Introduction
Dengue is an emerging arboviral infection with the potential to cause severe health complications, despite its relatively low mortality rate. The global burden of dengue has escalated dramatically in recent years, with the World Health Organization (WHO) reporting an eightfold increase in cases over the past two decades [
1]. In 2024 alone, over 14 million cases and more than 10,000 dengue-related deaths were reported globally, with unprecedented major outbreaks occurring in the Americas [
2]. This increase has been attributed to a complex interplay of factors, including rapid urbanisation, globalisation and increased human mobility, and persistent challenges in sustaining effective vector control. Climate change also contributed by expanding the geographic range of competent vectors and enhancing transmission suitability in many regions (3, 4). Although the overall case-fatality rate for dengue is low, the number of cases contributes to a substantial public health and societal burden, and among the subset of patients who progress to severe dengue, the risk of mortality and significant morbidity is considerably higher, impacting healthcare systems, particularly intensive care units. [
5].
The clinical evaluation of dengue interventions is complicated by the virus’s distinctive immunopathology. Pre-existing, non-neutralising antibodies from a prior infection with one serotype can paradoxically exacerbate the severity of a subsequent infection with a different serotype, a phenomenon known as antibody-dependent enhancement. Currently, there are no specific treatment modalities for dengue, but extensive research efforts are underway worldwide. Clinical trials are exploring various therapeutic candidates targeting different stages of the disease, including antivirals in the early viraemic phase and immunomodulatory therapies in late-stage/hospitalised patients with complications. While a dengue vaccine is available in some settings, its use remains limited due to constraints in efficacy, safety, and eligibility criteria, particularly in endemic regions. Meanwhile, a significant challenge in the impact of these trials is the heterogeneity of reported outcomes and the measurement instruments used. This variability complicates the comparison of results across studies, remains a barrier for meta-analyses, and hinders clinical guideline development.
To address this issue, Core Outcome Sets (COS) were proposed as a strategy to harmonise outcome reporting in clinical research [
6]. A COS is an agreed-upon, standardised selection of outcomes that should be measured and reported in all clinical trials for a specific condition. A Core Outcome Measurement Set (COMS) recommends how these outcomes should be measured. Implementing a COMS can reduce heterogeneity, enhance data quality, and facilitate evidence synthesis, thereby improving the overall quality of research in the field [
7].
This project is a part of the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) activities on research harmonisation and aimed to develop a COMS for Phase III/IV dengue clinical trials. The objective was to achieve consensus on the most critical outcomes to be assessed and the most appropriate measurement instruments for assessing these outcomes.
Methods
This study followed the Core Outcome Measures in Effectiveness Trials (COMET) Initiative framework [
6] and adhered to the Core Outcome Set–Standards for Development (COS-STAD) guidelines [
8]. The focus of this project was on the outcomes for Phase III/IV clinical trials evaluating intervention for dengue treatment irrespective of age. The study protocol was developed a priori, and the project was registered (
https://comet-initiative.org/Studies/Details/3029). Ethical approval for the study was obtained from the Oxford University Tropical Research Ethics Committee on 12
th June 2024 (533-24). We report this study according to the ACCORD (ACcurate Consensus Reporting Document) and Core Outcome Set–Standards for Reporting (COS-STAR) guidelines.
The WHO acted as a collaborator throughout the project. The “management group” consisting of SY, AD, XHSC, LM and DM was responsible for the study’s methodology and management. The steering committee that oversaw the project development and participated in key discussions throughout the project included LL, NM, BK, TJ, SM and PB (Appendix 1, p 4). The DEN-CORE Study Group consisted of healthcare professionals, researchers, industry representatives (in non-voting capacity throughout), methodologists, WHO representatives, and people with lived experience.
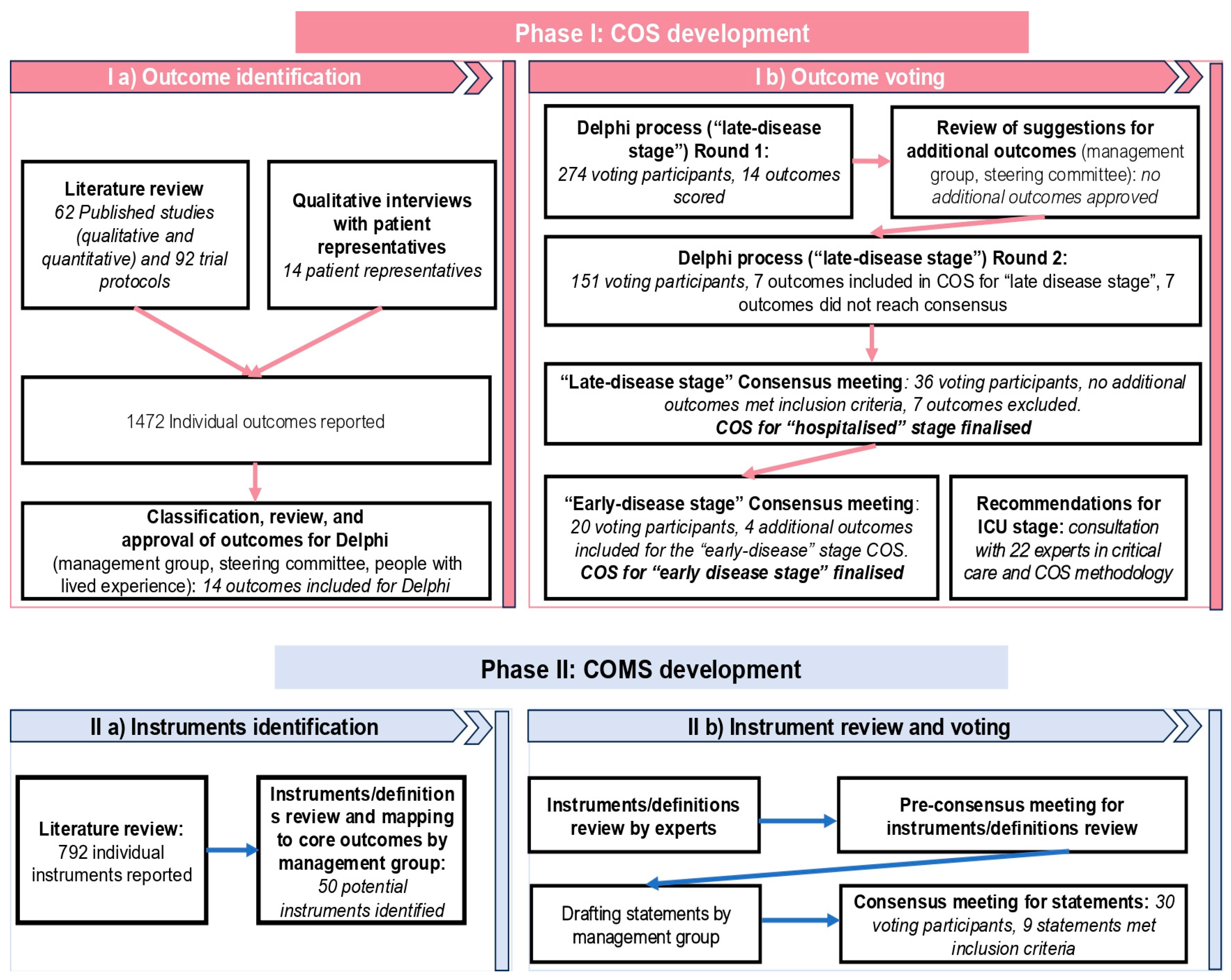
The study was conducted in two phases — phase I: COS development and phase II: COMS development (
Figure 1). The management group and steering committee decided a priori that outcomes should be reviewed for different stages of the disease separately (‘early’, ‘hospitalised’ and ‘ICU/HDU’) due to the specifics of dengue’s disease progression and inherent differenced in clinical management and standard of care.
Within this process these three disease stages were defined as follows:
‘early stage’ of the disease corresponding to the febrile or viraemic phase, prior to severe manifestations and the vast majority of patients are managed in outpatient settings.
‘hospitalised’ stage referred to the late or critical phase of the disease, when patients have developed complications.
Outcomes specific to trials that recruit individuals admitted to high-dependency units (HDU) or intensive care units (ICU) only were reviewed separately under the “ICU/HDU”.
Phase I: COS Development
Development of the COS involved: (a) outcome identification, and (b) outcome voting stage, which included a two-round online modified Delphi consensus process to rate the importance of the outcomes for the ‘hospitalised’ COS and an online interactive consensus meeting to review and agree upon the final ‘hospitalised’ COS and additional consensus meeting to ‘adapt or adopt’ ‘hospitalised’ COS for ‘early stage’. It was agreed that the need for dengue-specific COS for ‘ICU’ will be discussed with critical care physicians and methodologists.
Phase Ia: Outcome Identification
We conducted a comprehensive search of Medline and Embase, Clinical Trials.gov, and the International Clinical Trials Registry Platform from inception until September 11, 2023 (appendix 1, p 11).
Protocols and abstracts, then publications were screened for inclusion by pairs of team members (AHo, EKa, DBa, LXi, MKo, AAj, AMu) independently. Any disagreements between the screeners were resolved via consensus or a third reviewer. Outcome data were extracted by two reviewers independently and resolved for discrepancies. Outcomes were classified into domains using recommended taxonomy by Dodd and colleagues [
9]
The Oxford University Clinical Research Unit (OUCRU), Vietnam dengue patient representatives, provided perspectives on what outcomes they would like new treatments to target during semi-structured interviews (Appendix 1, p 25). Outcomes identified through the literature review and patient input were compiled and refined by the Management Group and Steering Committee, and accompanying descriptions were developed. The final list was then presented to people with lived experience to ensure the inclusion of relevant outcomes and the clarity of language used in outcome descriptions (Appendix 1, p 29).
Phase Ib: Outcome Voting
Delphi process (‘hospitalised’) and definitions
We conducted a two-round online modified Delphi consensus process for ‘hospitalised’ COS. The study team invited participants from published studies, professional organisations, research collaborators and professional networks and publicly available ISARIC webpage.
The Delphi survey was delivered in 11 languages (English, Spanish, Vietnamese, Portuguese, Bahasa, Thai, Malay, Nepali, Tagalog, Hindi, Sinhala) using the Qualtrics platform. Prior to its launch, the survey was piloted with members of the management group and native speakers of each translated version.
Participants were categorised into four stakeholder groups: [
1] people with lived experience of dengue and family members/carers; [
2] healthcare professionals/researchers with lived experience of dengue; [
3] healthcare professionals/researchers without lived experience of dengue; [
4] representatives of other dengue stakeholders including funding agencies, governmental and non-governmental bodies, industry, regulatory authorities. Responses from the fourth group were used to inform outcome selection and discussions but were not included in the analysis.
Delphi round 1
In the first round,participants anonymously rated each outcome using the nine-point Grading of Recommendations Assessment, Development and Evaluation (GRADE) scale [
9]. Each outcome had an "unable to rate" option. A free-text option was available to suggest additional outcomes. As per protocol novel outcomes suggested by ≥1% of participants were considered by the Steering Committee for inclusion in the second Delphi round. Collated feedback on the proposed outcomes was presented to participants during Round 2. All outcomes from the first round were included in the second round.
Delphi round 2
For each outcome, participants were shown their own round 1 rating alongside distribution of scores from each stakeholder group. They were then asked to rate each outcome again using the same scale.
Consensus for inclusion of an outcome in the COS was defined a priori as 70% or more of participants in each of the three analysis stakeholder groups rating the outcome as critically important (GRADE rating 7-9). Consensus for exclusion of an outcome from the COS was defined as 50% or less of respondents in each of three stakeholder groups rating the outcome as critically important. Outcomes that did not meet these criteria were discussed at the consensus meeting (appendix 1 p 49).
‘Hospitalised’ COS consensus meeting
Online consensus meeting was conducted by experienced independent facilitator. Outcomes that reached consensus for inclusion during the Delphi rounds were formally ratified. Outcomes that did not reach consensus were individually discussed following a structure of arguments in favour of its inclusion, followed by counterarguments. After the discussion, participants voted anonymously using the GRADE scale. Outcomes rated as critically important by 70% or more participants in all three stakeholder groups were included in the final ‘hospitalised’ COS (appendix 1 p 55).
‘Early stage’ COS consensus meeting
The ‘early-stage’ COS was developed using an ‘adapt or adopt’ methodology. Outcomes from the hospitalised COS were reviewed and considered for inclusion based on their relevance to the early, outpatient-managed phase of disease. An additional online consensus meeting was conducted to determine whether the ‘hospitalised’ COS should be adapted or adopted for dengue's 'early stage'(appendix 1, p 59).
Outcomes already included in the ‘hospitalised' COS were presented and considered for use in 'early stage' clinical trials, then additional outcomes relevant to the 'early stage' were discussed. Discussions, anonymous voting, and inclusion of outcomes voted above the defined thresholds occurred as described in the previous consensus meeting.
COS for ‘ICU/HDU’ trials.
Several COS have already been developed for ICU populations (appendix 1, p 73. According to best practices in outcome standardisation, researchers are encouraged to use existing COS where appropriate, to minimise duplication of efforts and promote consistency across studies (6, 8). To support this approach we developed a recommendation in consultation with experts in critical care and COS methodology.
Data Analysis
Descriptive statistics were used to show the overall GRADE scores of each stakeholder group at each stage, to determine whether they met the predefined criteria for inclusion or exclusion. The protocol defined a priori that only responses from Delphi participants who rated at least 50% of outcomes would be included in the analysis. Bar plots displaying the distribution of ratings for each outcome by stakeholder group were produced using R (version 4.2.1) and shown to participants in the second Delphi round.
Phase II: COMS developmentThe development of the COMS consisted of (a) instrument identification and (b) instrument review and voting stage.
Phase IIa: Instrument identification
The management group reviewed all measurement instruments identified through literature review. Instruments were mapped to the core outcomes voted in the first phase of the project. Instruments that did not map to any of the COS domains were excluded. Additional instruments not used in published research and clinical trial protocols were considered based on expert suggestions.
Phase IIb: Instruments review and voting
The management group compiled a comprehensive list of instruments derived from clinical trials. A group of independent international experts with extensive experience in dengue research and/or clinical care reviewed the identified instruments for feasibility. An online meeting was held to discuss the proposed instruments for investigator-reported outcomes. Based on this meeting, the core group developed a set of preliminary consensus statements. These statements were then presented during the consensus workshop. Due to resource constraints, patient-reported outcome measures will be separately assessed in a future consensus following COSMIN appraisal.
Consensus workshop
The consensus meeting was conducted in a hybrid format and chaired by an experienced facilitator. The statements were presented one by one. For each statement, discussions were held, and feasibility and potential modifications were extensively discussed.
We used the nominal group technique to present, discuss, refine and vote on each statement. Experts could ‘agree’, ‘disagree’ or ‘abstain’ on each vote. An additional option of ‘not having appropriate expertise’ was provided. The same level of 70% agreement was considered as per a priori defined threshold, indicating consensus. Votes of ‘abstained’ were included in the denominator, while votes of individuals declaring ‘not having appropriate expertise’ were excluded from the statistical analysis.
The voting was conducted anonymously using Mentimeter software. Industry representatives participated in discussions but were not allowed to vote.
Results
Phase I: Core Outcome Set development
Phase Ia: Outcome identification
Literature review and patient interviews.
This review included 62 studies and 92 protocols that met the inclusion criteria and reported a total of 1472 outcomes (appendix 1, p15).
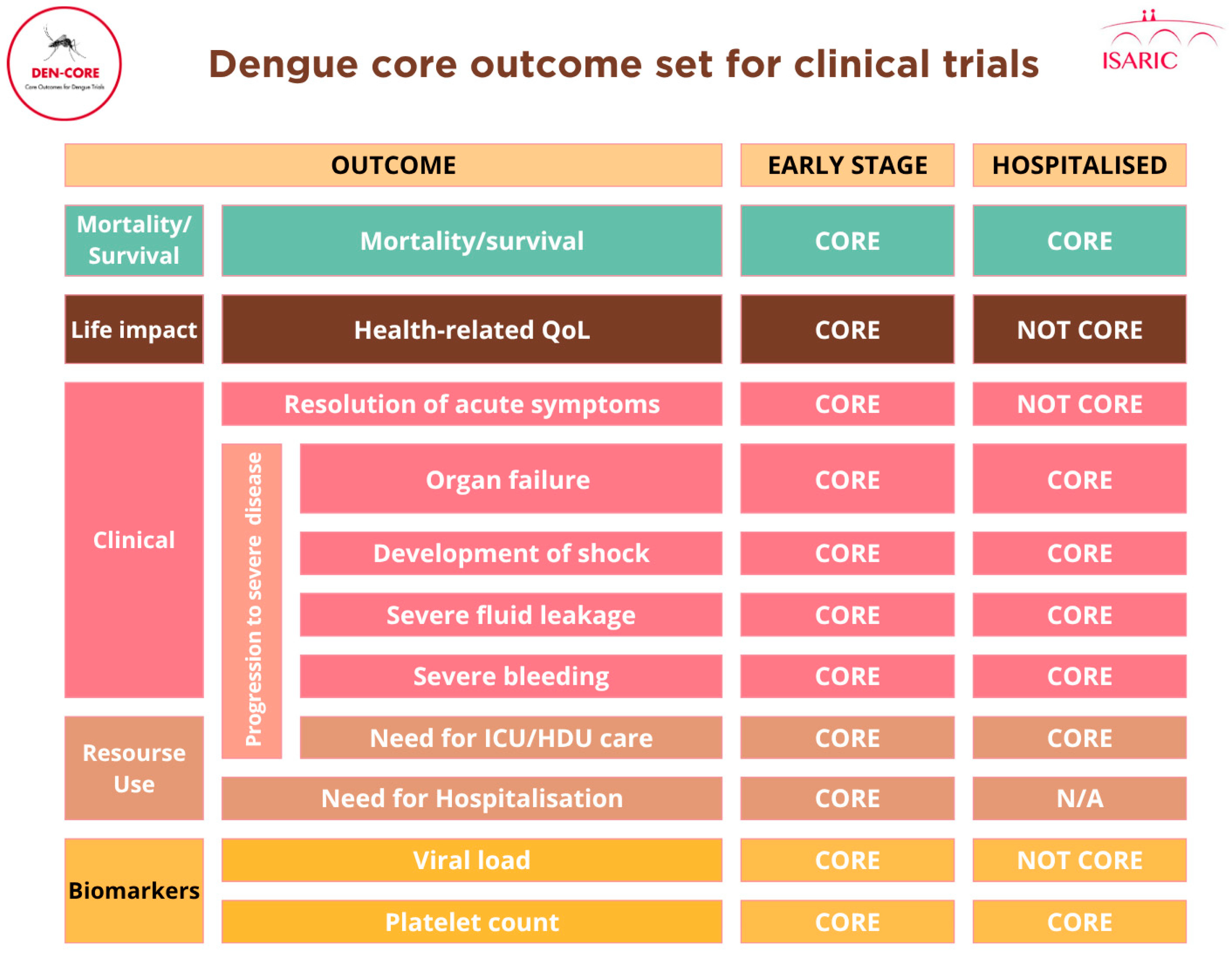
Upon classification and revision by the management group and steering committee members, 14 outcomes (appendix 1 p 29) were included for consideration in the Delphi. These outcomes were categorised into four domains: survival (1 outcome); physiological or clinical (11 outcomes); life impact (1 outcome); and resource use (1 outcome).
Phase Ib: Outcome voting
‘Hospitalised’ Delphi process
The first round of the online Delphi process was conducted from 19 June 2024 to 27 July 2024. A total of 291 participants from 36 countries completed the first round. Of these 274 (94%) were voting participants, representatives of other dengue stakeholders and their outcome rating was excluded from the analysis. In the second round, 160 participants (55%) from 30 countries participated. Of these, 151 were voting participants. Demographic characteristics of the participants for each Delphi round are presented in
Table 1. Further details about the Delphi participants can be found in Appendix 1 p 36.
The management group reviewed 115 submitted free-text responses related to additional outcomes, with no new outcomes added in the second Delphi round. Suggestions were discussed within the management group and steering committee (reasoning of decision-making was provided as a summary to the Delphi participants during the second round and is available in Appendix 1 p 45).
The second Delphi round occurred from 13 August 2024 to 24 September, with 151 participants assessing 14 outcomes. Subsequently, seven outcomes met criteria for inclusion, with one in mortality/survival domain, five in the physiological or clinical domain and one in the resource use domain (
Table 2). Seven other outcomes received mixed ratings across the stakeholder groups, which led to their discussion at a subsequent consensus meeting.
Table 2.
Summary of consensus process voting for outcomes and OMI.
Table 2.
Summary of consensus process voting for outcomes and OMI.
| |
“Hospitalised” stage COS |
“Early stage” COS
|
COMS
|
Results |
| Outcome |
%Stakeholders voting 7-9 in R2 online Delphi |
%Stakeholders voting 7-9 in consensus meeting |
% Stakeholders voting 7-9 in consensus meeting |
% Stakeholders voting for outcome measurement instruments during consensus workshop |
| P |
HCP / RS with lived experience |
HCP / RS without lived experience |
P
|
HCP/RS
|
| Mortality |
94% |
92% |
95% |
NA |
NA |
NA |
100% |
Outcome included in the COS both for “hospitalised” and “early-stage” dengue. Agreement has been reached on measurement instrument |
| Health-related Quality of life |
82% |
49% |
58% |
- |
33% |
84% |
NA |
Outcome excluded for the “hospitalised” dengue COS following consensus meeting. Outcome included in the COS for “early-stage” dengue. OMI were not discussed at the consensus workshop. Work ongoing |
| Time to recovery |
82% |
65% |
79% |
60% |
64% |
NA |
NA |
Outcome not included in the COS
|
| Resolution of acute symptoms |
Fatigue |
65% |
30% |
38% |
25% |
20% |
85% |
NA |
Individual outcomes were excluded for the “hospitalised” dengue COS following consensus meeting.
Outcome “Resolution of acute symptoms” included in the COS for “early-stage” dengue. OMI were not discussed at the consensus workshop. Work ongoing |
| Fever |
82% |
43% |
48% |
25% |
42% |
| Pain |
69% |
41% |
37% |
- |
13% |
| Severe gastrointestinal symptoms |
88% |
68% |
69% |
40% |
37% |
| Progression to severe disease |
Organ failure |
100% |
94% |
96% |
NA |
NA |
NA |
83% |
Outcomes included in the COS following Delphi survey both for “hospitalised” and “early-stage” dengue. Agreement has been reached on measurement instrument |
| Severe fluid/plasma leakage |
100% |
95% |
96% |
NA |
NA |
NA |
84% |
| Development of shock |
94% |
97% |
95% |
NA |
NA |
NA |
92% |
| Severe bleeding |
100% |
92% |
95% |
NA |
NA |
NA |
79% |
| Need for ICU/HDU care |
94% |
95% |
92% |
NA |
NA |
NA |
100% |
| Need for hospitalisation |
NA |
NA |
NA |
NA |
NA |
84% |
83% |
Outcome “Need for hospitalisation” was added to COS for “early stage” prior to the consensus meeting following core team discussions. Agreement has been reached on measurement instrument |
| Platelet count |
94% |
73% |
73% |
NA |
NA |
NA |
92% |
Outcome included in the COS following Delphi survey both for “hospitalised” and “early-stage” dengue. Agreement has been reached on measurement instrument |
| Viral load |
71% |
53% |
49% |
33% |
60% |
85% |
93% |
Outcome excluded for “hospitalised” COS. Outcome included in the COS for “early-stage” dengue. Agreement has been reached on measurement instrument |
COS, core outcome set; HCP, healthcare professionals; OMI, outcome measurement instrument; P, patients; R, researchers. Box 1. Recommendation for ICU dengue clinical trials*
We recommend using the agreed-upon DEN-CORE COS in clinical trials involving mixed populations (hospitalised/early-stage and patients requiring ICU/HDU care), where applicable.
For dengue clinical trials aiming at assessing efficacy of interventions restricted to patients in HDU/ICU settings only, we suggest using one of the existing ICU-specific COS listed in appendix 1 p 73 Selection of the most appropriate COS should depend on the specific needs of the trial.
We strongly encourage use of available critical care COS to ensure consistency across trials. |
Table 3.
Manuscript authors reflections regarding suitability of DEN-CORE Core Outcomes as Primary Endpoints in Phase III/IV Dengue Clinical Trials.
Table 3.
Manuscript authors reflections regarding suitability of DEN-CORE Core Outcomes as Primary Endpoints in Phase III/IV Dengue Clinical Trials.
| Core Outcome |
Suitability as Primary Endpoint* |
Rationale |
| Mortality |
High
(may be hindered by low prevalence) |
The most objective and robust outcome. Rare in most trial populations; requires large sample sizes. |
| Organ failure |
High |
Infrequent in general trial populations; better as a safety or severity indicator. |
| Development of shock |
High |
Clinically meaningful, treatment-sensitive, and recognised as a key severe disease indicator. |
| Health-related quality of life |
Moderate
(if validated PROMs are available) |
Applicable for phase III-IV trials of early stage, but requires culturally appropriate, validated tools, which are not yet agreed upon. |
| Resolution of acute symptoms |
Moderate |
Patient-relevant, responsive to treatment, and feasible to measure across phases of disease. Refinement of symptoms to be included, as it is still ongoing |
| Severe bleeding |
Moderate |
Relevant but less frequent and variable across settings; may require detailed adjudication. |
| Severe plasma leakage |
Moderate |
Specific endpoint in pathophysiology of dengue; useful in both hospital and research settings. Difficult to measure robustly. |
| Need for ICU/HDU admission |
Moderate |
Heavily influenced by health system capacity and thresholds for escalation of care. |
| Need for hospitalisation |
Moderate |
Pragmatic endpoint reflecting clinical worsening; feasible, observable, and patient-centred. |
| Platelet count |
Low |
Important biological marker but lacks direct clinical relevance as a standalone effectiveness/efficacy outcome. |
| Viral load |
Low |
More suitable for mechanistic/early-phase studies; does not directly reflect patient benefit. Lack of direct correlation of effect on viral load on clinical benefit currently precludes viral load as a standalone efficacy outcome for regulatory approval. While viral load is currently not considered a valid surrogate of clinical efficacy, regulators recommend to collect viral load data in pivotal clinical trials to potentially establish a correlation of clinical efficacy. |
‘Hospitalised’ COS consensus meeting
The consensus meeting was conducted online on 7 October 2024. Voting participants were divided into two stakeholder groups: (a) people with lived experience of dengue and family members/carers (n=5); (b) healthcare professionals/researchers (n=31). Detailed descriptions of the participants who attended the consensus meeting can be found in Appendix 1 p 49.
Upon ratification of outcomes that were voted “in” upon the Delphi process the seven outcomes were discussed in the following order: “Quality of life””; “Time to recovery”, “Severe gastrointestinal symptoms”, “Pain”, “Fever”, “Fatigue”; “Viral load” (
Figure 2A).
After discussions and subsequent voting, no additional outcomes met the predefined consensus definition for inclusion and the total number of outcomes in the COS for ‘hospitalised’ remained at seven (table 2).
‘Early stage’ COS ‘adapt or adopt’ consensus meeting
The “early stage” consensus meeting was conducted online on 9 December 2024, with a total of 20 voting participants. These participants voted as a single, collated group. Detailed descriptions of the participants who attended the consensus meeting can be found in appendix 1 p 59.
The meeting's purpose was to adopt the previously agreed ‘hospitalised’ COS to the ‘early stage’ and vote on the potential inclusion of additional outcomes. The management group decided a priori to include “need for hospitalisation” as a resource use outcome; this was discussed with participants at the meeting, with no disagreement.
The following four outcomes were discussed and met the predefined consensus definition for inclusion: “resolution of acute symptoms,” health-related quality of life, and “plasma viremia/viral load”. The fifth outcome discussed was “time to recovery”. In contrast to “resolution of acute symptoms” it was considered as a long-term outcome, and did not reach criteria for inclusion in the early-stage COS.
In total, 11 outcomes were included in the core outcome set for the “early stage” (table 2). Details of Delphi process and the consensus meeting are available in appendix 1 p 36-67.
COS for ‘ICU/HDU’ trials
Twenty-two experts in critical care and COS methodology provided feedback on the proposed approach to a Core Outcome Set for ‘ICU/HDU’ dengue trials (Box 1). The level of agreement with the recommendations formulated was 95%.
Phase II: Core Outcome Measurement Set Development
Phase IIa: Instrument Identification
A comprehensive literature review identified 792 instruments used across dengue studies and clinical trial protocols. After removing duplicates and mapping the instruments to the previously defined core outcomes, this number was narrowed down to 50. In addition to these, the study team considered outcome definitions developed by the NIH Tomashek et al. [
10], as well as instruments used in other COS initiatives. Based on this broader context, a final list of 41 measurement instruments was prepared for expert review. These instruments, were mapped to the 11 core outcomes identified in Phase I.
Phase IIb: Instruments Review and Voting
Three experts in dengue research and/or clinical care provided detailed feedback on the initial list of instruments, while others suggested additional relevant instruments. Prior to the consensus workshop, an online meeting was held to gather further insights on feasibility. Based on this discussion, preliminary statements providing recommendations on measuring each outcome were formulated and shared with all invited workshop participants.
The hybrid consensus workshop took place on 24 February 2025 in Ho Chi Minh City, Vietnam, with 47 participants, 37 participated onsite and 10 joined online. From these 29 participated in voting capacity. Full details of workshop participants are provided in Appendix 1 p.109.
At the beginning of the workshop, participants were briefed on the process and the pre-defined consensus criteria. Voting on each statement was conducted independently. Following discussion, consensus was reached regarding statements providing agreed-upon recommendations on measuring nine core outcomes discussed during the workshop. The median degree of consensus across statement voting was 92% (range 79 - 100%) (table 2). Details of all agreed upon statements are presented in
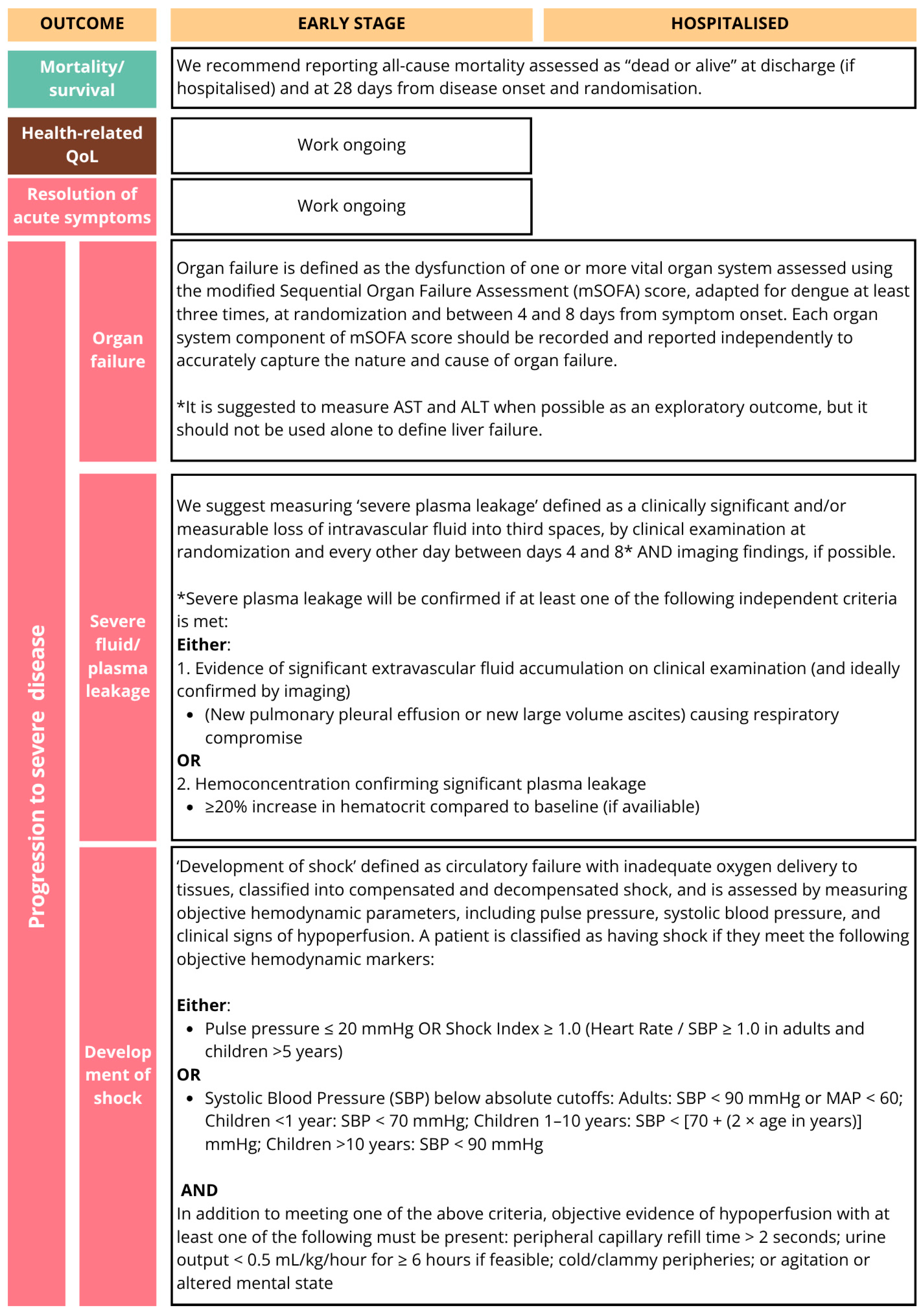
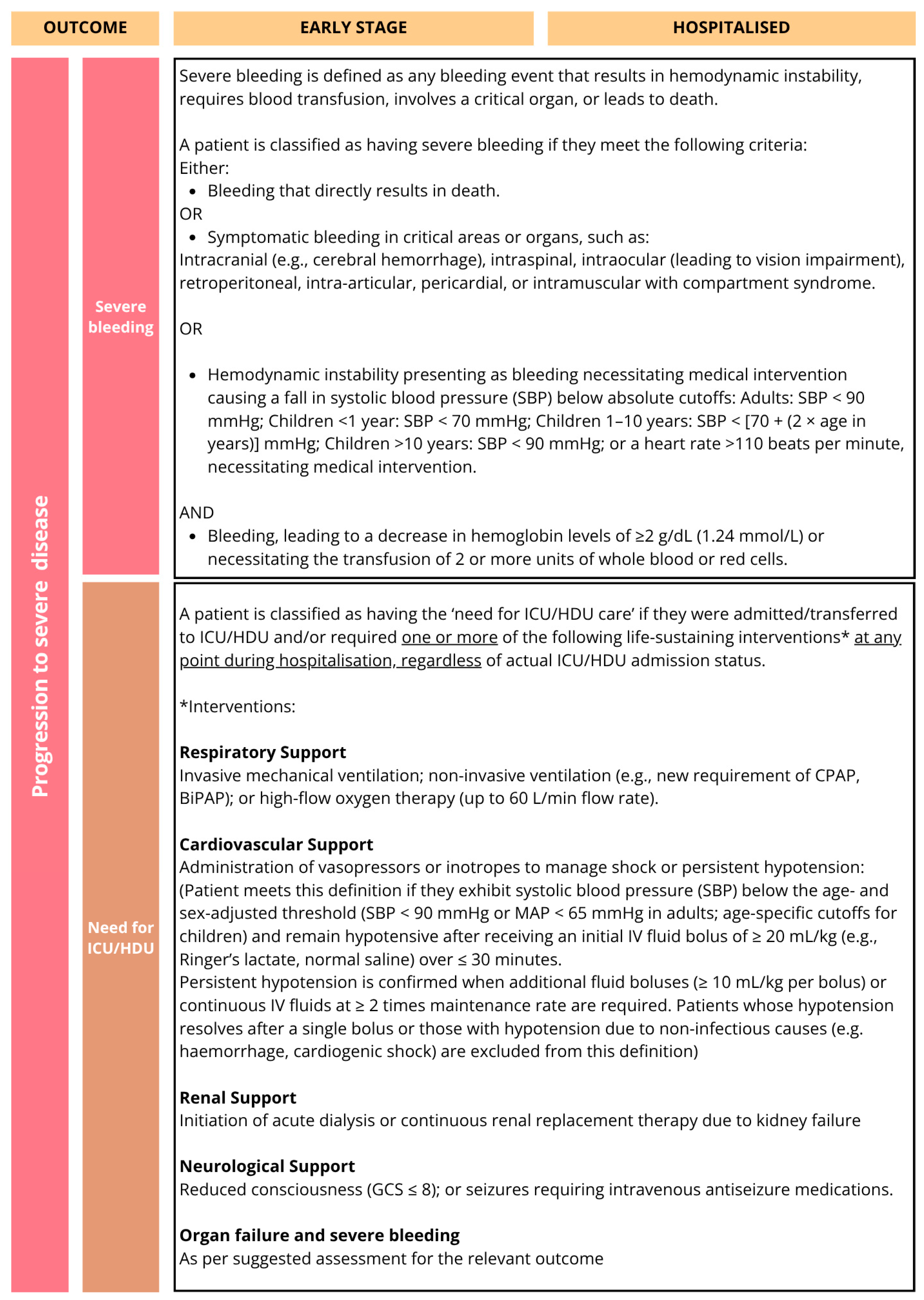
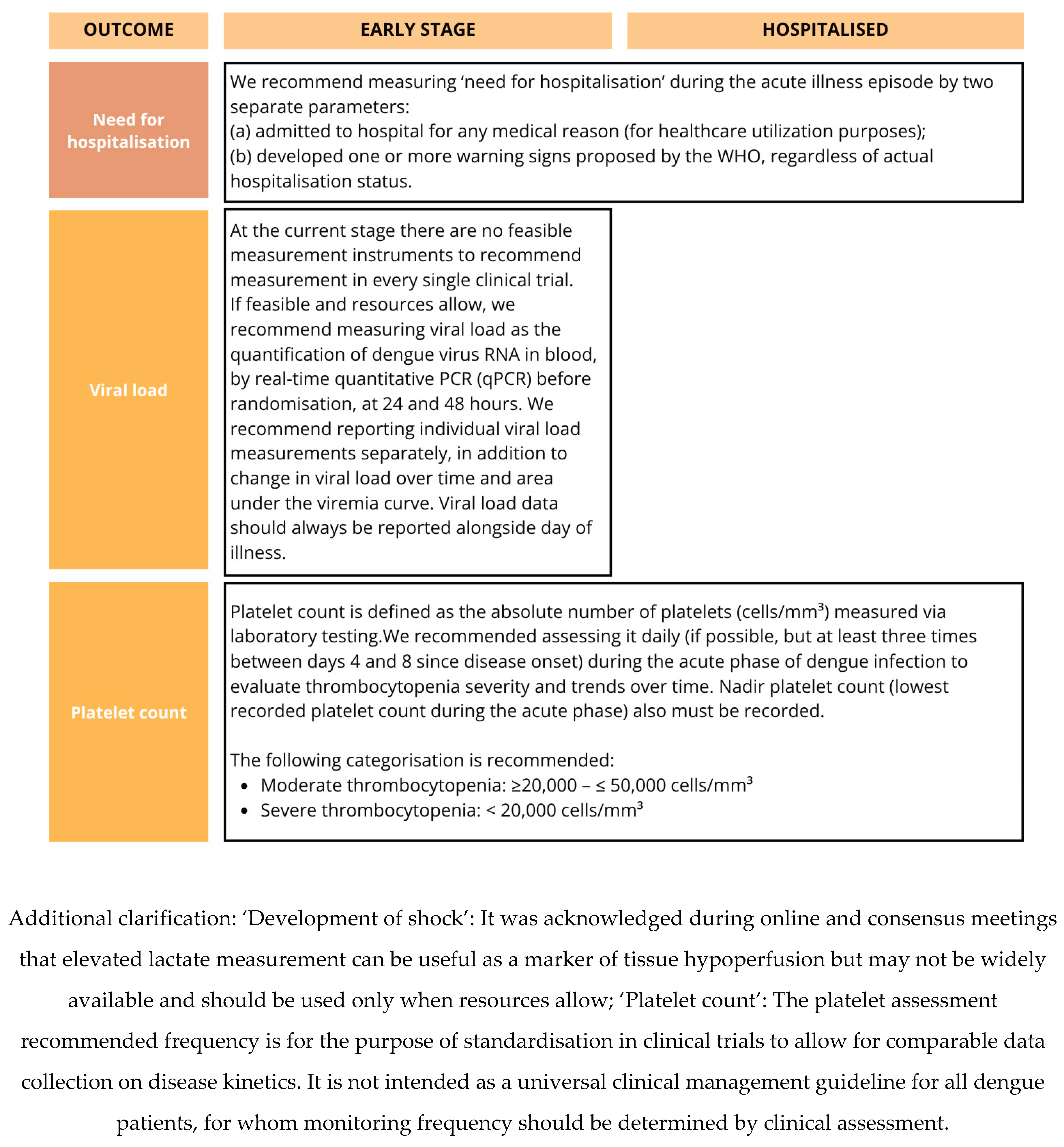
Figure 2B.
Two additional instruments—relating to the outcomes "Health-related Quality of Life" and "Resolution of Acute Symptoms"—were not discussed, as their evaluation requires COSMIN appraisal of all available instruments and additional meetings with heavy patient engagement. Detail of Core Outcome Measurement Set development can be found in appendix 1 p 75-109.
Figure 2.
A. Core Outcome Set for dengue clinical trials.
Figure 2.
A. Core Outcome Set for dengue clinical trials.
Figure 2.
B. Core Outcome Measurement Set for dengue clinical trials.
Figure 2.
B. Core Outcome Measurement Set for dengue clinical trials.
Discussion
This study represents the first global effort to establish a COMS for dengue clinical trials and covers all three disease stages. Through robust and inclusive process involving a diverse range of stakeholders we achieved consensus on core outcomes and corresponding measurement instruments. The DEN-CORE COMS addresses the variability in outcome selection and measurement, which has historically hindered data synthesis and the development of evidence-based clinical guidelines.
The core outcomes identified in the COMS reflect both the clinical priorities of healthcare providers, the biological relevance of established prognostic indicators in dengue, and outcomes of major public health relevance. Mortality, platelet count, development of shock, severe bleeding, organ failure, and plasma leakage were consistently prioritised by stakeholders, aligning with both the WHO dengue severity classification criteria [
10] and clinical endpoints proposed in earlier harmonisation efforts [
11]. Viremia and platelet count inclusion as ‘core’ outcomes for early-stage disease was discussed at length. Although these tests are resource-intensive and may not always be feasible or relevant in large phase III trials, where the focus is typically on more robust clinical outcomes, they were considered important to include, with standardised measurement instruments recommended.
Given the continuity of disease progression in dengue and the shared pathophysiological mechanisms across stages, outcomes from the hospitalised COS were reviewed as a logical foundation for early-stage trials. The early-stage COS was developed through a separate, structured consensus meeting using an “adapt or adopt” approach. This process allowed retention of relevant overlapping outcomes while tailoring the COS to the clinical realities and research priorities of early-stage dengue.
Including patient-centred outcomes, such as health-related quality of life and traditional clinical endpoints, such as ‘ICU admission’ and ‘need for hospitalisation’ balances feasibility with relevance. This dual focus aligns with emerging consensus from previous initiatives that have called for greater emphasis on functional and patient-reported endpoints alongside biomedical ones. While prioritising outcomes based on current clinical utility and measurement availability, COMS also acknowledges the need for ongoing evaluation and refinement, particularly for patient-reported outcomes and disease severity gradations [
12].
Outcomes prioritised in COMS provide a harmonised framework for reporting in dengue clinical trials. In Phase III trials, which serve as main source of evidence for regulatory approval, primary endpoints are typically selected to assess clinical efficacy and safety. Phase IV trials, by contrast, focus on evaluating real-world effectiveness, long-term safety, and health system relevance. Across both phases, selecting appropriate endpoints requires careful consideration of scientific validity and operational feasibility. Our COMS defines what should be measured in all trials but does not prescribe whether a given outcome should serve as a primary or secondary endpoint. This distinction should be guided by the intervention type, trial phase, and study objectives.
Outcomes such as mortality, progression to shock, need for hospitalisation, and resolution of clinical symptoms may be more relevant for phase III/IV trials, as they are meaningful to patients, sensitive to treatment effects, and commonly encountered in trial populations. These outcomes also reflect endpoints that regulators and clinicians prioritise for regulatory decision making. In contrast, laboratory-based outcomes like viral load and platelet count, though valuable for understanding disease pathophysiology and drug mechanisms, are often insufficiently patient-centred or clinically actionable in isolation. It is important to note, that although laboratory-based outcomes carry promise, there are currently no biomarkers that serve as highly accurate surrogate markers for clinical outcomes. These may be better suited as secondary endpoints or for inclusion in subgroup analyses, which could potentially inform about a correlation between viral load reduction and clinical outcomes for future clinical trials. Although this process was not aiming to provide recommendations on preferred primary endpoints, we would like to bring a word of caution and our suggestions in table 4 reflect our views on suitability of each core outcome to serve as a primary effectiveness endpoint in phase III/IV dengue trials, without precluding the broader reporting of the full COMS or regulatory decision making. Developing and validating novel biomarkers, including host immune response indicators and multiplex biosignatures, is important for future dengue research.
While instruments included in the DEN-CORE COMS represent the best-available tools based on current evidence and stakeholder consensus, we acknowledge that no single measurement instrument is perfect [
13]. Several outcomes prioritised in this process, such as ‘resolution of acute symptoms’ or ‘need for hospitalisation’, are highly meaningful to patients and clinicians, but may be context-dependent, subjective, or challenging to standardise across diverse settings. Their inclusion reflects their perceived clinical and public health importance, but further work may be required in the future to refine definitions and validate measurement instruments, particularly for subjective and patient-reported outcomes. All tools carry inherent limitations, whether related to feasibility in low-resource settings and/or accuracy of measurement. In a condition as heterogeneous and globally distributed as dengue, it is neither realistic nor desirable to expect universal agreement on every outcome or instrument [
14].
This study has several strengths. We used gold-standard methodology in line with COMET and COS-STAD recommendations. Throughout the project, our study engaged hundreds of stakeholders worldwide and included a broad range of perspectives from people with lived experience, healthcare professionals, researchers, and policy-makers, ensuring COMS global relevance and applicability. Inclusion of people with lived experience of dengue throughout the process ensured that outcomes reflect what matters most to patients, not just to professionals. Several outcomes were discussed but did not meet predefined thresholds for inclusion. Their exclusion reflects the structured consensus process rather than a lack of perceived clinical relevance.”
There are several limitations to this work. First, although the Delphi panel comprised participants from 36 countries, the majority were from Latin America and Southeast Asia. In contrast, representation from Africa and some parts of the Western Pacific was limited. This geographical imbalance may have influenced outcome prioritisation, particularly with respect to context-specific feasibility and relevance, which may potentially limit the immediate generalisability of findings across all endemic settings. Similarly, while we actively included people with lived experience of dengue, their numbers remained smaller than those of professionals, and further efforts are needed to strengthen patient representation, particularly from low-income settings. Second, while we aimed to identify measurement instruments for all core outcomes, the current COMS does not yet include validated PROMs, which limits its immediate applicability for capturing patient-centred outcomes. Until validated and culturally appropriate PROMs are developed and integrated, trialists may need to adapt existing tools pragmatically or transparently report PROM-related gaps. Future work will focus on identifying or developing such measures through COSMIN-guided appraisal and stakeholder engagement.. Another limitation is related to specific subgroups, such as immunocompromised individuals, pregnant women, and very young children, which may experience distinct manifestations or complications that are not adequately addressed by the current COMS. Finally, although the use of online Delphi methods and virtual meetings enabled broad participation, it may have limited engagement from stakeholders with restricted internet access and potentially constrained the depth of discussion typically afforded by in-person meetings.
We recognise that the implementation of COMS may vary substantially across settings due to differences in healthcare infrastructure, diagnostic capacity, and workforce availability. While some outcomes, such as mortality or hospital admission, are measurable in nearly all settings, others, such as serial laboratory markers, may not be feasible to collect in lower-resource environments. For this reason, the COMS is intended as a standard for clinical trials rather than clinical practice, with the understanding that its implementation may be partial in some contexts. Trialists are encouraged to report when specific outcomes could not be measured due to local resource limitations, as this transparency contributes to the broader interpretability and equity of the evidence base.Future work should focus on developing and validating appropriate patient-reported outcome measures for ‘health-related quality of life’ and ‘acute symptom resolution’. Validation within large-scale, pragmatic studies, such as platform trials that already incorporate most of the core outcome measures could support robust assessment of measurement properties and greatly facilitate uptake in future research and clinical practice. DEN-CORE COMS should be viewed as a foundational and rigorously developed starting point, not a static endpoint. A formal framework for periodic review and updating will be established, with a focus on further expansion of stakeholder representation in future work, especially from underrepresented countries and patient groups. Regulators encourage to consider available COS in clinical trial design to ensure consistency and comparability across clinical studies. By promoting consistency, relevance, and feasibility in outcome reporting, this COMS offers a critical step forward in standardising research practice, enabling better comparisons across studies, and ultimately informing the design and evaluation of future therapeutic interventions. Dissemination of the DEN-CORE COMS through global health networks, capacity-building workshops, and integration into trialist resources is essential to achieve its intended impact and ensure uptake. Ultimately, this COMS aims to support high-quality, patient-centered dengue research and contribute to improved outcomes for individuals affected by this globally important disease.
Search Strategy and Selection Criteria
Evidence from several sources was used to generate a comprehensive list of candidate outcomes and measurement instruments, which informed the initial round of the Delphi consensus process.
They were identified through structured searches of Ovid MEDLINE and Embase from inception to 8th of September 2023. We used a broad combination of search terms related to dengue (“dengue”, “DENV”, “dengue fever”, “dengue virus”, “severe dengue”, “dengue shock syndrome”, and synonyms including “breakbone fever”, “Thai haemorrhagic fever”) alongside terms related to clinical trials and outcome assessment (“clinical trial”, “outcome measurement”, “qualitative research”, “patient-reported outcomes”, “treatment outcome”, “expectation”, “patient experience”, “semi-structured interview”). No language restrictions were applied.
We also reviewed records from ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform and consulted guidelines and technical reports from relevant public health agencies, including the World Health Organization and regional dengue control programmes. Reference lists of included studies were manually screened for additional relevant articles.
Preclinical studies, animal experiments, and descriptive epidemiological reports without outcome assessment were excluded.
Funding
This work was supported by the Wellcome Trust [303666/Z/23/Z]; UK International Development [301542-403]; and the Gates Foundation [INV-063472] and Academy of Medical Sciences Networking Grant Scheme (NGR1\1953).
Acknowledgments
We thank all participants of the consensus development process, including Agus Rachmat, Alejandra Hernández Martínez, Ali Ait Hssain, Aliae AR Mohamed Hussein, Álvaro A. Faccini-Martínez, Anjanna Kukreja, Bunnet DIM, Carlos Arturo Alvarez-Moreno, Chamara Sarathchandra, Christian Owoo, David E. Castellanos M., David M Allen, Derwin Soler Dávila Guerra, Dinuka Ariyaratne, Dr A. LakKumar Fernando, Dr Anupama Hazarika, Dr. Ananda Wijewickrama., Dr.Ashok Kumar, Dr.Preshila Chandimali Samaraweera, Edward Sutanto, Erni J. Nelwan, Fabio Darío, Otero Arteaga, Favio Orlando Sarmiento Lopez, François Chappuis, Guilherme Amaral Calvet, Gunina Ksenia, Gustavo Balen Meneghel, Jose A. Calvache, Jose Forero-Mejia, Jose W Lopez Revilla, Jozennia Valenzuela Pactao, Justin S.G. Ooi, Kalaiarasu M Peariasamy, Lance Turtle, Lars Henning, Lee Marbill Peradilla, Li Zongchen, Lim Kah Chuan, Liziem Gabriela Valladares Duron, Manikka H M Thisara perera, Marco Antonio Lopes de Carvalho Netto, Margareta Oktaviani, Marise da Silva Mattos, Maryel Del Carmen Isuiza Rivas, Matthew Kain, Michele Fernanda Borges da Silva, Michelle Karman, Mohammad Muatasm Elmubark Gasmalla, Nguyen Hoan Phu, Nguyen Thanh Van, Novik Budiwardhana, Pamela Gamatan, Paul Ananth Tambyah, Perez diaz Carlos e, Permata Putri Karina, Pratheema Ramachandran, Priya Jarwar, Priyanki Shah, Professor Aneela Altaf Kidwai, Ranjith de Alwis, Rasidah Senian, Ririe Fachrina Malisie, Robert Dennis J. Garcia, Robert Stiward Corrales Llerena, Rosa Marina Margarita Guillermo Valdiviezo, Rosmery Gross Arteaga, Salaheddin M. Abdulhamid, Saman Man Pradhan, Sarah Bethencourt, Snigdha jain, Steven Chee Loon Lim, Dr Suraya Hanim Abdullah Hashim, Surbhi Jain, Tom E Fletcher, U P K Amarasekara, Wan Nurliyana Wan Ramli, Yaw Shin Ooi, Yesid Fabian Mantilla Florez, Yuen Jun Xian Paul; the translators who provided translation of all English materials to additional languages. We are particularly grateful to Professor Mike Clarke for his kind agreement to act as an independent facilitator of the online interactive consensus meeting and wonderful conduct of this meeting. We also thank all the volunteers who helped with meetings’ organization. We are grateful to Wajeeha Majik, Asia Mase and Ruslan Sharifullin for IT support during the project. We are very grateful to The Counter Coffee and Lounge, St. Paul’s, London management and staff, particularly wonderful Pani, Margo and Massimo, for their continuous support and patience.
Declaration of AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT in order to improve the grammatical structure and readability. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Contributions
LM: SY and DM conceived the idea for the study. DM led the methodological team and supervised the research teamwork throughout the project. LM and AD designed the study protocol. LM, DM, SY, AD and XHSC conducted the initial methodological discussions. Steering committee members: PB, LL, TJ, SM, NM, BKTV contributed to methodology discussions throughout the project. EP undertook the data analysis. AH, EKa, DB, LX, MK, AA, AM contributed to Phase I (Outcome Identification) by conducting the literature review, identifying outcome measures and measurement instruments, and categorising them for inclusion in the online Delphi survey. AD and DM coordinated the data revision process. JIVN and CLP led the qualitative component of Phase I, involving people with lived experience and conducting interviews. SY, XHSC, LM, DM, AU, LL, TJ, NM, BKTV, PB contributed to Phase Ia by providing expert feedback on the long list of outcomes and helped finalise the list for the Delphi survey participants. SB contributed to Phase Ia by refining the outcome list for the Delphi survey. AD developed the online Delphi surveys and was responsible for setting up the Delphi process, preparing the instructions and materials for the Delphi process, and communicating with stakeholders. AD, DM, SY, LM, XHSC organised the ‘Hospitalised” and “Early stage” consensus meetings. AA, AYC, AD, ASH, AT, AU, BKTV, CLP, CMF, DCBL, DM, DBP, DTHT, ER, GSSR, HTT, IMA, JDAA, JIVN, LL, LM, LX, LV, MPCR, MK, NM, NMN, PB, RMGR, RL, SA, SM, SY, TJ, XHSC participated in the phase I outcome voting phase of the process, including hospitalised and/or “early stage” consensus meeting. AA, AYC, AD, AMc, AMS, ASH, AT, ATW, BKTV, BAW, CFM, CPY, DM, DTHT, EEO, EK, EKa, ER, HCO, HQC, TBH, HTT, IMA, JR, JAW, KBA, TL, LL, LM, LV, MPCR, MR, MMT, NM, NMN, PA, PKL, PS, RC, RL, SFO, SJL, SLP, SY, TJ, VD, WHRS, XHSC, SB contributed to Phase II of the project, including participation in online and hybrid meetings, as well as providing suggestions and refining the statements. SB contributed to Phase II by suggesting and revising outcome statements. AD, DM, SY were responsible for designing the instrument/definition cards and their content. SY organised the hybrid consensus meeting for instruments and statements. DM, AD, EKa, AH, SY, LM, XHSC drafted the manuscript; all authors reviewed and approved the final manuscript.
Disclaimer/Publisher’s Note
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the regulatory agencies or organisations with which the authors are employed/affiliated.
Declaration of Interests
SY serves on the scientific committee for the Takeda dengue vaccine program, reports receiving consulting fees, and has participated in an advisory board for Novartis. She is also Chair of the DSMB for a Phase 2 antiviral trial. XHSC is a UK NIHR Academic Clinical Lecturer in Infectious Diseases at the University of Oxford. JIVN reports receiving grants from Wellcome Trust, UK, University of Oxford UK and ANRS, France. KBA reports receiving funding from the NIH (NIAID, Fogarty), the Wellcome Trust, and travel support from the PanDengue Summit organizers for a 2025 plenary address in Colombia. She is a member of the HealtheConnections Advisory Board, NY, USA. PA reports receiving the Chair Professor Research Grant (P16-50392) and co-funding research grants ((IO)R016536001 and P-20-52570) from the National Science and Technology Development Agency (NSTDA) and the Faculty of Medicine Siriraj Hospital, Mahidol University. She also received a joint MRC-NSTDA-TRF research grant ((IO)R015936005) and support from the RED program at the Faculty of Medicine Siriraj Hospital. She served on advisory boards for the PLATCOV and AD ASTRA trials. RC, RL and MR are employees of Novartis Pharmaceuticals. IMA is employed at Novartis as a Global Program Clinical Head. EEO reports receiving National Medical Research Council grants for studies on vaccines (MOH-000505-04), flaviviral host response (MOH-000601-00; NRF-CRP25-2020-0003), and dengue (MOH-000271-00). He has served on advisory boards on dengue vaccines for Takeda and Sanofi Pasteur, dengue therapeutics for Johnson & Johnson and Novartis, and on self-amplifying RNA vaccines for Arcturus Therapeutics. He also served as DSMB Chair for a J&J COVID vaccine trial by Mahidol University. SP holds research grants from the University of Minnesota, EDCTP-2, EDCTP-3, NIHR, Gilead Sciences, ViiV Healthcare, MRC, and Janssen-Cilag. She serves as a DSMB member for the TIPAL trial (UK NIHR-funded) and the IMPEDE-PKD trial (NIHR-funded, UK and international sites). GSR reports receiving a US NIH grant and a Malaysian Ministry of Health grant for work on Plasmodium knowlesi malaria. He has served as a speaker at ICTMM 2024 (Clinical Management of Dengue), ESCMID Global 2025 (Clinical Management of P. knowlesi Malaria), and the 7th Asia Dengue Summit (Kuala Lumpur), where he was also a committee member. He is Head of the Department of Medicine, Queen Elizabeth II Hospital, Ministry of Health Malaysia, and a member of the Malaysian Medical Council Specialty Education Committee. AT is an employee of Novartis Pharma AG, Basel, and a shareholder of Novartis. CPY reports receiving an honorarium from Takeda (to institution) for a lecture titled From Theory to Practice, Trials and Tribulations in Singapore, June 2024. She reports receiving grant funding to institution from Ministry of Health, Singapore ans a sponsored trial funding to institution from Astra Zeneca and Novartis. NM served in advisory roles on dengue for Johnson & Johnson, Novartis, and Abbott, and headed the dengue global program at Drugs for Neglected Diseases Initiative (DNDi) from June 2022 to 2024. BKTV reports receiving funding for a pilot trial of corticosteroids in patients hospitalized with dengue. DM reports receiving grants from the Bill & Melinda Gates Foundation [INV-063472] and the Academy of Medical Sciences Networking Grant Scheme (NGR1\1953). He also received support for attending meetings organized by the International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) and the World Health Organization. All other authors report no relevant conflicts of interest.
References
- WHO. Dengue and severe dengue 23 April 2024 [11.06.2025]. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- Control) EECfDPa. Dengue worldwide overview 2025 [updated 2025-03-03T09:17:53+0100. Available online: https://www.ecdc.europa.eu/en/dengue-monthly.
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLOS Neglected Tropical Diseases 2019, 13, e0007213. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Giovanetti, M.; Obolski, U.; Lourenço, J. Population at risk of dengue virus transmission has increased due to coupled climate factors and population growth. Communications Earth & Environment 2024, 5, 475. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.R.; Altman, D.G.; Bagley, H.; Barnes, K.L.; Blazeby, J.M.; Brookes, S.T.; et al. The COMET Handbook: version 1.0. Trials 2017, 18 (Suppl. 3), 280. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.J.; Clarke, M.; Williamson, P.R. A methodological approach for assessing the uptake of core outcome sets using ClinicalTrials.gov: findings from a review of randomised controlled trials of rheumatoid arthritis. BMJ 2017, 357, j2262. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.J.; Davis, K.; Altman, D.G.; Blazeby, J.M.; Clarke, M.; Tunis, S.; et al. Core Outcome Set-STAndards for Development: The COS-STAD recommendations. PLoS Med. 2017, 14, e1002447. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Clarke, M.; Becker, L.; Mavergames, C.; Fish, R.; Williamson, P.R. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018, 96, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. WHO Guidelines Approved by the Guidelines Review Committee. Geneva 2009.
- Tomashek, K.M.; Wills, B.; See Lum, L.C.; Thomas, L.; Durbin, A.; Leo, Y.S.; et al. Development of standard clinical endpoints for use in dengue interventional trials. PLoS Negl Trop Dis. 2018, 12, e0006497. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zambrano, M.; Torres-Hernandez, D.; Murillo-Ortiz, M.A.; Hurtado, I.C.; Davalos, D.M.; Cantor, E.; et al. Different Clinical Severity and Outcomes in a Cohort of Patients With Dengue With Warning Signs in an Endemic Latin American City. Open Forum Infect Dis. 2025, 12, ofaf227. [Google Scholar] [CrossRef] [PubMed]
- The Lancet P. A good enough measure. The Lancet Psychiatry 2020, 7, 825. [CrossRef] [PubMed]
- Krause, K.R.; Chung, S.; Adewuya, A.O.; Albano, A.M.; Babins-Wagner, R.; Birkinshaw, L.; et al. International consensus on a standard set of outcome measures for child and youth anxiety, depression, obsessive-compulsive disorder, and post-traumatic stress disorder. The Lancet Psychiatry 2021, 8, 76–86. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).