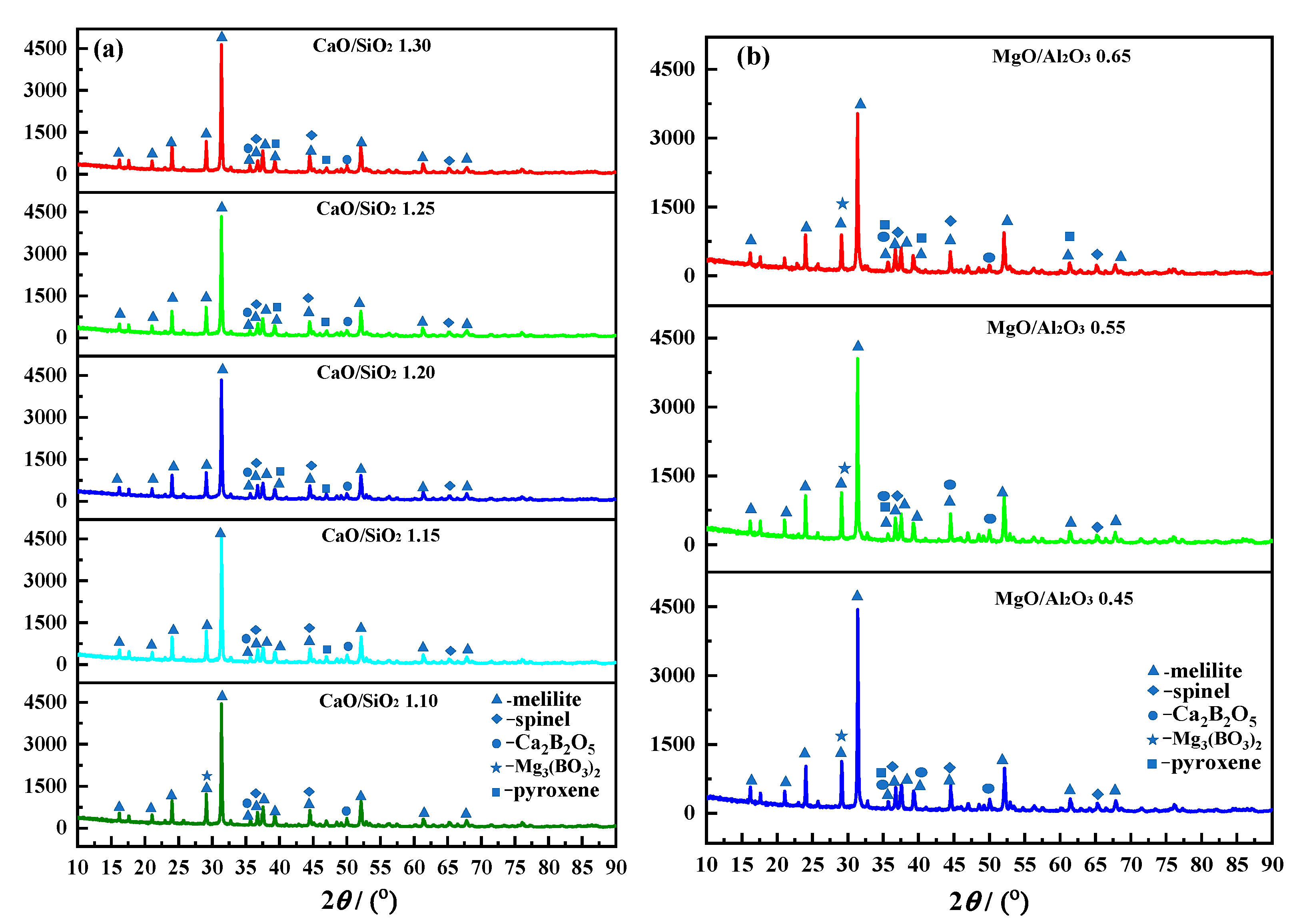
The XRD analysis results of different CaO/SiO
2 slag are shown in
Figure 11(a). The basic phase in different CaO/SiO
2 slag is melilite. When CaO/SiO
2 increases from 1.10 to 1.30, the diffraction peak intensity of melilite, spinel, and Ca
2B
2O
5 phases increases. When CaO/SiO
2 is 1.15, the Mg
3(BO
3)
2 phase disappears and pyroxene phase appears in the slag. The number of high melting point phases in the slag increase relatively and the crystallization ability of the slag increases under high temperature conditions, resulting in an increase in the
TBr and a decrease in fluidity.
3.2.2. Effects of MgO/Al2O3 on the Break Point Temperature of Slag
The effect of different MgO/Al
2O
3 on the break point temperature is shown in
Figure 8(b). With the increasing of MgO/Al
2O
3 from 0.40 to 0.65, the
TBr of the slag shows a downtrend, decreasing from 1570 K to 1542 K.
The phase diagram of CaO-SiO2-7.98%MgO-Al
2O
3-3.83%B
2O
3 slag system is caculated by the Phase Diagram module in FactSage. As shown in the
Figure 10, with the continuous decreasing of MgO/Al
2O
3, the composition of the slags is located in the area of melilite phase, and the liquidus temperature of melilite is relatively sparse, which demonstrates the phase is stable. According to FactSage, the liquidus temperature of slag with different MgO/Al
2O
3 was calculated as 1345.83, 1349.95, 1354.95, 1360.93, 1367.64, 1374.78 °C, and the liquidus temperature of slag increased. Thus, the crystallization capacity of slag is enhanced and the
TBr also increases. These results are agreement with the trend of the measurements of
TBr.
The XRD analysis results of different MgO/Al
2O
3 slag are shown in
Figure 11(b). There are melilite, spinel, pyroxene, Ca
2B
2O
5 and Mg
3(BO
3)
2 in the slag and the melilite is the basic phase. When MgO/Al
2O
3 increases from 0.40 to 0.65, the diffraction peak intensity of melilite, spinel, pyroxene and Mg
3(BO
3)
2 gradually weakens, while the diffraction peak intensity of Ca
2B
2O
5 and pyroxene slightly increases. This indicates that the Ca
2B
2O
5 and pyroxene in the slag are relatively increased, while the number of high melting point phases is relatively reduced, resulting in a decrease in the crystallization ability of the slag, a decrease in the
TBr and an improvement in fluidity under high temperature conditions.
Figure 10.
Phase diagram of five-element slag system CaO-SiO2-7.98%MgO-Al2O3-3.83%B2O3 with different MgO/Al2O3.
Figure 10.
Phase diagram of five-element slag system CaO-SiO2-7.98%MgO-Al2O3-3.83%B2O3 with different MgO/Al2O3.
Figure 11.
XRD analysis of different CaO/SiO2 and MgO/Al2O3 experimental slags. (a) - CaO/SiO2; (b) - MgO/Al2O3.
Figure 11.
XRD analysis of different CaO/SiO2 and MgO/Al2O3 experimental slags. (a) - CaO/SiO2; (b) - MgO/Al2O3.
3.3. Effects of CaO/SiO2 and MgO/Al2O3 on Activation Energy of Slag Viscous Flow
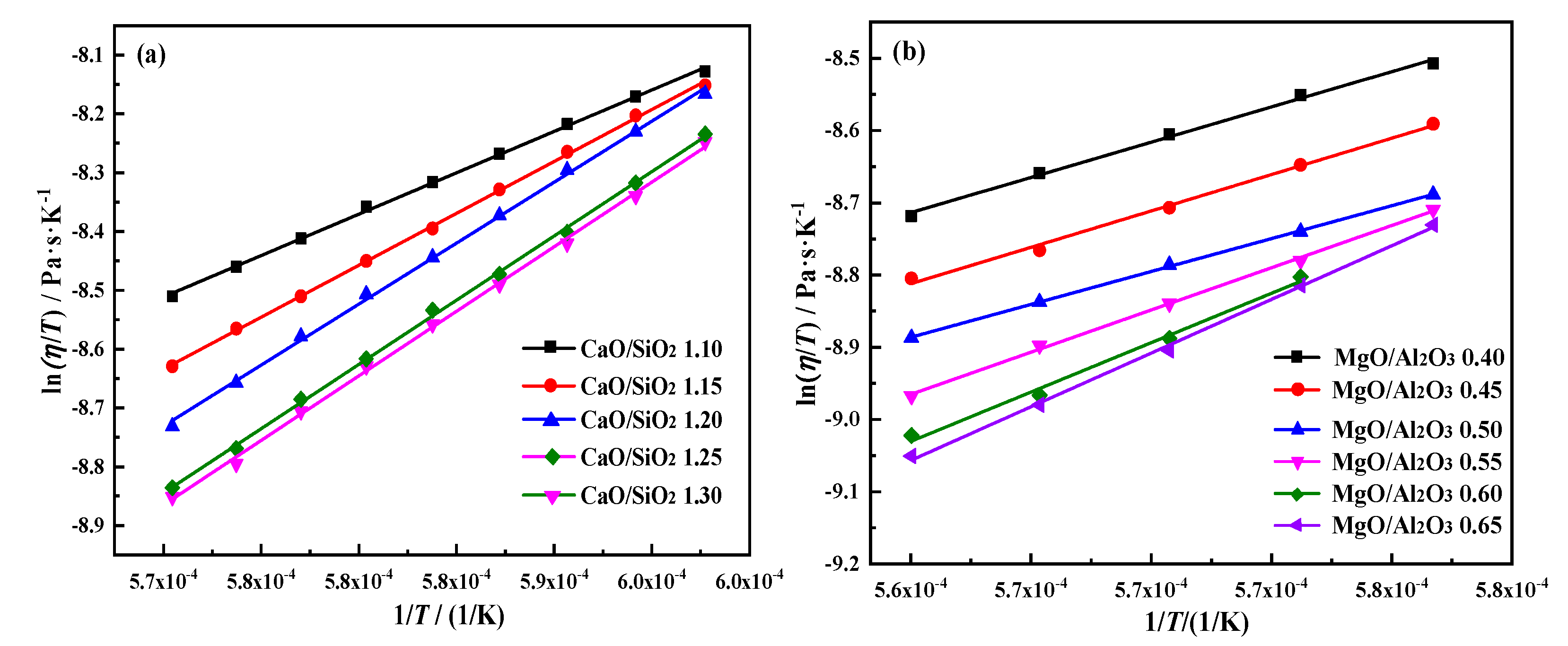
Viscous flow activation energy is a crucial viscosity characteristic of slag. The
Eη reflects the sensitivity of slag viscosity to temperature, representing the thermostability of slag [
21,
22,
23]. The calculation of viscous flow activation energy in this article adopts the modified Weymann-Frenkel equation by Urban, as shown in formula (1). Formula (2) can be obtained by taking the logarithm of both sides formula (1). The viscosity data measured in the experiment are calculated using linear regression method, and the slope is
Eη. Linear fitting results and the trend of
Eη with different CaO/SiO
2 and MgO/Al
2O
3 are shown in
Figure 12 and
Figure 13. The results indicate that there is a fine linear relationship between ln(
η/
T) and 1/
T. The linear correlation coefficients are all greater than 0.99. [
24,
25]
where, η is the viscosity, Pa·s;
A is the proportionality constant;
T is the temperature, K;
R is the gas constant, 8.314 J·(mol·K)
-1.
3.3.1. Effects of CaO/SiO2 on Activation Energy of Slag Viscous Flow
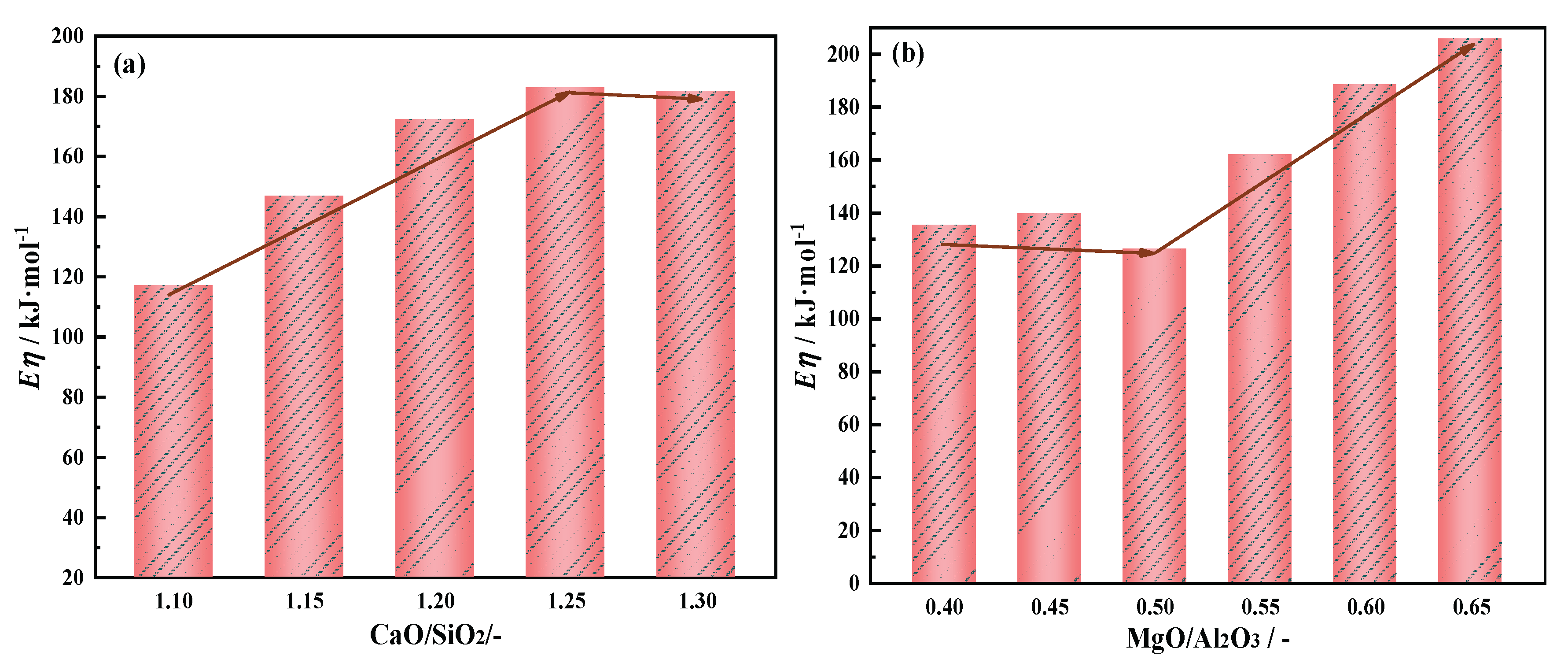
As shown in
Figure 13(a), when CaO/SiO
2 increases from 1.10 to 1.30,the
Eη increase from 117.01 to 182.86 kJ·mol
-1. This indicates that the sensitivity of slag viscosity to temperature is weakened. On the premise of ensuring better slag stability, the CaO/SiO
2 value of 1.25 is reasonable. From the perspective of slag structure, the complex slag structure is decomposed into simpler structure, the activation energy of slag is increased, and the stability is improved[
26,
27,
28]. The stability of the slag can also be characterized by the density of the isotherm in the phase diagram. The thinner the contour lines temperature and related subjects, the less the temperature affects the slag composition and the better the slag stability.
3.3.2. Effects of MgO/Al2O3 on Activation Energy of Slag Viscous Flow
As shown in
Figure 13(b), when MgO/Al
2O
3 increases from 0.40 to 0.50, the E
η chang inconspicuously. The E
η increase from126.20 to 205.86 kJ·mol
-1. When MgO/Al
2O
3 increases from 0.50 to 0.65, th the E
η significant increase. This indicates that the thermostability of slag to temperature is enhanced. The complex slag structure is decomposed into simpler structure, the activation energy of slag is increased.
As shown in
Figure 9 and
Figure 10, within the experimental value range, the isotherm becomes sparse with the increase of CaO/SiO
2 and MgO/Al
2O
3, indicating a better stability of the slag,which is consistent with the experimental fitting results [
29,
30].
Briefly, when CaO/SiO2 is 1.25, η1773K has a minimum of 0.227 Pa·s, a lower TBr is 1570 K, Eη is stable at a lower level, the slag has a good thermal stability performance. When MgO/Al2O3 is 0.55, the decreasing trend of η1773K begins to slow down to 0.226 Pa·s, TBr and Eη are 1570 K and 161.99 KJ·mol-1 respectively. Overall, when CaO/SiO2 is 1.25 and MgO/Al2O3 is 0.55, a good metallurgical properties of low boron-bearing high alumina slag system can be obtained, providing a good reference basis for blast furnace operation.
4. Conclusions
- (1)
With CaO/SiO2 increasing from 1.10 to 1.30, viscosity first decrease significantly and then slowed down. When CaO/SiO2 is 1.25, η1773K is 0.227 Pa·s. TBr shows an increasing trend, increasing from 1534 K to 1583 K. Eη increase from 117.01 to 182.86 kJ·mol-1 and the thermal stability of the slag deteriorates first and then improves. At this point, the slag system has a better performance.
- (2)
With MgO/Al2O3 increasing from 0.40 to 0.65, viscosity first decrease significantly and then slowed down. When MgO/Al2O3 is 0.55, η1773K is 0.226 Pa·s. TBr decrease from 1570 K to 1542 K. The Eη increase from 126.20 to 205.86 kJ·mol-1 and the thermal stability of the slag first improves and then deteriorates. At this point, the slag system has a better performance.
- (3)
Comprehensive considerations, when CaO/SiO2 is 1.25 and MgO/Al2O3 is 0.55, the η1773K, TBr and Eη are at a reasonable value. The low boron-bearing high alumina slag systems has the best metallurgical performance at this value.
Author Contributions
Ye Sun: Investigation, Writing-original draft. : Writing-review and editing.: Data curation. : Photo editing. : Methodology, Validation.
Acknowledgments
The authors are especially grateful to the Technological Project in Liaoning (2022JH2/101300124) and Basic Research Project of Education Department of Liaoning Province (2024JYTKYTD-16).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Zhang XY, Wang SZ, Zhao J et al. (2023) Influence of B2O3 on the thermophysical properties of molten blast furnace slag. J Non-Cryst Solids 615: 122429. [CrossRef]
- Bi Z, Li K, Jiang C et al. (2021) Effects of B2O3 on the structure and properties of blast furnace slag by molecular dynamics simulation. J Non-Cryst Solids 551: 120412. [CrossRef]
- Talapaneni T, Chaturvedi V (2022) Proposing a suitable slag composition by estimating the fusion behavior, viscosity and desulphurization ability for blast furnaces running with high alumina. Materials Today: Proc 67: 558-565. [CrossRef]
- Li, T. , Zhao, C., Sun, C. et al. (2020) Roles of MgO and Al2O3 in viscous and structural behavior of blast furnace primary slag with C/S = 1.4. Metall Mater Trans B 51: 2724-2734. [CrossRef]
- Liang HL, Chu MS, Feng C et al. (2018) Optimisation study and affecting mechanism of CaO/SiO2 and MgO on viscous behaviours of titanium-bearing blast furnace slag. Ironmak Steelmak 47: 106-117. [CrossRef]
- Gao, Ym. , Wang, Sb., Hong, C. et al. (2014) Effects of basicity and MgO content on the viscosity of the SiO2-CaO-MgO-9wt%Al2O3 slag system. Int J Miner Metall Mater 21: 353-362. [CrossRef]
- Huang, XH. , Liao JL., Zheng K.et al. (2014) Effect of B2O3 addition on viscosity of mould slag containing low silica content. Ironmak Steelmak 41:67-74. [CrossRef]
- Feng C, Chu MS, Tang J et al. (2016) Effects of MgO and TiO2 on the viscous behaviors and phase compositions of titanium-bearing slag. Int J Min Met Mater 23: 868-880. [CrossRef]
- Sukenaga S, Higo T, Shibata H et al. (2015) Effect of CaO/SiO2 ratio on surface tension of CaO-SiO2-Al2O3-MgO Melts. ISIJ Int 55: 1299-1304. [CrossRef]
- Saito N, Hori N, Nakashima K et al. (2003) Viscosity of blast furnace type slags. Metall Mater Trans B 34: 509-516. [CrossRef]
- Jiang C, Li K, Zhang J et al. (2018) Molecular dynamics simulation on the effect of MgO/Al2O3 ratio on structure and properties of blast furnace slag under different basicity conditions. Metall Mater Trans B 50: 367-375. [CrossRef]
- Qiu GX, Miao DJ, Wei XL et al. (2022) Effect of MgO/Al2O3 and CaO/SiO2 on the metallurgical properties of CaO-SiO2-Al2O3-MgO-TiO2 slag. J Non-Cryst Solids 585: 121545. [CrossRef]
- Yan Z, Lv X, Zhang J et al. (2016) Influence of MgO, Al2O3 and CaO/SiO2 on the viscosity of blast furnace type slag with high Al2O3 and 5%TiO2. Can Metall Quart 55: 186-194. [CrossRef]
- Wang C, Zhang J, Jiao K et al. (2017) Influence of basicity and MgO/Al2O3 ratio on the viscosity of blast furnace slags containing chloride. Metall Res Technol 114: 395. [CrossRef]
- Das K, Agrawal A, Reddy AS et al. (2021) FactSage studies to identify the optimum slag regime for blast furnace operation. T Indian I Metals 74: 419-428. [CrossRef]
- Jiang C, Li K, Zhang J et al. (2018) The effect of CaO(MgO) on the structure and properties of aluminosilicate system by molecular dynamics simulation. J Mol Liq 268: 762-769. [CrossRef]
- Jin HB, Yang SY, Liu H et al. (2023) Effect of CaO partial substituted by BaO on structure of aluminotitanate melts by molecular dynamics simulation. J Non-Cryst Solids 605: 122160. [CrossRef]
- Liu WG, Zuo HB (2021) Effect of MnO and CaO substitution for BaO on the viscosity and structure of CaO-SiO2-MgO-Al2O3-BaO-MnO slag. J Non-Cryst Solids 567: 120940. [CrossRef]
- Bi ZS, Li KJ, Jiang CH, Zhang JL, Ma SF (2021) Effects of amphoteric oxide (Al2O3 and B2O3) on the structure and properties of SiO2-CaO melts by molecular dynamics simulation. J Non-Cryst Solids 559: 120687. [CrossRef]
- Lei J, Yang W, Sheng GY et al. (2022) Effects of BaO Content and CaO/Al2O3 ratio on the properties and structure of aluminate slag. Metall Mater Trans B 53: 2239-2247. [CrossRef]
- Zhang C, Kong Y, Wu T, Bao GD et al. (2022) Effect of B2O3 on the structure and properties of aluminate slag. Metall Res Technol 119: 507. [CrossRef]
- Bo W, Jian X, Zhang HL, Sun S, Feng Z (2014) Influence of MgO on calcium aluminate slag system with lower calcium. Adv Mater Res 3226: 941-944. [CrossRef]
- Qin JH, Liu WG, Wu HJ et al. (2022) A comprehensive investigation on the viscosity and structure of CaO-SiO2-Al2O3-MgO-BaO slag with different Al2O3/SiO2 ratios. J Mol Liq 365: 120060. [CrossRef]
- Wang W, Dai S, Zhou L et al. (2020) Viscosity and structure of MgO-SiO2-based slag melt with varying B2O3 content. Ceram Int 46: 3631-3636. [CrossRef]
- Chen ZW, Meng Z, Liu LL et al. (2021) Structural and viscous insight into impact of MoO3 on molten slags. Metall Mater Trans B 52: 1-14. [CrossRef]
- Liu Hao, Qin YL, Yang YH et al. (2018) Influence of Al2O3 content on the melting and fluidity of blast furnace type slag with low TiO2 content. Journal of Chemistry 2018: 1-6. [CrossRef]
- Zhang W, He F, Xiao Y et al. (2020) Structure, crystallization mechanism, and properties of glass ceramics from molten blast furnace slag with different B2O3/Al2O3. Mater Chem Phys 243: 122664. [CrossRef]
- Jin, Z. , Wang, B., Liu, Z. et al. (2022) Effects of Fe/SiO2 ratio and MgO content on the viscous behaviors of the SiO2-FeO-MgO-12 wt pct Fe2O3-8wt pct CaO-3 wt pct Al2O3 slag system. Metall Mater Trans B 53: 902-915. [CrossRef]
- Kong WG, Liu JH, Yu YW et al. (2021) Effect of w(MgO)/w(Al2O3) ratio and basicity on microstructure and metallurgical properties of blast furnace slag. J Iron Steel Res Int 28: 1223-1232. [CrossRef]
- Yang D, Zhou H, Wang J et al. (2021) Influence of TiO2 on viscosity, phase composition and structure of chromium-containing high-titanium blast furnace slag. J Mater Res Technol 12: 1615-1622. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).