Introduction
Intensive Care Units (ICUs) are specialized units where advanced life-support systems are utilized to monitor and treat patients with critical health conditions [
1]. After major and specialized surgeries in general surgery or due to conditions such as acute mesenteric ischemia, intra-abdominal sepsis, severe pancreatitis, or trauma, patients often require monitoring in tertiary-level intensive care units. Therefore, general surgery and ICU services work closely together. Worldwide, approximately 310 million surgical procedures are performed annually. Around 7 million patients experience serious perioperative morbidity, and about 0.5% of surgeries result in mortality [
2,
3]. Advances in surgical techniques and postoperative care have allowed more complex surgeries to be performed, increasing the frequency of interventions in patients with severe health problems [
4,
5].
Septic shock and cardiogenic shock have been reported as leading causes of mortality in surgical ICU patients. Scoring systems such as APACHE, SAPS, and SOFA have been developed to predict mortality. Despite certain limitations, these scoring systems are widely used in clinical practice. Beyond scoring systems, factors such as low Glasgow Coma Scale (GCS) score (<9), prolonged mechanical ventilation, inotropic support, lactic acidosis, massive transfusion, presence of comorbidities, hypoalbuminemia, emergency surgical interventions, and advanced age have been reported to be associated with mortality in surgical ICUs [
6,
7]. Literature data specific to tertiary ICUs mainly investigate mortality-related factors in cardiovascular and neurosurgical patients. Reported mortality rates for ICU patients range between 20.5% and 43%, with the most common causes of death being sepsis, cardiopulmonary arrest, pneumonia, and malignant arrhythmias. Factors affecting mortality in general surgery-related pathologies requiring intensive care have not been sufficiently addressed.
Methods
Study Design and Ethical Approval
This retrospective case-control study was approved by the …. University Medical Research Ethics Committee on May 23, 2024 (Approval No: 24-5.1T/25).
This retrospective study was approved by the institutional ethics committee. Due to the retrospective nature of the study and the critical condition of many patients (including those who were intubated), obtaining informed consent was waived by the ethics committee.
Study Population and Inclusion Criteria
Patients aged 18 years and older with a history of general surgery-related pathology who were transferred from the General Surgery Clinic of …. University Faculty of Medicine Hospital to the tertiary Anesthesia Intensive Care Unit between January 2018 and December 2023 were included. Patients who had undergone solid organ transplantation (liver or kidney) were excluded due to differing clinical courses and prognostic outcomes. Patients admitted to the anesthesia ICU from other clinics or for non-general surgery-related pathologies and those under 18 years old were also excluded.
Data Collection
Demographic data, clinical status, vital signs at ICU admission, laboratory parameters, and scoring systems (SOFA, APACHE II, GCS) were retrospectively collected from electronic and physical patient records. All data were recorded using a standardized case form and transferred to an Excel spreadsheet.
Patients were divided into two groups based on mortality during ICU stay: the mortality group and the non-mortality group (discharged patients). Clinical, laboratory, and scoring parameters were compared between these groups. Demographic and descriptive parameters including age, sex, body mass index (BMI; weight in kg/height in m²), comorbidities, diagnosis, vital signs (blood pressure, pulse, urine output), respiratory support type (spontaneous breathing, oxygen mask, orotracheal intubation, tracheostomy), Glasgow Coma Scale (GCS), APACHE II, SOFA, HALP scores, neutrophil/lymphocyte ratio, neutrophil/platelet ratio, CRP/albumin ratio, platelet/lymphocyte ratio, need for inotropes, hemodialysis, transfusion requirements, and discharge status (alive or deceased) were evaluated. Subgroup analyses based on etiology (malignancy, sepsis, trauma) were performed to assess factors affecting mortality.
Statistical Analysis
Data were analyzed using SPSS version 29.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were presented as number (n) and percentage (%) for categorical variables, and mean ± standard deviation for normally distributed continuous variables. A 95% confidence interval was used, with p-values < 0.05 considered statistically significant. Normality of continuous data was assessed using Kolmogorov-Smirnov and Shapiro-Wilk tests. Normally distributed continuous variables were compared between groups using Student’s t-test or One-Way ANOVA, while non-normally distributed data were analyzed using Mann-Whitney U and Kruskal-Wallis tests. Categorical variables were compared with the Chi-square test; Spearman’s correlation was applied when appropriate. Survival analysis was conducted using Kaplan-Meier methods. For parameters showing significant differences between groups, cut-off values were identified via ROC curve analysis. Parameters with area under the curve (AUC) greater than 65% and significant p-values were further evaluated using the Youden index to determine optimal cut-off points. Patients were then dichotomized based on these cut-offs. Cox regression analysis was performed to evaluate the combined effect of these parameters on survival.
Results
Between January 2018 and December 2023, 259 patients transferred from the General Surgery Clinic to the Anesthesia Intensive Care Unit were screened. Sixteen patients with liver transplants, two with kidney transplants, and ten patients with missing data were excluded. A total of 231 patients were included, of whom 90 (39%) were female and 141 (61%) were male. The mean age was 59.49 ± 18.43 years, and the mean BMI was 28.85 ± 6.04. At ICU admission, 74.02% of patients had at least one comorbidity.
The primary diagnoses included gastrointestinal perforation in 57 patients (24.7%), mesenteric vascular disease in 19 (8.2%), mechanical bowel obstruction in 35 (15.2%), acute biliopancreatic infections (pancreatitis, cholecystitis, cholangitis) in 18 (7.8%), trauma in 44 (19%), necrotizing fasciitis in 5 (2.2%), gastrointestinal bleeding in 11 (4.8%), and elective cancer surgery requiring postoperative ICU care in 42 (18.2%). Among these patients, 150 (64.9%) died during ICU stay, while 81 (35.1%) were discharged alive. The ICU mortality rate for general surgery patients was 64.9%. The mean length of ICU stay was 11.38 ± 16.59 days . No significant differences were found between mortality and non-mortality groups in inflammatory indices including neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, neutrophil/platelet ratio, and HALP score (p=0.064, p=0.947, p=0.129, p=0.072, respectively). However, the CRP/albumin ratio was significantly higher in the mortality group (p=0.024). Decreased GCS scores at ICU admission were associated with mortality, while higher SOFA and APACHE II scores were significantly higher in the mortality group (p<0.001) (
Table 1).
Malignancy Group
Among 231 patients, 44 had a diagnosis of malignancy. Of these, 30 (68.18%) died, and 14 (31.81%) were discharged alive. Significant differences between mortality groups were found for APACHE II, HALP score, CRP/albumin ratio, neutrophil/lymphocyte ratio (NLR), and thrombocyte/lymphocyte ratio (TLR) (p=0.002, p=0.001, p=0.047, p=0.008, p=0.003, respectively) (
Table 2).
Sepsis Group
Sepsis was present in 134 patients (58.01%) due to diagnoses such as gastrointestinal perforation, mesenteric vascular disease, mechanical bowel obstruction, biliopancreatic infections, or necrotizing fasciitis. Among these, the mortality group had a higher mean BMI (30.33 ± 6.88) compared to the non-mortality group (27.13 ± 5.54) (p<0.05). Significant differences were observed between groups for APACHE II, SOFA, and thrombocyte/lymphocyte ratio (p<0.001, p=0.001, p=0.036 respectively). Other parameters showed no significant differences (
Table 3).
Trauma Group
There were 44 trauma patients requiring ICU care, of whom 14 (31.81%) died and 30 (68.18%) were discharged alive. The mean BMI was 28.45 ± 6.48 in the mortality group and 26.17 ± 2.58 in the non-mortality group (p=0.267). Significant differences between groups were noted for APACHE II and SOFA scores (p<0.001), but not for other parameters (
Table 4). Regarding blood transfusions, 13 patients (29.54%) did not require transfusion, while 31 (70.4%) received transfusions; among these, 10 (22.72%) had massive transfusions. No significant difference was found between groups regarding transfusion needs (p=0.130).
Factors Affecting Mortality
Sex was not found to be a significant factor affecting mortality. Mortality was significantly higher in patients who were intubated during ICU monitoring compared to those on oxygen mask or room air (p<0.001). Patients with impaired consciousness (closed or confused) had higher mortality compared to cooperative and oriented patients (p<0.001). Need for inotropic support, higher inotropic dose, and hypotension at ICU admission were significantly associated with increased mortality (p<0.05).
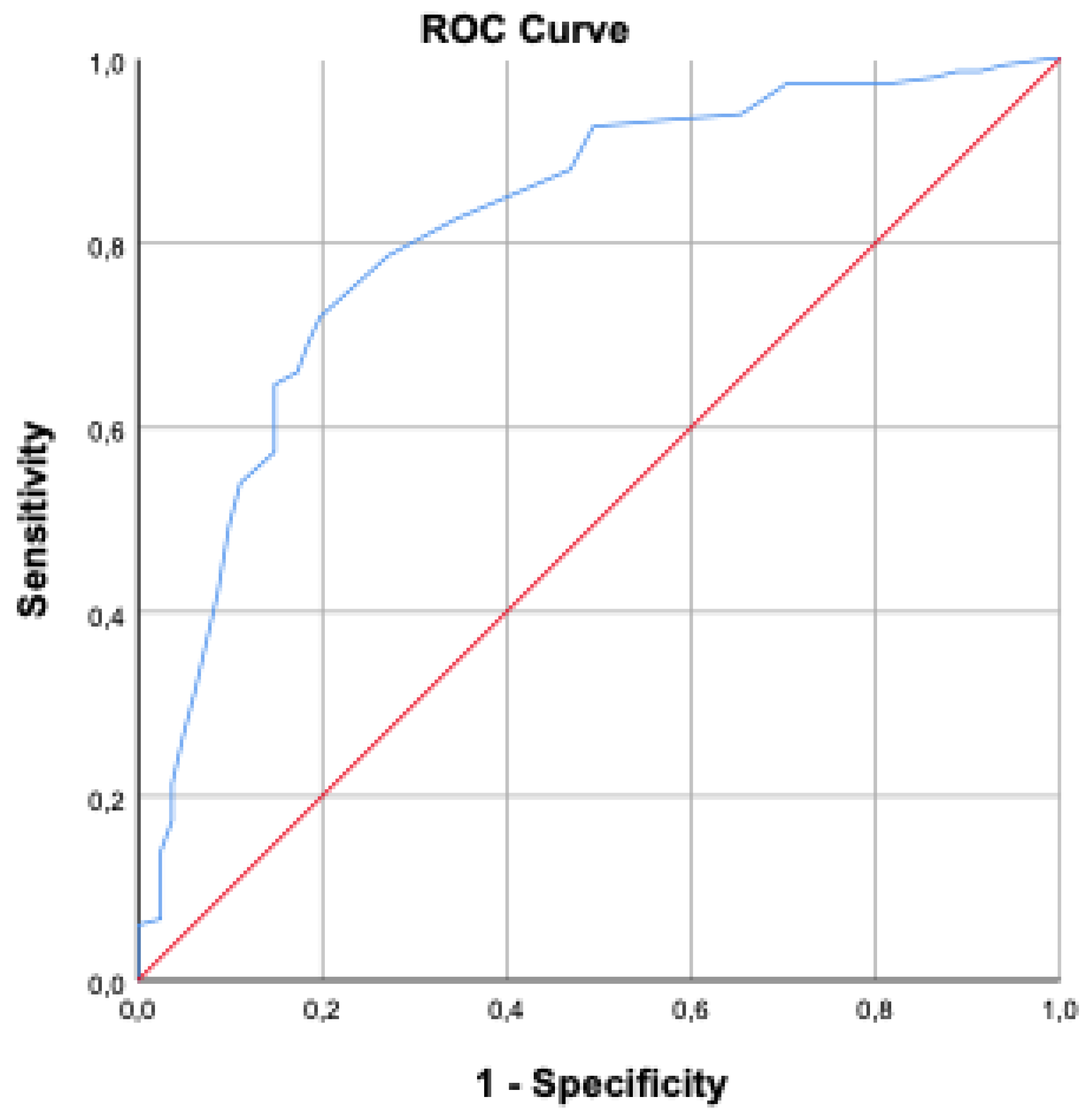
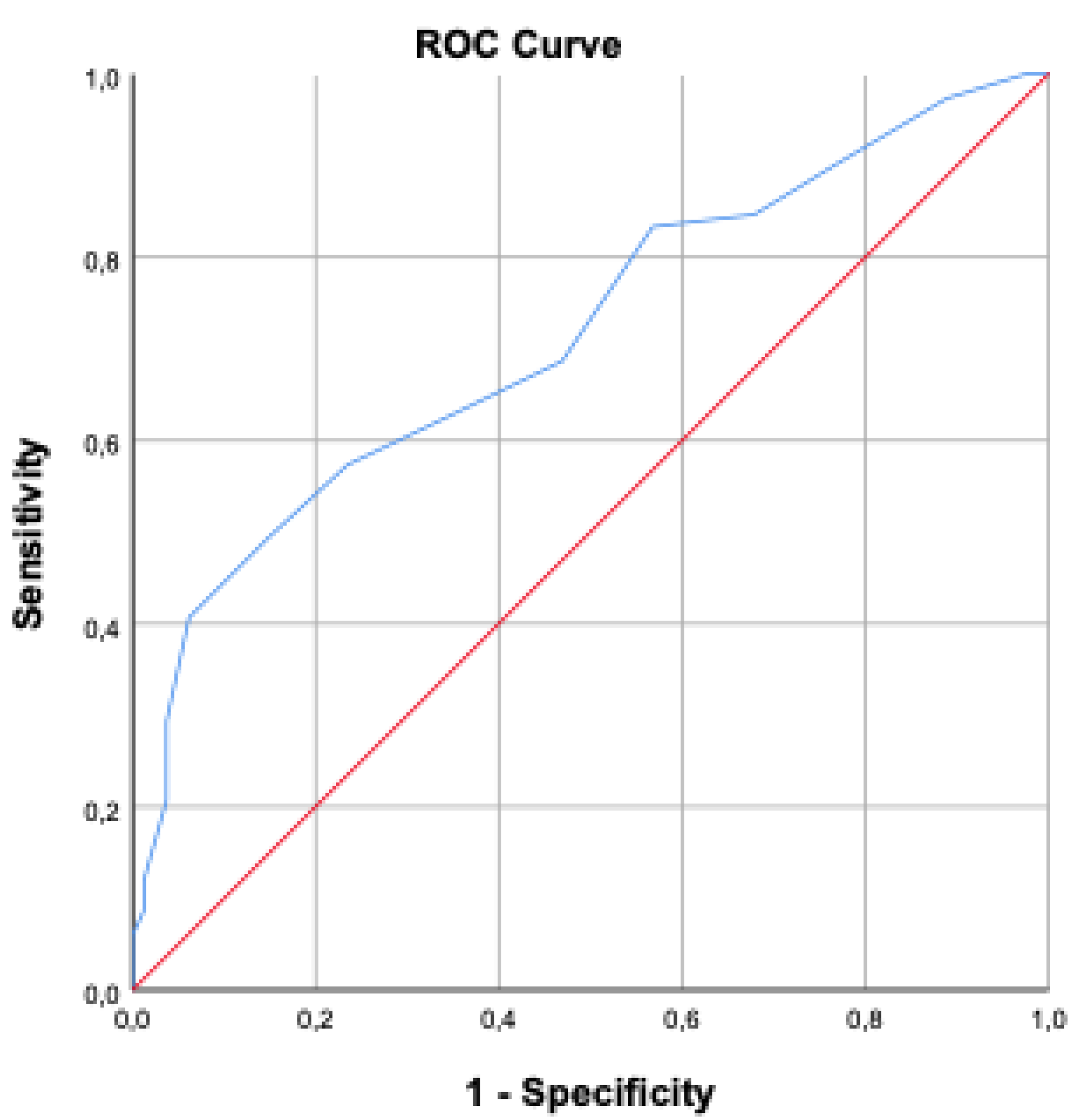
BMI was significantly higher in the mortality group compared to survivors (p<0.001). Oliguria and need for hemodialysis during ICU stay were significantly higher in the mortality group (p<0.001). No significant differences were found regarding blood transfusion or blood glucose levels (p=0.067 and p=0.487, respectively) . The average length of ICU stay was 11.65 ± 17.9 days in the mortality group and 10.87 ± 13.92 days in the non-mortality group (p=0.379). However, intubation duration was significantly longer in the mortality group (11.26 ± 16.97 days) compared to the non-mortality group (5.93 ± 12.93 days) (p<0.001). Age ≥ 58 years increased mortality risk by 4.56 times; BMI >30 increased it by 7.62 times; mean arterial pressure <70 mmHg increased it by 1.66 times; serum albumin <21.3 g/L increased it by 1.5 times; APACHE II score >18.5 increased it by 2.42 times; and SOFA score >9.5 increased it by 2.68 times. ROC analyses are presented in
Figure 1 and
Figure 2.
Discussion
This retrospective study evaluated surgical ICU patients over a five-year period, focusing on mortality rates and associated risk factors. Our findings revealed a high ICU mortality rate of 64.9%, which is influenced by the exclusive inclusion of general surgery patients with complex clinical profiles. Key factors such as advanced age, obesity, inflammatory markers including NLR and TLR in malignancy patients, as well as APACHE II and SOFA scores, were significantly associated with mortality. Additionally, the CRP/albumin ratio demonstrated prognostic value in predicting outcomes, especially among patients with malignancies. These results emphasize the multifactorial nature of mortality risk in surgical ICU patients and highlight important predictive parameters that can guide clinical management.
Advanced age, comorbidities, and disease severity represent independent risk factors for both short- and long-term mortality. Mortality rates increase significantly with age, especially in patients over 80 years old [
8,
9]. While advanced age alone does not prevent successful treatment, realistic expectations regarding outcomes require considering the overall health status and comorbidities of elderly patients. Obesity affects multiple systems and alters the expected metabolic response to conditions such as sepsis, trauma, and ischemia. Additional strain on cardiovascular and respiratory functions decreases the survival probability of obese patients during critical illness. Drug distribution and metabolism also differ in obese individuals, presenting further therapeutic challenges. Consequently, increased complications associated with obesity appear consistently across multiple studies [
10,
11,
12]. Advanced age and BMI were identified as significant risk factors for mortality in our study (p<0,001).
The neutrophil-to-lymphocyte ratio (NLR) has been utilized since the early 2000s as a parameter in endotoxemia, SIRS, and sepsis [
13]. The platelet-to-lymphocyte ratio (PLR) serves as a prognostic marker in inflammation, sepsis, trauma, pneumonia, colorectal cancer, and cardiac surgery [
14,
15,
16]. The neutrophil-to-platelet ratio (NPR) reflects an index of acute inflammatory response superimposed on chronic inflammation and acts as a prognostic biomarker in myocardial infarction and ulcerative colitis [
17,
18].
Across the entire cohort, these parameters did not exhibit statistically significant differences. Due to the heterogeneous patient population, subgroup analyses were conducted. In the malignancy subgroup, NLR and PLR demonstrated significance. A systematic meta-analysis indicates these ratios can predict outcomes in solid tumors and correlate with poor survival [
19]. Within this subgroup, average NLR was 12.64 ± 8.34 in non-survivors compared to 6.5 ± 4.15 in survivors, consistent with other studies [
20]. NLR and PLR ratios thus offer prognostic insights for surgical ICU patients with malignancies. In the sepsis subgroup, only PLR showed statistical significance; although differences in NLR appeared, these were not statistically significant, possibly due to inclusion of leukopenic and neutropenic patients affecting correlation. NPR did not show significance in any subgroup, suggesting the need for further research with alternative study designs.
The HALP score is an immunonutritional parameter developed for predicting survival, disease-free survival, and mortality risk in malignancy patients [
21]. It serves as a prognostic marker across several cancer types, including gastric, colorectal, bladder, prostate, kidney, esophageal, pharyngeal, lung, breast, and cervical cancers [
22,
23]. Despite its theoretical prognostic potential, it has not yet been widely integrated into clinical practice to guide nutritional interventions. Our study population included internal medicine, surgical, and emergency admissions; thus, heterogeneity likely contributed to a lack of significant difference in the overall cohort. However, HALP proved useful in predicting mortality within the malignancy subgroup. Its predictive capacity in acute inflammatory conditions such as sepsis and septic shock appears limited. Larger studies are required to validate these findings due to small sample sizes in subgroups.
The CRP-to-albumin ratio combines nutritional and inflammatory status and has been extensively examined as an independent prognostic marker in sepsis, infections, malignancies, and other diseases. However, few studies have specifically evaluated its prognostic value in critically ill surgical ICU patients [
24,
25]. The CRP/albumin ratio was significantly higher among non-survivors and aligned with literature in the malignancy subgroup. In the sepsis subgroup, expected significance was not observed, likely due to limited sample size and borderline values. Previous studies have suggested its independent predictive value for 28-day mortality in critical ICU patients [
26]. APACHE II and SOFA scores are widely recognized and accepted tools for mortality prediction in surgical ICU patients [
27]. Both scores were significantly higher among non-survivors. Subgroup analyses confirmed higher scores in the mortality groups, although the SOFA score did not differ significantly among malignancy patients. A notable limitation of APACHE II is the absence of variables related to hemodynamic support and mechanical ventilation, which suggests incorporating these parameters could enhance predictive accuracy. Some nonsignificant results in subgroups may be attributed to small sample sizes and heterogeneous distributions. Multivariate analyses support the use of both scores as independent predictors of mortality.
This study’s limitations include its single-center, retrospective design and the heterogeneity and limited sample size in certain subgroups, which may affect the generalizability of some results. Nevertheless, it provides a comprehensive overview of mortality-related factors in surgical ICU patients and lays a foundation for future research to validate and expand upon these findings in broader patient populations, ultimately aiming to improve intensive care management and patient outcomes.
Conclusion
The parameters examined in this study demonstrate significant predictive value in determining mortality rates among surgical intensive care patients and optimizing ICU resource utilization. Our findings highlight the complexity and high risk of mortality and morbidity in general surgical ICU patients, emphasizing the need for meticulous care. Age, obesity, comorbidities, hemodynamic status, and renal parameters all significantly impact mortality outcomes. Additionally, established scoring systems such as APACHE II and SOFA remain effective tools for mortality prediction.Furthermore, the CRP/albumin ratio, reflecting both inflammatory response and nutritional status, showed significant prognostic importance, particularly within the malignancy subgroup. Elevated CRP/albumin values correlated with higher mortality, suggesting its potential as an independent predictor and a useful parameter for risk stratification in critically ill surgical patients.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration 109 of Helsinki, and approved by the Institutional Review Board Clinical Research Ethics Committee of Ege University Faculty of Medicine. Ege University Medical Research Ethics Committee on May 23, 2024 (Approval No: 24-5.1T/25).
Data availability
Data are available from the corresponding author upon reasonable request.
Author Contributions
All authors meet the ICMJE authorship criteria. Tolga Girgin and Volkan Sayur contributed to the conception and design of the study. Erkan Güler and Can Uç were involved in data acquisition and analysis. Berk Göktepe and Volkan Sayur contributed to data interpretation and critical revision of the manuscript. Taylan Özgür Sezer and Sinan Ersin contributed to manuscript drafting and final editing. Mehmet Uyar and Tolga Girgin reviewed the manuscript for intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Funding
The authors received no fnancial support for the research and/or authorship of this article.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Tanaka, Y.; Masukawa, K.; Kawashima, A.; Hirayama, H.; Miyashita, M. Quality indicators for palliative care in intensive care units: a systematic review. Ann Palliat Med. 2023 May;12(3):584-599. [CrossRef]
- Weiser, T.G.; Regenbogen, S.E.; Thompson, K.D.; Haynes, A.B.; Lipsitz, S.R.; Berry, W.R.; et al. An estimation of the global volume of surgery: a modelling strategy based on available data. The Lancet. 2008 Jul;372(9633):139–44.
- Dobson, G.P. Trauma of major surgery: a global problem that is not going away. Vol. 81, International Journal of Surgery. Elsevier; 2020. p. 47–54.
- Di Nardo, M.; Tikkanen, J.; Husain, S.; Singer, L.G.; Cypel, M.; Ferguson, N.D.; Keshavjee, S.; Del Sorbo, L. Postoperative Management of Lung Transplant Recipients in the Intensive Care Unit. Anesthesiology. 2022 Mar 1;136(3):482-499. [CrossRef]
- Fontana, V.; Coureau, M.; Grigoriu, B.; Tamburini, N.; Lemaitre, J.; Meert, A.P. Place de la réanimation après chirurgie thoracique [The role of the intensive care unit after thoracic surgery]. Rev Mal Respir. 2022 Jan;39(1):40-54. French. [CrossRef]
- Zewudie, M.M.; Melesse, D.Y.; Filatie, T.D.; Zeleke, M.E. Variables associated to intensive care unit (ICU)-mortality among patients admitted to surgical intensive care unit in Ethiopia: a retrospective observational study. BMC Anesthesiol. 2023 Aug 18;23(1):279.
- Story, D.A.; Leslie, K.; Myles, P.S.; Fink, M.; Poustie, S.J.; Forbes, A.; et al. Complications and mortality in older surgical patients in Australia and New Zealand (the REASON study): a multicentre, prospective, observational study*. Anaesthesia. 2010 Oct 9;65(10):1022–30.
- Flaatten, H.; Beil, M.; Guidet, B. Elderly Patients in the Intensive Care Unit. Semin Respir Crit Care Med. 2021 Feb;42(1):10-19. [CrossRef]
- Brunker, L.B.; Boncyk, C.S.; Rengel, K.F.; Hughes, C.G. Elderly Patients and Management in Intensive Care Units (ICU): Clinical Challenges. Clin Interv Aging. 2023 Jan 22;18:93-112. [CrossRef]
- Tsekrekos, A.; Lovece, A.; Chrysikos, D.; Ndegwa, N.; Schizas, D.; Kumagai, K.; Rouvelas, I. Impact of obesity on the outcomes after gastrectomy for gastric cancer: A meta-analysis. Asian J Surg. 2022 Jan;45(1):15-26. [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; Rampulla, V.; Barni, S.; Cabiddu, M.; Bossi, A.; Ghidini, A.; Zaniboni, A. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021 Mar 1;4(3):e213520. [CrossRef]
- 12. Impact of visceral obesity on postoperative outcomes in colorectal cancer: a systematic review and meta-analysis. Front Oncol. 2025 May 6;15:1538073. [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474–88.
- Colloca, G.; Venturino, A.; Guarneri, D. Neutrophil-to-lymphocyte ratio predicts survival of patients with rectal cancer receiving neo-adjuvant chemoradiation followed by radical resection: a meta-analysis. Expert Rev Anticancer Ther. 2023 Apr;23(4):421-429. [CrossRef]
- 15. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio, and platelet-lymphocyte ratio in stroke-associated pneumonia: a systematic review and meta-analysis. Curr Med Res Opin. 2023 Mar;39(3):475-482. [CrossRef]
- Perry, L.A.; Liu, Z.; Loth, J.; Penny-Dimri, J.C.; Plummer, M.; Segal, R.; Smith, J. Perioperative Neutrophil-Lymphocyte Ratio Predicts Mortality After Cardiac Surgery: Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth. 2022 May;36(5):1296-1303. [CrossRef]
- Yamamoto-Furusho, J.K.; Mendieta-Escalante, E.A. Diagnostic utility of the neutrophil-platelet ratio as a novel marker of activity in patients with Ulcerative Colitis. PLoS One. 2020;15(4):e0231988.
- Somaschini, A.; Cornara, S.; Demarchi, A.; Mandurino-Mirizzi, A.; Fortuni, F.; Crimi, G.; Ferlini, M.; Camporotondo, R.; Gnecchi, M.; Visconti, L.O.; De Ferrari, G.M. Neutrophil to platelet ratio: A novel prognostic biomarker in ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. Eur J Prev Cardiol. 2020 Dec;27(19):2338-2340. [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. JNCI: Journal of the National Cancer Institute. 2014 Jun;106(6).
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020 Nov 20;18(1):360. [CrossRef]
- Chen, X.L.; Xue, L.; Wang, W.; Chen, H.N.; Zhang, W.H.; Liu, K.; et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. 2015 Dec 1;6(38):41370–82.
- Farag, C.M.; Antar, R.; Akosman, S.; Ng, M.; Whalen, M.J. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget. 2023 Feb 25;14:153–72.
- Xu, H.; Zheng, X.; Ai, J.; Yang, L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: A systematic review and meta-analysis of 13,110 patients. Int Immunopharmacol. 2023 Jan;114:109496. [CrossRef]
- Zhang, N.; Liu, Y.; Yang, C.; Li, X. Review of the Predictive Value of Biomarkers in Sepsis Mortality. Emerg Med Int. 2024 Jun 5;2024:2715606. [CrossRef]
- Li, J.; Zhang, S.; Hu, X.; Huang, T.; Chen, M. Correlation between the C-reactive protein (CRP)-albumin-lymphocyte (CALLY) index and the prognosis of gastric cancer patients after gastrectomy: a systematic review and meta-analysis. Surg Today. 2025 Apr;55(4):483-491. [CrossRef]
- Park, J.; Chung, K.; Song, J.; Kim, S.; Kim, E.; Jung, J.; et al. The C-Reactive Protein/Albumin Ratio as a Predictor of Mortality in Critically Ill Patients. J Clin Med. 2018 Oct 8;7(10):333.
- Cabrera Losada, A.; Correa Oviedo, M.A.; Herrera Villazón, V.C.; Gil-Tamayo, S.; Molina, C.F.; Gimenez-Esparza Vich, C.; Nieto Estrada, V.H. Towards better mortality prediction in cancer patients in the ICU: a comparative analysis of prognostic scales: systematic literature review. Med Intensiva (Engl Ed). 2024 Dec;48(12):e30-e40. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).