Submitted:
28 August 2025
Posted:
29 August 2025
You are already at the latest version
Abstract
Background/Objectives: Patients in palliative care often experience multifaceted forms of suffering that extend beyond physical symptoms, including existential distress, loss of meaning, and emotional pain. Ketamine-assisted psychotherapy (KAP) has emerged as a promising intervention for alleviating such complex forms of suffering, yet models specifically tailored to palliative populations remain scarce. This narrative review synthesizes current evidence on ketamine’s neurobiological, psychological, and experiential effects relevant to end-of-life care, and presents a novel, time-limited KAP model designed for use in palliative settings. Methods: Drawing from both biochemical and psychedelic paradigms, the review integrates findings from neuroscience, phenomenology, and clinical practice. In particular, it incorporates a dual-level experiential framework informed by recent models distinguishing ketamine’s differential effects on self-processing networks: the Salience Network (SN), related to embodied self-awareness, and the Default Mode Network (DMN), associated with narrative self-construction. This neurophenomenological perspective underpins the rationale for using two distinct dosing sessions. Results: The article proposes a short-course, time-limited KAP model that integrates preparatory and integrative psychotherapy, two ketamine dosing sessions (one low-dose and one moderate-dose), concurrent psychotherapy, goals of care discussion (GOCD), and optional pharmacological optimization. The model emphasizes psychological safety, meaning-making, and patient-centered care. The sequential dosing strategy leverages ketamine’s unique pharmacology and experiential profile to address both bodily and narrative dimensions of end-of-life distress. Conclusions: This dissociative-psychedelic model offers a compassionate, pragmatic, and theoretically grounded approach to relieving psychological and existential suffering in palliative care. By integrating neurobiological insights with psychotherapeutic processes, it provides a flexible and patient-centered framework for enhancing meaning, emotional resolution, and quality of life at the end of life. Further research is needed to evaluate its clinical feasibility, safety, and therapeutic efficacy.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Mechanisms of Action
2.1.1. -NMDA Antagonism
- Disinhibition Hypothesis: This hypothesis proposes that ketamine blocks NMDA receptors on GABAergic interneurons, reducing inhibitory control over pyramidal neurons. This disinhibition increases glutamate release in the medial prefrontal cortex (mPFC) and elevates the firing rate of pyramidal neurons. Such a “glutamate surge” promotes AMPA receptor activation relative to NMDA activation, enhancing synaptic activity and potentially contributing to antidepressant effects. Supporting this view, AMPA receptor antagonists or mGluR2/mGluR3 agonists can block ketamine’s behavioral effects, indicating the importance of elevated extracellular glutamate levels [18,19,20].
- Direct Inhibition Hypothesis: This hypothesis suggests that ketamine directly antagonizes NMDA receptors on resting pyramidal neurons, blocking tonic NMDA activation by ambient or spontaneously released glutamate. This blockade reduces suppression of protein synthesis and activates downstream synaptogenic cascades. Increased AMPA receptor activation through this mechanism is also thought to contribute to ketamine’s antidepressant effects [21,22].
2.1.2. BDNF and mTOR Signaling
2.1.3. Structural and Functional Changes
2.1.4. Functional Connectivity Changes
2.1.5. Lateral Habenula and Antidepressant Effects
2.1.6. Salience Network and Default Mode Network
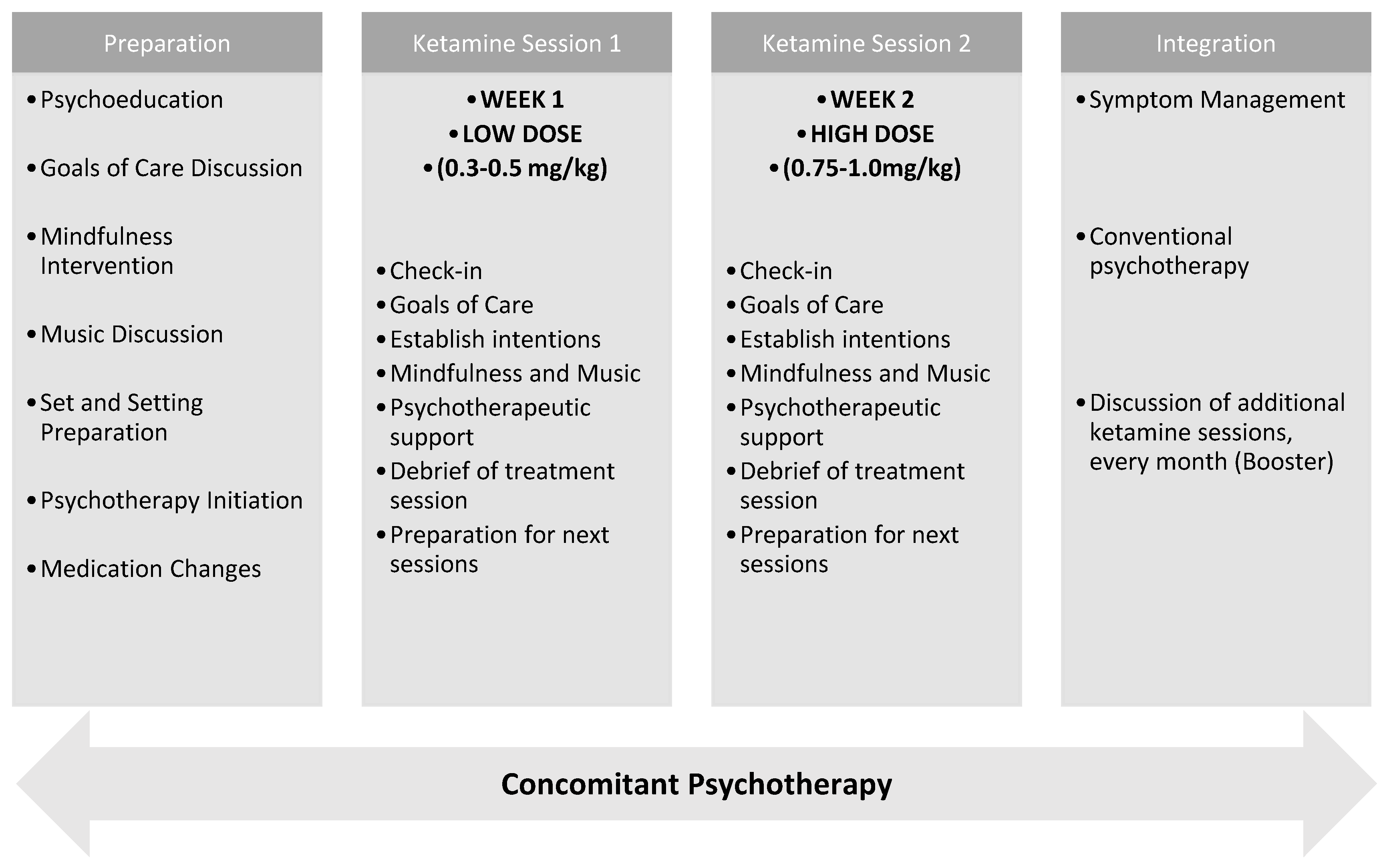
2.2. Short-Course Ketamine Protocol
2.2.1. Ketamine and Substance Use Disorders
2.2.2. Ketamine and Mood
2.2.3. Ketamine and Pain
2.3. Ketamine Models of Care
3. Results
3.1. Psychotherapy-Grade Therapeutic Frame
- A clear, time-limited treatment plan.
- Shared understanding of goals and expectations.
- Boundaries for communication and follow-up.
- Proactive exploration of treatment termination.
3.2. Treatment Phases and Timeline
3.2.1. Preparation Phase (2–3 Sessions)
- Psychoeducation about ketamine’s mechanisms, limitations, and potential benefits.
- Exploration and moderation of treatment expectations.
- Establishment of at least three goals of care tailored to the patient’s values and capacity.
- Orientation to potential psychological content or altered states during ketamine sessions.
3.2.2. Ketamine Sessions (2 Sessions)
- Session 1 (low-dose): ~0.3–0.5 mg/kg IV, primarily for acclimatization and to trigger dissociative experiences.
- Session 2 (moderate-dose): ~0.75–1.0 mg/kg IV, oriented toward deeper emotional or existential processing provided by psychedelic experiences.
3.2.3. Integration Phase (2–3 Sessions)
- Process psychological insights or unresolved emotional material.
- Revisit and refine GOCD.
- Translate subjective experiences into actionable, values-based changes.
- Address potential “termination reactions” and create closure.
3.3. Concomitant Psychotherapy
- Enhanced engagement due to ketamine’s rapid cognitive/emotional effects.
- Psychotherapeutic containment of challenging content.
- A continuity pathway for psychological support beyond the ketamine course.
3.4. Goals of Care
- Reconnecting with loved ones.
- Engaging in spiritually or personally meaningful activities.
- Establishing comfort-enhancing routines or rituals.
- Exploring creative expression or legacy documentation.
3.5. Bridging Biomedical and Psychedelic Paradigms
3.6. Clinical and Ethical Considerations
- Time-limited and feasible within palliative care settings.
- Flexible, allowing for individualized psychotherapy and diverse treatment goals.
- Ethically grounded, emphasizing autonomy, emotional safety, and informed consent.
- Integrated, with communication between ketamine providers, psychotherapists, and palliative care teams.
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sessa, B. The Psychedelic Renaissance: Reassessing the Role of Psychedelic Drugs in 21st Century Psychiatry and Society; Muswell Hill Press: London, UK, 2012. [Google Scholar]
- Nichols, D.E.; Johnson, M.W.; Nichols, C.D. Psychedelics as medicines: An emerging new paradigm. Clin. Pharmacol. Ther. 2017, 101, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Agrawal, M.; Griffiths, R.R.; Grob, C.; Berger, A.; Henningfield, J.E. Psychedelic-assisted psychotherapy to treat psychiatric and existential distress in life-threatening medical illnesses and palliative care. Neuropharmacology 2022, 216, 109174. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Giribaldi, B.; Watts, R.; et al. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Feduccia, A.A.; Jerome, L.; et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder in humans: Results of a phase 3 trial. Nat. Med. 2019, 27, 1025–1033. [Google Scholar]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; et al. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef]
- Roseman, L.; Nutt, D.J.; Carhart-Harris, R.L. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front. Pharmacol. 2018, 8, 974. [Google Scholar] [CrossRef]
- Hartogsohn, I. Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J. Psychopharmacol. 2016, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Riblet, N.; Larson, R.; Watts, B.V.; et al. Reevaluating the role of antidepressants in cancer-related depression: A systematic review and meta-analysis. Gen. Hosp. Psychiatry 2014, 36, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.; Boyle, A.B.; Rosenblum, A.M.; et al. Psychedelics for psychological and existential distress in palliative and cancer care. Curr. Oncol. 2019, 26, 225–226. [Google Scholar] [CrossRef]
- White, C.M.; Weisman, N.; Dalo, J. Psychedelics for patients with cancer: A comprehensive literature review. Ann. Pharmacother. 2023, 57, 1062–1075. [Google Scholar] [CrossRef]
- Schimmers, N.; Breeksema, J.J.; Smith-Apeldoorn, S.Y.; Veraart, J.; van den Brink, W.; Schoevers, R.A. Psychedelics for the treatment of depression, anxiety, and existential distress in patients with a terminal illness: A systematic review. Psychopharmacology (Berl.) 2022, 239, 15–33. [Google Scholar] [CrossRef]
- Kohtala, S. Ketamine–50 years in use: From anesthesia to rapid antidepressant effects and neurobiological mechanisms. Pharmacol. Rep. 2021, 73, 323–345. [Google Scholar] [CrossRef]
- Pham, T.H.; Gardier, A.M. Fast-acting antidepressant activity of ketamine: Highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies. Pharmacol. Ther. 2019, 199, 58–90. [Google Scholar] [CrossRef]
- Anand, A.; Mathew, S.J.; Sanacora, G.; et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N. Engl. J. Med. 2023, 388, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Sholevar, R.; Kromka, W.; Beaussant, Y. Ketamine and ketamine-assisted psychotherapy for psychiatric and existential distress in patients with serious medical illness: A narrative review. J. Palliat. Med. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.; et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef]
- Zarate, C.A. Jr.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Homayoun, H.; Moghaddam, B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 2007, 27, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, J.S.; Autio, H.; Vesa, L.; Antila, H.; Lindemann, L.; Hoener, M.C.; et al. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY451646 are abolished in BDNF conditional null mice. Psychopharmacology (Berl.) 2012, 220, 441–450. [Google Scholar]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Duman, R.S.; Li, N.; Liu, R.J.; Duric, V.; Aghajanian, G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012, 62, 35–41. [Google Scholar] [CrossRef]
- Li, N.; Liu, R.J.; Dwyer, J.M.; Banasr, M.; Lee, B.; Son, H.; et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry 2011, 69, 754–761. [Google Scholar] [CrossRef]
- Monteggia, L.M.; Gideons, E.; Kavalali, E.T. The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol. Psychiatry 2013, 73, 1199–1203. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Moda-Sava, R.N.; Murdock, M.H.; Parekh, P.K.; Fetcho, R.N.; Huang, B.S.; Huynh, T.N.; et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019, 364, eaat8078. [Google Scholar] [CrossRef]
- Duncan, W.C.; Sarasso, S.; Ferrarelli, F.; Selter, J.; Riedner, B.A.; Hejazi, N.S.; et al. Convergent evidence for abnormalities of rapid eye movement sleep in major depressive disorder. Biol. Psychiatry 2013, 73, e34–e35. [Google Scholar]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Scheidegger, M.; Walter, M.; Lehmann, M.; Metzger, C.; Grimm, S.; Boeker, H.; et al. Ketamine decreases resting state functional network connectivity in healthy subjects: Implications for antidepressant drug action. PLoS ONE 2012, 7, e44799. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Shaw, A.D.; Jackson, L.E.; Hall, J.; Moran, R.; Saxena, N. Electrophysiological signatures of the ketamine-induced psychedelic state. J. Psychopharmacol. 2015, 29, 313–322. [Google Scholar]
- Abdallah, C.G.; Averill, L.A.; Collins, K.A.; Geha, P.; Schwartz, J.; Averill, C.; et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 2017, 42, 1210–1219. [Google Scholar] [CrossRef]
- Kraus, C.; Kadriu, B.; Lanzenberger, R.; Zarate, C.A. Jr.; Kasper, S. Ketamine and beyond: Investigations into the mechanism of action of rapid-acting antidepressants. Eur. Neuropsychopharmacol. 2019, 29, 411–420. [Google Scholar]
- Dandash, O.; Daglas, R.; Cotton, S.M.; Allott, K.; Fornito, A.; Pantelis, C.; et al. Differential effect of a single dose of ketamine on fronto-striatal connectivity in healthy and depressed individuals. Transl. Psychiatry 2018, 8, 66. [Google Scholar]
- Yang, Y.; Cui, Y.; Sang, K.; Dong, Y.; Ni, Z.; Ma, S.; et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018, 554, 317–322. [Google Scholar] [CrossRef]
- Cui, Y.; Hu, S.; Hu, H. Lateral habenular burst firing as a target of the rapid antidepressant effects of ketamine. Trends Neurosci. 2019, 42, 179–191. [Google Scholar] [CrossRef]
- Ma, S.; Chen, M.; Jiang, Y.; Xiang, X.; Wang, S.; Wu, Z.; et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature 2023, 622, 802–809. [Google Scholar] [CrossRef]
- Marguilho, M.; Figueiredo, I.; Castro-Rodrigues, P. A unified model of ketamine’s dissociative and psychedelic properties. J. Psychopharmacol. 2022, 37, 14–32. [Google Scholar] [CrossRef]
- Bodnar, M.S.; Barber, S.; Jim, H.S.L.; Huang, J. The role of ketamine and its enantiomer in managing depression and pain in cancer patients: A narrative review. J Anesth Transl Med [Internet]. 2024, 3, 155–165. [Google Scholar] [CrossRef]
- Lee, W.; Sheehan, C.; Chye, R.; Chang, S.; Bayes, A.; Loo, C.; et al. Subcutaneous ketamine infusion in palliative patients for major depressive disorder (SKIPMDD)—Phase II single-arm open-label feasibility study. PLoS One [Internet] 2023, 18, 1–17. [Google Scholar] [CrossRef]
- Famuła, A.; Radoszewski, J.; Czerwiec, T.; Sobiś, J.; Więckiewicz, G. Ketamine in Substance Use Disorder Treatment: A Narrative Review. Alpha Psychiatry. 2024, 25, 206–211. [Google Scholar] [CrossRef]
- Grabski, M.; McAndrew, A.; Lawn, W.; Marsh, B.; Raymen, L.; Stevens, T.; et al. Adjunctive Ketamine With Relapse Prevention–Based Psychological Therapy in the Treatment of Alcohol Use Disorder. Am J Psychiatry. 2022, 179, 152–162. [Google Scholar] [CrossRef]
- Dakwar, E.; Nunes, E.V.; Hart, C.L.; Foltin, R.W.; Mathew, S.J.; Carpenter, K.M.; et al. A single ketamine infusion combined with mindfulness-based behavioral modification to treat cocaine dependence: A randomized clinical trial. Am J Psychiatry. 2019, 176, 923–930. [Google Scholar] [CrossRef]
- Anna, O.; Michael, A.; Apostolakis, M.; Mammadov, E.; Mitka, A.; Kalatta, M.A.; et al. Ketamine and hydroxynorketamine as novel pharmacotherapies for the treatment of Opioid-Use Disorders. Biol Psychiatry [Internet]. 2024, 563–379. [Google Scholar]
- Paice, J.A.; Bohlke, K.; Barton, D.; Craig, D.S.; El-Jawahri, A.; Hershman, D.L.; et al. Use of Opioids for Adults With Pain From Cancer or Cancer Treatment: ASCO Guideline. J Clin Oncol. 2023, 41, 914–930. [Google Scholar] [CrossRef]
- Preux, C.; Bertin, M.; Tarot, A.; Authier, N.; Pinol, N.; Brugnon, D.; et al. Prevalence of Opioid Use Disorder among Patients with Cancer-Related Pain: A Systematic Review. J Clin Med. 2022, 11. [Google Scholar] [CrossRef]
- Phillips, J.L.; Norris, S.; Talbot, J.; Birmingham, M.; Hatchard, T.; Ortiz, A.; et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: A randomized controlled trial. Am J Psychiatry. 2019, 176, 401–409. [Google Scholar] [CrossRef]
- Canuso, C.M.; Ionescu, D.F.; Li, X.; Qiu, X.; Lane, R.; Turkoz, I.; et al. Esketamine Nasal Spray for the Rapid Reduction of Depressive Symptoms in Major Depressive Disorder With Acute Suicidal Ideation or Behavior. J Clin Psychopharmacol. 2021, 41, 516–524. [Google Scholar] [CrossRef]
- Hossein, S.; Rengasamy, M.; Uzamere, A.; Spotts, C.; Howland, R.H.; Wallace, M.L.; et al. Effects of ketamine on individual symptoms and symptom networks of depression in a randomised controlled trial of ketamine for treatment-resistant depression. Br J Psychiatry. 2025, 1–10. [Google Scholar] [CrossRef]
- Salloum, N.C.; Fava, M.; Hock, R.S.; Freeman, M.P.; Flynn, M.; Hoeppner, B.; et al. Time to relapse after a single administration of intravenous ketamine augmentation in unipolar treatment-resistant depression. J Affect Disord [Internet]. 2020, 260, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.L.; Jollant, F.; Tritschler, L.; Colle, R.; Corruble, E.; Gardier, A.M. Pharmacological Mechanism of Ketamine in Suicidal Behavior Based on Animal Models of Aggressiveness and Impulsivity: A Narrative Review. Pharmaceuticals. 2023, 16, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.D.; DeVries, F.E.; Doyle, Z.; McIntyre, R.S.; Rodin, G.; Zimmermann, C.; et al. A Phase II, Open-Label Clinical Trial of Intranasal Ketamine for Depression in Patients with Cancer Receiving Palliative Care (INKeD-PC Study). Cancers (Basel) [Internet]. 2023, 15, 400. [Google Scholar] [CrossRef]
- Orhurhu, V.; Orhurhu, M.S.; Bhatia, A.; Cohen, S.P. Ketamine Infusions for Chronic Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Anesth Analg. 2019, 129, 241–254. [Google Scholar] [CrossRef]
- Thompson, T.; Whiter, F.; Gallop, K.; Veronese, N.; Solmi, M.; Newton, P.; et al. NMDA receptor antagonists and pain relief: A meta-analysis of experimental trials. Neurology. 2019, 92, E1652–62. [Google Scholar] [CrossRef]
- Jiao, J.; Fan, J.; Zhang, Y.; Chen, L. Efficacy and Safety of Ketamine to Treat Cancer Pain in Adult Patients: A Systematic Review. J Pain Symptom Manage. 2024, 67, e185–210. [Google Scholar] [CrossRef]
- Bennett, R.; Yavorsky, C.; Bravo, G. Ketamine for Bipolar Depression: Biochemical, Psychotherapeutic, and Psychedelic Approaches. Front. Psychiatry 2022, 13, 867484. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S. Ketamine and rapid-acting antidepressants: A window into a new neurobiology for mood disorder therapeutics. Annu. Rev. Med. 2021, 72, 3–16. [Google Scholar] [CrossRef]
- Krystal, J.H.; Sanacora, G.; Duman, R.S. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol. Psychiatry 2019, 86, 513–525. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Pedrelli, P.; Katz, J.; Sanacora, G. Ketamine in Major Depressive Disorder: An Update on Efficacy, Safety and Mechanisms of Action. Psychiatr. Clin. N. Am. 2020, 43, 145–156. [Google Scholar]
- Dakwar, E.; Levin, F.R. Ketamine: A Comprehensive Review of Pharmacokinetics, Pharmacodynamics, and Therapeutic Efficacy in Psychiatric Disorders. J. Clin. Psychiatry 2020, 81, 19rs12812. [Google Scholar]
- Wilkinson, S.T.; Ballard, E.D.; Bloch, M.H.; Murrough, J.W.; Feder, A.Y.; Zarate, C.A. Jr.; et al. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am. J. Psychiatry 2018, 175, 150–158. [Google Scholar] [CrossRef]
- Dore, J.; Turnbull, D.A.; Turnbull, S.J.; Smith, R.K.; Richards, V.H.; Richmond, N.K.; et al. Ketamine Assisted Psychotherapy (KAP): Patient Demographics, Clinical Data and Outcomes in Three Large Practices Implementing Ketamine-Assisted Psychotherapy. J. Psychoact. Drugs 2019, 51, 189–198. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Fasula, M.; Pavon, F.; Siegel, M. Ketamine for Treatment-Resistant Depression: Current Evidence and Future Directions. J. Affect. Disord. 2023, 298 Pt A, 765–780. [Google Scholar]
- LeMay, K.; Wilson, K.G. Treatment of existential distress in life threatening illness: A review of manualized interventions. Clin. Psychol. Rev. 2008, 28, 472–493. [Google Scholar] [CrossRef] [PubMed]
- Cornish, N.; Coles, T.; Cheng, M.J.; Sotomayor, C.R.; Wolfgang, A.; Spevak, C. Psychedelics, spirituality, and existential distress in patients at the end of life. Cleve. Clin. J. Med. 2025, 92, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Iglewicz, A.; Morrison, K.; Nelesen, R.A.; et al. Ketamine for the treatment of depression in patients receiving hospice care: A retrospective medical record review of thirty-one cases. Psychosomatics 2015, 56, 329–337. [Google Scholar] [CrossRef]
- Garel, N.; Drury, J.; Thibault Levesque, J.; Goyette, N.; Lehmann, A.; et al. The Montreal model: An integrative biomedical-psychedelic approach to ketamine for severe treatment-resistant depression. Front. Psychiatry 2023, 14, 1268832. [Google Scholar] [CrossRef]
| Model | Biochemical | Psychotherapeutic | Psychedelic |
| Name | Ketamine Infusion Therapy | Ketamine-Assisted Psychotherapy | Psychedelic Therapy |
| Objective | Symptom management | Catalyzing psychological change | Facilitating transformative experiences |
| Focus | Medication effects | Psychotherapy | Subjective experience |
| Number of Sessions | 6 to 12 sessions | 3 to 4 sessions | 1 to 2 sessions |
| Procedure | No preparation or integration | Includes preparation and integration | May or may not include preparation and integration |
| Administration Routes | Intravenous | Oral / Sublingual / Intramuscular / Subcutaneous | Intramuscular / Subcutaneous |
| Treatment Setting | Procedure room | Therapy office | Therapy offices & group settings |
| Component | Description |
| Population | Patients receiving palliative care; capable of engaging in brief psychological interventions |
| Dose & Administration | Two intravenous ketamine sessions (0.3–1. mg/kg over ~40 minutes), spaced ~5–10 days apart |
| Psychological Framework | Preparation, experience, and integration; emphasis on meaning-making, emotional exploration, and patient-centered care |
| Setting | Quiet, comfortable, non-medicalized room; minimal clinical cues |
| Sensory Modulation | Use of blindfolds and curated music playlists to enhance immersive and symbolic experience |
| Framing of Experience | Experiences presented as potentially meaningful reflections of inner states, not random or pathological |
| Therapeutic Stance | Emotional validation over interpretation; process emphasized over content |
| Patient Involvement | Collaborative selection of music, language, and therapeutic orientation; flexibility in session goals |
| Integration | Post-session reflection emphasizing emotional insights, symbolic coherence, and existential relevance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




