1. Introduction
Cervical spondylosis is a common disease characterized by degenerative changes in the cervical spine affecting the neurovascular structures, which is classified into vertebral artery,radiculopathy,myelopathy,and other subtypes[
1]. Cervical spinal canal stenosis caused by ligamentum flavum hypertrophy has a high risk of disability due to its dynamic compression mechanism. The most common level is C5-6, followed by C6-7, C4-5, C3-4, and C2-3[
2,
3]. With social progress and changes in lifestyle, the incidence of cervical spinal stenosis has shown a significant upward trend. Multilevel cervical spinal stenosis accounts for 8% to 10% of all cases of cervical spondylosis and is becoming more prevalent among younger individuals[
4]. This condition severely impacts patients' quality of life while presenting significant challenges to public health system[
5].
In the past, the treatment of cervical spinal stenosis—particularly ligamentum flavum hypertrophy—mainly relied on open posterior cervical surgery including open-door laminoplasty, double door laminoplasy and cervical laminectomy[
6,
7,
8].Among these, open-door laminoplasty has gradually been adopted by an increasing number of scholars worldwide due to its low surgical difficulty and good results. However, this operation could cause significant damage to the muscles and ligaments in the posterior cervical region during surgery and many patients experience axial pain and other symptoms after surgery[
9].
With the development of minimally invasive surgery, the application of spinal endoscopy in the cervical spine has become increasingly widespread. A typical example is the “keyhole” surgery for the treatment of cervical radiculopathy[
10]. In the case of cervical single-level ligamentum flavum hypertrophy, the spinal endoscopy offers significant advantages in terms of more precise localization and minimally invasive procedures. Additionally, the endoscopy group showed significant improvement in JOA scores after surgery and shorter hospital stays[
11]. The incidence of axial symptoms after surgery was also significantly lower in the endoscopic group than in the open-door laminoplasty group. In addition, the large-channel spinal endoscope offers unique advantages. First, the 10mm working channel provides a wider visualization compared to the 6.3mm working channel, reducing blind spots during surgery and lowering the potential risk of breaking through the ligamentum flavum and entering the spinal canal which could damage the spinal cord. Secondly, endoscopic surgery can achieve bilateral decompression through a unilateral incision by adjusting the angle of the working channel, which is particularly suitable for patients with bilateral spinal canal stenosis[
12,
13,
14]. However, applying this technique to multilevel spinal canal stenosis and its impact on postoperative cervical stability require further investigation.
We conducted a retrospective cohort study targeting patients with multilevel spinal canal stenosis primarily characterized by ligamentum flavum hypertrophy. We analyzed the clinical and imaging data of patients who underwent large-channel spinal endoscope unilateral laminotomy decompression at our hospital from January 2020 to December 2023, and compared them with patients who underwent open-door laminoplasty during the same period. The aim is to preliminarily assess the safety and efficacy of this surgical technique, providing a reference basis for technical improvements and widespread adoption.
2. Materials and Methods
This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Approval No. 2023-KY-1201) and was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Patient Inclusion and Exclusion Criteria
(1) Diagnosis of multilevel cervical spinal canal stenosis (involving ≥2 levels) and inadequate response to conservative treatment;(2) Radiographic evidence reveal cervical spinal canal stenosis characterized by ”clamp-type” compression or primarily due to ligamentum flavum hypertrophy(ligament thickness ≥3 mm on T2-weighted MRI with compressing the dural sac(3) Age ≥18 years;(4) Provision of written informed consent form;(5)Complete follow-up data is available.
Exclusion Criteria
(1) Spinal canal stenosis caused by other etiologies (e.g.disc herniation occupying >50% of the spinal canal cross-sectional area, traumatic injury, or neoplastic compression);(2) Patients with a history of cervical spine surgery;(3) Patients with cervical spine tumors, infections, or other conditions causing symptoms similar to cervical spondylosis;(4) Pregnancy or active lactation;(5) Patients who are unable or unwilling to comply with the study requirements
Baseline information
We collected data on 36 patients with multilevel cervical spinal canal stenosis who underwent surgical treatment in our hospital between January 2020 and December 2023.Patients were divided into two groups according to the surgical method: endoscopic group (n=16) and open group(n=20). All patients were strictly included according to the criteria.
Surgical procedure
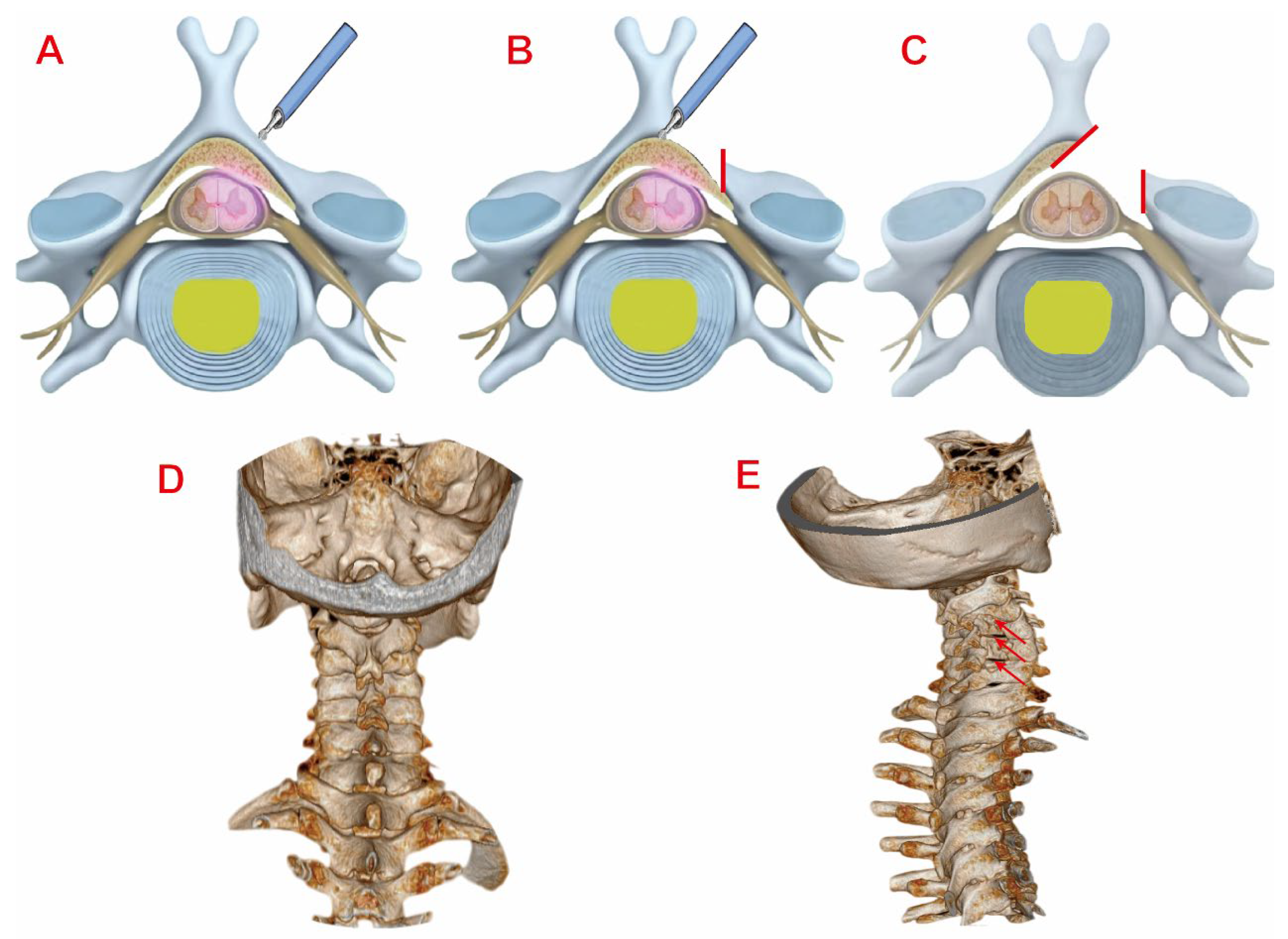
Endoscopic Group:Under general anesthesia, patients were positioned prone with cervical flexion. After head/shoulder fixation and sterile draping, the target levels were localized using C-arm fluoroscopy.Make a 1 cm skin incision (0.5 to 1 cm lateral to the midline of the spinous process).The dilation catheter and working tube were inserted in sequence to reach the surface of the lamina with blunt soft-tissue dissection. Under fluoroscopic guidance, the position of the working tube was confirmed to be accurate before connecting to the endoscopic system. The soft tissues on the surface of the vertebral lamina and the root of the spinous process were cleaned and the bleeding was stopped by radiofrequency electrocoagulation, which exposed the root of the spinous process, the same-side lamina.Using diamond abrasor to polish lamina to a transparent thin layer- initially addressing the rostral margin of the inferior vertebral lamina in an inside-out trajectory, followed by the caudal margin of the superior vertebral lamina. After fully exposing the ligamentum flavum, remove it with nucleus pulposus forceps and use laminectomy rongeur to fully decompress. The same method is used for handling other areas of responsibility. In cases of bilateral cervical spinal canal stenosis, the contralateral ligamentum flavum could be resected via the "over-the-top" technique. After adequate decompression, the spinal cord and nerve roots are completely relaxed and pulsation is good.The endoscope system is then withdrawn. Skin incisions are closed in layers after standard antisepsis. The surgical illustration is demonstrated in
Figure 1.
Open Group:After general anesthesia induction, patients were positioned prone with head and shoulders immobilized.Following sterile preparation and draping, a 10 cm midline incision was made from C3 to C7 level. Skin, subcutaneous tissue, and deep fascia were incised sequentially.Paraspinal muscles were bluntly dissected bilaterally to expose spinous processes and laminae. After confirming vertebral levels: C3-C7 spinous process bases were resected.The side with more severe symptoms is the open-side, and the other side is the hinged-side.Hinged-side laminae were thinned to unicortical bone using a rongeur. Open-side laminae underwent full-thickness resection.Drive anchors with wires into the hinged side,then lift the vertebral lamina on the side of the open-side and secure it with wire.After fully expanding the spinal canal and thoroughly decompressing it, pulsation of the dura mater was observed, with no obvious compressive objects. The incision was irrigated, bleeding was thoroughly stopped, gelatin sponges were placed, and pre-trimmed autologous bone was inserted on the hinge side. After placing the drainage tube, the incision was closed layer by layer.
Efficacy evaluation
Demographic and operative parameters were recorded for both groups: including Age, sex, body mass index (BMI),Comorbidities (e.g., hypertension), Number of involved vertebral levels, Incision length, operative time, intraoperative blood loss, hospital stay.Clinical outcomes were assessed preoperatively and at 1, 3, 6, 12 months postoperatively using: Visual Analog Scale (VAS) for neck/upper extremity pain,Neck Disability Index (NDI), Japanese Orthopaedic Association (JOA) score.Neurological recovery rate was calculated at final follow-up: .
All radiographic parameters were independently measured by two orthopaedic surgeons to ensure reproducibility of the results.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics(version 26.0; IBM Corp.) Continuous data are presented as mean ± standard deviatio (±s), while categorical data are expressed as frequencies and percentages.Normality of continuous variables was assessed using the Shapiro-Wilk test. Intergroup comparisons of normally distributed data were conducted with independent samples t-tests, and multigroup analyses used one-way ANOVA. Non-normally distributed quantitative data were analyzed with the Wilcoxon rank-sum test and reported as median.Categorical data were evaluated using Pearson's χ² or Fisher's exact tests for nominal variables and Mann-Whitney U tests for ordinal variables. P < 0.05 was defined a statistically significant difference.
3. Results
Basic parameters and perioperative information
A total of 36 patients were enrolled, including 16 in the endoscopic group and 20 in the open group. Basic parameters and perioperative information of the two groups are summarized in
Table 1. No significant differences were observed in demographic or clinical baseline parameters between the two groups (P > 0.05). All procedures were successfully completed without intraoperative complications and post-operative infections. All incisions achieved good healing.The endoscopic group had significantly shorter surgical times(1.6±0.6h vs 2.1±0.2h), smaller incision lengths(1.3±0.1cm vs 9.5±0.7cm), less blood loss(12.4±7.4ml vs 64.3±19.5ml), and shorter hospital stays(6.6±1.1 days vs 8.6±1.4 days) than the open surgery group, with statistically significant differences(P<0.05).Ligamentum flavum hypertrophy predominantly involved:C4–C5(81.3%),C3–C4(62.5%), C5–C6( 56.2%).Preoperative T2-weighted MRI showed a mean thickness of 3.8 ± 0.6 mm (range: 3.1–5.2 mm).
Clinical Outcomes
Serial assessments of pain and functional outcomes are detailed in
Table 2. Both groups showed significant improvements in VAS, JOA, and NDI scores at all postoperative time points (1, 3, 6, 12 months and final follow-up) compared with preoperative baselines (P < 0.05). Interestingly, the VAS score was better in the endoscopic group than in the open group at 1-month follow-up(VAS score 2.69 ± 0.79 vs. 4.40 ± 0.88 P < 0.05). There was no statistically significant difference in the rate of neurological improvement between the two groups at each follow-up time point.
Changes in Radiographic Parameters
Radiographic parameters of both groups preoperatively, postoperatively, and at final follow-up are summarized in
Table 3 and
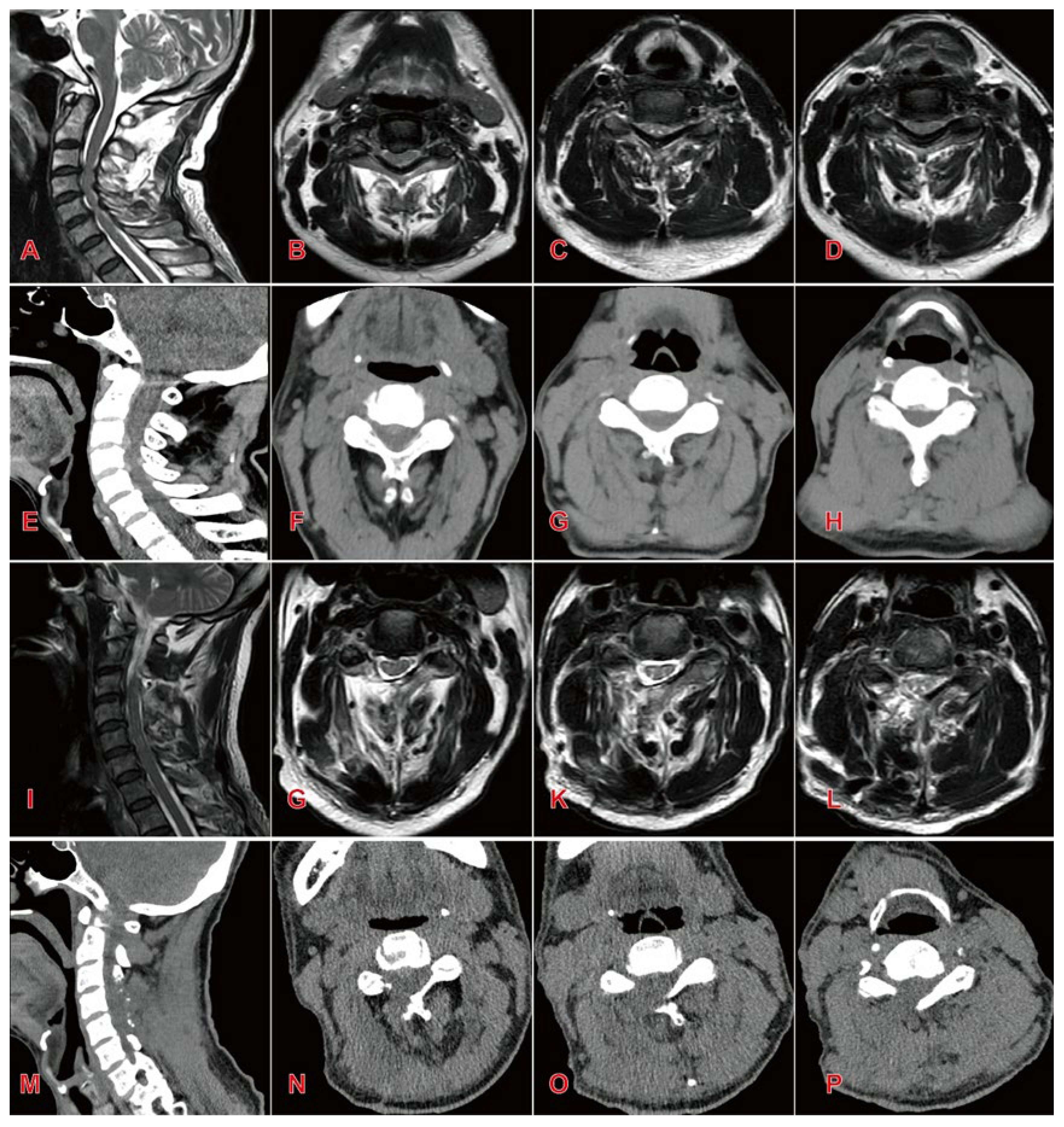
Table 4. Both groups showed significant improvement in spinal canal area at the affected levels postoperatively (P < 0.05). However,the open group exhibited significantly higher segmental Pavlov ratios than the endoscopic group at both postoperative and final follow-up assessments (P < 0.05).No statistically significant intergroup differences were detected in C2–C7 Cobb angles pre- or postoperatively (P > 0.05). Notably, at final follow-up, the Endoscopic Group demonstrated significantly smaller C2–C7 Cobb angles compared to the Open Group (13.57 ± 2.29° vs. 16.34 ± 2.95°, P < 0.05).Similarly, T1 slope angles showed no significant intergroup differences pre- or postoperatively (P > 0.05). However, at final follow-up, the endoscopic group maintained significantly reduced T1 slopes relative to the open group (22.62 ± 1.51° vs. 25.24 ± 2.41°, P < 0.05). Representative case 1 images are presented in
Figure 2.
Complications
Two patients in the open group developed postoperative unilateral deltoid and biceps weakness with pain in the C5 dermatome, consistent with C5 nerve root palsy. One additional patient in this group experienced axial pain. All three received pharmacological management with corticosteroids, dehydrating agents, and anti-inflammatory analgesics to alleviate nerve root edema and irritation.Postoperative management included drainage system placement combined with therapeutic interventions: intravenous hydration, neurotrophic pharmacotherapy, and bed rest. In the Endoscopic Group, one patient exhibited transient bilateral upper limb weakness, which resolved with dehydrating agents and neurotrophic drugs by discharge. We considered that factors such as water pressure impact during surgery and prolonged surgery time may have caused transient nerve damage.At the 6-month follow-up, complete neurological recovery was confirmed. No revision surgery was required in either cohort.
4. Discussion
The open-door laminoplasty was first pioneered by Hirabayashi and has been continuously improved as an effective surgical procedure for treating multilevel spinal canal stenosis[
7,
15]. However, this approach carries inherent limitations that rongeur removal of the outer cortical bone at the hinged-side compromises structural integrity, elevating fracture risk[
16]. Subsequent segmental instability may manifest as postoperative neck pain or spinal cord dysfunction in patients. As spinal endoscopy theory and equipment have continuously improved, the range of applications is growing. Previous literature reported a 56-year-old male patient with C2-3 cervical spinal canal stenosis caused by calcification of the ligamentum flavum, who underwent large-channel spinal endoscope laminotomy decompression and recovered well after the operation[
17]. Similarly, D. Carr et al. demonstrated a 90.7% overall excellent rate following endoscopic unilateral laminotomy for bilateral decompression in 10 severe cervical stenosis cases[
18]. These studies indicate that large-channel spinal endoscope laminotomy decompression techniques is feasible in the treatment of single-level cervical spinal stenosis, effectively alleviating symptoms of cervical spinal cord compression, and laying the foundation for its application in multi-levels cervical spinal stenosis.
This study compared the efficacy and differences between large-channel spinal endoscope cervical laminotomy decompression and open-door laminoplasty for multilevels cervical stenosis cases. The endoscopic approach minimizes iatrogenic injury through enhanced intraoperative visualization and precision, preserving posterior muscular attachments and facet joint integrity which is particularly critical given that more than 50% compromise of the facet joints significantly destabilises the cervical spine.Radiographically, the endoscopic group exhibited reduced T1 slopes at final follow-up versus preoperative baselines, whereas open group demonstrated increased T1 slopes (P < 0.05). This divergence reflects open group's substantially greater trauma to posterior musculoligamentous complexes and joints, predisposing to postoperative kyphosis deformity, which is consistent with the research conducted by Fu et al[
19]. Importantly, although the spinal canal area in the endoscopic group was smaller than that in the open group after surgery and the Pavlov ratio at each segment was also lower than that in the open group, it is interesting to note that both groups demonstrated significant improvements in VAS, JOA, and NDI scores at all post-operative time points compared with their pre-operative status, with no significant difference observed in the JOA improvement rate between the two groups after surgery.This indicates that both surgical approaches are effective in relieving symptoms and promoting functional recovery. However, when considering other factors such as hospital stay duration and hospitalization costs, the endoscopic group patients exhibits superiority over the open group in terms of patients' overall post-operative satisfaction.
Open-door laminoplasty for multilevel cervical spinal canal stenosis requires resection of ≥3 segments of the laminae to utilize the bowstring biomechanical effect for spinal cord decompression[
15,
20]. However, this extensive approach inherently risks iatrogenic structural compromise, correlating with higher complication rates. In this study, two patients in the open group developed C5 nerve root palsy and one patient developed axial pain symptoms after surgery. Following Open-door laminoplasty, the spinal cord was adequately decompressed, and the dura mater drifted backward, which results in traction on the nerve roots. C5 nerve root paralysis occurred because the C5 nerve root is relatively short[
21,
22].Pharmacological management (corticosteroids + dehydrating agents) effectively reduced root edema. Studies have found that more than 70% of patients with C5 nerve root palsy can fully recover muscle strength within 4-5 months after surgery[
23].Large-channel spinal endoscope cervical laminotomy decompression can precisely remove compressive tissue and expand the spinal canal area through visual guidance. At the same time, it reduces intraoperative bleeding and injury. It has the advantages of minimal injury, less bleeding, quick recovery, and high patient satisfaction.
Precise decompression of spinal endoscopy is achieved by resecting the caudal margin of the superior lamina and rostral margin of the inferior lamina for the treatment of single-level cervical spinal stenosis. For Multi-level cervical spinal stenosis, it is usually necessary to completely remove the unilateral lamina of the middle segments. This not only helps to fully expand the spinal canal area, but also reduces the risk of residual bone fractures and inadvertent spinal cord injury.
In addition, I have the following insights regarding the details of the surgical procedure: (1)The size of the incision is critical to the success of the surgery. We typically select a 1 cm incision. An incision that is too large may affect the tightness of the large channel. When inserting the working channel, it should be rotated clockwise to avoid displacement or instability. (2) When removing the lamina, we should make initial lamina thinning with horizontal burring force. Then use the laminar rongeur to gently remove the inner layer of cortical bone, gradually exposing the ligamentum flavum. This is very important and can effectively prevent diamond abrasor damage to the spinal cord. It should be emphasized that spinal endoscopic surgery entails a steep learning curve and demands advanced surgical skills for the operator. In addition, the indications for this surgical operation are relatively limited. The surgical indications should be strictly controlled to avoid poor postoperative efficacy. This study has several limitations: First, the small sample size and short follow-up duration constrain statistical power. Second, the assessment of cervical stability mainly relies on two-dimensional measurement methods. Future investigations should employ three-dimensional reconstruction to quantify dynamic changes in spinal canal volume and biomechanical parameters. Furthermore, as a single-center retrospective study, the generalizability of findings may be limited. Prospective randomized controlled trials are warranted to corroborate these conclusions.
5. Conclusions
In summary, for the treatment of multilevel cervical spinal stenosis, large-channel spinal endoscope unilateral laminotomy decompression demonstrates comparable short-term efficacy to open-door laminoplasty. Furthermore, the endoscopic approach offers distinct advantages including minimally invasive access, reduced intraoperative blood loss, accelerated postoperative recovery, and relatively higher patient satisfaction.
Author Contributions
Conceptualization, Yiping Zheng. and Luyang Wang.; methodology, Donglin Yang.and Xiaoxin Chen; software, Mingwang Zhao and. Yongchun Zhang; validation, Xingchen Li; writing—original draft preparation, Yiping Zheng; writing—review and editing, Xingchen Li.and Yusheng Xu ; funding acquisition, Yusheng Xu. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Approval No. 2023-KY-1201).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VAS |
Visual Analog Scale |
| JOA |
Japanese Orthopaedic Association score |
| NDI |
Neck Disability Index |
| BMI |
Body mass index |
References
- Theodore, N. Degenerative Cervical Spondylosis. N Engl J Med 2020, 383, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Okada, E.; et al. Modic changes in the cervical spine: prospective 10-year follow-up study in asymptomatic subjects. J Bone Joint Surg Br 2012, 94, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Hansson, T.; Suzuki, N.; et al. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. European spine journal 2009, 18, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Hoti, Y.U.D.; Aziz, A.; et al. Clinical Outcome of Laminoplasty in Cervical Myelopathy. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP 2018, 28, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Lv Y, Tian W, et al. The prevalence and associated factors of symptomatic cervical Spondylosis in Chinese adults: a community-based cross-sectional study. BMC musculoskeletal disorders 2018,19(1),325.
- Wang, L.; Liu, S.Y.; et al. Comparative study of Kurokawa's double door laminoplasty and modified Kurokawa's double door laminoplasty for the treatment of cervical disorders. Zhonghua Wai Ke Za Zhi 2013, 51, 508–512. [Google Scholar] [PubMed]
- Hirabayashi, K.; Watanabe, K.; et al. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976) 1983, 8, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Kirita, Y. Extensive simultaneous multisegment laminectomy for myelopathy due to the ossification of the posterior longitudinal ligament in the cervical region. Spine (Phila Pa 1976) 1986, 11, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Lin CR, Tsai SHL, et al. What is the best strategy for C3 in open-door laminoplasty: laminectomy versus laminoplasty-a systematic review and meta-analysis. Spine J 2025,25(7),1440-54.
- Wu PF, Li YW, et al. Posterior Cervical Foraminotomy Via Full-Endoscopic Versus Microendoscopic Approach for Radiculopathy: A Systematic Review and Meta-analysis. Pain Physician 2019,22(1),41-52.
- Song, C.; Wei, Z.; et al. Clinical study of posterior percutaneous large channel full-endoscopic cervical laminectomy and decompression in the treatment of single-segment cervical spondylotic myelopathy. Chinese Journal of Anatomy and Clinics 2021, 26, 61–67. [Google Scholar]
- Han, S.; Zeng, X.; et al. Clinical Application of Large Channel Endoscopic Systems with Full Endoscopic Visualization Technique in Lumbar Central Spinal Stenosis: A Retrospective Cohort Study. Pain Ther 2022, 11, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Siepe CJ, Bridts AL, et al. Full-endoscopic bilateral over-the-top decompression in lumbar central stenosis: surgical technique and outcomes. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2023,32(8),2918-23.
- Huang, Y.H.; Lien, F.C.; et al. Full Endoscopic Uniportal Unilateral Laminotomy for Bilateral Decompression in Degenerative Lumbar Spinal Stenosis: Highlight of Ligamentum Flavum Detachment and Survey of Efficacy and Safety in 2 Years of Follow-up. World neurosurgery 2020, 134, e672–e681. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, K.; Watanabe, K. A Review of My Invention of Expansive Laminoplasty. Neurospine 2019, 16, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Vedantam A, Harinathan B, et al. Differences in spinal cord biomechanics after laminectomy, laminoplasty, and laminectomy with fusion for degenerative cervical myelopathy. J Neurosurg Spine 2023,39(1),28-39.
- Lin, Y.; Rao, S.; et al. Posterior Percutaneous Full-Endoscopic Cervical Laminectomy and Decompression for Cervical Stenosis with Myelopathy: A Technical Note. World neurosurgery 2019, 124, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Carr DA, Abecassis IJ, et al. Full endoscopic unilateral laminotomy for bilateral decompression of the cervical spine: surgical technique and early experience. Journal of spine surgery (Hong Kong) 2020,6(2),447-56.
- xin FJ, Yi J, et al. Relationship between T1 slope angle and cervical curvature change after posterior cervical single open-door lamin-oplasty. Orthopedic Journal of China 2015,23(15),1360-4.
- Cho SK, Kim JS, et al. Cervical Laminoplasty: Indications, Surgical Considerations, and Clinical Outcomes. J Am Acad Orthop Surg 2018,26(7),e142-e52.
- Usami, Y.; Yokota, A.; et al. Morphology of cervical periradicular fibrous sheath and nerve roots in relation to postoperative C5 palsy. Spine J 2022, 22, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Currier, B.L. Neurological complications of cervical spine surgery: C5 palsy and intraoperative monitoring. Spine (Phila Pa 1976) 2012, 37, E328–E334. [Google Scholar] [CrossRef] [PubMed]
- Thompson SE, Smith ZA, et al. C5 Palsy After Cervical Spine Surgery: A Multicenter Retrospective Review of 59 Cases. Global Spine J 2017,7(1 Suppl),64s-70s.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).