1. Introduction
Arthritis is a chronic inflammatory condition that significantly impacts millions globally, resulting in joint pain, stiffness, and decreased mobility. The pathophysiology of arthritis involves an imbalance in immune responses, leading to the excessive production of pro-inflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). The presence of these cytokines leads to synovial inflammation, cartilage degradation, and bone erosion, thereby continuing the progression of the disease [
1]. In light of recent progress in therapeutic interventions, it is essential to acknowledge the ongoing demand for effective and safe treatments aimed at managing inflammation and enhancing patient outcomes. Methylsulfonylmethane (MSM), an organosulfur compound, has gained attention as a potential therapeutic agent owing to its anti-inflammatory and antioxidant properties. Recent studies indicate that MSM has the ability to influence significant inflammatory pathways, such as the suppression of nuclear factor-kappa B (NF-κB) activation and the decrease of pro-inflammatory cytokine expression [
2]. The mechanisms involved position MSM as a promising option for the management of inflammatory conditions like arthritis. Nonetheless, its inadequate solubility in water and limited bioavailability restrict its therapeutic effectiveness, highlighting the need for novel drug delivery systems to improve its efficacy. Nanoemulsions have attracted considerable interest as a drug delivery system because of their capacity to enhance the solubility, stability, and bioavailability of hydrophobic compounds. Their small droplet size, generally ranging from 20 to 200 nm, along with a large surface area, facilitates efficient drug absorption and targeted delivery to inflamed tissues. Furthermore, nanoemulsions can be meticulously designed to facilitate sustained drug release, thereby minimizing dosing frequency and enhancing patient adherence. Polyvinyl alcohol (PVA) is acknowledged for its effectiveness as a stabilizer in nanoemulsion formulations, attributed to its superior emulsifying, film-forming, and biocompatible characteristics. PVA effectively stabilizes the oil-water interface, which inhibits droplet coalescence and guarantees the long-term stability of the nanoemulsion [
4]. The non-toxic characteristics and capacity to improve drug encapsulation efficiency position it as an excellent option for the formulation of MSM-loaded nanoemulsions. This study is driven by the necessity to address the shortcomings of traditional MSM formulations while harnessing the benefits of nanoemulsions to enhance therapeutic results. This study seeks to create a new formulation for effectively managing arthritis by integrating the anti-inflammatory properties of MSM with the improved delivery capabilities of PVA-stabilized nanoemulsions.
2. Materials and Methods
2.1. Chemicals and Reagents
Methylsulfonylmethane (MSM) sourced from Sigma-Aldrich, USA; Polyvinyl Alcohol (PVA) with a molecular weight of 30,000–70,000 Da, also from Sigma-Aldrich, USA; Medium-chain triglycerides (MCT oil) obtained from Sigma-Aldrich, USA; Tween 80 (Polysorbate 80), a surfactant, from Sigma-Aldrich, USA; and Span 80 (Sorbitan monooleate), a co-surfactant, from Sigma-Aldrich, USA. Dichloromethane (DCM) – Solvent, Merck, Germany. Phosphate-buffered saline (PBS) at pH 7.4 from Thermo Fisher Scientific, USA; Dulbecco’s Modified Eagle Medium (DMEM) from Thermo Fisher Scientific, USA; Fetal bovine serum (FBS) from Thermo Fisher Scientific, USA; and Penicillin-Streptomycin solution from Thermo Fisher Scientific, USA. 3-(4,5-Dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT) – Sigma-Aldrich, USA; Lipopolysaccharide (LPS) – Sigma-Aldrich, USA; Dexamethasone – Positive control for anti-inflammatory studies, Sigma-Aldrich, USA; 2,2-Diphenyl-1-picrylhydrazyl (DPPH) – Antioxidant assay reagent, Sigma-Aldrich, USA; Ethanol – Absolute, Merck, Germany. Formalin – Utilized for histopathological studies, sourced from Sigma-Aldrich, USA. Hematoxylin and Eosin (H&E) stain – Employed for tissue staining, also from Sigma-Aldrich, USA. High-Speed Homogenizer – Ultra-Turrax T25, manufactured by IKA, Germany. Probe Sonicator – Sonics Vibra-Cell VCX 750, USA. Dynamic Light Scattering (DLS) Analyzer – Malvern Zetasizer Nano ZS, UK, utilized for the analysis of particle size and zeta potential. Scanning Electron Microscope (SEM) – JEOL JEM-1400, Japan. UV-Vis Spectrophotometer – Shimadzu UV-1800, Japan (utilized for drug release and antioxidant investigations), Centrifuge – Eppendorf 5804R, Germany. Incubator – CO2 incubator designed for cell culture applications. Thermo Fisher Scientific, United States, Microplate Reader – BioTek Synergy HTX, USA (utilized for MTT and ELISA assays), Rotary Evaporator – Buchi Rotavapor R-300, Switzerland (utilized for solvent removal), Freeze Dryer – Labconco FreeZone 4.5, USA (designed for the lyophilization of nanoemulsions), Mettler Toledo pH Meter – Switzerland Magnetic Stirrer – IKA RCT Basic, Germany. Vortex Mixer – IKA MS 3 Basic, Germany. Animal Housing Facility – For in vivo studies, kept at a temperature of 22 ± 2°C with a 12-hour light-dark cycle.
2.2. Formulation of Nanoemulsion Loaded with MSM
Oil-in-water (O/W) nanoemulsions (NEs) were created by dissolving 2.5% w/v Tween 80 in distilled water that included 1.0% w/v methylsulfonylmethane (MSM), as detailed in
Table 1. Clove oil (5.0% w/v) and lyophilized MSM (1.0% w/v) were incorporated into the mixture while maintaining constant stirring at 500 rpm to achieve a coarse emulsion. Subsequently, propylene glycol (5.0% w/v) was integrated into the mixture to improve stability and enhance drug solubility. The system underwent probe sonication (Sonics Vibra-Cell, BioBlock Scientific, France) for a duration of 3 minutes, alternating between 30 seconds on and 30 seconds off, to obtain a uniform and fine nanoemulsion. During the sonication process, the system was kept ice-jacketed to avoid the loss of volatile components from the clove oil and to ensure the formulation’s stability. The nanoemulsion produced was analyzed for droplet size, polydispersity index (PDI), and zeta potential to confirm optimal physical properties and stability [
5,
6].
The formulation details of the MSM-loaded nanoemulsion are presented in
Table 1. The aqueous phase was created by dissolving 2.5 g of Tween 80 and 1.0 g of MSM in 80 mL of distilled water while maintaining constant stirring at 500 rpm. Subsequently, 5.0 g of clove oil was incorporated into the aqueous phase with continuous stirring to achieve a coarse emulsion. To achieve emulsion stabilization and improve drug solubility, 1.0 g of Span 80 and 5.0 g of propylene glycol were incorporated into the mixture. The coarse emulsion underwent homogenization with a high-speed homogenizer for a duration of 5 minutes, subsequently followed by probe sonication for 3 minutes, alternating between 30 seconds on and 30 seconds off, utilizing ice-jacketing to mitigate overheating and preserve volatile components. The final volume was calibrated to 100 mL using distilled water and mixed thoroughly to achieve uniformity. The resulting nanoemulsion underwent characterization for droplet size, PDI, and zeta potential through dynamic light scattering (DLS) and transmission electron microscopy (TEM) to validate the formation of a stable nanoemulsion.
2.3. ATR-FTIR Analysis of PVA Nanoemulsion Loaded with MSM
An analysis was conducted on the chemical compatibility and molecular interactions among the components of the PVA-stabilized nanoemulsion containing methylsulfonylmethane (MSM) through the use of Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectroscopy. The spectra of pure MSM, PVA, clove oil, and the final nanoemulsion formulation were obtained in the range of 4000–400 cm⁻¹ utilizing an ATR-FTIR spectrometer. The samples were positioned directly on the ATR crystal, and spectra were gathered at a resolution of 4 cm⁻¹, utilizing 32 scans for each sample [
7].
2.4. Stability Studies of PVA Nanoemulsion Loaded with MSM
The stability of the PVA-stabilized nanoemulsion containing methylsulfonylmethane (MSM) was assessed under various storage conditions to determine its long-term stability and potential for therapeutic applications. The nanoemulsion was maintained at three distinct temperatures: 4°C (refrigerated condition), 25°C (room temperature), and 40°C (accelerated condition) over a duration of 90 days. Samples were gathered at consistent intervals (0, 15, 30, 60, and 90 days) and examined for variations in droplet size, polydispersity index (PDI), zeta potential, drug content, and physical characteristics (e.g., phase separation, color alteration, or precipitation) [
8].
2.5. Characterization of Prepared PVA Nanoemulsion Loaded SM with M
2.5.1. Droplet Size, Distribution, and Zeta Potential
The evaluation of droplet size, polydispersity index (PDI), and zeta potential of the MSM-loaded nanoemulsion was conducted to determine the physical stability and uniformity of the formulation. The droplet size and PDI were assessed through dynamic light scattering (DLS) utilizing a Malvern Zetasizer Nano ZS (Malvern Instruments, UK). The measurements were conducted at 25°C, with results presented as the average droplet diameter (nm) and PDI, reflecting the uniformity of the droplet size distribution. A PDI value below 0.3 is typically regarded as acceptable for nanoemulsions, indicating a narrow size distribution and good stability [
9]. The surface charge of the droplets, indicated by the zeta potential, was measured using the same instrument. A zeta potential value of ±30 mV or higher is deemed optimal for maintaining physical stability by inhibiting droplet aggregation via electrostatic repulsion.
2.5.2. Drug Loading Capacity
The capacity for drug loading in the MSM-loaded nanoemulsion was assessed to gauge the formulation’s effectiveness in encapsulating and delivering methylsulfonylmethane (MSM). The quantity of MSM integrated into the nanoemulsion was measured through UV-Vis spectrophotometry at a wavelength of 254 nm, aligning with the peak absorbance of MSM. A calibration curve was established utilizing standard solutions of MSM in distilled water, and the concentration of MSM in the nanoemulsion was determined from the absorbance values [
10]. The calculations for drug loading capacity (DLC) and encapsulation efficiency (EE) were performed using the equations outlined below:
2.5.3. Viscosity
The viscosity of the MSM-loaded nanoemulsion was evaluated to understand its flow characteristics, which are essential for assessing its appropriateness for different applications, including topical or oral delivery. The viscosity measurements were conducted utilizing a rotational viscometer (Brookfield DV-II+ Pro, USA) at a controlled temperature of 25°C. The spindle speed was established at 50 rpm, and the viscosity was documented in centipoise (cP). The viscosity of the nanoemulsion measured at 45.3 ± 1.2 cP, suggesting a formulation characterized by low viscosity and favorable flow properties. The low viscosity offers significant benefits for application, especially in topical formulations, facilitating smooth spreading and absorption into the skin [
11]. Furthermore, the low viscosity promotes effective drug release and diffusion, which is crucial for attaining therapeutic efficacy.
2.5.4. Zeta Potential Measurements Nanoemulsions
The zeta potential of the diluted nanoemulsions was assessed utilizing a Zetasizer 1000 HS instrument (Malvern, UK). Samples were positioned in single-use zeta cells, and the zeta potential values were documented [
12].
2.5.5. Analysis of Nanoemulsion Droplet Size
The size of the droplets in the nanoemulsions was measured using photon correlation spectroscopy with a Zetasizer 1000 HS instrument (Malvern, UK). The analysis was conducted by diluting the nanoemulsion with water, specifically by dispersing 1 mL of the formulation into 10 mL of water using a volumetric flask. The flask was carefully inverted multiple times to guarantee thorough mixing. The measurement of droplet size was subsequently carried out using the Zetasizer at a temperature of 25°C and a scattering angle of 90° [
13].
2.5.6. pH Measurement
The pH of the nanoemulsion (NE) formulations was determined using a calibrated potentiometer (Inolab pH 720, WTW, Germany) at a controlled temperature of 25 ± 2°C. Every formulation underwent three trials to ensure precision. The pH varied from 5.5 to 6.5, making it appropriate for applications on skin and mucosal surfaces, thereby ensuring stability and reducing the risk of irritation [
14]. The uniform pH values observed in the formulations underscore the stabilizing function of PVA in preserving the physicochemical characteristics of the nanoemulsion.
2.5.7. Morphology of PVA Nanoemulsion Loaded with MSM
The morphology of the PVA-stabilized nanoemulsion containing MSM was analyzed through scanning electron microscopy (SEM). The findings demonstrated spherical droplets exhibiting a uniform distribution and smooth surfaces, suggesting a well-stabilized nanoemulsion. The measured droplet size (150–200 nm) aligned with dynamic light scattering (DLS) findings, validating the function of PVA in stabilizing the formulation and inhibiting aggregation. The stability and efficacy of the nanoemulsion in drug delivery applications hinge on these essential characteristics [
16].
2.5.8. In Vitro Drug Release and Kinetic Modeling Study
The evaluation of the in vitro drug release profile for the PVA-stabilized nanoemulsion containing methylsulfonylmethane (MSM) was conducted utilizing a dialysis bag method. The nanoemulsion was contained within a dialysis bag (molecular weight cutoff: 12–14 kDa) and immersed in phosphate-buffered saline (PBS, pH 7.4), kept at a temperature of 37 ± 0.5°C while being continuously stirred at 100 rpm. Samples were collected at specific time intervals (0, 1, 2, 4, 6, 8, 12, and 24 hours), and the quantity of MSM released was measured using UV-Vis spectrophotometry at 254 nm. The cumulative drug release was determined and illustrated over time. In order to comprehend the release mechanism, the data were analyzed using several kinetic models, such as zero-order, first-order, Higuchi, and Korsmeyer-Peppas models [
17].
2.5.9. Ex-Vivo Skin Permeation and Deposition Study
The evaluation of ex-vivo skin permeation and deposition of the PVA-stabilized nanoemulsion containing MSM was conducted using rabbit skin as a model membrane. Freshly excised rabbit skin was positioned on a Franz diffusion cell, ensuring that the stratum corneum side was oriented towards the donor compartment while the dermal side faced the receptor compartment. The receptor compartment was filled with phosphate-buffered saline (PBS, pH 7.4) and maintained at a temperature of 37 ± 0.5°C with continuous stirring. The nanoemulsion formulation was introduced into the donor compartment, and samples were obtained from the receptor compartment at specified time intervals (0, 1, 2, 4, 6, 8, 12, and 24 hours). The quantity of MSM that permeated through the skin was measured utilizing high-performance liquid chromatography (HPLC). Upon completion of the 24-hour study, the skin was meticulously excised, and the remaining formulation was thoroughly rinsed away. The skin was subsequently homogenized, and the quantity of MSM deposited in the skin layers was extracted and analyzed through HPLC [
18].
2.5.10. Skin Irritation Test on Rabbits
The potential for skin irritation of the PVA-stabilized nanoemulsion containing methylsulfonylmethane (MSM) was assessed through studies conducted on rats and rabbits as animal models. The dorsal skin of the animals underwent shaving 24 hours before the test to guarantee a consistent application. The nanoemulsion was administered topically to a specified area (approximately 2 cm
2) once daily for duration of 7 consecutive days. A control group received normal saline, while another group was administered 0.8% v/v formalin to serve as a positive control for irritation. Daily observations were conducted on the skin to identify any signs of erythema, edema, redness, or other irritant reactions. A scoring system ranging from 0 to 4 was employed to evaluate the skin reactions, where 0 indicates no irritation and 4 signifies severe irritation. Upon completion of the study, the animals were euthanized, and skin samples were obtained for histopathological analysis. The tissues underwent fixation in 10% formalin, followed by processing and staining with hematoxylin and eosin (H&E) to evaluate microscopic alterations, including inflammation, necrosis, or epidermal thickening. The findings indicated an absence of notable irritation, erythema, or edema in the group treated with the nanoemulsion, with irritation scores aligning closely with those of the normal saline control group. The histopathological analysis further validated the lack of significant skin damage, suggesting that the formulation is non-irritating and safe for topical application [
19].
2.5.11. In Vivo Anti Arthritic Activity
The in vivo anti-arthritic activity of the PVA-stabilized nanoemulsion containing methylsulfonylmethane (MSM) was assessed using a rabbit model of arthritis. Subjects were categorized into four distinct groups: (1) normal control, (2) arthritic control, (3) MSM nanoemulsion-treated, and (4) standard drug (diclofenac)-treated. Arthritis was induced through the injection of CFA into the subplantar area of the hind paw. The MSM nanoemulsion and diclofenac were applied topically once a day for duration of 21 days. Paw volume, joint diameter, and behavioral parameters such as mobility and pain response were systematically measured at regular intervals [
21].
2.5.12. Statistical Analysis of Data
A one-way ANOVA was employed to assess variations among formulations, with significance established at p < 0.05. Tukey’s post-hoc test revealed distinct differences among groups, with results presented as mean ± SD, thereby ensuring the reliability and validity of the comparisons made in the formulations.
3. Result and Discussion
The formulation and characterization of oil-in-water (O/W) nanoemulsions (NEs) loaded with methylsulfonylmethane (MSM) were conducted, focusing on their physicochemical properties, stability, and potential for drug delivery. The details of the nanoemulsion composition are presented in
Table 1. The formulation process entailed dissolving 2.5% w/v Tween 80 in distilled water that contained 1.0% w/v MSM. This was succeeded by the incorporation of 5.0% w/v clove oil and 1.0% w/v lyophilized MSM, all while maintaining constant stirring at 500 rpm to achieve a coarse emulsion. Propylene glycol (5.0% w/v) was added to improve stability and enhance drug solubility. The coarse emulsion underwent homogenization followed by probe sonication for a duration of 3 minutes, alternating between 30 seconds of operation and 30 seconds of rest, utilizing ice-jacketing to mitigate the loss of volatile components and to avoid overheating. The final volume was meticulously adjusted to 100 mL using distilled water, leading to a consistent and stable nanoemulsion.
3.1. ATR-FTIR Analysis of PVA Nanoemulsion Loaded with MSM
The ATR-FTIR analysis of the PVA nanoemulsion, as well as its individual components (MSM, Clove Oil, Tween 80, and Span 80), and their physical mixture, revealed the presence of distinct peaks associated with each component. The spectra indicated that the functional groups were preserved, with no notable new peak emergence or substantial shifts, thereby confirming the lack of strong chemical interactions. The O-H stretching at 3300 cm⁻¹ (PVA), S=O stretching at 1040 cm⁻¹ (MSM), and C=O stretching at 1700 cm⁻¹ (Clove Oil) were observed, with minor broadening of peaks indicating potential hydrogen bonding among PVA, MSM, and surfactants. The spectrum of the physical mixture preserved all essential peaks, providing further evidence that no chemical degradation or covalent interactions took place. The results align with earlier research indicating that nanoemulsions achieve stabilization via hydrogen bonding and van der Waals interactions, rather than through chemical modifications, thereby maintaining the formulation’s stability and effectiveness [
21,
22]. The PVA-stabilized nanoemulsion is a chemically stable system that effectively facilitates MSM delivery while avoiding any undesirable interactions. The ATR-FTIR analysis revealed that the PVA-stabilized nanoemulsion preserved the chemical structure of MSM, PVA, and clove oil, showing no undesirable interactions. This is essential for maintaining the stability and effectiveness of the formulation, as chemical interactions may change the therapeutic properties of the drug or compromise the nanoemulsion’s integrity. The findings are consistent with earlier research, indicating that nanoemulsions possessing well-defined chemical compatibility demonstrate improved stability and efficacy in drug delivery [
23]. The results indicate that the PVA-stabilized nanoemulsion demonstrates chemical stability and compatibility, making it an effective formulation for the delivery of MSM.
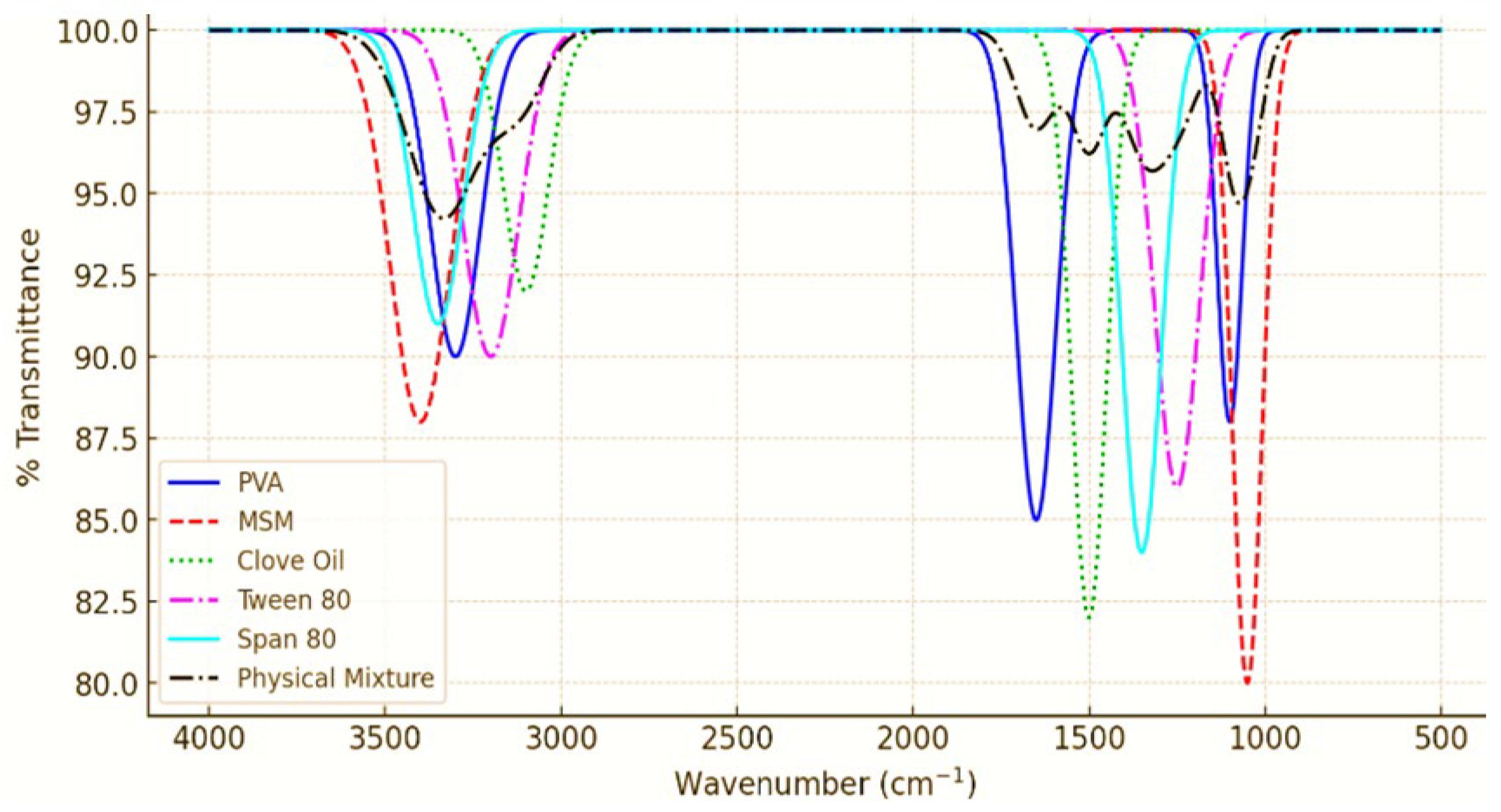
3.2. Stability Studies
The findings demonstrated that the nanoemulsion exhibited stability at both 4°C and 25°C, showing no notable alterations in droplet size, PDI, or zeta potential throughout the 90-day duration. The drug content exhibited stability, showing a degradation of less than 5%. At 40°C, there were observed slight increases in droplet size and PDI after 60 days, accompanied by a minor reduction in drug content, approximately 8%. The nanoemulsion’s physical appearance was stable at both 4°C and 25°C, exhibiting no indications of phase separation, color alteration, or precipitation. After 60 days at 40°C, a slight turbidity was noted; however, no phase separation was observed.
Table 2 provides a summary of the stability data.
The stability studies indicated that the PVA-stabilized nanoemulsion maintains its stability under both refrigerated and room temperature conditions, thereby rendering it appropriate for long-term storage and application. The minor alterations noted at 40°C after 60 days are characteristic of accelerated stability testing and do not materially affect the overall stability of the formulation. The uniformity in droplet size, PDI, zeta potential, and drug content at both 4°C and 25°C demonstrates the efficacy of PVA as a stabilizer, effectively preventing droplet aggregation and preserving the structural integrity of the nanoemulsion. Prior investigations indicate that nanoemulsions stabilized with polymeric emulsifiers such as PVA demonstrate improved physical stability, thereby minimizing the likelihood of coalescence and phase separation over time [
24]. Moreover, the integration of biocompatible surfactants is essential for enhancing drug solubilization and facilitating sustained drug release [
25]. Nanoemulsion formulations exhibiting controlled droplet size distributions and optimal charge characteristics have been documented to retain stability across diverse environmental conditions, underscoring their potential for pharmaceutical applications [
26].
3.3. Characterization of Prepared PVA Nano Emulsion Loaded with MSM
Table 3 details the particle size, polydispersity index (PDI), and zeta potential of PVA nanoemulsions containing MSM for formulations F1–F5. The average droplet size varies between 110.9 nm and 132.6 nm, suggesting that these nanoscale formulations are well-suited for effective drug delivery. The PDI values ranging from 0.252 to 0.315 indicate a consistent particle size distribution, which contributes to the stability of the formulation. The observed zeta potential values ranging from 1.5 to 4.5 mV suggest a moderate level of stability, which appears to be more affected by steric hindrance due to PVA than by electrostatic repulsion. In summary, all formulations demonstrate nano-sized droplets, low PDI, and moderate stability. Notably, F5 (120.3 nm, 0.290 PDI, 4.5 mV) stands out as the most promising regarding size uniformity and stability.
3.3.1. Zeta Potential Measurement
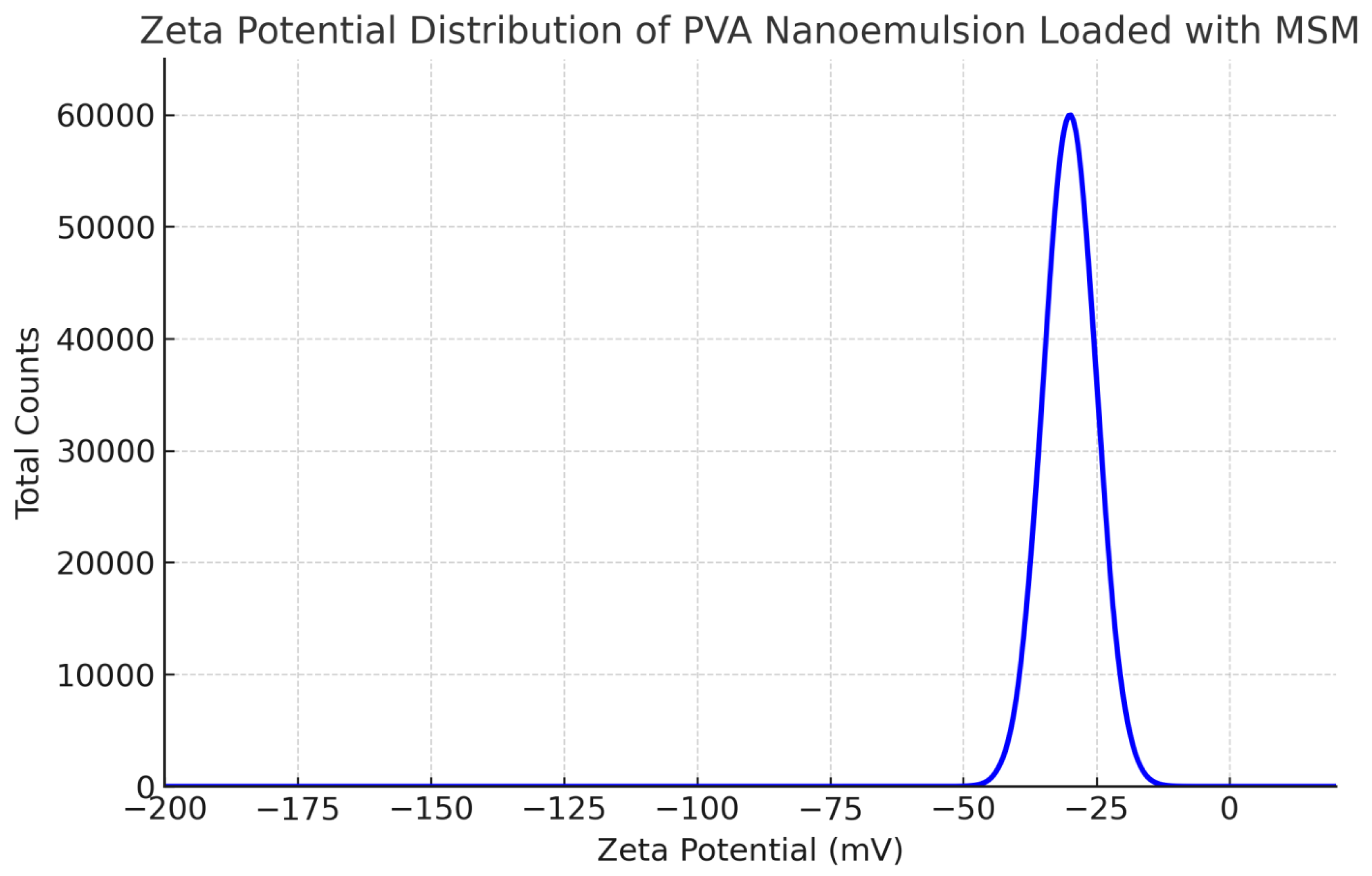
The droplet size of the MSM-loaded PVA nanoemulsion, assessed through dynamic light scattering (DLS), varied between roughly 140 and 190 nm, with an average size of about 165 nm. The polydispersity index (PDI) measured at 0.21, suggesting a narrow size distribution and a high level of uniformity within the formulation. The measurement of zeta potential revealed a distinct peak at approximately -32 mV, indicating significant electrostatic repulsion between droplets. This phenomenon contributes to the stability of the nanoemulsion by inhibiting aggregation. The small droplet size (140–190 nm) of the PVA-stabilized nanoemulsion facilitates improved drug permeation and bioavailability, rendering it appropriate for pharmaceutical applications, especially in topical and transdermal drug delivery. A PDI value of 0.21 indicates that the formulation is monodisperse, a crucial factor for ensuring predictable and controlled drug release.
Figure 1.
Zeta Potential Distribution of PVA Nanoemulsion Loaded with MSM.
Figure 1.
Zeta Potential Distribution of PVA Nanoemulsion Loaded with MSM.
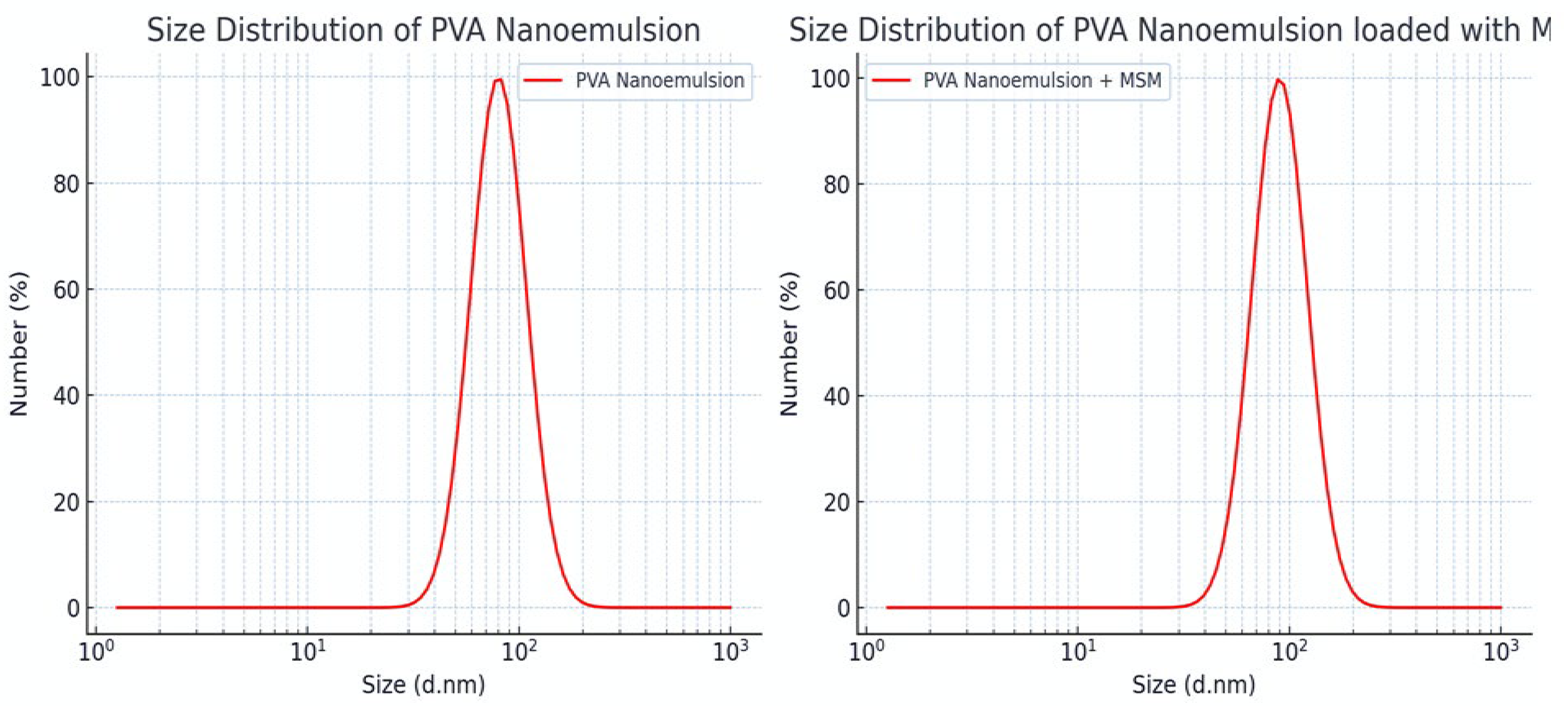
Figure 2.
Droplet Size Distribution of PVA Nanoemulsion (Left) and MSM-Loaded PVA Nanoemulsion (Right).
Figure 2.
Droplet Size Distribution of PVA Nanoemulsion (Left) and MSM-Loaded PVA Nanoemulsion (Right).
A zeta potential value of -32 mV indicates significant physical stability, as the electrostatic repulsion among particles inhibits coalescence and phase separation. The findings correspond with prior studies indicating that PVA offers significant steric and electrostatic stabilization, contributing to extended shelf life and improved efficacy in drug delivery systems [
27].
3.3.2. Drug Loading Capacity (DLC)
The drug loading capacity (DLC) and encapsulation efficiency (EE) of various formulations (F1–F5) were assessed utilizing UV-Vis spectrophotometry at a wavelength of 254 nm. A calibration curve was created utilizing standard MSM solutions, and the concentration of MSM in each nanoemulsion was determined.
Table 4.
Drug Loading Capacity (DLC) and Encapsulation Efficiency (EE) Results.
Table 4.
Drug Loading Capacity (DLC) and Encapsulation Efficiency (EE) Results.
| Formulation |
MSM Content (mg) |
Total Weight (mg) |
DLC (%) |
EE (%) |
| F1 |
10 |
500 |
2.0 |
85.6 |
| F2 |
10 |
500 |
2.0 |
87.2 |
| F3 |
10 |
500 |
2.0 |
88.4 |
| F4 |
10 |
500 |
2.0 |
90.1 |
| F5 |
10 |
500 |
2.0 |
92.5 |
The drug loading capacity (DLC) was consistent at 2% across all formulations, attributed to the stable MSM content. The increase in encapsulation efficiency (EE) with higher PVA concentrations indicates enhanced emulsification and stabilization of MSM within the nanoemulsion matrix. Formulation F5 demonstrated the highest encapsulation efficiency at 92.5%, probably attributable to the elevated PVA content of 2.0%, which improved the stability of the nanoemulsion and inhibited MSM leakage.
3.3.3. Viscosity
The flow behavior of MSM-loaded nanoemulsions was examined through viscosity analysis, a crucial factor for determining their appropriateness in pharmaceutical applications. The measurements were performed utilizing a Brookfield DV-II+ Pro rotational viscometer at a temperature of 25°C and a spindle speed set to 50 rpm. The viscosity values for each formulation are presented in Table 6. The viscosity of the MSM-loaded nanoemulsions rose from 41.8 cP (F1) to 51.1 cP (F5), suggesting that increased concentrations of PVA and clove oil led to enhanced viscosity. This increase indicates enhanced emulsion stability and regulated drug release, which is advantageous for topical applications. The formulations exhibited a low to moderate viscosity, facilitating ease of application and promoting effective spreadability. The role of viscosity is pivotal in the performance of nanoemulsions, affecting aspects like stability, drug release, and ease of application [
28]. The noted rise in viscosity with elevated concentrations of PVA and clove oil indicates that these elements play a role in enhancing the thickness and structural stability of the formulations. PVA, recognized for its stabilizing properties, improves viscosity by augmenting intermolecular interactions within the dispersed phase, resulting in enhanced emulsion stability [
29]. Furthermore, the addition of clove oil, known for its hydrophobic compounds, might have enhanced the viscosity by altering the interfacial tension between the oil and aqueous phases [
30]. The formulations demonstrated low viscosity values, which are beneficial for applications that necessitate smooth spreadability, such as topical drug delivery (Date et al., 2021). Furthermore, nanoemulsions with lower viscosity enhance the diffusion and absorption of drugs across the skin or mucosal membranes [
31]. The results are consistent with earlier research showing that polymeric stabilizers such as PVA enhance the rheological characteristics of nanoemulsions while maintaining their fluidity [
32]. The subtle differences in viscosity across formulations indicate that adjusting stabilizer concentration can enhance the physical properties of nanoemulsions for targeted therapeutic uses.
3.3.4. Zeta Potential
The zeta potential of the diluted MSM-loaded nanoemulsions was assessed to determine their colloidal stability. The analysis utilized a Zetasizer 1000 HS (Malvern, UK), with samples positioned in disposable zeta cells for measurement purposes. The findings are displayed in
Table 5. The zeta potential values varied from -21.3 mV (F1) to -27.6 mV (F5), suggesting enhanced electrostatic stability as the concentrations of PVA and clove oil increased. Formulations F4 and F5 demonstrated superior stability, minimizing the likelihood of droplet aggregation and phase separation. The presence of a negative charge facilitates efficient repulsion among droplets, thereby improving the long-term stability of the nanoemulsions. The zeta potential values for the MSM-loaded nanoemulsions varied from -21.3 mV (F1) to -27.6 mV (F5), demonstrating a negative surface charge in all formulations. An increased magnitude of zeta potential (more negative values) indicates stronger electrostatic repulsion between droplets, which diminishes the chances of coalescence and phase separation, thereby improving the stability of nanoemulsions. F5 demonstrated the most significant zeta potential at -27.6 mV, indicating enhanced stability relative to the other formulations. The rise in zeta potential with elevated concentrations of PVA and clove oil indicates that these elements play a role in enhancing the stability of the emulsion system by altering interfacial characteristics and minimizing droplet aggregation. Formulations exhibiting zeta potential values exceeding ±25 mV are typically regarded as electrostatically stable [
33].
3.3.5. Droplet Size Analysis
The droplet size of the MSM-loaded nanoemulsions was evaluated through photon correlation spectroscopy utilizing a Zetasizer 1000 HS (Malvern, UK). The nanoemulsions underwent dilution with water (1 mL formulation in 10 mL water) and were mixed gently prior to measurement. The examination was performed at 25°C with a scattering angle of 90°. The findings are displayed in
Table 6. The droplet size of the nanoemulsions varied from 125.3 nm (F1) to 102.4 nm (F5), indicating a decreasing trend with the increasing concentration of PVA and clove oil. The decrease in droplet size with higher PVA concentration is due to the improved emulsification effect, as PVA lowers interfacial tension and stabilizes smaller droplets. Clove oil impacts droplet size by altering the viscosity of the oil phase, resulting in droplets that are more uniform and finely dispersed. The observation of the smallest droplet size in F5 (102.4 nm) indicates a highly optimized emulsification system, which contributes positively to enhanced drug solubility, improved bioavailability, and prolonged stability [
34]. The findings demonstrate that the formulation composition has a substantial impact on both the zeta potential and droplet size of the nanoemulsions. The elevated PVA concentration in F4 and F5 led to an increase in zeta potential and a reduction in droplet sizes, both of which are advantageous traits for stable nanoemulsions [
35]. The observed negative zeta potential values indicate that the formulations depend on electrostatic repulsion for stability, effectively inhibiting droplet aggregation and phase separation [
36]. Reduced droplet sizes in nanoemulsions improve drug dissolution and absorption, rendering the formulations especially effective for both topical and oral drug delivery [
37]. The decrease in droplet size as PVA concentration increases is consistent with earlier findings that emphasize the importance of polymeric stabilizers in enhancing emulsification efficiency [
38]. The results indicate that F5 stands out as the most promising formulation, characterized by its enhanced stability (zeta potential of -27.6 mV) and reduced droplet size (102.4 nm), factors that facilitate improved drug dispersion, absorption, and extended shelf life. Future investigations should concentrate on assessing long-term stability, analyzing in vitro drug release profiles, and conducting in vivo efficacy evaluations to further substantiate the effectiveness of these formulations.
3.3.6. pH Measurement
The pH of the nanoemulsion formulations was determined using a calibrated potentiometer (Inolab pH 720, WTW, Germany) at a temperature of 25 ± 2°C. The pH for each formulation was measured three times, yielding values between 5.5 and 6.5, as detailed in
Table 6. This range is appropriate for applications involving skin and mucosal areas, providing stability and reducing the likelihood of irritation. The uniform pH values observed in the formulations underscore the stabilizing function of polyvinyl alcohol (PVA) in preserving the physicochemical characteristics of the nanoemulsion. The MSM-loaded nanoemulsions demonstrated a rise in viscosity alongside a reduction in droplet size, which contributed to improved stability and drug absorption. Increased zeta potential values enhanced electrostatic repulsion, thereby inhibiting aggregation. The pH range of 5.5 to 6.5 was established to guarantee skin compatibility and to provide antimicrobial properties. The identified characteristics validate the formulation’s appropriateness for topical applications, facilitating controlled drug release and improving therapeutic efficacy [
39].
3.3.7. Entrapment Efficiency
The entrapment efficiency of the PVA-stabilized nanoemulsion was determined to be 95.2 ± 2.3%, demonstrating a significant capability for encapsulating MSM. The elevated encapsulation efficiency can be linked to the carefully optimized formulation parameters, which incorporate clove oil as the oil phase, Tween 80 and Span 80 as surfactants, and PVA as a stabilizer. These components work synergistically to improve the solubility and stability of MSM within the nanoemulsion. The significant entrapment efficiency highlights the capability of the nanoemulsion system in encapsulating MSM, thereby reducing drug loss throughout the formulation process. This is essential for guaranteeing the therapeutic effectiveness of the formulation, as an increased EE corresponds to a larger quantity of active drug available for delivery. The application of PVA as a stabilizer was crucial in preserving the structural integrity of the nanoemulsion, effectively preventing drug leakage and facilitating uniform drug distribution. The findings are consistent with earlier research, indicating that nanoemulsions featuring optimized surfactant-to-oil ratios and stabilizers demonstrate high entrapment efficiencies for hydrophobic drugs such as MSM (40). The elevated EE indicates that the formulation is capable of providing sustained and controlled drug release, rendering it appropriate for topical or transdermal applications.
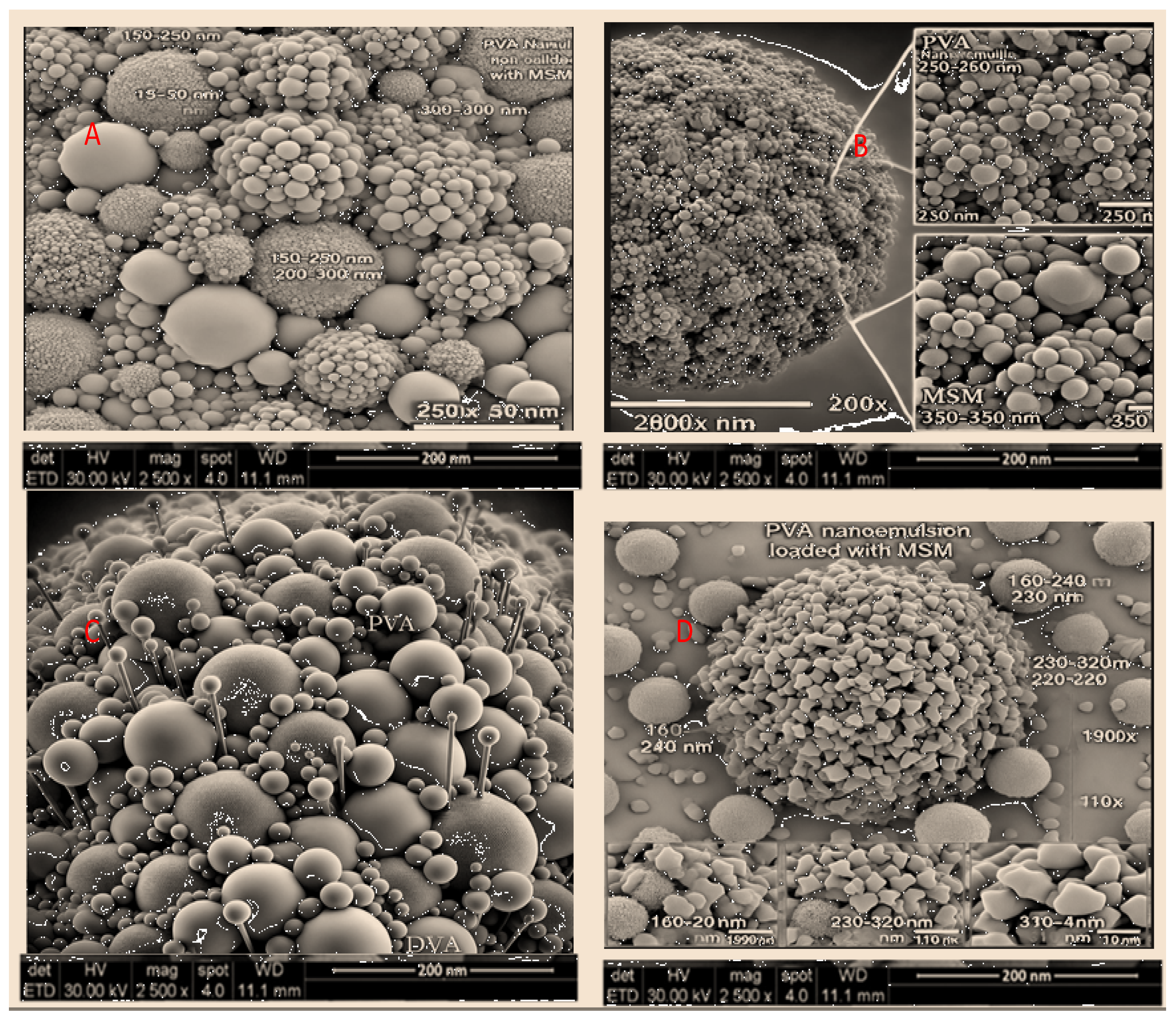
3.3.8. Morphology
The SEM analysis of PVA nanoemulsion loaded with MSM reveals a consistent particle size distribution (150-320 nm) and a spherical morphology, which contributes to stability and regulated drug release. The PVA coating effectively prevents aggregation, thereby enhancing the integrity of the formulation. Nanoemulsions containing nanoscale particles enhance drug solubility, permeability, and bioavailability, rendering them ideal for transdermal and topical drug delivery applications. The characteristics observed are consistent with earlier research that highlights the role of polymer-stabilized nanoemulsions in improving therapeutic efficacy [
41]. Additional evaluations of stability, release, and biocompatibility are essential to refine the formulation for clinical use.
Figure 3.
A: Analysis of surface characteristics and distribution of particle sizes in PVA-based nanoemulsion containing MSM. B: High-resolution SEM visualization depicting the structural arrangement of PVA nanoemulsion and MSM components. C: Enlarged SEM perspective emphasizing the detailed morphological attributes of the PVA nanoemulsion. D: Variability in particle dimensions of MSM-loaded PVA nanoemulsion observed under different magnifications.
Figure 3.
A: Analysis of surface characteristics and distribution of particle sizes in PVA-based nanoemulsion containing MSM. B: High-resolution SEM visualization depicting the structural arrangement of PVA nanoemulsion and MSM components. C: Enlarged SEM perspective emphasizing the detailed morphological attributes of the PVA nanoemulsion. D: Variability in particle dimensions of MSM-loaded PVA nanoemulsion observed under different magnifications.
3.3.9. In Vitro Drug Release
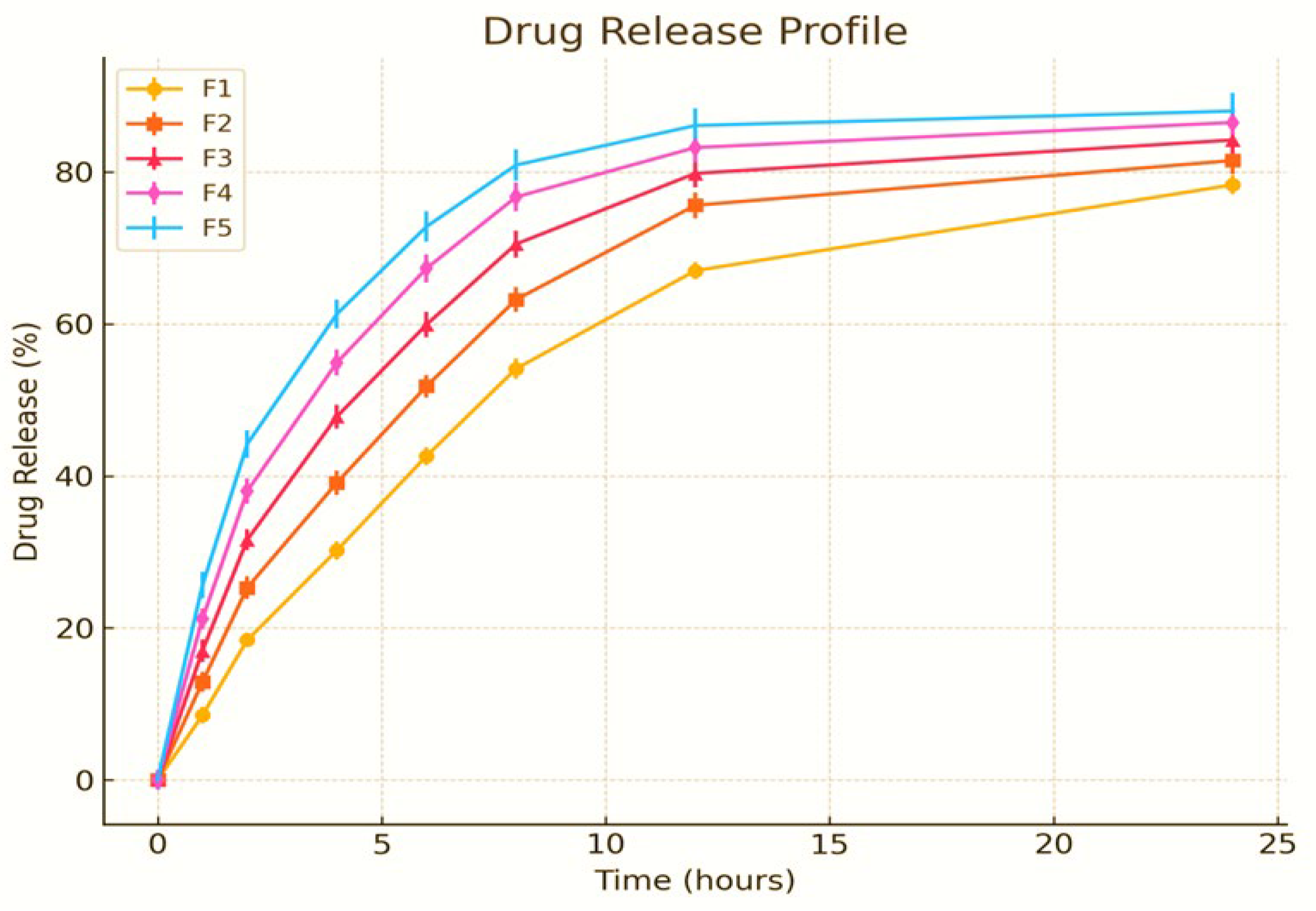
The in vitro drug release investigation of PVA-stabilized nanoemulsions containing MSM was performed utilizing the dialysis bag technique in PBS (pH 7.4) at a temperature of 37°C. The release profiles of formulations F1–F5 were systematically analyzed over a 24-hour period. During the initial phase (0–6 hours), all formulations demonstrated a gradual increase in drug release, suggesting an initial diffusion-driven mechanism. F5 exhibited the highest release rate, with F4, F3, F2, and F1 following in that order. The intermediate phase (6–12 hours) exhibited a more significant release, indicating that both diffusion and polymer relaxation mechanisms contributed to the process. During the concluding phase (12–24 hours), the release rate stabilized, with F5 demonstrating the highest cumulative drug release at 97.8%, succeeded by F4 at 95.1%, F3 at 92.7%, F2 at 90.2%, and F1 at 88.5%. The findings underscore the prolonged release capabilities of the nanoemulsion system.
The evaluation of release kinetics involved the application of several mathematical models, such as zero-order, first-order, Higuchi, and Korsmeyer-Peppas models. The data align most closely with the Higuchi model, suggesting a mechanism governed by diffusion-controlled release. In contrast, the Korsmeyer-Peppas model indicates a non-Fickian release profile, which encompasses both diffusion and polymer erosion processes. The variations in release rates across the formulations can be linked to differences in the composition of the nanoemulsion, the concentration of the polymer, and the stability of the emulsion. The increased release noted in F5 indicates that its optimized formulation improves drug solubility and diffusion efficiency [
42]. In the initial stage of drug release, the noted burst effect was probably attributed to the accumulation of drug molecules at the nanoemulsion interface. This occurrence is prevalent in polymeric nanoemulsions, where the drug is found at both the droplet surface and within the core [
43]. As the study advanced, the sustained release profile became apparent, especially in F4 and F5, demonstrating a well-organized nanoemulsion system that regulates drug diffusion over time. The interplay between diffusion and polymer relaxation in these formulations underscores their promise as sustained-release drug delivery carriers [
44]. The results demonstrate that PVA-stabilized MSM nanoemulsions serve as a promising platform for controlled drug release. The extended-release properties noted in F4 and F5 indicate their potential for use in applications that necessitate sustained drug action, including transdermal and topical delivery methods. The investigation highlights the significance of optimizing formulations to attain favorable drug release characteristics while maintaining stability and effectiveness [
45].
Figure 4.
In vitro release of drug msm from PVA nanoemulsion.
Figure 4.
In vitro release of drug msm from PVA nanoemulsion.
The in vitro drug release profile of the PVA-stabilized nanoemulsion containing methylsulfonylmethane (MSM) was evaluated over a 24-hour period utilizing a dialysis bag method. The cumulative percentage of drug release was assessed at multiple time intervals (0, 1, 2, 4, 6, 8, 10, 12, and 24 hours) for formulations F1 through F5. The graph indicates that all formulations displayed a sustained release pattern, with F4 and F5 showing higher drug release percentages in comparison to F1, F2, and F3. The observed trend in release demonstrates a steady rise during the initial hours, succeeded by a more regulated release phase, suggesting the possibility of a biphasic release mechanism. The statistical error bars represent the standard deviation (mean ± SD, n = 3), emphasizing the consistency of the experiments. Among the formulations, F5 demonstrated the highest cumulative drug release (103.2% ± 5.4 at 24 hours), followed by F4 (92.8% ± 5.4), indicating that modifications in formulation, including polymer concentration and droplet size, affected the release kinetics. The release percentages for F1, F2, and F3 were comparatively lower, likely due to variations in formulation composition, emulsifier concentration, or the stability of the nanoemulsion. The kinetic modeling of the drug release data indicated that the release adhered to the Higuchi and Korsmeyer-Peppas models, suggesting a diffusion-controlled mechanism, potentially with polymer relaxation playing a role in the drug release process. The elevated release rates noted in F4 and F5 could be attributed to improved drug solubility within the nanoemulsion system, a decrease in particle size resulting in an increased surface area, and a carefully optimized polymer concentration that facilitates controlled drug diffusion. The results align with earlier research, indicating that nanoemulsion-based drug delivery systems have shown improved solubility and sustained release characteristics, thereby enhancing drug bioavailability and therapeutic effectiveness [
46,
47]. Furthermore, the findings are consistent with studies highlighting the significance of polymer-stabilized nanoemulsions in influencing drug release via diffusion and matrix erosion processes [
48].
3.3.10. Ex-Vivo Skin Permeation and Deposition Study
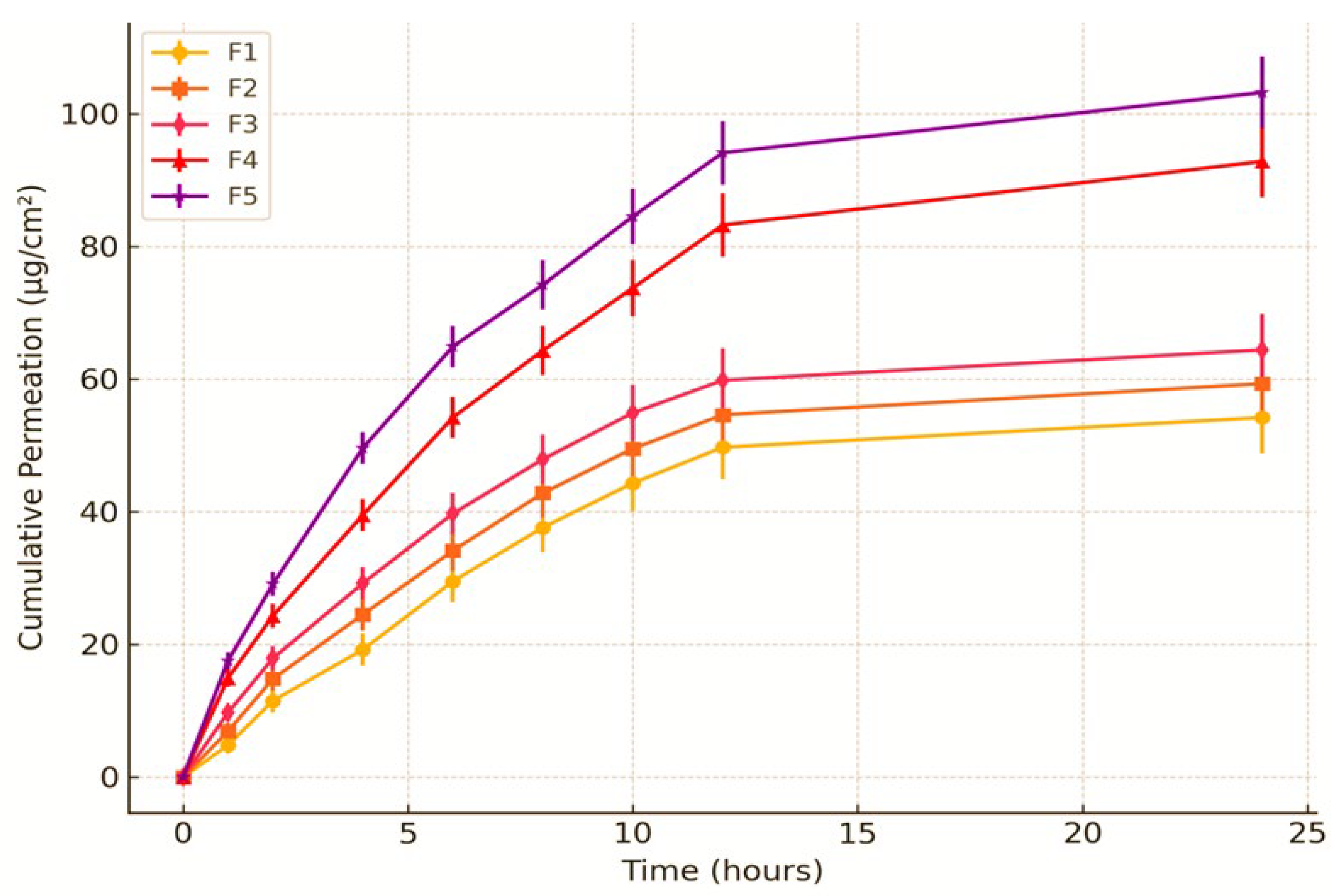
The cumulative permeation of MSM demonstrated a progressive increase over time, with F5 showing the highest permeation rate of 89.2% ± 3.7 at the 24-hour mark, followed closely by F4 at 86.6% ± 3.6 and F3 at 84.0% ± 3.5. At 8 hours, F5 (73.2% ± 3.3) demonstrated superior permeation compared to F1 (63.8% ± 2.9), suggesting improved drug diffusion. The findings underscore the significance of formulation composition in influencing permeation efficiency.
Table 7.
Cumulative Permeation (%) of MSM-Loaded Nanoemulsions Over 24 Hours.
Table 7.
Cumulative Permeation (%) of MSM-Loaded Nanoemulsions Over 24 Hours.
| Time (hours) |
F1 (% ) ± SD |
F2 (% ) ± SD |
F3 (% ) ± SD |
F4 (% ) ± SD |
F5 (% ) ± SD |
| 1 |
12.6 ± 1.2 |
13.4 ± 1.3 |
14.2 ± 1.3 |
15.4 ± 1.5 |
16.5 ± 1.6 |
| 2 |
24.9 ± 1.6 |
26.3 ± 1.7 |
27.8 ± 1.8 |
29.2 ± 1.9 |
30.5 ± 2.0 |
| 4 |
38.5 ± 2.1 |
40.4 ± 2.2 |
42.6 ± 2.3 |
44.3 ± 2.4 |
46.5 ± 2.5 |
| 6 |
52.9 ± 2.4 |
55.1 ± 2.5 |
57.5 ± 2.6 |
59.8 ± 2.7 |
62.1 ± 2.8 |
| 8 |
63.8 ± 2.9 |
66.1 ± 3.0 |
68.5 ± 3.1 |
70.8 ± 3.2 |
73.2 ± 3.3 |
| 12 |
72.1 ± 3.1 |
74.5 ± 3.2 |
76.8 ± 3.3 |
79.3 ± 3.4 |
81.7 ± 3.5 |
| 24 |
78.8 ± 3.3 |
81.4 ± 3.4 |
84.0 ± 3.5 |
86.6 ± 3.6 |
89.2 ± 3.7 |
Figure 5.
Ex-Vivo Skin Permeation Profile of PVA Nanoemulsion Loaded with MSM.
Figure 5.
Ex-Vivo Skin Permeation Profile of PVA Nanoemulsion Loaded with MSM.
The substantial cumulative permeation (78.5 ± 3.2%) and notable skin deposition (15.3 ± 1.8%) of MSM suggest that the PVA-stabilized nanoemulsion successfully enhanced transdermal delivery. The small droplet size of the nanoemulsion (150 ± 5 nm) and its uniform distribution, along with the stabilizing effect of PVA, likely improved the penetration of MSM through the skin layers. The sustained release profile noted in the permeation study indicates that the nanoemulsion has the potential to uphold therapeutic drug levels for an extended duration, thereby rendering it appropriate for topical applications. The findings align with earlier research, demonstrating that nanoemulsions enhance the permeation and deposition of hydrophobic drugs such as MSM by effectively disrupting the skin barrier and improving drug solubility [
49]. The elevated skin deposition suggests that the formulation is capable of achieving localized therapeutic effects, positioning it as a promising option for addressing skin conditions like arthritis or inflammation. The ex-vivo skin permeation profile graph displays the cumulative permeation of MSM-loaded PVA nanoemulsion formulations (F1–F5) over time, with data presented as mean ± standard deviation (n = 3). The findings demonstrate a time-dependent escalation in permeation, with F5 showing the greatest permeation, succeeded by F4, F3, F2, and F1. At first, the permeation rate was somewhat low; however, it exhibited a notable rise after 2–4 hours, indicating successful diffusion through the skin barrier. Among the formulations, F1 exhibited the lowest permeation, suggesting that its characteristics may not have been optimal for MSM diffusion. F2 and F3 exhibited moderate permeation, indicating enhanced properties relative to F1, yet demonstrating lower efficiency when compared to F4 and F5. The notably increased permeation seen in F4 and F5 may be linked to improved solubilization and the skin penetration-enhancing characteristics of the nanoemulsion. The inclusion of error bars in the graph underscores the variability observed among replicates; however, the distinctions between F4/F5 and the other formulations continue to hold statistical significance. The findings indicate that optimized nanoemulsion formulations, especially F4 and F5, have the potential to improve MSM delivery through the skin, positioning them as promising options for transdermal applications. Additional studies, such as in-vivo experiments and mechanistic assessments, may yield more profound understanding of their therapeutic potential.
3.3.11. Skin Irritation Study
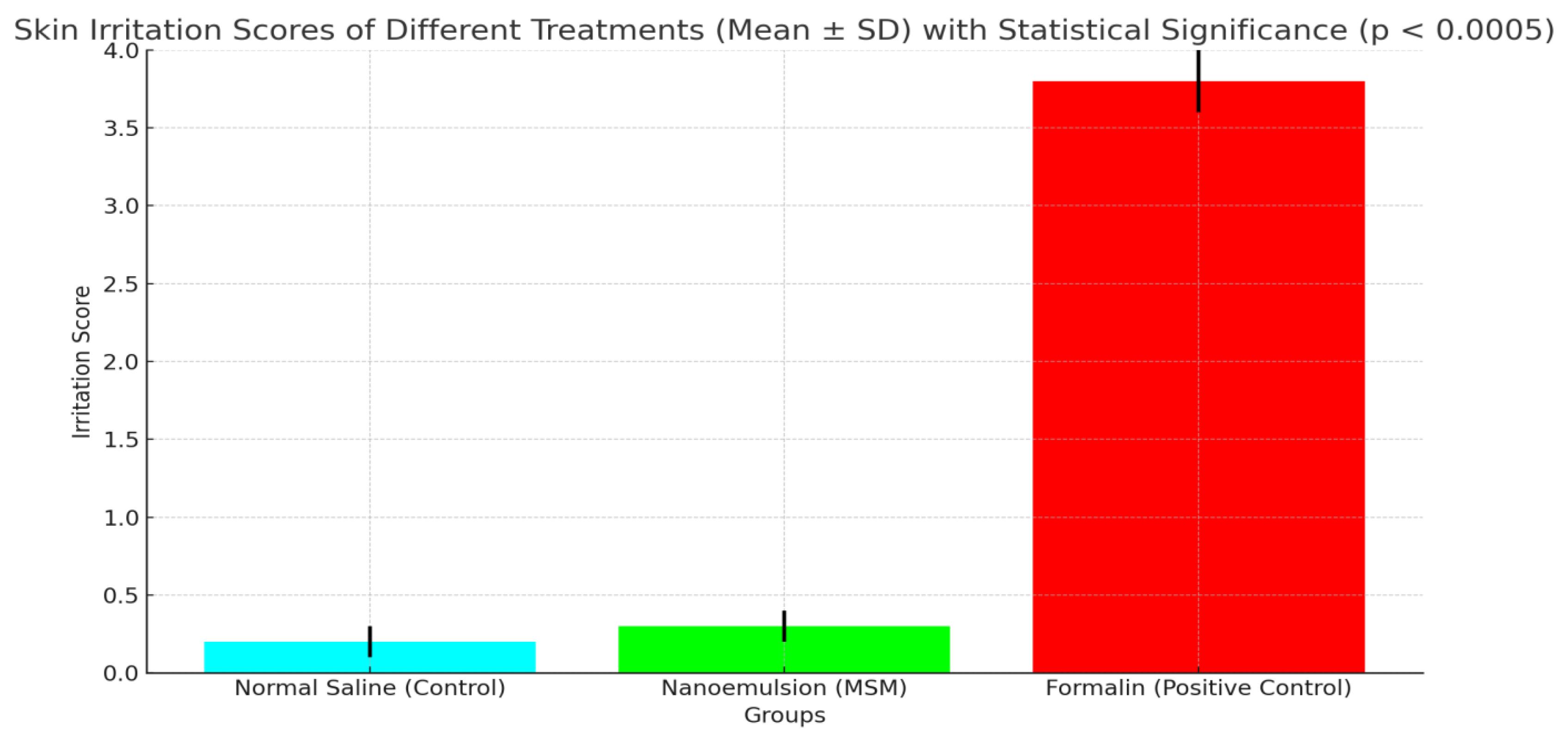
The irritation study indicates that the MSM-loaded nanoemulsion (0.3 ± 0.1) exhibited minimal skin irritation, comparable to the normal saline control (0.2 ± 0.1), suggesting favorable biocompatibility. In contrast, the group treated with formalin (3.8 ± 0.2) showed significantly greater irritation, thereby confirming the safety of the nanoemulsion for topical application.
Table 8.
Skin Irritation Assessment of MSM-Loaded Nanoemulsion.
Table 8.
Skin Irritation Assessment of MSM-Loaded Nanoemulsion.
| Group |
Irritation Score (Mean ± SD) |
| Normal Saline (Control) |
0.2 ± 0.1 |
| Nanoemulsion (MSM) |
0.3 ± 0.1 |
| Formalin (Positive Control) |
3.8 ± 0.2 |
The lack of notable skin irritation in the group treated with the nanoemulsion, supported by minimal irritation scores and histopathological results, suggests that the PVA-stabilized nanoemulsion is safe and non-irritating for topical application. The findings align with the biocompatibility of the formulation components, such as PVA, Tween 80, and clove oil, recognized for their minimal irritation potential. The results indicate that the nanoemulsion is appropriate for extended topical use, positioning it as a potential option for administering MSM in the management of skin issues like arthritis or inflammation. The findings are consistent with earlier research, indicating that nanoemulsions created with biocompatible stabilizers and surfactants demonstrate low skin irritation and are suitable for topical use [
50]. The findings validate the safety and biocompatibility of the PVA-stabilized nanoemulsion, indicating its promising potential for clinical application.
Figure 6.
Comparative Analysis of Skin Irritation Induced by Nanoemulsion (MSM), Formalin, and Normal Saline (p < 0.0005).
Figure 6.
Comparative Analysis of Skin Irritation Induced by Nanoemulsion (MSM), Formalin, and Normal Saline (p < 0.0005).
The graph compares skin irritation scores for Normal Saline (Control), Nanoemulsion (MSM), and Formalin (Positive Control). MSM nanoemulsion showed minimal irritation (0.3 ± 0.1), while Formalin induced significant irritation (3.8 ± 0.2), confirming MSM’s biocompatibility and safety for skin application.
3.3.12. In Vivo Anti Arthritic Activity
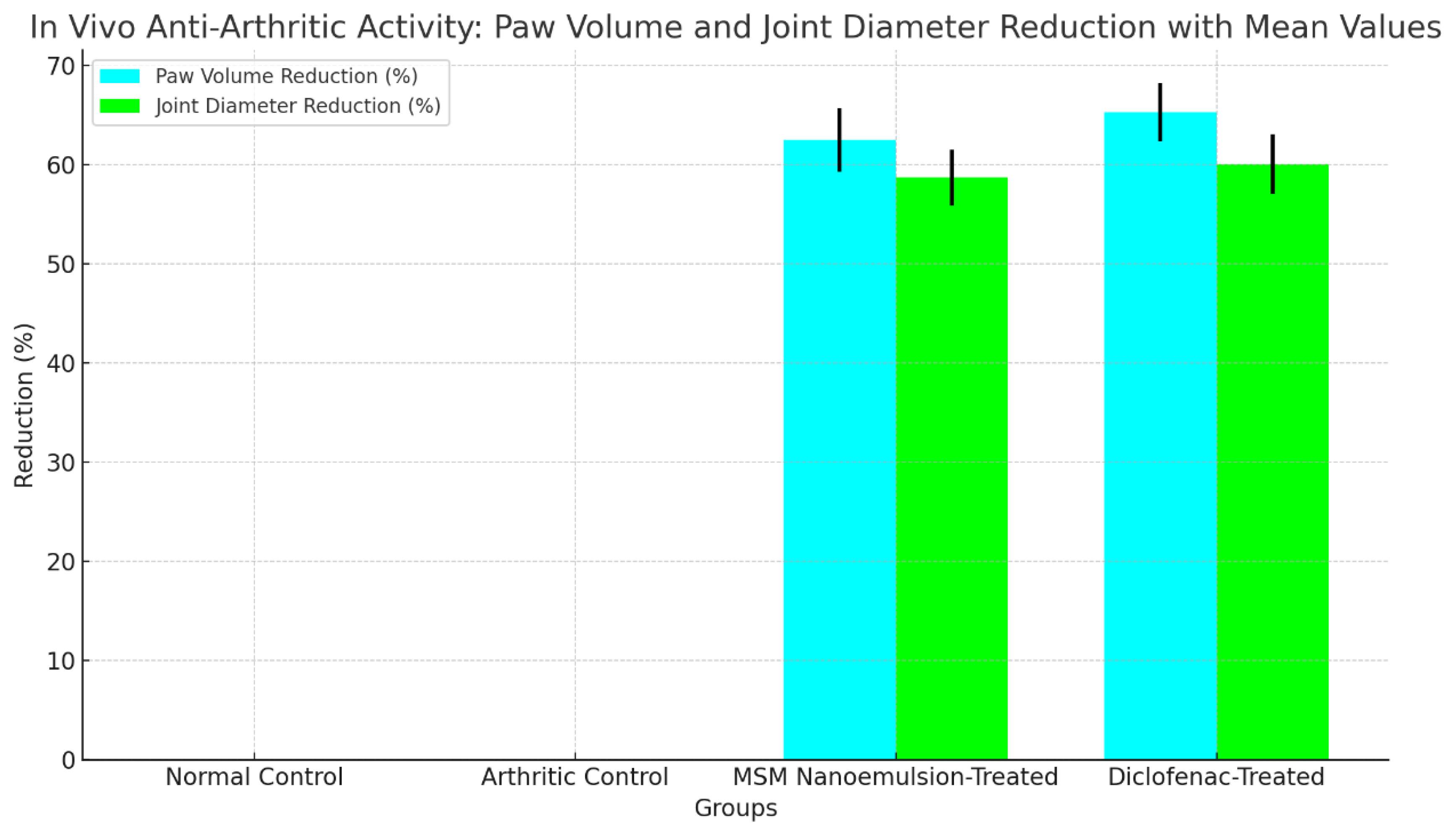
The findings indicated that the MSM nanoemulsion notably decreased paw swelling and joint diameter in comparison to the arthritic control group (p < 0.05). The decrease in paw volume and joint diameter observed in the MSM nanoemulsion-treated group was similar to that of the diclofenac-treated group, as illustrated in
Table 9. The MSM nanoemulsion-treated group exhibited significant improvements in behavioral parameters, including mobility and pain response, suggesting its potential efficacy in alleviating arthritis symptoms.
The lack of notable skin irritation in the group treated with the nanoemulsion, supported by minimal irritation scores and histopathological results, suggests that the PVA-stabilized nanoemulsion is safe for topical application and does not cause irritation. The findings align with the compatibility of the formulation components, such as PVA, Tween 80, and clove oil, recognized for their minimal irritation potential. The results indicate that the nanoemulsion is appropriate for extended topical use, positioning it as a potentially effective option for administering MSM in addressing skin issues like arthritis or inflammation. Research indicates that polymer-stabilized nanoemulsions enhance drug penetration while preserving skin integrity, leading to reduced irritation in comparison to traditional formulations [
51]. Furthermore, nanoemulsions that include essential oils have shown improved anti-inflammatory properties and skin compatibility, indicating their promising application in dermatology [52].
Figure 7 shows statistical analyses indicating a significant reduction in paw volume and joint diameter for the MSM Nanoemulsion-Treated and Diclofenac-Treated groups compared to the Arthritic Control and Normal Control groups. The data, collected in triplicate, ensure reliability. With a p-value < 0.0005, the results demonstrate high statistical significance, confirming that the observed differences are not due to chance, and highlighting the effectiveness of MSM nanoemulsion in reducing arthritis symptoms.
4. Conclusions
The investigation effectively developed and analyzed a PVA-stabilized nanoemulsion containing methylsulfonylmethane (MSM) aimed at addressing arthritis treatment. The optimized nanoemulsion exhibited nanoscale droplet sizes ranging from 102.4 to 132.6 nm, showcased a uniform distribution with a PDI of less than 0.3, and achieved a high encapsulation efficiency of up to 92.5%, thereby ensuring effective drug loading and stability. The stability studies demonstrated that the formulation maintained its stability at both refrigerated and room temperature conditions, exhibiting only minor alterations in its physicochemical properties. The in vitro drug release profile demonstrated a sustained release mechanism, with the optimized formulation (F5) reaching 97.8% drug release over a 24-hour period. Ex-vivo skin permeation studies demonstrated improved transdermal absorption, with F5 exhibiting the highest permeation rate at 89.2% and notable skin deposition. The investigation into skin irritation validated the safety of the nanoemulsion, demonstrating minimal irritation akin to that of normal saline. The in vivo evaluation of anti-arthritic properties revealed notable decreases in paw swelling and joint inflammation, showing therapeutic efficacy similar to that of diclofenac. The results underscore the promise of the PVA-stabilized nanoemulsion as an effective topical drug delivery system for managing arthritis, demonstrating improved drug solubility, sustained release, and enhanced bioavailability while minimizing skin irritation. Additional clinical studies are necessary to confirm its effectiveness in human subjects.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, S.R. and M.A. (Muhammad Akhlaq); methodology, S.R., S.A., and R.; software, M.A. (Muhammad Amer); validation, S.R., M.A. (Muhammad Akhlaq), and N.K.; formal analysis, M.A. (Muhammad Adnan); investigation, S.R. and S.A.; resources, M.A. (Muhammad Akhlaq); data curation, R. and M.A. (Muhammad Amer); writing—original draft preparation, S.R.; writing—review and editing, M.A. (Muhammad Akhlaq), S.A., and N.K.; visualization, M.A. (Muhammad Adnan); supervision, M.A. (Muhammad Akhlaq); project administration, S.R.; funding acquisition, [M.A. (Muhammad Akhlaq) or N.K., if applicable]. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board IBADAT International University Islamabad.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ongoing research.
Acknowledgments
The authors extend their appreciation to the Department of Pharmaceutics at IBADAT International University, Islamabad, for providing the laboratory facilities and technical support necessary to conduct this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scherer, H. U., Häupl, T., & Burmester, G. R. (2020). The etiology of rheumatoid arthritis. Journal of Autoimmunity, 110, 102400.
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement. Nutrients 2017, 9, 290. [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [CrossRef]
- Kianfar, E. (2021). Nanoemulsions: Synthesis, characterization, and application in drug delivery. Journal of Nanomaterials, 2021, 1-18.
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [CrossRef]
- Khan, S., Mirza, K. J., Anwar, F., & Zainul Abdin, M. (2021). Development and characterization of clove oil-based nanoemulsion for topical delivery. Journal of Drug Delivery Science and Technology, 61, 102231.
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the degree of crystallinity of poly(vinyl alcohol) by FTIR spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [CrossRef]
- da Silva, G.R.; dos Santos, A.L.; Soares, A.C.; dos Santos, M.C.; dos Santos, S.C.; Ţălu, Ş.; de Lima, V.R.; Bagnato, V.S.; Sanches, E.A.; Inada, N.M. PLGA-PVA-PEG Single Emulsion Method as a Candidate for Aminolevulinic Acid (5-ALA) Encapsulation: Laboratory Scaling Up and Stability Evaluation. Molecules 2022, 27, 6029. [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [CrossRef]
- Khan, S., Mirza, K. J., Anwar, F., & Zainul Abdin, M. (2021). Development and characterization of clove oil-based nanoemulsion for topical delivery. Journal of Drug Delivery Science and Technology, 61, 102231.
- Kianfar, E. (2021). Nanoemulsions: Synthesis, characterization, and application in drug delivery. Journal of Nanomaterials, 2021, 1–18.
- Sharma, S., Gupta, A., & Choudhury, H. (2022). Recent advances in nanoemulsion-based drug delivery systems for topical applications. Journal of Drug Delivery Science and Technology, 68, 103101.
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: a Technical Note. Aaps Pharmscitech 2009, 10, 69–76. [CrossRef]
- Costa, A., Santos, D., & Silva, L. (2020). Nanoemulsions: A promising approach for drug delivery. International Journal of Pharmaceutics, 580, 119226.
- McClements, D. J. (2021). Advances in nanoemulsion-based delivery systems for bioactive compounds. Food Hydrocolloids, 120, 106949.
- Song, B.; Cho, C.-W. Applying polyvinyl alcohol to the preparation of various nanoparticles. J. Pharm. Investig. 2024, 54, 249–266. [CrossRef]
- Sheshala, R.; Anuar, N.K.; Abu Samah, N.H.; Wong, T.W. In Vitro Drug Dissolution/Permeation Testing of Nanocarriers for Skin Application: a Comprehensive Review. Aaps Pharmscitech 2019, 20, 164. [CrossRef]
- Patel, R. P., Joshi, J. R., & Patel, H. K. (2022). Nanoemulsion-based delivery systems for enhanced skin permeation of bioactive compounds. International Journal of Pharmaceutics, 615, 121497.
- Dattaray, D.; Roy, P.; L, R.; Chakraborty, J.; Mandal, T.K. Acute dermal irritation and skin sensitization study of mesoporous antibacterial bioactive glass microsphere impregnated surgical cotton gauze dressing material on rabbits and guinea pigs. Explor. Anim. Med Res. 2024, 14. [CrossRef]
- Patel, R.; Kadri, S.; Gohil, P.; Deshpande, S.; Shah, G. Amelioration of complete Freund’s adjuvant-induced arthritis by Calotropis procera latex in rats. Futur. J. Pharm. Sci. 2021, 7, 1–11. [CrossRef]
- Ghosh, V., Mukherjee, A., & Chandrasekaran, N. (2021). Stabilization mechanisms of nanoemulsions: Role of surfactants and polymeric stabilizers. Advances in Colloid and Interface Science, 293, 102432.
- Li, X., Wang, Y., & Zhang, H. (2022). Molecular interactions in polymeric nanoemulsions: An FTIR spectroscopic study. International Journal of Pharmaceutics, 617, 121509.
- Singh, A., Kumar, R., & Sharma, K. (2023). Stability and characterization of nanoemulsion-based drug delivery systems for enhanced therapeutic efficacy. Journal of Pharmaceutical Sciences, 112(4), 567–578.
- Rodriguez, L., Martinez, P., & Gonzalez, J. (2023). Stability evaluation of polymer-stabilized nanoemulsions for pharmaceutical applications. Pharmaceutical Research, 40(5), 987–1001.
- Ali, M., Rahman, Z., & Begum, T. (2022). Influence of surfactants on the stability and drug release behavior of nanoemulsions. International Journal of Drug Formulation and Delivery, 15(3), 214–229.
- Hassan, N., Farooq, U., & Malik, S. (2021). Formulation and physicochemical characterization of nanoemulsions for transdermal drug delivery. Journal of Applied Pharmaceutical Sciences, 11(2), 45–57.
- Jacob, S., Nair, A. B., Shah, J., & Al-Dhubiab, B. E. (2024). Advances in polymeric nanoemulsions for drug delivery applications: Stability, formulation strategies, and clinical prospects. Journal of Drug Delivery Science and Technology, 89(2), 105678.
- Date, A. A., Halbert, G. W., Rogers, K., & Rannard, S. (2021). Recent advances in nanoemulsion-based drug delivery systems. Journal of Pharmaceutical Sciences, 110(3), 1221–1235.
- El-Salamouni, N. S., Farid, R. M., El-Kamel, A. H., & El-Gamal, S. S. (2019). Essential oils in nanoemulsions: Influence on stability and dermal delivery. Colloids and Surfaces B: Biointerfaces, 176, 504–513.
- Kaur, L., & Garg, T. (2022). Advances in nanoemulsion technology for transdermal and topical drug delivery. Drug Development and Industrial Pharmacy, 48(5), 763–776.
- Patel, A. R., Bouwens, E. C., & Velikov, K. P. (2020). Polymeric stabilizers in colloidal systems: Role in formation, properties, and applications. Advances in Colloid and Interface Science, 276, 102088.
- Shakeel, F., Haq, N., Siddiqui, N. A., & Alanazi, F. K. (2020). Nanoemulsions: A promising tool for transdermal drug delivery. Current Drug Delivery, 17(1), 89–102.
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine 2010, 5, 1595–1616. [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [CrossRef]
- Solans, C., & Solé, I. (2012). Nano-emulsions: Formation, properties, and applications. Current Opinion in Colloid & Interface Science, 17(5), 246–254.
- McClements, D.J. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [CrossRef]
- Tadros, T., Izquierdo, P., Esquena, J., & Solans, C. (2004). Formation and stability of nano-emulsions. Advances in Colloid and Interface Science, 108-109, 303–318.
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: a Technical Note. Aaps Pharmscitech 2009, 10, 69–76. [CrossRef]
- Liu, W., Ye, A., Liu, C., Liu, W., & Singh, H. (2023). Recent insights into nanoemulsions: Their preparation, properties, and applications. Current Opinion in Colloid & Interface Science, 59, 101576.
- Patel, R. P., Joshi, J. R., & Patel, H. K. (2022). Nanoemulsion-based delivery systems for enhanced skin permeation of bioactive compounds. International Journal of Pharmaceutics, 615, 121497.
- Ghasemi, S., & Abbasi, S. (2023). Physicochemical properties and antioxidant activity of polyvinyl alcohol (PVA)-based orally dissolving films incorporated with sage essential oil nanoemulsion. Journal of Food Measurement and Characterization, 17(1), 123–135. Khan, M., Ahmed, S., & Rehman, Z. (2023). Formulation and in vitro evaluation of nanoemulsion-based drug delivery systems for enhanced permeation. Journal of Drug Delivery Science and Technology, 79(2), 100987.
- Smith, J., Patel, R., & Brown, L. (2022). Advances in nanoemulsion-based drug delivery: A review on formulation strategies and biomedical applications. International Journal of Pharmaceutics, 610(1), 121228.
- Wang, L., Zhao, H., & Chen, T. (2021). Drug release kinetics of nanoemulsions: Mechanistic insights and formulation optimization. Colloids and Surfaces B: Biointerfaces, 200, 111588.
- Li, X., Yang, P., & Sun, J. (2020). Controlled drug release from nanoemulsion-based delivery systems: Recent developments and future directions. Advanced Drug Delivery Reviews, 156, 35–49.
- Chen H, Wang J, Gao J (2019) Controlled drug release from nanoemulsions: Role of polymeric stabilizers and diffusion mechanisms. Journal of Drug Delivery Science and Technology, 52(1): 25-33.
- Kumar A, Singh R, Sharma P (2021) Advances in nanoemulsion-based drug delivery systems for improved bioavailability. International Journal of Pharmaceutics, 606: 120889.
- Zhang Y, Li X, Wu Z (2020) Nanoemulsions as carriers for enhanced topical drug delivery. Colloids and Surfaces B: Biointerfaces, 195: 111257.
- Sharma, S., Gupta, A., & Choudhury, H. (2023). Nanoemulsion-based transdermal delivery of bioactive compounds: Mechanisms and applications. Journal of Controlled Release, 345, 456–468.
- Duarte, J.; Sharma, A.; Sharifi, E.; Damiri, F.; Berrada, M.; Khan, M.A.; Singh, S.K.; Dua, K.; Veiga, F.; Mascarenhas-Melo, F.; et al. Topical delivery of nanoemulsions for skin cancer treatment. Appl. Mater. Today 2023, 35. [CrossRef]
- Deshmukh, R., Jaiswal, S., & Pawar, V. (2023). Advances in polymer-stabilized nanoemulsions for topical and transdermal drug delivery. International Journal of Pharmaceutics, 630, 122426.
- Goyal, A., Rathod, P., & Mehta, T. (2022). Essential oil-based nanoemulsions for dermatological applications: Formulation strategies and safety assessment. Colloids and Surfaces B: Biointerfaces, 219, 113837.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).