Submitted:
04 September 2025
Posted:
05 September 2025
You are already at the latest version
Abstract
Keywords:
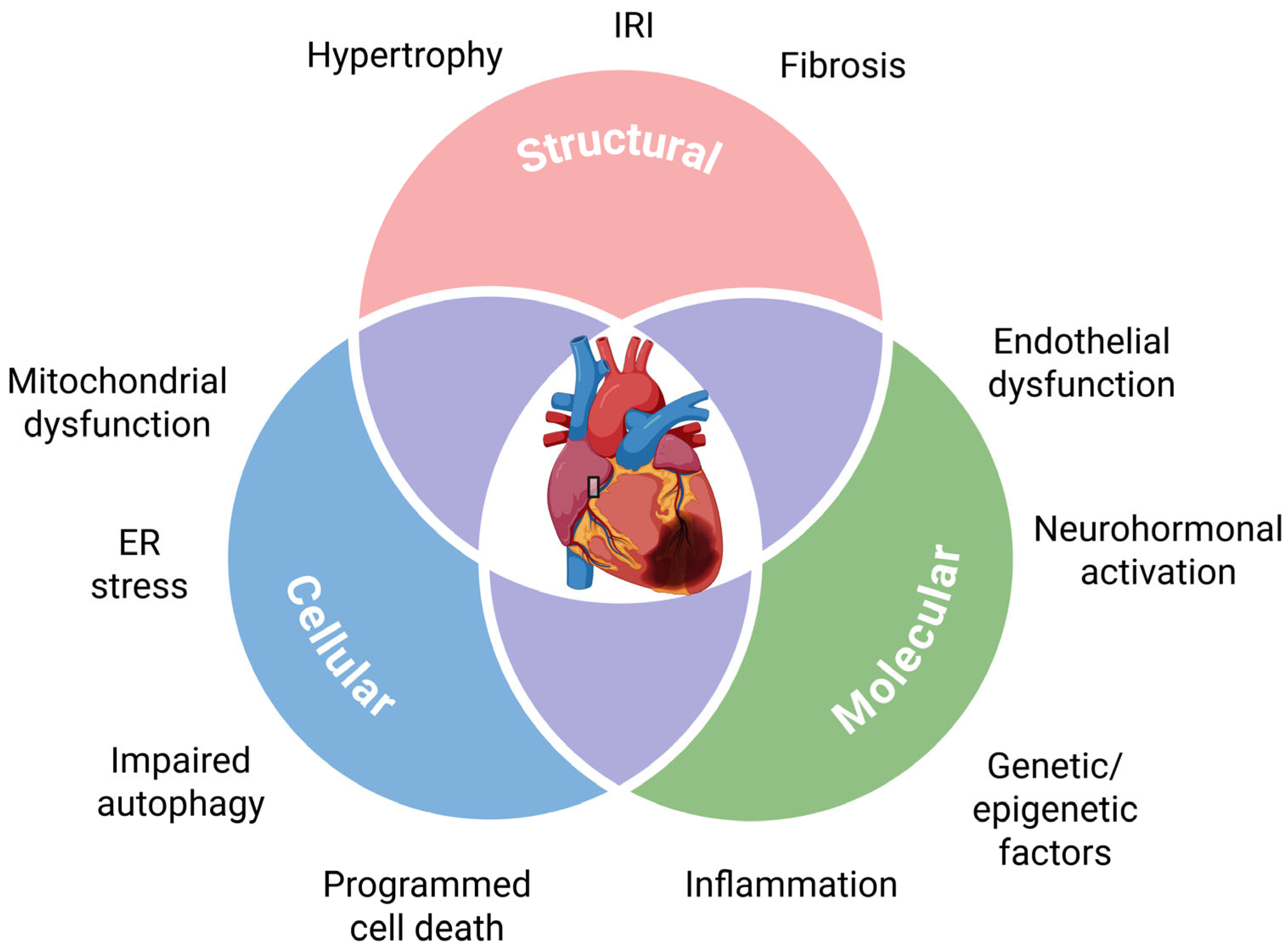
1. Introduction
2. Energy Metabolism, Oxidative Stress and Mitochondrial Dysfunction in Heart Failure
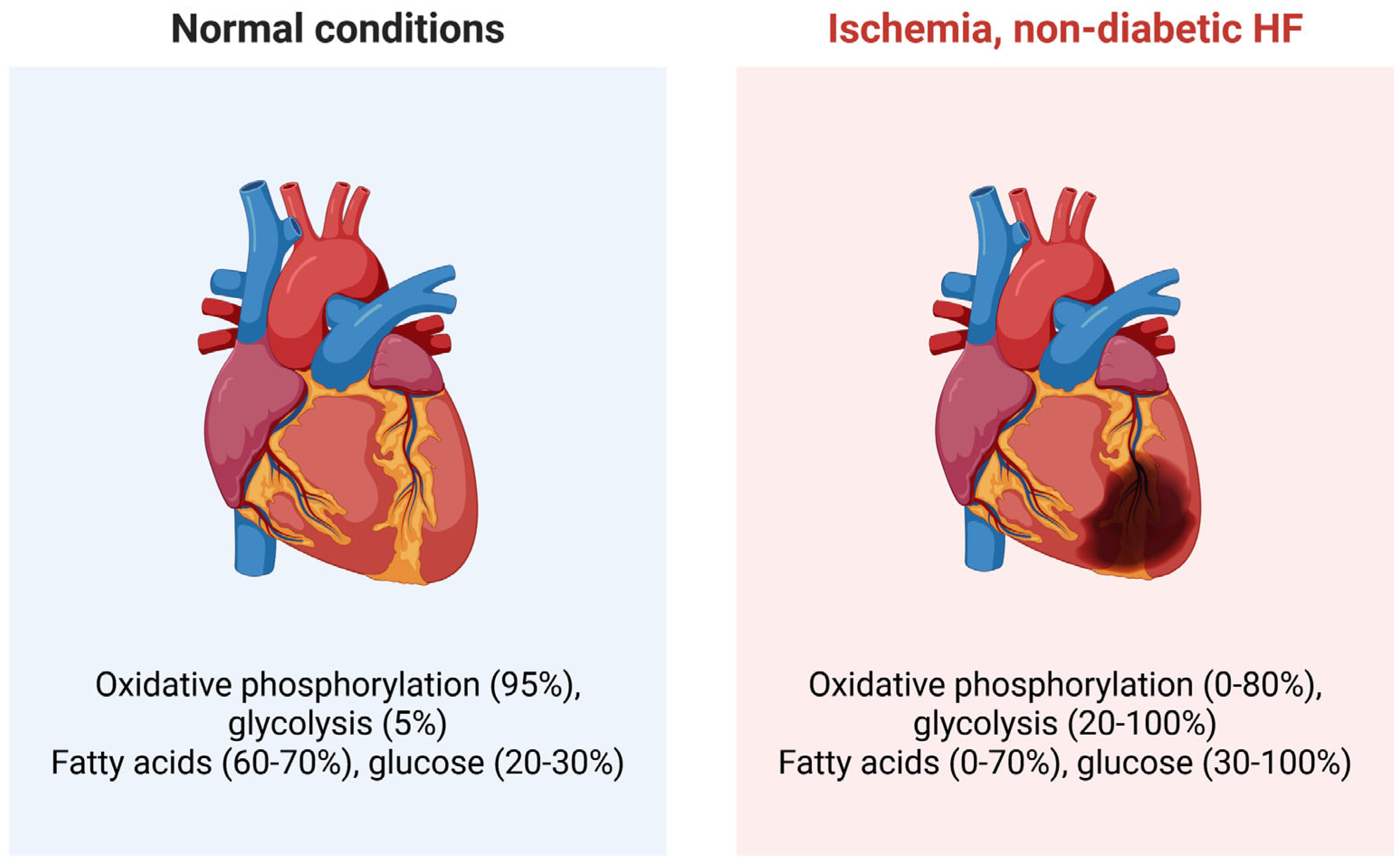
2.1. Energy Metabolism
2.2. Oxidative Stress
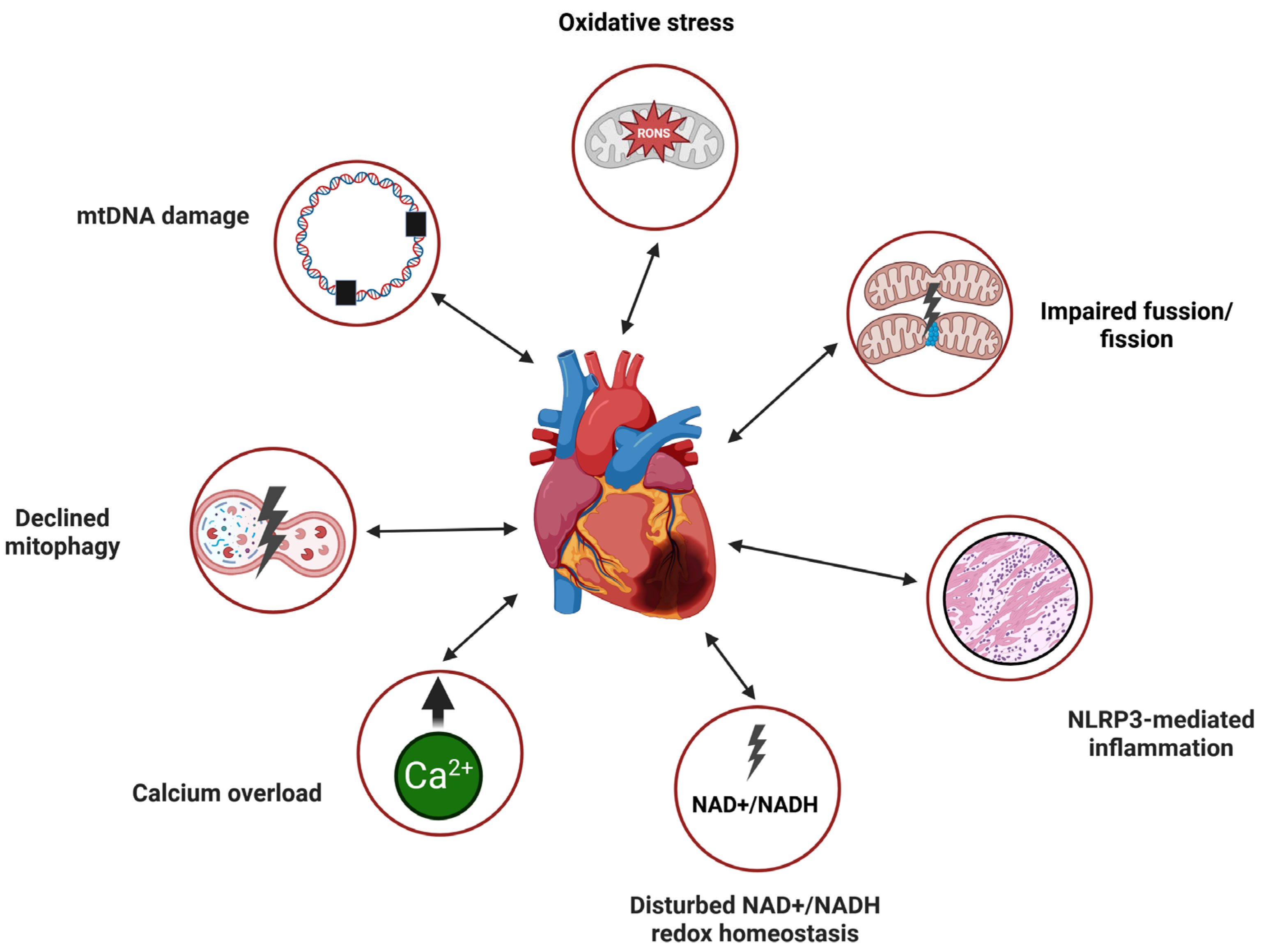
2.3. Mitochondrial Dysfunction
3. Sirtuins in Heart Failure
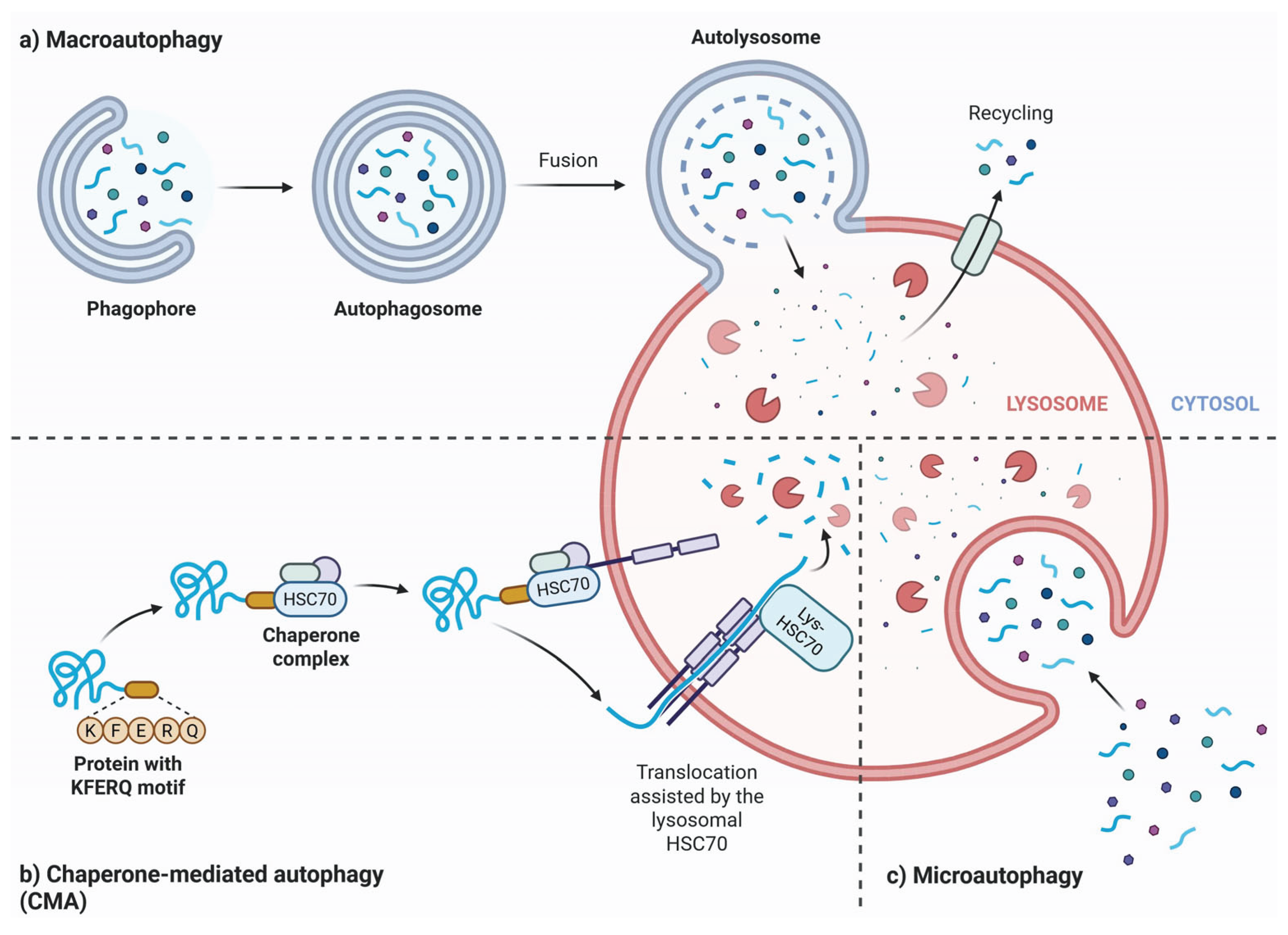
4. Autophagy in Heart Failure
5. Interplay between Mitochondrial Quality Control, Sirtuins, and Autophagy in Heart Failure
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Braunwald, E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. The New England journal of medicine 1997, 337, 1360–1369. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2025, 151, e41–e660. [Google Scholar] [CrossRef]
- Bozkurt, B.; Ahmad, T.; Alexander, K.; Baker, W.L.; Bosak, K.; Breathett, K.; Carter, S.; Drazner, M.H.; Dunlay, S.M.; Fonarow, G.C.; et al. HF STATS 2024: Heart Failure Epidemiology and Outcomes Statistics An Updated 2024 Report from the Heart Failure Society of America. Journal of Cardiac Failure 2025, 31, 66–116. [Google Scholar] [CrossRef]
- Levy, D.; Kenchaiah, S.; Larson, M.G.; Benjamin, E.J.; Kupka, M.J.; Ho, K.K.; Murabito, J.M.; Vasan, R.S. Long-term trends in the incidence of and survival with heart failure. The New England journal of medicine 2002, 347, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Weston, S.A.; Redfield, M.M.; Hellermann-Homan, J.P.; Killian, J.; Yawn, B.P.; Jacobsen, S.J. Trends in heart failure incidence and survival in a community-based population. Jama 2004, 292, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.A.; Zaccardi, F.; Squire, I.; Okhai, H.; Davies, M.; Huang, W.; Mamas, M.; Lam, C.S.P.; Khunti, K.; Kadam, U.T. Risk Factors for Heart Failure. Circulation: Heart Failure 2020, 13, e006472. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.S.; Cogswell, R.; Thenappan, T. The heterogeneity of heart failure: will enhanced phenotyping be necessary for future clinical trial success? J Am Coll Cardiol 2014, 64, 1775–1776. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc Diagn Ther 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Zile, M.R.; Bennett, T.D.; St. John Sutton, M.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition From Chronic Compensated to Acute Decompensated Heart Failure. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef]
- Mann, D.L.; Felker, G.M. Mechanisms and Models in Heart Failure. Circulation Research 2021, 128, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Roman-Pepine, D.; Serban, A.M.; Capras, R.-D.; Cismaru, C.M.; Filip, A.G. A Comprehensive Review: Unraveling the Role of Inflammation in the Etiology of Heart Failure. Heart Failure Reviews 2025, 30, 931–954. [Google Scholar] [CrossRef]
- Patel, J.; Rassekh, N.; Fonarow, G.C.; Deedwania, P.; Sheikh, F.H.; Ahmed, A.; Lam, P.H. Guideline-Directed Medical Therapy for the Treatment of Heart Failure with Reduced Ejection Fraction. Drugs 2023, 83, 747–759. [Google Scholar] [CrossRef]
- Lund, L.H.; Crespo-Leiro, M.G.; Laroche, C.; Zaliaduonyte, D.; Saad, A.M.; Fonseca, C.; Čelutkienė, J.; Zdravkovic, M.; Bielecka-Dabrowa, A.M.; Agostoni, P.; et al. Heart failure in Europe: Guideline-directed medical therapy use and decision making in chronic and acute, pre-existing and de novo, heart failure with reduced, mildly reduced, and preserved ejection fraction - the ESC EORP Heart Failure III Registry. Eur J Heart Fail 2024, 26, 2487–2501. [Google Scholar] [CrossRef]
- Jaarsma, T.; Hill, L.; Bayes-Genis, A.; La Rocca, H.B.; Castiello, T.; Čelutkienė, J.; Marques-Sule, E.; Plymen, C.M.; Piper, S.E.; Riegel, B.; et al. Self-care of heart failure patients: practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2021, 23, 157–174. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Gustafsson, F.; Mebazaa, A.; Steffel, J.; Witte, K.K.; Delgado, V.; Linde, C.; Vernooy, K.; Anker, S.D.; et al. Integration of implantable device therapy in patients with heart failure. A clinical consensus statement from the Heart Failure Association (HFA) and European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC). European Journal of Heart Failure 2024, 26, 483–501. [Google Scholar] [CrossRef]
- Argiro, A.; Bui, Q.; Hong, K.N.; Ammirati, E.; Olivotto, I.; Adler, E. Applications of Gene Therapy in Cardiomyopathies. JACC: Heart Failure 2024, 12, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Roussoulières, A.; Farrero, M.; Gustafsson, F.; Kittleson, M.; Munagala, M.; Stehlik, J. Heart Transplant and Durable Mechanical Circulatory Support for Specific Less-Common Cardiomyopathies. CJC Open 2025, 7, 813–820. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. The Journal of clinical investigation 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J. Autophagy: from phenomenology to molecular understanding in less than a decade. Nature reviews. Molecular cell biology 2007, 8, 931–937. [Google Scholar] [CrossRef]
- Li, L.; Xi, R.; Gao, B.; Zeng, Y.; Ma, Q.; Gong, T.; Wang, J. Research progress of autophagy in heart failure. American journal of translational research 2024, 16, 1991–2000. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Energy metabolism in heart failure. J Physiol 2004, 555, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zou, Y.; Song, C.; Cao, K.; Cai, K.; Wu, Y.; Zhang, Z.; Geng, D.; Sun, W.; Ouyang, N.; et al. The role of glycolytic metabolic pathways in cardiovascular disease and potential therapeutic approaches. Basic Res Cardiol 2023, 118, 48. [Google Scholar] [CrossRef] [PubMed]
- Kolwicz, S.C.; Purohit, S.; Tian, R. Cardiac Metabolism and its Interactions With Contraction, Growth, and Survival of Cardiomyocytes. Circulation Research 2013, 113, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ Res 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Zhu, X.; Hu, J.; Liu, C.; Liu, L. Energy metabolism and redox balance: How phytochemicals influence heart failure treatment. Biomedicine & Pharmacotherapy 2024, 171, 116136. [Google Scholar] [CrossRef]
- Shehadeh, L.A.; Robleto, E.; Lopaschuk, G.D. Cardiac energy substrate utilization in heart failure with preserved ejection fraction: reconciling conflicting evidence on fatty acid and glucose metabolism. Am J Physiol Heart Circ Physiol 2025, 328, H1267–H1295. [Google Scholar] [CrossRef]
- Carvalho, R.A. Chapter 12 - The glycolytic pathway to heart failure. In Glycolysis; Ferreira, R., Oliveira, P.F., Nogueira-Ferreira, R., Eds.; Academic Press, 2024; pp. 235–266. [Google Scholar]
- Smith, C.S.; Bottomley, P.A.; Schulman, S.P.; Gerstenblith, G.; Weiss, R.G. Altered Creatine Kinase Adenosine Triphosphate Kinetics in Failing Hypertrophied Human Myocardium. Circulation 2006, 114, 1151–1158. [Google Scholar] [CrossRef]
- Weiss, R.G.; Gerstenblith, G.; Bottomley, P.A. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proceedings of the National Academy of Sciences of the United States of America 2005, 102, 808–813. [Google Scholar] [CrossRef]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ Res 2023, 132, 882–898. [Google Scholar] [CrossRef] [PubMed]
- Sb, H.; X, J.; Qh, Y.; Xr, Z.; Bb, Z.; Kh, W.; Xy, S.; Yt, C.; Xr, R.; Jf, M.; et al. The vicious circle between mitochondrial oxidative stress and dynamic abnormality mediates triethylene glycol dimethacrylate-induced preodontoblast apoptosis. Free Radical Biology and Medicine 2019, 134, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). International journal of molecular medicine 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Hewitt, O.H.; Degnan, S.M. Antioxidant enzymes that target hydrogen peroxide are conserved across the animal kingdom, from sponges to mammals. Scientific reports 2023, 13, 2510. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Experimental & molecular medicine 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Schwemmlein, J.; Maack, C.; Bertero, E. Mitochondria as Therapeutic Targets in Heart Failure. Current Heart Failure Reports 2022, 19, 27–37. [Google Scholar] [CrossRef]
- Teixeira, R.B.; Albro, J.H.; Sabra, M.; Abedin, T.; Tucker, A.N.; Sidharth, R.; Sellke, F.W.; Wipf, P.; Abid, M.R. Mitochondria-targeted ROS scavenger JP4-039 improves cardiac function in a post-myocardial infarction animal model and induces angiogenesis in vitro. PloS one 2025, 20, e0320703. [Google Scholar] [CrossRef]
- Ku, H.J.; Ahn, Y.; Lee, J.H.; Park, K.M.; Park, J.W. IDH2 deficiency promotes mitochondrial dysfunction and cardiac hypertrophy in mice. Free Radic Biol Med 2015, 80, 84–92. [Google Scholar] [CrossRef]
- Nickel, A.G.; von Hardenberg, A.; Hohl, M.; Löffler, Joachim R.; Kohlhaas, M.; Becker, J.; Reil, J.-C.; Kazakov, A.; Bonnekoh, J.; Stadelmaier, M.; et al. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metabolism 2015, 22, 472–484. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, R.; Liao, X.; Castillero, E.; Kennel, P.J.; Brunjes, D.L.; Franz, M.; Möbius-Winkler, S.; Drosatos, K.; George, I.; et al. MicroRNA-195 Regulates Metabolism in Failing Myocardium Via Alterations in Sirtuin 3 Expression and Mitochondrial Protein Acetylation. Circulation 2018, 137, 2052–2067. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Experimental & molecular medicine 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Borchi, E.; Bargelli, V.; Stillitano, F.; Giordano, C.; Sebastiani, M.; Nassi, P.A.; d’Amati, G.; Cerbai, E.; Nediani, C. Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochimica et biophysica acta 2010, 1802, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sam, F.; Kerstetter, D.L.; Pimental, D.R.; Mulukutla, S.; Tabaee, A.; Bristow, M.R.; Colucci, W.S.; Sawyer, D.B. Increased Reactive Oxygen Species Production and Functional Alterations in Antioxidant Enzymes in Human Failing Myocardium. Journal of Cardiac Failure 2005, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, S.; Bieligk, U.; Beulich, K.; Hasenfuss, G.; Prestle, J. Gene Expression of Antioxidative Enzymes in the Human Heart. Circulation 2000, 101, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality control of the mitochondrion. Developmental cell 2021, 56, 881–905. [Google Scholar] [CrossRef]
- Held, N.M.; Houtkooper, R.H. Mitochondrial quality control pathways as determinants of metabolic health. Bioessays 2015, 37, 867–876. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases. Signal Transduction and Targeted Therapy 2024, 9, 50. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants (Basel, Switzerland) 2023, 12. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Y.; Liu, B.; Gao, M.; Li, A.; Li, X.; Gong, G. PGC-1α-Mediated Mitochondrial Quality Control: Molecular Mechanisms and Implications for Heart Failure. Frontiers in cell and developmental biology 2022, Volume 10 - 2022. [Google Scholar] [CrossRef]
- Bhat, S.; Chin, A.; Shirakabe, A.; Ikeda, Y.; Ikeda, S.; Zhai, P.; Hsu, C.P.; Sayed, D.; Abdellatif, M.; Byun, J.; et al. Recruitment of RNA Polymerase II to Metabolic Gene Promoters Is Inhibited in the Failing Heart Possibly Through PGC-1α (Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α) Dysregulation. Circ Heart Fail 2019, 12, e005529. [Google Scholar] [CrossRef]
- Kärkkäinen, O.; Tuomainen, T.; Mutikainen, M.; Lehtonen, M.; Ruas, J.L.; Hanhineva, K.; Tavi, P. Heart specific PGC-1α deletion identifies metabolome of cardiac restricted metabolic heart failure. Cardiovasc Res 2019, 115, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Lehman, J.J.; Kelly, D.P. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol 2002, 29, 339–345. [Google Scholar] [CrossRef]
- Russell, L.K.; Mansfield, C.M.; Lehman, J.J.; Kovacs, A.; Courtois, M.; Saffitz, J.E.; Medeiros, D.M.; Valencik, M.L.; McDonald, J.A.; Kelly, D.P. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 2004, 94, 525–533. [Google Scholar] [CrossRef]
- Gwon, J.G.; Lee, S.M. Role of PTEN-Induced Protein Kinase 1 as a Mitochondrial Dysfunction Regulator in Cardiovascular Disease Pathogenesis. Vasc Specialist Int 2024, 40, 9. [Google Scholar] [CrossRef]
- Billia, F.; Hauck, L.; Konecny, F.; Rao, V.; Shen, J.; Mak, T.W. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proceedings of the National Academy of Sciences 2011, 108, 9572–9577. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Bi, Y.; Zhao, Z.; Wang, S.; Lin, S.; Yang, Z.; Wang, X.; Mao, J. Emerging role of mitophagy in heart failure: from molecular mechanism to targeted therapy. Cell cycle (Georgetown, Tex.) 2023, 22, 906–918. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Hayashidani, S.; Kang, D.; Suematsu, N.; Nakamura, K.; Utsumi, H.; Hamasaki, N.; Takeshita, A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 2001, 88, 529–535. [Google Scholar] [CrossRef]
- Vela-Guajardo, J.E.; Pérez-Treviño, P.; Rivera-Álvarez, I.; González-Mondellini, F.A.; Altamirano, J.; García, N. The 8-oxo-deoxyguanosine glycosylase increases its migration to mitochondria in compensated cardiac hypertrophy. J Am Soc Hypertens 2017, 11, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, K.H.; Kleppa, L.; Aronsen, J.M.; Eide, L.; Carlsen, H.; Haugen Ø, P.; Sjaastad, I.; Klungland, A.; Rasmussen, L.J.; Attramadal, H.; et al. Impaired dynamics and function of mitochondria caused by mtDNA toxicity leads to heart failure. Am J Physiol Heart Circ Physiol 2015, 309, H434–H449. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Otsu, K. Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases. The Biochemical journal 2018, 475, 839–852. [Google Scholar] [CrossRef]
- Misawa, T.; Takahama, M.; Kozaki, T.; Lee, H.; Zou, J.; Saitoh, T.; Akira, S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nature Immunology 2013, 14, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.R.; Payne, R.M. Mitochondrial Acetylation and Diseases of Aging. Journal of aging research 2011, 2011, 234875. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Szekely, K.; Zhang, K.; Lanfear, D.E.; Sabbah, H.N. Evidence of Hyperacetylation of Mitochondrial Regulatory Proteins in Left Ventricular Myocardium of Dogs with Chronic Heart Failure. International journal of molecular sciences 2025, 26. [Google Scholar] [CrossRef]
- Castillo, E.C.; Morales, J.A.; Chapoy-Villanueva, H.; Silva-Platas, C.; Treviño-Saldaña, N.; Guerrero-Beltrán, C.E.; Bernal-Ramírez, J.; Torres-Quintanilla, A.; García, N.; Youker, K.; et al. Mitochondrial Hyperacetylation in the Failing Hearts of Obese Patients Mediated Partly by a Reduction in SIRT3: The Involvement of the Mitochondrial Permeability Transition Pore. Cell Physiol Biochem 2019, 53, 465–479. [Google Scholar] [CrossRef]
- Grillon, J.M.; Johnson, K.R.; Kotlo, K.; Danziger, R.S. Non-histone lysine acetylated proteins in heart failure. Biochimica et biophysica acta 2012, 1822, 607–614. [Google Scholar] [CrossRef]
- Pei, Z.; Dong, M.; Meng, X.; Yao, W.; Guo, Y.; Wang, F. Effects of Nicotinamide Adenine Dinucleotide on Older Patients with Heart Failure. Rev Cardiovasc Med 2024, 25, 297. [Google Scholar] [CrossRef] [PubMed]
- Nasuhidehnavi, A.; Zarzycka, W.; Górecki, I.; Chiao, Y.A.; Lee, C.F. Emerging interactions between mitochondria and NAD+ metabolism in cardiometabolic diseases. Trends in Endocrinology & Metabolism 2025, 36, 176–190. [Google Scholar] [CrossRef]
- Johnson, E.; Albakri, J.S.; Allemailem, K.S.; Sultan, A.; Alwanian, W.M.; Alrumaihi, F.; Almansour, N.M.; Aldakheel, F.M.; Khalil, F.M.A.; Abduallah, A.M.; et al. Mitochondrial dysfunction and calcium homeostasis in heart failure: Exploring the interplay between oxidative stress and cardiac remodeling for future therapeutic innovations. Current Problems in Cardiology 2025, 50, 102968. [Google Scholar] [CrossRef]
- Modesti, L.; Danese, A.; Angela Maria Vitto, V.; Ramaccini, D.; Aguiari, G.; Gafà, R.; Lanza, G.; Giorgi, C.; Pinton, P. Mitochondrial Ca(2+) Signaling in Health, Disease and Therapy. Cells 2021, 10. [Google Scholar] [CrossRef]
- Pathak, T.; Trebak, M. Mitochondrial Ca(2+) signaling. Pharmacol Ther 2018, 192, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; He, P.; Liu, W.; Wu, H.; Wang, Z. Unraveling mitochondrial crosstalk: a new frontier in heart failure pathogenesis. Front Cardiovasc Med 2025, 12, 1641023. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Verdin, E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome biology 2004, 5, 224. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduction and Targeted Therapy 2022, 7, 402. [Google Scholar] [CrossRef]
- Gupta, R.; Ambasta, R.K.; Kumar, P. Multifaced role of protein deacetylase sirtuins in neurodegenerative disease. Neurosci Biobehav Rev 2022, 132, 976–997. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Song, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Z.; Wang, Y. The dual role of sirtuins in cancer: biological functions and implications. Frontiers in oncology 2024, 14, 1384928. [Google Scholar] [CrossRef] [PubMed]
- Ianni, A.; Yuan, X.; Bober, E.; Braun, T. Sirtuins in the Cardiovascular System: Potential Targets in Pediatric Cardiology. Pediatric Cardiology 2018, 39, 983–992. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Wu, Y.T.; Tsai, C.L.; Wei, Y.H. Current understanding and future perspectives of the roles of sirtuins in the reprogramming and differentiation of pluripotent stem cells. Experimental biology and medicine (Maywood, N.J.) 2018, 243, 563–575. [Google Scholar] [CrossRef]
- Lipphardt, M.; Dihazi, H.; Müller, G.A.; Goligorsky, M.S. Fibrogenic Secretome of Sirtuin 1-Deficient Endothelial Cells: Wnt, Notch and Glycocalyx Rheostat. Frontiers in physiology 2018, 9, 1325. [Google Scholar] [CrossRef]
- Ronnebaum, S.M.; Patterson, C. The FoxO family in cardiac function and dysfunction. Annu Rev Physiol 2010, 72, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Sadoshima, J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol 2015, 309, H1375–H1389. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.L. Energy metabolism in health and diseases. Signal Transduct Target Ther 2025, 10, 69. [Google Scholar] [CrossRef]
- Ding, Y.-N.; Wang, H.-Y.; Chen, X.-F.; Tang, X.; Chen, H.-Z. Roles of Sirtuins in Cardiovascular Diseases: Mechanisms and Therapeutics. Circulation Research 2025, 136, 524–550. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. Jama 2016, 315, 36–46. [Google Scholar] [CrossRef]
- Corbi, G.; Conti, V.; Troisi, J.; Colucci, A.; Manzo, V.; Di Pietro, P.; Calabrese, M.C.; Carrizzo, A.; Vecchione, C.; Ferrara, N.; et al. Cardiac Rehabilitation Increases SIRT1 Activity and β-Hydroxybutyrate Levels and Decreases Oxidative Stress in Patients with HF with Preserved Ejection Fraction. Oxidative medicine and cellular longevity 2019, 2019, 7049237. [Google Scholar] [CrossRef]
- Russomanno, G.; Corbi, G.; Manzo, V.; Ferrara, N.; Rengo, G.; Puca, A.A.; Latte, S.; Carrizzo, A.; Calabrese, M.C.; Andriantsitohaina, R.; et al. The anti-ageing molecule sirt1 mediates beneficial effects of cardiac rehabilitation. Immunity & ageing : I & A 2017, 14, 7. [Google Scholar] [CrossRef]
- Costantino, S.; Mengozzi, A.; Velagapudi, S.; Mohammed, S.A.; Gorica, E.; Akhmedov, A.; Mongelli, A.; Pugliese, N.R.; Masi, S.; Virdis, A.; et al. Treatment with recombinant Sirt1 rewires the cardiac lipidome and rescues diabetes-related metabolic cardiomyopathy. Cardiovasc Diabetol 2023, 22, 312. [Google Scholar] [CrossRef] [PubMed]
- Alhasaniah, A.H.; Alissa, M.; Elsaid, F.G.; Alsugoor, M.H.; AlQahtani, M.S.; Alessa, A.; Jambi, K.; Albakri, G.S.; Albaqami, F.M.K.; Bennett, E. The enigmatic role of SIRT2 in the cardiovascular system: Deciphering its protective and detrimental actions to unlock new avenues for therapeutic intervention. Curr Probl Cardiol 2025, 50, 102929. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chang, H.C.; Tatekoshi, Y.; Mahmoodzadeh, A.; Balibegloo, M.; Najafi, Z.; Wu, R.; Chen, C.; Sato, T.; Shapiro, J.; et al. SIRT2 inhibition protects against cardiac hypertrophy and ischemic injury. eLife 2023, 12. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.F.; Wang, N.Y.; Wang, X.M.; Liang, S.T.; Zheng, W.; Lu, Y.B.; Zhao, X.; Hao, D.L.; Zhang, Z.Q.; et al. SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation 2017, 136, 2051–2067. [Google Scholar] [CrossRef]
- Zheng, M.; Du, X.; Zhao, L.; Sun, H.; Chen, M.; Yang, X. Elevated plasma Sirtuin2 level predicts heart failure after acute myocardial infarction. J Thorac Dis 2021, 13, 50–59. [Google Scholar] [CrossRef]
- Sarikhani, M.; Maity, S.; Mishra, S.; Jain, A.; Tamta, A.K.; Ravi, V.; Kondapalli, M.S.; Desingu, P.A.; Khan, D.; Kumar, S.; et al. SIRT2 deacetylase represses NFAT transcription factor to maintain cardiac homeostasis. Journal of Biological Chemistry 2018, 293, 5281–5294. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ Res 2021, 128, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Schiattarella, G.G.; Jiang, N.; Altamirano, F.; Szweda, P.A.; Elnwasany, A.; Lee, D.I.; Yoo, H.; Kass, D.A.; Szweda, L.I.; et al. NAD(+) Repletion Reverses Heart Failure With Preserved Ejection Fraction. Circ Res 2021, 128, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Jheng, J.R.; Bai, Y.; Noda, K.; Huot, J.R.; Cook, T.; Fisher, A.; Chen, Y.Y.; Goncharov, D.A.; Goncharova, E.A.; Simon, M.A.; et al. Skeletal Muscle SIRT3 Deficiency Contributes to Pulmonary Vascular Remodeling in Pulmonary Hypertension Due to Heart Failure With Preserved Ejection Fraction. Circulation 2024, 150, 867–883. [Google Scholar] [CrossRef]
- Wang, Y.C.; Koay, Y.C.; Pan, C.; Zhou, Z.; Tang, W.; Wilcox, J.; Li, X.S.; Zagouras, A.; Marques, F.; Allayee, H.; et al. Indole-3-Propionic Acid Protects Against Heart Failure With Preserved Ejection Fraction. Circ Res 2024, 134, 371–389. [Google Scholar] [CrossRef]
- Bergmann, L.; Lang, A.; Bross, C.; Altinoluk-Hambüchen, S.; Fey, I.; Overbeck, N.; Stefanski, A.; Wiek, C.; Kefalas, A.; Verhülsdonk, P.; et al. Subcellular localization and mitotic interactome analyses identify SIRT4 as a centrosomally localized and microtubule associated protein. bioRxiv 2020, 2020.2002.2017.940692. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Witt, C.N.; Bode, C. Mitochondrial sirtuins in the heart. Heart Failure Reviews 2016, 21, 519–528. [Google Scholar] [CrossRef]
- Luo, Y.X.; Tang, X.; An, X.Z.; Xie, X.M.; Chen, X.F.; Zhao, X.; Hao, D.L.; Chen, H.Z.; Liu, D.P. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J 2017, 38, 1389–1398. [Google Scholar] [CrossRef]
- Byrne, N.J.; Koentges, C.; Khan, E.; Pfeil, K.; Sandulescu, R.; Bakshi, S.; Költgen, C.; Vosko, I.; Gollmer, J.; Rathner, T.; et al. Sirtuin 4 accelerates heart failure development by enhancing reactive oxygen species-mediated profibrotic transcriptional signaling. Journal of Molecular and Cellular Cardiology Plus 2025, 12, 100299. [Google Scholar] [CrossRef]
- Guo, A.H.; Baliira, R.; Skinner, M.E.; Kumar, S.; Andren, A.; Zhang, L.; Goldsmith, R.S.; Michan, S.; Davis, N.J.; Maccani, M.W.; et al. Sirtuin 5 levels are limiting in preserving cardiac function and suppressing fibrosis in response to pressure overload. Scientific reports 2022, 12, 12258. [Google Scholar] [CrossRef]
- Liu, B.; Che, W.; Zheng, C.; Liu, W.; Wen, J.; Fu, H.; Tang, K.; Zhang, J.; Xu, Y. SIRT5: A Safeguard Against Oxidative Stress-Induced Apoptosis in Cardiomyocytes. Cellular Physiology and Biochemistry 2013, 32, 1050–1059. [Google Scholar] [CrossRef]
- Li, S.; Shen, S.; Hong, Y.; Ding, K.; Chen, S.; Chen, J.; Wang, C.; Wen, Y.; Mo, G.; Yu, L.; et al. Sirt5 preserves cardiac function in ischemia-reperfusion injury by inhibiting ANT2 lactylation. bioRxiv 2024, 2024.2011.2025.625148. [Google Scholar] [CrossRef]
- Khan, D.; Ara, T.; Ravi, V.; Rajagopal, R.; Tandon, H.; Parvathy, J.; Gonzalez, E.A.; Asirvatham-Jeyaraj, N.; Krishna, S.; Mishra, S.; et al. SIRT6 transcriptionally regulates fatty acid transport by suppressing PPARγ. Cell reports 2021, 35, 109190. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Brooks, A.; Xu, S.; Luo, J.; Steiner, R.; Mickelsen, D.M.; Moravec, C.S.; Jeffrey, A.D.; Small, E.M.; et al. SIRT6 Mitigates Heart Failure With Preserved Ejection Fraction in Diabetes. Circ Res 2022, 131, 926–943. [Google Scholar] [CrossRef]
- Li, Y.; Meng, X.; Wang, W.; Liu, F.; Hao, Z.; Yang, Y.; Zhao, J.; Yin, W.; Xu, L.; Zhao, R.; et al. Cardioprotective Effects of SIRT6 in a Mouse Model of Transverse Aortic Constriction-Induced Heart Failure. Frontiers in physiology 2017, Volume 8 - 2017. [Google Scholar] [CrossRef]
- Kiran, S.; Chatterjee, N.; Singh, S.; Kaul, S.C.; Wadhwa, R.; Ramakrishna, G. Intracellular distribution of human SIRT7 and mapping of the nuclear/nucleolar localization signal. Febs j 2013, 280, 3451–3466. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Qi, R.-Q.; Song, J.-W.; Wang, S.-Y.; Dong, Z.-J.; Chen, Y.-H.; Liu, Y.; Zhou, X.-Y.; Li, J.; Liu, X.-Y.; et al. Sirtuin 7 ameliorates cuproptosis, myocardial remodeling and heart dysfunction in hypertension through the modulation of YAP/ATP7A signaling. Apoptosis 2024, 29, 2161–2182. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Izumiya, Y.; Araki, S.; Nakamura, T.; Kimura, Y.; Hanatani, S.; Yamada, T.; Ishida, T.; Yamamoto, M.; Onoue, Y.; et al. Cardiomyocyte Sirt (Sirtuin) 7 Ameliorates Stress-Induced Cardiac Hypertrophy by Interacting With and Deacetylating GATA4. Hypertension 2020, 75, 98–108. [Google Scholar] [CrossRef]
- Vakhrusheva, O.; Smolka, C.; Gajawada, P.; Kostin, S.; Boettger, T.; Kubin, T.; Braun, T.; Bober, E. Sirt7 Increases Stress Resistance of Cardiomyocytes and Prevents Apoptosis and Inflammatory Cardiomyopathy in Mice. Circulation Research 2008, 102, 703–710. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ding, H.; Li, D.; Shen, W.; Zhang, X. The Current State of Research on Sirtuin-Mediated Autophagy in Cardiovascular Diseases. Journal of Cardiovascular Development and Disease 2023, 10, 382. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nature Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, G.; Liu, H.; Wang, L.; Lu, R.; Li, L. Molecular mechanisms of secretory autophagy and its potential role in diseases. Life sciences 2024, 347, 122653. [Google Scholar] [CrossRef] [PubMed]
- Jahn, R.; Cafiso, D.C.; Tamm, L.K. Mechanisms of SNARE proteins in membrane fusion. Nature Reviews Molecular Cell Biology 2024, 25, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A.; Ernst, A.; Dikic, I. Cargo recognition and trafficking in selective autophagy. Nature cell biology 2014, 16, 495–501. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Chiong, M.; Lavandero, S.; Klionsky, D.J.; Ren, J. Mitophagy in cardiovascular diseases: molecular mechanisms, pathogenesis, and treatment. Trends in molecular medicine 2022, 28, 836–849. [Google Scholar] [CrossRef]
- De Meyer, G.R.Y.; De Keulenaer, G.W.; Martinet, W. Role of autophagy in heart failure associated with aging. Heart Failure Reviews 2010, 15, 423–430. [Google Scholar] [CrossRef]
- Nalbandian, A.; Llewellyn, K.J.; Nguyen, C.; Yazdi, P.G.; Kimonis, V.E. Rapamycin and chloroquine: the in vitro and in vivo effects of autophagy-modifying drugs show promising results in valosin containing protein multisystem proteinopathy. PloS one 2015, 10, e0122888. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Li, M.; Zhang, B.; Shen, P.; Liu, P.; Zheng, D.; Chen, Y.; Jiang, J. Autophagy activation attenuates angiotensin II-induced cardiac fibrosis. Archives of biochemistry and biophysics 2016, 590, 37–47. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, D.; Zheng, J.; Wu, X.; Wang, J.; Liu, X.; He, Y.; Zhang, C.; Liu, C.; Wang, T.; et al. Chikusetsu saponin IVa attenuates isoprenaline-induced myocardial fibrosis in mice through activation autophagy mediated by AMPK/mTOR/ULK1 signaling. Phytomedicine 2019, 58, 152764. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Yang, X. Functions of Autophagy in Pathological Cardiac Hypertrophy. International Journal of Biological Sciences 2015, 11, 672–678. [Google Scholar] [CrossRef]
- Sehrawat, A.; Mishra, J.; Mastana, S.S.; Navik, U.; Bhatti, G.K.; Reddy, P.H.; Bhatti, J.S. Dysregulated autophagy: A key player in the pathophysiology of type 2 diabetes and its complications. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2023, 1869, 166666. [Google Scholar] [CrossRef]
- He, H.; Liu, X.; Lv, L.; Liang, H.; Leng, B.; Zhao, D.; Zhang, Y.; Du, Z.; Chen, X.; Li, S.; et al. Calcineurin suppresses AMPK-dependent cytoprotective autophagy in cardiomyocytes under oxidative stress. Cell death & disease 2014, 5, e997. [Google Scholar] [CrossRef]
- Shaikh, S.; Troncoso, R.; Criollo, A.; Bravo-Sagua, R.; García, L.; Morselli, E.; Cifuentes, M.; Quest, A.F.; Hill, J.A.; Lavandero, S. Regulation of cardiomyocyte autophagy by calcium. American journal of physiology. Endocrinology and metabolism 2016, 310, E587–E596. [Google Scholar] [CrossRef]
- Sugden, P.; Fuller, S.; Weiss, S.; Clerk, A. Glycogen synthase kinase 3 (GSK3) in the heart: A point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. British journal of pharmacology 2008, 153 Suppl 1, S137–S153. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.; Jang, S.; Agostini, B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: therapeutic perspectives. Pharmacol Ther 2014, 144, 202–225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhou, X.; Yang, T.; Wang, L.; Feng, L.; Wang, Z.; Xu, J.; Jing, W.; Wang, T.; Su, H.; et al. The role of autophagy in cardiovascular disease: Cross-interference of signaling pathways and underlying therapeutic targets. Frontiers in Cardiovascular Medicine 2023, Volume 10 - 2023. [Google Scholar] [CrossRef]
- Ljubojević-Holzer, S.; Kraler, S.; Djalinac, N.; Abdellatif, M.; Voglhuber, J.; Schipke, J.; Schmidt, M.; Kling, K.M.; Franke, G.T.; Herbst, V.; et al. Loss of autophagy protein ATG5 impairs cardiac capacity in mice and humans through diminishing mitochondrial abundance and disrupting Ca2+ cycling. Cardiovasc Res 2022, 118, 1492–1505. [Google Scholar] [CrossRef]
- Mongirdienė, A.; Skrodenis, L.; Varoneckaitė, L.; Mierkytė, G.; Gerulis, J. Reactive Oxygen Species Induced Pathways in Heart Failure Pathogenesis and Potential Therapeutic Strategies. Biomedicines 2022, 10, 602. [Google Scholar] [CrossRef]
- Peng, Y.; Liao, B.; Zhou, Y.; Zeng, W. Ginsenoside Rb2 improves heart failure by down-regulating miR-216a-5p to promote autophagy and inhibit apoptosis and oxidative stress. J Appl Biomed 2023, 21, 180–192. [Google Scholar] [CrossRef]
- Denton, D.; Xu, T.; Kumar, S. Autophagy as a pro-death pathway. Immunol Cell Biol 2015, 93, 35–42. [Google Scholar] [CrossRef]
- Huang, D.; Wen, Q.; Su, Y.; Li, X. miR-17-5p Inhibits BNIP3-Mediated Mitochondrial Autophagy to Attenuate Pathological Cardiac Fibrosis. Balkan Med J 2025. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 2012, 3, 1078. [Google Scholar] [CrossRef]
- Chaanine, A.H.; Kohlbrenner, E.; Gamb, S.I.; Guenzel, A.J.; Klaus, K.; Fayyaz, A.U.; Nair, K.S.; Hajjar, R.J.; Redfield, M.M. FOXO3a regulates BNIP3 and modulates mitochondrial calcium, dynamics, and function in cardiac stress. Am J Physiol Heart Circ Physiol 2016, 311, H1540–H1559. [Google Scholar] [CrossRef]
- Dai, D.F.; Rabinovitch, P. Mitochondrial oxidative stress mediates induction of autophagy and hypertrophy in angiotensin-II treated mouse hearts. Autophagy 2011, 7, 917–918. [Google Scholar] [CrossRef]
- Mann, D.L. Basic mechanisms of left ventricular remodeling: the contribution of wall stress. Journal of Cardiac Failure 2004, 10, S202–S206. [Google Scholar] [CrossRef]
- Nishida, K.; Otsu, K. Autophagy during cardiac remodeling. Journal of Molecular and Cellular Cardiology 2016, 95, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Z.; Li, Q.; Trammell, S.A.; Schmidt, M.S.; Pires, K.M.; Cai, J.; Zhang, Y.; Kenny, H.; Boudina, S.; et al. Control of NAD<sup>+</sup> homeostasis by autophagic flux modulates mitochondrial and cardiac function. The EMBO journal 2024, 43, 362–390. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Tam, E.; Song, E.; Xu, A.; Sweeney, G. Crosstalk between myocardial autophagy and sterile inflammation in the development of heart failure. Autophagy Reports 2024, 3, 2320605. [Google Scholar] [CrossRef]
- Chiu, B.; Jantuan, E.; Shen, F.; Chiu, B.; Sergi, C. Autophagy-Inflammasome Interplay in Heart Failure: A Systematic Review on Basics, Pathways, and Therapeutic Perspectives. Ann Clin Lab Sci 2017, 47, 243–252. [Google Scholar] [PubMed]
- Pavillard, L.E.; Cañadas-Lozano, D.; Alcocer-Gómez, E.; Marín-Aguilar, F.; Pereira, S.; Robertson, A.A.B.; Muntané, J.; Ryffel, B.; Cooper, M.A.; Quiles, J.L.; et al. NLRP3-inflammasome inhibition prevents high fat and high sugar diets-induced heart damage through autophagy induction. Oncotarget 2017, 8, 99740–99756. [Google Scholar] [CrossRef]
- Tang, L.Q.; Wang, L.L.; Tang, Q.F.; Wang, W. SLC26A4 regulates autophagy and activates the NLRP3 inflammasome to mediate pathological cardiac hypertrophy. Scientific reports 2025, 15, 12511. [Google Scholar] [CrossRef]
- Tang, L.; Yu, X.; Zheng, Y.; Zhou, N. Inhibiting SLC26A4 reverses cardiac hypertrophy in H9C2 cells and in rats. PeerJ 2020, 8, e8253. [Google Scholar] [CrossRef]
- Oka, T.; Hikoso, S.; Yamaguchi, O.; Taneike, M.; Takeda, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; Nakayama, H.; Nishida, K.; et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 2012, 485, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail 2021, 8, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Izumiya, Y.; Rokutanda, T.; Ianni, A.; Hanatani, S.; Kimura, Y.; Onoue, Y.; Senokuchi, T.; Yoshizawa, T.; Yasuda, O.; et al. Sirt7 Contributes to Myocardial Tissue Repair by Maintaining Transforming Growth Factor-β Signaling Pathway. Circulation 2015, 132, 1081–1093. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischemia/reperfusion: Translational pathophysiology of ischemic heart disease. Med 2024, 5, 10–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Sala-Mercado, J.A.; Wider, J.; Undyala, V.V.; Jahania, S.; Yoo, W.; Mentzer, R.M., Jr.; Gottlieb, R.A.; Przyklenk, K. Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation 2010, 122, S179–S184. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, C.; Wang, W.; Li, M.; Ma, C.; Gao, B. SIRT1 is a regulator of autophagy: Implications for the progression and treatment of myocardial ischemia-reperfusion. Pharmacological Research 2024, 199, 106957. [Google Scholar] [CrossRef]
- Luo, G.; Jian, Z.; Zhu, Y.; Zhu, Y.; Chen, B.; Ma, R.; Tang, F.; Xiao, Y. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. International journal of molecular medicine 2019, 43, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E. Hypoxic Stress-Dependent Regulation of Na,K-ATPase in Ischemic Heart Disease. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef]
- Hariharan, N.; Maejima, Y.; Nakae, J.; Paik, J.; Depinho, R.A.; Sadoshima, J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res 2010, 107, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martinez, O.; de Leon-Oliva, D.; Boaru, D.L.; Lopez-Gonzalez, L.; García-Montero, C.; Alvarez-Mon, M.A.; Guijarro, L.G.; Torres-Carranza, D.; Saez, M.A.; et al. Autophagy in Its (Proper) Context: Molecular Basis, Biological Relevance, Pharmacological Modulation, and Lifestyle Medicine. Int J Biol Sci 2024, 20, 2532–2554. [Google Scholar] [CrossRef]
- Gan, T.; Qu, S.; Zhang, H.; Zhou, X.J. Modulation of the immunity and inflammation by autophagy. MedComm (2020) 2023, 4, e311. [Google Scholar] [CrossRef]
- Yapryntseva, M.A.; Maximchik, P.V.; Zhivotovsky, B.; Gogvadze, V. Mitochondrial sirtuin 3 and various cell death modalities. Frontiers in cell and developmental biology 2022, 10, 947357. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxidants & redox signaling 2018, 28, 711–732. [Google Scholar] [CrossRef]
| Sirtuin | Subject | Effect | Mediators/mechanism | Reference |
|---|---|---|---|---|
| SIRT1a | HFpEF patients | Reduced systolic blood pressure, increased EF | Increased levels of SIRT1 and NAD+ in caloric restriction and exercise program; increased antioxidant capacity | [86,87] |
| Mouse model of HFpEF | Protection against harmful effects related to heart remodeling | Regulation of lipid metabolism and inflammation | [88] | |
| SIRT2 | SIRT2-/- mice and mice lacking SIRT1 in cardiomyocytes | Improved cardiac function after I/R and PO, reduced cardiac hypertrophy | Maladaptive effects in response to stress, decrease in antioxidant protection due to NRF2 inhibition | [90] |
| SIRT2-/- mice | Cardiac hypertrophy and fibrosis, reduced EF and fractional shortening | SIRT2-mediated AMPK activity through deacetylating LKB1 | [91] | |
| Mice with overexpressed SIRT2 | Protection against hypertrophy and fibrosis caused by age and ANGII | |||
| Patients after acute myocardial infarction | Positive correlation between SIRT2 and HF | [92] | ||
| SIRT2-/- mice | Heart hypertrophy, remodeling, fibrosis, and age-related dysfunction | SIRT2 interacted with and deacetylated the NFATc2 transcription factor | [93] | |
| SIRT3 | Mouse model of HFpEF | Fibrosis | hyperacetylation of mitochondrial proteins, resulting in enhanced production of interleukins IL1B and IL18 and increased assembly of NLRP3 | [94] |
| Mouse model of HFpEF | Impairment in mitochondrial fatty acid oxidation | Hyperacetylation of key enzymes of fatty acid oxidation, SIRT3 downregulation, NAD+ deficiency | [95] | |
| Mouse model of HFpEF | Improved glucose uptake and metabolism | Activation of the skeletal muscle SIRT3-5’-AMPK pathway | [96] | |
| Mouse model of HFpEF with SIRT3 deficiency | Pulmonary vascular remodeling | Increased secretion of lysyl oxidase homolog 2 and β2-microglobulin | [96] | |
| HFpEF patients | Heart remodeling | Reduction of indole-3-propionic acid, activating NNMT-SIRT3 axis | [97] | |
| SIRT4 | Mice with ANGII infusion | Progression from compensated to decompensated cardiac hypertrophy | SIRT4 overexpression | [100] |
| Mice with heart-specific SIRT4 overexpression | sped up heart failure development in response to pressure overload | Mitochondrial RONS-mediated increase in profibrotic transcriptional signaling | [101] | |
| SIRT5 | Mice with SIRT5 overexpression | Protection against TAC consequences | Suppression of metabolic switch from fatty acid oxidation to glycolysis, immune activation, and fibrotic signaling pathways | [102] |
| Mouse cardiomyocytes | Reduction in the cell viability, and an increase in the number of apoptotic cells and the caspase 3/7 activity | Direct interaction between B2CL1 and SIRT5 | [103] | |
| mouse model of cardiac IR injury | reduced mitochondrial damage and alleviated cardiac injury | Increasing SIRT5 levels reduced mitochondrial damage and alleviated cardiac injury through interaction with ANT2, inhibiting its lactylation and enhancing its interaction with VDAC1 | [104] | |
| SIRT6 | Mouse model of HFpEF | HF mitigation | restoring endothelial SIRT6 function and it was underlined by the deacetylation of histone H3K9 around the PPARG promoter | [105] |
| Diabetic HF patients | Decreased level of SIRT6 | [106] | ||
| Diabetic mouse model of HFpEF | Improvements in diastolic dysfunction and decreased cardiac lipid buildup | Suppression of endothelial PPARγ expression via SIRT6-dependent deacetylation of histone H3 near the PPARγ gene promoter. | [106] | |
| TAC mice | Mitigated TAC-induced heart dysfunction and decreased cardiac inflammation, resulting in reduced cardiac fibrosis and smaller infarcts | Overexpression of SIRT6 elevated TERT and TRF1 levels | [107] | |
| SIRT7 | TAC mice | Increase in heart weight relative to tibial length, and they demonstrated a reduced cardiac contractile function | Interaction between SIRT7 and the transcription factor GATA-4 was identified, and GATA4 knockdown lessened the severity of phenylephrine-induced cardiac hypertrophy. SIRT7 deacetylated GATA4 in cardiomyocytes, influencing its transcriptional activity. | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





