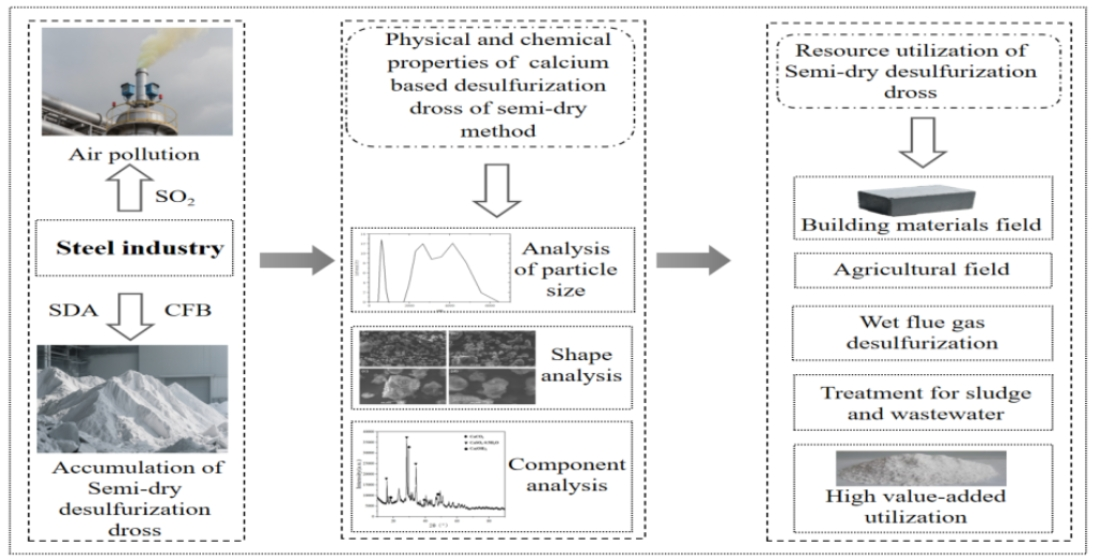
1. Introduction
With the continuous intensification of the global warming trend, climate change has become a hot issue of focus for all countries in the 21st century. Currently, we are facing severe challenges posed by climate change, and we also need to address the serious harm caused by air pollution [
1,
2]. Sulfur dioxide (SO
2) belongs to the category of atmospheric pollutants, it is a colorless, pungent odor, toxic gas, which can a significant impact on human health and the atmospheric environment [
3]. The Green Environment Trust (GET) reported in its 2019 report that China was the third largest emitter of SO
2 in the world, accounting for 8% of the global total of anthropogenic SO
2 emissions [
4]. The 2021 China Environmental Status Report, which was released, showed that 98.2% of the cities in China had reached the national-level standard for sulfur dioxide, and the area of acid rain regions accounted for 3.8% of the total national land area [
5]. McLinden et al [
6] identified previously unreported sulfur dioxide emission sources via satellite observations, which are predominantly located in developing countries and account for approximately 6-12% of global anthropogenic emissions. Acid rain in China mostly of the sulfurous type, which mainly originated from the large emissions of SO
2. The hazards of acid rain were widespread, and it would damage soil, vegetation, human health and buildings, etc. The emission problem of SO
2 cannot be ignored [
7]. When SO
2 is emitted into the atmosphere, it is oxidized by air to form sulfate particles, which can adsorb water vapor, thus forming acid rain, causing multi-faceted harm to the ecological environment. Firstly, acid rain accelerates the erosion rate of the vegetation surface, destroys the tissue structure and chlorophyll of leaves, and thus inhibit the photosynthesis of plants, affecting their normal growth and development [
8]; acid rain also causes damage to microorganisms in the soil, making the interaction between soil microorgan more frequent, not only inhibiting their cooperative behavior but also intensifying their competition [
9]; acid rain caused by industrial emissions, when it falls into rivers, lakes and other waters will cause water acidification, which will increase the solubility of some heavy metal ions, and this has a great impact on the content of heavy metals in aquatic sediments thus poisoning aquatic organisms and destroying the balance of the aquatic ecosystem [
10]; The sulfate ions in acid rain will degrade cement hydration products in concrete, and acid rain through roads, buildings and other structures will produce corrosive effects, accelerating aging and shortening their service life [
11,
12]; In addition, SO
2 also poses serious hazards to humans, long-term exposure to sulfur dioxide can irritate the respiratory system, and also increase the risk of cancer.
According to the National Bureau of Statistics' "Statistical Bulletin on National Economic and Social Development in 2021", the steel production in China in2021 was 1.337 billion tons [
13]. The steel industry, as a high energy-consuming and high-emission industry, released a large amount of SO
2 in the production process, and the emitted SO
2 accounted for more than 30% of the total industrial emissions, becoming one of the main sources of SO
2 emissions in China. With the continuous and rapid development of China's economy, the extensive application of new technologies in the industrial field had significantly improved the production efficiency. In recent years along with the continuous improvement of national requirements for environmental protection, flue gas desulfurization technology had developed rapidly, and the integrated and collaborative treatment of desulfurization,itrification, and dust removal had become the mainstream management model for iron and steel enterprises. The Law of the People's Republic of China on the Prevention and Control of Environmental Pollution by Solid Waste explicitly stipulates that industrial waste management must adhere to the principles of reduction, resource recovery, and harmless treatment, generating units are required to establish a whole-process pollution prevention responsibility system to minimize waste generation at the source [
14]. Among various desulfurization technologies, semi-dry desulfurization was widely used with relatively low investment cost, small footprint, and dry by- from desulfurization, etc. [
15,
16]. According to statistics, the output of desulfurization by-products in China reached 11 million tons in 2020, and the output of dry desulfurization dross exceeded 30 million tons [
17]. The steel industry emited about 20 million tons of semi-dry desulfurization dross every year nationwide, and the semi-dry desulfization dross, as a by-product of desulfurization after processing, had become a bulk solid waste, urgently needing mature and reliable treatment technology to achieve comprehensive utilization of Semi--dry desulfurization dross [
18]. Semi-dry flue gas desulfurization technology mainly included circulating fluidized bed method (CFB) and spray drying absorption (SDA) [
19]. The CFB system used powdery Ca(OH)
2 as the absorbent, and the flue gas and absorbent were mixed and reacted in the tower. The temperature was controlled by adjusting the amount of water sprayed, no wastewater was produced, the adaptability was strong, the load adjustment range was wide, the desulfurization dross was recycled, and the desulfurization efficiency can reach more than 98%. SDA sprayed the lime slurry into the absorption tower to make it contact and reacted with the flue gas. However, the process had some disadvantages: core equipment, the rotating atomizer, was prone to wear, resulting in high maintenance costs; the system was prone to scaling and blockage problems, and the digested sludge to be discharged outside; moreover, the process had a relatively poor adaptability to changes in flue gas load and SO
2 concentration, and the desulfurization efficiency was usually less than 90%. The flue gas circulating fluidized bed desulfurization process (CFB-FGD) had become one of the best semi-dry desulfur processes with comprehensive performance after more than thirty years of continuous improvement [
20]. However, the Ca/S ratio of the semi-dry process was higher than that of the process, which would lead to an increase in the production of desulfurization dross with low utilization, resulting in more difficult treatment of desulfurization dross and the need to study on the treatment of desulfurization dross [
21].
Due to harmful gases such as sulfur dioxide emitted by industries, it had become an important source of atmospheric pollution. With the promotion of national environmental protection policies, a large number of enterprises had introduced sintering flue gas desulfurization devices, which not only effectively reduced pollutant emissions, but also brought about new problems that urgent need to be solved. At present, its main disposal methods were abandonment or stacking, and such practices will not only cause secondary pollution to water and soil, but also severely damage atmospheric environment [
22]. During the natural leaching of desulfurization dross, the leached mass concentrations of heavy metals such as Pb, Ni, and Cd exceeded the limits set by the West German landfill standards for solid waste by 0.4 to 1.47 times [
23]. If disposal sites lack protective measures such as impermeable layers and rain shelters, leachate can mobilize heavy metals from the dross, compromising the safety of its subsequent resource utilization. The environmental risks of semi-dry desulfurization dross represent a core challenge that must be addressed in its resource recovery, primarily due to its complex physicochemical properties. First, the risk of heavy metal pollution is particularly prominent. The desulfurization process effectively captures and enriches trace heavy metal elements from flue gas. Studies indicate that desulfurization dross from iron and steel sintering processes is a key carrier for Tl enrichment, far exceeding levels commonly found in general industrial solid waste [
24]. In addition, various heavy metals such as As, Cr, lead Pb, and Zn are frequently detected [
25]. Under natural storage conditions, these toxic elements are highly prone to leaching and migration through rainwater, posing long-term and hidden threats to surrounding soil and groundwater ecosystems, and ultimately endangering human health through the food chain. Second, desulfurization dross typically contains large amounts of unreacted absorbents, such as CaO and Ca(OH)
2, rendering it highly alkaline, along with elevated Cl
- content [
26]. These properties can alter the pH of adjacent soil upon direct exposure, disrupt soil aggregate structure and microbial community balance, and potentially exacerbate soil salinization. In early wet desulfurization processes, wastewater enriched with these pollutants exhibited thallium concentrations more than ten times above regulatory standards, highlighting its significant potential for environmental mobility [
24].
Therefore, the safe disposal and resource utilization of desulfurization dross are not merely considerations for economic benefit but essential measures to block the pathways of pollutant diffusion and uphold the principle of environmental risk prevention. Although semi-dry desulfurization dross had been preliminarily applied in the fields of building materials and agriculture due to its advantages such as cohes and high CaO content, there were still many problems: large component fluctuation leading to unstable product performance; incomplete oxidation of calcium sulfite affecting utilization safety; and imperfect environmental risk system restricting large-scale application. This paper focuses on the resource utilization of semi-dry calcium-based desulfurization dross, firstly analyzing its physical and chemical properties, identifying restrictive factors such as complex composition and poor stability; and then systematically sorting out the application research in the fields of building materials, agriculture, and wet flue gas desulfurization analyzing the application potential in various fields, aiming to provide technical reference for the large-scale and high-value utilization of semi-dry desulfurization dross, and to have environmental benefits and economic benefits.
2. Physical and Chemical Properties of Desulphurization Dross of Semi-Dry Method
Semi-dry desulfurization dross was a high-calcium and high-sulfur dross residue, and its composition was relatively complex with high content of CaSO
3, low permeability, and unstable properties, which restricted the extensive application of desulfurization dross and were not conducive to the healthy of semi-dry desulfurization technology [
18,
27]. The desulfurization dross generated by the semi-dry desulfurization process was powder, and the composition of the desulfurization dross was significantly affected by the flue gas, and its color showed different states, such as reddish brown, light yellow or grayish white. For instance, when treating sintering flue gas containing iron ore dust, the desulfurization dross absorbs approximately 1.57% Fe
2O
3 and 94.08% calcium sulfides, often resulting in a yellowish-white color; In cases where the iron content in the flue gas is lower, the dross contains only about 1.35% Fe
2O
3 and 41.25% calcium sulfides, typically appearing off-white; Further variations in iron content may lead to other colors, such as reddish-brown, directly correlated to the differing concentrations of iron compounds (e.g., Fe
2O
3) in the desulfurization dross depending on the flue gas source [
19,
28]. Over the past decades, various desulfurization processes had been developed by various industries, institute, and universities, which had enabled the emission of pollutants in the flue gas of the iron and steel industry to meet the standard, and had contributed to reduction of sulfur dioxide emissions and the reduction of the area of acid rain in China [
29]. The type of desulfurizing agent directly determines the core composition of desulfurization dross, which can be classified as follows:Calcium-based desulfurization dross, produced using calcium-based sorbents such as limestone, quicklime, or Ca(OH)
2; Magnesium-based desulfurization dross, generated with MgO or Mg(OH)
2 as the sorbent; Ammonia-based desulfurization dross, resulting from the use of ammonia water or liquid ammonia, primarily containing ammonium salts (e.g., ammonium sulfate and ammonium sulfite); Sodium-based desulfurization dross, formed with sodium carbonate (Na
2CO
3) or sodium hydroxide (NaOH) as the sorbent. Due to the high cost of sodium resources, its industrial application is relatively limited. Calcium-based desulfurization dross is the most common type, mainly composed of calcium sulfates/sulfites (such as CaSO
4 and CaSO
3). The classification basis of calcium-based desulfurization dross in the iron and steel industry were shown in
Table 1.
2.1. Analysis of Particle Size of Desulphurization Dross provided by a company in Ma'anshan
The particle size distribution of desulfurization dross can be analyzed to optimize its pretreatment processes (such as grinding), thereby improving its stability and applicability [
30]. The desulfurization dross sample used in the test was provided by a certain company Ma’anshan by the method of SDA. The particle size of the desulfurization dross was detected by the particle size analyzer (Nano ZS90, and the results are shown in
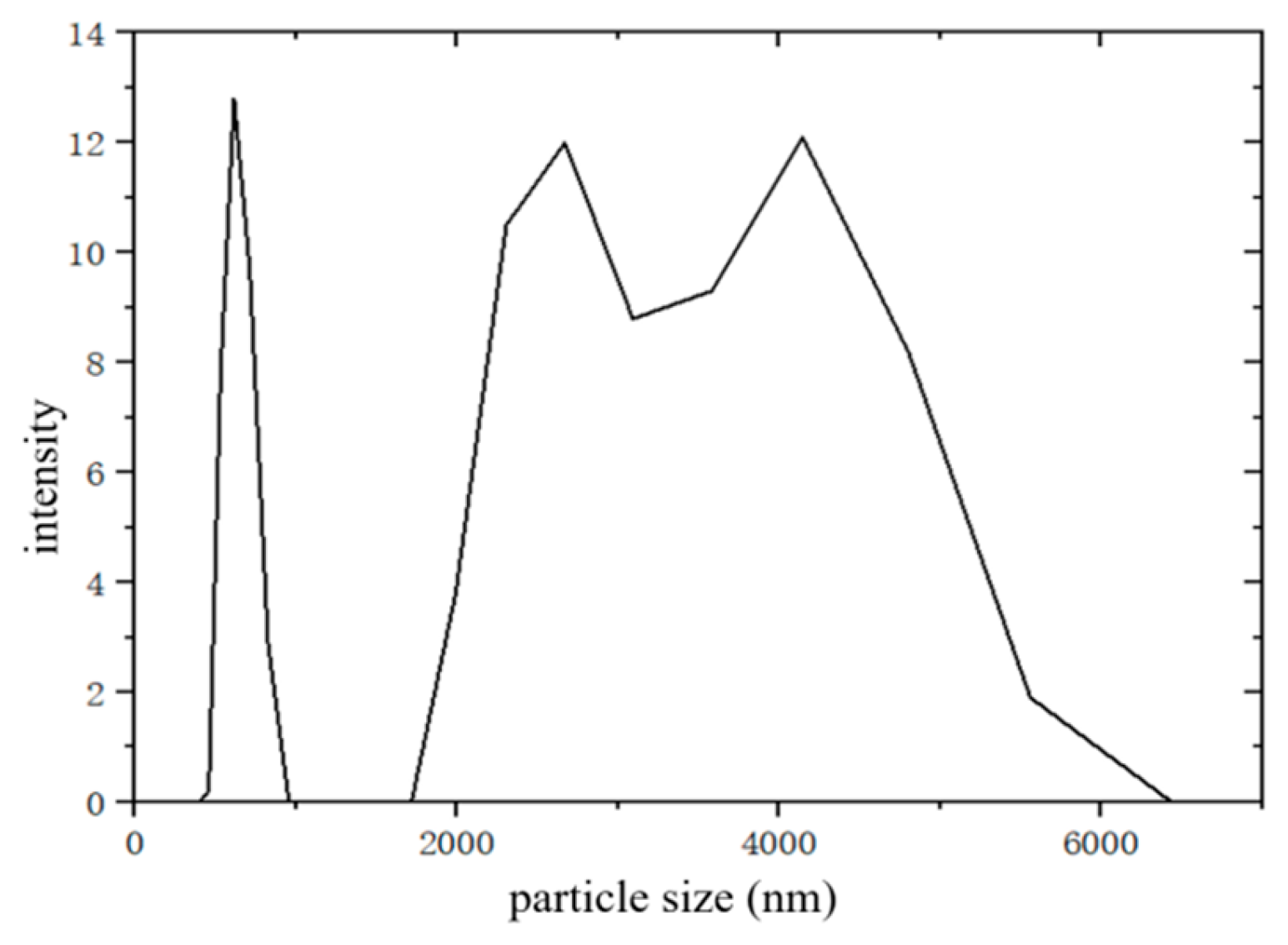
Figure 1.
It can be seen from
Figure 1 that the average particle size of the desulfurization dross obtained by the SDA method was 2446 nm, with three peaks, that is, three groups of nanoparticles with different particle sizes, and the three peaks were located at 641.1 nm, 2591 nm, and 3984 nm, respectively. Li et al [
31] detected the desulfurization dross produced by the SDA method in the Anshan Iron and Steel, and the data show that: the median particle size (D50) of the desulfurization dross was 6460 nm, the volume average diameter was 8650 nm, and the area average diameter was 2960 nm. Qian et al [
19] detected the desulfurization dross obtained by the DFA method, showed that 80% of the desulfurization dross particle size was above 1039 nm, and the median particle size (D50) was 4521 nm. It can be seen that the process of the desulfurization dross produced by different enterprises was the same, the law of particle size distribution was roughly the same, but the particle size of the desulfurization dross produced by the SDA method was significantly larger than that of the DFA method. The comparison between SDA and DFA methods is shown in
Table 2.
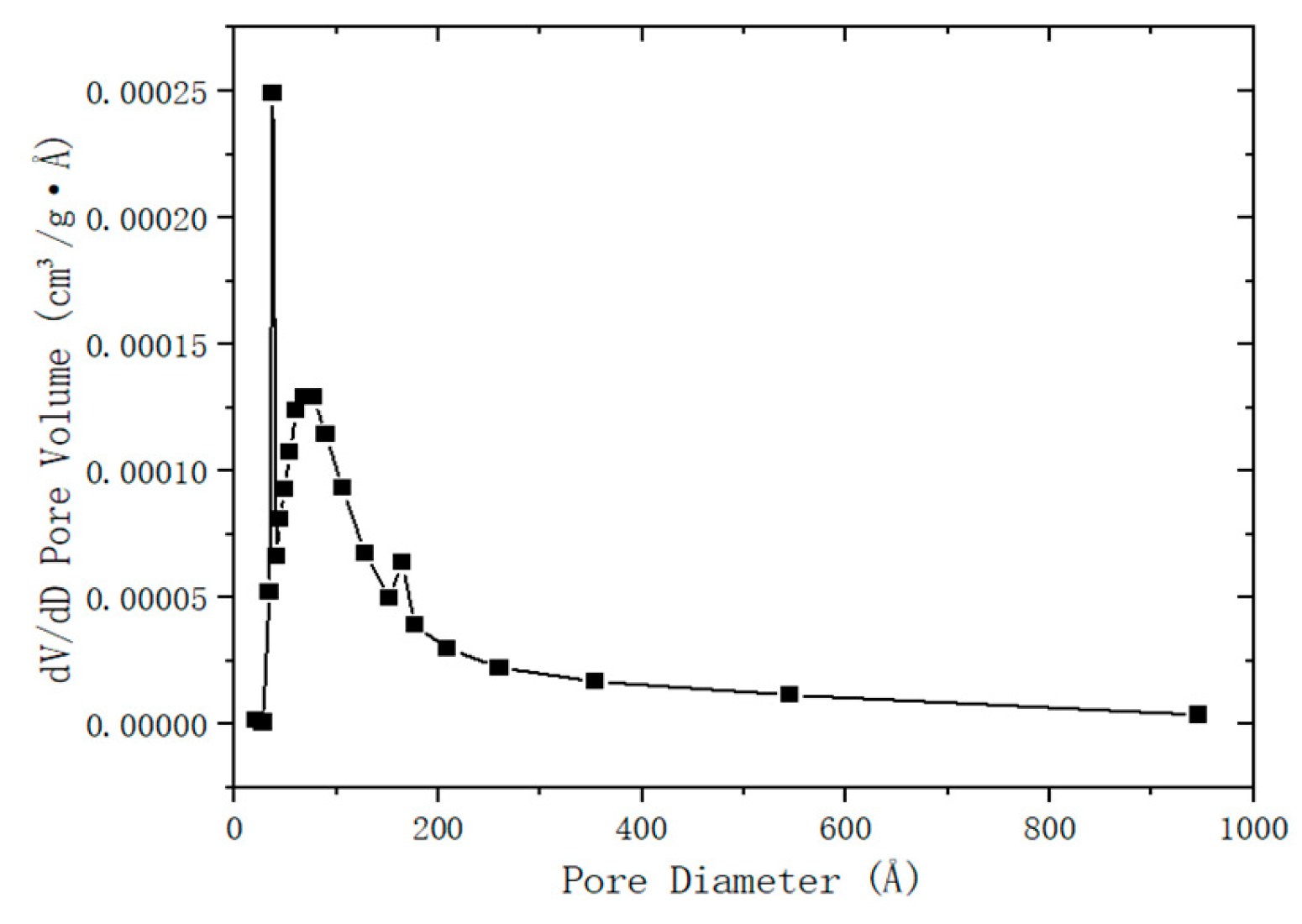
The desulfurization dross was detected by the nitrogen adsorption desorption instrument (BET), and the detection results of the pore size of the desurization dross were shown in
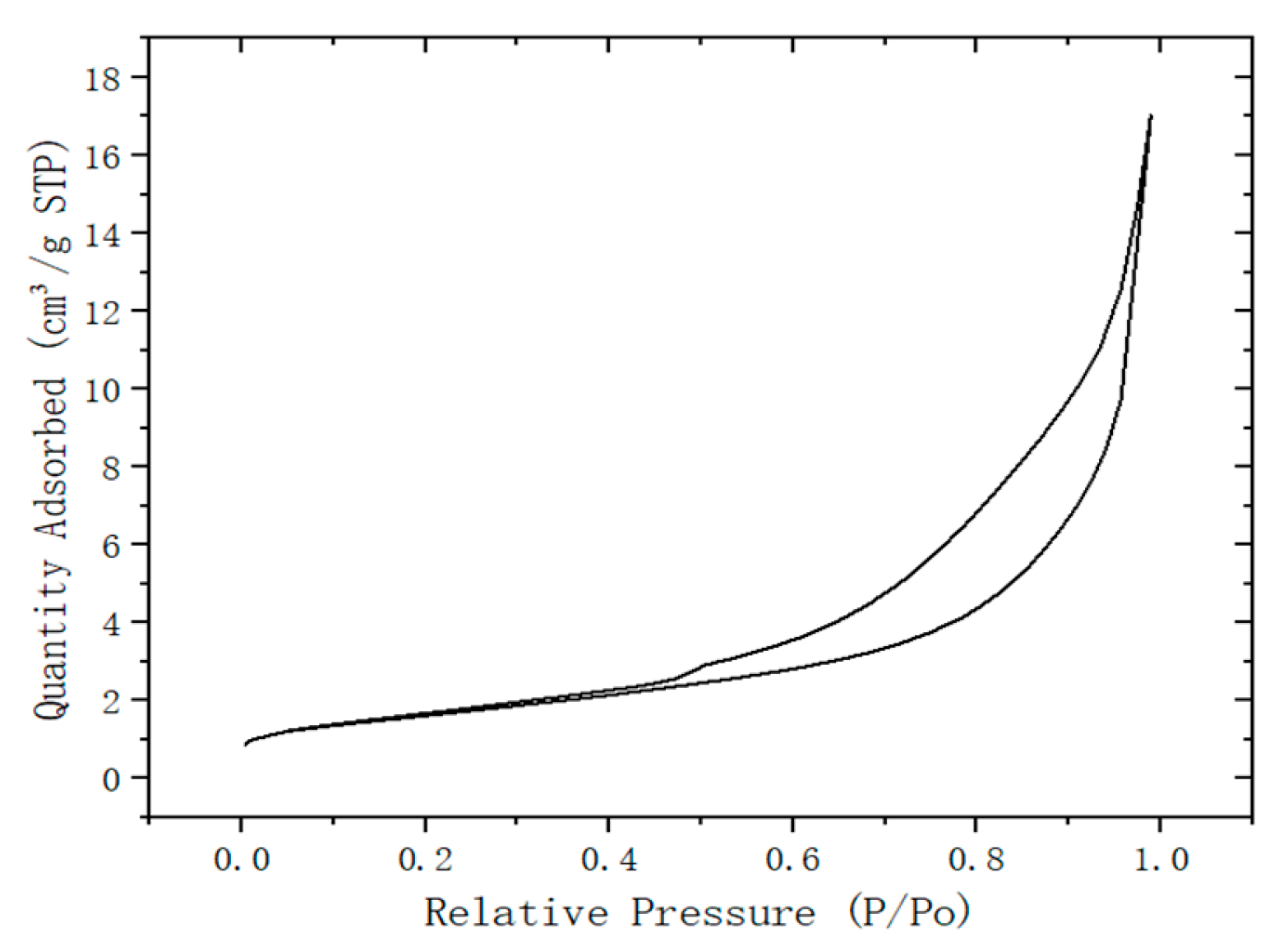
Figure 2, and the adsorption and desorption curves of the desulfurization dross were shown in
Figure 3.
Qian et al [
19] tested the specific surface area of DFA method desulfurization dross as 2.25 m
2/g. The measured results showed that the specific surface area of the SDA method desulfurization dross provided by a certain company in Ma'anshan was 5.868 m
2/g, which was relatively large. The total pore volume of the desulfurized dross is 0.026282 cm
3/g. The average pore diameter of the desulfurization dross was 13.1 nm, which belonged to mesores. It can be seen from
Figure 2 that most mesopores were located within 2-50 nm, and there were few large pores larger than 50nm. The mesoporous structure endowed the desulfurization dross with a large specific surface area, and this property made it have potential in the field of adsorption, such being used as an adsorbent to remove heavy metal ions in wastewater treatment. However, the smaller pore size may also affect its performance in road base backfill, and was necessary to optimize the pore structure by pretreatment (such as grinding) to improve the applicability [
30].
From
Figure 3, it can be seen that in the low pressure area, the adsorption amount increased slowly, or even may decrease slightly; the high pressure area, the adsorption amount increased sharply with the increase of pressure, and there was no obvious saturation platform. During the adsorption process, the interaction between solid surface gas molecules was relatively weak, and the interaction between adsorption molecules was stronger than that between molecules and surfaces. In combination with
Figure 2, the larger the specific surface area desulfurization dross, the more efficient physical and chemical adsorption capacity can be achieved, and the surface activity and catalytic ability were stronger [
32,
33]. This adsorption characteristic showed that the desulfurization dross had a certain adsorption capacity for small molecular substances in gas (such as SO2) or liquid, which can be to assist the absorption of SO
2 in wet flue gas desulfurization, or to improve the dewatering performance by adsorbing water during the sludge dewater process.
2.2. Shape Analysis of Desulfurization Dross
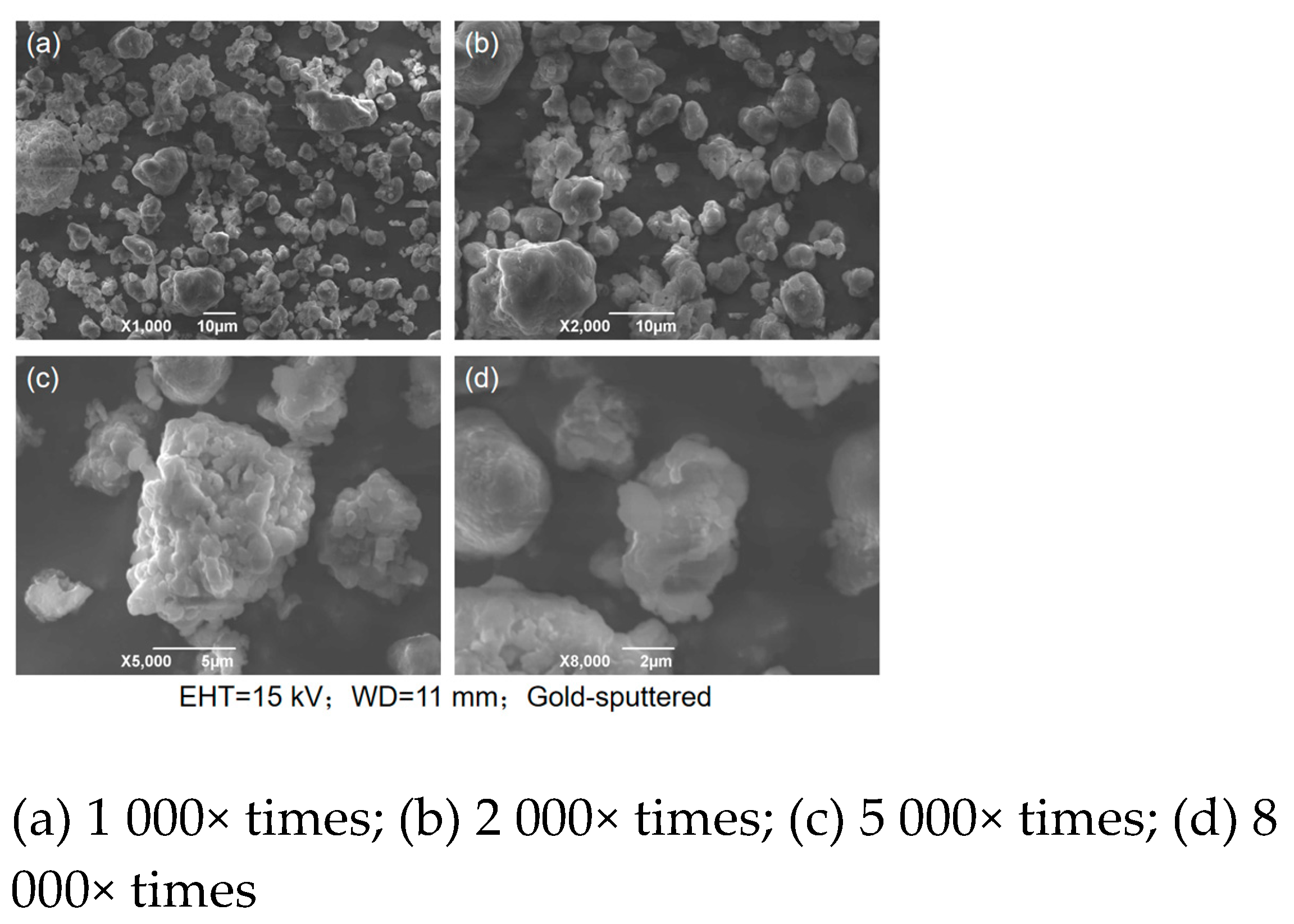
The desulfurization dross was detected by scanning electron microscope (SEM), and
Figure 4 showed the SEM images of the desulfurization dross ground and sieved to 200 mesh at different magnifications. The coefficient of variation of desulfurization dross particles gradually decreases with increasing magnification, ranging from 0.14 to 0.24. This indicates higher uniformity in particle morphology at smaller scales. At larger scales (e.g., 1 000× times), increased morphological variability is observed, likely due to particle agglomeration or surface attachments.
From
Figure 4, it can be seen that the desulfurization dross particles were irregular in shape, porous, and agomerated. The reasons for the above may be: 1) In the process of desulfurization, there were differences in reaction conditions such as temperature, reactant concentration reaction time, etc., in different areas. In areas with high local temperature and large reaction contact area, the reaction rate was fast, and the desulfurization dross particles were larger, and vice versa; 2) The desulfurization reaction was a complex multiphase reaction, and the newly formed desulfurization product gradually deposited on the of the original particles. Due to the difference in reaction sites and reaction rates, the surface growth was uneven, forming an irregular shape; 3) It was difficult for desulfization products to form a liquid phase, and even if a significant solid-phase diffusion effect can be produced, it was impossible to achieve strong densification, so the surface structure of semi-dry desulfurization dross was loose and porous [
33]; 4) Under the action of surface tension, the surface energy reached a minimum, resulting in the desulfurization dross particles becoming spherical; 5) There was a charge on the surface of the desulfurization dross particles, and particles with opposite charged attract each other, in agglomeration. The particles of semi-dry desulfurization dross were mostly close to spherical, with a relatively dense structure, rougher surface, and stronger hesion. During the desulfurization process, the surface of these desulfurization dross particles may adsorb desulfurization products, and then form a dense structure, impete the continuation of the desulfurization reaction, leaving effective desulfurization substances inside. Therefore, the semi-dry desulfurization dross can be recycled and for wet flue gas desulfurization [
33,
34,
35]. The desulfurization dross sample was ground prior to analysis to eliminate interference from factors such as particle agglomeration and uneven size distribution, thereby ensuring clear observation of the microscopic morphology and surface structure of individual particles. The desulfurization dross detected by Zhang Yuanyao et al. was shown in
Figure 5, and it can be found that the microscopic morphology of different semi-dry desulfurization dross particles was generally the same, which was spherical with an irregular surface [
25]. The sphericity indices of the two types of desulfurization dross are relatively low, ranging from 0.3 to 0.7, while the roughness indices range between 2 and 20 μm. Sample (c) exhibits a sphericity of 0.4, which is significantly higher than that of other samples due to its more regular morphology. In terms of roughness: Sample (a) shows the highest roughness (20 μm), attributed to its porous and protruding surface features; Sample (c) has the smoothest surface, with the lowest roughness value of 2 μm.
The desulfurization dross of semi-dry method had the characteristics of fine particle size, loose and porous surface, and large specific surface area, etc., the reaction activity was better. The desulfurization dross can be reused better by pulverizing the particles [
36,
37].
2.3. Component Analysis of Desulfurization Dross provided by a company in Ma'anshan
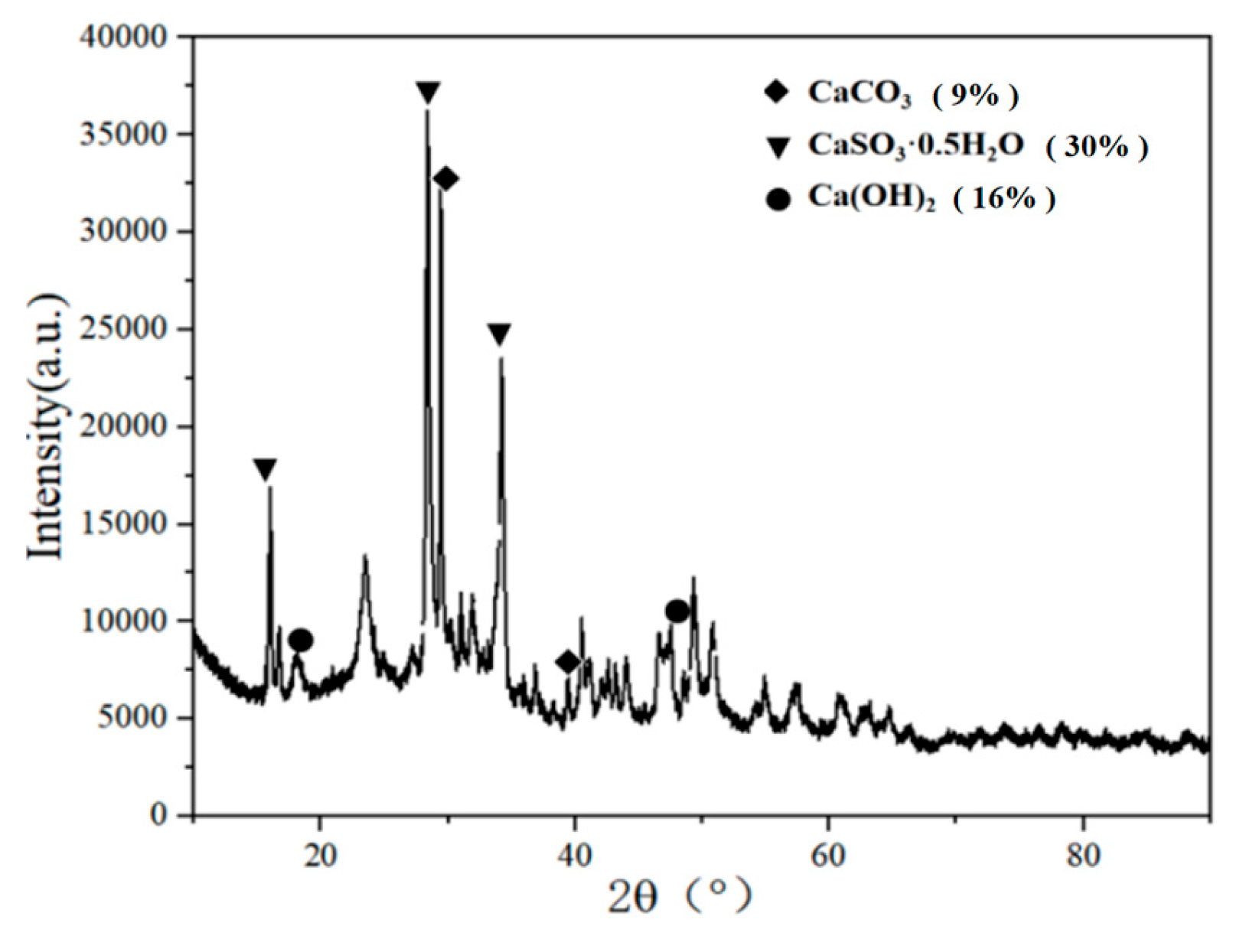
The component of desulfurization dross from semi-dry method was analyzed by XRD and XRF, and the two testing results were corroborated each other. According to the results of XRD and XRF tests, it can be judged whether the desulfurization dross belonged to high-sulfur and high-cal desulfurization dross. According to the characteristics of the composition, the desulfurization dross from the semi-dry method can be reused [
17]. The-ray diffraction instrument (XRD) was used to detect the desulfurization dross, and the detection results were shown in
Figure 6. X-ray fluorescence spectrometerXRF) was used to detect the components of desulfurization dross, and the components of desulfurization dross were shown in
Table 3.
It can be seen from
Figure 6 that the mineral phase of the desulfurization dross was mainly composed of CaSO
3∙0.5H
2O, CaCO
3, and Ca(OH)
2.
It can be seen from
Table 2 that the main components in the desulfurization dross were CaO, SO
3, Cl, Fe
2O
3, K
2O, MgO, SiO
2, TiO
2, Na
2O, Al
2O
3, in addition to other metal oxides. Them, the most abundant was CaO, which accounted for 55.49%. SO
3 was the second most abundant, with a content of 31.3%, indicating that the self-tested desulfurization dross belonged to a typical high-calcium and high-sulfur complex compound. Chen et al. [
17] tested three types of desulfurization dross and also concluded that the desulfurization dross belonged to a high-calcium and high-sulf complex compound. Combining
Figure 3, it can be seen that calcium oxide in the desulfurization dross mainly existed in the form of CaSO
3∙0.5H
2O. The semi-dry desulfurization dross also contained small amounts of Cl, Fe, Si, and other elements, which will waste its utilization value in agriculture, construction and other fields if it was directly buried [
25]. During the desulfurization process, the calcium-based desulfurizer reacted with sulfur dioxide in the sintering flue gas, eventually generating calcium sulfite. If the calcium-based desulfurizer was excessive during the desulfurization process or the reaction was incomplete, there may be a amount of calcium carbonate and calcium hydroxide remaining in the desulfurization dross. It can be seen from
Figure 6 and
Table 3 that the desulfur dross submitted for inspection was not completely desulfurized. In addition to the product of the desulfurization reaction CaSO
3∙0.5H
2O, there still unreacted limestone (CaCO
3), Ca(OH)
2, and other substances in the desulfurization dross. The desulfurization dross also impurities generated due to the reaction of impurities in the desulfurizing agent or other components in the flue gas. The main elements for preparing sulfoaluminate cement clinker contained Ca, S, O, Si, etc. [
38]. According to the composition of the semi-dry desulfurization dross, it can be used as main production raw material for sulfoaluminate cement.
3. Resource Utilization for Semi-Dry Desulfurization Dross
The semi-dry desulfurization dross was high-calcium and high-sulfur, with fine particle size, porous surface desulfurization dross particles, and large specific surface area. Because of these characteristics, the semi-dry desulfurization dross can be used as binding for building materials; because of the high content of Ca(OH)2 in the desulfurization dross, the desulfurization dross is alkaline can be used to improve acidic soil; the desulfurization dross also contained residual desulfurizer, which can be used instead of part of the desulfurizer for wet desulfurization; due to the porosity of the desulfurization dross, it can adsorb and purify sludge wastewater; the desurization dross contained a large amount of CaSO3∙0.5H2O, CaCO3, Ca(OH)2, CaO, and these can be used for high-value utilization of the desulfurization dross. Therefore, the research on the resource utilization of semi-dry desulfurization gyps mainly focused on building materials, agriculture, wet desulfurization, sludge wastewater treatment and high-value utilization and other fields.
3.1. Building Materials Field
The construction materials sector represents the largest and most technologically mature avenue for the utilization of semi-dry desulfurization dross. Leveraging its cementitious properties, filling capacity, and alkalinity, it can partially replace natural raw materials or industrial additives. Pretreatment processes are employed to address compositional instability, ensuring compatibility with the performance requirements of various building materials. Zhu et al. [
23] found that when using deionized water to simulate natural leaching of desulfurization dross, the leached heavy metal concentrations were as follows: Cu
2+ ≤ 0.021 mg/L, Zn
2+ ≤ 0.180 mg/L, Pb
2+ ≤ 0.494 mg/L, Cd
2+ ≤ 0.070 mg/L, and Ni
2+ ≤ 0.352 mg/L. All values were below the limits specified in the Identification Standards for Hazardous Wastes, indicating that the material does not qualify as hazardous waste. This provides a critical environmental safety basis for its resource utilization.
In road engineering applications, the cementitious properties and economic advantages of desulfurization dross are particularly significant. The semi-dry desulfurization dross can be used for road filling backfill because of its characteristics: (1) the desulfurization dross can cementitious reactions similar to cement, generating calcium-silicate hydrate and forming strength, so it can be used as a high-quality road base material; (2) theulfurization dross, as an industrial combustion waste residue, was low-priced, only one-tenth the price of market expanders, and using it as road filling back for recycling can not only reduce engineering costs but also reduce the waste of mineral resources. Liu et al. [
39] applied desulfurization dross to the base course of road cement stabilized crushed stone, using the expansion performance of desulfurization dross itself to partially replace the expansion agent. Gao et al. [
40] found that the use of desulfization slag to partially or fully replace mineral powder as the fine filler of asphalt mixture had high feasibility. When the particle size was the same as that of the mineral powder, desulfurization slag can replace the mineral powder, and the desulfurization slag with high calcium content had a favorable effect on the adhesion performance of asphalt. Currently, the market price of slag micropowder ranges from 200 to 300 yuan/t, while desulfurization dross can be acquired at a minimum cost of 20 yuan/t [
41]. By incorporating oxidatively modified desulfurization dross into slag micropowder applications, not only can it meet substantial market demand, but it also adds significant value, generating considerable economic benefits for enterprises. Zuo et al. [
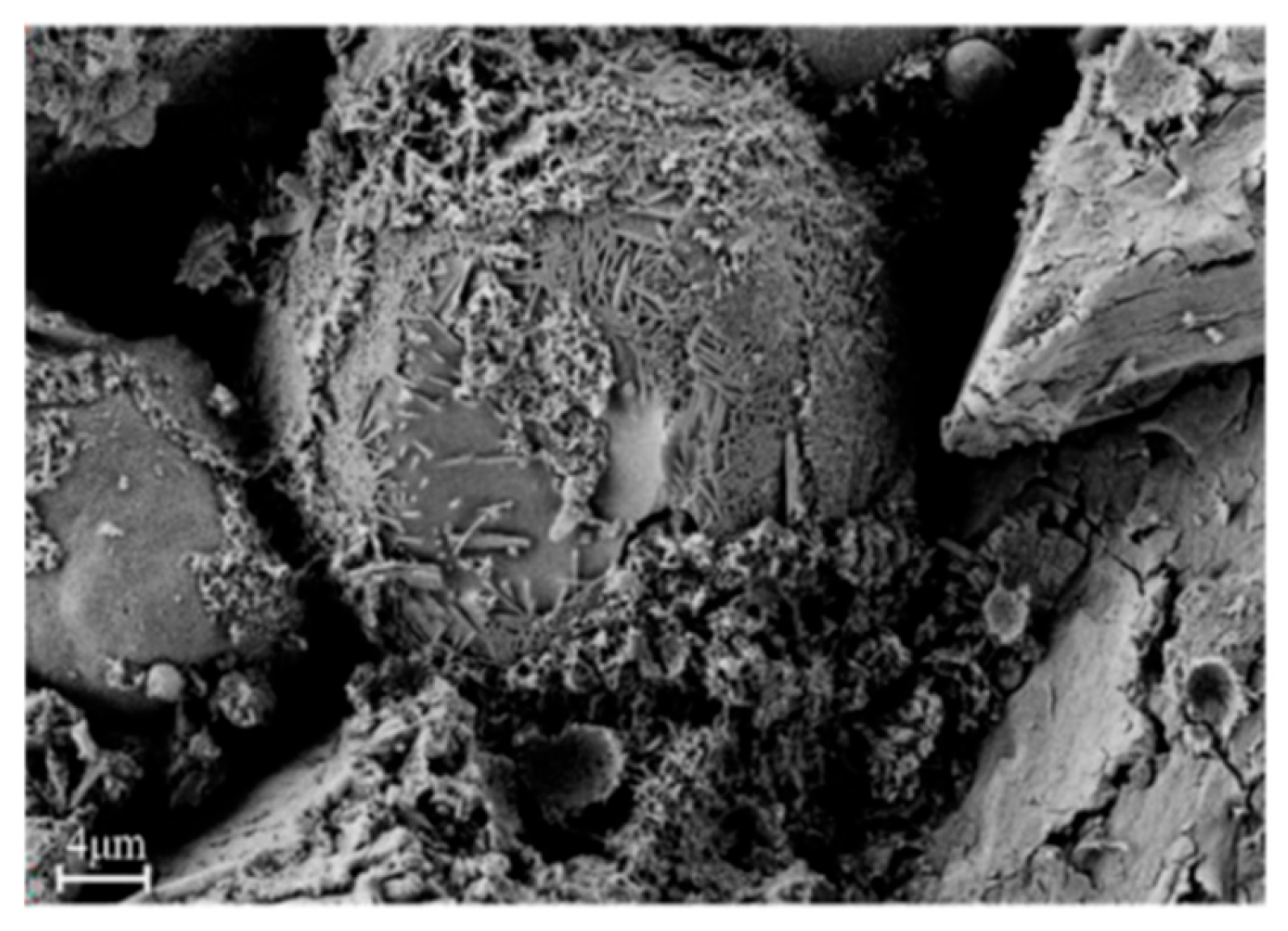
42] prepared light-weight soil for road backfill with desulfurization slag and fly dross as raw materials. Through the promotion desulfurization dross to the disintegration of fly dross, the desulfurization slag particles had been partially wrapped in flocculent and needle-like substances 7 days of curing, which enhanced the interlocking force between particles and the formation of cementitious materials, thus further improving the strength. The SEM chart of light- soil solidified for 7 days of curing was shown in
Figure 7.
Zhang et al. [
43] developed a desulfurization slag-based soil solidifier and found that as the dosage ofulfurization slag increased, the setting time of the desulfurization slag-based soil solidifier showed an increasing trend and the mortar strength showed a decreasing trend, the addition of desulphurization dross should be less than or equal to 30%. Desulfurization dross can also partially replace cement in cement production. This approach not only enables efficient utilization of industrial solid waste and reduces environmental pollution, but also enhances corporate economic benefits, achieving a win-win outcome for both environmental protection and economic development. It provides an effective solution for waste utilization and pollution control. Compared with traditional cement mortar, it had advantages such as crack resistance, no hollowing, and good thermal insulation [
44]. Desulfization dross had a large recycling value because it was often used as raw materials for cement production due to its cementitious properties [
45]. Zhong et al. [
46] utilized desulfurization dross to replace a portion of cement raw materials, which can reduce clinker burning energy consumption. When the sulfur content in clinker increased by 0.14%, the 28 d strength increased by 2 MPa. The process of adding desulfurization dross to Granulated blast furnace slag powder and then mixing it into cement is mature and stable. Su et al. [
47] added 1%-3% desulfurization dross to ground and dried slag micropowder and conducted repeatability tests every 2 hours to determine the chloride ion content in the ground granulated blast furnace slag powder with different blending ratios. As shown in the accompanying table 4, the repeatability verification demonstrated that the chemical composition of the slag micropowder remained stable with the incorporation of 1%-2% desulfurization dross, with no significant adverse effects on its physicochemical properties. The 28 d activity index exceeded 95%, and all measured indicators complied with the requirements specified in the Chinese National Standard GB/T 18046-2017 for slag micropowder. The mechanical strength of the mixtures without desulfurization dross and with desulfurization dross is shown in
Table 4, which includes three sets of data: Group A (without desulfurization dross added), Group B (with 12% desulfurization dross added), and Group C (with 11.25% desulfurization dross added).
The desulfurization dross contained a large amount of f-CaO and SO
32–, which can enhance the bonding performance, and it can be as a binder and mixed with cement [
48]. Wu et al. [
26] found that the high content of CaO in the desulfurization dross could effectively activate the activity of mineral powder, promote its hydration and produce certain strength, and the strength could reach 70% of cementitious materials. Carro-López et al. [
49] found that the compressive strength of cement mortar with ratio of desulphurization dross to cement of 2:3 exceeded 52.5 MPa at 28 d. Chi et al. [
50] found that desulphurization dross can replace part of the cement or can be used as a substitute for fly dross but in ordinary Portland cement, its addition amount needs to be controlled within 20%. Li et al. [
51] found that when the addition of superfine desphurization dross did not exceed 30%, the mixture of this cement and superfine desulphurization dross had better rheological properties than pure cement paste. Shi et al. [
52] used desulphurization dross to activate mineral powder to prepare modified admixtures, and its 7 d and 28d compressive strength was significantly improved, especially when the addition of desulphurization dross was 10%, its 7 d compressive strength was increased by about 3% compared with the mineral powder-cement mortar group without adding desulphurization dross. Fan et al. [
53] improved the strength activity of desulphurization dross residue by mechanical grinding, and compounded the ground desulphurization dross with fly dross according to the ratio of 3:7 to prepare compositeixtures. The composite admixture of desulphurization dross residue can replace 20% of cement, and the C30 desulphurization dross residue concrete prepared, and the 28 d compressive strength reached 41.1 MPa, which was far higher than the requirements for preparing C30 concrete.
Desulfurization dross can also be used in the field of concrete. Yang et al. [
54] found that adding a proper amount of desulfurization dross to concrete played a filling effect, reduced the proportion of pores inside the matrix, and improved its degree of compaction, thereby improving the compressive strength. The replacement of cement with desulfization dross can improve the dry shrinkage performance of the mixture, but it will reduce the compressive strength. Compared with the pure cement stabilized recycled aggregate mixture, the frost resistance of cement desulfurization dross stabilized recycled aggregate mixture was poor, but it can improve the dry shrinkage performance [
55]. Chen et al. [
56] found that the incorporation of 30% desulfurization dross could improve the 7 d compressive strength and flexural strength of plaster dross mortar, but Ca(OH)
2 in the desulfurization dross would shorten the setting time, and it was necessary to select alkali-resistantarders to improve fluidity. Appropriate amount of desulfurization dross was added to the autoclaved aerated concrete, which can only improve the gasification effect, but also enhance the compressive strength to a certain extent. Xu et al. [
57] found that autoclaved aerated concrete mixed with appropriate amount of sintered desulfurization dross had higher content of tobermorite and the crystallinity was improved, which had higher strength. The components of desulfurization dross fluctuated greatly, and the unstable calcium sulfite could be improved by pretreatment (such as grinding, oxidation). Cao et al. [
58] used semi-dry desulphurization dross after water digestion, which can improve the compressive of aerated concrete and reduce its dry density at the same time; the use of ball milling to grind the semi-dry desulphurization dross improved the compressive of aerated concrete, reduced its dry density, and reduced the content of CaSO
4 in the chemical composition, which made the crystallinity of tobermorite improve and its distribution more compact. Based on the concept of multi-source solid waste synergistic utilization, Zhang et al. [
59] prepared high-value lightweight energysaving wall materials with semi-dry sintering desulphurization dross, fly dross and cement as the main raw materials under non-autoclaved curing conditions, a compressive strength of 8.64 MPa at 28 d; this process does not require pre-oxidation treatment of desulphurization dross and provides technical support for its simple and high-value utilization.
The direct resource utilization of semi-dry desulfurization dross is constrained by its chemical instability, primarily due to the potential risks of volume expansion and strength degradation caused by free calcium oxide (f-CaO) and calcium sulfite. Studies have shown that hydration digestion can effectively convert f-CaO into Ca(OH)
2 in advance, significantly improving the volume stability of the material and avoiding the risk of delayed expansion in products. Mechanical grinding, on the other hand, optimizes particle size distribution, increases specific surface area, disrupts dense structures, releases internal active components, and enhances its compactness as a filler in matrices. Additionally, low-temperature and humid storage conditions must be strictly avoided to prevent the formation of non-strengthening thaumasite, which can lead to deterioration in product performance [
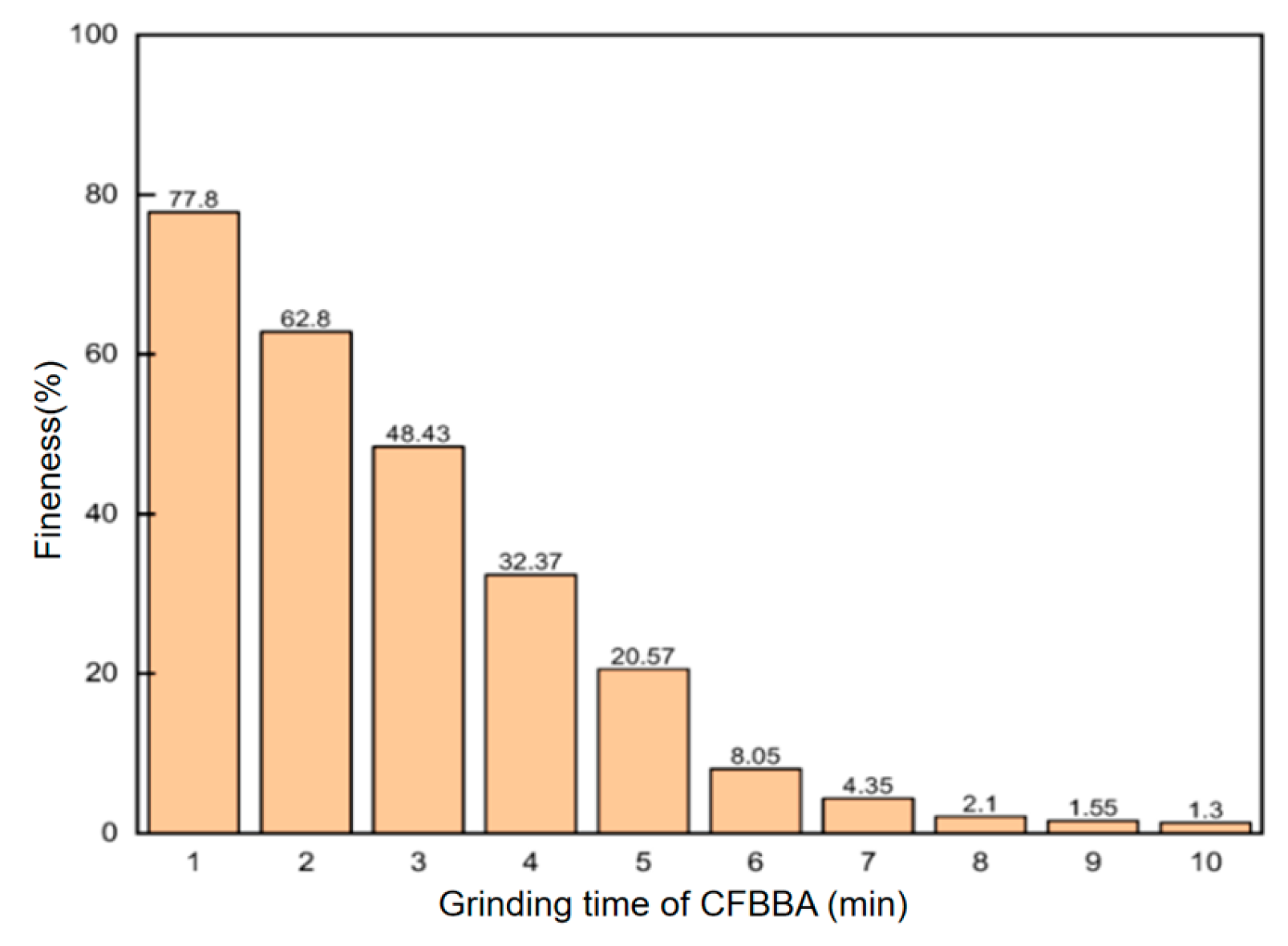
57]. Therefore, targeted pretreatment is a crucial preliminary step for achieving efficient and safe utilization. Dud et al. [
60] used the grinding process to treat CFB method desulfurization dross (CFBBA) to produce cement admixture, the fin of desulfurization dross had met the standard GB/T 1596-2017 after grinding for 5 min, and the effect of grinding time fineness can be seen in
Figure 8.
The effectiveness of semi-dry desulfurization dross in building materials is strongly influenced by the interplay of pretreatment process, dosage ratio, and type of matrix material. In applications with relatively lenient strength requirements, such as road subgrades or cement raw meal, the allowable incorporation ratio of desulfurization dross can be higher. In contrast, for high-performance materials like autoclaved aerated concrete or dross plaster, the dosage must be carefully controlled and combined with pretreatment methods such as hydration digestion or mechanical grinding to ensure performance stability. This situation also highlights a current research gap: the absence of a unified “composition-process-performance” correlation model. As a result, parameters must be repeatedly determined through trial experiments for different application scenarios, which hinders large-scale promotion.
3.2. Agricultural Field
Soil acidification was a major problem that had hindered the development of agricultural planting in recent years. It was mainly caused by the loss of a large of alkaline bases such as calcium, magnesium, and potassium due to rainfall leaching, coupled with the lack of traditional agricultural measures and the long-term use of a large amount chemical fertilizers, which led to an imbalance of soil nutrients [
61]. Soil acidification not only exacerbated soil compaction and reduces fertilizer utilization efficiency but also led to slow growth of crops, an increase in diseases, and a decrease in crop yield and quality. Phoungthong et al.[
62] found that desulphurization dross can be reused as an alternative material for civil and applications, but the pollutants contained in the desulphurization dross may pollute the local environment, hindering its material reuse. Before use, the phytotoxicity leachate produced by desulphurization dross can be assessed. However, the harmful heavy metals contained in the desulphurization dross may accumulate in the environment and cause poisoning to animals or humans through the food chain, and may also indirectly harm animals, plants and human health through the pollution of groundwater [
63]. The semi- desulphurization dross was alkaline and can improve the acidity of the soil, increase the pH value, release the mineral elements needed by plants, and at the same reduce the accumulation of toxic soluble metals in the soil, and improve the soil properties. The agricultural sector can utilize the strong alkalinity and nutrient-releasing capacity of semi-dry desulfurization dross for acidic soil amelioration and cultivation substrate preparation. However, it is essential to strictly control heavy metal risks to ensure agricultural environmental safety.
Dong et al. [
64] found that the application of bioactive carbon and desulfurization dross could enhance the rice wound flow, root crown ratio and biomass. Liu et al. [
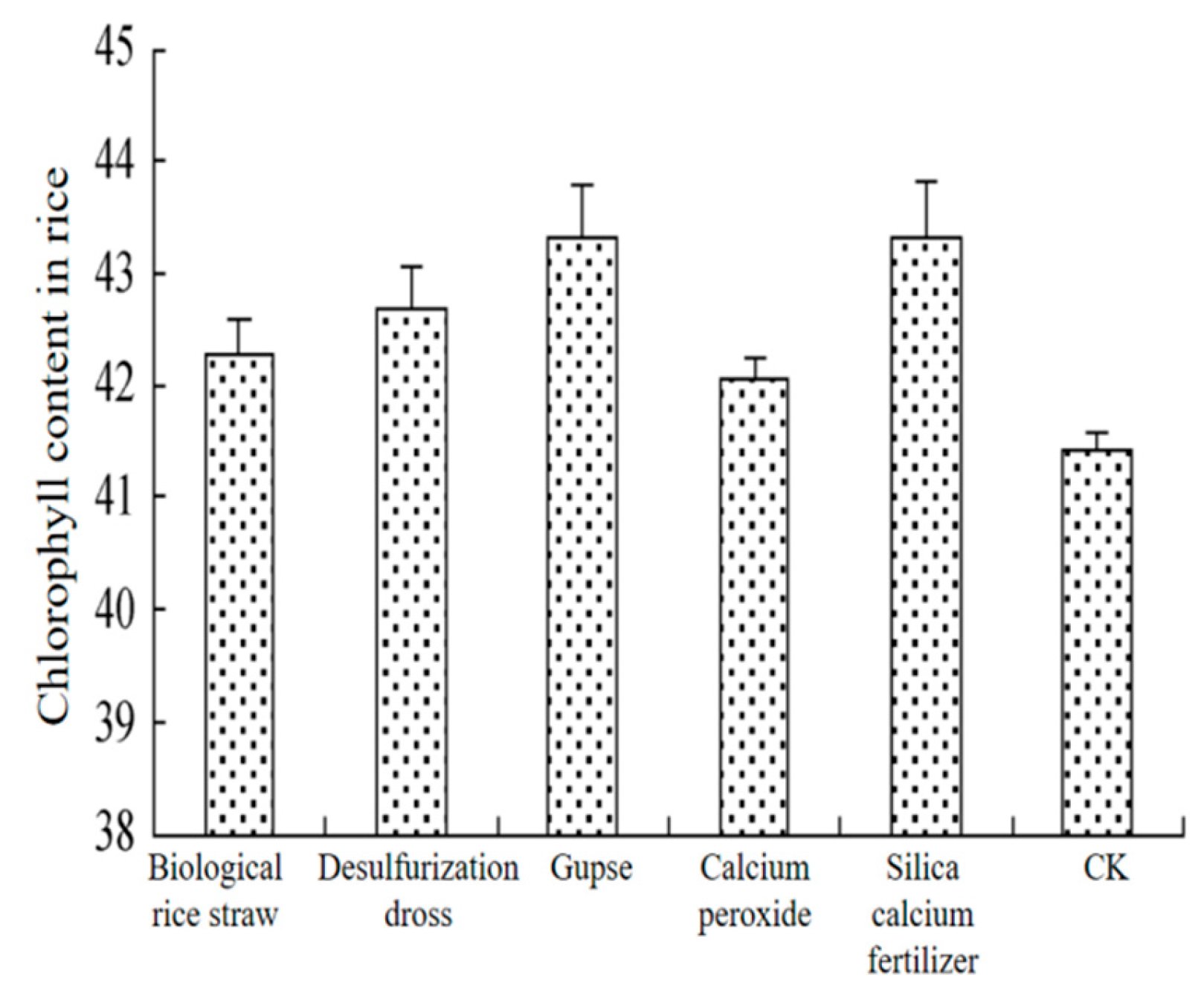
65] found through field trials that the application of desulfurization dross as a soil conditioner in cold paddies significantly increased the effective phosphorus and effective potassium of the soil, and at the same time increased the chlorophyll content of rice leaves by 3%, and use of desulfurization dross in agriculture was less expensive than other conditioners such as dross, and the effects of different conditioners on the chlorophyll content of leaves were shown in
Figure 9.
Zhou et al. [
66] used fly dross and desulphurization dross as the main raw materials to prepare non-fired expanded perlite through steam curing. The perlite had a developed internal pore structure, large water absorption rate, and was rich in elements such as Si, Ca and S which was suitable for plant growth needs; the non-fired planting expanded perlite was a colloidal aluminosilicate network structure geopolymer binder, which had a structure and strong durability.
3.3. Wet Flue Gas Desulfurization
As a by-product of desulfurization, the semi-dry desulfurization dross contained a certain amount of effective desulfization components. By adopting a "waste-treats-waste" approach, the residual active desulfurization components in the dross can be utilized to partially replace limestone-based desulfurizing agents, thereby reducing desulfurization costs while enabling the cyclic utilization of the desulfurization dross. Li et al. [
67] found that it was feasible to partially replace limestone powder with calcium-based desulfurization dross in wet flue gas desulfurization systems. While pure limestone powder achieved a desulfurization efficiency of 99%, when the substitution ratio of desulfurization dross was controlled within 50%, the desulfurization efficiency of the system can maintained above 95%. Yang et al. [
68] used semi-dry desulfurization dross in wet flue gas desulfurization, the desulfurization efficiency decreased with the increase of flue gas temperature, flow rate, and SO
2 concentration, and increased with the increase of liquid-gas ratio and pH; and compared with the traditional wet flue gas desulfurization agent limestone, the difference in desulfurization efficiency was no more than 5%, and the-dry desulfurization dross had a desulfurization efficiency of more than 90% and strong desulfurization activity, which could meet the emission index of desulfurization efficiency not less than 95%. As a desulfurization agent in wet flue gas desulfurization, semi-dry desulfurization will have problems such as poor desulfurization efficiency, small desulfurization capacity, and high slurry concentration [
67]. Zhang et al. [
32] found that when the mixing ratio of coke oven desulfurization dross and semi-dry desulfurization dross was 5%, the desulfurization effect was significantly improved, and the average desulfurization efficiency could be maintained above 96% under the conditions of low temperature, high SO
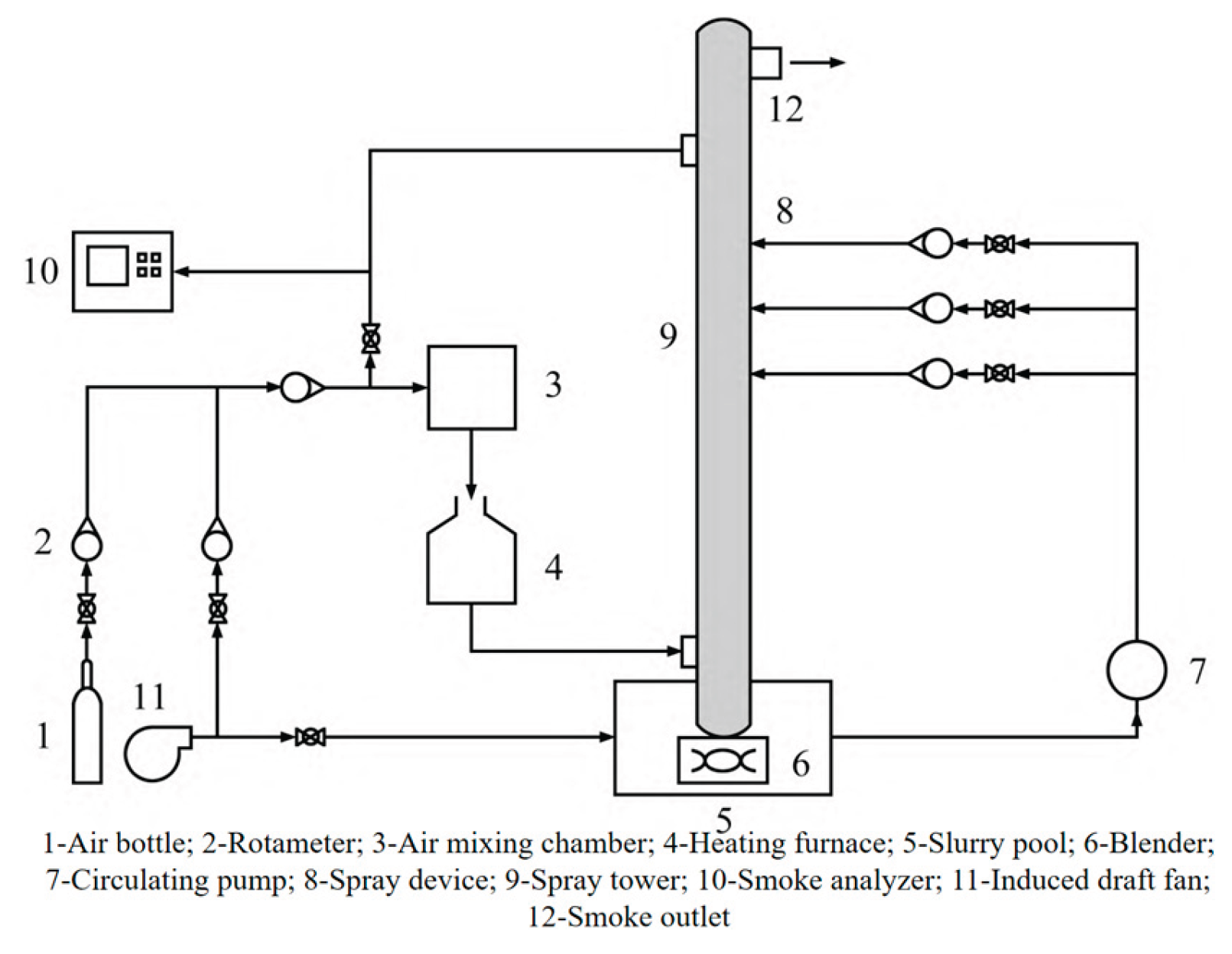
2 concentration, and fast flue gas flow rate, and the maximum could reach 99%, which greatly improved the desulfurization efficiency of semi-dry desulfurization dross. The spraying device for wet flue gas desulfurization is shown in
Figure 10. The application of semi-dry desulfurization dross in wet flue gas desulfurization systems requires comprehensive consideration of process compatibility, operational stability, and potential risks. In practice, excessively large particle sizes in the dross may lead to clogging of spray nozzles in the absorption tower, reducing desulfurization efficiency. Meanwhile, high Cl
- content can accelerate equipment corrosion and shorten service life. By optimizing pretreatment processes—such as grinding for particle size reduction and wdrossing for chloride removal—the economic and environmental benefits of its utilization can be fully realized.
The content of CaSO
3 was one of the reasons affecting the dissolution rate of desulfurization dross, and the higher the content of CaSO
3, the lower the dissolution rate. The semi-dry desulfurization dross was alkaline due to the presence of a large amount of CaO and Ca(OH)
2, and can partially replace limestone for wet flue gas desulfurization, and its dissolution characteristics affect the desulfurization performance. Jia et al. [
69] found that reducing the pH value of the solution and appropriately increasing the proportion of desulfurization dross addition were beneficial to the dissolution of the desurization agent. Due to the relatively poor oxidation conditions of the semi-dry desulfurization dross, the content of calcium sulfite in the desulfurization dross higher. Wang et al. [
70] conducted an experiment of adding desulfurization dross to limestone for wet desulfurization, and found that addition of 25% desulfurization dross could effectively oxidize calcium sulfite in the desulfurization dross. Baek et al. [
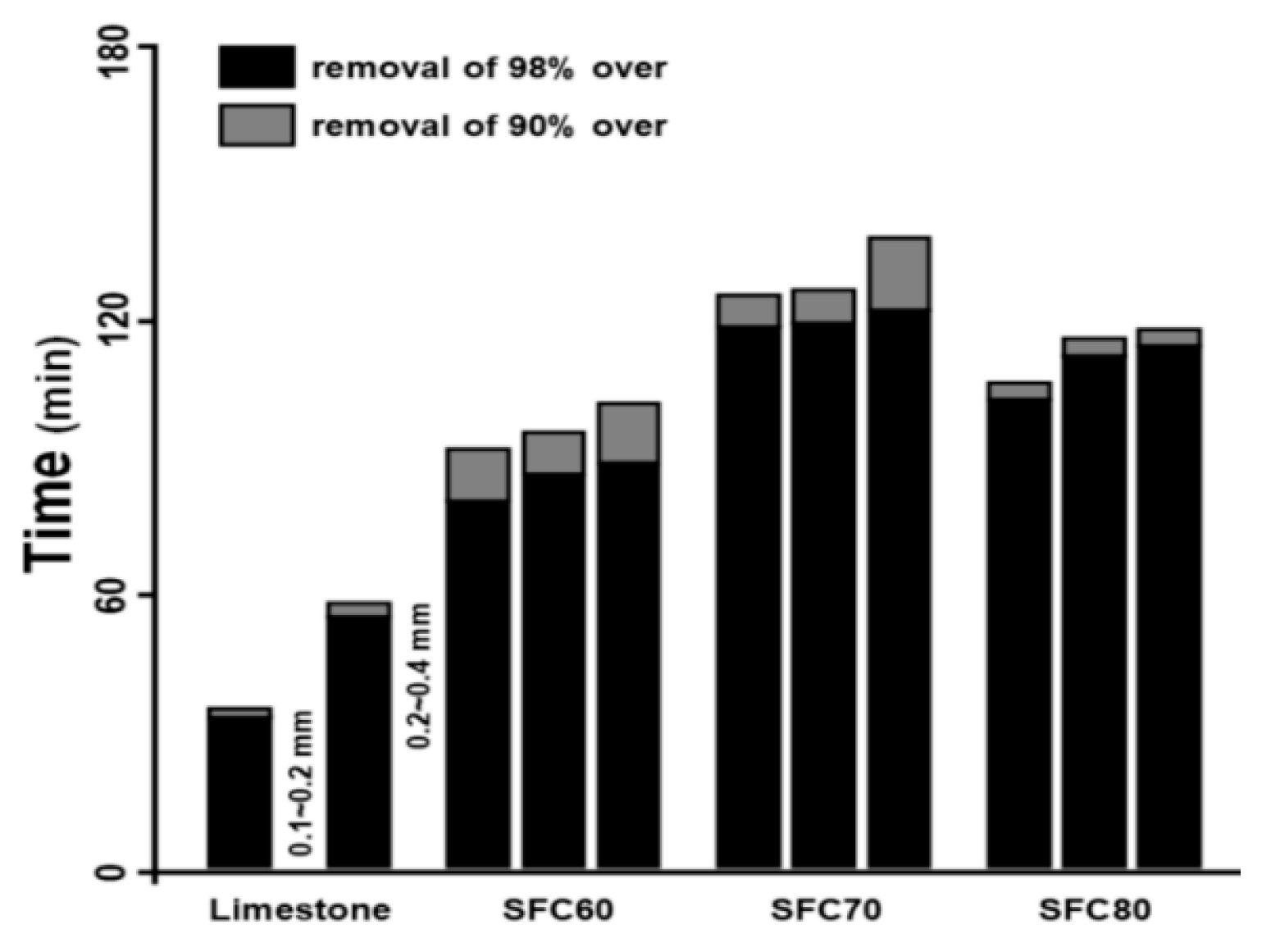
71] tested SO
2 concentration of the fluidized bed reactor filled with limestone and calcium-based desulfurization adsorbents (SFC60, SFC70, and FC80), and the time for desulfurization rate of 90% was less than 2 h, and the adsorption time measurement of calcium-basedulfurization adsorbents was shown in
Figure 11. As an industrial by-product, desulfurization dross is generated in large quantities and boasts raw material costs significantly lower than those of traditional coagulants. Utilizing desulfurization dross in the production of coagulants can also reduce landfill disposal expenses, further amplifying its economic advantages.
Studies indicate that the core value of semi-dry desulfurization dross in sludge and wastewater treatment lies in its multi-functional synergy: In sludge treatment, it simultaneously provides skeletal support, moisture adsorption, and structural conditioning; In wastewater treatment, it offers alkaline adjustment, coagulation, and heavy metal adsorption capabilities. Sulfuric acid modification has been identified as a key pretreatment method for enhancing wastewater treatment efficiency, significantly improving turbidity and heavy metal removal rates. While current research has covered applications in municipal sludge and mine wastewater, its effectiveness in treating complex effluents such as high-salinity wastewater and dyeing wastewater remains unexplored, indicating a need for broader application scenarios.
3.4. Treatment for Sludge and Wastewater
Leveraging the porous adsorption capacity, alkalinity regulation ability, and skeletal support function of semi-dry desulfurization dross, it can be applied in sludge and wastewater treatment. This approach addresses challenges such as poor sludge dewaterability and low removal efficiency of pollutants in wastewater, enabling the synergistic treatment of solid waste and wastewater. Sludge from cities had the characteristics of large production, high water content, complex composition, difficult dewatering, and contained a large amount of plant nutrients. The treatment cost was relatively high, and how to dewater the sludge at low cost had become the key to sludge treatment. The oxidation by potassium permanganate, the skeleton construction by desulphurized dross, and the improvement of sludge dewatering performance can achieved by strengthening the thermal effect and chemical oxidation effect of the combined technology of microwave, ultrasonic wave, and ultraviolet ray (PUWU) in turn [
72,
73,
74]. Tai et al. [
74] found that after coupling treatment with PUWU, potassium ferrate and desulphurization dross, the sludge structure becomes more dense, a continuous morphology was formed on the surface, the porosity was reduced and the solid particles were arranged closely, which helped the of water. The SEM of the original sludge and the treated sludge were shown in
Figure 12. Ouyang et al. [
75] used desulphurization dross and oily sludge to prepare pellets. After high-temperature firing, pellets had a good solidification effect on heavy metals such as Cd, Cr, Pb, and Zn, with a large density and low water absorption.
The desulfurization dross modified by sulfuric acid will form a complex with complex structure and composition, which can reduce the surface potential of suspended particles in water weaken the same charge repulsion between charged particles, and promote the collision and agglomeration of particles to form flocs, so that the particles in water can be precipitated.Therefore, it can be used as a coagulant to treat wastewater [
76]. Jiu Yudan [
77] modified the desulfurization dross from coal-fired power plants with sulfuric acid, and the research conclusion showed the desulfurization dross after modification with sulfuric acid had good performance in removing turbidity from mine wastewater. Fang et al. [
78] utilized semi-dry desulfurization dross to remove chromium and vanadium from wastewater, and the residual mass concentration of Cr(VI), total Cr, and V were 0.63 mg·L
–1, 0.395 mg·L
–1, and 0.155 mg·L
–1, respectively.
3.5. High Value-Added Utilization
High value-added utilization is a crucial direction for upgrading semi-dry desulfurization dross from low-value consumption to high-value conversion. Through chemical transformation or modification, it can be processed into high-purity and highly functional products, thereby enhancing its economic value. Zeng et al. [
79] successfully prepared high-purity calcium sulfate powder by reacting 70 g of desulfurization dross, 300 mL of water, and 45 g of hydrogen peroxide for 90 min, and then adding 550 g of dilute sulfuric for 60 min. The conversion rate of calcium sulfite was 97.86%, and the content of calcium sulfate in the product was 98.4%. The process flow diagram for the preparation of high-value-added calcium sulfate from desulfurization dross was shown in
Figure 13 [
80].
Zhang et al. [
81] modified the desulfurization dross through chemical modification treatment, and used the modified desulfurization dross to replace part of the carbon black to prepare modified desulfurization dross-based ecological rubber; the preparation process was analyzed by XRD and SEM, etc. It was found after the modified desulfurization dross was added, the maximum torque of the ecological rubber compound decreased significantly, and the scorching time and the normal vulcanization time were, and the vulcanization process was divided into the induction period, the reaction period and the flat period, and different cross-linked network structures were formed in each stage. Lu et al. [
82] used desulfurization dross as reinforcing filler and prepared natural rubber (NR)/desulfurization dross composite by direct mixing, which shortened the scorching period of NR and improved the efficiency of vulcanization, and also improved the thermal stability and mechanical properties of the composite, and improved the comprehensive of NR in a low-cost way. Lv et al. [
83] used a boiling furnace to catalytically decompose desulphurization dross with a composite catalyst composed ferrous sulfide and ferrous sulfate at a reaction temperature of 900℃, achieving a desulphurization rate of 91.41% The generated SO
2 can be used to produce sulphuric acid, and the roasting product can be used as raw material for sintering plants. Mao et al. [
84] reduced and calcined the sinter SDA desulphurization dross, and analyzed out SO
2 for acid production, and the solid product can recycled.
Du et al. [
85] used desulphurized dross as a catalyst to catalyze the pyrolysis of cotton stalk at 900℃ and found that the desulphurized dross could promote the pyrolysis of cotton stalk, improve the yield of pyrolysis gas and the loworific value, and promote the generation of H
2 from cellulose and CH
4 from lignin, and inhibit the generation of CO and CO
2 from cellulose.
In terms of industrial scalability, most current research on high-value applications remains at the laboratory or pilot stage, and transitioning to large-scale industrial implementation still faces multiple bottlenecks. The variability in the composition of desulfurization dross from different production sources poses a significant challenge to chemical conversion processes, which require highly stable input materials.
3.6. Environmental risk
The environmental risks associated with semi-dry calcium-based desulfurization dross stem from its complex physicochemical properties. During its reuse in agriculture, construction, and other fields, these risks may be released through various pathways of toxicity, necessitating targeted identification and management.
Although the construction sector mitigates risks through solidification, long-term use or improper disposal still poses potential hazards. When used in wall materials, desulfurization dross must undergo sufficient pretreatment to meet the particle size distribution and harmful substance content requirements specified in Recycled Coarse Aggregate for Concrete (GB/T 25177-2010). The Technical Guidelines for Construction of Highway Roadbase Bases (JTG/T 3650-2020) require that the pH of grouting materials be between 6.5 and 8.0. Therefore, when desulfurization dross is used in roadbase materials, its dosage must be controlled to avoid strong alkalinity causing corrosion to the pavement.
In agricultural applications, desulfurization dross may leach heavy metals such as Pb, Ni, and Cd. Testing should be conducted according to the methods specified in Fertilizers and Soil Conditioners - Determination of Arsenic, Cadmium, Chromium, Lead, and Mercury Contents (BS ISO 17318:2015)[
23,
25,
86]. If heavy metal leaching concentrations are excessively high, these metals can migrate through the "soil - crop root absorption - plant accumulation" pathway. The accumulation of heavy metals in crops may exceed the limits set in the National Food Safety Standard - Limits of Contaminants in Food, ultimately endangering human health through the food chain [
23,
25,
87]. Moreover, current research predominantly focuses on the short-term effects of desulfurization dross on crop growth, while long-term data tracking its impact on soil microbial communities and the evolution of soil physicochemical properties remain scarce. Addressing this knowledge gap represents a critical direction for future studies.
In addition to conventional uses, the high-value extraction and targeted conversion of calcium resources from this dross align with the core principles of the circular economy-reduce, reuse, recycle—and can generate significant economic and environmental benefits. Although technical and economic challenges remain, increasingly stringent environmental policies, rising resource costs, and carbon neutrality goals provide a strong impetus for innovation. These emerging pathways hold considerable potential and long-term significance, enabling a genuine integration of environmental and economic benefits.
4. Conclusions and Outlook
(1) The resource utilization of semi-dry calcium based desulfurization dross is an important direction to promote the treatment of industrial solid waste and the development of circular economy. Desulfurization dross had shown the feasibility of replacing natural materials in building materials, agriculture and other fields, such as reducing cement energy consumption improving acidic soil, and its economy was better than traditional methods. desulfurization dross can also be used for wet desulfurization and wastewater treatment and high valueadded utilization, which can achieve "waste treatment".
(2) The primary technical challenge stems from the highly volatile composition of materials, which leads to inconsistent product performance. Existing pretreatment processes are constrained by high costs and energy consumption, making them economically unviable. There is an urgent need to develop scalable pretreatment technologies. On the regulatory front, the absence of targeted product standards, environmental access regulations, and comprehensive risk management systems prevents effective assessment of long-term environmental impacts and ecological accumulation effects, thereby increasing uncertainties in large-scale implementation.
(3) To systematically advance development in this field, we should establish long-term environmental behavior monitoring and risk assessment models based on real-world application scenarios. Accelerate the formulation of classification standards, usage specifications, and environmental release thresholds for desulfurization dross-based products to build a comprehensive standard system. Develop a "cascade utilization" model for desulfurization dross: initially replace limestone in wet flue gas desulfurization processes, then repurpose the resulting secondary desulfurization dross as construction filler to enhance resource efficiency. Only through coordinated efforts in technological innovation, standard refinement, and regulatory mechanism innovation can we break current bottlenecks and achieve high-value, large-scale, and environmentally safe utilization of semi-dry desulfurization dross.