1. Introduction
Heart failure (HF) is a complex clinical syndrome that results from structural or functional impairments of the ventricles, ultimately leading to compromised systolic and/or diastolic performance and symptomatic left ventricular dysfunction. Clinically, it is primarily manifested through cardinal symptoms such as exertional dyspnea, fatigue, reduced exercise tolerance, and signs of fluid overload including peripheral edema and pulmonary congestion [
1].
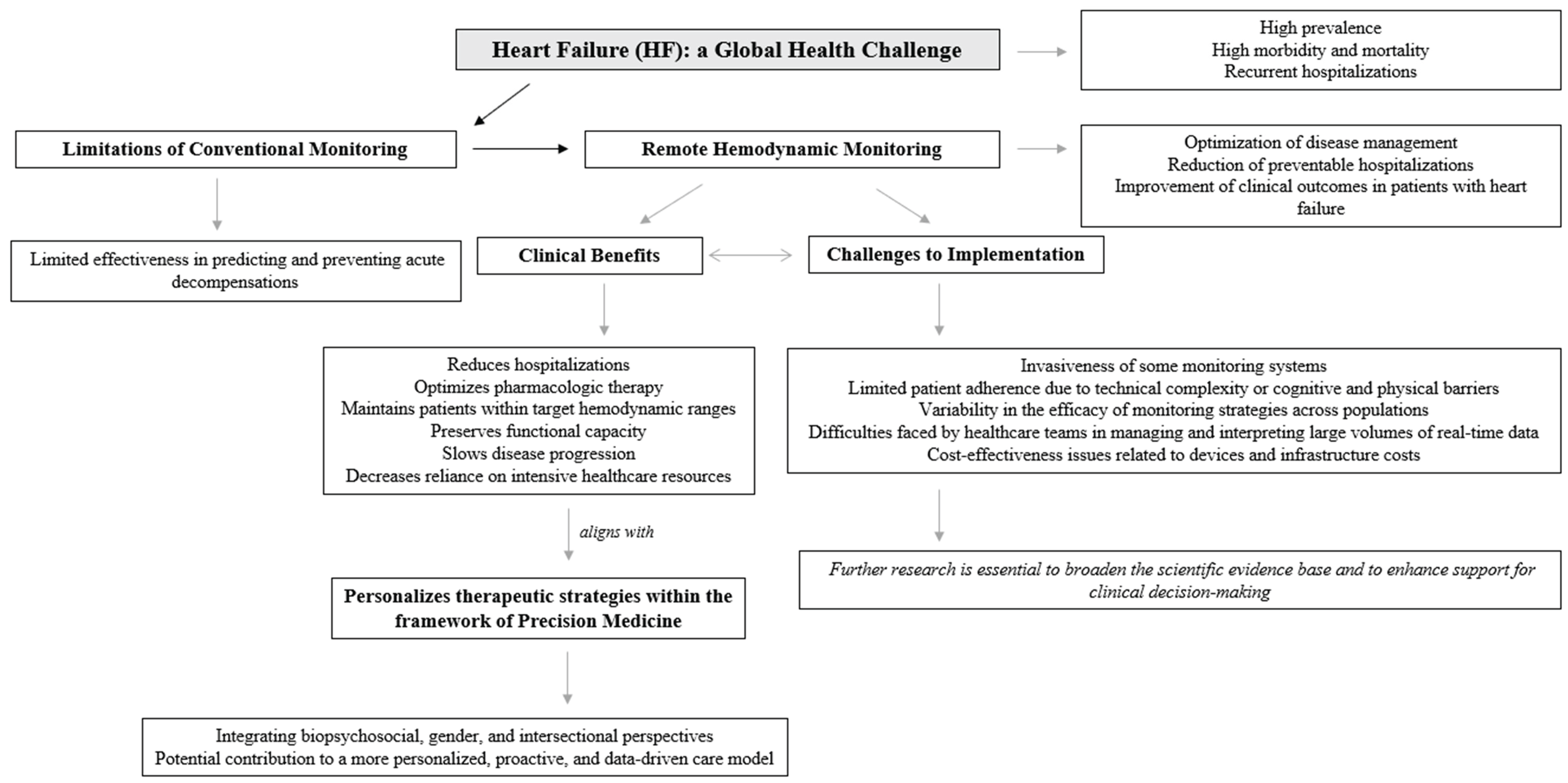
Beyond its clinical complexity, heart failure represents a major global and worldwide health challenge due to its high prevalence, significant morbidity and mortality, and recurrent hospitalizations. From an epidemiological perspective, it exerts a substantial burden on healthcare systems, particularly in aging populations, and is associated with poor long-term prognosis despite advances in pharmacological and device-based therapies. The progressive nature of heart failure and its frequent exacerbations highlight the urgent need for innovative strategies aimed at early detection, close monitoring, and individualized management [
2].
Heart failure represents a critical public health concern in the world, requiring urgent attention and the implementation of effective healthcare policies [
1,
2,
3]. Cardiovascular diseases are the leading cause of mortality worldwide, accounting for an estimated 17.9 million deaths in 2019. In Spain, more than 770,000 individuals are currently living with heart failure, with prevalence rates ranging from 4.7% to 6.8% among adults over the age of 45. Heart failure is responsible for over 25% of all hospital admissions due to cardiovascular conditions, underscoring its significant clinical and systemic impact. In-hospital mortality associated with heart failure exceeds 10%, rising to nearly 20% within the first-year post-discharge, and reaching 40% to 50% at five years [
3].
From an economic perspective, heart failure imposes a substantial burden on the Spanish healthcare system. The estimated annual cost is approximately €2.5 billion, accounting for around 3.8% of total national health expenditure, with hospital-related costs alone surpassing €470 million. These figures highlight the urgent need to strengthen coordination across levels of care, develop robust preventive strategies, promote healthy lifestyle behaviors, and allocate sufficient resources to support the accreditation and operation of specialized heart failure units. In this context, advancing the use of remote hemodynamic monitoring may offer a promising strategy to optimize disease management, reduce preventable hospitalizations, and improve clinical outcomes in patients with heart failure [
3].
Hemodynamic monitoring has emerged as a valuable strategy in reducing hospital readmissions among patients with heart failure by enabling early detection of decompensation and guiding proactive management. One of its primary advantages is related to its ability to detect subclinical congestion. Techniques such as pulmonary artery pressure monitoring can identify elevations in cardiopulmonary pressures well before the onset of overt symptoms, providing an essential timeframe for early therapeutic intervention. This early detection allows for timely optimization of treatment regimens, as hemodynamic data can inform clinicians’ decisions regarding adjustments in pharmacologic therapy based on predefined pressure thresholds. By maintaining patients within target hemodynamic ranges, this approach mitigates the progression of heart failure and decreases the likelihood of acute exacerbations requiring hospitalization [
4].
In the same line, CardioMEMS is an FDA-approved remote hemodynamic monitoring system that uses a wireless sensor implanted in a pulmonary artery to measure hemodynamic pressures. This system has proven effective in reducing heart failure hospitalizations, as demonstrated in the CHAMPION trial, which showed a 28% reduction in heart failure admissions at 6 months, with sustained benefits beyond 2 years. The system enables patient management based on hemodynamic goals, independent of symptoms, providing an opportunity for early therapeutic interventions. It is applicable to both preserved and reduced ejection fraction patients, broadening its use across the heart failure population. However, it faces challenges such as the need for daily patient compliance to transmit data, the system’s cost, and the required infrastructure to monitor large numbers of patients. Additionally, it involves an invasive procedure for sensor implantation [
4,
5].
Similarly, evidence from clinical trials, such as the CHAMPION study [
5,
6], supports a protocol-driven model of care in which management decisions are based on hemodynamic targets rather than symptom severity alone. This strategy has been shown to significantly reduce hospital admissions by maintaining pulmonary artery pressures below high-risk thresholds. Moreover, by intervening during the early stages of hemodynamic deterioration, clinicians can prevent the development of symptomatic decompensation and avoid the need for acute hospital-based care. Notably, the benefits of hemodynamic monitoring extend across the heart failure spectrum, showing efficacy in patients with both preserved and reduced ejection fraction. Furthermore, these positive outcomes appear to be lasting over time, with sustained reductions in hospitalization rates and improved long-term clinical trajectories [
4,
5,
6].
Remote monitoring, particularly wireless hemodynamic monitoring, has emerged as a valuable tool in the comprehensive management of heart failure. By enabling early detection of physiological deterioration, these technologies contribute to optimized patient care through timely therapeutic adjustments, ultimately reducing hospitalizations and enhancing quality of life. Clinical trials have demonstrated notable benefits in select populations; however, the heterogeneity of trial results underscores the relevance of linking remote device data to structured and effective clinical decision-making pathways. The utility of remote monitoring depends not only on technological capabilities but also on the ability to act upon the data in a timely and personalized manner [
4,
5,
6,
7].
Recent advances in cardiac device technology -characterized by increased miniaturization, improved automation, and greater patient usability- have positioned remote monitoring as a potentially indispensable component of modern heart failure care system. This evolution has been further accelerated by the shift toward virtual consultations and telehealth models, particularly during and following the COVID-19 pandemic. Looking ahead, future developments in artificial intelligence, machine learning, and real-time data integration are expected to enhance the predictive power and clinical utility of remote monitoring systems. These innovations will likely play a central role in enabling more proactive, personalized, and scalable approaches to heart failure management [
7].
Despite its positive results up to date, the broader implementation of hemodynamic monitoring is challenged by factors such as the invasive nature of certain monitoring systems, patient adherence to monitoring protocols, and concerns about cost-effectiveness. Addressing these barriers will be essential to fully realizing the potential of hemodynamic-guided management in heart failure care [
4,
5,
6].
Therefore, these comprehensive reviews aim to analyse the challenges and strategies of remote monitoring to reduce rehospitalizations in heart failure patients, highlighting its effectiveness and clinical relevance.
4. Discussion
Monitoring hemodynamic status and congestion plays a critical role in preventing hospital readmissions among patients with heart failure. Elevated intracardiac filling pressures, particularly in the pulmonary circulation, frequently precede the onset of clinical symptoms, providing a valuable opportunity for early detection and intervention before overt decompensation occurs. Traditional approaches based on symptom reporting or periodic assessments often fail to capture these early hemodynamic changes, resulting in delayed therapeutic action and increased risk of hospitalization. In this context, remote hemodynamic monitoring technologies have emerged as a promising strategy to enable continuous assessment of congestion and guide timely, individualized management. Clinical evidence suggests that such systems can significantly reduce heart failure-related hospital admissions by facilitating early therapeutic adjustments based on objective physiological data rather than relying solely on patient-reported outcomes or physical examination findings [
8,
9].
Reducing hospital readmissions appears to remain as a major challenge for the heart failure community, despite advances in pharmacological management and care coordination. While several remote monitoring strategies have been introduced with the aim of decreasing rehospitalization rates, their overall impact has been inconsistent across patient populations and clinical settings [
4,
10]. Differences in efficacy, limited patient access, technological complexity, and the financial burden associated with certain interventions-particularly those requiring invasive implantation or specialized infrastructure- represent significant barriers to widespread implementation [
4]. Furthermore, disparities in digital literacy, adherence, and healthcare resource availability exacerbate these challenges, particularly among vulnerable or underserved populations. The development of more accessible, accurate, and cost-effective monitoring solutions that integrate physiological, psychological, and contextual data will be essential to overcoming these limitations. Future research should focus not only on technological innovation but also on health system integration, equity of access, and personalized care pathways. Ongoing progress in this area is indispensable for transforming remote monitoring from an emerging intervention into a clinically validated and widely implementable component of standard heart failure care [
4].
Therefore, reducing hospital readmissions in heart failure remains a persistent and multifaceted clinical challenge. This difficulty stems largely from the progressive and relapsing nature of the disease, as well as the limited capacity to fully prevent recurrent decompensations. Heart failure is marked by high rates of early readmission following hospitalization, often within 30 days of discharge, and is associated with a steady decline in functional status and quality of life. The transition from stable chronic heart failure to acute decompensated heart failure is particularly concerning, as it dramatically increases the risk of adverse outcomes. Epidemiological data indicate that all-cause mortality rises from 6.4% to 23.6% within one year of an acute decompensation episode, reflecting the severity and fragility of this clinical trajectory [
9,
16,
17,
18].
Notably, recent analyses have challenged the assumption that readmissions are largely preventable. Estimates suggest that fewer than 25% of heart failure readmissions can be avoided through current interventions, highlighting the need to recalibrate expectations and strategies in managing post-discharge care. Moreover, although initiatives aimed at reducing 30-day readmission rates have yielded modest improvements in hospitalization metrics, they have coincided with a paradoxical 1.3% increase in short-term mortality. This unintended consequence raises important concerns about the validity of readmission-based performance benchmarks and underscores the need for more nuanced, patient-centered outcome measures that capture the complexity of disease progression and care transitions in heart failure [
9,
16,
17,
18].
These challenges underscore the necessity of strategic planning, targeted training, and appropriate resource allocation to support the successful integration of remote monitoring technologies into routine clinical practice. The adoption of such systems is not merely a matter of technological acquisition but requires a fundamental reconfiguration of existing care pathways. This includes the development of clear implementation protocols, the designation of clinical responsibilities within multidisciplinary teams, and the establishment of infrastructure for real-time data analysis and timely clinical decision-making. Additionally, healthcare professionals must receive adequate training to interpret hemodynamic data, adjust treatment regimens accordingly, and engage patients in the monitoring process to ensure adherence and optimize outcomes. From a systems perspective, adequate funding, institutional support, and alignment with broader health policy goals are essential to ensure long-term sustainability. Without addressing these organizational and operational dimensions, the clinical benefits demonstrated in controlled trials may not be fully achieved in real-world settings [
12,
19].
Moreover, given the multifactorial nature of heart failure and the complexity of remote hemodynamic monitoring, there is a growing need to adopt a comprehensive biopsychosocial approach to patient management. While current strategies emphasize physiological parameters such as pulmonary artery pressure and cardiac output, long-term adherence and clinical success are also related to behavioral, psychological, and social factors. Patient engagement with self-monitoring technologies, understanding of their clinical condition, emotional responses to chronic disease management, and the presence of supportive healthcare and social networks also play a critical role in the effective implementation of remote monitoring strategies. Integrating these psychosocial dimensions into heart failure care models not only enhances patient adherence and outcomes but also aligns monitoring interventions with the principles of personalized and precision medicine [
20]. Future research and clinical protocols should therefore prioritize transdisciplinary collaboration and the inclusion of psychological science frameworks alongside technological innovation.
In addition to the biopsychosocial perspective, incorporating a gender-sensitive and intersectional approach [
21] is essential for advancing equity and effectiveness in remote hemodynamic monitoring for heart failure. Evidence increasingly indicates that sex and gender differences influence not only the epidemiology and pathophysiology of heart failure but also patients’ access to care, engagement with health technologies, and clinical outcomes. Women, for instance, are often underrepresented in clinical trials and may experience different symptom profiles and treatment responses compared to men. Moreover, intersectional factors such as age, socioeconomic status, ethnicity, education, and caregiving responsibilities can further shape how individuals experience heart failure and interact with monitoring systems. Ignoring these dimensions risks reinforcing existing disparities and limiting the applicability of monitoring interventions across diverse patient populations. Therefore, research and clinical implementation efforts must systematically consider gender and intersectionality to ensure that remote monitoring strategies are inclusive, equitable, and responsive to the complex realities of those living with heart failure.
These considerations are closely aligned with the Sustainable Development Goals (SDGs), particularly Goal 3, which advocates for universal health coverage and access to quality essential healthcare services, and Goal 5, which emphasizes gender equality and the empowerment of all women and girls. By integrating biopsychosocial, gender-sensitive, and intersectional frameworks into remote hemodynamic monitoring strategies, healthcare systems can contribute to reducing health disparities and promoting equitable, person-centred care in line with global development priorities.
Related to the clinical implications of these findings, the integration of remote hemodynamic monitoring into clinical practice has the potential to significantly transform the management of heart failure by shifting from a reactive to a proactive care model. Clinical implementation of these technologies enables early identification of hemodynamic deterioration, facilitating timely pharmacological adjustments and reducing the incidence of acute decompensations and hospital readmissions. For clinicians, this means moving beyond symptom-driven management toward data-informed decision-making that is tailored to the individual physiological profile of each patient. To achieve these benefits, healthcare systems must establish dedicated protocols for incorporating remote monitoring into routine workflows, including appropriate patient selection; particularly those with persistent congestion, recurrent hospitalizations, or functional class III symptoms-and clear pathways for clinical response.
Furthermore, successful adoption requires the development of interdisciplinary care teams trained in the interpretation of remote hemodynamic data and supported by digital infrastructures that ensure efficient communication between patients and providers. Clinical practice must also address challenges related to patient engagement, digital literacy, and adherence, particularly in vulnerable populations such as older adults or people with limited access to technology. Additionally, the incorporation of remote monitoring into heart failure management aligns with the broader goals of precision medicine by enabling individualized care based on real-time physiological data. Accordingly, clinicians should be equipped not only to interpret this information but also to act upon it in a timely and appropriate manner, supported by evolving clinical guidelines, institutional infrastructures, and reimbursement frameworks that acknowledge the value of technology-enabled chronic disease management.
In summary, remote hemodynamic monitoring is currently one of the most effective strategies for reducing heart failure readmissions, nevertheless, all remote monitoring methods face challenges related to adherence, sensitivity, invasiveness, and cost. Future research needed to address these barriers and improve outcomes for the broader heart failure population.
Future research also should prioritize the integration of remote hemodynamic monitoring into standardized, multidisciplinary care pathways to determine its effectiveness across broader patient populations and healthcare settings. Large-scale, pragmatic clinical trials are needed to evaluate not only hospitalization and mortality outcomes, but also long-term effects on quality of life, functional capacity, and healthcare resource utilization. A particular emphasis should be placed on identifying which patient subgroups derive the greatest benefit from remote monitoring technologies, considering variables such as age, sex, comorbidities, socioeconomic status, and digital health literacy. Additionally, efforts should focus on improving the clinical interpretability and actionability of remotely acquired data, including the development of dynamic algorithms that integrate hemodynamic parameters with biomarkers, symptom reports, and behavioral metrics.
Emerging opportunities include the application of artificial intelligence to enhance predictive modeling and real-time clinical decision support, as well as the continued miniaturization and automation of devices to improve usability and patient adherence. Future studies should also explore the cost-effectiveness of remote monitoring in routine clinical practice and assess its alignment with value-based care models. Notably, an intersectional and gender-sensitive approach must be incorporated into future research frameworks to ensure equitable access, reduce disparities, and enhance the relevance of interventions across diverse populations. As remote monitoring technologies evolve, their success will ultimately depend on efficient integration into personalized care strategies supported by digital infrastructure, training, and policy-level engagement.
5. Conclusions
Remote hemodynamic monitoring has emerged as a promising strategy to reduce hospitalizations related to heart failure and to improve overall patient outcomes. Despite significant advancements in pharmacological and device-based therapies, conventional approaches to heart failure management have shown limited effectiveness in predicting and preventing acute decompensations. Traditional monitoring, often reliant on symptoms and periodic clinical evaluations, frequently fails to identify early hemodynamic deterioration, resulting in missed opportunities for timely intervention. In contrast, remote hemodynamic monitoring offers a proactive model of care by detecting subtle increases in intracardiac filling pressures; often weeks before the onset of clinical symptoms that typically prompt hospitalization. This early physiological insight enables clinicians to initiate tailored pharmacological adjustments and optimize volume status before patients reach a critical point of decompensation. As such, remote monitoring represents a key advancement in the transition toward anticipatory, individualized, and data-driven heart failure management.
The integration of biopsychosocial, gender-sensitive, and intersectional perspectives into remote hemodynamic monitoring strategies aligns closely with the principles of precision medicine. Precision medicine seeks to tailor healthcare interventions to the unique biological, psychological, and social characteristics of each individual, moving beyond the traditional “one-size-fits-all” or “generic treatment” strategy. By considering not only hemodynamic and physiological parameters but also behavioral patterns, gender-related differences, and socio-contextual factors, clinicians can more accurately predict risk, personalize therapeutic targets, and improve adherence to monitoring technologies. Such multidimensional profiling enhances the capacity to deliver interventions that are both clinically effective and contextually relevant, thereby advancing a truly patient-centered model of care. Remote monitoring technologies, when embedded within this precision framework, offer a powerful platform for dynamic, individualized, and equitable heart failure management. Although recent advances have introduced promising alternatives, further investigation remains necessary to strengthen the scientific evidence base and to provide more robust support for clinical decision-making in this field.