Submitted:
25 August 2025
Posted:
26 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Finding a Solution for Digestate Disposal Is an Urgent Task
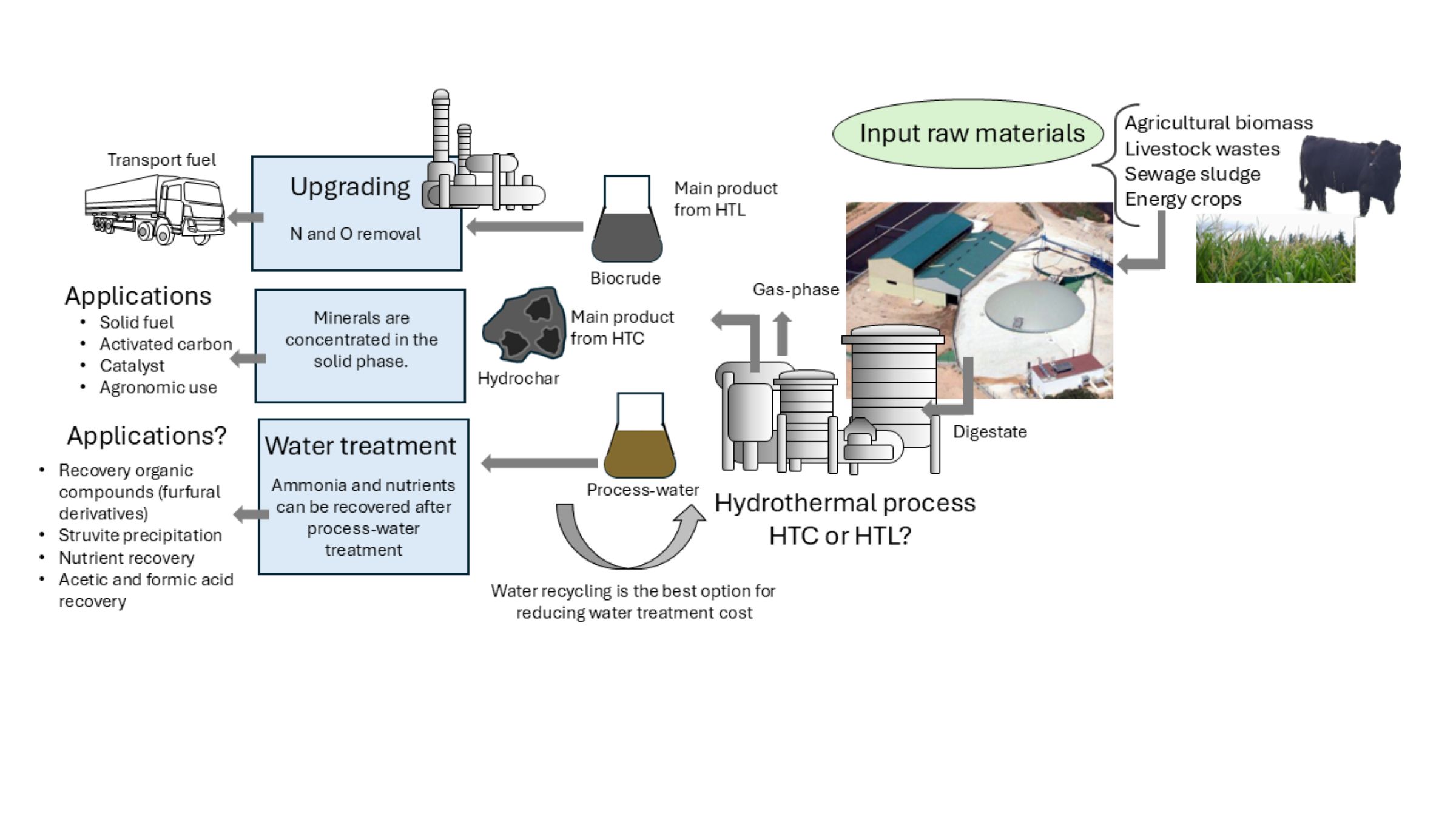
2. Materials and Methods
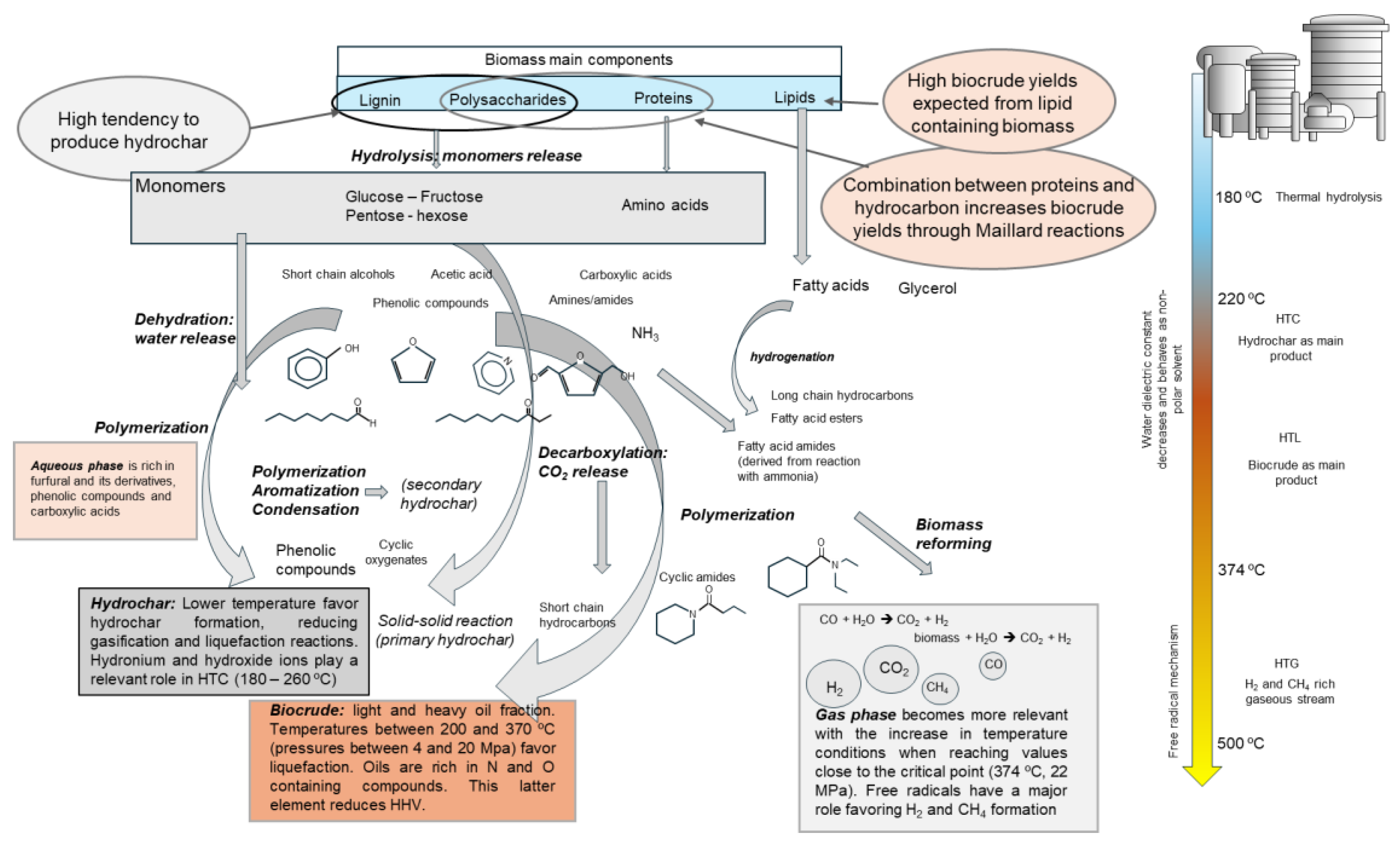
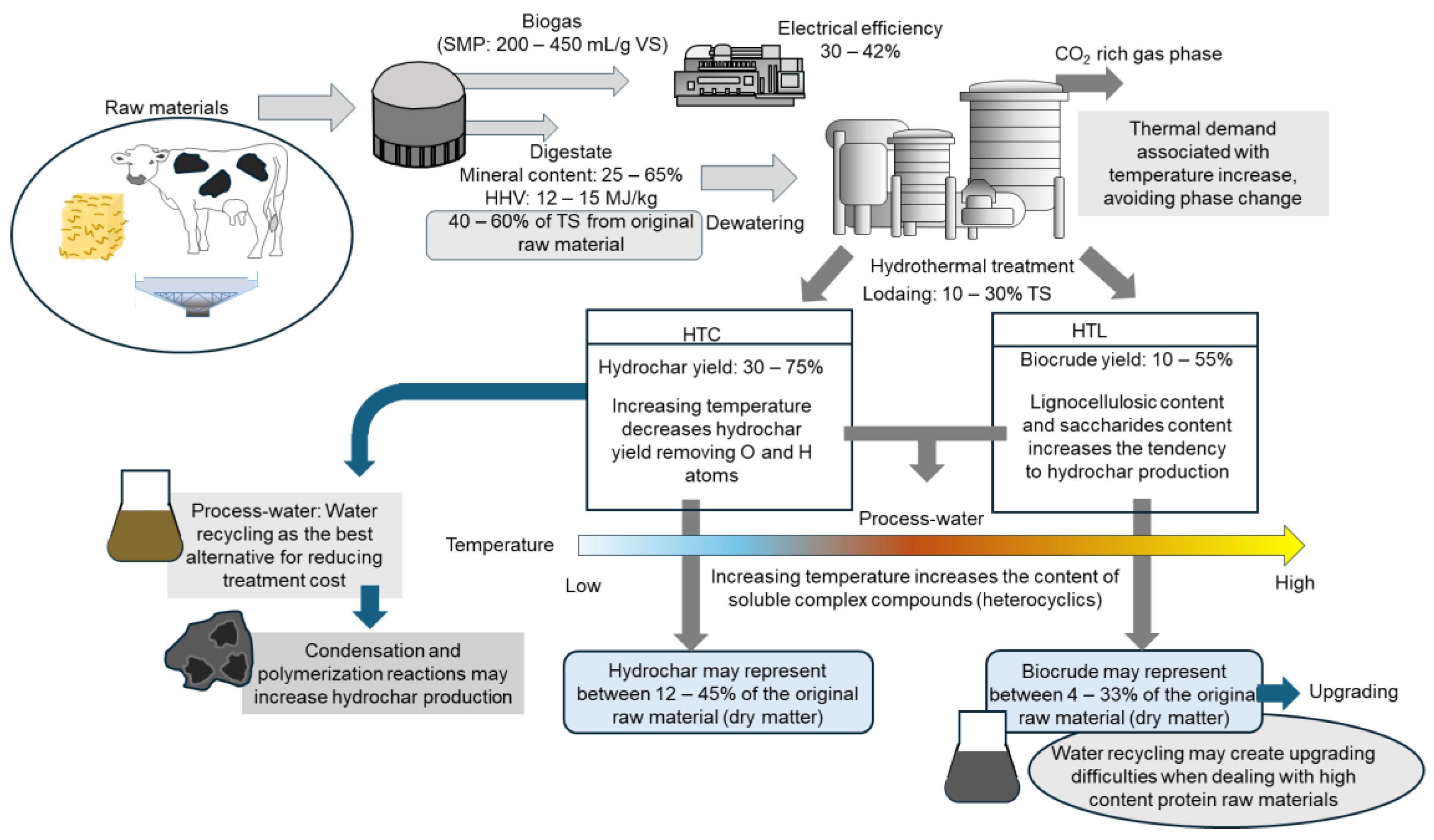
3. Hydrothermal Processes
3.1. Large Demonstration Projects Are Needed
4. Hydrothermal Conversion of Digested Material
5. Aqueous Phase
6. Techno-Economic Assessment
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Czekała, W.; Jasiński, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas Plant Operation: Digestate as the Valuable Product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- REPowerEU: Joint European Action for more affordable, secure and sustainable energy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:52022DC0108 (accessed on 1st August 2025).
- Brunetti, A.; Barbieri, G. Membrane Engineering for Biogas Valorization. Front. Chem. Eng. 2021, 3, 775788. [Google Scholar] [CrossRef]
- Francisco López, A.; Lago Rodríguez, T.; Faraji Abdolmaleki, S.; Galera Martínez, M.; Bello Bugallo, P.M. From Biogas to Biomethane: An In-Depth Review of Upgrading Technologies That Enhance Sustainability and Reduce Greenhouse Gas Emissions. Appl. Sci. 2024, 14, 2342. [Google Scholar] [CrossRef]
- Czekała, W. Social Aspects of Agricultural Biogas Plants. In: Biogas Plants: Waste Management, Energy Production and Carbon Footprint Reduction. 2024. Wiley. 279-290. [CrossRef]
- Bourdin, S.; Chassy, A. Are Citizens Ready to Make an Environmental Effort? A Study of the Social Acceptability of Biogas in France. Environ. Manag. 2023, 71, 1228–1239. [Google Scholar] [CrossRef]
- Szymańska, M.; Ahrends, H.E.; Srivastava, A.K.; Sosulski, T. Anaerobic Digestate from Biogas Plants—Nuisance Waste or Valuable Product? Appl. Sci. 2022, 12, 4052. [Google Scholar] [CrossRef]
- Fernández-Domínguez, D.; Guilayn, F.; Patureau, D.; Jimenez, J. Characterising the stability of the organic matter during anaerobic digestion: A selective review on the major spectroscopic techniques. Rev. Environ. Sci. Bio/Technol. 2022, 21, 691–726. [Google Scholar] [CrossRef]
- González-Rojo, S.; Carrillo-Peña, D.; González, R.G.; Gómez, X. Assessing Digestate at Different Stabilization Stages: Application of Thermal Analysis and FTIR Spectroscopy. Eng 2024, 5, 1499–1512. [Google Scholar] [CrossRef]
- Alburquerque, J.; De la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eu. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- García-López, A.M.; Delgado, A.; Anjos, O.; Horta, C. Digestate Not Only Affects Nutrient Availability but Also Soil Quality Indicators. Agronomy 2023, 13, 1308. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Nag, R.; Whyte, P.; Markey, B.K.; O'Flaherty, V.; Bolton, D.; Fenton, O.; Richards, K.G.; Cummins, E. Ranking hazards pertaining to human health concerns from land application of anaerobic digestate. Sci. Total Environ. 2020, 710, 136297. [Google Scholar] [CrossRef]
- Cucina, M.; Castro, L.; Escalante, H.; Ferrer, I.; Garfí, M. Benefits and risks of agricultural reuse of digestates from plastic tubular digesters in Colombia. Waste Manage. 2021, 135, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.T.; Banjade, S.; Murthy, S.N. Combined anaerobic and aerobic digestion for increased solids reduction and nitrogen removal. Water Res. 2011, 45, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, J.; Wachemo, A.C.; Yuan, H.; Zuo, X.; Li, X. Mass conversion pathway during anaerobic digestion of wheat straw. RSC adv. 2020, 10, 27720–27727. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Blanco, D.; Cascallana, J.G.; Carrillo-Peña, D.; Gómez, X. Anaerobic Co-Digestion of Sheep Manure and Waste from a Potato Processing Factory: Techno-Economic Analysis. Fermentation 2021, 7, 235. [Google Scholar] [CrossRef]
- Parra-Orobio, B.A.; Cruz-Bournazou, M.N; Torres-Lozada, P. Single-Stage and Two-Stage Anaerobic Digestion of Food Waste: Effect of the Organic Loading Rate on the Methane Production and Volatile Fatty Acids. Water Air Soil Pollut. 2021, 232, 105. [Google Scholar] [CrossRef]
- Sayara, T.; Sánchez, A. A Review on Anaerobic Digestion of Lignocellulosic Wastes: Pretreatments and Operational Conditions. Appl. Sci. 2019, 9, 4655. [Google Scholar] [CrossRef]
- Poddar, B.J.; Nakhate, S.P.; Gupta, R.K.; Chavan, A.R.; Singh, A.K.; Khardenavis, A.A.; Purohit, H.J. A comprehensive review on the pretreatment of lignocellulosic wastes for improved biogas production by anaerobic digestion. Int. J. Environ. Sci. Technol. 2022, 19, 3429–3456. [Google Scholar] [CrossRef]
- Delzeit, R.; Kellner, U. The impact of plant size and location on profitability of biogas plants in Germany under consideration of processing digestates. Biomass Bioenerg. 2013, 52, 43–53. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Z.; Isaguirre, C.; Liu, Y.; Liao, W. Fungal fermentation on anaerobic digestate for lipid-based biofuel production. Biotechnol. Biofuels 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Jasinska, A.; Wojciechowska, E.; Stoknes, K.; Roszak, M. Bioconversion of Agricultural Wastes into a Value-Added Product: Straw of Norwegian Grains Composted with Dairy Manure Food Waste Digestate in Mushroom Cultivation. Horticulturae 2022, 8, 331. [Google Scholar] [CrossRef]
- Fernandes, F.; Silkina, A.; Gayo-Peláez, J.I.; Kapoore, R.V.; de la Broise, D.; Llewellyn, C.A. Microalgae Cultivation on Nutrient Rich Digestate: The Importance of Strain and Digestate Tailoring under PH Control. Appl. Sci. 2022, 12, 5429. [Google Scholar] [CrossRef]
- Sobolewska, E.; Borowski, S.; Jurczak, T. Growth of microalgae and cyanobacteria consortium in a photobioreactor treating liquid anaerobic digestate from vegetable waste. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Elbl, P.; Baláš, M.; Lisý, M.; Lisá, H. Sewage sludge and digestate gasification in an atmospheric fluidized bed gasifier. Biomass Conv. Bioref. 2024, 14, 21821–21829. [Google Scholar] [CrossRef]
- Rathnayake, N.; Patel, S.; Hakeem, I.G.; Veluswamy, G.; Al-Waili, I.; Agnihotri, S.; Vuppaladadiyam, A.K.; Surapaneni, A.; Bergmann, D.; Shah, K. The Pyrolysis of Biosolids in a Novel Closed Coupled Pyrolysis and Gasification Technology: Pilot Plant Trials, Aspen Plus Modelling, and a Techno-Economic Analysis. Water 2024, 16, 3399. [Google Scholar] [CrossRef]
- Winchell, L.J.; Wells, M.J.; Ross, J.J.; Kakar, F.; Teymouri, A.; Gonzalez, D.J.; Dangtran, K.; Bessler, S.M.; Carlson, S.; Almansa, X.F.; Norton, J.W.; Bell, K.Y. Fate of perfluoroalkyl and polyfluoroalkyl substances (PFAS) through two full-scale wastewater sludge incinerators. Water Environ. Res. 2024, 96, e11009. [Google Scholar] [CrossRef] [PubMed]
- Freda, C.; Nanna, F.; Villone, A.; Barisano, D.; Brandani, S.; Cornacchia, G. Air gasification of digestate and its co-gasification with residual biomass in a pilot scale rotary kiln. Int. J. Energy Environ. Eng. 2019, 10, 335–346. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Z.; Cao, L.; Ma, L.; Wang, F.; Li, J.; Li, W. Interaction and Kinetics Study of the Co-Gasification of High-solid Anaerobic Digestate and Lignite. Molecules 2020, 25, 459. [Google Scholar] [CrossRef]
- Yang, H.; Guo, Y.; Fang, N.; Dong, B. Life cycle assessment of greenhouse gas emissions of typical sewage sludge incineration treatment route based on two case studies in China. Environ. Res. 2023, 231, 115959. [Google Scholar] [CrossRef]
- González-Arias, J.; Fernández, C.; Rosas, J.G.; Bernal, M.P.; Clemente, R.; Sanchez, M.E.; Gómez, X. Integrating anaerobic digestion of pig slurry and thermal valorisation of biomass. Waste Biomass Valor. 2020, 11, 6125–6137. [Google Scholar] [CrossRef]
- Petrovič, A.; Vohl, S.; Cenčič Predikaka, T.; Bedoić, R.; Simonič, M.; Ban, I.; Čuček, L. Pyrolysis of Solid Digestate from Sewage Sludge and Lignocellulosic Biomass: Kinetic and Thermodynamic Analysis, Characterization of Biochar. Sustainability 2021, 13, 9642. [Google Scholar] [CrossRef]
- Chang, H.; Yuan, J.; Zhao, Y.; Bisinella, V.; Damgaard, A.; Christensen, T.H. Carbon footprints of incineration, pyrolysis, and gasification for sewage sludge treatment. Resour. Conserv. Recycl. 2025, 212, 107939. [Google Scholar] [CrossRef]
- Abdelfatah-Aldayyat, E.; González-Rojo, S.; Gómez, X. Reviewing Digestate Thermal Valorization: Focusing on the Energy Demand and the Treatment of Process Water. Environments 2024, 11, 239. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal Jr, M.J.; Tester, J. W. Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Leland, A.; Felix, L. Hydrothermal carbonization (HTC) of biomass for energy applications. In Biomass preprocessing and pretreatments for production of biofuels, 2018. Editor: Tumuluru, J.S. (pp. 196-254). CRC Press. [CrossRef]
- Zhang, B.; Biswal, B.K.; Zhang, J.; Balasubramanian, R. Hydrothermal treatment of biomass feedstocks for sustainable production of chemicals, fuels, and materials: progress and perspectives. Chem. Rev. 2023, 123, 7193–7294. [Google Scholar] [CrossRef]
- Farru, G.; Scheufele, F.B.; Moloeznik Paniagua, D.; Keller, F.; Jeong, C.; Basso, D. Business and Market Analysis of Hydrothermal Carbonization Process: Roadmap toward Implementation. Agronomy 2024, 14, 541. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.; Delgenès, J.; Steyer, J.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. [CrossRef]
- Svensson, K.; Kjørlaug, O.; Higgins, M.J.; Linjordet, R.; Horn, S.J. Post-Anaerobic Digestion Thermal Hydrolysis of Sewage Sludge and Food Waste: Effect on Methane Yields, Dewaterability and Solids Reduction. Water Res. 2018, 132, 158–166. [Google Scholar] [CrossRef]
- García-Cascallana, J.; Barrios, X.G.; Martinez, E.J. Thermal Hydrolysis of Sewage Sludge: A Case Study of a WWTP in Burgos, Spain. Appl. Sci. 2021, 11, 964. [Google Scholar] [CrossRef]
- Dwyer, J.; Starrenburg, D.; Tait, S.; Barr, K.; Batstone, D.J.; Lant, P. Decreasing activated sludge thermal hydrolysis temperature reduces product colour, without decreasing degradability. Water Res. 2008, 42, 4699–4709. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenès, J.P.; Carrère, H. Effects of thermal treatments on five different waste activated sludge samples solubilisation, physical properties and anaerobic digestion. Chem. Eng. J. 2008, 139, 236–244. [Google Scholar] [CrossRef]
- Boel, M.J.; Wang, H.; Farra, A.A.; Megido, L.; González-LaFuente, J.M.; Shiju, N.R. Hydrothermal liquefaction of plastics: a survey of the effect of reaction conditions on the reaction efficiency. React. Chem. Eng. 2024, 9, 1014–1031. [Google Scholar] [CrossRef]
- Li, Y.; Leow, S.; Fedders, A.C.; Sharma, B.K.; Guest, J.S.; Strathmann, T.J. Quantitative multiphase model for hydrothermal liquefaction of algal biomass. Green Chem. 2017, 19, 1163–1174. [Google Scholar] [CrossRef]
- Ischia, G.; Sudibyo, H.; Miotello, A.; Tester, J.W.; Fiori, L.; Goldfarb, J.L. Identifying the transition from hydrothermal carbonization to liquefaction of biomass in a batch system. ACS Sustainable Chem. Eng. 2024, 12, 11–4539. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Bertucco, A. Severity Factor as an Efficient Control Parameter to Predict Biomass Solubilization and Saccharification During Acidic Hydrolysis of Microalgal Biomass. Bioenerg. Res. 2018, 11, 491–504. [Google Scholar] [CrossRef]
- Jeder, A.; Sanchez-Sanchez, A.; Gadonneix, P.; Masson, E.; Ouederni, A.; Celzard, A.; Fierro, V. The severity factor as a useful tool for producing hydrochars and derived carbon materials. Environ. Sci. Pollut. Res. 2018, 25, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Querejeta, N.; Gil, M.V.; Rubiera, F.; Pevida, C. Sustainable coffee-based CO2 adsorbents: Toward a greener production via hydrothermal carbonization. Greenh. Gases: Sci. Technol. 2018, 8, 309–323. [Google Scholar] [CrossRef]
- Kim, Y.; Kreke, T.; Mosier, N.S.; Ladisch, M.R. Severity factor coefficients for subcritical liquid hot water pretreatment of hardwood chips. Biotechnol. Bioeng. 2014, 111, 254–263. [Google Scholar] [CrossRef]
- Zhang, Y.; Hawboldt, K.; MacQuarrie, S.; Thomas, R. Hydrothermal valorization of beach-cast brown seaweed Ascophyllum nodosum into bioactive compounds and hydrochar using severity factor as a design tool. Chem. Eng. Sci. 2026, 319, 122301. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Galbe, M.; Garrote, G.; Ramirez-Gutierrez, D.M.; Ximenes, E.; Sun, S.; Lachos-Perez, D.; Rodríguez-Jasso, R.M.; Sun, R.; Yang, B.; Ladisch, M.R. Severity factor kinetic model as a strategic parameter of hydrothermal processing (steam explosion and liquid hot water) for biomass fractionation under biorefinery concept. Bioresour. Technol. 2021, 342, 125961. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Savage, P. E. Fast and isothermal hydrothermal liquefaction of sludge at different severities: Reaction products, pathways, and kinetics. Appl. Energy 2020, 260, 114312. [Google Scholar] [CrossRef]
- Faeth, J.L.; Valdez, P.J.; Savage, P.E. Fast hydrothermal liquefaction of Nannochloropsis sp. to produce biocrude. Energy Fuels 2013, 27, 3–1391. [Google Scholar] [CrossRef]
- Möller, M.; Harnisch, F.; Schröder, U. Hydrothermal liquefaction of cellulose in subcritical water—the role of crystallinity on the cellulose reactivity. Rsc Adv. 2013, 3, 11035–11044. [Google Scholar] [CrossRef]
- Coronella, C.J.; Lynam, J.G.; Reza, M.T.; Uddin, M.H. (2014). Hydrothermal Carbonization of Lignocellulosic Biomass. In: Jin, F. (eds) Application of Hydrothermal Reactions to Biomass Conversion. Green Chemistry and Sustainable Technology. Springer, Berlin, Heidelberg. [CrossRef]
- Gollakota, A.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sust. Energ. Rev. 2017, 81, 1378–1392. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal carbonization of biomass for energy and crop production. Appl. Bioenerg. 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Abdeldayem, O.M.; Dupont, C.; Ferras, D.; Kennedy, M. An experimental and numerical investigation of secondary char formation in hydrothermal carbonization: revealing morphological changes via hydrodynamics. RSC adv. 2025, 15, 12723–12738. [Google Scholar] [CrossRef] [PubMed]
- Bühler, W.; Dinjus, E.; Ederer, H.; Kruse, A.; Mas, C. Ionic reactions and pyrolysis of glycerol as competing reaction pathways in near- and supercritical water. J. Supercritic. Fluids 2001, 22, 37–53. [Google Scholar] [CrossRef]
- Wei, N.; Xu, D.; Hao, B.; Guo, S.; Guo, Y.; Wang, S. Chemical reactions of organic compounds in supercritical water gasification and oxidation. Water Res. 2021, 190, 116634. [Google Scholar] [CrossRef]
- Moghaddam, E.M.; Goel, A.; Siedlecki, M.; Michalska, K.; Yakaboylu, O.; de Jong, W. Supercritical water gasification of wet biomass residues from farming and food production practices: lab-scale experiments and comparison of different modelling approaches. Sustain. Energ. Fuels 2021, 5, 1521–1537. [Google Scholar] [CrossRef]
- Yin, F.; Chen, H.; Xu, G.; Wang, G.; Xu, Y. A detailed kinetic model for the hydrothermal decomposition process of sewage sludge. Bioresour. Technol. 2015, 198, 351–357. [Google Scholar] [CrossRef]
- Urrea, J.L.; Collado, S.; Oulego, P.; Díaz, M. Effect of wet oxidation on the fingerprints of polymeric substances from an activated sludge. Water Res. 2016, 105, 282–290. [Google Scholar] [CrossRef]
- Kruse, A.; Bernolle, P.; Dahmen, N.; Dinjus, E.; Maniam, P. Hydrothermal gasification of biomass: consecutive reactions to long-living intermediates. Energy Environ. Sci. 2010, 3, 136–143. [Google Scholar] [CrossRef]
- Khandelwal, K.; Boahene, P.; Nanda, S.; Dalai, A.K. A Review of the Design and Performance of Catalysts for Hydrothermal Gasification of Biomass to Produce Hydrogen-Rich Gas Fuel. Molecules 2023, 28, 5137. [Google Scholar] [CrossRef]
- Lynam, J.G.; Reza, M.T.; Yan, W.; Vásquez, V.R.; Coronella, C.J. Hydrothermal carbonization of various lignocellulosic biomass. Biomass Conv. Bioref. 2015, 5, 173–181. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Rodríguez, R.; Morales, V.; Nasirian, N.; Bautista, L.F.; Vicente, G. Hydrothermal Liquefaction of Microalga Using Metal Oxide Catalyst. Processes 2020, 8, 15. [Google Scholar] [CrossRef]
- Tsarpali, M.; Kuhn, J.N.; Philippidis, G.P. Hydrothermal Carbonization of Residual Algal Biomass for Production of Hydrochar as a Biobased Metal Adsorbent. Sustainability 2022, 14, 455. [Google Scholar] [CrossRef]
- McGaughy, K.; Toufiq Reza, M. Hydrothermal carbonization of food waste: simplified process simulation model based on experimental results. Biomass Conv. Bioref. 2018, 8, 283–292. [Google Scholar] [CrossRef]
- Aierzhati, A.; Watson, J.; Si, B.; Stablein, M.; Wang, T.; Zhang, Y. Development of a mobile, pilot scale hydrothermal liquefaction reactor: Food waste conversion product analysis and techno-economic assessment. Energy Convers. Manag.: X, 1000. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, F.; Djandja, J.O.; Zhang, S.; Xu, Y.; Duan, P. Hydrothermal liquefaction of crop straws: Effect of feedstock composition. Fuel 2020, 265, 116946. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Optimization and modeling of process parameters during hydrothermal gasification of biomass model compounds to generate hydrogen-rich gas products. Int. J. Hydrogen Energy 2020, 45, 18275–18288. [Google Scholar] [CrossRef]
- Reza, M.T.; Freitas, A.; Yang, X.; Hiibel, S.; Lin, H.; Coronella, C.J. Hydrothermal carbonization (HTC) of cow manure: Carbon and nitrogen distributions in HTC products. Environ. Prog. Sustain. Energy 2016, 35, 1002–1011. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Cheng, Z.; Dong, H.; Yan, B.; Chen, G. Synergetic effect and primary reaction network of corn cob and cattle manure in single and mixed hydrothermal liquefaction. J. Anal. Appl. Pyrolysis 2021, 155, 105076. [Google Scholar] [CrossRef]
- Tavasoli, A.; Aslan, M.; Salimi, M.; Balou, S.; Pirbazari, S.; Hashemi, H.; Kohansal, K. Influence of the blend nickel/porous hydrothermal carbon and cattle manure hydrochar catalyst on the hydrothermal gasification of cattle manure for H2 production. Energy Convers. Manage. 2018, 173, 15–28. [Google Scholar] [CrossRef]
- Roslan, S.Z.; Zainudin, S.F.; Mohd Aris, A.; Chin, K.B.; Musa, M.; Mohamad Daud, A.R.; Syed Hassan, S.S.A. Hydrothermal Carbonization of Sewage Sludge into Solid Biofuel: Influences of Process Conditions on the Energetic Properties of Hydrochar. Energies 2023, 16, 2483. [Google Scholar] [CrossRef]
- Marrone, P.A.; Elliott, D.C.; Billing, J.M.; Hallen, R.T.; Hart, T.R.; Kadota, P.; Moeller, J.C.; Randel, M.A.; Schmidt, A.J. Bench-Scale Evaluation of Hydrothermal Processing Technology for Conversion of Wastewater Solids to Fuels. Water Environ. Res. 2018, 90, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Liu, S.; Zhang, H.; Zheng, R.; Cui, J.; Wang, D.; Rahim, D.A.; Kanchanatip, E. Syngas production and heavy metal dynamics during supercritical water gasification of sewage sludge. Front. Environ. Sci. Eng. 2024, 18, 149. [Google Scholar] [CrossRef]
- Cheng, F.; Cui, Z.; Chen, L.; Jarvis, J.; Paz, N.; Schaub, T.; Nirmalakhandan, N.; Brewer, C.E. Hydrothermal liquefaction of high- and low-lipid algae: Bio-crude oil chemistry. Appl. Energy 2017, 206, 278–292. [Google Scholar] [CrossRef]
- Graz, Y.; Bostyn, S.; Richard, T.; Bocanegra, P.E.; De Bilbao, E.; Poirier, J.; Gokalp, I. Hydrothermal conversion of Ulva macro algae in supercritical water. J. Supercritic. Fluids 2015, 107, 182–188. [Google Scholar] [CrossRef]
- Jensen, C.U.; Hoffmann, J.; Rosendahl, L.A. Co-processing potential of HTL bio-crude at petroleum refineries. Part 2: A parametric hydrotreating study. Fuel 2016, 165, 536–543. [Google Scholar] [CrossRef]
- Yu, J.; Biller, P.; Mamahkel, A.; Klemmer, M.; Becker, J.; Glasius, M.; Iversen, B.B. Catalytic hydrotreatment of bio-crude produced from the hydrothermal liquefaction of aspen wood: a catalyst screening and parameter optimization study. Sustain. Energ. Fuels 2017, 1, 832–841. [Google Scholar] [CrossRef]
- Patel, B.; Arcelus-Arrillaga, P.; Izadpanah, A.; Hellgardt, K. Catalytic Hydrotreatment of algal biocrude from fast Hydrothermal Liquefaction. Renew. Energy 2017, 101, 1094–1101. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Gómez, B.R.; Hornung, U.; Kruse, A.; Prins, W. Hydrothermal liquefaction of microalgae in a continuous stirred-tank reactor. Energy Fuels 2015, 29, 6422–6432. [Google Scholar] [CrossRef]
- Kapusta, K. Effect of ultrasound pretreatment of municipal sewage sludge on characteristics of bio-oil from hydrothermal liquefaction process. Waste Manage. 2018, 78, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Long, C.; Sun, S.; Zhao, Y.; Luo, J.; Wu, D. Catalytic hydrothermal liquefaction of peanut shell for the production aromatic rich monomer compounds. J. Energy Inst. 2021, 96, 90–96. [Google Scholar] [CrossRef]
- Wu, B.; Berg, S.M.; Remucal, C.K.; Strathmann, T.J. Evolution of N-containing compounds during hydrothermal liquefaction of sewage sludge. ACS Sustainable Chem. Eng. 2020, 49, 18303–18313. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Chen, W.; Haque, M.A.; Lu, T.; Aierzhati, A.; Reimonn, G. A perspective on hydrothermal processing of sewage sludge. Curr. Opin. Env. Sci. Health 2020, 14, 63–73. [Google Scholar] [CrossRef]
- Ischia, G.; Fiori, L. Hydrothermal Carbonization of Organic Waste and Biomass: A Review on Process, Reactor, and Plant Modeling. Waste Biomass Valor. 2021, 12, 2797–2824. [Google Scholar] [CrossRef]
- Zaccariello, L.; Battaglia, D.; Morrone, B.; Mastellone, M.L. Hydrothermal carbonization: a pilot-scale reactor design for bio-waste and sludge pre-treatment. Waste Biomass Valor. 2022, 13, 3865–3876. [Google Scholar] [CrossRef]
- Terranova Energy. Available online: https://www.terranova-energy.com/en/technology/ (accessed on 2nd August 2025).
- Ingelia. Available online: https://www.ingelia.com/en/plantas-htc (accessed on 2nd August 2025).
- HTCycle: A closed cycle solution for waste management. Available online: https://htcycle.ag/ (accessed on 2nd August 2025).
- HTCycle. HTCycle EU Project. Available online: https://htcycle.ag/en/eu-project_53 (accessed on 2nd August 2025).
- Horizon 2020. Sewage sludge reuse with Phosphate recovery and heavy metal absorption with an innovative HTC technology. Available online: https://cordis.europa.eu/project/id/823124/results (accessed on 2nd August 2025).
- Ferrentino, R.; Langone, M.; Fiori, L.; Andreottola, G. Full-Scale Sewage Sludge Reduction Technologies: A Review with a Focus on Energy Consumption. Water 2023, 15, 615. [Google Scholar] [CrossRef]
- Lucian, M.; Merzari, F.; Messineo, A.; Volpe, M. Hydrothermal carbonization of sludge residues via carborem C700 industrial scale continuous operating plant. Chem. Eng. Trans. 2022, 92, 19–24. [Google Scholar]
- Greenthesis group. CarboREM – Recovery of Energy and Materials. Available online: https://www.greenthesisgroup.com/tecnologie-innovative/carborem-recovery-of-energy-and-materials/ (accessed on 2nd August 2025).
- LicellaTM. Available online: https://www.licella.com/ (accessed on 2nd August 2025).
- Arbios. Available online: https://arbiosbiotech.com/ (accessed on 2nd August 2025).
- MuraTechnology. Available online: https://www.muratechnology.com/hydroprt/ (accessed on 2nd August 2025).
- Gómez, X.; Diaz, M.C.; Cooper, M.; Blanco, D.; Morán, A.; Snape, C.E. Study of biological stabilization processes of cattle and poultry manure by thermogravimetric analysis and 13C NMR. Chemosphere 2007, 68, 1889–1897. [Google Scholar] [CrossRef]
- Gómez, X.; Blanco, D.; Lobato, A.; Calleja, A.; Martínez-Núñez, F.; Martin-Villacorta, J. Digestion of cattle manure under mesophilic and thermophilic conditions: characterization of organic matter applying thermal analysis and 1H NMR. Biodegradation 2011, 22, 623–635. [Google Scholar] [CrossRef]
- Pizzanelli, S.; Calucci, L.; Forte, C.; Borsacchi, S. Studies of Organic Matter in Composting, Vermicomposting, and Anaerobic Digestion by 13C Solid-State NMR Spectroscopy. Appl. Sci. 2023, 13, 2900. [Google Scholar] [CrossRef]
- Bargmann, I.; Rillig, M.C.; Kruse, A.; Greef, M.; Kücke, M. Effects of hydrochar application on the dynamics of soluble nitrogen in soils and on plant availability. J. Plant Nutr. Soil Sci. 2014, 177, 48–58. [Google Scholar] [CrossRef]
- Saber, M.; Takahashi, F.; Yoshikawa, K. Characterization and application of microalgae hydrochar as a low-cost adsorbent for Cu (II) ion removal from aqueous solutions. Environ. Sci. Pollut. Res. 2018, 25, 32721–32734. [Google Scholar] [CrossRef] [PubMed]
- Oumabady, S.; Selvaraj, P.S.; Kamaludeen, S.P.; Ettiyagounder, P.; Suganya, K. Application of sludge-derived KOH-activated hydrochar in the adsorptive removal of orthophosphate. RSC adv. 2021, 11, 6535–6543. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Qin, Q.; Chen, G.; Wang, W. Alkali Etching Hydrochar-Based Adsorbent Preparation Using Chinese Medicine Industry Waste and Its Application in Efficient Removal of Multiple Pollutants. Processes 2023, 11, 412. [Google Scholar] [CrossRef]
- Khan, L.A.; Liaquat, R.; Aman, M.; Kanan, M.; Saleem, M.; khoja, A.H.; Bahadar, A.; Khan, W.U.H. Investigation of Novel Transition Metal Loaded Hydrochar Catalyst Synthesized from Waste Biomass (Rice Husk) and Its Application in Biodiesel Production Using Waste Cooking Oil (WCO). Sustainability 2024, 16, 7275. [Google Scholar] [CrossRef]
- Khosravi, A.; Yuan, Y.; Liu, Q.; Zheng, H.; Hashemi, M.; Tang, Y.; Xing, B. Hydrochars as slow-release phosphorus fertilizers for enhancing corn and soybean growth in an agricultural soil. Carbon Res. 2024, 3, 7. [Google Scholar] [CrossRef]
- Subramanya, S.M.; Rios, N.; Kollar, A.; Stofanak, R.; Maloney, K.; Waltz, K.; Powers, L.; Rane, C.; Savage, P.E. Statistical models for predicting oil composition from hydrothermal liquefaction of biomass. Energy Fuels 2023, 37, 9–6619. [Google Scholar] [CrossRef]
- Borbolla-Gaxiola, J.E.; Ross, A.B.; Dupont, V. Multi-Variate and Multi-Response Analysis of Hydrothermal Carbonization of Food Waste: Hydrochar Composition and Solid Fuel Characteristics. Energies 2022, 15, 5342. [Google Scholar] [CrossRef]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal carbonization of biogas digestate: Effect of digestate origin and process conditions. Waste Manage. 2019, 100, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, U.; Ross, A.; Camargo-Valero, M.; Williams, P. A comparison of product yields and inorganic content in process streams following thermal hydrolysis and hydrothermal processing of microalgae, manure and digestate. Bioresour. Technol. 2016, 200, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Urbanowska, A.; Kabsch-Korbutowicz, M.; Wnukowski, M.; Seruga, P.; Baranowski, M.; Pawlak-Kruczek, H.; Serafin-Tkaczuk, M.; Krochmalny, K.; Niedzwiecki, L. Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation. Energies 2020, 13, 262. [Google Scholar] [CrossRef]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Oechsner, H.; Lautenbach, A.; Kruse, A. Effect of residence time during hydrothermal carbonization of biogas digestate on the combustion characteristics of hydrochar and the biogas production of process water. Bioresour. Technol. 2021, 333, 125110. [Google Scholar] [CrossRef]
- Belete, Y.Z.; Mau, V.; Yahav Spitzer, R.; Posmanik, R.; Jassby, D.; Iddya, A.; Kassem, N.; Tester, J.W.; Gross, A. Hydrothermal carbonization of anaerobic digestate and manure from a dairy farm on energy recovery and the fate of nutrients. Bioresour. Technol. 2021, 333, 125164. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Niedzwiecki, L.; Sieradzka, M.; Mlonka-Mędrala, A.; Baranowski, M.; Serafin-Tkaczuk, M.; Magdziarz, A. Hydrothermal carbonization of agricultural and municipal solid waste digestates – Structure and energetic properties of the solid products. Fuel 2020, 275, 117837. [Google Scholar] [CrossRef]
- Zhao, X.; Becker, G.C.; Faweya, N.; Rodriguez Correa, C.; Yang, S.; Xie, X.; Kruse, A. Fertilizer and activated carbon production by hydrothermal carbonization of digestate. Biomass Convers. Biorefin. 2018, 8, 423–436. [Google Scholar] [CrossRef]
- Charnnok, B.; Khompatara, K.; Chaiprapat, S.; Krishnan, S. Hydrothermal Carbonization of Digestate from Lignocellulosic Biogas Power Plants for Sustainable Soil Improvement and Low Carbon Emissions. BioEnergy Res. 2025, 18, 61. [Google Scholar] [CrossRef]
- Farru, G.; Asquer, C.; Cappai, G.; De Gioannis, G.; Melis, E.; Milia, S.; Muntoni, A.; Piredda, M.; Scano, E.A. Hydrothermal carbonization of hemp digestate: influence of operating parameters. Biomass Convers. Biorefin. 2024, 14, 6999–7010. [Google Scholar] [CrossRef]
- Sharma, K.; Rosendahl, L.A.; Pedersen, T.H. Evaluating direct use fertilizer potential of hydrothermal liquefaction solid mineral products: Integrating anaerobic digestion and hydrothermal liquefaction. Waste Manag. 2024, 191, 203–211. [Google Scholar] [CrossRef]
- Tito, E.; Landi, D.; Demichelis, F.; Pipitone, G.; Bensaid, S.; Pirone, R. Hydrothermal liquefaction of digestate from the organic fraction of municipal solid waste: Optimization of operating parameters. Energy Convers. Manag. 2025, 336, 119881. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Thermal depolymerization of biogas digestate as a viable digestate processing and resource recovery strategy. In Advances in Eco-Fuels for a Sustainable Environment 2019; pp. 277-308. [CrossRef]
- Posmanik, R.; Martinez, C.M.; Cantero-Tubilla, B.; Cantero, D.A.; Sills, D.L.; Cocero, M.J.; Tester, J.W. Acid and alkali catalyzed hydrothermal liquefaction of dairy manure digestate and food waste. ACS Sustainable Chem. Eng. 2018, 6, 2–2724. [Google Scholar] [CrossRef]
- Sudibyo, H.; Tester, J.W. Sustainable resource recovery from dairy waste: A case study of hydrothermal co-liquefaction of acid whey and anaerobic digestate mixture. Energy Fuels 2023, 37, 4–2897. [Google Scholar] [CrossRef]
- Mikusińska, J.; Kuźnia, M.; Czerwińska, K.; Wilk, M. Hydrothermal Carbonization of Digestate Produced in the Biogas Production Process. Energies 2023, 16, 5458. [Google Scholar] [CrossRef]
- Parmar, K.R.; Brown, A.E.; Hammerton, J.M.; Camargo-Valero, M.A.; Fletcher, L.A.; Ross, A.B. Co-Processing Lignocellulosic Biomass and Sewage Digestate by Hydrothermal Carbonisation: Influence of Blending on Product Quality. Energies 2022, 15, 1418. [Google Scholar] [CrossRef]
- Sudibyo, H.; Pecchi, M.; Tester, J.W. Experimental-based mechanistic study and optimization of hydrothermal liquefaction of anaerobic digestates. Sustain. Energ. Fuels 2022, 6, 2314–2329. [Google Scholar] [CrossRef]
- Xu, D.; Lin, G.; Liu, L.; Wang, Y.; Jing, Z.; Wang, S. Comprehensive evaluation on product characteristics of fast hydrothermal liquefaction of sewage sludge at different temperatures. Energy 2018, 159, 686–695. [Google Scholar] [CrossRef]
- Roslan, S.Z.; Zainol, M.M.; Bikane, K.; Syed-Hassan, S.S.A. Hydrothermal carbonization of sewage sludge for hydrochar production: optimization of operating conditions using Box-Behnken design coupled with response surface methodology. Biomass Convers. Biorefin. 2024, 1–17. [Google Scholar] [CrossRef]
- Mazariegos, I.; Abdelfath-Aldayyat, E.; González-Rojo, S.; Gómez, X. Reducing fossil fuel demand by using biofuels as an alternative hydrothermal liquefaction is a promising process for transforming biomass into drop-in fuels. RSC Sustain. 2025, 3, 3228–3265. [Google Scholar] [CrossRef]
- Albrecht, K.O.; Zhu, Y.; Schmidt, A.J.; Billing, J.M.; Hart, T.R.; Jones, S. B.; Maupin, G.; Hallen, R.; Ahrens, T.; Anderson, D. Impact of heterotrophically stressed algae for biofuel production via hydrothermal liquefaction and catalytic hydrotreating in continuous-flow reactors. Algal Res. 2016, 14, 17–27. [Google Scholar] [CrossRef]
- Haider, M.S.; Castello, D.; Rosendahl, L.A. Two-stage catalytic hydrotreatment of highly nitrogenous biocrude from continuous hydrothermal liquefaction: A rational design of the stabilization stage. Biomass Bioenerg. 2020, 139, 105658. [Google Scholar] [CrossRef]
- Sudibyo, H.; Budhijanto, B.; Celis, C.; Mahannada, A.; Suparmin, A.; Wintoko, J.; Prasetyo, D.J.; Anwar, M. Hydrothermal coliquefaction of anaerobic digestate with polyphenolic extracts from agricultural byproducts producing nearly nitrogen-free biocrude oil. Sustain. Energ. Fuels 2024, 8, 4533–4549. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Blazina, G.V.; Rajagopalan, K.; Strathmann, T.J. Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour. Technol. 2012, 109, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ender, T.; Ekanthalu, V.S.; Jalalipour, H.; Sprafke, J.; Nelles, M. Process Waters from Hydrothermal Carbonization of Waste Biomasses like Sewage Sludge: Challenges, Legal Aspects, and Opportunities in EU and Germany. Water 2024, 16, 1003. [Google Scholar] [CrossRef]
- Kulikova, Y.; Klementev, S.; Sirotkin, A.; Mokrushin, I.; Bassyouni, M.; Elhenawy, Y.; El-Hadek, M.A.; Babich, O. Aqueous Phase from Hydrothermal Liquefaction: Composition and Toxicity Assessment. Water 2023, 15, 1681. [Google Scholar] [CrossRef]
- Melo, T.M.; Bottlinger, M.; Schulz, E.; Leandro, W.M.; de Aguiar Filho, A.M.; Ok, Y.S.; Rinklebe, J. Effect of biosolid hydrochar on toxicity to earthworms and brine shrimp. Environ. Geochem. Health 2017, 39, 1351–1364. [Google Scholar] [CrossRef]
- Pham, M.; Schideman, L.; Scott, J.; Rajagopalan, N.; Plewa, M.J. Chemical and biological characterization of wastewater generated from hydrothermal liquefaction of Spirulina. Environ. Sci. Technol. 2013, 47, 2131–2138. [Google Scholar] [CrossRef]
- Tommaso, G.; Chen, W.; Li, P.; Schideman, L.; Zhang, Y. Chemical characterization and anaerobic biodegradability of hydrothermal liquefaction aqueous products from mixed-culture wastewater algae. Bioresour. Technol. 2015, 178, 139–146. [Google Scholar] [CrossRef]
- Basar, I.A.; Liu, H.; Eskicioglu, C. Effects of municipal sludge composition on hydrothermal liquefaction products: Aqueous phase characterization and biodegradability assessment. Bioresour. Technol. 2024, 400, 130671. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Shakya, R. Nutrient removal and energy production from aqueous phase of bio-oil generated via hydrothermal liquefaction of algae. Bioresour. Technol. 2017, 230, 43–48. [Google Scholar] [CrossRef]
- Matayeva, A.; Biller, P. Hydrothermal liquefaction aqueous phase treatment and hydrogen production using electro-oxidation. Energy Convers. Manag. 2021, 244, 114462. [Google Scholar] [CrossRef]
- Reza, M.T.; Freitas, A.; Yang, X.; Coronella, C.J. Wet air oxidation of hydrothermal carbonization (HTC) process liquid. ACS Sustainable Chem. Eng. 2016, 4, 6–3250. [Google Scholar] [CrossRef]
- González-Arias, J.; De la Rubia, M.; Sánchez, M.; Gómez, X.; Cara-Jiménez, J.; Martínez, E. Treatment of hydrothermal carbonization process water by electrochemical oxidation: Assessment of process performance. Environ. Res. 2023, 216, 114773. [Google Scholar] [CrossRef] [PubMed]
- Bhatwadekar, S.; Boruah, B.; Riedel, N.W.; Royer, N.D.; Pedersen, T.H.; Lopez-Ruiz, J.A. Electrocatalytic oxidation of hydrothermal liquefaction-derived aqueous phase for on-site wastewater treatment and H2 production. Cell Rep. Phys. Sci. 2025, 6, 102555. [Google Scholar] [CrossRef]
- Silva Thomsen, L.B.; Anastasakis, K.; Biller, P. Wet oxidation of aqueous phase from hydrothermal liquefaction of sewage sludge. Water Res. 2022, 209, 117863. [Google Scholar] [CrossRef]
- Xu, D.; Liu, L.; Wei, N.; Guo, Y.; Wang, S.; Wu, Z.; Duan, P. Catalytic supercritical water gasification of aqueous phase directly derived from microalgae hydrothermal liquefaction. Int. J. Hydrog. Energy 2019, 44, 26181–26192. [Google Scholar] [CrossRef]
- Kilgore, U.J.; Subramaniam, S.; Fox, S.P.; Cronin, D.J.; Guo, M.F.; Schmidt, A.J.; Ramasamy, K.K.; Thorson, M.R. Wet air oxidation of HTL aqueous waste. Biomass Bioenerg. 2023, 176, 106889. [Google Scholar] [CrossRef]
- Davidson, S.D.; Lopez-Ruiz, J.A.; Zhu, Y.; Cooper, A.R.; Albrecht, K.O.; Dagle, R.A. Strategies to valorize the hydrothermal liquefaction-derived aqueous phase into fuels and chemicals. ACS Sustainable Chem. Eng. 2019, 7, 24, 19889–19901. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Messineo, A. Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges. Energies 2021, 14, 2962. [Google Scholar] [CrossRef]
- Kambo, H.S.; Minaret, J.; Dutta, A. Process water from the hydrothermal carbonization of biomass: a waste or a valuable product? Waste Biomass Valori. 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Wang, R.; Jin, Q.; Ye, X.; Lei, H.; Jia, J.; Zhao, Z. Effect of process wastewater recycling on the chemical evolution and formation mechanism of hydrochar from herbaceous biomass during hydrothermal carbonization. J. Clean. Prod. 2020, 277, 123281. [Google Scholar] [CrossRef]
- Xu, Z.; Song, H.; Li, P.; He, Z.; Wang, Q.; Wang, K.; Duan, P. Hydrothermal carbonization of sewage sludge: Effect of aqueous phase recycling. Chem. Eng. J. 2020, 387, 123410. [Google Scholar] [CrossRef]
- Jensen, C.U.; Rosendahl, L.A.; Olofsson, G. Impact of nitrogenous alkaline agent on continuous HTL of lignocellulosic biomass and biocrude upgrading. Fuel Process. Technol. 2017, 159, 376–385. [Google Scholar] [CrossRef]
- Shah, A.A.; Toor, S.S.; Seehar, T.H.; Nielsen, R.S.; H. Nielsen, A.; Pedersen, T.H.; Rosendahl, L.A. Bio-Crude Production through Aqueous Phase Recycling of Hydrothermal Liquefaction of Sewage Sludge. Energies 2020, 13, 493. [Google Scholar] [CrossRef]
- Harisankar, S.; Vishnu Mohan, R.; Choudhary, V.; Vinu, R. Effect of water quality on the yield and quality of the products from hydrothermal liquefaction and carbonization of rice straw. Bioresour. Technol. 2022, 351, 127031. [Google Scholar] [CrossRef]
- Kassem, N.; Hockey, J.; Lopez, C.; Lardon, L.; Angenent, L.T.; Tester, J. W. Integrating anaerobic digestion, hydrothermal liquefaction, and biomethanation within a power-to-gas framework for dairy waste management and grid decarbonization: a techno-economic assessment. Sustain. Energ. Fuels 2020, 4, 4644–4661. [Google Scholar] [CrossRef]
- Ho, T.T.-T.; Nadeem, A.; Choe, K. A Review of Upscaling Hydrothermal Carbonization. Energies 2024, 17, 1918. [Google Scholar] [CrossRef]
- Thermal Hydrolysis Processes. Veolia Water Technologies. Available online: https://www.asia.veoliawatertechnologies.com/sites/g/files/dvc3516/files/document/2019/01/thermal_hydrolisis_process.pdf (accessed on 3rd August 2025).
- Veolia. Sludge treatment. Available online: https://www.veoliawatertechnologies.fi/fi/sludge-treatment (accessed on 3rd August 2025).
- Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef]
- Ahmad, S.; Daddi, T.; Novi, A.; Marrucci, L. Evaluating environmental impacts and techno-economic feasibility of an integrated and novel wastewater and sludge treatment system for circular economy objectives. Comput. Ind. Eng. 2025, 204, 111035. [Google Scholar] [CrossRef]
- Nadarajah, K.; Rodriguez-Narvaez, O.M.; Ramirez, J.; Bandala, E.R.; Goonetilleke, A. Lab-scale engineered hydrochar production and techno-economic scaling-up analysis. Waste Manage. 2024, 174, 568–574. [Google Scholar] [CrossRef]
- Carrasco, S.; Pino-Cortés, E.; Barra-Marín, A.; Fierro-Gallegos, A.; León, M. Use of Hydrochar Produced by Hydrothermal Carbonization of Lignocellulosic Biomass for Thermal Power Plants in Chile: A Techno-Economic and Environmental Study. Sustainability 2022, 14, 8041. [Google Scholar] [CrossRef]
- Potrč, S.; Petrovič, A.; Egieya, J.M.; Čuček, L. Valorization of Biomass Through Anaerobic Digestion and Hydrothermal Carbonization: Integrated Process Flowsheet and Supply Chain Network Optimization. Energies 2025, 18, 334. [Google Scholar] [CrossRef]
- Hydorthermal Carbonization (HTC): Valorisation of organic waste and sludges for hydrochar production and biofertilizers. Available online: https://www.ieabioenergy.com/wp-content/uploads/2021/10/HTC-Valorisation-of-organic-wastes-and-sludges-for-hydrochar-production-and-biofertilizers-Full-Report.pdf (accessed on 3rd August 2025).
- Bautista-Peñuelas, E.; Macías, J.D.; Villafán-Vidales, H.I.; Valadés-Pelayo, P.J.; Arcelus-Arrillaga, P.; Ayala-Cortés, A.; Cedano-Villavicencio, K.; Arancibia-Bulnes, C.A.; Peña-Cruz, M.I. Hydrothermal liquefaction of wood wastes in a concentrating solar plant: A techno-economic analysis. Energy Convers. Manag. 2023, 282, 116861. [Google Scholar] [CrossRef]
- Funkenbusch, L.T.; Mullins, M.E.; Vamling, L.; Belkhieri, T.; Srettiwat, N.; Winjobi, O.; Shonnard, D.R.; Rogers, T.N. Technoeconomic assessment of hydrothermal liquefaction oil from lignin with catalytic upgrading for renewable fuel and chemical production. Wiley Interdiscip. Rev.: Energy Environ. 2018, 8, e319. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Hansen, N.H.; Pérez, O.M.; Villamar Cabezas, D.E.; Rosendahl, L.A. Renewable hydrocarbon fuels from hydrothermal liquefaction: A techno-economic analysis. Biofuel. Bioprod. Bior. 2018, 12, 213–223. [Google Scholar] [CrossRef]
- Snowden-Swan, L.; Li, S.; Jiang, Y.; Thorson, M.; Schmidt, A.; Seiple, T.; Billing, J.; Santosa, M.; Hart, T.; Fox, S.; Cronin, D.; Ramasamy, K.; Anderson, D.; Hallen, R.; Almansa, X.F.; Norton, J. Pacific Northwest National Laboratory,OSTI.GOV, 2022. Available online: https://www.osti.gov/servlets/purl/1863608 (accessed on 20 December 2024).
- Hussain, A.; Anastasakis, K. Technoeconomic evaluation of integrating hydrothermal liquefaction in wastewater treatment plants. Bioresour. Technol. 2025, 419, 132030. [Google Scholar] [CrossRef]
- González, R.; Ellacuriaga, M.; Aguilar-Pesantes, A.; Carrillo-Peña, D.; García-Cascallana, J.; Smith, R.; Gómez, X. Feasibility of Coupling Anaerobic Digestion and Hydrothermal Carbonization: Analyzing Thermal Demand. Appl. Sci. 2021, 11, 11660. [Google Scholar] [CrossRef]
- Bacci di Capaci, R.; Tasca, A.L.; Gori, R.; Vitolo, S.; Puccini, M.; Pannocchia, G. An Integrated Approach to the Hydrothermal Carbonization of Sewage Sludge: Simulation, Modeling, and Life Cycle Assessment. ChemEngineering 2023, 7, 44. [Google Scholar] [CrossRef]
- Xie, S.; Yu, G.; Li, C.; You, F.; Li, J.; Tian, R.; Wang, G.; Wang, Y. Dewaterability enhancement and heavy metals immobilization by pig manure biochar addition during hydrothermal treatment of sewage sludge. Environ. Sci. Pollut. Res. 2019, 26, 16537–16547. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Urbanowska, A.; Niedzwiecki, L.; Czerep, M.; Baranowski, M.; Aragon-Briceño, C.; Kabsch-Korbutowicz, M.; Arora, A.; Seruga, P.; Wnukowski, M.; et al. Hydrothermal Carbonisation as Treatment for Effective Moisture Removal from Digestate—Mechanical Dewatering, Flashing-Off, and Condensates’ Processing. Energies 2023, 16, 5102. [Google Scholar] [CrossRef]
- Ha, D.T.; Tong, H.D.; Tran, K.; Trinh, T.T. The role of chemical functional groups in dewaterability of hydrochar: A molecular simulation study. J. Mol. Liq. 2024, 400, 124482. [Google Scholar] [CrossRef]
- Yue, Y.; Yao, Y.; Lin, Q.; Li, G.; Zhao, X. The change of heavy metals fractions during hydrochar decomposition in soils amended with different municipal sewage sludge hydrochars. J. Soils Sediments 2017, 17, 763–770. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Liu, Q. Fate and distribution of nutrients and heavy metals during hydrothermal carbonization of sewage sludge with implication to land application. J. Clean. Prod. 2019, 225, 972–983. [Google Scholar] [CrossRef]
- Cavali, M.; Hennig, T.B.; Libardi Junior, N.; Kim, B.; Garnier, V.; Benbelkacem, H.; Bayard, R.; Woiciechowski, A.L.; Matias, W.G.; de Castilhos Junior, A.B. Co-Hydrothermal Carbonization of Sawdust and Sewage Sludge: Assessing the Potential of the Hydrochar as an Adsorbent and the Ecotoxicity of the Process Water. Appl. Sci. 2025, 15, 1052. [Google Scholar] [CrossRef]
- Chiguano-Tapia, B.; Diaz, E.; de la Rubia, M.A.; Mohedano, A.F. Co-Hydrothermal Carbonization of Swine Manure and Soybean Hulls: Synergistic Effects on the Potential Use of Hydrochar as a Biofuel and Soil Improver. Sustainability 2025, 17, 5022. [Google Scholar] [CrossRef]
- Ye, T.; Gou, L.; Wang, Y.; Liu, N.; Dai, L.; Wang, Y. Co-hydrothermal carbonization of pretreated sludge and polyethylene terephthalate for the preparation of low-nitrogen clean solid fuels. RSC Adv. 2024, 14, 17326–17337. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, K.; Liu, Q.; Han, L.; Zhang, X. A Comprehensive Hydrothermal Co-Liquefaction of Diverse Biowastes for Energy-Dense Biocrude Production: Synergistic and Antagonistic Effects. Int. J. Environ. Res. Public Health 2022, 19, 10499. [Google Scholar] [CrossRef]
- Herdlevær, K.M.; Barth, T. Optimizing Formic Acid-Assisted Co-HTL of Digested Sewage Sludge and Lignocellulosic Waste for Enhanced Bio-Crude Yield and Energy Recovery. Energies 2024, 17, 258. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Li, A.; Wei, X.; Bai, J.; Li, D.; Huang, G.; Chang, C.; Li, P. Co-liquefaction of municipal sludge and tobacco stems: a novel strategy for crafting bio-oil enriched with high value-added nitrogen-containing compounds and amide entities. Biomass Conv. Bioref. 2025, 15, 6463–6481. [Google Scholar] [CrossRef]
- De Carolis, C.; Iori, V.; Narciso, A.; Gentile, D.; Casentini, B.; Pietrini, F.; Grenni, P.; Barra Caracciolo, A.; Iannelli, M.A. The Effects of Different Combinations of Cattle Organic Soil Amendments and Copper on Lettuce (cv. Rufus) Plant Growth. Environments 2024, 11, 134. [Google Scholar] [CrossRef]
- Prasad, M.; Foster, P. Comprehensive Evaluation and Development of Irish Compost and Digestate Standards for Heavy Metals, Stability and Phytotoxicity. Environments 2023, 10, 166. [Google Scholar] [CrossRef]
| Severity factor | Parameters | Specific considerations | References |
|---|---|---|---|
| HTC process | |||
| Log(R0) |
t: residence time in minutes T: temperature (°C) R0: reaction ordinate |
Equation considers only residence time at desired temperature. | [49,50] |
| Log(R0) |
n: number of treatment stages ti: residence time in minutes of each stage Ti: temperature of each stage (°C) R0: reaction ordinate ω = empirical parameter representing first-order approximation (12.1 for hardwood chips, 4.6 for enzymatic hydrolysis) Tf = temperature in the middle of the range of experimental conditions R = Universal gas constant (8.314 J mol/K) Ea = apparent activation energy |
Considering several treatment stages. | [48,51] |
| Log(R0) R0 = R0 (heating period) + R0 (holding period) |
t: residence time in minutes T: temperature (°C) T(t): Temperature profile during the heating period R0: reaction ordinate |
Equation considers the heating stage and the residence time at desired temperature. | [52] |
| Log(R0) R0 = R0 (heating period) + R0 (holding period) + R0 (cooling period) |
t: residence time in minutes T: temperature (ºC) T(t): Temperature profile during the heating/cooling period R0: reaction ordinate |
Equation considers the heating stage, the residence time at desired temperature and cooling stage. | [53] |
| HTL process | |||
| SI: empirical severity index |
t: residence time in seconds T: temperature (K) Eb = reference activation energy (2x105 J/mol) R = Universal gas constant (8.314 J mol/K) T0 = Reference temperature (700 K) |
Equation considers the reaction period. | [54] |
| R0 |
t: residence time in minutes T(t): Temperature profile during the heating period R0: reaction ordinate |
Equation considers the temperature profile | [55] |
| Biomass | HTC | HTL | HTG |
|---|---|---|---|
| Lignocellulosic biomass | Materials: corn stover, Tahoe mix, switch grass, rice hulls, Loblolly pine Temperature: 200 – 280 °C, pressure: 1.4 – 6.9 MPa, water to biomass ratio: 5:1 and 10:1. Heating time: 15 – 30 min, duration: 5 – 20 min. Increasing temperature reduces char yield. The increase in lignocellulosic content enhances char production. Recycling of process water does not increase char production at high temperature [68]. |
Materials: corn, peanut, soybean and rice straw. Temperature: 320 ºC, duration: 60 min. Poor bio-oil yields, with best results obtained from soybean straw. High char yield (24.5 – 35.5 %) [73]. |
Materials: synthetic biomass containing cellulose, xylose and lignin as model compounds. Temperature: 300 – 500 ºC, duration: 30 – 60 min. Feed stock concentration: 10 – 30 wt%. Hydrogen yield increases with temperature and reaction time. Xylose produced the highest yield (2.26 mmol H2/g), whereas lignin showed the lowest (0.73 mmol H2/g) [74]. |
| Manures | Material: cow manure Temperature: 180, 220 and 260 °C, duration: 5 and 30 min, water to biomass ratio: 5:1. Increasing temperature decreases char yield. A decrease in the liquid phase was observed at temperatures below 260 °C. The liquid pH became more acidic with the increase of HTC severity. Hydrochar with high ash content [75] |
Material: cow manure Temperature: 300 – 360 °C, duration: 30 min, water to biomass ratio: 5.7:1. Biocrude yield was 36.5% with the best performance obtained at 340 °C. Char yield was high (40 – 45%) due to the high ash content of manure [76]. |
Material: cow manure Temperature: 380 - 440 °C, pressure: 230 – 294 bars, duration: 5 – 30 min. Syngas yield: 16.1 – 25.9 mmol gas/ g TSfeed [77]. |
| Sewage sludge | Material: sewage sludge Temperature: 150 – 300 °C, duration: 30 – 150 min, solid load: 10 – 30%. Solid yield was maximum at 150 °C with hydrochar having high volatile and low ash content. Therefore, HHV 1 was also at its maximum at the lowest temperature tested [78]. |
Material: sewage sludge (primary, secondary and digested sludge were tested individually). Temperature: 276 – 358 °C, duration: 18 – 30 min, loading: 1.5 L/h at 10 – 16% TS. Experiments were conducted in a pilot plant using a 1.0 L CSTR 2. Biocruede yields range from 25 – 37% [79]. |
Material: sewage sludge Temperature: 380 – 420 °C, duration: 15 – 60 min, water to sludge ratio: 9:1. Increasing temperature increases syngas yields and hydrogen production (yields obtained ranged 7.2 - 10.9 mmol gas/g TS) [80]. |
| Algal biomass | Material: Picochlorum oculatum UTEX LB 1998 (lipid extracted algae) Temperature: 180 – 220 °C, heating rate: 5 °C/min, duration: 1 – 3 h, solid load: 8 – 15% TS. Hydrochar yield ranged from 26.8% to 36.4%, with values decreasing as the temperature increased. Increasing solid loading favored hydrochar formation [70]. |
Material: Galdieria sulphuraria and Nannochloropsis salina Temperature: 310 – 350 °C, duration: 5 – 60 min, solid loading: 5 – 10 %TS. N. salina required milder conditions, whereas the maximum temperature tested was required for G. sulphuraria. Biocrude yield was in the range of 40 to 54.3% for N. salina, whereas yields for G. sulphuraia were much lower (18.1 – 27.5%) [81]. |
Material: Ulva rotundata and armoricana Temperature: 400 - 550 °C, duration: 7 – 120 min, solid load: 7 and 16.4% TS. The experiment with the maximum duration yielded better results regarding methane content (3.8 mmol/g TS). High solid loading reduces hydrogen and methane yields. Hydrogen yield improved with an increase in temperature, reaching an approximate value of 1.8 mmol/g TS at 550 °C [82]. |
| Food waste | Material: Food waste derived from campus dining halls. Temperature: 200 – 260 °C, heating time to reach desired temperature: 25 min, duration: 30 min. Solid load: 9.5 – 10% TS. Hydrochar yield was between 68.5 and 75%, with a low ash content [71]. |
Material: Food waste Temperature: 300 °C, flow rate of 56.7 L/min. Feed TS content: 13.3%. One of the few works carried out under continuous conditions using a plug flow reactor (Pilot plant). The biocrude yield was 29.5%. The process energy consumption ratio was 0.53 (reported as the ratio between heating demand and energy contained in hydrochar) [72]. |
Material: Fruit and vegetable waste Temperature: 530 – 600 °C, duration: 45 min. The small reactor was removed from the oven and cooling was made by using a fan until the ambient temperature was reached. Hydrogen yield of 7.5 mmol/g TS. Hydrogen yield increases with increasing temperature. Methane yield decreases at temperatures higher than 400 °C due to the backward methanation reaction, which consumes CH4 and H2O to form H2 and CO [63]. |
| Type of digested material | Experimental conditions and main results | Reference |
|---|---|---|
| HTC experiments | ||
| Digestate derived from a thermophilic plant digesting household waste (OHWD), a mesophilic plant digesting cow manure with a great proportion of bedding material (wheat straw) (CMD), and a mesophilic plant treating cow manure and silage (ECD) | Temperature: 170 – 250 °C Reaction time: 2 - 5 h (50 – 80 min were spent in heating the reactor to the working temperature). 15 min cooling after reaction ends. Reactor volume: 250 mL The ash content of digestate was high for OHWD (35.8%) and ECD (28.7%), but not for CMD thanks to their high content in wheat straw (15.7%), therefore hydrochar yields kept relation with the raw material characteristics, with higher ash content digestates reporting hydrochar with lower HHVs (in the range of 11.9 – 16.1 MJ/kg) whereas for CMD the hydrochar HHV ranged 17.1 - 20.7 MJ/kg. The operating conditions applied resulted in lower hydrochar yields with increasing temperature but improved fuel quality in contrast. The authors recommended CMD as the only digestate adequate for HTC. |
[116] |
| Digestate from sewage sludge with high ash content (61.2%) and low HHV (7.8 MJ/kg) | Temperature: HTC: 250 °C, reaction time: 1 h. HTL: 350 °C, reaction time: 1 h. Reactor volume: 75 mL. The high ash content of digestate was the factor responsible for the high solid residue obtained from any of the thermal processes tested. However, the HHV of hydrochar was low because of the high ash content of the raw material (4.3 MJ/kg and ash content of hydrochar of 81.4%). |
[117] |
| Digestate derived from a rural digestion plant with low ash content (8.1%, HHV of 19.74 MJ/kg) | Temperature: 200 °C, reaction time: 270 min. Water to biomass ratio of 12:1 The dewaterability of HTC solids improved compared to raw digestate. Hydrochar had an HHV of 23.63 MJ/kg and an ash content of 9.7%. |
[118] |
| Energy crop digestate (29.2% ash content) | Temperature: 210 °C, reaction time: 30 min to 5 h. HHV of digestate was around 16.0 MJ/kg, a value similar to that of the raw material; therefore, no energetic condensation was observed. Hydrochar yield was between 70% and 75.4%, with values decreasing as the reaction time increased. | [119] |
| Cow manure digestate with high ash content (44.8%) | Temperature: 180, 210 and 240 °C, reaction time: 60 min. Cooling: Quenching in an ice bath. Reactor volume: 50 mL, biomass to liquid ratio: 1:3. Whey was used as a carbon supplement. Hydrochar derived from digestate had an ash content between 49.5% and 58.6%. The addition of whey favored the incorporation of carbon into hydrochar, lowering the ash content to 43.4 – 49.9% (HHV of 11.4 - 15.5 MJ/kg). Energy ratios were higher when testing fresh manure compared to digestate. |
[120] |
| Agricultural feedstock digestate (8.1% ash content) and MSW digestate (55.9% ash content) | Temperature: 200 °C, reaction time: 4.5 h Reactor volume: 4 L, biomass to water ratio: 1:12. Best performance obtained for the digestate with lower ash content, yielding a hydrochar with a HHV of 23.2 MJ/kg. Dewatering was improved after HTC treatment with a slight enhancement (14%) in the case of agricultural feedstock digestate, but a 44% enhancement in the case of MSW digestate. |
[121] |
| Digestate from a plant using maize silage, liquid manure and grass silage as feed (27% ash content). | Temperature: 190, 220, 250 °C, reaction time: 3 h. Heating time to reach process temperature: 45 min. Cooling time: 5 min. Reactor volume: 250 mL. Hydrochar produced was intended for phosphate recovery and the remaining fraction after leaching, as activated carbon. Hydrochar yield showed a reversing trend with temperature. Lower yields were obtained at higher temperatures. Most of the phosphorus was retained in hydrochar, being positively correlated with temperature. |
[122] |
| Digestate from a plant using Napier grass, corn residue and rice husk as feed (30.8% ash content). | Temperature: 225, 245, 265 °C, reaction time: 1 h. Reactor volume: 250 mL. Biomass to water ratio: 1:10 Hydrochar yield reported was between 45.6% and 49.5%, with higher values obtained at lower HTC temperatures. Hydrochar phosphorus content increased with HTC temperature. Heavy metals accumulated in hydrochar without leaching to the water-soluble fraction. |
[123] |
| Digestate from hemp straw (17% ash content) | Temperature: 180, 200 °C, reaction time: 1, 3, 6 h Reactor volume: 1.5 L, solid loading: 13% TS Hydrochar yield ranged from 60 to 88.7%, with lower values obtained at higher temperatures and when increasing the reaction time at a constant temperature. |
[124] |
| Sewage sludge digestate, organic fraction of solid waste digestate, agro-industrial waste digestate | Temperature: 190 °C, reaction time: 1 h Industrial demonstration plant: C700 with treatment capacity of 5000 t/year (flow: 0.7 t/h). Heat consumption: 8.5 m3 CH4/t reactor input material Electricity consumption: 4.6 kWh/t reactor input material Hydrochar yield: 55 – 63% Phosphorus was concentrated in hydrochar (3.2 – 3.4 wt.%) derived from sewage sludge and agro-industrial waste. High levels of Cu and Zn were reported for sludge-derived hydrochar |
[100] |
| HTL experiments | ||
| Digestate from biogas plant (Nature Energy A/S, Denmark) (ash content: 36.2 – 39.5%) | HTL continuous plant Temperature 380 °C Hydrochar derived from the process was studied as fertilizer. The extraction of phosphate was evaluated using different leaching media and toxicity of hydrochar and the hydrochar filtered from the aqueous phase was tested as well. The presence of low molecular weight polycyclic aromatic hydrocarbon was the main cause of toxicity, with the filtered hydrochar presenting higher toxic effects. |
[125] |
| Digestate from a biogas plant treating OFMSW 1 and green waste (Pinerolo, Italy), (42% ash content) | Temperature: 300 – 360 °C, reaction time: 10 – 60 min, solid loading: 5 – 30 wt% Heating rate: 83 – 93 ºC/min, Cooling time: 50 s Reactor volume: 20 mL Maximum biocrude yield: 31.5% (HHV: 31 MJ/kg) Solid yield: 45 – 50% expressed on dry basis. |
[126] |
| Digestate derived from laboratory experiment treating a mixture of hydrolyzed dissolved air flotation sludge and stockyard waste (from animal pens) (ash content 39.5%) | Temperature: 250 – 350 °C, reaction time: 0 – 60 min, initial pressure: 0.1 – 5 MPa Reactor volume: 500 mL, heating rate: 3.5 °C/min. Maximum biocrude yield: 7% (at 290 °C, and 5 MPa of initial pressure, holding time of 83 min – including the heating time in this value) Hydrochar yield: 44.6% |
[127] |
| Manure digestate | Temperature: 300 °C, reaction time 60 min Heating time: 20 min Reactor volume: 500 mL Testing performance under acidic and alkaline conditions. Dehydration reactions were enhanced by the acidic pH, increasing biocrude yields. |
[128] |
| Manure digestate (MD) (ash content 26%) and acid whey (AW) | Temperature: 280 – 360 °C, reaction time: 10 – 50 min, AW:MD ratio: 0 – 2. Maximum biocrude yield (45.6%) obtained at 354 °C with a reaction time of 21 min and an AW:MD ratio of 1.21 |
[129] |
| Raw material treated | Hydrothermal process | Water treatment | Reference |
|---|---|---|---|
| Olive tree pruning | HTC: 2-L batch reactor at 250 °C and 3 h of reaction time. Process water characteristics: pH = 3.25, TOC 1 = 7.11 g/L |
Electro-oxidation using BDD 2 cell (25 V), current densities from 1.38 to 11.79 mA/cm2. TOC removal was between 30% and 40% |
[149] |
| Wheat straw | HTL Pilot plant at 350 °C with flow rate of 58.5 L/h. Slurry TS content: 12.5% Process water characteristics: pH = 5.4, TOC = 18.7 g/L |
Electro-oxidation using BDD cell. Constant current supplied (0 – 64 V, 0 – 10 A), current densities: 45.7, 95.2 and 147.2 mA/cm2. COD removal was between 14.4% and 81.6 % | [147] |
| Sewage sludge | HTL Pilot plant at 325 °C with flow rate of 43.0 L/h. Slurry TS content: 16.0% Process water characteristics: pH = 4.4, TOC = 12.2 g/L |
Electro-oxidation using BDD cell. Constant current supplied (0 – 64 V, 0 – 10 A), current densities: 45.7, 95.2 and 147.2 mA/cm2. COD removal was between 20.6% and 99.7% | [147] |
| Sewage sludge, food waste, spirulina, digestate | HTL Pilot plant at 325 °C with flow rate of 43.0 L/h. Slurry TS content: 16.0% Process water characteristics: COD = 12 – 120 g/L |
Electro-oxidation using RuO2/Ti anode and Ru/CF cathode (1.5 – 2.5 V) Current densities: 0 – 60 mA/cm2 The energy required for water treatment (COD reduction) and producing H2 is about twice that of commercial H2 electrolyzers |
[150] |
| Sewage sludge | HTL Pilot plant at 325 °C with flow rate of 43.0 L/h. Slurry TS content: 16.0% Process water characteristics: pH = 3.85, TOC = 11.9 g/L |
Wet oxidation with O2 Temperature: 200 – 350 °C Load pressure: 20 and 90 bar Final pressure: 50 – 190 bar Time: 2 – 180 min Treated process water at 350 °C and a reaction time of 180 min results in TOC concentrations of 470 mg/L and 704.5 mg/L of ammonium. Minimum energy requirement is 9.6 kWh/kg COD |
[151] |
| Microalgae (N. chlorella) | HTL: 410 mL batch reactor, 11 wt.% microalgae loading, 320 °C, reaction time 30 min, heating ramp 3.5 °C/min Process water characteristics: TOC = 12.4 g/L, TN 3= 8.1 g/L |
Supercritical water gasification (SCWG). 4.1 mL mini-batch reactor. Temperature: 450 – 500 °C Holding time: 10 min, including heating time. Catalyst testing: Pt-Pd/C, Ru/C, Pd/C, Na2CO3 and NaOH. Increasing temperature favored H2 production. Best performance found for Na2CO3 and NaOH catalyst addition |
[152] |
| Sewage sludge, food waste | HTL: continuous flow HTL plug flow reactor (300 mL), 350 °C. Process water characteristics: 1.7 – 3.1% C content, 0.5 – 0.9 wt.% N content |
Catalytic wet air oxidation, catalyst screening including HTL residual solids. Temperature: 175 and 225 °C (13.4 – 14.6 MPa Operating pressure) Air charging pressure 7.4 – 8.5 MPa An increased conversion of COD was achieved when using WO3 and ZrO2 as catalysts, resulting in higher yields of acetic and formic acid. Testing of trickle bed reactor using CeZr catalyst (4.1 MPa, 225 ºC, 0.6 h-1 of WHSV 4) achieved 50% COD reduction. |
[153] |
| Raw material | Technology | Description | Reference |
|---|---|---|---|
| Out of specification compost (OSC) and grape marc (GM) | HTC Treatment capacity 20,000 t/year (dry biomass: 14,000 t OSC/year, 7000 t GM/year Main product: Hydrochar |
Equipment: high pressure pump, HTC reactor, heat exchangers, decanter, burner, depressurizing tanks, dryer, blower. No treatment considered for the process-water Plant cost estimate: 1.77 M€ 1 Break even value of hydrochar: 200 €/t |
[166] |
| Sewage sludge | HTC Treatment capacity 16,000 t/year (no specification of sludge TS content) Main product: Hydrochar (activated coal), Phosphate (struvite), sulphates Revenues obtained from waste treatment fees (main revenue) |
Equipment: HTC reactor, Recycling system for HTC process water, Separator, Activation of hydrochar to produce active carbon, Struvite precipitator, metal sulphate production unit Plant cost estimate: 10 M€ |
[98] |
| Sewage sludge | HTC Treatment capacity: sludge from a WWTP with 212,697 m3/d. Revenues obtained from hydrochar |
Equipment: Boiler, HTC reactor, filter press, dryer, pelletizer, purification unit for process water treatment (not specified) Plant cost estimate: 425,000 € |
[167] |
| Rice straw | HTC Treatment capacity: 84,000 t/year. Revenues obtained from hydrochar as activated coal. Process water treatment was not considered, neither recycling |
Equipment: Crusher, heat exchanger, HTC reactor, Filter press, solid washer, dryer. Plant cost estimate: 5.3 MUSD 2 Operational costs: 3.71 MUSD/year Minimum sale price: US$ 76/t |
[168] |
| Forest residue | HTC Treatment capacity: 235,153 - 783,862 t/year Water recycling is not considered. Process water treatment is not specified |
Equipment: Shredder, Mixer, HTC reactor, flashing tower, heat exchangers, storage tank, cooling towers, rotary filter, rotary dryer, pelletizer Plant cost estimate: 19.48 - 51.87 MUSD Operational costs: 13.7 – 33.2 MUSD/year |
[169] |
| Cattle manure and co-substrates (corn silage, sunflower cake, whey) | AD + HTC Treatment capacity: Cattle manure: 307,325 t/year Corn silage: 42,046 t/year Sunflower cake: 77 t/year Process water treatment not specified Whey: 590 t/year Revenues obtained from electricity, heat, digestate and hydrochar. |
Equipment: HTC reactor, filtration, dryer Annual profit: 23.13 MUSD/ year Electricity production: 245.7 GWh/year, heat production: 298.8 GWh/year, hydrochar production: 185 kt/year Hydrochar selling price: 150 USD/t Electricity selling price: 0.155 USD/kWh |
[170] |
| Paper sludge, sewage sludge, biowaste | HTC Treatment capacity: Biowaste 78,000 t/year (70% moisture content). Process water is partially recirculated, and remaining water is treated by reverse osmosis. Treated water is suitable for soil irrigation. |
Equipment: Mixer, high pressure pumping, heat exchangers, HTC reactor, Ash reduction tank, filter press, dryer, reverse osmosis, pelletizer. Hydrochar yield: 15,400 t/year (65.8%) Revenues from hydrochar selling and fees from waste treatment (50 €/t). Plant cost estimate: 27.3 M€ Operational costs: 3.08 M€/year Hydrochar selling price: 180 €/t |
[171] |
| Forestry residues | HTL Treatment capacity 3653 t/year (10% TS content) equivalent to 365.3 t/year dry biomass. Main product: biocrude. No upgrading considered |
Equipment: HTL reactor, heat exchangers, boiler, high pressure pumps, solar parabolic trough collectors, cyclone separator. Process water recirculation Plant cost estimate: 0.83 – 1.02 MUSD. Operational costs: 13% of CAPEX 3 Biocrude yield: 31% MFSP 4: 0.97 – 1.02 (USD/kg), biocrude. |
[172] |
| Kraft pulp mill waste | HTL + up-grading Treatment capacity 146,000 t/year dry lignin. Process water recycling |
Equipment: HTL reactor, centrifuge decanter, hydrotreatment/hydro-deoxygenation of biocrude, distillation, phenolic extraction Plant cost estimate: 114 – 124 MUSD Operational costs: 43.5 – 48.8 MUSD/year MFSP: 0.93 – 1.0 (USD/kg), gasoline |
[173] |
| Wood biomass (Aspen wood) + crude glycerol | HTL Treatment capacity 182,500 t/year (wood biomass), 365,000 t/year (wood + biomass) with composition of 17.8% wood, 16.5% glycerol, 65.7% water. Process water recycling, no information regarding the treatment of process water. |
Equipment: Pretreatment, HTL reactor, Hydrotreating, cracking unit. Plant cost estimate: 225.8 MUSD Fuel yield (gasoline equivalent): 27.8% MFSP: 0.56 – 1.87 (USD/L), gasoline |
[174] |
| Sewage sludge | HTL Treatment capacity 40,150 t/year (dry sludge) Revenues from fee sludge treatment |
Equipment: Fee sludge treatment tariff: 44 USD/t (wet sludge) Plant cost estimate: 32.9 MUSD Operational costs: 2.7 MUSD/year Upgrading is considered to take place in an independent facility MFSP: 2.27 USD/gal (biocrude gallon). |
[175] |
| Sewage sludge | HTL HTL plant base capacity: 14,000 t/year sludge (20% dry matter). WWTP capacity of 0.1 – 1.0 million population equivalents. Sludge with a TS content of 20%. Bio-oil upgrading is not considered. |
Equipment: High pressure pumping, heat exchangers, HTL plug flow reactor, Filter, Flashing unit, decanter Process water treated by back recirculation to the conventional waste activated sludge system. Plant cost estimate: 4.4 MUSD (base case 150,000 PE WWTP) Operational costs: 0.9 MUSD/year MFSP: 1.4 €/kg, biocrude. |
[176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





