1. Introduction
Timely and effective management during an in-hospital code blue event is a key determinant of patient survival and subsequent quality of life. Cardiopulmonary resuscitation (CPR), the appropriate administration of medications, and the accurate identification of the initial cardiac rhythm significantly influence immediate outcomes and the likelihood of hospital discharge [
1,
2].
The effectiveness of an in-hospital code blue team depends on multiple factors. One critical aspect is the clear assignment of roles and responsibilities among professionals from various disciplines, which has been identified as a key contributor to successful interventions [
3,
4]. Likewise, strong leadership is essential, as it facilitates rapid decision-making and precise coordination of team actions, minimizing errors and optimizing critical interventions [
5,
6].
Simulation-based strategies have proven effective in strengthening both technical and non-technical skills, leading to improved team performance and better clinical outcomes, such as increased survival rates [
7,
8]. Furthermore, a culture of interprofessional collaboration fosters the early activation of code blue protocols, reducing delays and enabling a more effective response [
9].
Strict adherence to resuscitation algorithms and operational standards during code blue events has been associated with improved outcomes, including reduced neurological morbidity and increased survival [
7]. Additionally, structured review and analysis of code blue management can uncover areas for improvement, promoting organizational learning and continuous optimization of team performance [
3,
8].
One of the primary indicators used to evaluate the effectiveness of code blue management is the return of spontaneous circulation (ROSC) rate. According to the literature, the average ROSC rate in adult patients experiencing in-hospital cardiac arrest ranges from 66% to 72%, with variability across hospitals and patient subgroups [
10,
11]. For events occurring in intensive care units (ICUs), this rate decreases to approximately 62% [
12]. Overall hospital discharge survival following in-hospital cardiac arrest is estimated at around 24%, according to international guidelines, with variations depending on the initial rhythm, event location, patient age, and comorbidities [
13].
Moreover, the Charlson Comorbidity Index (CCI) is a validated tool that quantifies the burden of comorbid conditions and provides prognostic value in the context of in-hospital cardiac arrest [
14]. The In-Hospital Cardiac Arrest–Return of Spontaneous Circulation (IHCA-ROSC) score, validated in a multicenter cohort, enables standardized estimation of ROSC. It integrates pre-arrest clinical variables, facilitating outcome comparisons and performance assessment during resuscitation efforts. However, its predictive value for individual prognosis remains limited [
10]. The Risk Score for QTc Prolongation (RISQ-PATH), on the other hand, lacks evidence supporting its use as a prognostic tool for clinical outcomes [
15]. Nevertheless, it is included in this analysis on an exploratory basis to evaluate potential associations.
The initial cardiac rhythm also has a significant impact on survival. In arrests with shockable rhythms, survival exceeds 35%, while in non-shockable rhythms, such as asystole or pulseless electrical activity, survival ranges between 10% and 12%. Patients with shockable rhythms also tend to achieve better neurological outcomes upon discharge [
16].
A key rationale for conducting this study is the limited and heterogeneous body of literature available in Latin America regarding code blue management. Most published studies focus on out-of-hospital cardiac arrest, while those addressing team-based management, ROSC rates, in-hospital cardiac arrest, and clinical outcomes are scarce. Additionally, there is a persistent lack of evaluations assessing protocol adherence and providing a comprehensive characterization of how these events are managed within the regional hospital context [
17].
Aligned with the concept of a “learning health system,” multidisciplinary analysis of code blue events enables the implementation of protocol modifications aimed at improving the quality of resuscitative care and clinical outcomes [
18]. Within this context, the role of pharmacists in recording and documenting events has gained relevance and has been implemented at Clínica Bíblica.
Retrospective analysis of code blue events not only enables the identification of key indicators such as ROSC, in-hospital mortality, and discharge rates, but also helps uncover gaps in clinical processes that may impact patient outcomes [
18]. In this context, the Joint Commission International (JCI) standard COP.04.00 underscores the importance of internally reviewing resuscitation events, incorporating both clinical outcomes and the processes involved in patient care [
16].
The objective of this study is to analyze the management of code blue events at Clínica Bíblica through a retrospective review of cases that occurred between January 2020 and December 2024. The findings will contribute to strengthening the regional characterization of code blue team performance in in-hospital cardiac arrest and will serve as a foundation for proposing recommendations to improve the institutional protocol. These improvements aim to promote safer and more efficient care, while reinforcing the role and performance of each member of the resuscitation team.
2. Materials and Methods
This retrospective observational study was designed to analyze the clinical outcomes of code blue events recorded at Clínica Bíblica between January 2020 and December 2024. In addition, patterns within the care processes potentially associated with these outcomes were identified, with the aim of generating institutional recommendations aligned with the continuous improvement standards set forth by the Joint Commission International.
2.1. Study Design and Setting
All patients who experienced an in-hospital code blue activation during the defined period were included, while those with incomplete medical records or who were deceased prior to the arrival of the response team were excluded. Data collection was conducted between February and May 2025 through a thorough and systematic review of clinical and operational records.At Clínica Bíblica, Code Blue activations are initiated by healthcare personnel who identify signs of cardiac arrest—either through continuous monitoring or clinical observation (e.g., unresponsiveness, apnea, pulselessness). Once recognized, the event is immediately reported via the centralized Code Blue alarm system, which is broadcast hospital-wide through speakers and digital displays. This system allows for the rapid mobilization of a pre-designated multidisciplinary response team, including medical, nursing, pharmacy, and technical staff. Due to the strategic distribution of team members and clearly established response protocols, arrival times are extremely short—typically within seconds—enabling prompt and high-quality clinical intervention.
A structured instrument was used to collect sociodemographic variables, comorbidities, event-related data (initial rhythm, medications administered, interventions performed), immediate outcomes (ROSC), subsequent mortality, and hospital discharge status. The following prognostic scores were applied: Charlson Comorbidity Index (CCI), In-Hospital Cardiac Arrest–Return of Spontaneous Circulation (IHCA-ROSC), and RISQ-PATH.
Statistical analysis included measures of central tendency, proportions, and subgroup comparisons using Fisher’s exact tests and relative risk calculations with 95% confidence intervals. The study was approved by the Scientific Ethics Committee of the University of Costa Rica (CEC-143-2019).
2.2. Inclusion and Exclusion Criteria
The study included all patients who experienced a code blue event at Clínica Bíblica between January 2020 and December 2024, regardless of whether a pharmacist participated during the resuscitation. Cases were excluded if the medical records were incomplete or lacked sufficient information regarding the code blue intervention.
2.3. Institutional Information
The Hospital Clínica Bíblica is a private, high-complexity medical center located in San José, Costa Rica, with an average capacity of 61 beds in recent years. The average daily volume of outpatient discharges, considering the period from 2020 to 2024, is approximately 6 patients. Although there is no systematic record of the number of visitors, the hospital maintains a constant flow of patients and companions, consistent with its role as one of the country’s main referral centers.
2.4. Data Collection
Data collection was conducted between February 1 and May 15, 2025, through an exhaustive review of electronic medical records and code blue activation logs from Clínica Bíblica. A standardized instrument developed by the research team was used to systematically gather information, ensuring consistency across data points.
The instrument captured sociodemographic variables such as age, sex, year of the event, and the location within the hospital where the code blue was activated. It also documented clinical admission conditions and relevant comorbidities, which were used to calculate the Charlson Comorbidity Index (CCI). This index estimates the comorbidity burden and predicts one-year mortality risk by assigning weighted scores to specific pre-existing conditions. In this study, the CCI was applied retrospectively to each patient according to criteria established in the literature. In addition to comorbidities, the instrument captured whether patients received mechanical ventilation prior to the event, including both invasive mechanical ventilation (MV) and non-invasive ventilation (NIV), as documented in the medical record.
The In-Hospital Cardiac Arrest–Return of Spontaneous Circulation (IHCA-ROSC) score was calculated to estimate the likelihood of achieving ROSC after an in-hospital cardiac arrest, based on various pre-event clinical variables. The Risk Score for QTc Prolongation (RISQ-PATH) was also applied on an exploratory basis to estimate the risk of QTc interval prolongation based on individual clinical factors. Notably, the RISQ-PATH was calculated using clinical variables only, excluding QT-prolonging medication exposure, due to the retrospective nature of the study and the lack of standardized data on medication use in the reviewed records. All variables used are defined in Annex S1.
Regarding pharmacologic management, all medications administered during the events were recorded, along with the number of doses. Cardiac rhythm tracings documented during the code blue events were analyzed to determine the presenting rhythm at the time of arrest and to evaluate the appropriateness of defibrillation decisions. This also allowed for assessment of whether pharmacological interventions aligned with the identified cardiac rhythm. The aim of this component was to identify opportunities to improve real-time clinical decision-making, particularly in relation to rhythm recognition and the selection of appropriate medications.
Additionally, the roles performed by different healthcare professionals during each code blue event were described, with particular attention to the role of the clinical pharmacist. Finally, the primary clinical outcomes were recorded, including whether ROSC was achieved, in-hospital mortality, and the final disposition of the patient following the event. All collected data were consolidated into a structured database for subsequent analysis.
2.5. Statistical Analysis
Statistical analysis was conducted using a quantitative, retrospective approach, aiming to describe the clinical outcomes observed during code blue events and to identify potential associations between clinical variables and outcomes. Descriptive statistics were first applied to characterize the cohort. Continuous variables, such as age, were summarized using measures of central tendency and dispersion, specifically the median and interquartile range. Categorical variables, such as sex, initial cardiac rhythm, presence of comorbidities, and clinical outcomes, were presented as absolute frequencies and proportions.
Subsequently, Kaplan–Meier survival curves were generated to analyze the cumulative probability of two primary outcomes: return of spontaneous circulation (ROSC) and in-hospital survival. These curves allowed for the evaluation of how the duration of the code blue event, measured in minutes, correlated with a progressive decline in the probability of achieving these clinical outcomes.
Comparisons between subgroups were conducted using Fisher’s exact test, due to the small sample size and the categorical nature of the variables. These tests were used to compare outcomes based on comorbidity burden (Charlson Comorbidity Index), estimated risk from the RISQ-PATH score, and other clinical variables. In addition, relative risks (RR) with 95% confidence intervals were calculated to estimate the magnitude of observed associations.
To predict ROSC, the validated IHCA-ROSC model was used. This score integrates multiple clinical variables to estimate the adjusted probability of achieving ROSC. Each variable was assigned a value based on the β coefficients reported in the literature, and the corresponding logistic formula was applied. The resulting logit (p) was transformed into a probability using the logistic function. This individual probability was calculated for each patient using an automated system.
2.6. Ethical Approval and Consent to Participate
This study was approved by the Scientific Ethics Committee of the University of Costa Rica on June 16, 2025 (Approval Reference Number: CEC-347-2025). Written informed consent was not required, as no direct interventions were performed on the patients.
3. Results
3.1. Demographic Characteristics, Comorbidities, and Clinical Outcomes
A total of 77 patients who experienced in-hospital code blue events between January 2020 and December 2024 were included in the analysis.
Table 1 summarizes the demographic characteristics and distribution of comorbidities observed in these patients. The median age was 70 years, with an interquartile range of 52–83 years, and 61% of the patients treated for cardiopulmonary arrest during this period were male.
Regarding immediate outcomes, 43 patients (55.84%) achieved return of spontaneous circulation (ROSC), while 34 (44.16%) died during the code blue event. Of the 43 patients who achieved ROSC, 10 (12.99%) were transferred to another healthcare facility, and the remainder were admitted to the intensive care unit (ICU). Among those admitted to the ICU, 17 patients (22.08%) died during their stay, and 16 patients (20.78%) were eventually discharged.
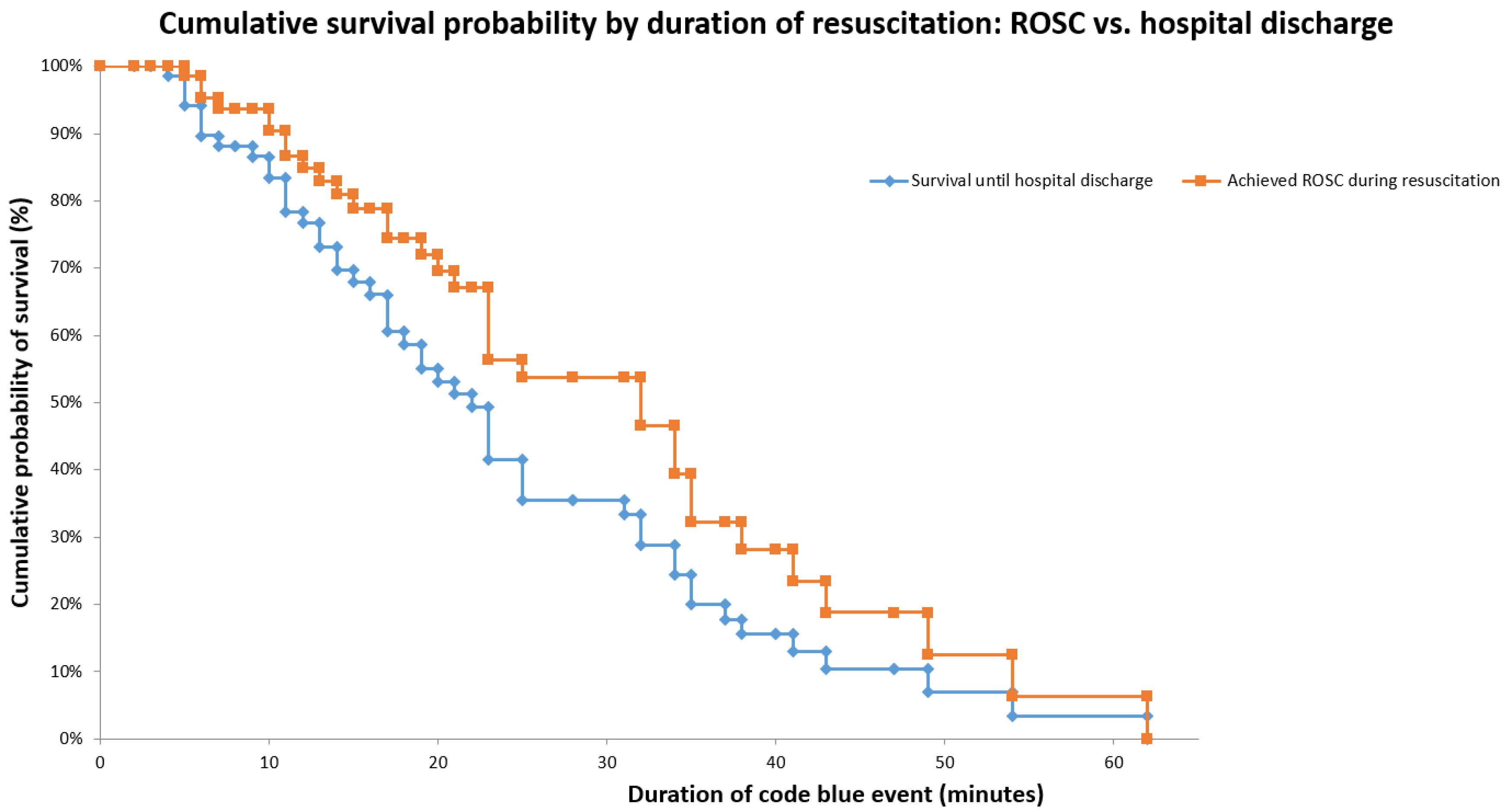
Figure 1 displays two Kaplan–Meier curves illustrating survival among patients who experienced a code blue (n = 77). The survival curve up to ROSC shows that while the initial probability of survival begins at 100%, it progressively declines as the duration of the resuscitation effort increases. A particularly steep drop is observed between minutes 10 and 20, with the curve stabilizing around 53% by minute 30. This decline reflects mortality occurring during resuscitation, underscoring the importance of minimizing code duration to improve survival outcomes.
In contrast, the in-hospital survival curve continues to decline beyond the ROSC curve, following a similar downward trend and reaching approximately 35% at 30 minutes. This progressive decline highlights that, although ROSC is achieved, a significant proportion of patients subsequently die due to complications during hospitalization. These findings demonstrate that achieving ROSC alone is not sufficient to ensure in-hospital survival, emphasizing the critical need for appropriate post-resuscitation care.
3.2. Association Between Comorbidity and Clinical Outcomes
According to the Charlson Comorbidity Index (CCI), the most prevalent conditions among the 77 patients analyzed were: moderate or severe renal disease (25.97%), myocardial infarction (20.78%), diabetes with end-organ damage (18.18%), tumor without metastasis (12.99%), and peptic ulcer disease (11.69%). Based on the CCI scores (Annex S1), 15 patients (19.48%) had a high comorbidity burden, 12 (15.58%) had moderate comorbidity, and 50 (64.94%) had low comorbidity, the latter being the most frequent.
Table 2 summarizes the relationship between the level of comorbidity and the main clinical outcomes.
The frequency of shockable rhythms was higher in the high-comorbidity group (40.0%) compared to the low-comorbidity group (24.0%), although this difference was not statistically significant (p = 0.3235; RR: 0.60; 95% CI: 0.27–1.32). ROSC rates were similar between the two groups (56.0% vs. 53.3%; p > 0.9999; RR: 1.05; 95% CI: 0.62–1.79). In terms of hospital discharge, a significant difference was observed in favor of the low-comorbidity group (65.0%) compared to the high-comorbidity group (14.3%) (p = 0.0329; RR: 4.55; 95% CI: 0.72–28.73).
3.3. Association Between RISQ-PATH and Clinical Outcomes
According to the RISQ-PATH score classification, 38 patients (49.4%) were categorized as low risk and 39 (50.6%) as high risk. The association between this classification and the main clinical outcomes is presented in
Table 3. Baseline clinical characteristics were comparable between the two groups, particularly with respect to age and comorbidities. The proportion of shockable rhythms was low in both groups but slightly higher in the low-risk group (7.89% vs. 2.56%), without reaching statistical significance (p = 0.3584; RR: 3.08; 95% CI: 0.33–28.31).
The ROSC rate was 60.53% in the low-risk group and 51.28% in the high-risk group (p = 0.4936; RR: 1.18; 95% CI: 0.79–1.76), suggesting a non-significant trend toward better outcomes in patients with lower QTc prolongation risk scores.
Notably, when stratifying by initial cardiac rhythm, 23 out of 35 patients (65.7%) in the low-risk group with non-shockable rhythms achieved ROSC, compared to 20 out of 38 (52.6%) in the high-risk group. Although this was not the primary purpose of applying the RISQ-PATH tool, this finding may reflect a clinically meaningful difference in physiological resilience or comorbidity burden—even among patients presenting with non-shockable rhythms.
As shown in
Table 3, only 4 patients presented with shockable rhythms (VT/VF), consistent with the detailed breakdown presented in
Table 4, where asystole was the predominant initial rhythm in 76.6% of cases.
Regarding hospital discharge among patients who achieved ROSC and remained hospitalized, more favorable outcomes were observed in the low-risk group (63.16%) compared to the high-risk group (28.57%) (p = 0.0799; RR: 2.21; 95% CI: 0.90–5.42). While not statistically significant, this trend suggests a potential association that may reach significance in studies with larger sample sizes.
3.4. Association Between IHCA-ROSC and Clinical Outcomes
As summarized in
Table 4, most patients included in the study presented with unfavorable clinical conditions that, according to the IHCA-ROSC model, are associated with a low likelihood of successful resuscitation. Asystole was the predominant initial rhythm (76.6%), and only 5.2% of patients presented with a shockable rhythm (ventricular fibrillation or ventricular tachycardia).
Regarding the context of the event, 33.8% occurred in the emergency department, 28.6% in the ICU, 14.3% on inpatient wards, and 23.4% in procedural areas. A high proportion of urgent admissions was identified (63.6%), along with a considerable level of functional dependence: only 41.6% of patients were functionally independent, while 35.1% were totally dependent. As for primary diagnoses, the majority of patients had no specific diagnosis documented (63.6%), although 10.4% had liver failure or neoplasms. In addition, a relevant proportion of patients had received mechanical ventilation (including both invasive and non-invasive modalities) (20.8%) and vasopressors (39.0%) prior to the event. Taken together, these clinical characteristics contributed to the low probability of successful resuscitation predicted by the IHCA-ROSC model.
Regarding age, patients aged 80 years or older represented 29.9% of the cohort, followed by those aged 70–79 years (22.1%). Older patients showed a significantly lower proportion of ROSC, and none of them survived to hospital discharge.
Based on these predictors, 98.7% of patients were classified as having a low probability of ROSC according to the IHCA-ROSC model. As shown in
Table 5, the mean predicted probability was 5.53%, whereas the actual ROSC rate achieved was 55.84%, with an absolute difference of 50.3 percentage points (p < 0.000001). This finding highlights a marked discrepancy between the predicted and observed outcomes. While this may suggest a favorable clinical performance by the resuscitation team, the small sample size and cohort heterogeneity limit the strength of this interpretation and prevent definitive conclusions.
3.5. Operational Evaluation of the Code Blue Team
Analysis of the procedures executed by the code blue team revealed overall adherence to institutional standards for emergency response. In all recorded events (n = 77), the full multidisciplinary team was present, including medical staff, nursing personnel, pharmacists, and technical support. Each member performed specific roles, contributing to coordinated and efficient care.
All audited records contained complete documentation of critical time points: initiation of chest compressions, drug administration, defibrillation (when applicable), and event resolution. The responsibility for timing and time tracking was fully assumed by the pharmacy service, which also recorded drug administration during resuscitation. In addition to these responsibilities, pharmacists supported pharmacotherapeutic decision-making during the code.
Epinephrine was administered with a median time of 2 minutes from the start of the event and was used in over 94% of cases across all rhythm types. Among patients with ventricular tachycardia (VT) or ventricular fibrillation (VF), amiodarone was administered in 55.6%, consistent with its role as the antiarrhythmic of choice in resuscitation protocols. Lidocaine use was minimal (11.1% in VT/VF), reflecting a deliberate clinical decision, as this drug is readily available at all times within the hospital.
Norepinephrine was used in 41.2% of patients with pulseless electrical activity (PEA) and 38.9% of those with VT/VF. Its use was exclusively reserved for the post-ROSC phase as vasopressor support during post-resuscitation care in the ICU.
Defibrillation was applied promptly in patients with shockable rhythms, and rhythm identification was performed by trained physicians. Regarding chest compressions, the majority were performed by patient care assistants (38.04%), followed by nursing staff (34.90%) and general physicians (10.59%), demonstrating active participation from both technical and professional personnel in basic life support. All members of the resuscitation team, including leaders and support personnel, were certified in Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS), which ensured a rapid and high-quality response during each event.
Ventilation was primarily managed by respiratory therapists (53.25%), followed by nursing staff (32.47%) and anesthesia personnel (10.39%), reflecting an appropriate distribution of responsibilities based on technical competencies. In terms of recording medication administration times, participation beyond the pharmacist was minimal, with nursing staff (1.30%) and general physicians (1.30%) assisting in a few cases. A similar pattern was observed in the documentation of chest compression timing, where nurses were responsible in 5.19% of events, alongside the pharmacist.
Leadership during code events was primarily assumed by physicians from critical care specialties. Intensive care physicians led 33.77% of the codes, followed by internal medicine (29.87%), cardiology (11.69%), and surgery (7.79%). Other specialties that led events included general physicians (5.19%), family and community medicine (2.60%), and with one event each (1.30%), gynecology, emergency medicine, anesthesia, pediatrics, and nursing. Due to this wide variation in team leadership and the limited number of cases per specialty, it is not possible to determine which combination of team composition is most effective in achieving favorable outcomes.
Intravenous (IV) access was the primary route used in 98.7% of cases (n = 76), establishing it as the preferred method for medication and fluid administration. Intraosseous (IO) access was used in only one case (1.3%), reserved for situations where venous access could not be achieved in a timely manner. Advanced airway management via orotracheal intubation was performed in 32.47% of patients (n = 25). In the remaining 63.64% (n = 49), this intubation procedure was not performed, likely due to rapid ROSC, poor prognosis upon arrival of the team, or because adequate ventilation was achieved with the use of a bag-valve-mask without the need for intubation.
4. Discussion
4.1. Clinical Outcomes Observed vs. International Literature
Of the 77 patients who experienced cardiopulmonary arrest, 51 died—this figure includes both deaths during the event and those occurring after ROSC. Therefore, the total mortality in the cohort was 66.23%, which aligns with reported in-hospital mortality rates ranging from 68% to 76% in the literature [
13,
19].
The Charlson Comorbidity Index (CCI), used to assess patients’ comorbidity burden, revealed that the most prevalent conditions were renal disease, myocardial infarction, diabetes with end-organ damage, and non-metastatic tumors. These findings are consistent with previous studies that have shown cardiovascular and renal comorbidities to be significant predictors of mortality and ROSC in in-hospital cardiac arrest [
20,
21,
22].
Regarding the relationship between CCI and clinical outcomes, our results did not show statistically significant differences in ROSC rates between low and high comorbidity groups, which could suggest that the CCI does not always accurately reflect a patient’s likelihood of successful resuscitation. However, studies such as that by Piscator et al. demonstrated that patients with moderate and high CCI scores had a markedly reduced probability of survival compared to those with a low CCI [
14].
The CCI remains a valuable prognostic indicator, although some studies suggest that its ability to predict post-ROSC outcomes may be limited when compared to other factors such as immediate response during the event and early intervention. Evidence indicates that the CCI’s predictive value is more relevant in the short term, serving as a guide for decision-making during resuscitation [
14].
Overall, analysis of the CCI in our cohort shows that, despite similar ROSC rates between low and high comorbidity groups, significant differences were observed in hospital discharge outcomes. This underscores the importance of considering a patient's comorbidity profile not only to predict the immediate success of a code blue but also to guide optimal post-resuscitation care and long-term recovery planning. These considerations are well complemented by using an age-adjusted version of the CCI (ACCI), which has been significantly associated with 30-day survival and supports its prognostic value in early outcomes and clinical decision-making post-resuscitation [
14].
In the analysis of the RISQ-PATH model data, no statistically significant differences were found between low- and high-risk patients in terms of likelihood of presenting with a shockable initial rhythm, achieving ROSC, or being discharged from the hospital. This suggests that in this cohort, the presence of risk factors associated with QTc prolongation did not correlate directly with the emergence of malignant arrhythmias such as Torsades de Pointes (TdP), nor with increased risk of sudden cardiac death [
23].
Moreover, current scientific evidence supports the usefulness of applying this score in hospitalized patients, as it has demonstrated high sensitivity (87–88%) and moderate specificity (44–46%) for detecting a QTc interval ≥ 450 ms in men or ≥ 470 ms in women, and particularly ≥ 500 ms, which is considered high-risk, on baseline ECG. Its integration into clinical decision support systems (CDSS) in various hospital settings is critical for reducing unnecessary alerts and minimizing the incidence of reported adverse events in hospitalized patients [
23].
4.2. Performance higher than estimated by the IHCA-ROSC models
According to the IHCA-ROSC results, 98.7% of patients at Clínica Bíblica were classified as having a low probability of achieving ROSC. Nevertheless, 55.86% of patients successfully achieved ROSC. These findings indicate that code blue management at Clínica Bíblica is both comprehensive and effective, with the interdisciplinary team attaining ROSC rates notably higher than those predicted by validated models based on scoring systems.
However, when compared to the literature, some differences emerge—particularly related to patient populations and the models employed. For instance, Chan and Tang (2020), analyzing 82,279 cases from the Get With The Guidelines–Resuscitation (GWTG-R) registry, reported a ROSC rate of 71.9%. In our study, the observed rate was 55.86%, and this discrepancy may be attributed to several factors: differences in patient demographics and comorbidity profiles, variations in care protocols and team response times, resource availability (equipment and personnel), the time period analyzed (e.g., the influence of the pandemic), and potential data capture biases [
10].
Nevertheless, achieving ROSC does not guarantee survival, as patients may develop post-cardiac arrest complications such as anoxic brain injury, myocardial dysfunction, ischemia-reperfusion-induced systemic inflammatory response, multi-organ failure, and metabolic or electrolyte imbalances. These conditions may result in the development of post-cardiac arrest syndrome (PCAS), one of the most severe complications in this patient population [
24].
Consequently, patients who achieve ROSC often face low chances of hospital discharge. In our dataset, only 33% of all patients survived to hospital discharge following a code blue. This figure is higher than global averages—particularly in countries such as the United States, where overall survival-to-discharge rates after in-hospital cardiac arrest (IHCA) range from 17% to 22.3% [
10,
25].
4.3. Operational Evaluation of the Resuscitation Team
From an operational perspective, the data reported in this study show that the resuscitation team functioned in a structured and interdisciplinary manner in 100% of the observed events. During these code blue activations, pharmacists were responsible for time tracking and ensuring the traceability of medication administration. Physicians—particularly those from critical care specialties—led the event, identified cardiac rhythms, and made decisions regarding medication administration. Nurses were actively involved in chest compressions and drug delivery, while patient care assistants performed compressions. Respiratory therapists led ventilation efforts, supported by nursing staff and anesthesiology personnel.
This in-hospital approach aligns with the International Standards for Hospital Emergency Medical Management Guidelines, which clearly define the key roles of each team member. Specifically, the guidelines emphasize that the physician should lead the team and make clinical decisions; nurses are responsible for continuously monitoring vital signs and administering non-pharmacological treatments while supporting medical procedures under physician supervision. Pharmacists contribute with pharmacotherapy recommendations, ensuring correct dosing and administration of medications during resuscitation. Finally, other team members, such as technicians and support personnel, play a critical role in procedures such as defibrillation and compressions during the event [
26].
4.4. Medication Use Profile During Code Blue Events
Epinephrine was the most frequently used drug, with a mean administration time of 2 minutes from the onset of the event. Lidocaine was used only marginally, and norepinephrine was initiated exclusively after return of spontaneous circulation (ROSC) had been achieved. These findings differ slightly from standard resuscitation protocols, which recommend administering epinephrine every 3–5 minutes for both shockable and non-shockable rhythms. This variation may indicate an opportunity to improve adherence to guidelines; however, it may also reflect the immediate clinical judgment required in each situation, in which epinephrine was deemed the most appropriate medication during the critical moments of the event [
27].
4.5. Additional Improvements to the Code Blue Protocol
A potential improvement following code blue events involves obtaining a laboratory panel that includes arterial blood gas analysis to assess acid–base balance and oxygenation; serum electrolytes (Na⁺, K⁺, Ca²⁺, and Mg²⁺) and glucose, to rule out imbalances that could trigger arrhythmias or hypoglycemia; and lactate, as a marker of tissue perfusion and resuscitation effectiveness. In addition, measuring troponins and natriuretic peptides (BNP or NT-proBNP) helps quantify myocardial injury and guide differential diagnoses such as acute coronary syndrome or endocarditis, while a toxicology panel supports the detection of recreational drugs or medications with proarrhythmic or respiratory depressant potential [
27,
28].
The early integration of these parameters—especially pH and lactate—not only supports the correction of reversible causes but also enables the accurate application of prognostic scores such as NULL-PLEASE, rCAST, MIRACLE₂, CAHP, and TOMAHAWK Risk Score, thereby enhancing the prediction of both in-hospital and out-of-hospital survival, as well as neurological prognosis [
29,
30,
31,
32,
33].
In relation to data collection, the consistent presence of a clinical pharmacist during code blue events is also of critical importance. Studies have shown pharmacist involvement in up to 69.7% of hospitals in the United States during such events (34). Throughout the entire event, the pharmacist contributes to data collection, preparation and administration of medications, compatibility evaluation, and optimal dosing recommendations. They also advise the team on correct drug usage and provide key information for real-time adjustments. This comprehensive approach enhances patient safety, helps reduce mortality, and improves the efficiency of hospital resources [
34,
35].
Moreover, in the post-cardiac arrest setting, therapeutic hypothermia has been shown to provide not only neurological protection but also reduced mortality in patients with an initial shockable rhythm. However, both AHA and European guidelines agree that the current evidence is not yet robust enough to support routine use. Therefore, its application should be assessed on a case-by-case basis, weighing the clinical profile and individual circumstances of each patient [
36].
4.6. Study Limitations
This study presents several limitations that should be acknowledged. First, its retrospective design exposes the analysis to underreporting and information bias, which may compromise the accuracy of the findings. Second, the sample size was small and the patient population was heterogeneous in terms of age, comorbidities, and arrest severity, limiting the external validity and comparability of the results.
Moreover, due to the limited number of code blue cases analyzed and the variability in team composition, it is not possible to draw definitive conclusions about the clinical performance of the resuscitation team or to confirm that outcomes were substantially better than those predicted by prognostic models.
Additionally, the quality of resuscitation maneuvers and the number of CPR cycles administered were not quantified—factors that are critical to protocol success and whose absence hinders the accurate interpretation of intervention effectiveness.
Furthermore, the lack of key data such as height, weight, lactate levels, and arterial blood gases prevented the evaluation of important risk variables (e.g., obesity and acidosis) and precluded the use of complementary prognostic scores. The study also had limited geographic coverage within Latin America, which constrains the generalizability of the findings to the broader region.
Finally, in the Kaplan–Meier survival analysis, transferred patients were treated as censored data due to the absence of post-transfer follow-up information; this methodological decision was made to more accurately reflect observed in-hospital survival.
Taken together, these data gaps and methodological limitations underscore the need for future prospective, multicenter studies that incorporate standardized resuscitation protocols and systematic collection of clinical and laboratory variables in order to generate more robust and generalizable evidence.
5. Conclusions
In this analysis of code blue events, the most frequent comorbidities were renal disease, myocardial infarction, diabetes with end-organ damage, and non-metastatic neoplasms. A higher comorbidity burden was associated with an increased incidence of post-resuscitation complications. Stratification using the RISQ-PATH score did not reveal significant differences between low- and high-risk patients in terms of shockable rhythms, ROSC achievement, or hospital discharge.
At Hospital Clínica Bíblica, a ROSC rate of 55.86% and a hospital discharge rate of 21% were achieved, with structured adherence to the multidisciplinary protocol in 100% of events and earlier administration of epinephrine compared to what is typically reported in the literature. Finally, we identified opportunities for improvement in the immediate post-event collection of laboratory tests, the expansion of clinical pharmacist-led data documentation, and the systematic implementation of therapeutic hypothermia in patients presenting with shockable rhythms.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Annex S1
Author Contributions
Research J.M.-J, A.F.-M and K.C.-M.; Methodology: E.Z.-M., A.F.-M, J.C.-F, and G.F.-A.; Project management: E.Z.-M, A.F-M, J.C-.F and G.F.-A.; Supervision: E.Z.-M., J.D.-M., and A.F.-M.; Verification: A.F.-M., E.Z.- M., J.M.-J., and K.C.-M.; validation, Writing—original draft: J.M.-J, A.F-M and K.C-M.; Writing—review and editing: J.M.-J, A.F-M and K.C.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions Clínica Bíblica.
Acknowledgments
The authors would like to thank Dr. Arguedas, Dra. Ortiz, and Dra. Carrillo for their valuable guidance and support throughout the development of this study. Their clinical insight and encouragement were instrumental in shaping the analysis and final interpretation of the findings. We also acknowledge the administrative and academic support provided by Clínica Bíblica and the University of Costa Rica.
Conflicts of Interest
The authors declare no conflicts of interest
Abbreviations
The following abbreviations are used in this manuscript:
| ACCI |
Age-Combined Charlson Comorbidity Index |
| AHA |
American Heart Association |
| BNP |
Brain Natriuretic Peptide |
| CAHP |
Cardiac Arrest Hospital Prognosis |
| CCI |
Charlson Comorbidity Index |
| CDSS |
Clinical Decision Support Systems |
| CI |
Confidence Interval |
| CPR |
Cardiopulmonary Resuscitation |
| ECG |
Electrocardiogram |
| ICU |
Intensive Care Unit |
| IHCA |
In-Hospital Cardiac Arrest |
| IHCA-ROSC |
In-Hospital Cardiac Arrest Return of Spontaneous Circulation Score |
| IO |
Intraosseous |
| IV |
Intravenous |
| JCI |
Joint Commission International |
| PCAS |
Post-Cardiac Arrest Syndrome |
| QTc |
Corrected QT Interval |
| RISQ-PATH |
Risk Score for QTc Prolongation |
| ROSC |
Return of Spontaneous Circulation |
| TdP |
Torsades de Pointes |
| VF |
Ventricular Fibrillation |
| VT |
Ventricular Tachycardia |
References
- Leo, W.Z.; Chua, D.; Tan, H.C.; Ho, V.K. Chest Compression Quality and Patient Outcomes with the Use of a CPR Feedback Device: A Retrospective Study. Sci. Rep. 2023, 13, 19852. [Google Scholar] [CrossRef]
- Meaney, P.A.; Bobrow, B.J.; Mancini, M.E.; Christenson, J.; De Caen, A.R.; Bhanji, F.; Abella, B.S.; Kleinman, M.E.; Edelson, D.P.; Berg, R.A.; et al. Cardiopulmonary Resuscitation Quality: Improving Cardiac Resuscitation Outcomes Both Inside and Outside the Hospital: A Consensus Statement From the American Heart Association. Circulation 2013, 128, 417–435. [Google Scholar] [CrossRef]
- Nallamothu, B.K.; Guetterman, T.C.; Harrod, M.; Kellenberg, J.E.; Lehrich, J.L.; Kronick, S.L.; Krein, S.L.; Iwashyna, T.J.; Saint, S.; Chan, P.S. How Do Resuscitation Teams at Top-Performing Hospitals for In-Hospital Cardiac Arrest Succeed?: A Qualitative Study. Circulation 2018, 138, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Abu Fraiha, Y.; Shafat, T.; Codish, S.; Frenkel, A.; Dolfin, D.; Dreiher, J.; Konstantino, Y.; Abu Abed, S.; Schwartz, D.; Fichman, A.; et al. Outcomes of In-Hospital Cardiac Arrest Managed with and without a Specialized Code Team: A Retrospective Observational Study. PLOS ONE 2024, 19, e0309376. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, S.; Johansson, A.C.; Tschan, F.; Semmer, N.K.; Rock, L.; Howell, M.D.; Marsch, S. Teamwork and Leadership in Cardiopulmonary Resuscitation. J. Am. Coll. Cardiol. 2011, 57, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Morriello, F.; Quirion, J.; Yee, H.; Narendrula, R. Empowering Senior Medical Residents as Resuscitation Team Leaders. BMC Med. Educ. 2025, 25, 662. [Google Scholar] [CrossRef]
- Cheng, A.; Magid, D.J.; Auerbach, M.; Bhanji, F.; Bigham, B.L.; Blewer, A.L.; Dainty, K.N.; Diederich, E.; Lin, Y.; Leary, M.; et al. Part 6: Resuscitation Education Science: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142. [Google Scholar] [CrossRef]
- Spitzer, C.R.; Evans, K.; Buehler, J.; Ali, N.A.; Besecker, B.Y. Code Blue Pit Crew Model: A Novel Approach to in-Hospital Cardiac Arrest Resuscitation. Resuscitation 2019, 143, 158–164. [Google Scholar] [CrossRef]
- Dukes, K.; Bunch, J.L.; Chan, P.S.; Guetterman, T.C.; Lehrich, J.L.; Trumpower, B.; Harrod, M.; Krein, S.L.; Kellenberg, J.E.; Reisinger, H.S.; et al. Assessment of Rapid Response Teams at Top-Performing Hospitals for In-Hospital Cardiac Arrest. JAMA Intern. Med. 2019, 179, 1398. [Google Scholar] [CrossRef]
- Chan, P.S.; Tang, Y. ; for the American Heart Association’s Get With the Guidelines-Resuscitation Investigators Risk-Standardizing Rates of Return of Spontaneous Circulation for In-Hospital Cardiac Arrest to Facilitate Hospital Comparisons. J. Am. Heart Assoc. 2020, 9, e014837. [Google Scholar] [CrossRef]
- Okubo, M.; Komukai, S.; Andersen, L.W.; Berg, R.A.; Kurz, M.C.; Morrison, L.J.; Callaway, C.W. Duration of Cardiopulmonary Resuscitation and Outcomes for Adults with In-Hospital Cardiac Arrest: Retrospective Cohort Study. BMJ 2024, e076019. [Google Scholar] [CrossRef]
- Cagino, L.M.; Moskowitz, A.; Nallamothu, B.K.; McSparron, J.; Iwashyna, T.J.; Grossestreuer, A.; Moskowitz, A.; Edelson, D.; Ornato, J.; Ann Peberdy, M.; et al. Trends in Return of Spontaneous Circulation and Survival to Hospital Discharge for In–Intensive Care Unit Cardiac Arrests. Ann. Am. Thorac. Soc. 2023, 20, 1012–1019. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar] [CrossRef]
- Piscator, E.; Hedberg, P.; Göransson, K.; Djärv, T. Survival after In-Hospital Cardiac Arrest Is Highly Associated with the Age-Combined Charlson Co-Morbidity Index in a Cohort Study from a Two-Site Swedish University Hospital. Resuscitation 2016, 99, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Grandbois Van Ravenhorst, C.; Schluep, M.; Endeman, H.; Stolker, R.-J.; Hoeks, S.E. Prognostic Models for Outcome Prediction Following In-Hospital Cardiac Arrest Using Pre-Arrest Factors: A Systematic Review, Meta-Analysis and Critical Appraisal. Crit. Care 2023, 27, 32. [Google Scholar] [CrossRef]
- Morrison, L.J.; Neumar, R.W.; Zimmerman, J.L.; Link, M.S.; Newby, L.K.; McMullan, P.W.; Hoek, T.V.; Halverson, C.C.; Doering, L.; Peberdy, M.A.; et al. Strategies for Improving Survival After In-Hospital Cardiac Arrest in the United States: 2013 Consensus Recommendations.
- Schnaubelt, S.; Monsieurs, K.G.; Semeraro, F.; Schlieber, J.; Cheng, A.; Bigham, B.L.; Garg, R.; Finn, J.C.; Greif, R.; Bray, J.; et al. Clinical Outcomes from Out-of-Hospital Cardiac Arrest in Low-Resource Settings — A Scoping Review. Resuscitation 2020, 156, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.M.; Cheng, A.; Panchal, A.R.; Topjian, A.A.; Aziz, K.; Bhanji, F.; Bigham, B.L.; Hirsch, K.G.; Hoover, A.V.; Kurz, M.C.; et al. Part 7: Systems of Care: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142. [Google Scholar] [CrossRef] [PubMed]
- Schluep, M.; Hoeks, S.E.; Blans, M.; Van Den Bogaard, B.; Koopman-van Gemert, A.; Kuijs, C.; Hukshorn, C.; Van Der Meer, N.; Knook, M.; Van Melsen, T.; et al. Long-Term Survival and Health-Related Quality of Life after in-Hospital Cardiac Arrest. Resuscitation 2021, 167, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.M.; Tran, A.; Cheng, W.; Rochwerg, B.; Taljaard, M.; Vaillancourt, C.; Rowan, K.M.; Harrison, D.A.; Nolan, J.P.; Kyeremanteng, K.; et al. Pre-Arrest and Intra-Arrest Prognostic Factors Associated with Survival after in-Hospital Cardiac Arrest: Systematic Review and Meta-Analysis. BMJ 2019, l6373. [Google Scholar] [CrossRef]
- Israelsson, J.; Koistinen, L.; Årestedt, K.; Rooth, M.; Bremer, A. Associations between Comorbidity and Health-Related Quality of Life among in-Hospital Cardiac Arrest Survivors – A Cross-Sectional Nationwide Registry Study. Resuscitation 2023, 188, 109822. [Google Scholar] [CrossRef]
- Nolan, J.P.; Berg, R.A.; Andersen, L.W.; Bhanji, F.; Chan, P.S.; Donnino, M.W.; Lim, S.H.; Ma, M.H.-M.; Nadkarni, V.M.; Starks, M.A.; et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Template for In-Hospital Cardiac Arrest: A Consensus Report From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Circulation 2019, 140. [Google Scholar] [CrossRef]
- Vandael, E.; Vandenberk, B.; Vandenberghe, J.; Van den Bosch, B.; Willems, R.; Foulon, V. A Smart Algorithm for the Prevention and Risk Management of QTc Prolongation Based on the Optimized RISQ-PATH Model. Br. J. Clin. Pharmacol. 2018, 84, 2824–2835. [Google Scholar] [CrossRef]
- Lazzarin, T.; Tonon, C.R.; Martins, D.; Fávero, E.L.; Baumgratz, T.D.; Pereira, F.W.L.; Pinheiro, V.R.; Ballarin, R.S.; Queiroz, D.A.R.; Azevedo, P.S.; et al. Post-Cardiac Arrest: Mechanisms, Management, and Future Perspectives. J. Clin. Med. 2022, 12, 259. [Google Scholar] [CrossRef]
- Girotra, S.; Nallamothu, B.K.; Spertus, J.A.; Li, Y.; Krumholz, H.M.; Chan, P.S. Trends in Survival after In-Hospital Cardiac Arrest. N. Engl. J. Med. 2012, 367, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Peberdy, M.A.; Cretikos, M.; Abella, B.S.; DeVita, M.; Goldhill, D.; Kloeck, W.; Kronick, S.L.; Morrison, L.J.; Nadkarni, V.M.; Nichol, G.; et al. Recommended Guidelines for Monitoring, Reporting, and Conducting Research on Medical Emergency Team, Outreach, and Rapid Response Systems: An Utstein-Style Scientific Statement: A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiopulmonary, Perioperative, and Critical Care; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Resuscitation 2007, 75, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Marino, B.S.; Tabbutt, S.; MacLaren, G.; Hazinski, M.F.; Adatia, I.; Atkins, D.L.; Checchia, P.A.; DeCaen, A.; Fink, E.L.; Hoffman, G.M.; et al. Cardiopulmonary Resuscitation in Infants and Children With Cardiac Disease. Circulation 2018. [Google Scholar] [CrossRef] [PubMed]
- Stiles, M.K.; Wilde, A.A.M.; Abrams, D.J.; Ackerman, M.J.; Albert, C.M.; Behr, E.R.; Chugh, S.S.; Cornel, M.C.; Gardner, K.; Ingles, J.; et al. 2020 APHRS/HRS Expert Consensus Statement on the Investigation of Decedents with Sudden Unexplained Death and Patients with Sudden Cardiac Arrest, and of Their Families. Heart Rhythm 2021, 18, e1–e50. [Google Scholar] [CrossRef]
- Byrne, C.; Barcella, C.A.; Krogager, M.L.; Pareek, M.; Ringgren, K.B.; Andersen, M.P.; Mills, E.H.A.; Wissenberg, M.; Folke, F.; Gislason, G.; et al. External Validation of the Simple NULL-PLEASE Clinical Score in Predicting Outcomes of out-of-Hospital Cardiac Arrest in the Danish Population - A Nationwide Registry-Based Study. Resuscitation 2022, 180, 128–136. [Google Scholar] [CrossRef]
- Kim, N.; Kitlen, E.; Garcia, G.; Khosla, A.; Miller, P.E.; Johnson, J.; Wira, C.; Greer, D.M.; Gilmore, E.J.; Beekman, R. Validation of the rCAST Score and Comparison to the PCAC and FOUR Scores for Prognostication after Out-of-Hospital Cardiac Arrest. Resuscitation 2023, 188, 109832. [Google Scholar] [CrossRef]
- Aldous, R.; Roy, R.; Cannata, A.; Abdrazak, M.; Mohanan, S.; Beckley-Hoelscher, N.; Stahl, D.; Kanyal, R.; Kordis, P.; Sunderland, N.; et al. MIRACLE2 Score Compared With Downtime and Current Selection Criterion for Invasive Cardiovascular Therapies After OHCA. JACC Cardiovasc. Interv. 2023, 16, 2439–2450. [Google Scholar] [CrossRef]
- Sauneuf, B.; Dupeyrat, J.; Souloy, X.; Leclerc, M.; Courteille, B.; Canoville, B.; Ramakers, M.; Goddé, F.; Beygui, F.; du Cheyron, D.; et al. The CAHP (Cardiac Arrest Hospital Prognosis) Score: A Tool for Risk Stratification after out-of-Hospital Cardiac Arrest in Elderly Patients. Resuscitation 2020, 148, 200–206. [Google Scholar] [CrossRef]
- Thevathasan, T.; Spoormans, E.; Akin, I.; Fuernau, G.; Tebbe, U.; Haeusler, K.G.; Oeff, M.; Hassager, C.; Fichtlscherer, S.; Zeymer, U.; et al. Early Risk Stratification of Patients After Successfully Resuscitated Out-of-Hospital Cardiac Arrest Without ST-Segment Elevation-The Angiography After Out-of-Hospital Cardiac Arrest Without ST-Segment Elevation (TOMAHAWK) Risk Score. Crit. Care Explor. 2025, 7, e1221. [Google Scholar] [CrossRef]
- Bond, C.A.; Raehl, C.L. Clinical Pharmacy Services, Pharmacy Staffing, and Hospital Mortality Rates. Pharmacotherapy 2007, 27, 481–493. [Google Scholar] [CrossRef]
- McAllister, M.W.; Chestnutt, J.G. Improved Outcomes and Cost Savings Associated With Pharmacist Presence in the Emergency Department. Hosp. Pharm. 2017, 52, 433–437. [Google Scholar] [CrossRef]
- Meta-Analysis Comparing Hypothermia Versus Normothermia in Patients After a Cardiac Arrest. Am. J. Cardiol. 2023, 201, 158–165. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).