1. Introduction
According to the latest data from the Joint United Nations Programme on AIDS (UNAIDS), there were 1.3 million new HIV infection in 2024 [
1]. Most of these infections happened in sub-Saharan Africa and with women and girls accounting for 44% [
2]. Cameroon’s HIV epidemic is generalised, meaning it is not primarily restricted to high-risk population groups. Studies of HIV prevalence in the country have been limited and have typically focused either on specific high-risk population groups or residents of major cities. For example, in the early 1990s, HIV infection rates in Yaoundé were 1.2%, 2.2%, 2.6% and 7.6% among blood donors, pregnant women, patients with sexually transmitted diseases (STD) and patients with tuberculosis (TB), respectively [
3,
4]. The prevalence rapidly increased thereafter, reaching 21.6% in TB patients and 34% among female sex workers (FSW) by 1996 [
5,
6].
In 2000, we assessed HIV prevalence in certain rural communities across four administrative regions of the equatorial rain forest of Cameroon [
7]. Fifty-three villages were visited and an overall prevalence of 5.8% was found [
7]. These regions included localities where four cross-species transfer of simian immunodeficiency virus (SIV) from non-human primates to humans occurred [
8,
9,
10]. One of these transfers gave rise to HIV-1 group M (HIV-1M), which is today responsible of the global HIV pandemic. It is plausible that after sustaining human-to-human transmission, the HIV-1 M progenitor spread throughout Cameroon’s equatorial rainforest region and sparked localised epidemics [
11]. This suggests that these regions host some of the oldest and most mature HIV-1 M epidemics.
In 2016, Cameroon began implementing the “Universal Test and Treat” (UTT) [
12] policy to advance towards the UNAIDS target of 95-95-95 by 2025, whereby by 2025, we should have diagnosed, treated and suppressed the viral loads in 95% of people living with HIV. As a result, the number of people living with HIV (LWH) on antiretroviral treatment (ART) increased from 168,431 (27.1% ART coverage) in 2015 to 350,818 (70.6% ART coverage) in 2020 [
13]. The most recent Demographic and Health Survey (DHS) conducted in 2018 indicated an overall HIV prevalence of 2.8% [
14], decreased from 5.3% in 2014 [
15].
Bekolo and collaborators (2023) reviewed the three DHS organised in the country in 2018, 2014 and 2011 and found that there were some persistent disparities in prevalence by age, regions and gender among HIV-infected individuals. For instance, women showed higher infection rates than men (3.5%, versus 1.9%) and adults aged 35-39 years being the most affected. Regional differences ranged from 5.8% in the South to 1% in the Far North region [
16]. In addition, their analyses also indicated that the decline in HIV prevalence in Cameroon over the study period was associated with a reduction in sexual behaviors. Notably, the proportion of men and women receiving an HIV test and result increased by more than 30% [
16]. Furthermore, among the 20 locations that were identified as HIV hot spot clusters during the 2018 survey, 13 were rural or semi-rural localities [
17]. Therefore, understanding the socio-cultural behind higher rural HIV infection is critical for the development of targeted interventions.
2. Materials and Methods
2.1. Villages and Study Population
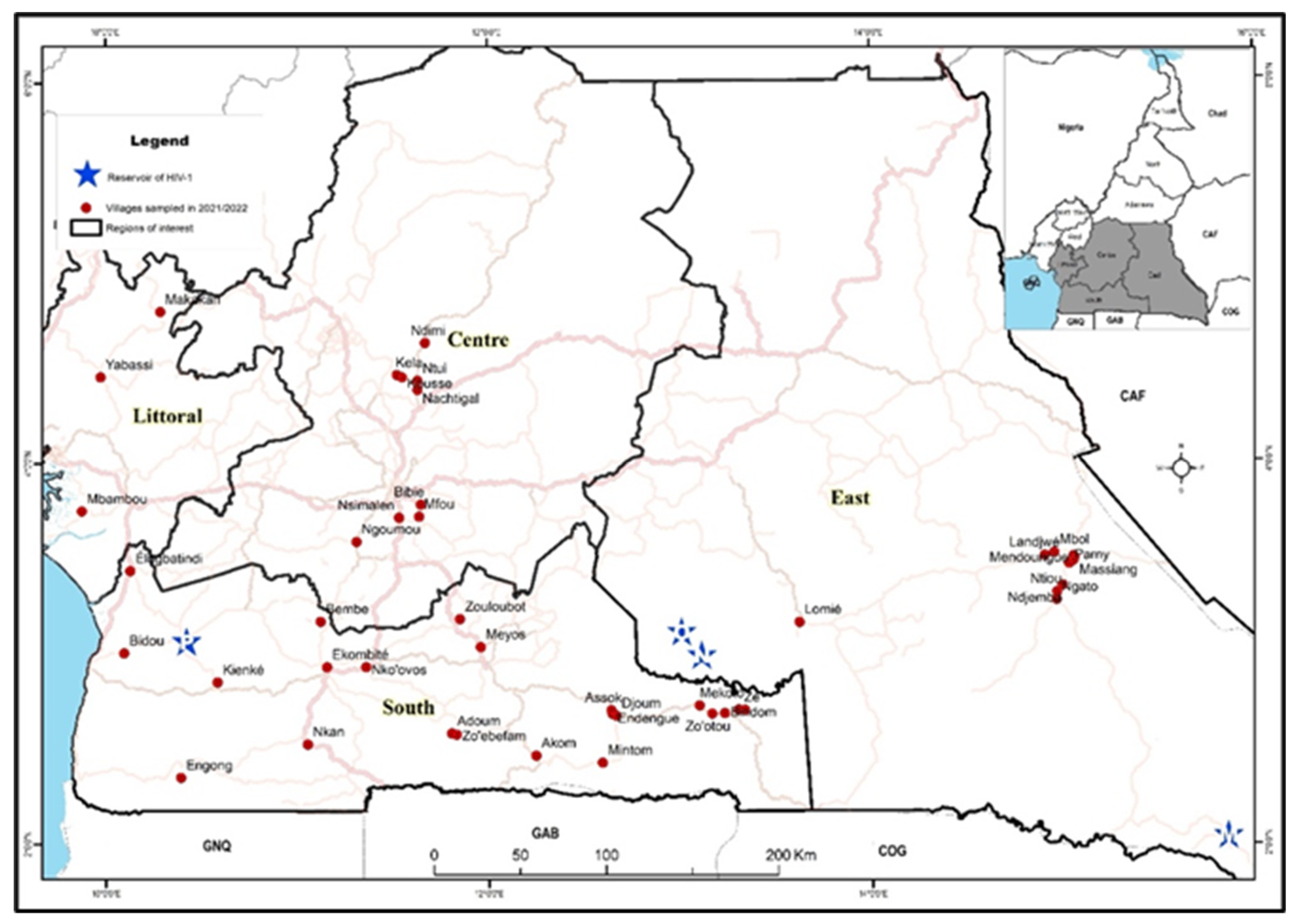
We conducted a health campaign in 2021 and 2022 in remote communities of four administrative regions within Cameroon’s equatorial rainforest (
Figure 1). The campaign addressed general health issues (STD, hypertension, malaria, food safety) and included voluntary HIV testing. Inclusion criteria comprised individuals aged 15 years or older, without obvious comorbidities, who wished to know their HIV status; while the exclusion criteria involved any individuals with obvious comorbidities, who were unwilling to know their HIV status and/or who were under the age of 15 years. The campaign started with door-to-door awareness by local health workers, followed by a mobile clinic in a public village space over two days. Demographic and behavioural information was collected via face-to-face interviews using a structured questionnaire. Pre- and post-test counselling was performed per Cameroonian national guidelines [
12]. Blood samples were collected after pre-counselling for HIV testing. The survey was conducted in conjunction with local public health personnel who also ensured that individuals diagnosed with HIV were referred to the nearest healthcare centre for access to ART according to national guidelines and procedures [
12]. Five ml of blood was drawn from all participants for HIV testing.
2.2. HIV Testing
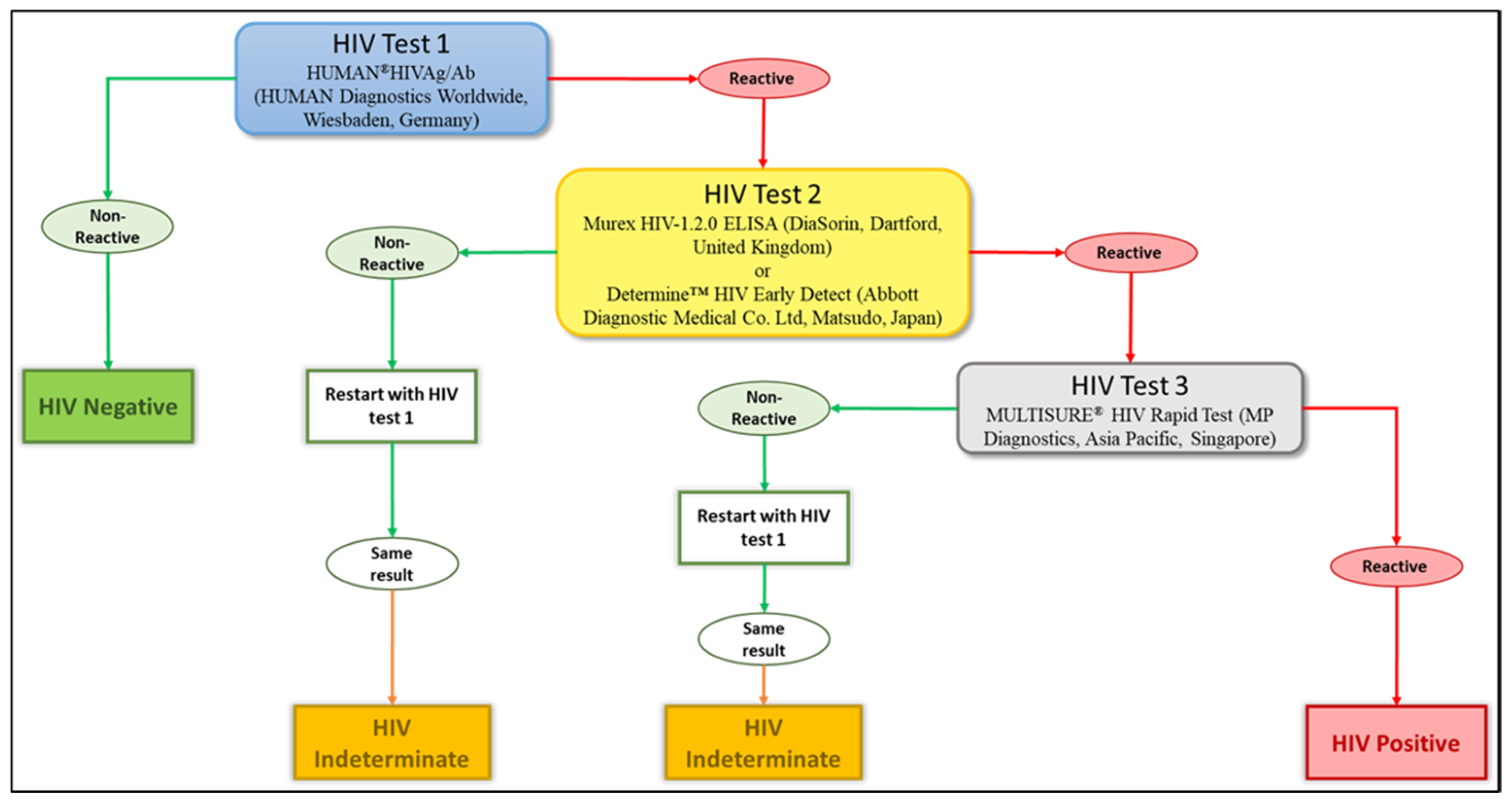
Blood samples were transported to a specialized HIV-testing laboratory, where they were tested for HIV antibodies. Four different tests were used to diagnose the HIV infection: two ELISA tests, the HUMAN
®HIVAg/Ab (HUMAN Diagnostics Worldwide, Wiesbaden, Germany) and the Murex HIV-1.2.0 ELISA (DiaSorin, Dartford, United Kingdom), along with two rapid tests, the Determine™ HIV Early Detect (Abbott Diagnostic Medical Co. Ltd., Matsudo, Japan) and the MULTISURE
® HIV Rapid Test (MP Diagnostics, Asia Pacific, Singapore). We adopted the WHO three tests strategy as the reported national HIV prevalence in the country is below 5% [
18]. The testing algorithm is summarised in
Figure 2. The HUMAN
®HIVAg/Ab was used to pre-screen samples, with non-reactive results considered HIV-negative. Reactive samples (potentially HIV-positive) were then retested using either the Murex HIV-1.2.0 ELISA or the Determine™ HIV Early Detect. Plasma samples reactive to these tests were finally tested using the MULTISURE
® HIV Rapid Test and reactive samples were classified as HIV-positive. On the other hand, discordant samples (reactive to one of these three tests) were repeated and if the same results were obtained, there were categorised as “indeterminate” and were excluded from further analyses (
Figure 2). Participants with such a result were requested to perform another test in the nearest health centre after one month while abstaining from any risky behaviour.
2.3. Statistical Analyses
Data were analysed using Stata version 19 [
19]. Frequencies and percentages (%) were used to describe qualitative variables. Pearson’s Chi-square test was used to establish relationships between qualitative variables. To quantify the associations, Poisson regression models were used, however, due to overdispersion, Negative Binomial regression models were preferred because they incorporated an additional term to account for the excess variance, as recommended by Barros and Hirakata [
20]. These allowed to obtain unadjusted prevalence ratio (PR) for univariable (bivariate) analysis and adjusted prevalence ratio (aPR) for multivariate analysis with 95% confidence interval (CI). Variables used for adjustment were those with p-values less than 0.05 at univariate analysis, these included age group, sex, region, marital status, and education. Finally, variables with p-values less than 0.05 were considered statistically significant.
2.4. Ethics Considerations
The study was approved by the Cameroon Ministry of Public Health through its National Ethics Committee (N° 2022/12/1510/CE/CNERSH/SP). Written informed consent was obtained from all participants prior to participation in line with the Declaration of Helsinki. HIV-positive individuals received counselling and referral to the nearest ART center.
3. Results
3.1. Description of Serosurveys
During January 2021 and September 2022, we visited 46 villages and enrolled 5,631 participants from four administrative regions of the equatorial rainforest areas of Cameroon: Center, East, Littoral and South (
Figure 1). A total of 55 participants were excluded from the study due to indeterminate HIV results (defined in the methods section) leaving a number of 5,576 study participants. In the Center region, 9 villages were visited and 829 individuals enrolled (15% of the total participants); in Littoral, 3 villages and 859 individuals (15%); in South, 23 villages and 1,905 individuals (34%); and in East, 11 villages and 1,983 individuals (36%).
3.2. Characteristics of Study Participants
Among the participants who responded to the questionnaire, the majority (60.6%) of them were male. Participant ‘ages varied from 15 to 101 years with median age 31 (IQR: 22-42) years. The age group 15–19 years was the most represented, accounting for 18.7%. The majority (90%) of the study participants had attended at least a primary school and 58% of them were in a living relationship (officially married or not).
3.3. HIV Prevalence in Remote Communities of Cameroon
The overall HIV prevalence among our study participants was 3.6% with notable variation among the four administrative regions (p<0.001). Compared to the Center region which has the lowest prevalence at 2.3%, the East has a similar prevalence at 2.8% (p=0.09). However, the Littoral (3.3%) and the South (5.2%) had a significant higher prevalence (p=0.01) and (p<0.001), respectively (
Table 1). Among the visited villages, the prevalence ranged from 0.5% in Ngoumou in the Center region to 14.0% in Mekoto in the South region.
3.4. Bivariate Analyses
Table 1 also detailed the prevalence according to gender, age and aged groups, marital status and level of education. As shown in this table, women were 1.5 times more infected than men [PR (95%CI): 1.48(1.30-1.69)], and this difference was statistically significant (p<0.001).
Marital status was categorized as single, married, divorced/separated or living together. HIV prevalence was similar among single individuals and those in stable unions (both married or living together), but significantly higher in individuals who were divorced or separated (p<0.001). Individuals with primary education were 1.8 times more likely to be infected than those with no education [PR (95%CI): 1.70 (1.08-2.69)]. However, the prevalence among those with secondary or higher education was similar to those with no education (3.6% vs 3.1%).
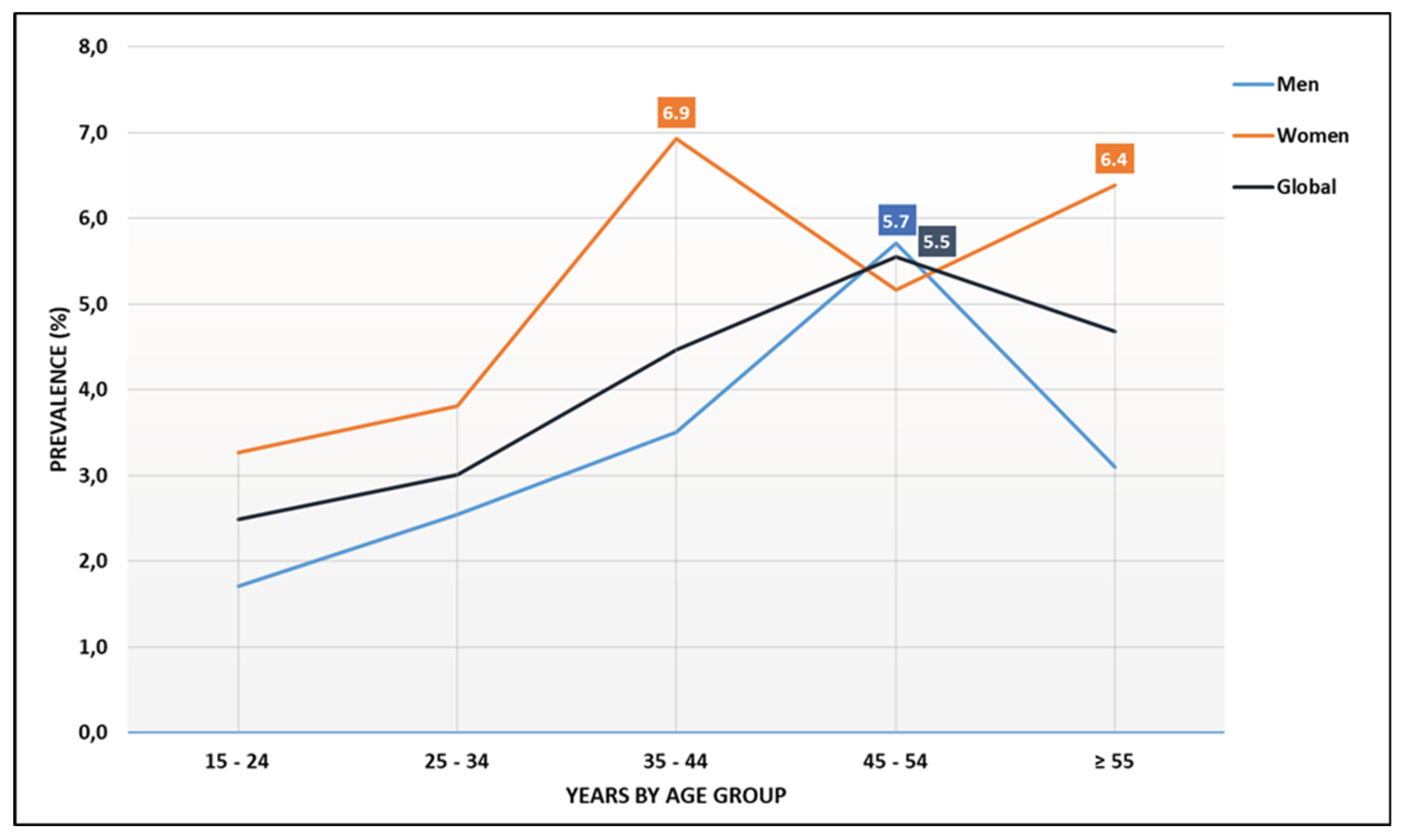
Analysis of age groups showed the lowest prevalence in the 15-19 years group (1.8%). Apart from the 30-34 years group, all older cohorts had a significantly higher HIV prevalence compared to this baseline. In addition, we observed a striking contrast between genders. Peak prevalence was observed among women aged 35-44 years (over 6%) and 55 years and older (over 5%), while among men, the most affected group were those aged 45-54 years (over 5%) (
Table 1 and
Figure 3). Furthermore, when participants were grouped into standard epidemiological strata of 15-49 years (generally used in studies or surveys to determine the prevalence in Cameroon) vs ≥50 years, prevalence was significantly higher in the later (4.9%), vs 3.4% in the former (p=<0.001;
Table 1), emphasizing the importance of including older adults in surveillance studies.
3.5. Multivariate Analyses
Table 2 presents results from the multivariate analysis. After adjustment, the following factors remained significantly associated with HIV seropositivity. The multivariate analysis revealed a higher HIV prevalence ratio among individuals aged 50-54 years compared to those aged 15-19 years (aPR=2.47, 95% CI: [1.63, 3.74], p<0.001). Women had higher odds (aPR=1.48, 95% CI: [1.26, 1.74], p<0.001) than male. Compared to the Center region, risk was significantly higher in East (aPR=1.63, 95% CI: [1.19, 2.23], p<0.001), Littoral (aPR=1.99, 95% CI: [1.41, 2.81], p<0.001), and South (aPR=2.27, 95% CI: [1.65, 3.13], p<0.001). Divorced/separated/widowed individuals (10.2%) had increased risk (aPR=2.16, 95% CI: [1.48, 3.16], p<0.001) compared to single individuals (3.2%). It is also noteworthy that both primary (aPR=2.00, 95% CI: [1.26, 3.14], p=0.003) and secondary-or-higher education (aPR=2.15, 95% CI: [1.36, 3.85], p<0.001) were associated with increased risk compared to no education.
4. Discussion
The present study shows differences in HIV infection and associated risk factors in remote communities of four administrative regions of the equatorial rainforest of Cameroon: a country where HIV-1 group M (HIV-1M) has likely been circulating since the start of the global HIV-1 pandemic and with one of the highest HIV-1 M genetic diversity [
21]. We observed significant regional differences with the lowest prevalence found in the Center region and the highest in the South. Furthermore, women were significantly more likely to be infected than men, and HIV prevalence was highest among individuals with only primary education. Additionally, our data show that adults (aged 35 years and above) have markedly higher HIV prevalence than adolescents and young adults. Notably, HIV prevalence in the population aged 50 years and older was 4.9%, significantly higher than the 3.4% reported among individuals aged 15 to 49 years, a group typically used as the standard denominator in national HIV surveys in Cameroon.
The overall prevalence among the individuals aged between 15 to 49 years at 3.4% was similar to the most recent data, published in 2018 and organized in urban and semi-urban locations, that indicated a rate of HIV infection of 2.7% [
22] and 2.8% [
14]. Nevertheless, as observed with the surveys organised in urban and semi-urban settings, significant differences exist among the visited regions. This difference might be attributed to the availability and quality of health services for the management of HIV/AIDS across the country. Specifically when the national AIDS control committee assessed factors like the accessibility of HIV testing and counselling, the intensity of efforts directed towards prevention of mother-to-child-transmissions, care and support of people living with HIV, and the provision of laboratory and medication management throughout health facilities of Cameroon, they found high discrepancies among health facilities [
23]. It is also worth noting that many of the visited communities are located along the road network used by truck drivers, especially in the East, Littoral and South regions; and many studies have described this specific group of individuals as high-risk population for HIV infection [
24,
25]. Despite the discrepancy of HIV prevalence observed here and previously across the different regions of Cameroon, one key observation should be mentioned; rural communities in the South region experienced the highest HIV prevalence (5.2%), as was the case with individuals living in urban areas of this same region (6.9%) [
22]. In addition, we also found a similar prevalence of HIV infection in remote localities of the South in 2000 (4.5%) [
7] and in 2012/2013 (5.5%) [
26]. This observation seems to indicate that HIV prevalence in the South region of Cameroon has been stable over time at around 5%. The South region of Cameroon is well connected to other parts of the country, and more importantly to many neighbouring countries (Gabon, Equatorial Guinea and Republic of Congo). This high connectivity certainly lower barriers to HIV spread by increasing mixing of diverse population.
The main finding of this study is that HIV circulates mainly among adults and older individuals. Two peaks in prevalence were observed among women: those aged 35–45 years and those aged 55–64 years and above. Among men, the peak occurred in the 45–54 years group, which suggests a mature and possibly long-standing epidemic. In contrast, the most recent Demographic and Health Survey (DHS) conducted in 2018 in urban and semi-urban areas found that adults aged 35–39 years were the most affected group [
14]. Three main hypotheses may explain the relatively high prevalence in older individuals observed in our cohort. First, sample bias may play a role: fewer individuals (ranging from 2% to 9%) were enrolled in the 40–65 years and more age groups compared with those aged 15–39 years (12%–19%), and imprecise estimates are more likely with smaller sample sizes [
27]. Second, expansion of ART may have increased the life expectancy of PLWH, allowing those infected as adults to survive into older age. Third, demographic shifts such as rural exodus may have led to a higher proportion of older adults remaining in rural areas, as younger people migrate to cities for employment. Nevertheless, and assuming that old women are less sexually active as their younger counterparts, one should worry about old men aged between 45-54 years who can be a source of new infections in the communities.
5. Conclusions
This study demonstrates that HIV remains a significant health concern in remote rural communities of Cameroon’s equatorial regions, with patterns distinct from those in urban areas. Older adults, particularly those aged 35 years and above, and women bear a disproportionate burden of infection. Regional disparities were evident, with the South region consistently showing the highest prevalence, suggesting persistent local drivers of transmission. Divorce, widowhood, and lower educational attainment were also associated with elevated risk, underscoring the role of social vulnerability. The concentration of cases among older age groups, likely influenced by ART-driven survival and demographic shifts, challenges the conventional focus on the 15–49 years age bracket in surveillance. Expanding monitoring to include older adults and tailoring prevention and care strategies to high-prevalence regions and mobile populations are critical steps. Addressing these gaps is essential to curb ongoing transmission and achieve equitable HIV control in rural Cameroon.
Author Contributions
YFN, UCT, OHG, BE, D-DK: performed all the experiments. YFN, GN-T: performed data analyses. RFT, MTch and MT: supervised field work. MT, RFT and GN-T: designed and supervised the study. YFN, RFT, GN-T and MT: wrote the original draft of the manuscript. All authors contributed to subsequent revisions and approved the final version submitted for publication.
Funding
This research was supported by the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE) which is funded by the Science for Africa Foundation to the Developing Excellence in Leadership, Training and Science in Africa (DELTAS Africa) programme [Del-22-007] with support from the Wellcome Trust and the UK Foreign, Commonwealth & Development Office. The latter is part of the EDCPT2 programme supported by the European Union; the Bill & Melinda Gates Foundation [INV-033558]; and Gilead Sciences Inc., [19275]. All content contained within is that of the authors and does not necessarily reflect positions or policies of any SANTHE funder. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Informed Consent Statement
The study was approved by the Cameroon Ministry of Public Health through its National Ethics Committee (N° 2022/12/1510/CE/CNERSH/SP). Written informed consent was obtained from all participants prior to participation in line with the Declaration of Helsinki. HIV-positive individuals received counselling and referral to the nearest ART center.
Data Availability Statement
Non applicable
Conflicts of Interest
All the authors declared no conflict of interest.
References
-
AIDS, Crisis and the Power to Transform: UNAIDS Global AIDS Update 2025; Joint United Nations Programme on HIV/AIDS: Geneva, 2025.
- Global Statistics. Available online: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics (accessed on 22 July 2025).
-
2004: Report on the Global AIDS Epidemic: 4th Global Report; Joint United Nations Programme on HIV, AIDS, Ed.; UNAIDS: Geneva, 06; ISBN 978-92-9173-355-2.
- Zekeng, L.; Yanga, D.; Trebucq, A.; Sokal, D.; Salla, R.; Kaptue, L. HIV Prevalence in Patients with Sexually Transmitted Diseases in Yaounde, (Cameroon) in 1989 and 1990: Necessity of an STD Control Programme. Sexually Transmitted Infections 1992, 68, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Kuaban, C.; Bercion, R. [HIV seroprevalence in adults with pulmonary tuberculosis in Yaounde, Cameroon]. Med Trop (Mars) 1996, 56, 357–360. [Google Scholar] [PubMed]
- Buvé, A.; Caraël, M.; Hayes, R.J.; Auvert, B.; Ferry, B.; Robinson, N.J.; Anagonou, S.; Kanhonou, L.; Laourou, M.; Abega, S.; et al. Multicentre Study on Factors Determining Differences in Rate of Spread of HIV in Sub-Saharan Africa: Methods and Prevalence of HIV Infection. AIDS 2001, 15 (Suppl. S4), S5–S14. [Google Scholar] [CrossRef] [PubMed]
- Nyambi, P.; Zekeng, L.; Kenfack, H.; Tongo, M.; Nanfack, A.; Nkombe, I.; Ndonko, F.; Shang, J.; Burda, S.; Mbah, H.; et al. HIV Infection in Rural Villages of Cameroon. J Acquir Immune Defic Syndr 2002, 31, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Keele, B.F.; Van Heuverswyn, F.; Li, Y.; Bailes, E.; Takehisa, J.; Santiago, M.L.; Bibollet-Ruche, F.; Chen, Y.; Wain, L.V.; Liegeois, F.; et al. Chimpanzee Reservoirs of Pandemic and Nonpandemic HIV-1. Science 2006, 313, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Van Heuverswyn, F.; Li, Y.; Neel, C.; Bailes, E.; Keele, B.F.; Liu, W.; Loul, S.; Butel, C.; Liegeois, F.; Bienvenue, Y.; et al. Human Immunodeficiency Viruses: SIV Infection in Wild Gorillas. Nature 2006, 444, 164. [Google Scholar] [CrossRef] [PubMed]
- D’arc, M.; Ayouba, A.; Esteban, A.; Learn, G.H.; Boué, V.; Liegeois, F.; Etienne, L.; Tagg, N.; Leendertz, F.H.; Boesch, C.; et al. Origin of the HIV-1 Group O Epidemic in Western Lowland Gorillas. Proc Natl Acad Sci USA 2015, 112, E1343–E1352. [Google Scholar] [CrossRef] [PubMed]
- Tongo, M.; Martin, D.P.; Dorfman, J.R. Elucidation of Early Evolution of HIV-1 Group M in the Congo Basin Using Computational Methods. Genes (Basel) 2021, 12, 517. [Google Scholar] [CrossRef] [PubMed]
-
Directives Nationales sur la Prise en Charge du VIH; Ministère de la Santé Publique: Yaoundé, Cameroun, 2021.
-
Plan Stratégique National de Lutte contre le VIH/Sida et les IST 2021-2023 du Cameroun; Ministère de la Santé Publique/Comité National de Lutte contre le Sida: Yaoundé, Cameroun, 2020.
-
Enquête Démographique et de Santé du Cameroun 2018; Institut National de la Statistique (INS) et ICF: Yaoundé, Cameroun et Rockville, Maryland, USA : INS et ICF, 2020.
-
Enquête Démographique et de Santé et à Indicateurs Multiples du Cameroun 2011; Institut National de la Statistique (INS) et ICF. International: Calverton, Maryland, USA: INS et ICF International, 2012.
- Bekolo, C.E.; Kouanfack, C.; Ateudjieu, J.; Bechem, E.T.; Ndeso, S.A.; Tendengfor, N.; Nsagha, D.S.; Choukem, S.P. The Declining Trend in HIV Prevalence from Population-Based Surveys in Cameroon between 2004 and 2018: Myth or Reality in the Universal Test and Treat Era? BMC Public Health 2023, 23, 479. [Google Scholar] [CrossRef] [PubMed]
- Sandie, A.B.; Tchatchueng Mbougua, J.B.; Nlend, A.E.N.; Thiam, S.; Nono, B.F.; Fall, N.A.; Senghor, D.B.; Sylla, E.H.M.; Faye, C.M. Hot-Spots of HIV Infection in Cameroon: A Spatial Analysis Based on Demographic and Health Surveys Data. BMC Infectious Diseases 2022, 22, 334. [Google Scholar] [CrossRef] [PubMed]
-
Consolidated Guidelines on HIV Testing Services, 2019; World Health Organization: Geneva, 2020.
- Statistical Software for Data Science|Stata. Available online: https://www.stata.com/ (accessed on 25 August 2025).
- Barros, A.J.; Hirakata, V.N. Alternatives for Logistic Regression in Cross-Sectional Studies: An Empirical Comparison of Models That Directly Estimate the Prevalence Ratio. BMC Medical Research Methodology 2003, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Godwe, C.; Goni, O.H.; San, J.E.; Sonela, N.; Tchakoute, M.; Nanfack, A.; Koro, F.K.; Butel, C.; Vidal, N.; Duerr, R.; et al. Phylogenetic Evidence of Extensive Spatial Mixing of Diverse HIV-1 Group M Lineages within Cameroon but Not between Its Neighbours. Virus Evol 2024, 10, veae070. [Google Scholar] [CrossRef] [PubMed]
-
Évaluation de l’impact du VIH sur la population au Cameroun (CAMPHIA) 2017-2018 : Rapport final.; Ministère de la santé (MINSANTE), Division de la Recherche Opérationnelle en Santé (DROS): Yaoundé : MINSANTE, DROS, 2020.
-
Epidémiologie de l’infection à VIH au Cameroun : Premier semestre 2018; Comité National de Lutte Contre le Sida, 2018.
- Mosoko, J.J.; Macauley, I.B.; Zoungkanyi, A.-C.B.; Bella, A.; Koulla-Shiro, S. Human Immunodeficiency Virus Infection and Associated Factors among Specific Population Subgroups in Cameroon. AIDS Behav 2009, 13, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Edoul, G.; Chia, J.E.; Vidal, N.; Guichet, E.; Montavon, C.; Delaporte, E.; Mpoudi Ngole, E.; Ayouba, A.; Peeters, M. High HIV Burden and Recent Transmission Chains in Rural Forest Areas in Southern Cameroon, Where Ancestors of HIV-1 Have Been Identified in Ape Populations. Infection, Genetics and Evolution 2020, 84, 104358. [Google Scholar] [CrossRef] [PubMed]
- Ngoume, Y.F.; Teagho, U.C.; Eselacha, B.; Goni, O.H.; Kenfack, D.-D.; Tchakoute, M.; Nguefack-Tsague, G.; Tongo, M. Differences in HIV Infection Trends in Two Regions of Cameroon with a Longstanding HIV Epidemic: Insights from 2012 and 2022. Front Public Health 2025, 13, 1517213, fpubh.2025.1517213. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Sample Size and Its Importance in Research. Indian J Psychol Med 2020, 42, 102–103. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).