Submitted:
21 August 2025
Posted:
22 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Sensing Surfaces and PEN Detection
2.2.1. Sensor Surface Functionalization
2.2.2. AuNP Immobilization
2.2.3. Aptamer Functionalization of the Sensors
2.2.4. Biosensing
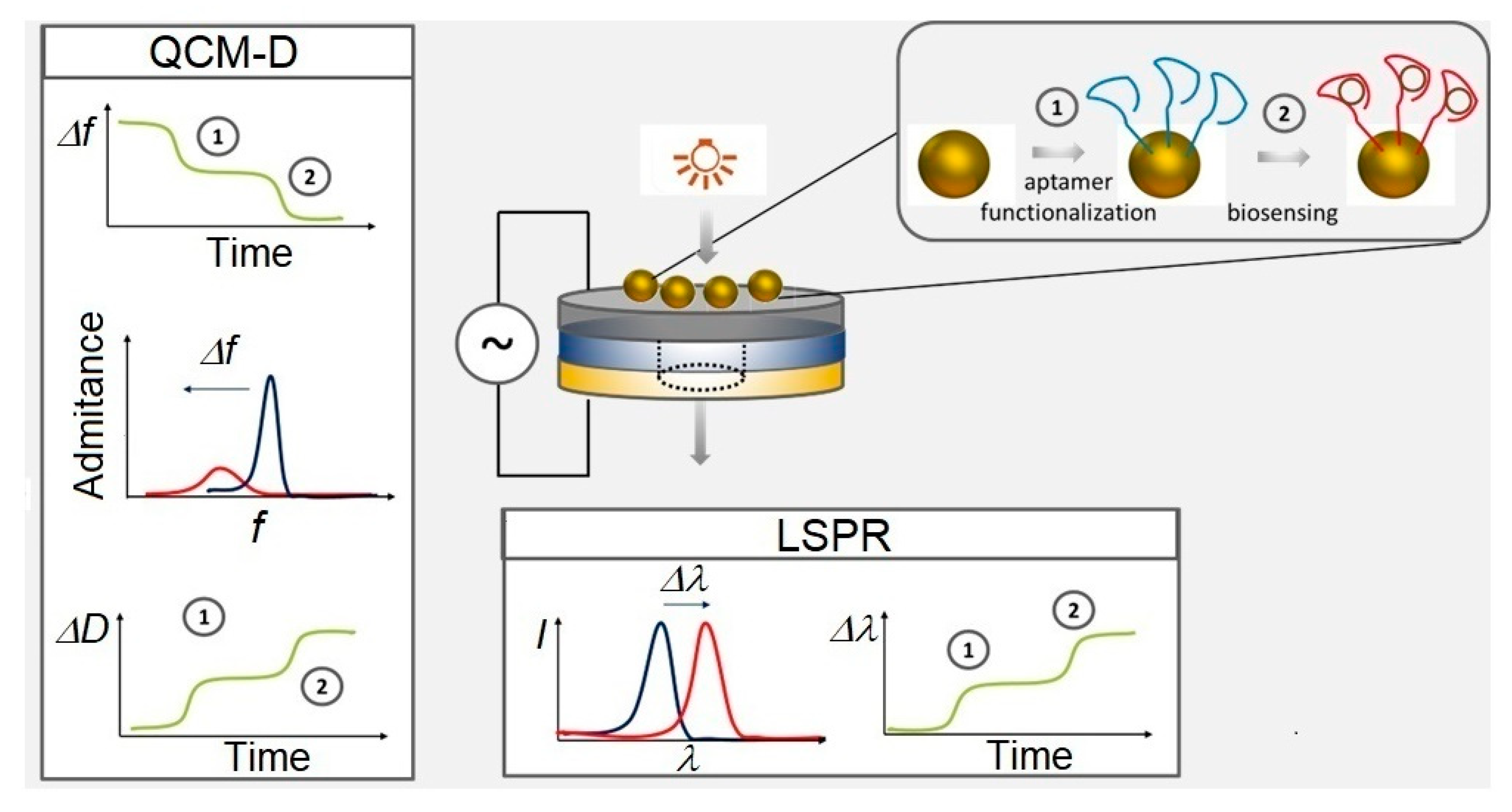
2.3. Experimental Apparatus and Data Analysis
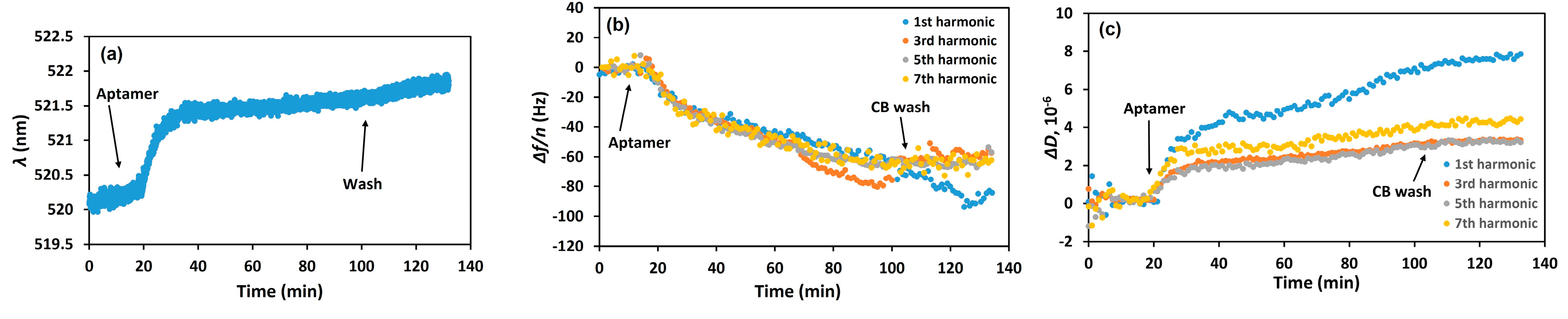
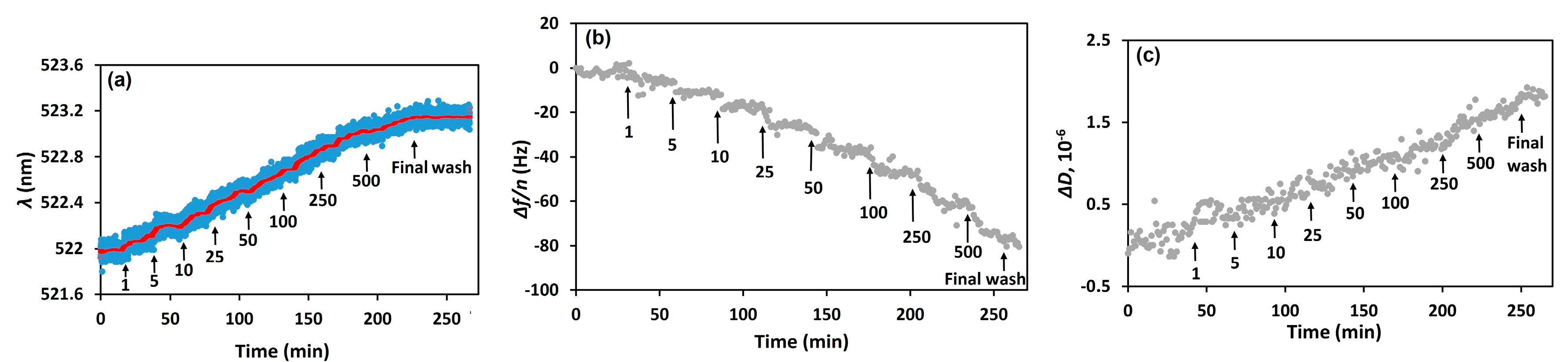
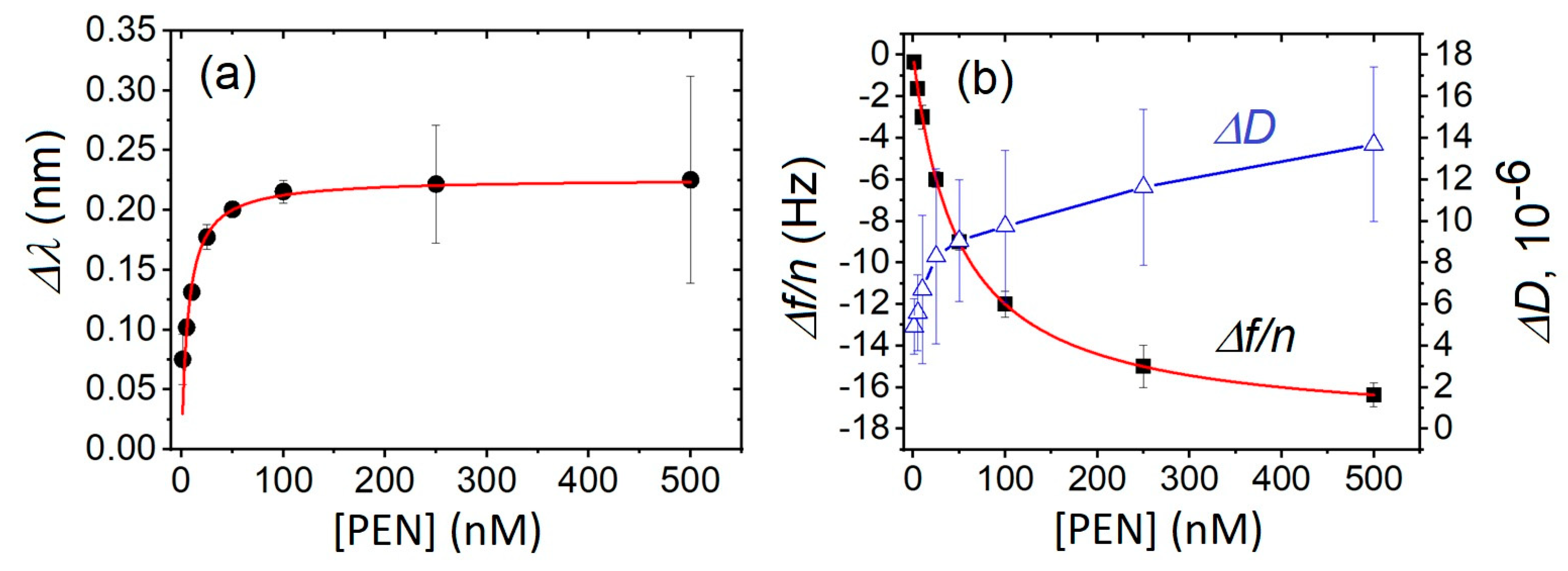
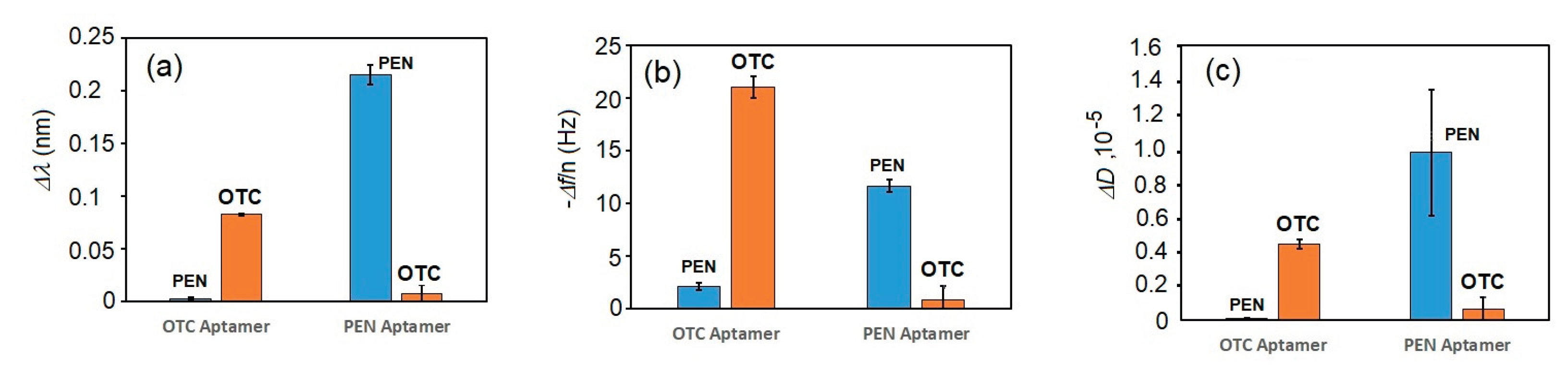
3. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banan, K.; Hatamabadi, D.; Afsharara, H.; Mostafiz, H.; Sadeghi, H.; Rashidi, S.; Beirami, A.D.; Shahbazi, M.-A.; Kecili, R.; Hussain, C.M.; Ghorbani-Bidkorbeh, F. MIP-based extraction techniques for the determination of antibiotic residues in edible meat samples: Design, performance and recent developments. Trends Food Sci. Technol. 2022, 199, 164–179. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Tsekenis, G.; Oravczova, V.; Hianik, T. Electrochemical aptasensors for antibiotic detection: Recent achievements and application for monitoring food safety. Sensors 2022, 22, 3584. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Antibiotic use in heavy pigs: comparison between urine and muscle samples from food chain animals analysed by HPLC-MS/MS. Food Chem. 2017, 235, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hlabangana, L.; Memeza, S. Ion-pair isocratic simultaneous determination of broad spectrum antibiotics in environmental samples by HPLC with UV detection. Environ. Nanotechnol. Monit. Manag. 2018, 10, 104–111. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Monette, C.E.; Bracero, S.; Saha, M.S. Methods for field measurement of antibiotic concentrations: limitations and outlook. FEMS Microbiol. Ecology 2018, 94, fiy105. [Google Scholar] [CrossRef]
- Cháfer-Pericás, C.; Maquieira, A.; Puchades, R. Fast screening methods to detect antibiotic residues in food samples. Trends Anal. Chem. 2010, 29, 1038–1049. [Google Scholar] [CrossRef]
- Aga, D.S.; O’Connor, S.; Ensley, S.; Payero, J.O.; Snow, D.; Tarkalson, D. Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography−mass spectrometry. J. Agric. Food Chem. 2005, 53, 7165–7171. [Google Scholar] [CrossRef]
- Madej, M.; Knihnicki, P.; Porada, R.; Kochana, J. (Bio)electroanalysis of tetracyclines: Recent developments. Biosensors 2025, 15, 101. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, Y.; Liu, Y.; Hu, X.; Chen, H. Current strategies of plasmonic nanoparticles assisted surface-enhanced Raman scattering toward biosensor studies. Biosens. Bioelectron. 2023, 228, 115231. [Google Scholar] [CrossRef]
- Tian, R.; Sun, J.; Ye, Y.; Lu, X.; Sun, X. Screening strategy of aptamer and its application in food contaminants determination. Trends Anal. Chem. 2024, 175, 117710. [Google Scholar] [CrossRef]
- Ballantine, D.S.; White, R.M.; Martin, S.J. , Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohltjen, H. Acoustics Wave Sensors. Theory, Design, and Physico-Chemical Applications, 1st ed.; Academic Press: San Diego, USA, 1997; pp. 36–149. [Google Scholar]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chemical Reviews 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Yu, N.; Wu, J. Rapid and reagentless detection of thrombin in clinic samples via microfluidic aptasensors with multiple target-binding sites. Biosens. Bioelectron. 2019, 146, 111726. [Google Scholar] [CrossRef]
- Hao, D.; Hu, C.; Grant, J.; Glidle, A.; Cumming, D.R.S. Hybrid localized surface plasmon resonance and quartz crystal microbalance sensor for label free biosensing. Biosens. Bioelectron. 2018, 100, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Jatschka, J.; Dathe, A.; Csáki, A.; Fritzsche, W.; Stranik, O. Propagating and localized surface plasmon resonance sensing — A critical comparison based on measurements and theory. Sensing and Bio-Sensing Research 2016, 7, 62-70. [CrossRef]
- Zhao, J.; Guo, W.; Peia, M.; Ding, F. GR–Fe3O4NPs and PEDOT–AuNPs composite based electrochemical aptasensor for the sensitive detection of penicillin. Anal. Methods 2016, 8, 4391. [Google Scholar] [CrossRef]
- Niazi, J.H.; Lee, S.J.; Gu, M.B. Single-stranded DNA aptamers specific for antibiotics tetracyclines. Bioorganic and Medicinal Chem. 2008, 16, 7245–7253. [Google Scholar] [CrossRef] [PubMed]
- Nobusawa, K.; Okamoto, N.; Chong, K.S.L.; Lin, X.; Iwahori, K.; Yamashita, I. Dispersed gold nanoparticle array produced by apoferritins utilizing biomineralization and chemical conversion. ACS Omega 2017, 2, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.M.; Cho, N.J.; Kanazawa, K. Analyzing spur-distorted impedance spectra for the QCM. J. Sensors, 2009, 2009, 259746. [Google Scholar] [CrossRef]
- Johannsmann, D.; Langhoff, A.; Leppin, C.; Reviakine, I.; Maan, A.M.C. Effect of noise on determining ultrathin-film parameters from QCM-D data with the viscoelastic model. Sensors 2023, 23, 1348. [Google Scholar] [CrossRef]
- Muckley, E.S.; Rama Vasudevan, R.; Sumpter, B.G.; Advincula, R.C.; Ivanov, I.N. Machine intelligence-centered system for automated characterization of functional materials and interfaces. ACS Appl. Mater. Interfaces 2023, 15, 2329–2340. [Google Scholar] [CrossRef]
- Sakti, S.P.; Lucklum, R.; Hauptmann, P.; Bühling, F.; Ansorge, S. Disposable TSM-biosensor based on viscosity changes of the contacting medium. Biosens. Bioelectron. 2001, 16, 1101–1108. [Google Scholar] [CrossRef]
- Ellis, J.S.; Thompson, M. Acoustic coupling at multiple interfaces and the liquidphase response of the thickness shear-mode acoustic wave sensor. Chem. Commun. 2024, 11, 1310–1311. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localised surface plasmon resonance biosensing: Current challenges and approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Physik 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Voinova, M.V.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: Continuum mechanic’s approach. Physica Scripta 1999, 59, 391–396. [Google Scholar] [CrossRef]
- Reviakine, I.; Johannsmann, D.; Richter, R.P. Hearing what you cannot see and visualizing what you hear: Interpreting quartz crystal microbalance data from solvated interfaces. Anal. Chem. 2011, 83, 8838–8848. [Google Scholar] [CrossRef] [PubMed]
- Osypova, A.; Thakar, D.; Dejeu, J.; Bonnet, H.; Van der Heyden, A.; Dubacheva, G.V.; Richter, R.P.; Defrancq, E.; Spinelli, N.; Coche-Guérente, K.; Labbé, P. Sensor based on aptamer folding to detect low-molecular weight analytes. Anal. Chem. 2015, 87, 7566–7574. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar]
- Karaseva, N.A.; Ermolaeva, T.N. Piezoelectric immunosensors for the detection of individual antibiotics and the total content of penicillin antibiotics in foodstuffs. Talanta 2014, 120, 312–317. [Google Scholar] [CrossRef]
- Cappi, G.; Spiga, F.M.; Moncada, Y.; Ferretti, A.; Beyeler, M.; Bianchessi, M.; Decosterd, L.; Buclin, T.; Guiducci, C. Label-free detection of tobramycin in serum by transmission-localized surface plasmon resonance. Anal. Chem. 2015, 87, 5278–5285. [Google Scholar] [CrossRef]
- Blidar, A.; Feier, B.; Tertis, M.; Galatus, R.; Cristea, C. Electrochemical surface plasmon resonance (EC-SPR) aptasensor for ampicillin detection. Anal. Bioanal. Chem. 2019, 411, 1053–1065. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Guan, J.; He, K.; Gunasekaran, S. Selection of ssDNA aptamer using GO-SELEX and development of DNA nanostructure-based electrochemical aptasensor for penicillin. Biosens. Bioelectron. X 2022, 12, 100220. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




