Submitted:
18 August 2025
Posted:
20 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Demographic and Clinicopathological Features
3.3. Histological Findings
3.4. Estrogen Receptors (ER) /Progesterone Receptors (PR) /HER-2 Status
3.5. Treatment
3.6. Outcome
3.5. Patient Survival
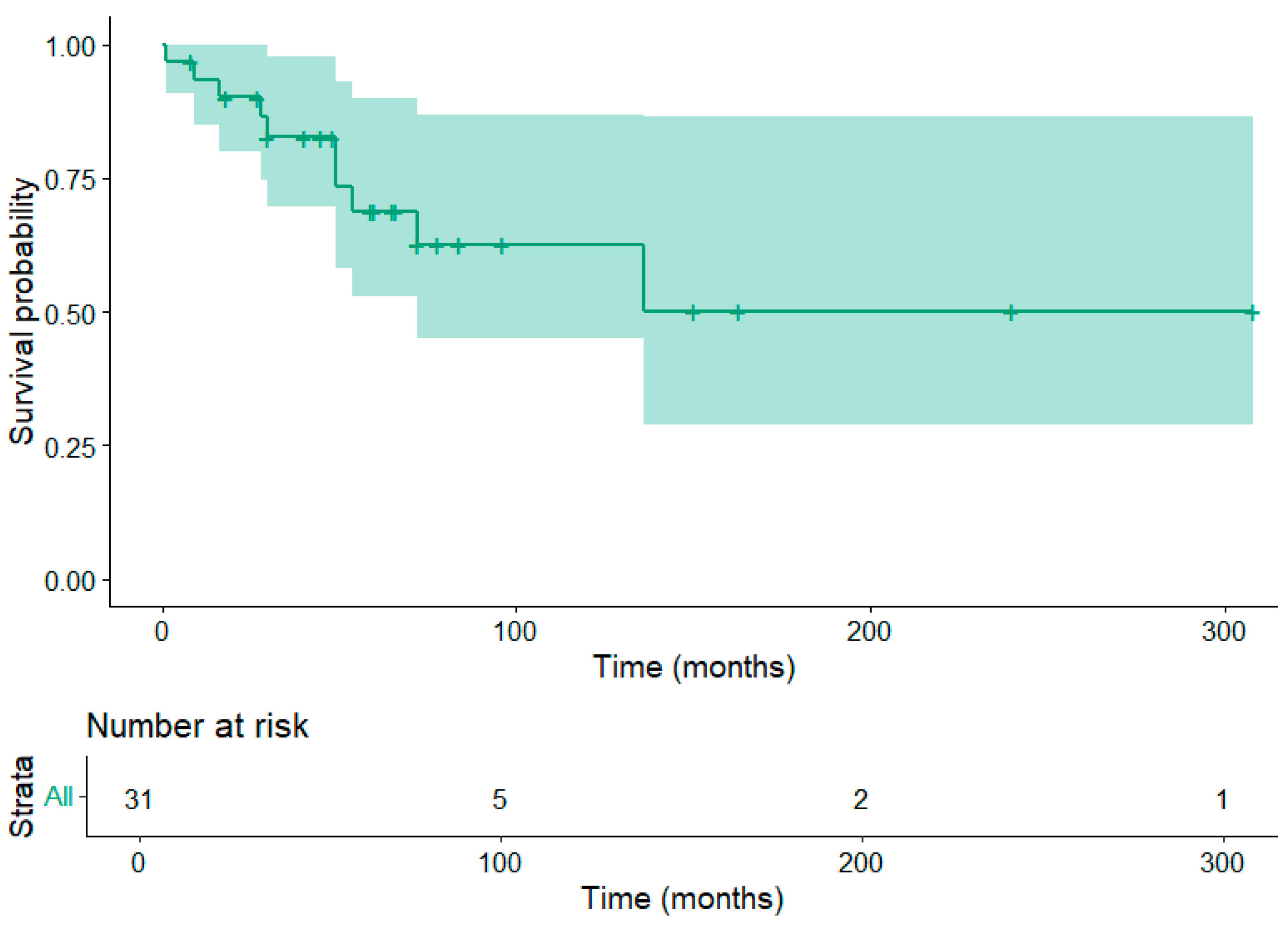
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ellis, I.; Galea, M.; Broughton, N.; Locker, A.; Blamey, R.; Elston, C.: Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 1992, 20, 479-489. [CrossRef]
- Foote Jr, F.W.; Stewart, F.W.: Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol. 1941, 17, 491. [CrossRef]
- Koufopoulos, N.; Pateras, I.S.; Gouloumis, A.R.; Ieronimaki,A.I.;, Zacharatou, A.; Spathis, A.; Leventakou, D.; Economopoulou, P.; Psyrri, A.; Arkadopoulos, N.; Panayiotides I.G.: Diagnostically Challenging Subtypes of Invasive Lobular Carcinomas: How to Avoid Potential Diagnostic Pitfalls. Diagnostics 2022, 12, 2658. [CrossRef]
- Reed, A.E.M.; Kutasovic, J.R.; Lakhani, S.R.; Simpson, P.T.: Invasive lobular carcinoma of the breast: morphology, biomarkers and’omics. Breast Cancer Res. 2015, 17, 1-11. [CrossRef]
- Arpino, G.; Bardou, V.J.; Clark, G.M.; Elledge, R.M.: Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004, 6, 1-8. [CrossRef]
- Mathew, A.; Rajagopal, P.S.; Villgran, V.; Sandhu, G.S.; Jankowitz, R.C.; Jacob, M.; Rosenzweig, M.; Oesterreich, S.; Brufsky, A.: Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkund. 2017, 77, 660-666. [CrossRef]
- Inoue, M.; Nakagomi, H.; Nakada, H.; Furuya, K.; Ikegame, K.; Watanabe, H.; Omata, M.; Oyama, T.: Specific sites of metastases in invasive lobular carcinoma: a retrospective cohort study of metastatic breast cancer. Breast Cancer. 2017, 24, 667-672. [CrossRef]
- Kioleoglou, Z.; Georgaki, E.; Koufopoulos, N.; Kostek, O.; Volakakis, N.; Dimitriadou, A.; Kokkali, S.: Gastrointestinal metastases from lobular breast carcinoma: a literature review. Cureus. 2024, 16, e65852. [CrossRef]
- de Leeuw, W.J.; Berx, G.; Vos, C.B.; Peterse, J.L.; Van de Vijver, M.J.; Litvinov, S.; Van Roy, F.; Cornelisse, C.J.; Cleton-Jansen, A.M.: Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol. 1997, 183, 404-411. [CrossRef]
- Martinez, V.; Azzopardi, J.: Invasive lobular carcinoma of the breast: incidence and variants. Histopathology. 1979, 3, 467-488. [CrossRef]
- Fisher, E.R.; Gregorio, R.M.; Redmond, C.; Fisher, B.: Tubulolobular invasive breast cancer: a variant of lobular invasive cancer. Hum Pathol. 1977, 8, 679-683. [CrossRef]
- Fechner RE: Histologic variants of infiltrating lobular carcinoma of the breast. Hum Pathol. 1975, 6, 373-378. [CrossRef]
- Koufopoulos, N.I.; Boutas, I.; Pouliakis, A.; Samaras M.G.; Kotanidis, C.; Kontogeorgi, A.; Dimas, D.T.; Ieronimaki, A.I.; Leventakou, D.; Spathis, A.; Zanelli, M.; Palicelli, A.; Zizzo, M.; Goutas, D.; Pateras, I.S.; Panayiotides, I.G.: The “forgotten” subtypes of breast carcinoma: a systematic review of selected histological variants not included or not recognized as distinct entities in the current World Health Organization classification of breast tumors. Int J of Mol Sci. 2024, 25, 8382. [CrossRef]
- Eusebi, V.; Magalhaes, F.; Azzopardi, J.G.: Pleomorphic lobular carcinoma of the breast: an aggressive tumor showing apocrine differentiation. Hum Pathol. 1992, 23, 655-662. [CrossRef]
- Christgen, M.; Steinemann, D.; Kühnle, E.; Länger, F.; Gluz, O.; Harbeck, N.; Kreipe, H.: Lobular breast cancer: Clinical, molecular and morphological characteristics. Pathol Res Pract. 2016, 212, 583-597. [CrossRef]
- Aranda, F.I.; Laforga, J.B.; Martinez, M.A.: Metastasis from breast lobular carcinoma to an endometrial polyp Report of a case with immunohistochemical study. Acta Obstet Gynecol Scand. 1993, 72, 585-587. [CrossRef]
- Sugiyama, T.; Toyoda, N.; Nose, J.; Kihira, N.; Ando, Y.; Ishihara, A.: Breast cancer metastatic to uterine leiomyoma: A case report. J Obstet Gynaecol. 1995, 21, 349-355. [CrossRef]
- Menzin, A.W.; De Risi, D.; Smilari, T.F.; Kalish, P.E.; Vinciguerra, V.: Lobular breast carcinoma metastatic to the vulva: a case report and literature review. Gynecol Oncol. 1998, 69, 84-88. [CrossRef]
- Arnould, L.; Franco, N.; Soubeyrand, M.S.; Mege, F.; Belichard, C.; Lizard-Nacol, S.; Collin, F.: Breast carcinoma metastasis within granulosa cell tumor of the ovary: morphologic, immunohistologic, and molecular analyses of the two different tumor cell populations. Hum Pathol. 2002, 33, 445-448. [CrossRef]
- Alvarez, C.; Ortiz-Rey, J.; Estévez, F.; De La Fuente, A.: Metastatic lobular breast carcinoma to an endometrial polyp diagnosed by hysteroscopic biopsy. Obstet Gynecol. 2003, 102, 1149-1151.
- Ogino, A.; Nomizu, T.; Gonnda, K.; Okouchi, C.; Sakuma, T.; Yamada, M.; Katagata, N.; Watanabe, F.; Yamaguchi, Y.; Yoshida, T.: A case of breast cancer metastasizing to cervix after resection of pancreatic metastasis. Breast Cancer. 2003, 10, 284-288. [CrossRef]
- Rau, A.R.; Saldanha, P.; Raghuveer, C.: Metastatic lobular mammary carcinoma diagnosed in cervicovaginal smears: a case report. Diagn Cytopathol. 2003, 29, 300-302. [CrossRef]
- Blecharz, P.; Szpor, J.; Karolewski, K.; Ryś, J.: Breast cancer metastases to uterine leiomyomas-a clinical and patomorphological analysis of two cases. Nowotwory J Oncol. 2004, 54, 488-488.
- Famoriyo, A.; Sawant, S.; Banfield, P.J.: Abnormal uterine bleeding as a presentation of metastatic breast disease in a patient with advanced breast cancer on tamoxifen therapy. Arch Gynecol Obstet. 2004, 270, 192-193. [CrossRef]
- Sheen-Chen, S.M.; Eng, H.L.; Huang, C.C.: Breast cancer metastatic to the vulva. Gynecol Oncol. 2004, 94, 858-860. [CrossRef]
- Al-Brahim, N.; Elavathil, L.J.: Metastatic breast lobular carcinoma to tamoxifen-associated endometrial polyp: case report and literature review. Ann Diagn Pathol. 2005, 9, 166-168. [CrossRef]
- Lee, T.F.; Wang, Y.L.; Wei, T.S.; Chen, C.P.: Incidental detection of metastatic lobular breast carcinoma in the female internal genital organs 2 years following modified radical mastectomy. Taiwan J Obstet Gynecol. 2005, 44, 368-371. doi: 10.1016/S1028-4559(09)60175-3.
- Scopa, C.D.; Aletra, C.; Lifschitz-Mercer, B.; Czernobilsky, B.: Metastases of breast carcinoma to the uterus. Report of two cases, one harboring a primary endometrioid carcinoma, with review of the literature. Gynecol Oncol. 2005, 96, 543-547. [CrossRef]
- Chen, P.; Hu, W.M.; Wang, P.H.; Suen, J.H.: Recurrent breast cancer presents as a single solid ovarian mass and ascites. Taiwan J Obstet Gynecol. 2006, 45, 356-359. [CrossRef]
- Erkanli, S.; Kayaselcuk, F.; Kuscu, E.; Bolat, F.; Sakalli, H.; Haberal, A.: Lobular carcinoma of the breast metastatic to the uterus in a patient under adjuvant anastrozole therapy. Breast. 2006, 15, 558-561. [CrossRef]
- Perišić, D.; Jančić, S.; Kalinović, D.; Čekerevac, M.: Metastasis of lobular breast carcinoma to the cervix. J Obstet Gynaecol Res. 2007, 33, 578-580. [CrossRef]
- Manci, N.; Marchetti, C.; Esposito, F.; Graziano, M.; Tomao, F.; Pastore, M.; Bellati, F.; Panici, P.B.: Late breast cancer recurrence to the uterine cervix with a review of the literature. Int J Gynecol Pathol. 2008, 27, 113-117. [CrossRef]
- Manipadam, M.; Walter, N.; Selvamani, B.: Lobular carcinoma metastasis to endometrial polyp unrelated to tamoxifen: Report of a case and review of the literature. APMIS. 2008, 116, 538-540. [CrossRef]
- Bogliolo, S.; Morotti, M.; Valenzano Menada, M.; Fulcheri, E.; Musizzano, Y.; Casabona, F.: Breast cancer with synchronous massive metastasis in the uterine cervix: a case report and review of the literature. Arch Gynecol Obstet. 2010, 281, 769-773. [CrossRef]
- Ustaalioglu, B.B.; Bilici, A.; Seker, M.; Salman, T.; Gumus, M.; Barisik, N.O.; Salepci, T.; Yaylaci, M.: Metastasis of lobular breast carcinoma to the uterus in a patient under anastrozole therapy. Oncologie. 2009, 32, 424-426. [CrossRef]
- Engelstaedter, V.; Mylonas, I.: Lower genital tract metastases at time of first diagnosis of mammary invasive lobular carcinoma. Arch Gynecol Obstet. 2011, 283, 93-95. [CrossRef]
- Hooker, A.; Radder, C.; van De Wiel, B.; Geenen, M.: Metastasis from breast cancer to an endometrial polyp; treatment options and follow-up. Report of a case and review of the literature. Eur J Gynaecol Oncol. 2011, 32, 228-230.
- Işçi, H.; Güdücü, N.; Basgul, A.; Aydınlı, K.; Calay, Z.; Dünder, I.: Lobular carcinoma of the breast metastasızıng to leiomyoma in a patient under letrozole treatment. Eur J Gynaecol Oncol. 2011, 32, 560-562.
- Horikawa, M.; Mori, Y.; Nagai, S.; Tanaka, S.; Saito, S.; Okamoto, T.: Metastatic breast cancer to the uterine cervix mimicking a giant cervical leiomyoma. Nagoya J Med Sci. 2012, 74, 347.
- Komeda, S.; Furukawa, N.; Kasai, T.; Washida, A.; Kobayashi, H.: Uterine metastasis of lobular breast cancer during adjuvant letrozole therapy. J Obstet Gynaecol. 2013, 33, 100-101. [CrossRef]
- Vicioso, L.; Ortega, M.V.; Cívico, V.; López-Beltrán, A.: Synchronous metastasis from lobular carcinoma and primary carcinoma of the endometrium in a patient after tamoxifen therapy. Int J Gynecol Pathol. 2013, 32, 66-70. [CrossRef]
- Alligood-Percoco, N.R.; Kessler, M.S.; Willis, G.: Breast cancer metastasis to the vulva 20 years remote from initial diagnosis: a case report and literature review. Gynecol Oncol Rep. 2015, 13, 33-35. [CrossRef]
- Bezpalko, K.; Mohamed, M.A.; Mercer, L.; McCann, M.; Elghawy, K.; Wilson, K.: Concomitant endometrial and gallbladder metastasis in advanced multiple metastatic invasive lobular carcinoma of the breast: a rare case report. Int J Surg Case Rep. 2015, 14, 141-145. [CrossRef]
- Lokadasan, R.; Ratheesan, K.; Sukumaran, R.; Nair, S.P.: Metastatic lobular carcinoma of breast mimics primary cervix carcinoma: two case reports and a review of the literature. Ecancermedicalscience. 2015, 9. [CrossRef]
- Toyoshima, M.; Iwahashi, H.; Shima, T.; Hayasaka, A.; Kudo, T.; Makino, H.; Igeta, S.; Matsuura, R.; Ishigaki, N.; Akagi, K.; Sakurada, J.; Suzuki, H.; Yoshinaga, K.: Solitary uterine metastasis of invasive lobular carcinoma after adjuvant endocrine therapy: a case report. J Med Case Rep. 2015, 9, 1-4. [CrossRef]
- Waks, A.G.; Lennon, J.; Yadav, B.S.; Hwang, H.; dSchapirael Carmen, M.; Johnson, N.B.; Reynolds, K.; Schapira, L.; Gilman, P.B.; Overmoyer, B.: Metastasis to the Cervix Uteri 15 Years After Treatment of Lobular Carcinoma of the Breast. Semin Oncol. 2015 42, e81-94. [CrossRef]
- Lai, M.J.; Lai, C.L.; Huang, I.H.; Yu, J.C.; Lee, H.S.; Dai, M.S.: Synchronous endometrial and gastric metastases of invasive lobular breast carcinomas. Taiwan J Obstet Gynecol. 2016, 55, 131-134. [CrossRef]
- Makris, G.M.; Marinelis, A.; Battista, M.J.; Chrelias, C.; Papantoniou, N.: An ovarian mass after breast cancer: Metachronous carcinoma or metastasis? A case report. Int J Ssurg Case Rep. 2017, 31, 106-108. [CrossRef]
- Martinez, M.R.; Marazuela, M.A.; Vallejo, M.R.; Bernabeu, R.Á.; Medina, T.P.: Metastasis of lobular breast cancer to endometrial polyps with and without the presence of vaginal bleeding. Int J Gynecol Obstet. 2016, 134, 101-102. [CrossRef]
- Akhtar, A.; Ratra, A.; Puckett, Y.; Sheikh, A.B.; Ronaghan, C.A.: Synchronous uterine metastases from breast cancer: case study and literature review. Cureus. 2017, 9, e1840. [CrossRef]
- Bennett, J.A.; Young, R.H.; Chuang, A.Y.; Lerwill, M.F.: Ovarian metastases of breast cancers with signet ring cells: a report of 17 cases including 14 Krukenberg tumors. Int J Gynecol Pathol. 2018, 37, 507-515. [CrossRef]
- Razia, S.; Nakayama, K.; Tsukao, M.; Nakamura, K.; Ishikawa, M.; Ishibashi, T.; Ishikawa, N.; Sanuki, K.; Yamashita, H.; Ono, R.; Hossain, M.M.; Minamoto, T.; Kyo, S.: Metastasis of breast cancer to an endometrial polyp, the cervix and a leiomyoma: A case report and review of the literature. Oncol Lett. 2017, 14, 4585-4592. [CrossRef]
- Seo, S.O.; Shin, J.Y.; Ji, Y.I.: Metastatic uterine cancer looking as cervical fibroid in recurrent breast cancer woman: a case report. Obstet Gynecol Sci. 2017, 60, 481-484. [CrossRef]
- Aytekin, A.; Bilgetekin, I.; Ciltas, A.; Ogut, B.; Coskun, U.; Benekli, M.: Lobular breast cancer metastasis to uterus during adjuvant tamoxifen treatment: A case report and review of the literature. J Cancer Res Ther. 2018, 14, 1135-1137. [CrossRef]
- Briki, R.; Cherif, O.; Bannour, B.; Hidar, S.; Boughizane, S.; Khairi, H.: Uncommon metastases of invasive lobular breast cancer to the endometrium: a report of two cases and review of the literature. Pan Afr Med J. 2018, 30. [CrossRef]
- Franco-Márquez, R.; Torres-Gaytán, A.G.; Narro-Martinez, M.A.; Carrasco-Chapa, A.; Núñez, B.G.; Boland-Rodriguez, E.: Metastasis of Breast Lobular Carcinoma to Endometrium Presenting as Recurrent Abnormal Uterine Bleeding: A Case Report and Review of Literature. Case Rep Pathol. 2019, 2019, 5357194. [CrossRef]
- Kachi, A.; Nicolas, G.; Semaan, D.B.; Hashem, M.; Abou Sleiman, C.: Unusual pattern of invasive lobular carcinoma metastasis: a case report. Am J Case Rep. 2019, 20, 1659. [CrossRef]
- Fontinele, D.R.S.; Vieira, S.C.; da Silva Júnior, R.G.; Rodrigues, T.S.: Lobular carcinoma of the breast with metastasis to the uterine cervix. J Cancer Res Ther. 2019, 15, 1411-1414. [CrossRef]
- Abdallah, H.; Elwy, A.; Alsayed, A.; Rabea, A.; Magdy, N.: Metastatic breast lobular carcinoma to unusual sites: a report of three cases and review of literature. J Med Cases. 2020, 11, 292. [CrossRef]
- Arif, S.H.; Mohammed, A.A.; Mohammed, F.R.: Metastatic invasive lobular carcinoma of the breast to the endometrium presenting with abnormal uterine bleeding; Case report. Ann Med Surg. 2020, 51, 41-43. [CrossRef]
- Gomez, M.; Whitting, K.; Naous, R.: Lobular breast carcinoma metastatic to the endometrium in a patient under tamoxifen therapy: A case report. SAGE Open Med Case Rep. 2020, 8, 2050313X20907208. [CrossRef]
- Yuan, L.; Oshilaja, O.; Sierk, A.; et al.: Metastatic breast cancer diagnosed on cervical cytology. Cytopathology. 2021, 32, 127-131. [CrossRef]
- Akizawa, Y.; Kanno, T.; Horibe, Y.; Shimizu, Y.; Noguchi, E.; Yamamoto, T.; Okamoto, T.; Nagashima, Y.; Tabata, T.: Ovarian metastasis from breast cancer mimicking a primary ovarian neoplasm: a case report. Mol Clin Oncol. 2021, 15, 135. [CrossRef]
- Awazu, Y.; Fukuda, T.; Imai, K.; Yamauch, M.; Kasai, M.; Ichimura, T.; Yasui, T.; Sumi, T.: Uterine metastasis of lobular breast carcinoma under tamoxifen therapy: A case report. Mol Clin Oncol. 2021, 15, 266. [CrossRef]
- Lim, L.; Wang, T.Y.; Lam, H.B.; Chang, C.L.: Massive metastasis of breast cancer to female genital organs. Taiwan J Obstet Gynecol. 2021, 60, 563-566. [CrossRef]
- Kong, D.; Dong, X.; Qin, P.; Sun, D.; Zhang, Z.; Zhang, Y.; Hao, F.; Wang, M.: Asymptomatic uterine metastasis of breast cancer: Case report and literature review. Medicine. 2022, 101, e31061. [CrossRef]
- Li, T.; Jiang, X.; Zhang, Z.; Chen, X.; Wang, J.; Zhao, X.; Zhang, J.: Case Report: 68Ga-FAPI PET/CT, a more advantageous detection mean of gastric, peritoneal, and ovarian metastases from breast cancer. Front Oncol. 2022, 12, 1013066. [CrossRef]
- Benlghazi, A.; Messaoudi, H.; Benali, S.; Tazi, I.; Elhassani, M.M.; Kouach, J.: Lobular carcinoma metastasis to endometrial polyps: Insights from a case report and literature analysis. Int J Surg Case Rep. 2024, 124, 110463. [CrossRef]
- Faur, A.C.; Gurban, C.V.; Dăescu, E.; Tîrziu, R.V.; Lazăr, D.C.; Ghenciu, L.A.: Mucin-Producing Lobular Breast Carcinoma Metastasis to an Ovarian Fibroma: Histopathological and Immunohistochemical Analysis of a Rare Case and Literature Review. Diagnostics. 2024, 14, 953. [CrossRef]
- Cserni, G.; Floris, G.; Koufopoulos, N.; Kovács, A.; Nonni, A.; Regitnig, P.; Stahls, A.; Varga, Z.: Invasive lobular carcinoma with extracellular mucin production—a novel pattern of lobular carcinomas of the breast. Clinico-pathological description of eight cases. Virchows Arch. 2017, 471, 3-12. [CrossRef]
- Koufopoulos, N.; Antoniadou, F.; Kokkali, S.; Pigadioti, E.; Khaldi, L.: Invasive lobular carcinoma with extracellular mucin production: description of a case and review of the literature. Cureus. 2019, 11, e5550. [CrossRef]
- Mazur, M.T.; Hsueh, S.; Gersell, D.J.: Metastases to the female genital tract: analysis of 325 cases. Cancer. 1984, 53, 1978-1984. [CrossRef]
- Borst, M.J.; Ingold, J.A.: Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993, 114, 637-642.
- Harris, M.; Howell, A.; Chrissohou, M.; Swindell, R.; Hudson, M.; Sellwood, R.: A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer. 1984, 50, 23-30. [CrossRef]
- Lamovec, J.; Bračkko, M.: Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol. 1991, 48, 28-33. [CrossRef]
- Winston, C.B.; Hadar, O.; Teitcher, J.B.; et al.: Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol. 2000, 175, 795-800. [CrossRef]
- Ayhan, A.; Guvenal, T.; Salman, M.; Ozyuncu, O.; Sakinci, M.; Basaran, M.: The role of cytoreductive surgery in nongenital cancers metastatic to the ovaries. Gynecol Oncol. 2005, 98, 235-241. [CrossRef]
- Bruchim, I.; Ben-Harim, Z.; Piura, E.; Tepper, R.; Fishman, A.: Preoperative clinical and radiological features of metastatic ovarian tumors. Arch Gynecol Obstet. 2013, 288, 615-619. [CrossRef]
- Tian, W.; Zhou, Y.; Wu, M.; Yao, Y.; Deng, Y.: Ovarian metastasis from breast cancer: a comprehensive review. Clin Transl Oncol. 2019, 21, 819-827. [CrossRef]
- Demopoulos, R.I.; Touger, L.; Dubin, N.: Secondary ovarian carcinoma: a clinical and pathological evaluation. Int J Gynecol Pathol. 1987, 6, 166-175. [CrossRef]
- Bigorie, V.; Morice, P.; Duvillard, P.; Antoine, M.; Cortez, A.; Flejou, J.F.; Uzan, S.; Darai, E.; Barranger, E.: Ovarian metastases from breast cancer: report of 29 cases. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2010, 116, 799-804. [CrossRef]
- Tserkezoglou, A.; Kontou, S.; Hadjieleftheriou, G.; Apostolikas, N.; Vassilomanolakis, M.; Sikiotis, K.; Salamalekis, E.; Tseke, P.; Magiakos, G.: Primary and metastatic ovarian cancer in patients with prior breast carcinoma. Pre-operative markers and treatment results. Anticancer Res. 2006, 26, 2339-2344.
- Rosendahl, M.; Wielenga, V.T.; Nedergaard, L.; Kristensen, S.G.; Ernst, E.; Rasmussen, P.E.; Anderson, M.; Schmidt, K.T.; Andersen, C.Y.: Cryopreservation of ovarian tissue for fertility preservation: no evidence of malignant cell contamination in ovarian tissue from patients with breast cancer. Fertil Steril. 2011, 95, 2158-2161. [CrossRef]
- Bastings, L.; Beerendonk, C.; Westphal, J.; Massuger, L.F.; Kaal, S.E.; van Leeuwen, F.E.; Braat, D.D.; Peek, R.: Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update. 2013, 19, 483-506. [CrossRef]
- Peters, I.T.; van Zwet, E.W.; Smit, V.T.; Liefers, G.J.; Kuppen, P.J.; Hilders, C.G.; Trimbos, J.B.: Prevalence and risk factors of ovarian metastases in breast cancer patients< 41 years of age in the Netherlands: a nationwide retrospective cohort study. PLoS One. 2017, 12, e0168277. [CrossRef]
- He, H.; Gonzalez, A.; Robinson, E.; Yang, W.T.: Distant metastatic disease manifestations in infiltrating lobular carcinoma of the breast. AJR Am J Roentgenol. 2014, 202, 1140-1148. [CrossRef]
- Guerriero, S.; Alcazar, J.; Pascual, M.; Ajossa, S.; Olartecoechea, B.; Hereter, L.: Preoperative diagnosis of metastatic ovarian cancer is related to origin of primary tumor. Ultrasound Obstet Gynecol. 2012, 39, 581-586. [CrossRef]
- Boutas, I.; Kontogeorgi, A.; Koufopoulos, N.; Dimas, D.T.; Sitara, K.; Kalantaridou, S.N.; Dimitrakakis, K.: Breast cancer and fertility preservation in young female patients: a systematic review of the literature. Clinics and Practice. 2023, 13, 1413-1426. [CrossRef]
- Webb, M.J.; Decker, D.G.; Mussey, E.: Cancer metastatic to the ovary: factors influencing survival. Obstet Gynecol. 1975, 45, 391-396.
- Bumpers, H.L.; Hassett, J.M.; Penetrante, R.B.; Hoover, E.L.; Holyoke, E.D.: Endocrine organ metastases in subjects with lobular carcinoma of the breast. Arch Surg. 1993, 128, 1344-1347. [CrossRef]
- Ferlicot, S.; Vincent-Salomon, A.; Medioni, J.; Genin, P.; Rosty, C.; Sigal-Zafrani, B.; Fréneaux, P.; Jouve, M.; Thiery, J.P.; Sastre-Garau, X.: Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer. 2004, 40, 336-341. [CrossRef]
- Weigelt, B.; Peterse, J.L.; Van't Veer, L.J.: Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005, 5, 591-602. [CrossRef]
- Fujiwara, K.; Ohishi, Y.; Koike, H.; Sawada, S.; Moriya, T.; Kohno, I.: Clinical implications of metastases to the ovary. Gynecol Oncol. 1995, 59, 124-128. [CrossRef]
- Moore, E.; Roylance, R.; Rosenthal, A.: Breast cancer metastasising to the pelvis and abdomen: what the gynaecologist needs to know. BJOG. 2012, 119, 788-794. [CrossRef]
- Nandy, S.B.; Gangwani, L.; Nahleh, Z.; Subramani, R.; Arumugam, A.; de la Rosa, J.M.; Lakshmanaswamy, R.: Recurrence and metastasis of breast cancer is influenced by ovarian hormone’s effect on breast cancer stem cells. Future Oncology. 2015, 11, 983-995. [CrossRef]
- de la Monte, S.M.; Hutchins, G.M.; Moore, G.W.: Influence of age on the metastatic behavior of breast carcinoma. Hum Pathol. 1988, 19, 529-534. [CrossRef]
- Pimentel, C.; Becquet, M.; Lavoué, V.; Hénno, S.; Lévêque, J.; Ouldamer, L.: Ovarian metastases from breast cancer: a series of 28 cases. Anticancer Res. 2016, 36, 4195-4200.
- Hann, L.E.; Lui, D.M.; Shi, W.; Bach, A.M.; Selland, D.L.; Castiel, M.: Adnexal masses in women with breast cancer: US findings with clinical and histopathologic correlation. Radiology. 2000, 216, 242-247. [CrossRef]
- Abd El hafez, A.; Monir, A.: Diagnostic spectrum of ovarian masses in women with breast cancer; magnetic resonance imaging: histopathology correlation. Ann Diagn Pathol. 2013, 17, 441-447. [CrossRef]
- Curtin, J.P.; Barakat, R.R.; Hoskins, W.J.: Ovarian disease in women with breast cancer. Obstet Gynecol. 1994, 84, 449-452.
- Abu-Rustum, N.R.; Aghajanian, C.A.; Venkatraman, E.S.; Feroz, F.; Barakat, R.R.: Metastatic breast carcinoma to the abdomen and pelvis. Gynecol Oncol. 1997, 66, 41-44. [CrossRef]
- Rabban, J.T.; Barnes, M.; Chen, L.M.; Powell, C.B.; Crawford, B.; Zaloudek, C.J.: Ovarian pathology in risk-reducing salpingo-oophorectomies from women with BRCA mutations, emphasizing the differential diagnosis of occult primary and metastatic carcinoma. Amer J Surg Pathol. 2009, 33, 1125-1136. [CrossRef]
- Gagnon, Y.; Tětu, B.: Ovarian metastases of breast carcinoma. A clinicopathologic study of 59 cases. Cancer. 1989, 64, 892-898. [CrossRef]
- De Waal, Y.R.; Thomas, C.M.; Oei, A.L.; Sweep, F.C.; Massuger, L.F.: Secondary ovarian malignancies: frequency, origin, and characteristics. Int J Gynecol Cancer. 2009, 19, 1160-1165. [CrossRef]
- Kondi-Pafiti, A.; Kairi-Vasilatou, E.; Iavazzo, C.; Dastamani, C.; Bakalianou, K.; Liapis, A.; Hassiakos, D.; Fotiou, S.: Metastatic neoplasms of the ovaries: a clinicopathological study of 97 cases. Arch Gynecol Obstet. 2011, 284, 1283-1288. [CrossRef]
- Perrotin, F.; Marret, H.; Bouquin, R.; Fischer-Perrotin, N.; Lansac, J.; Body, G.: Incidence, diagnosis and prognosis of ovarian metastasis in breast cancer. Gynecol Obstetr Fertil. 2001, 29, 308-315. [CrossRef]
- Antila, R.; Jalkanen, J.; Heikinheimo, O.: Comparison of secondary and primary ovarian malignancies reveals differences in their pre-and perioperative characteristics. Gynecol Oncol. 2006, 101, 97-101. [CrossRef]
- Bruls, J.; Simons, M.; Overbeek, L.I.; Bulten, J.; Massuger, L.F.; Nagtegaal, I.D.: A national population-based study provides insight in the origin of malignancies metastatic to the ovary. Virchows Arch 2015, 467, 79-86. [CrossRef]
- Kubeček, O.; Laco, J.; Špaček, J.; Petera, J.; Kopecký, J.; Kubečková, A.; Filip, S.: The pathogenesis, diagnosis, and management of metastatic tumors to the ovary: a comprehensive review. Clin Exp Metastasis. 2017, 34, 295-307. [CrossRef]
- Yadav, B.S.; Sharma, S.; Robin, T.P.; Sams, S.; Elias, A.D.; Kaklamani, V.; Kelly Marcom, P.; Schaefer, S.; Morris, G.J.: Synchronous primary carcinoma of breast and ovary versus ovarian metastases. Semin Oncol. 2015, 42, e13-e24. [CrossRef]
- Tamás, J.; Vereczkey, I.; Tóth, E.: Metastatic tumors in the ovary, difficulties of histologic diagnosis. Magy Onkol. 2015, 59, 205-213.
- Sal, V.; Demirkiran, F.; Topuz, S.; Kahramanoglu, I.; Yalcin, I.; Bese, T.; Sozen, H.; Tokgozoglu, N.; Salihoglu, Y.; Turan, H.; Iyibozkurt, C.; Kolomuc, T.; Sofiyeva, N.; Berkman, S.; Arvas, M.: Surgical treatment of metastatic ovarian tumors from extragenital primary sites. Int J Gynecol Cancer. 2016, 26, 688-696. [CrossRef]
- Pauer, H.U.; Viereck, V.; Burfeind, P.; Emons, G.; Krauss, T.: Uterine cervical metastasis of breast cancer: a rare complication that may be overlooked. Oncologie. 2003, 26, 58-60. [CrossRef]
- Pérez-Montiel, D.; Serrano-Olvera, A.; Salazar, L.C.; Cetina-Pérez, L.; Candelaria, M.; Coronel, J.; Montalvo, L.A.; de León, D.C.: Adenocarcinoma metastatic to the uterine cervix: a case series. J Obstet Gynaecol Res. 2012, 38, 541-549. [CrossRef]
- Abell, M.R.; Gosling, J.R.: Gland cell carcinoma (adenocarcinoma) of the uterine cervix. Am J Obstet Gynecol. 1962, 83, 729-755. [CrossRef]
- Scott, K.; Bryson, G.; Jamison, J.; Coutts, M.; McCluggage, W.G.: Cervical squamous carcinomas with prominent acantholysis and areas resembling breast lobular carcinoma: an aggressive form of dedifferentation. Int J Gynecol Pathol. 2018, 37, 74-81. [CrossRef]
- Mansor, S.; McCluggage, W.G.: Cervical adenocarcinoma resembling breast lobular carcinoma: a hitherto undescribed variant of primary cervical adenocarcinoma. Int J Gynecol Pathol. 2010, 29, 594-599. [CrossRef]
- Hermi, A.; Chakroun, M.; Saadi, A.; Saidani, B.; Kacem, L.B.; Chebil, M.: Upper urinary tract urothelial carcinoma diagnosis by biopsy of a vaginal metastasis. Urol Case Rep. 2022, 43, 102114. [CrossRef]
- Yan, Y.; Guo, T.; Zhang, M.; Cui, G.: Vaginal metastasis from breast cancer: A case report. Open Life Sci. 2023, 18, 20220623. [CrossRef]
- Gandhi, A.K.; Roy, S.; Mridha, A.R.; Sharma, D.N.: Vulvar metastasis from carcinoma breast unveiling distant metastasis: Exploring an unusual metastatic pattern. J Egypt Natl Canc Inst. 2015, 27, 243-246. [CrossRef]
| Characteristic | Measure |
| Number of cases | 61 |
| Age | |
| Mean±SD | 57.4±12.2 |
| Median [Min, Max] | 56.0 [32.0, 86.0] |
| Tumor Size (cm) | |
| Mean±SD | 3.65±2.11 |
| Median [Min, Max] | 3.65 [0.900, 10.0] |
| Missing | 39 (63.9%) |
| Primary tumor ER status | |
| Negative | 2 (3.3%) |
| Positive | 34 (55.7%) |
| Missing | 25 (41.0%) |
| Primary tumor PR status | |
| Negative | 2 (3.3%) |
| Positive | 34 (55.7%) |
| Missing | 25 (41.0%) |
| HER-2/Cc-erbB-2 status | |
| Negative | 21 (34.4%) |
| Positive | 3 (4.9%) |
| Missing | 37 (60.7%) |
| Secondary tumor ER status | |
| Negative | 3 (4.9%) |
| Positive | 31 (50.8%) |
| Missing | 27 (44.3%) |
| Secondary tumor PR status | |
| Negative | 6 (9.8%) |
| Positive | 24 (39.3%) |
| Missing | 31 (50.8%) |
| HER-2 status | |
| Negative | 9 (14.8%) |
| Positive | 3 (4.9%) |
| Missing | 49 (80.3%) |
| Stage pT component | |
| pT1 | 1 (1.6%) |
| pT1b | 1 (1.6%) |
| pT1c | 6 (9.8%) |
| pT2 | 15 (24.6%) |
| pT3 | 6 (9.8%) |
| pT4 | 1 (1.6%) |
| Missing | 31 (50.8%) |
| Stage pN component | |
| N0 | 8 (13.1%) |
| N1 | 10 (16.4%) |
| N2 | 5 (8.2%) |
| N3 | 9 (14.7%) |
| Missing | 29 (47.5%) |
| Stage pM component | |
| M0 | 22 (36.1%) |
| M1 | 6 (9.8%) |
| Missing | 33 (54.1%) |
| Radiotherapy | |
| No | 24 (39.3%) |
| Yes | 27 (44.3%) |
| Missing | 10 (16.4%) |
| Interval to Met (in months) | |
| Mean±SD | 65.6±70.0 |
| Median [Min, Max] | 48.0 [2.00, 360] |
| Missing | 20 (32.8%) |
| Last, follow up (in months) | |
| Mean±SD | 71.1±67.6 |
| Median [Min, Max] | 54.0 [1.00, 308] |
| Missing | 30 (49.2%) |
| Last, follow up status | |
| ANED | 19 (31.1%) |
| AWD | 9 (14.8%) |
| DOD | 10 (16.4%) |
| Missing | 23 (37.7%) |
| Tumor grade | |
| Grade II | 10 (16.4%) |
| Grade III | 2 (3.3%) |
| Missing | 49 (80.3%) |
| Authors | Year | Age | Clinical Presentation | Tumor Size (cm) | Stage | Site of metastasis FGT[16–69 | Other metastatic sites |
| Aranda et al. | 1993 | 76 | Asymptomatic | NM | pTx, N0 | Endometrial polyps | - |
| Sugiyama et al. | 1995 | 51 | Hypermenorrhea | 4 | pT2, N1, M1 | Uterine leiomyoma | - |
| Menzin et al. | 1998 | 53 | Vulvar tumor | 2,5 | pT2, N0, M1 | Vulva | Vertebrae and pelvic bones. |
| Arnould et al. | 2002 | 59 | Abdominal and pelvic pain | - | - | Ovarian granulosa cell tumor | - |
| Alvarez et al. | 2003 | 69 | Metrorrhagia | - | pT2, N1, M0 | Endometrial polyps and uterus | Skull and spine. |
| Ogino et al. | 2003 | 49 | Abnormal genital bleeding | 3.5 | pT2, N0, M0 | Cervix | Pancreas, stomach |
| Rau et al. | 2003 | 55 | Abdominal pain | 5 | pT4, N1, M0 | Cervix | - |
| Blecharz et al. | 2004 | 46 | Enlarged uterus | 1 | pT1c, N2 | Uterine leiomyoma | Bone |
| Famoriyo et al. | 2004 | 78 | Postmenstrual bleeding | - | NM | Uterus and cervix | - |
| Sheen-Chen et al. | 2004 | 32 | Vulvar tumor | 3 | pT2, N0, M0 | Vulva | - |
| Al-brachim et al. | 2005 | 53 | Vaginal bleeding | NM | pTxN1 | Endometrial polyps | - |
| Lee et al. | 2005 | 76 | Asymptomatic | NM | NM | Uterine leiomyoma, myometrium, endometrial polyps, cervical stroma and soft tissue adjacent to the uterus and the cervix |
- |
| Scopa et al. | 2005 | 50 | Vaginal bleeding | 6 | pT3N3 | Endometrium, endocervix, ovaries, and fallopian tubes. | - |
| Scopa et al. | 2005 | 81 | Vaginal bleeding | 1,6 | pT1c, N3 | Endometrium | - |
| Chen et al. | 2006 | 47 | Poor appetite and abdominal fullness | pT3, N0, M0 | Ovary | - | |
| Erkanli et al. | 2006 | 63 | Asymptomatic | NM | pT2, N3, M0 | Endometrium, cervix, and ovaries. | - |
| Perisic et al. | 2007 | 65 | Asymptomatic | NM | pT2, N1, M0 | Cervix | - |
| Manci et al. | 2008 | 41 | Asymptomatic | 1 | pT1c, N0, M0 | Cervix | - |
| Manipadan et al. | 2008 | 70 | Vaginal bleeding | NM | NM | Endometrial polyps | - |
| Bogliolo et al. | 2009 | 78 | Asymptomatic | 0,9 | pT1b, N2, M1 | Cervix | - |
| Ustaalioglu et al. | 2009 | 56 | Vaginal bleeding | NM | pT2, N2, M0 | Endometrium, myometrium, cervix, left uterine tube and left ovary. | - |
| Engelstaedter et al. | 2011 | 64 | Asymptomatic | 4 | pT3, N3, M1 | uterus, adnexa | Liver |
| Hooker et al. | 2011 | 83 | Postmenopausal uterine bleeding | NM | NM | Endometrial polyps, vulva | Pleura, peritoneum, stomach |
| Isci et al. | 2011 | 48 | Increased abdominal girth urinary incontinence | NM | NM | Both the ovaries, tubes, abdominal washing fluid, myometrium, and the huge leiomyoma | Liver, bone |
| Horikawa et al. | 2012 | 52 | abdominal discomfort, polyuria. | 5 | pT2, N3, M1 | Cervix, endometrium | - |
| Komeda et al. | 2012 | 59 | Asymptomatic | 3,8 | pT3, N2, M0 | Uterus | multiple metastases |
| Vicioso et al. | 2013 | 67 | Metrorrhagia | 3,2 | pT2, N2, M0 | Uterus | Left femur, calvarial skull, axial skeleton, and rib cage. |
| Alligood-Percoco et al. | 2015 | 36 | Abdominal bloating | NM | NM | Ovaries / vulva | Peritoneum, lymph nodes |
| Bezpalko et al. | 2015 | 47 | Vaginal bleeding | 1,8 | pT1c, Nx, M1 | Endometrium | gallbladder, bone marrow, lymph nodes, and peritoneum. |
| Lokadasan et al. | 2015 | 49 | Menorrhagia | NM | NM | Endometrium, myometrium, fibroid, cervix, bilateral ovaries. | - |
| Lokadasan et al. | 2015 | 49 | Abdominal distention, pain | NM | pT3, N3, M0 | Cervix, bilateral adnexa | Omentum |
| Toyoshima et al. | 2015 | 62 | Abdominal compression. | NM | pT2, N1, M0 | Uterine leiomyomata, myometrium | - |
| Waks et al. | 2015 | 53 | Postcoital bleeding | NM | pT2, N1, M0 | Cervix, corpus uteri | Pelvic and para-aortic lymph nodes |
| Lai et al. | 2016 | 54 | Asymptomatic | NM | pT3, N3, M0 | Endometrium | Stomach |
| Makris et al. | 2016 | 45 | Asymptomatic | NM | pT2, N1, M0 | Ovary | - |
| Martinez et al. | 2016 | 40 | Vaginal bleeding | NM | NM | Endometrium | Orbit, bone |
| Martinez et al. | 2016 | 48 | Asymptomatic | NM | NM | Endometrium, myometrium | - |
| Akhtar et al. | 2017 | 62 | Asymptomatic | 2,9 | NM | Endometrium, cervix | - |
| Bennett et al. | 2017 | 53 | Asymptomatic | NM | Bilateral ovaries | - | |
| Bennett et al. | 2017 | 64 | Vaginal fullness and discomfort | NM | Bilateral ovaries, uterus, fallopian tubes | - | |
| Bennett et al. | 2017 | 54 | Adnexal mass | NM | - | - | |
| Bennett et al. | 2017 | 41 | Adnexal mass | NM | Bilateral ovaries, fallopian tube | - | |
| Bennett et al. | 2017 | 48 | NM | NM | Left ovary, uterine serosa | - | |
| Razia et al. | 2017 | 58 | Abnormal uterine bleeding | NM | NM | Endometrial polyps, uterine leiomyoma, cervix | - |
| Seo et al. | 2017 | 46 | Vaginal bleeding | 4 | pT1, N0, M0 | Uterine corpus, endocervix, left ovary | - |
| Aytekin et al. | 2018 | 38 | Vaginal bleeding | NM | pT2, N3, M0 | Uterus, bilateral ovaries, vaginal cuff, cervix | - |
| Briki et al. | 2018 | 50 | Postmenopausal uterine bleeding | NM | pT2, N1, M0 | Endometrium | - |
| Franko-Marquez et al. | 2019 | 86 | Abnormal uterine bleeding. | NM | NM | Endometrium | - |
| Kachi et al. | 2019 | 58 | Altered bowel habits, abdominal pain, bloating. | NM | NM | Bilateral ovaries | large bowel, appendix |
| Fontinele et al. | 2019 | 57 | Abnormal uterine bleeding | 10 | ypT0, N0, M0 | Cervix | - |
| Abdallah et al. | 2020 | 59 | Lower abdominal pain | 5 | NM | Endometrium, myometrium, leiomyoma, cervix | Scapula |
| Arif et al. | 2020 | 55 | Vaginal bleeding, lower abdominal pain. | NM | NM | Endometrial polyp | - |
| Gomez et al. | 2020 | 69 | Post menopausal bleeding | NM | NM | Endometrium | - |
| Yuan et al. | 2020 | 64 | Asymptomatic | NM | NM | Endometrium, cervix | Bones |
| Akizawa et al. | 2021 | 49 | Abdominal distention | 5 | NM | Ovary | Bones |
| Awazu et al. | 2021 | 66 | Abnormal genital bleeding | NM | NM | Endometrium | Bones |
| Lim et al. | 2021 | 57 | Vaginal bleeding | 5,6 | NM | Uterus, cervix, bilateral ovaries, fallopian tubes | - |
| Kong et al. | 2022 | 64 | Right shoulder pain | 1,5 | pT1c, N3 M0 | Uterus, cervix, bilateral ovaries, fallopian tubes | Bones |
| Li et al. | 2022 | 61 | stomach discomfort | NM | pT1c, N1, M0 | Bilateral ovaries, peritoneum | Stomach |
| Benlghazi et al. | 2024 | 56 | abnormal uterine bleeding | NM | NM | Endometrial polyps | - |
| Faur et al. | 2024 | 82 | abdominal distension and pain | NM | NM | Ovarian fibroma | - |
| Authors | Primary tumor | Metastatic tumor | Tumor grade | ||||
| ER status | PR status | HER-2 status | ER status | PR status | HER-2 status | ||
| Aranda et al. | NM | NM | NM | NM | NM | NM | NM |
| Sugiyama et al. | NM | NM | NM | NM | NM | NM | NM |
| Menzin et al. | + | + | NM | + | + | NM | NM |
| Arnould et al. | NM | NM | NM | + | + | NM | NM |
| Alvarez et al. | + | + | NM | + | + | NM | NM |
| Ogino et al. | - | + | NM | - | + | NM | NM |
| Rau et al. | NM | NM | NM | NM | NM | NM | NM |
| Blecharz et al. | + | + | NM | + | - | NM | NM |
| Famoriyo et al. | NM | NM | NM | NM | NM | NM | NM |
| Sheen-Chen et al. | NM | NM | NM | NM | NM | NM | NM |
| Al-brachim et al. | NM | NM | NM | + | + | NM | NM |
| Lee et al. | NM | NM | NM | + | + | NM | Grade II |
| Scopa et al. | - | + | NM | - | + | + | NM |
| Scopa et al. | NM | NM | NM | + | + | NM | NM |
| Chen et al. | + | + | - | + | + | - | Grade III |
| Erkanli et al. | + | + | - | NM | NM | NM | - |
| Perisic et al. | + | + | NM | NM | NM | NM | Grade II |
| Manci et al. | + | + | - | + | + | + | NM |
| Manipadan et al. | Not done | Not done | Not done | NM | NM | NM | NM |
| Bogliolo et al. | + | + | - | NM | NM | NM | Grade II |
| Ustaalioglu et al. | + | + | - | + | + | - | NM |
| Engelstaedter et al. | + | + | Grade II | ||||
| Hooker et al. | + | + | - | + | - | NM | NM |
| Isci et al. | + | - | - | NM | NM | NM | NM |
| Horikawa et al. | + | + | - | + | NM | NM | NM |
| Komeda et al. | + | + | - | - | - | NM | NM |
| Vicioso et al. | + | + | - | + | - | Grade II | |
| Alligood-Percoco et al. | + | + | NM | + | NM | NM | NM |
| Bezpalko et al. | + | + | - | NM | NM | NM | Grade II |
| Lokadasan et al. | + | + | - | NM | NM | NM | NM |
| Lokadasan et al. | + | + | NM | + | + | NM | Grade II |
| Toyoshima et al. | + | + | + | + | - | NM | NM |
| Waks et al. | + | + | + | + | - | NM | |
| Lai et al. | + | + | - | + | + | - | Grade II |
| Makris et al. | + | + | NM | + | + | NM | NM |
| Martinez et al. | NM | NM | NM | NM | NM | NM | |
| Martinez et al. | NM | NM | NM | NM | NM | NM | NM |
| Akhtar et al. | + | + | - | + | + | NM | NM |
| Bennett et al. | NM | NM | NM | NM | NM | NM | NM |
| Bennett et al. | NM | NM | NM | NM | NM | NM | NM |
| Bennett et al. | NM | NM | NM | NM | NM | NM | NM |
| Bennett et al. | NM | NM | NM | NM | NM | NM | NM |
| Bennett et al. | NM | NM | NM | NM | NM | NM | NM |
| Razia et al. | NM | NM | NM | + | + | + | NM |
| Seo et al. | + | + | NM | + | + | NM | NM |
| Aytekin et al. | + | + | - | NM | NM | NM | NM |
| Briki et al. | + | + | - | NM | NM | NM | NM |
| Franko-Marquez et al. | + | + | + | + | NM | NM | NM |
| Kachi et al. | NM | NM | NM | + | + | NM | NM |
| Silva Fontinele et al. | + | + | - | + | + | - | Grade II |
| Abdallah et al. | + | + | - | + | + | NM | NM |
| Arif et al. | NM | NM | NM | NM | NM | NM | NM |
| Gomez et al. | NM | NM | NM | NM | NM | NM | Grade II |
| Yuan et al. | NM | NM | NM | + | + | - | NM |
| Akizawa et al. | + | + | - | + | NM | NM | NM |
| Awazu et al. | NM | NM | NM | + | + | - | NM |
| Lim et al. | + | + | + | NM | NM | NM | NM |
| Kong et al. | + | - | - | + | + | - | NM |
| Li et al. | NM | NM | NM | NM | NM | NM | NM |
| Benlghazi et al. | + | + | - | + | - | - | Grade III |
| Faur et al. | NM | NM | NM | NM | NM | NM | NM |
| Authors | Surgery | CHT | RT | Hormonal therapy | Interval to Met (Mo) |
Second-Line Therapy |
Outcome |
| Aranda et al. | NM | NM | NM | NM | 36 | HBSO | NM |
| Sugiyama et al. | MRM | 5-FU, mitomycin C, and pirarubicin |
N | Tamoxifen | Concomitant | - | 48 ANED |
| Menzin et al. | Quadrantectomy, ALND, and partial vulvectomy. | NM | N | Tamoxifen | Concomitant | - | 18 AWD |
| Arnould et al. | NM | CHT | Y | Tamoxifen | 48 | Bilateral oophorectomy, letrozole | 60 ANED |
| Alvarez et al. | MRM | 6 x CMF | Y | Tamoxifen | 48 | Biopsy | NM |
| Ogino et al. | MRM | No | N | Tamoxifen | 128 | Pancreatoduodenectomy, HBSO, ADM-TXL CHT and anastrozole. | 136 DOD |
| Rau et al. | MRM | 6x CHT | Y | Tamoxifen | 48 | Patient refused | Lost to follow-up |
| Blecharz et al. | MRM, HBSO | 6 x ADR, CTX, 5-FU. | N | Tamoxifen | Concomitant | Aromatase inhibitors and biphosphonates and palliative radiotherapy of the thoracic and lumbar vertebrae. | 59 AWD |
| Famoriyo et al. | NM | NM | NM | Tamoxifen | NM | NM | NM |
| Sheen-Chen et al. | MRM | 6 x CMF | N | Tamoxifen | 40 | Wide excision of the tumor, cyclophosphamide, epirubicin, and 5-FU. | 40 AWD |
| Al-brachim et al. | MRM | No | N | Tamoxifen | 48 | NM | NM |
| Lee et al. | MRM | CHT | Y | Tamoxifen | 18 | HBSO | |
| Scopa et al. | MRM | 4 x epirubicin | Y | Tamoxifen and LH-RH agonist | 36 | HBSO | 54 DOD |
| Scopa et al. | MRM | 4 x cyclophosphamide and adriamycin. | N | Tamoxifen and LH-RH agonist | 24 | HBSO | 30 DOD |
| Chen et al. | MRM | 6 x CHT | Y | Tamoxifen | 56 | HBSO, partial OM, and pelvic lymph node sampling |
NM |
| Erkanli et al. | MRM | Patient refused CHT | Patient refused RT | - | 8 | HBSO, omentectomy, and pelvic lymphadenectomy. Cyclophosphamide, epirubicin, and 5-FU | 8 AWD |
| Perisic et al. | MRM | 6 x CMF | Y | Tamoxifen | 52 | The patient refused CHT | 72 DOD |
| Manci et al. | quadrentectomy | No | Y | Tamoxifen | 130 | HBSO, pelvic lymphadenectomy, CHT, Femara. | 150 ANED |
| Manipadan et al. | Biopsy | 6 x docetaxel and zoledronic acid | N | No | 2 | Polypectomy | NM |
| Bogliolo et al. | Quadrantectomy, SLNB, endometrial and cervical biopsy. | 6 x 5-FU, epirubicin, cyclophosphamide, and docetaxel | Y | Letrozole | Concomitant | - | 30 AWD |
| Ustaalioglu et al. | MRM | 4 x doxorubicine, cyclophosphamide / 4 x docetaxel | Y | Anastrozole | 36 | HBSO, exemestane | 45 ANED |
| Engelstaedter et al. | HBSO, OM, lumpectomy, ALND. | Navelbine. | N | Tamoxifen | Concomitant | - | 65 ANED |
| Hooker et al. | No | No | N | Letrozole, Tamoxifen, Fulvestrant | 60 | Polypectomy | 72 ANED |
| Isci et al. | No | CHT | N | Letrozole and ibandronate. | 15 | HBSO, CHT | 27 ANED |
| Horikawa et al. | HBSO, MRM | No | N | Anastrozole, S-1 | Concomitant | - | 84 ANED |
| Komeda et al. | MRM | 4 x doxorubicin, cyclophosphamide/ 6 x paclitaxel | N | Letrozole | 15 | The patient refused HBSO, doxorubicin, and cyclophosphamide. | 28 DOD |
| Vicioso et al. | Quadrantectomy, ALND. | 6 x taxotere, adriamycin, and cyclophosphamide. | N | Tamoxifen | 72 | HBSO, letrozole, RT. | 163 AWD |
| Alligood-Percoco et al. | MRM | 4 x doxorubicin, 8 x CMF. | Y | Tamoxifen | 116 / 240 | HBSO, appendectomy, debulking, Taxotere, and Xeloda / Arimidex | 240 AWD |
| Bezpalko et al. | Biopsy | CHT | N | Hormonal therapy | Concomitant | - | 1 DOD |
| Lokadasan et al. | Biopsy | 5-FU, epirubicin, cyclophosphamide | N | Hormonal therapy | Concomitant | - | NM |
| Lokadasan et al. | MRM | 3 x 5-FU, adriamycin, cyclophosphamide / 3 x docetaxel | Y | Tamoxifen | 48 | Carboplatin, gemcitabine. | NM |
| Toyoshima et al. | Breast conserving surgery | 6 x 5-FU, epirubicin, cyclophosphamide. | Y | Anastrozole | 84 | HBSO, exemestane | ANED |
| Waks et al. | Breast conserving surgery, ALND | methotrexate, cyclophosphamide, 5-FU | Y | Tamoxifen | 180 | Biopsy, CHT | NM |
| Lai et al. | MRM | CHT | Y | Tamoxifen / anastrozole / exemestane | 84 | endometrial biopsy, CHT | AWD |
| Makris et al. | Lumpectomy, ALND | 6 x docetaxel, doxorubicin, cyclophosphamide | Y | No | 24 | HBSO, omentectomy, peritoneal biopsies, 6 x carboplatin, paclitaxel | 18 ANED |
| Martinez et al. | MRM, endometrial biopsy | CHT | N | Tamoxifen | Concomitant | - | |
| Martinez et al. | MRM | CHT | Y | Tamoxifen | 18 | HBSO | NM |
| Akhtar et al. | Biopsy | Patient refused CHT | N | N | Concomitant | - | Lost to follow-up |
| Bennett et al. | NM | NM | NM | NM | NM | ΝΜ | 49 DOD |
| Bennett et al. | NM | NM | NM | NM | NM | ΝΜ | 84 AWD |
| Bennett et al. | NM | NM | NM | NM | NM | ΝΜ | 9 DOD |
| Bennett et al. | NM | NM | NM | NM | NM | ΝΜ | NM |
| Bennett et al. | NM | NM | NM | NM | NM | ΝΜ | NM |
| Razia et al. | Surgery | doxifluridine, cyclophosphamide, docetaxel | Y | Goserelin acetate, Tamoxifen, toremifene citrate | 108 | HBSO, a partial colectomy, an aromatase inhibitor | ANED |
| Seo et al. | Breast conserving surgery, ALND | 2 x neoadj. Cyclophosphamide, adriamycin 4 x adj. cyclophosphamide, adriamycin |
Y | Goserelin, TamoxifenTamoxifenTamoxifen | 24 | HBSO | ANED |
| Aytekin et al. | MRM | 4 x adriamycin, cyclophosphamide / 12 x paclitaxel | Y | tamoxifen and luteinizing hormone-releasing hormone analog | 10 | HBSO, CHT | 16 DOD |
| Briki et al. | MRM | CHT | Y | Tamoxifen | 24 | HBSO | NM |
| Franko-Marquez et al. | MRM | CHT | N | NM | 360 | Biopsy, CHT | NM |
| Kachi et al. | MRM | 9 x CHT | Y | Tamoxifen | 60 | Anterior resection, BO, appendectomy | NM |
| Silva Fontinele et al. | MRM | Neoadj. 4x doxorubicin, cyclophosphamide/ 12 x paclitaxel | Y | Tamoxifen | 39 | HBSO, anastrozole | 66 ANED |
| Abdallah et al. | HBSO | 6 x cycolphosphamide, epirubicin 5-FU | N | Hormonal therapy | Concomitant | - | NM |
| Arif et al. | NM | NM | N | Tamoxifen | 84 | HBSO | 96 ANED |
| Gomez et al. | MRM | NM | NM | Tamoxifen | 60 | Biopsy | NM |
| Yuan et al. | MRM | CHT | Y | Hormonal therapy | 132 | Biopsy | ANED |
| Akizawa et al. | HBSO, OM | Palbociclib, denosumab | N | Letrozole | Concomitant | - | ANED |
| Awazu et al. | Surgery | CHT | NM | aromatase inhibitors/tamoxifen | 276 | HBSO, partial OM, a biopsy of the peritoneum, fulvestrant, toremifene citrate, and tegafur | 308 ANED |
| Lim et al. | MRM | 4 x cyclophosphamide, adriamycin, 5-FU/ 4 x taxotere | Y | Tamoxifen | 30 | HBSO, fulvestrant, ribociclib | NM |
| Kong et al. | MRM | 4 x epirubicin, cyclophosphamide / 4 x paclitaxel | patient refused RT | Letrozole | 29 | Radiotherapy, zoledronate / HBSO, 6 x paclitaxel, capecitabine, radiotherapy, zoledronate / 2 x gemcitabine, cisplatin | 49 DOD |
| Li et al. | MRM | 6 x docetaxel, doxorubicin, cyclophosphamide | Y | Anastrozole | 36 | hyperthermic perfusion chemotherapy (paclitaxel) | ANED |
| Benlghazi et al. | MRM | 3 x Epirubicine, cyclophosphamide, 5FU / 3 x Docetaxel | Y | Tamoxifen | 60 | HBSO, hormonal treatment | 78 ANED |
| Faur et al. | NM | NM | NM | NM | NM | NM | NM |
| Characteristic | HR and 95% CI | p-value | N |
| Primary tumor ER status | 0.41 (0.08-2.18) | 0.283 | 22 |
| Primary tumor PR status | 0.27 (0.03-2.6) | 0.223 | 22 |
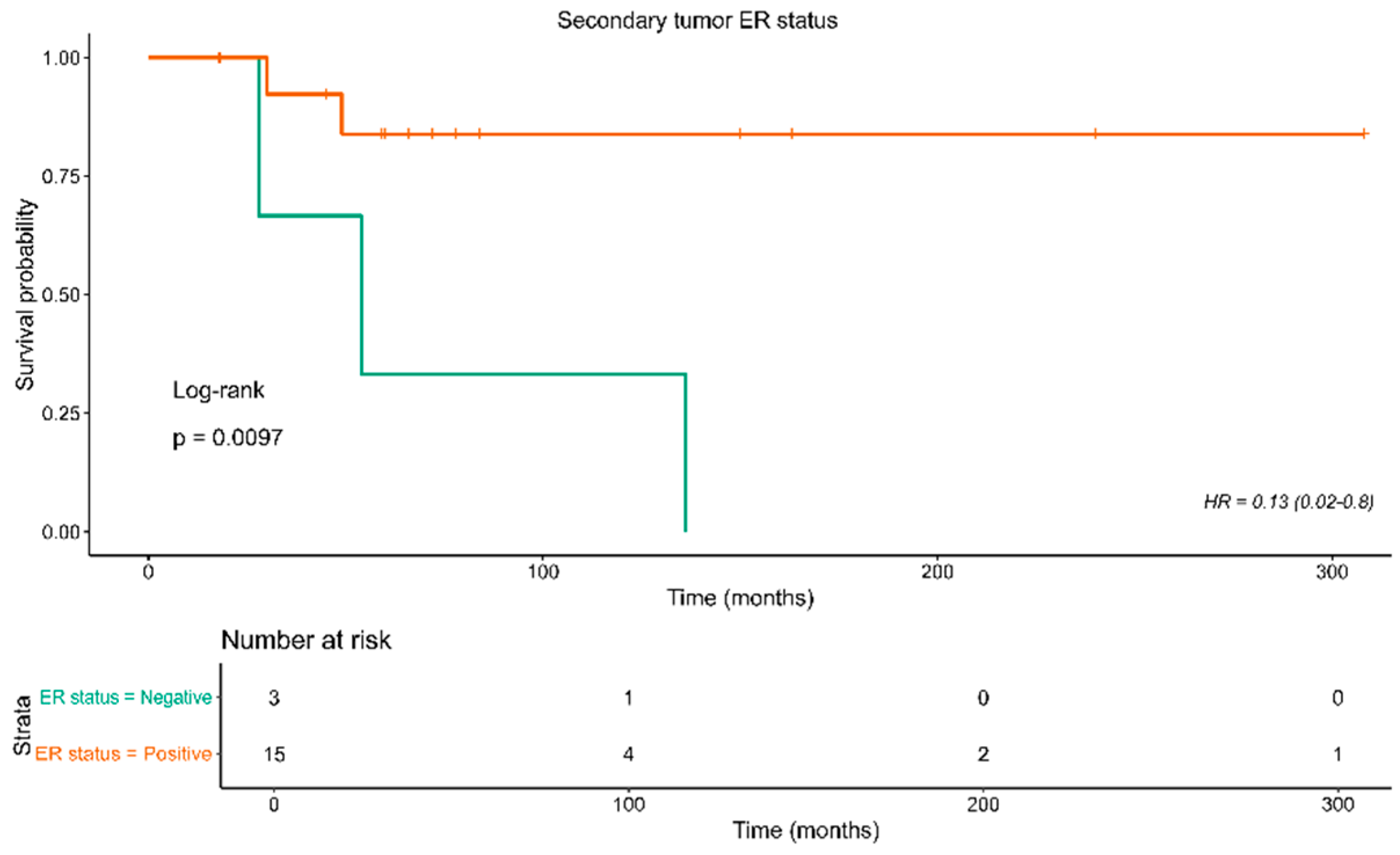
| Secondary tumor ER status | 0.13 (0.02-0.8) | 0.01 | 18 |
| Secondary tumor PR status | 2.04 (0.23-18.39) | 0.518 | 16 |
| HER-2 status | 1.73 (0.11-27.89) | 0.695 | 7 |
| Stage pN | 4.91 (0.2-118.15) | 0.31 | 20 |
| Stage pM | 0.64 (0.07-5.76) | 0.686 | 18 |
| Other metastasis | 0.82 (0.22-3.03) | 0.76 | 31 |
| RT | 0.72 (0.17-3.02) | 0.65 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





