Submitted:
16 August 2025
Posted:
19 August 2025
You are already at the latest version
Abstract
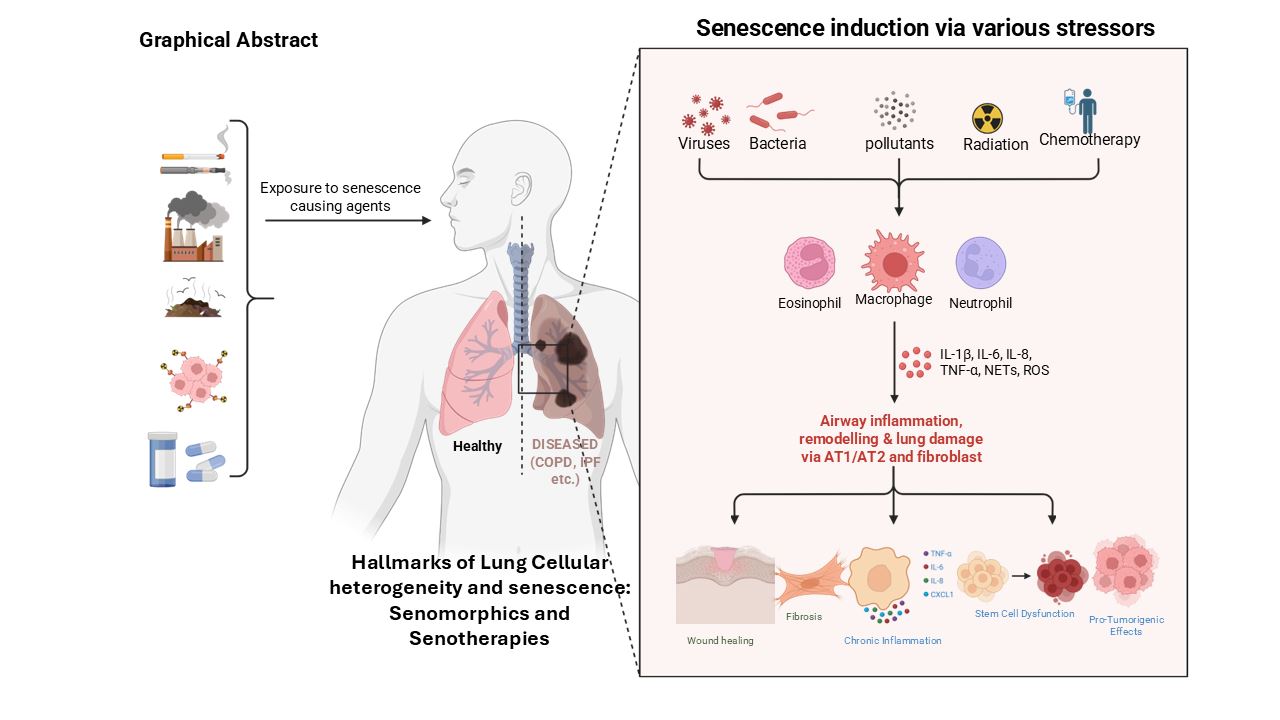
Keywords:
1. Introduction
2. Molecular Mechanisms of Cellular Senescence.
3. The Dichotomous Role of Senescence in Health and Disease
- Beneficial Roles
- Detrimental Roles
4. Roles of Senescence in Lung Diseases
- Role of cellular senescence in lung diseases based on cellular heterogeneity
- Age-dependent biomarkers in lung diseases
- Disease-dependent biomarkers
4.1. Senescence in COPD
Senescence Across Different COPD Stages and Severities
4.2. Senescence in Idiopathic Pulmonary Fibrosis (IPF)
4.3. Lung Cancer
4.4. Senescence in Acute Lung Injury and LARDS (Including COVID-19)
4.5. Cystic Fibrosis (CF)
4.6. Pulmonary Hypertension
- Key proteins implicated in Lung cellular senescence
5.1. Senomorphics
5.2. Mechanisms of Senomorphic Action: Targeting SASP Regulation
5.3. Major Classes and Examples of Senomorphic Agents
- Natural Compounds and Derivatives
5.4. Repurposed Drugs
- Novel Synthetic Compounds
5.5. Therapeutic Potential and Limitations of Senomorphism
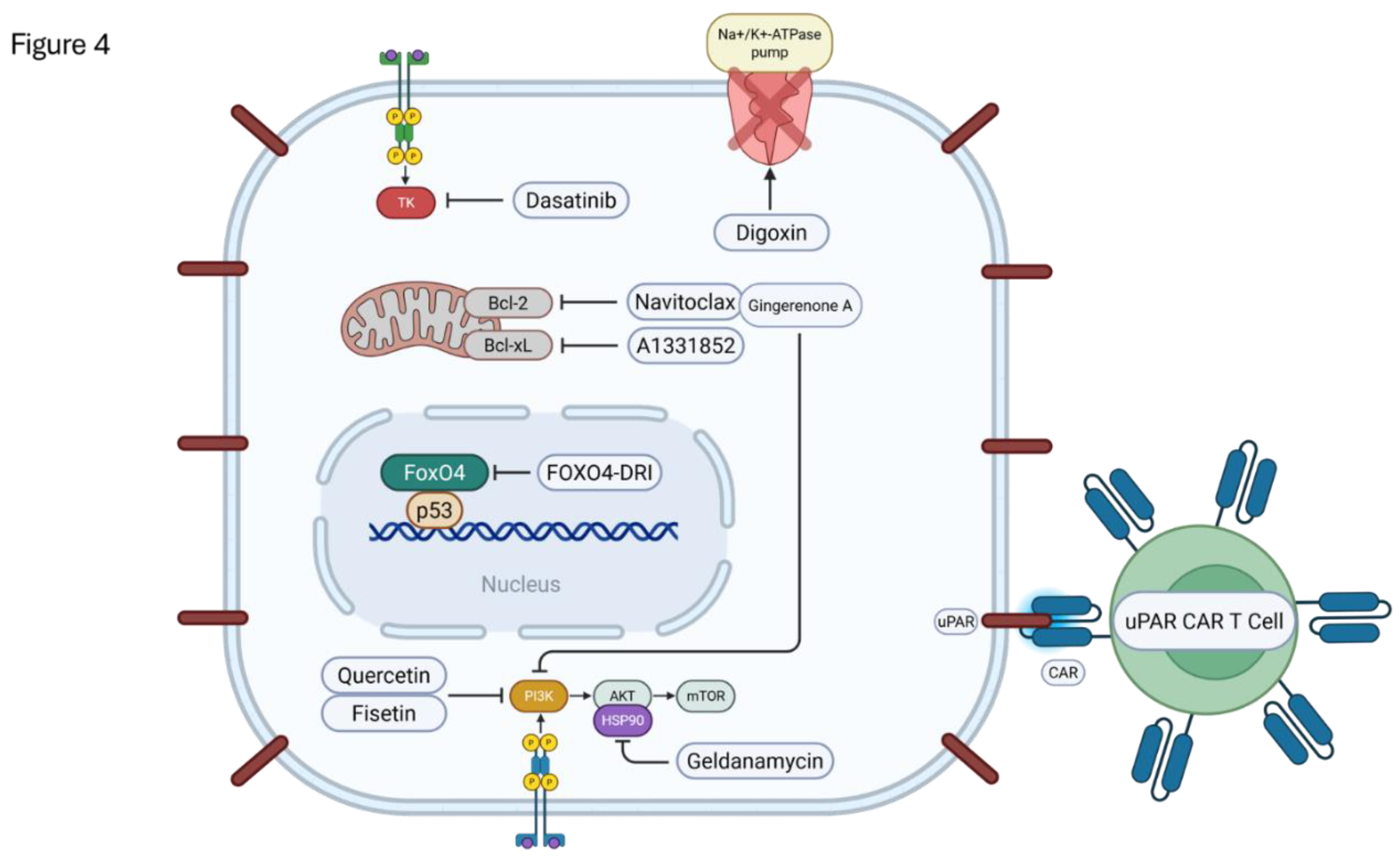
5.6. Senolytics
5.7. Mechanisms of Senolytic Action
- Major Classes and Examples of Senolytics
- Early Senolytics (Dasatinib and Quercetin)
- Natural Products
- Bcl-2
- l-2 Family Inhibitors
- HSP90
- P90 Inhibitors:
- Novel Therapeutic Modalities
- Other Classes:
- Delivery Mechanisms for Senotherapeutics
- Therapeutic Applications and Strategies
- Current Status of Clinical Trials
- Senotherapeutics Challenges and Opportunities
- Biomarker gaps: Need for in vivo markers of senescence in lungs
6. Underexplored Aspects
6.1. Senescence and Immune Cell Crosstalk in the Lung
6.2. Senescence Heterogeneity Across Lung Cell Types
6.3. Reversibility of Senescence and Plasticity
6.4. Role of Mechanical Stress and ECM Stiffness
6.5. Senescence-Associated Metabolic Reprogramming
7. Future Directions and Unmet Needs
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| DDR | DNA Damage Response |
| SASP | Senescence-Associated Secretory Phenotype |
| COPD | Chronic Obstructive Pulmonary Disease |
| IPF | Idiopathic Pulmonary Fibrosis |
| OIS | Oncogene-Induced Senescence |
| TIS | Therapy-Induced Senescence |
| MiDAS | Mitochondrial Dysfunction-Associated Senescence |
| AMPK | AMP-activated Protein Kinase |
| AT2 | Alveolar Type II Cells |
| ECM | Extracellular Matrix |
| ROS | Reactive Oxygen Species |
| PDGF-AA | Platelet-Derived Growth Factor-AA |
| MMPs | Matrix Metalloproteinases |
| p53 | Tumor Suppressor Protein 53 |
| p21 | Cyclin-Dependent Kinase Inhibitor 1 |
| p16 | Cyclin-Dependent Kinase Inhibitor 4A |
| Rb | Retinoblastoma |
| ALI | Acute Lung Injury |
| ARDS | Acute Respiratory Distress Syndrome |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| EVs | Extracellular Vesicles |
| rhCC16 | Recombinant Human Clara Cell Protein 16 |
| SIRT1 | Sirtuin 1 |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| TGF-β | Transforming Growth Factor Beta |
| CTGF | Connective Tissue Growth Factor |
| PDGF | Platelet-Derived Growth Factor |
| MMP | Matrix Metalloproteinase |
| PAECs | Pulmonary Artery Endothelial Cells |
| PASMCs | Pulmonary Artery Smooth Muscle Cells |
| iPAH | Idiopathic Pulmonary Arterial Hypertension |
| JAG1 | Jagged-1 |
| DLL4 | Delta-Like 4 |
| YWHAZ | Tyrosine 3-Monooxygenase/Tryptophan 5-Monooxygenase Activation Protein Zeta |
| ABT-737 | Senolytic Therapy Drug |
| FOXO4-DRI | Forkhead Box O4-Drug Resistance Inhibitor |
| SA-β-Gal | Senescence-Associated β-Galactosidase |
| γH2A.X | Gamma-Histone H2A Variant X |
| DNAmAge | DNA Methylation Age |
| AgeAcc | Age Acceleration |
| WGCNA | Weighted Gene Co-Expression Network Analysis |
| IL-10 | Interleukin 10 |
| TP53 | Tumor Protein 53 |
| H2AX | H2A.X Variant Histone |
| CDKN2A | Cyclin-Dependent Kinase Inhibitor 2A |
| GDF15 | Growth Differentiation Factor 15 |
| CDKN1A | Cyclin-Dependent Kinase Inhibitor 1A |
| TNFRSF1B | Tumor Necrosis Factor Receptor Superfamily Member 1B |
| Bcl2 L1 | BCL2 Like 1 |
| CXCL8 | C-X-C Motif Chemokine Ligand 8 |
| IL1A | Interleukin 1 Alpha |
| MMP12 | Matrix Metallopeptidase 12 |
| SERPINE1 | Serine Protease Inhibitor, Clade E, Member 1 (Plasminogen Activator Inhibitor-1) |
| TGFβ1 | Transforming Growth Factor Beta 1 |
| TNF | Tumor Necrosis Factor |
| IL-6 | Interleukin 6 |
| IL-1beta | Interleukin 1 Beta |
| MMP-8 | Matrix Metallopeptidase 8 |
| VEGFA | Vascular Endothelial Growth Factor A |
| SnCs | Senescent Cells |
| NF-κB | Nuclear Factor Kappa B |
| mTOR | Mammalian Target of Rapamycin |
| p38 MAPK | p38 Mitogen-Activated Protein Kinase |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| ATM | Ataxia Telangiectasia Mutated |
| STACs | Sirtuin-Activating Compounds |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| DAMPs | Damage-Associated Molecular Patterns |
| TAME | Targeting Aging with Metformin |
| eNOS | Endothelial Nitric Oxide Synthase |
| Ruxolitinib | JAK Inhibitor |
| Rapalogs | Rapamycin Analogs |
| SR12343 | NF-κB Inhibitor |
References
- Gorgoulis, V. , et al. , Cellular Senescence: Defining a Path Forward. Cell, 2019, 179, 813–827. [Google Scholar]
- Herranz, N. and J. Gil, Mechanisms and functions of cellular senescence. J Clin Invest, 2018, 128, 1238–1246. [Google Scholar] [PubMed]
- Wei, W. and S. Ji, Cellular senescence: Molecular mechanisms and pathogenicity. J Cell Physiol, 2018, 233, 9121–9135. [Google Scholar] [PubMed]
- Calabrò, A. , et al., Senotherapeutics to Counteract Senescent Cells Are Prominent Topics in the Context of Anti-Ageing Strategies. Int J Mol Sci, 2024, 25(3).
- Dimri, G.P. , et al. , A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A, 1995, 92, 9363–7. [Google Scholar] [PubMed]
- Bitencourt, T.C. , et al. , Subcellular structure, heterogeneity, and plasticity of senescent cells. Aging Cell, 2024, 23, e14154. [Google Scholar]
- Coppé, J.P. , et al., The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol, 2010, 5: 99-118.
- Wang, B. , et al. , The senescence-associated secretory phenotype and its physiological and pathological implications. Nat Rev Mol Cell Biol, 2024, 25, 958–978. [Google Scholar]
- Basisty, N. , et al. , A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol, 2020, 18, e3000599. [Google Scholar]
- Borghesan, M. , et al. , A Senescence-Centric View of Aging: Implications for Longevity and Disease. Trends Cell Biol, 2020, 30, 777–791. [Google Scholar]
- d'Adda di Fagagna, F. , et al. , A DNA damage checkpoint response in telomere-initiated senescence. Nature, 2003, 426, 194–8. [Google Scholar]
- Hayflick, L. and P.S. Moorhead, The serial cultivation of human diploid cell strains. Exp Cell Res, 1961, 25: 585-621.
- Parimon, T. , et al. , Senescence of alveolar epithelial progenitor cells: a critical driver of lung fibrosis. Am J Physiol Cell Physiol, 2023, 325, C483–c495. [Google Scholar]
- Okuda, R. , et al. , Cellular senescence and senescence-associated secretory phenotype: comparison of idiopathic pulmonary fibrosis, connective tissue disease-associated interstitial lung disease, and chronic obstructive pulmonary disease. J Thorac Dis, 2019, 11, 857–864. [Google Scholar] [PubMed]
- Sundar, I.K. , et al. , Genetic Ablation of p16(INK4a) Does Not Protect against Cellular Senescence in Mouse Models of Chronic Obstructive Pulmonary Disease/Emphysema. Am J Respir Cell Mol Biol, 2018, 59, 189–199. [Google Scholar]
- Rashid, K. , et al. , Lung cellular senescence is independent of aging in a mouse model of COPD/emphysema. Sci Rep, 2018, 8, 9023. [Google Scholar]
- Serrano, M. , et al. , Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell, 1997, 88, 593–602. [Google Scholar]
- Pribluda, A. , et al. , A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell, 2013, 24, 242–56. [Google Scholar]
- Ewald, J.A. , et al. , Therapy-induced senescence in cancer. J Natl Cancer Inst, 2010, 102, 1536–46. [Google Scholar] [CrossRef]
- Schmitt, C.A. , et al. , A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell, 2002, 109, 335–46. [Google Scholar]
- Bajtai, E. , et al. , Therapy-induced senescence is a transient drug resistance mechanism in breast cancer. Mol Cancer, 2025, 24, 128. [Google Scholar]
- Wiley, C.D. , et al. , Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab, 2016, 23, 303–14. [Google Scholar] [PubMed]
- Muñoz-Espín, D. , et al. , Programmed cell senescence during mammalian embryonic development. Cell, 2013, 155, 1104–18. [Google Scholar] [PubMed]
- Acosta, J.C. , et al. , A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol, 2013, 15, 978–90. [Google Scholar] [PubMed]
- Rattanavirotkul, N., K. Kirschner, and T. Chandra, Induction and transmission of oncogene-induced senescence. Cell Mol Life Sci, 2021, 78, 843–852. [Google Scholar]
- Mastri, M. , et al. , A Transient Pseudosenescent Secretome Promotes Tumor Growth after Antiangiogenic Therapy Withdrawal. Cell Rep, 2018, 25, 3706–3720. [Google Scholar]
- Reimann, M., S. Lee, and C.A. Schmitt, Cellular senescence: Neither irreversible nor reversible. J Exp Med, 2024, 221(4).
- Campisi, J. and F. d'Adda di Fagagna, Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol, 2007, 8, 729–40. [Google Scholar] [PubMed]
- Chang-Chien, J. , et al. , Particulate matter causes telomere shortening and increase in cellular senescence markers in human lung epithelial cells. Ecotoxicol Environ Saf, 2021, 222, 112484. [Google Scholar]
- Eckhardt, C.M. and H. Wu, Environmental Exposures and Lung Aging: Molecular Mechanisms and Implications for Improving Respiratory Health. Curr Environ Health Rep, 2021, 8, 281–293. [Google Scholar]
- Tsuji, T., K. Aoshiba, and A. Nagai, Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol, 2004, 31, 643–9. [Google Scholar]
- Miwa, S. , et al., Mitochondrial dysfunction in cell senescence and aging. J Clin Invest, 2022, 132(13).
- O'Reilly, S., E. Markiewicz, and O. C. Idowu, Aging, senescence, and cutaneous wound healing-a complex relationshiFront Immunol, 2024, 15, 1429716. [Google Scholar]
- Yao, H. , et al. , Timing and cell specificity of senescence drives postnatal lung development and injury. Nat Commun, 2023, 14, 273. [Google Scholar]
- Dennery, P.A. and H. Yao, Emerging role of cellular senescence in normal lung development and perinatal lung injury. Chin Med J Pulm Crit Care Med, 2024, 2, 10–16. [Google Scholar] [PubMed]
- Schafer, M.J. , et al. , Cellular senescence mediates fibrotic pulmonary disease. Nat Commun, 2017, 8, 14532. [Google Scholar]
- Woldhuis, R.R. , et al. , COPD-derived fibroblasts secrete higher levels of senescence-associated secretory phenotype proteins. Thorax, 2021, 76, 508–511. [Google Scholar]
- Yao, C. , et al. , Senescence of Alveolar Type 2 Cells Drives Progressive Pulmonary Fibrosis. Am J Respir Crit Care Med, 2021, 203, 707–717. [Google Scholar]
- Barkauskas, C.E. , et al. , Type 2 alveolar cells are stem cells in adult lung. J Clin Invest, 2013, 123, 3025–36. [Google Scholar]
- Ruysseveldt, E., K. Martens, and B. Steelant, Airway Basal Cells, Protectors of Epithelial Walls in Health and Respiratory Diseases. Front Allergy, 2021, 2, 787128. [Google Scholar] [PubMed]
- Desai, T.J., D. G. Brownfield, and M.A. Krasnow, Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature, 2014, 507, 190–4. [Google Scholar]
- Han, S., G. R.S. Budinger, and C.J. Gottardi, Alveolar epithelial regeneration in the aging lung. J Clin Invest, 2023, 133(20).
- Wisman, M. , et al. , Lower levels of senescence in human lung mesenchymal stromal cells compared with lung fibroblasts: implications for tissue regeneration in COPD. Am J Physiol Lung Cell Mol Physiol, 2025, 328, L858–l865. [Google Scholar]
- Tsuji, T., K. Aoshiba, and A. Nagai, Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med, 2006, 174, 886–93. [Google Scholar]
- Yanai, H. , et al. , Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging (Albany NY), 2015, 7, 664–72. [Google Scholar] [PubMed]
- Nyunoya, T. , et al. , Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol, 2006, 35, 681–8. [Google Scholar]
- Alder, J.K. , et al. , Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A, 2008, 105, 13051–6. [Google Scholar]
- Baek, K.H. , et al. , Thrombospondin-1 mediates oncogenic Ras-induced senescence in premalignant lung tumors. J Clin Invest, 2013, 123, 4375–89. [Google Scholar]
- Tripathi, U. , et al. , SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging (Albany NY), 2021, 13, 21838–21854. [Google Scholar]
- Hendrickson, C.M. and M. A. Matthay, Viral pathogens and acute lung injury: investigations inspired by the SARS epidemic and the 2009 H1N1 influenza pandemic. Semin Respir Crit Care Med, 2013, 34, 475–86. [Google Scholar]
- Kellogg, D.L. , et al. , Cellular Senescence in Idiopathic Pulmonary Fibrosis. Curr Mol Biol Rep, 2021, 7, 31–40. [Google Scholar]
- Woldhuis, R.R. , et al. , Link between increased cellular senescence and extracellular matrix changes in COPD. Am J Physiol Lung Cell Mol Physiol, 2020, 319, L48–l60. [Google Scholar]
- Gurkar, A.U. , et al. , Spatial mapping of cellular senescence: emerging challenges and opportunities. Nat Aging, 2023, 3, 776–790. [Google Scholar] [PubMed]
- Cohn, R.L. , et al. , The heterogeneity of cellular senescence: insights at the single-cell level. Trends Cell Biol, 2023, 33, 9–17. [Google Scholar] [PubMed]
- Sanborn, M.A. , et al. , Unveiling the cell-type-specific landscape of cellular senescence through single-cell transcriptomics using SenePy. Nat Commun, 2025, 16, 1884. [Google Scholar] [PubMed]
- Bueno, M. , et al. , Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol, 2020, 33, 101509. [Google Scholar]
- Liu, S. , et al. , Mitochondrial dysfunction and alveolar type II epithelial cell senescence: The destroyer and rescuer of idiopathic pulmonary fibrosis. Front Cell Dev Biol, 2025, 13, 1535601. [Google Scholar]
- Cuevas-Mora, K. , et al. , Hermansky-Pudlak syndrome-2 alters mitochondrial homeostasis in the alveolar epithelium of the lung. Respir Res, 2021, 22, 49. [Google Scholar]
- Su, W. , et al. , YAP1 inhibits the senescence of alveolar epithelial cells by targeting Prdx3 to alleviate pulmonary fibrosis. Exp Mol Med, 2024, 56, 1643–1654. [Google Scholar]
- Yu, Y. , et al. , YAP/TAZ activation mediates PQ-induced lung fibrosis by sustaining senescent pulmonary epithelial cells. Respir Res, 2024, 25, 212. [Google Scholar] [PubMed]
- Ovadya, Y. , et al. , Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun, 2018, 9, 5435. [Google Scholar]
- Parikh, P. , et al. , Cellular senescence in the lung across the age spectrum. Am J Physiol Lung Cell Mol Physiol, 2019, 316, L826–l842. [Google Scholar] [CrossRef]
- Freund, A., C. K. Patil, and J. Campisi, p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. Embo j, 2011, 30, 1536–48. [Google Scholar]
- Ajoolabady, A. , et al. , Hallmarks and mechanisms of cellular senescence in aging and disease. Cell Death Discovery, 2025, 11, 364. [Google Scholar]
- Di Micco, R. , et al. , Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology, 2021, 22, 75–95. [Google Scholar]
- Zhang, L. , et al., Cellular senescence: a key therapeutic target in aging and diseases. J Clin Invest, 2022, 132(15).
- Paez-Ribes, M. , et al. , Targeting senescent cells in translational medicine. EMBO Mol Med, 2019, 11, e10234. [Google Scholar]
- Demaria, M. , et al. , An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell, 2014, 31, 722–33. [Google Scholar]
- Krizhanovsky, V. , et al. , Senescence of activated stellate cells limits liver fibrosis. Cell, 2008, 134, 657–67. [Google Scholar]
- Ferrucci, L. and M. Zampino, A mitochondrial root to accelerated ageing and frailty. Nat Rev Endocrinol, 2020, 16, 133–134. [Google Scholar]
- Cho, H.J. , et al. , Nintedanib induces senolytic effect via STAT3 inhibition. Cell Death Dis, 2022, 13, 760. [Google Scholar] [PubMed]
- Palmer, A.K. , et al. , Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell, 2019, 18, e12950. [Google Scholar]
- Du, D. , et al. , Senotherapy Protects against Cisplatin-Induced Ovarian Injury by Removing Senescent Cells and Alleviating DNA Damage. Oxid Med Cell Longev, 2022, 2022, 9144644. [Google Scholar] [PubMed]
- Lee, S. , et al. , Molecular programs of fibrotic change in aging human lung. Nat Commun, 2021, 12, 6309. [Google Scholar]
- Wu, J. , et al. , Central role of cellular senescence in TSLP-induced airway remodeling in asthma. PLoS One, 2013, 8, e77795. [Google Scholar]
- Cottage, C.T. , et al. , Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice. Commun Biol, 2019, 2, 307. [Google Scholar]
- Sinha, S. , et al. , COVID-19 lung disease shares driver AT2 cytopathic features with Idiopathic pulmonary fibrosis. EBioMedicine, 2022, 82, 104185. [Google Scholar]
- Alder, J.K. , et al. , Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci U S A, 2015, 112, 5099–104. [Google Scholar]
- Orjalo, A.V. , et al. , Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A, 2009, 106, 17031–6. [Google Scholar]
- Ortiz-Montero, P., A. Londoño-Vallejo, and J. Vernot, Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal, 2017, 15, 17. [Google Scholar]
- Rana, T. , et al. , PAI-1 Regulation of TGF-β1-induced Alveolar Type II Cell Senescence, SASP Secretion, and SASP-mediated Activation of Alveolar Macrophages. Am J Respir Cell Mol Biol, 2020, 62, 319–330. [Google Scholar]
- Rivas, M. , et al., Senescence: Pathogenic Driver in Chronic Obstructive Pulmonary Disease. Medicina (Kaunas), 2022, 58(6).
- Zhou, F. , et al. , Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury. Respir Res, 2011, 12, 78. [Google Scholar] [PubMed]
- Kuźnar-Kamińska, B. , et al. , Serum from patients with chronic obstructive pulmonary disease induces senescence-related phenotype in bronchial epithelial cells. Sci Rep, 2018, 8, 12940. [Google Scholar]
- Wendisch, D. , et al. , SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell, 2021, 184, 6243–6261. [Google Scholar]
- Tsuji, T., K. Aoshiba, and A. Nagai, Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration, 2010, 80, 59–70. [Google Scholar] [PubMed]
- Bateman, G. , et al., Airway Epithelium Senescence as a Driving Mechanism in COPD Pathogenesis. Biomedicines, 2023, 11(7).
- Aghali, A. , et al. , Cellular senescence is increased in airway smooth muscle cells of elderly persons with asthma. Am J Physiol Lung Cell Mol Physiol, 2022, 323, L558–l568. [Google Scholar] [PubMed]
- Hernandez-Gonzalez, F. , et al., Cellular Senescence in Lung Fibrosis. Int J Mol Sci, 2021, 22(13).
- Kyi, P. , et al. , Endothelial senescence mediates hypoxia-induced vascular remodeling by modulating PDGFB expression. Front Med (Lausanne), 2022, 9, 908639. [Google Scholar]
- Schmitt, C.A. , et al. , COVID-19 and cellular senescence. Nat Rev Immunol, 2023, 23, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M. , et al., Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J, 2017, 50(2).
- Evangelou, K. , et al., Pulmonary infection by SARS-CoV-2 induces senescence accompanied by an inflammatory phenotype in severe COVID-19: possible implications for viral mutagenesis. Eur Respir J, 2022, 60(2).
- Justice, J.N. , et al. , Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine, 2019, 40, 554–563. [Google Scholar]
- Childs, B.G. , et al. , Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov, 2017, 16, 718–735. [Google Scholar]
- MacNee, W. , Is Chronic Obstructive Pulmonary Disease an Accelerated Aging Disease? Ann Am Thorac Soc, 2016, 13 Suppl 5: S429-s437.
- Wang, T. , et al. , The association between leukocyte telomere length and chronic obstructive pulmonary disease is partially mediated by inflammation: a meta-analysis and population-based mediation study. BMC Pulm Med, 2022, 22, 320. [Google Scholar]
- Devulder, J.V., et al., COPD Airway Epithelial Cell-derived Extracellular Vesicles Spread Cellular Senescence via MicroRNA-34a. Am J Respir Cell Mol Biol, 2025.
- Ren, Y.J. , et al. , rhCC16 Suppresses Cellular Senescence and Ameliorates COPD-Like Symptoms by Activating the AMPK/Sirt1-PGC-1-α-TFAM Pathway to Promote Mitochondrial Function. J Cell Mol Med, 2025, 29, e70566. [Google Scholar] [PubMed]
- Xiaofei, Y. , et al. , Erythromycin attenuates oxidative stress-induced cellular senescence via the PI3K-mTOR signaling pathway in chronic obstructive pulmonary disease. Front Pharmacol, 2022, 13, 1043474. [Google Scholar]
- Kaur, G., T. Muthumalage, and I. Rahman, Clearance of senescent cells reverts the cigarette smoke-induced lung senescence and airspace enlargement in p16-3MR mice. Aging Cell, 2023, 22, e13850. [Google Scholar]
- Norheim, K.L. , et al. , Effect of nicotinamide riboside on airway inflammation in COPD: a randomized, placebo-controlled trial. Nat Aging, 2024, 4, 1772–1781. [Google Scholar]
- Luo, X. , et al. , Multi-modal transcriptomic analysis reveals metabolic dysregulation and immune responses in chronic obstructive pulmonary disease. Sci Rep, 2024, 14, 22699. [Google Scholar]
- Alfaro, E. , et al. , Effect of physical activity in lymphocytes senescence burden in patients with COPD. Am J Physiol Lung Cell Mol Physiol, 2024, 327, L464–l472. [Google Scholar] [PubMed]
- Campisi, M. , et al. , DNA Methylation-Based Age Prediction and Telomere Length Reveal an Accelerated Aging in Induced Sputum Cells Compared to Blood Leukocytes: A Pilot Study in COPD Patients. Front Med (Lausanne), 2021, 8, 690312. [Google Scholar]
- He, Y. , et al. , Cellular senescence and radiation-induced pulmonary fibrosis. Transl Res, 2019, 209, 14–21. [Google Scholar]
- Borie, R., B. Crestani, and H. Bichat, Prevalence of telomere shortening in familial and sporadic pulmonary fibrosis is increased in men. Am J Respir Crit Care Med, 2009, 179, 1073. [Google Scholar]
- Oldham, J.M. , Interstitial Lung Abnormalities and Aging Biomarkers: A Mediation. Am J Respir Crit Care Med, 2021, 203, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.L. , et al. , The Association of Aging Biomarkers, Interstitial Lung Abnormalities, and Mortality. Am J Respir Crit Care Med, 2021, 203, 1149–1157. [Google Scholar]
- Álvarez, D. , et al. , IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol, 2017, 313, L1164–l1173. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C. , et al. , Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol, 2001, 24, 591–8. [Google Scholar]
- Yosef, R., et al., Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun, 2016, 7: 11190.
- Sanders, Y.Y. , et al. , Histone deacetylase inhibition promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in mice. Eur Respir J, 2014, 43, 1448–58. [Google Scholar]
- Sisson, T.H. , et al., PAI-1 interaction with sortilin-related receptor 1 is required for lung fibrosis. JCI Insight, 2025, 10(11).
- Marudamuthu, A.S. , et al. , Plasminogen activator inhibitor-1 suppresses profibrotic responses in fibroblasts from fibrotic lungs. J Biol Chem, 2015, 290, 9428–41. [Google Scholar] [PubMed]
- Wiley, C.D. , et al., Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight, 2019, 4(24).
- Jun, J.I. and L.F. Lau, CCN2 induces cellular senescence in fibroblasts. J Cell Commun Signal, 2017, 11, 15–23. [Google Scholar]
- Chien, Y. , et al. , Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev, 2011, 25, 2125–36. [Google Scholar] [PubMed]
- Tsukui, T. , et al. , Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun, 2020, 11, 1920. [Google Scholar]
- Habermann, A.C. , et al. , Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv, 2020, 6, eaba1972. [Google Scholar]
- Reyfman, P.A. , et al. , Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am J Respir Crit Care Med, 2019, 199, 1517–1536. [Google Scholar]
- Muñoz-Espín, D. and M. Serrano, Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol, 2014, 15, 482–96. [Google Scholar]
- Prasanna, P.G. , et al. , Therapy-Induced Senescence: Opportunities to Improve Anticancer Therapy. J Natl Cancer Inst, 2021, 113, 1285–1298. [Google Scholar]
- Saleh, T. , et al., Therapy-Induced Senescence: An "Old" Friend Becomes the Enemy. Cancers (Basel), 2020, 12(4).
- Coppé, J.P. , et al. , Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol, 2008, 6, 2853–68. [Google Scholar]
- Wang, L., L. Lankhorst, and R. Bernards, Exploiting senescence for the treatment of cancer. Nat Rev Cancer, 2022, 22, 340–355. [Google Scholar]
- Lei, Y. , et al. , Senescent lung fibroblasts in idiopathic pulmonary fibrosis facilitate non-small cell lung cancer progression by secreting exosomal MMP1. Oncogene, 2025, 44, 769–781. [Google Scholar] [PubMed]
- Demaria, M. , et al. , Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov, 2017, 7, 165–176. [Google Scholar] [PubMed]
- Haston, S. , et al. , Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell, 2023, 41, 1242–1260. [Google Scholar]
- Huidobro, C. , et al. , Cellular and molecular features of senescence in acute lung injury. Mech Ageing Dev, 2021, 193, 111410. [Google Scholar]
- Wissler Gerdes, E.O. , et al. , Role of senescence in the chronic health consequences of COVID-19. Transl Res, 2022, 241, 96–108. [Google Scholar]
- Lee, S. , et al. , Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature, 2021, 599, 283–289. [Google Scholar] [PubMed]
- Torres Acosta, M.A. and B.D. Singer, Pathogenesis of COVID-19-induced ARDS: implications for an ageing population. Eur Respir J, 2020, 56(3).
- Künzi, L., et al., Cystic Fibrosis Lung Disease in the Aging Population. Front Pharmacol, 2021, 12: 601438.
- Bezzerri, V. , et al. , Is cellular senescence involved in cystic fibrosis? Respir Res, 2019, 20, 32. [Google Scholar] [PubMed]
- Fischer, B.M. , et al. , Increased expression of senescence markers in cystic fibrosis airways. Am J Physiol Lung Cell Mol Physiol, 2013, 304, L394–400. [Google Scholar] [CrossRef]
- Easter, M. , et al., FGF receptors mediate cellular senescence in the cystic fibrosis airway epithelium. JCI Insight, 2024, 9(15).
- Laucirica, D.R., L. W. Garratt, and A. Kicic, Progress in Model Systems of Cystic Fibrosis Mucosal Inflammation to Understand Aberrant Neutrophil Activity. Front Immunol, 2020, 11, 595. [Google Scholar]
- Voynow, J.A. and M. Shinbashi, Neutrophil Elastase and Chronic Lung Disease. Biomolecules, 2021, 11(8).
- Barnes, P.J., J. Baker, and L.E. Donnelly, Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am J Respir Crit Care Med, 2019, 200, 556–564. [Google Scholar]
- Wang, S. , et al. , Inflammatory Activity of Epithelial Stem Cell Variants from Cystic Fibrosis Lungs Is Not Resolved by CFTR Modulators. Am J Respir Crit Care Med, 2023, 208, 930–943. [Google Scholar]
- Roger, I. , et al., Senescence Alterations in Pulmonary Hypertension. Cells, 2021, 10(12).
- Culley, M.K. and S. Y. Chan, Endothelial Senescence: A New Age in Pulmonary Hypertension. Circ Res, 2022, 130, 928–941. [Google Scholar]
- Safaie Qamsari, E. and D. J. Stewart, Cellular senescence in the pathogenesis of pulmonary arterial hypertension: the good, the bad and the uncertain. Front Immunol, 2024, 15, 1403669. [Google Scholar]
- Born, E. , et al. , Eliminating Senescent Cells Can Promote Pulmonary Hypertension Development and Progression. Circulation, 2023, 147, 650–666. [Google Scholar]
- Wang, A.P. , et al. , Pulmonary Artery Smooth Muscle Cell Senescence Promotes the Proliferation of PASMCs by Paracrine IL-6 in Hypoxia-Induced Pulmonary Hypertension. Front Physiol, 2021, 12, 656139. [Google Scholar]
- Ramadhiani, R. , et al. , Endothelial cell senescence exacerbates pulmonary hypertension by inducing juxtacrine Notch signaling in smooth muscle cells. iScience, 2023, 26, 106662. [Google Scholar] [PubMed]
- Meng, Z.Y. , et al. , Identification and experimental verification of senescence-related gene signatures and molecular subtypes in idiopathic pulmonary arterial hypertension. Sci Rep, 2024, 14, 22157. [Google Scholar] [PubMed]
- van der Feen, D.E. , et al., Cellular senescence impairs the reversibility of pulmonary arterial hypertension. Sci Transl Med, 2020, 12(554).
- Malinina, A. , et al. , IL10 deficiency promotes alveolar enlargement and lymphoid dysmorphogenesis in the aged murine lung. Aging Cell, 2020, 19, e13130. [Google Scholar] [PubMed]
- Gessner, C. , et al. , Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir Med, 2005, 99, 1229–40. [Google Scholar]
- Lim, S. , et al., Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am J Respir Crit Care Med, 2000, 162(4 Pt 1): 1355-60.
- Blázquez-Prieto, J. , et al. , Activation of p21 limits acute lung injury and induces early senescence after acid aspiration and mechanical ventilation. Transl Res, 2021, 233, 104–116. [Google Scholar]
- Kobayashi, Y. , et al. , Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol, 2020, 22, 934–946. [Google Scholar]
- Jiang, C. , et al. , Serpine 1 induces alveolar type II cell senescence through activating p53-p21-Rb pathway in fibrotic lung disease. Aging Cell, 2017, 16, 1114–1124. [Google Scholar]
- Chen, H. , et al. , TGF-β1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency. Exp Mol Med, 2020, 52, 130–151. [Google Scholar]
- Kaur, G., I. K. Sundar, and I. Rahman, p16-3MR: A Novel Model to Study Cellular Senescence in Cigarette Smoke-Induced Lung Injuries. Int J Mol Sci, 2021, 22(9).
- Yamada, Z. , et al. , Senescence of alveolar epithelial cells impacts initiation and chronic phases of murine fibrosing interstitial lung disease. Front Immunol, 2022, 13, 935114. [Google Scholar]
- Reyes, N.S. , et al. , Sentinel p16(INK4a+) cells in the basement membrane form a reparative niche in the lung. Science, 2022, 378, 192–201. [Google Scholar]
- Zhou, Y. , et al. , Expression of p16 and p53 in non-small-cell lung cancer: clinicopathological correlation and potential prognostic impact. Biomark Med, 2019, 13, 761–771. [Google Scholar]
- Keow, J. , et al. , Digital quantification of p16-positive foci in fibrotic interstitial lung disease is associated with a phenotype of idiopathic pulmonary fibrosis with reduced survival. Respir Res, 2022, 23, 147. [Google Scholar]
- Verhamme, F.M. , et al. , Elevated GDF-15 contributes to pulmonary inflammation upon cigarette smoke exposure. Mucosal Immunol, 2017, 10, 1400–1411. [Google Scholar] [PubMed]
- Jiang, G., C. T. Liu, and W.D. Zhang, IL-17A and GDF15 are able to induce epithelial-mesenchymal transition of lung epithelial cells in response to cigarette smoke. Exp Ther Med, 2018, 16, 12–20. [Google Scholar]
- Radwanska, A. , et al., Increased expression and accumulation of GDF15 in IPF extracellular matrix contribute to fibrosis. JCI Insight, 2022, 7(16).
- Zhang, Y. , et al. , GDF15 is an epithelial-derived biomarker of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol, 2019, 317, L510–l521. [Google Scholar] [CrossRef] [PubMed]
- Lv, X. , et al. , The cell cycle inhibitor P21 promotes the development of pulmonary fibrosis by suppressing lung alveolar regeneration. Acta Pharm Sin B, 2022, 12, 735–746. [Google Scholar]
- Ma, J.H. , et al., K63 Ubiquitination of P21 Can Facilitate Pellino-1 in the Context of Chronic Obstructive Pulmonary Disease and Lung Cellular Senescence. Cells, 2022, 11(19).
- Russo, R.C. , et al. , Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol, 2009, 40, 410–21. [Google Scholar]
- Kristan, S.S. , et al. , Airway angiogenesis in stable and exacerbated chronic obstructive pulmonary disease. Scand J Immunol, 2012, 75, 109–14. [Google Scholar]
- Shaghaghi, H. , et al. , A model of the aged lung epithelium in idiopathic pulmonary fibrosis. Aging (Albany NY), 2021, 13, 16922–16937. [Google Scholar]
- Khan, S.S. , et al. , A null mutation in SERPINE1 protects against biological aging in humans. Sci Adv, 2017, 3, eaao1617. [Google Scholar]
- Betsuyaku, T. , et al. , Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med, 1999, 159, 1985–91. [Google Scholar]
- Zhang, L. , et al. , Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. Febs j, 2023, 290, 1362–1383. [Google Scholar] [PubMed]
- Robbins, P.D. , et al. , Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span. Annu Rev Pharmacol Toxicol, 2021, 61, 779–803. [Google Scholar] [PubMed]
- Di Micco, R. , et al. , Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol, 2021, 22, 75–95. [Google Scholar]
- Laberge, R.M. , et al. , Author Correction: MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol, 2021, 23, 564–565. [Google Scholar] [PubMed]
- Xu, M. , et al. , JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A, 2015, 112, E6301–10. [Google Scholar]
- Van Nguyen, T. , et al. , DNA damage-induced cellular senescence is sufficient to suppress tumorigenesis: a mouse model. J Exp Med, 2007, 204, 1453–61. [Google Scholar]
- Lewinska, A. , et al. , AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe(3)O(4) nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol, 2020, 28, 101337. [Google Scholar]
- Bogdanowicz, P. , et al. , Senomorphic activity of a combination of niacinamide and hyaluronic acid: correlation with clinical improvement of skin aging. Sci Rep, 2024, 14, 16321. [Google Scholar]
- Moiseeva, O. , et al. , Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell, 2013, 12, 489–98. [Google Scholar]
- Zhu, Y. , et al. , Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell, 2016, 15, 428–35. [Google Scholar]
- Zhu, Y. , et al. , The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell, 2015, 14, 644–58. [Google Scholar] [PubMed]
- Wang, W. , et al. , The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends in Food Science & Technology, 2016, 56, 21–38. [Google Scholar]
- Zhu, Y. , et al. , New agents that target senescent cells: the flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463, Aging (Albany NY), 2017, 9, 955–963. [Google Scholar]
- Yousefzadeh, M.J. , et al. , Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine, 2018, 36, 18–28. [Google Scholar] [PubMed]
- Moaddel, R. , et al. , Identification of gingerenone A as a novel senolytic compound. PLoS One, 2022, 17, e0266135. [Google Scholar]
- Rawat, L. , et al. , Piperlongumine induces ROS mediated cell death and synergizes paclitaxel in human intestinal cancer cells. Biomed Pharmacother, 2020, 128, 110243. [Google Scholar]
- Chang, J. , et al. , Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med, 2016, 22, 78–83. [Google Scholar]
- Fuhrmann-Stroissnigg, H., L. J. Niedernhofer, and P.D. Robbins, Hsp90 inhibitors as senolytic drugs to extend healthy aging. Cell Cycle, 2018, 17, 1048–1055. [Google Scholar]
- Fuhrmann-Stroissnigg, H. , et al. , Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun, 2017, 8, 422. [Google Scholar]
- Amor, C. , et al. , Senolytic CAR T cells reverse senescence-associated pathologies. Nature, 2020, 583, 127–132. [Google Scholar]
- Amor, C. , et al. , Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat Aging, 2024, 4, 336–349. [Google Scholar]
- Baar, M.P. , et al. , Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell, 2017, 169, 132–147. [Google Scholar]
- González-Gualda, E. , et al. , A guide to assessing cellular senescence in vitro and in vivo. Febs j, 2021, 288, 56–80. [Google Scholar] [PubMed]
- Kotschy, A. , et al. , The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature, 2016, 538, 477–482. [Google Scholar]
- Partridge, L., M. Fuentealba, and B. K. Kennedy, The quest to slow ageing through drug discovery. Nat Rev Drug Discov, 2020, 19, 513–532. [Google Scholar]
- Gomari, H., M. Forouzandeh Moghadam, and M. Soleimani, Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. Onco Targets Ther, 2018, 11, 5753–5762. [Google Scholar]
- Cai, Y. , et al. , Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res, 2020, 30, 574–589. [Google Scholar]
- Poblocka, M. , et al. , Targeted clearance of senescent cells using an antibody-drug conjugate against a specific membrane marker. Sci Rep, 2021, 11, 20358. [Google Scholar] [PubMed]
- Wissler Gerdes, E.O., et al., Strategies for late phase preclinical and early clinical trials of senolytics. Mech Ageing Dev, 2021, 200: 111591.
- Hickson, L.J. , et al. , Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine, 2019, 47, 446–456. [Google Scholar] [PubMed]
- de Vos, S. , et al. , Safety and efficacy of navitoclax, a BCL-2 and BCL-X(L) inhibitor, in patients with relapsed or refractory lymphoid malignancies: results from a phase 2a study. Leuk Lymphoma, 2021, 62, 810–818. [Google Scholar]
- Cai, Y. , et al. , The landscape of aging. Sci China Life Sci, 2022, 65, 2354–2454. [Google Scholar] [CrossRef]
- Muñoz-Espín, D. , et al., A versatile drug delivery system targeting senescent cells. EMBO Mol Med, 2018, 10(9).
- Schafer, M.J. , et al., The senescence-associated secretome as an indicator of age and medical risk. JCI Insight, 2020, 5(12).
- Zhou, L. , et al. , Senescence as a dictator of patient outcomes and therapeutic efficacies in human gastric cancer. Cell Death Discov, 2022, 8, 13. [Google Scholar]
- Bian, R., et al., CDKN1A as a target of senescence in heart failure: insights from a multiomics study. Front Pharmacol, 2024, 15: 1446300.
- Suryadevara, V. , et al. , SenNet recommendations for detecting senescent cells in different tissues. Nat Rev Mol Cell Biol, 2024, 25, 1001–1023. [Google Scholar]
- Prieto, L.I. , et al. , Senescent alveolar macrophages promote early-stage lung tumorigenesis. Cancer Cell, 2023, 41, 1261–1275,e6. [Google Scholar]
- Smith, R. , et al. , A new model and precious tool to study molecular mechanisms of macrophage aging. Aging (Albany NY), 2024, 16, 12697–12725. [Google Scholar]
- Maus, M. , et al. , Iron accumulation drives fibrosis, senescence and the senescence-associated secretory phenotype. Nat Metab, 2023, 5, 2111–2130. [Google Scholar] [PubMed]
- Su, L. , et al. , Potential role of senescent macrophages in radiation-induced pulmonary fibrosis. Cell Death Dis, 2021, 12, 527. [Google Scholar]
- Zhou, B.W. , et al. , The role of macrophage polarization and cellular crosstalk in the pulmonary fibrotic microenvironment: a review. Cell Commun Signal, 2024, 22, 172. [Google Scholar]
- Campbell, R.A. , et al. , The Role of Ageing and Parenchymal Senescence on Macrophage Function and Fibrosis. Front Immunol, 2021, 12, 700790. [Google Scholar]
- Salminen, A. , Inhibitory immune checkpoints suppress the surveillance of senescent cells promoting their accumulation with aging and in age-related diseases. Biogerontology, 2024, 25, 749–773. [Google Scholar] [CrossRef]
- Majewska, J. , et al. , p16-dependent increase of PD-L1 stability regulates immunosurveillance of senescent cells. Nat Cell Biol, 2024, 26, 1336–1345. [Google Scholar] [PubMed]
- Marin, I., M. Serrano, and F. Pietrocola, Recent insights into the crosstalk between senescent cells and CD8 T lymphocytes. NPJ Aging, 2023, 9, 8. [Google Scholar]
- Melo-Narváez, M.C. , et al., Lung regeneration: implications of the diseased niche and ageing. Eur Respir Rev, 2020, 29(157).
- Ruhland, M.K. , et al. , Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat Commun, 2016, 7, 11762. [Google Scholar] [PubMed]
- Li, X. , et al. , Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther, 2023, 8, 239. [Google Scholar]
- Favaretto, G. , et al. , Neutrophil-activating secretome characterizes palbociclib-induced senescence of breast cancer cells. Cancer Immunol Immunother, 2024, 73, 113. [Google Scholar] [PubMed]
- Rolas, L. , et al. , Senescent endothelial cells promote pathogenic neutrophil trafficking in inflamed tissues. EMBO Rep, 2024, 25, 3842–3869. [Google Scholar]
- Cai, C. , et al. , The role of fibroblast-neutrophil crosstalk in the pathogenesis of inflammatory diseases: a multi-tissue perspective. Front Immunol, 2025, 16, 1588667. [Google Scholar]
- Fulop, T. , et al. , Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol, 2017, 8, 1960. [Google Scholar]
- Wynn, T.A. and K. M. Vannella, Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity, 2016, 44, 450–462. [Google Scholar]
- Simmons, S.R. , et al., Older but Not Wiser: the Age-Driven Changes in Neutrophil Responses during Pulmonary Infections. Infect Immun, 2021, 89(4).
- Yang, S.C. , et al. , Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed J, 2021, 44, 439–446. [Google Scholar] [PubMed]
- Wang, Y. , et al. , The aging lung: microenvironment, mechanisms, and diseases. Front Immunol, 2024, 15, 1383503. [Google Scholar]
- Soto-Heredero, G. , et al. , KLRG1 identifies regulatory T cells with mitochondrial alterations that accumulate with aging. Nat Aging, 2025, 5, 799–815. [Google Scholar] [PubMed]
- Liu, Z. , et al. , Immunosenescence: molecular mechanisms and diseases. Signal Transduct Target Ther, 2023, 8, 200. [Google Scholar] [PubMed]
- Han, S., Q. Lu, and X. Liu, Advances in cellular senescence in idiopathic pulmonary fibrosis (Review). Exp Ther Med, 2023, 25, 145. [Google Scholar]
- Wang, Y. , et al. , Immunosenescence, aging and successful aging. Front Immunol, 2022, 13, 942796. [Google Scholar]
- Devulder, J.V. , Unveiling mechanisms of lung aging in COPD: A promising target for therapeutics development. Chin Med J Pulm Crit Care Med, 2024, 2, 133–141. [Google Scholar] [CrossRef]
- Xie, C. , et al., Role of cellular senescence in inflammatory lung diseases. Cytokine Growth Factor Rev, 2023, 70: 26-40.
- Wan, R. , et al., Cellular Senescence: A Troy Horse in Pulmonary Fibrosis. Int J Mol Sci, 2023, 24(22).
- Sun, Y. , An updated landscape of cellular senescence heterogeneity: Mechanisms, technologies and senotherapies. Translational Medicine of Aging, 2023, 7, 46–51. [Google Scholar] [CrossRef]
- Neri, F., et al., Senescent cell heterogeneity and responses to senolytic treatment are related to cell cycle status during cell growth arrest. bioRxiv, 2024.
- D'Agnillo, F. , et al. , Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med, 2021, 13, eabj7790. [Google Scholar]
- Zhou, S. , et al. , Alveolar type 2 epithelial cell senescence and radiation-induced pulmonary fibrosis. Front Cell Dev Biol, 2022, 10, 999600. [Google Scholar]
- Najari Beidokhti, M. , et al. , Lung endothelial cell senescence impairs barrier function and promotes neutrophil adhesion and migration. Geroscience, 2025, 47, 2655–2671. [Google Scholar]
- Mebratu, Y.A. , et al. , The aged extracellular matrix and the profibrotic role of senescence-associated secretory phenotype. Am J Physiol Cell Physiol, 2023, 325, C565–c579. [Google Scholar]
- Tao, W., Z. Yu, and J. J. Han, Single-cell senescence identification reveals senescence heterogeneity, trajectory, and modulators. Cell Metab, 2024, 36, 1126–1143. [Google Scholar]
- Saul, D. , et al. , A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat Commun, 2022, 13, 4827. [Google Scholar] [PubMed]
- Melo-Narváez, M.C. , et al., Stimuli-Specific Senescence of Primary Human Lung Fibroblasts Modulates Alveolar Stem Cell Function. Cells, 2024, 13(13).
- Liao, Y.L. , et al. , Senescent endothelial cells: a potential target for diabetic retinopathy. Angiogenesis, 2024, 27, 663–679. [Google Scholar] [PubMed]
- Morton, L. , et al. , Pericytes and Extracellular Vesicle Interactions in Neurovascular Adaptation to Chronic Arterial Hypertension. J Am Heart Assoc, 2025, 14, e038457. [Google Scholar] [PubMed]
- Blackburn, J.B. , et al. , An update in club cell biology and its potential relevance to chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol, 2023, 324, L652–l665. [Google Scholar]
- Jia, M. , et al. , Transcriptional changes of the aging lung. Aging Cell, 2023, 22, e13969. [Google Scholar]
- Jia, H. , et al. , A single-cell atlas of lung homeostasis reveals dynamic changes during development and aging. Commun Biol, 2024, 7, 427. [Google Scholar]
- Kasmani, M.Y. , et al. , A spatial sequencing atlas of age-induced changes in the lung during influenza infection. Nat Commun, 2023, 14, 6597. [Google Scholar] [PubMed]
- Wang, J. , et al. , A transcriptome-based human universal senescence index (hUSI) robustly predicts cellular senescence under various conditions. Nat Aging, 2025, 5, 1159–1175. [Google Scholar] [PubMed]
- Nguyen, N.D. , et al. , scDOT: optimal transport for mapping senescent cells in spatial transcriptomics. Genome Biol, 2024, 25, 288. [Google Scholar]
- Goyal, A. , et al., Targeting p53-p21 signaling to enhance mesenchymal stem cell regenerative potential. Regen Ther, 2025, 29: 352-363.
- Martínez-Zamudio, R.I. , et al. , AP-1 imprints a reversible transcriptional programme of senescent cells. Nat Cell Biol, 2020, 22, 842–855. [Google Scholar]
- Ashraf, H.M., B. Fernandez, and S. L. Spencer, The intensities of canonical senescence biomarkers integrate the duration of cell-cycle withdrawal. Nat Commun, 2023, 14, 4527. [Google Scholar]
- Nacarelli, T. , et al. , NAD(+) metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol, 2019, 21, 397–407. [Google Scholar]
- Ritschka, B. , et al. , The senotherapeutic drug ABT-737 disrupts aberrant p21 expression to restore liver regeneration in adult mice. Genes Dev, 2020, 34, 489–494. [Google Scholar]
- Saleh, T. , et al., Tumor cell escape from therapy-induced senescence. Biochem Pharmacol, 2019, 162: 202-212.
- Sladitschek-Martens, H.L. , et al. , YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING. Nature, 2022, 607, 790–798. [Google Scholar]
- Blokland, K.E.C. , et al., Senescence of IPF Lung Fibroblasts Disrupt Alveolar Epithelial Cell Proliferation and Promote Migration in Wound Healing. Pharmaceutics, 2020, 12(4).
- Suki, B., J. H. T. Bates, and E. Bartolák-Suki, Remodeling of the Aged and Emphysematous Lungs: Roles of Microenvironmental Cues. Compr Physiol, 2022, 12, 3559–3574. [Google Scholar]
- Zhang, Y. , et al. , Metabolic reprogramming in cancer and senescence. MedComm (2020), 2025, 6, e70055. [Google Scholar]
- Lian, H. , et al., Fatty acid synthase inhibition alleviates lung fibrosis via β-catenin signal in fibroblasts. Life Sci Alliance, 2025, 8(2).
- Schneider, J.L. , et al. , The aging lung: Physiology, disease, and immunity. Cell, 2021, 184, 1990–2019. [Google Scholar]
- Liu, B. , et al. , Lipid and glucose metabolism in senescence. Front Nutr, 2023, 10, 1157352. [Google Scholar]
- Correia-Melo, C. , et al. , Mitochondria are required for pro-ageing features of the senescent phenotype. Embo j, 2016, 35, 724–42. [Google Scholar]
- Kang, Y. , et al. , Telomere Dysfunction Disturbs Macrophage Mitochondrial Metabolism and the NLRP3 Inflammasome through the PGC-1α/TNFAIP3 Axis. Cell Rep, 2018, 22, 3493–3506. [Google Scholar] [PubMed]
- Sabbatinelli, J., et al., Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front Physiol, 2019, 10: 1523.
- Wiley, C.D. and J. Campisi, The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat Metab, 2021, 3, 1290–1301. [Google Scholar] [PubMed]
- Singh, R. , et al. , 2-Deoxy-D-Glucose: A Novel Pharmacological Agent for Killing Hypoxic Tumor Cells, Oxygen Dependence-Lowering in Covid-19, and Other Pharmacological Activities. Adv Pharmacol Pharm Sci, 2023, 2023, 9993386. [Google Scholar] [PubMed]
- Zong, Y. , et al. , Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct Target Ther, 2024, 9, 124. [Google Scholar]
- Jiménez-Loygorri, J.I. , et al. , Mitophagy curtails cytosolic mtDNA-dependent activation of cGAS/STING inflammation during aging. Nat Commun, 2024, 15, 830. [Google Scholar]
- Duran, I. , et al. , Detection of senescence using machine learning algorithms based on nuclear features. Nat Commun, 2024, 15, 1041. [Google Scholar] [PubMed]
- Abdelmohsen, K. , et al. , Identification of senescent cell subpopulations by CITE-seq analysis. Aging Cell, 2024, 23, e14297. [Google Scholar]
- Viswanathan, V.S. , et al. , The state of the art for artificial intelligence in lung digital pathology. J Pathol, 2022, 257, 413–429. [Google Scholar]
- Kokosi, M.A., G. A. Margaritopoulos, and A.U. Wells, Personalised medicine in interstitial lung diseases: Number 6 in the Series "Personalised medicine in respiratory diseases" Edited by Renaud Louis and Nicolas Roche. Eur Respir Rev, 2018, 27(148).
- Misawa, T. , et al., Identification of Novel Senescent Markers in Small Extracellular Vesicles. Int J Mol Sci, 2023, 24(3).
- Waters, D.W. , et al. , Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol, 2018, 315, L162–l172. [Google Scholar]
- Wu, Q. , et al. , Cigarette Smoke Induces Human Airway Epithelial Senescence via Growth Differentiation Factor 15 Production. Am J Respir Cell Mol Biol, 2016, 55, 429–38. [Google Scholar]
- Xu, S. , et al. , Assessment of cellular senescence potential of PM2.5 using 3D human lung fibroblast spheroids in vitro model. Toxicol Res (Camb), 2024, 13, tfae037. [Google Scholar]
- Chew, S. , et al. , Impairment of mitochondrial function by particulate matter: Implications for the brain. Neurochem Int, 2020, 135, 104694. [Google Scholar]
- Martic, I. , Jansen-Dürr, and M. Cavinato, Effects of Air Pollution on Cellular Senescence and Skin Aging. Cells, 2022, 11(14).
- Yang, D. , et al. , The impact of lung microbiota dysbiosis on inflammation. Immunology, 2020, 159, 156–166. [Google Scholar] [PubMed]
- Jang, D.H. , et al. , The connection between aging, cellular senescence and gut microbiome alterations: A comprehensive review. Aging Cell, 2024, 23, e14315. [Google Scholar]
- Zhang, W. , et al. , Emerging Insight Into the Role of Circadian Clock Gene BMAL1 in Cellular Senescence. Front Endocrinol (Lausanne), 2022, 13, 915139. [Google Scholar] [PubMed]
- Chhunchha, B., E. Kubo, and D.Singh, Clock Protein Bmal1 and Nrf2 Cooperatively Control Aging or Oxidative Response and Redox Homeostasis by Regulating Rhythmic Expression of Prdx6. Cells, 2020, 9(8).
- Wang, Q. , et al. , Circadian clock molecule REV-ERBα regulates lung fibrotic progression through collagen stabilization. Nat Commun, 2023, 14, 1295. [Google Scholar]
- Fu, T.E. and Z. Zhou, Senescent cells as a target for anti-aging interventions: From senolytics to immune therapies. J Transl Int Med, 2025, 13, 33–47. [Google Scholar]
- Nambiar, A. , et al. , Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine, 2023, 90, 104481. [Google Scholar]
- Mansfield, L. , et al. , Emerging insights in senescence: pathways from preclinical models to therapeutic innovations. NPJ Aging, 2024, 10, 53. [Google Scholar] [PubMed]
- Bendani, H. , et al. , Revolutionizing breast cancer immunotherapy by integrating AI and nanotechnology approaches: review of current applications and future directions. Bioelectron Med, 2025, 11, 13. [Google Scholar]
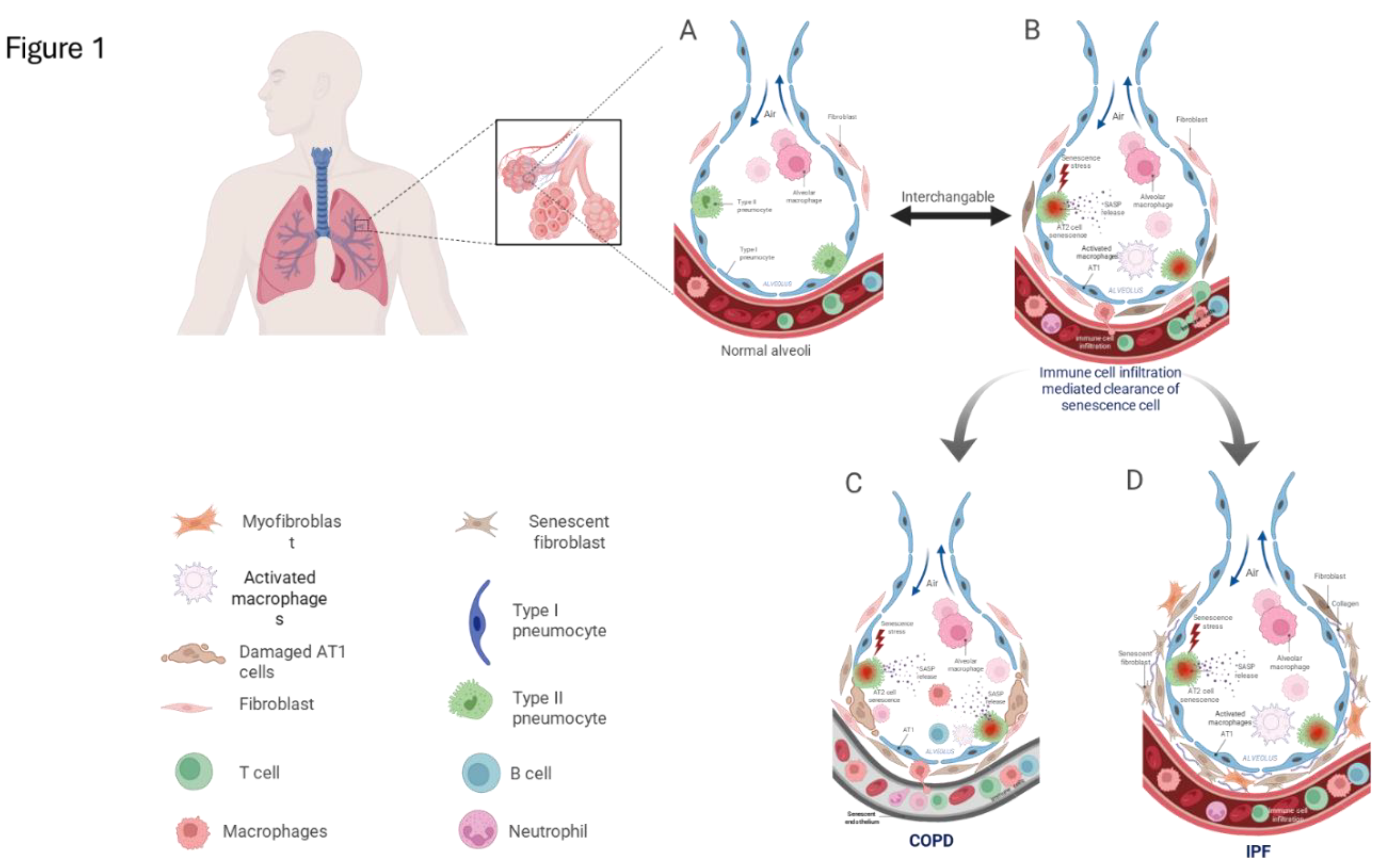
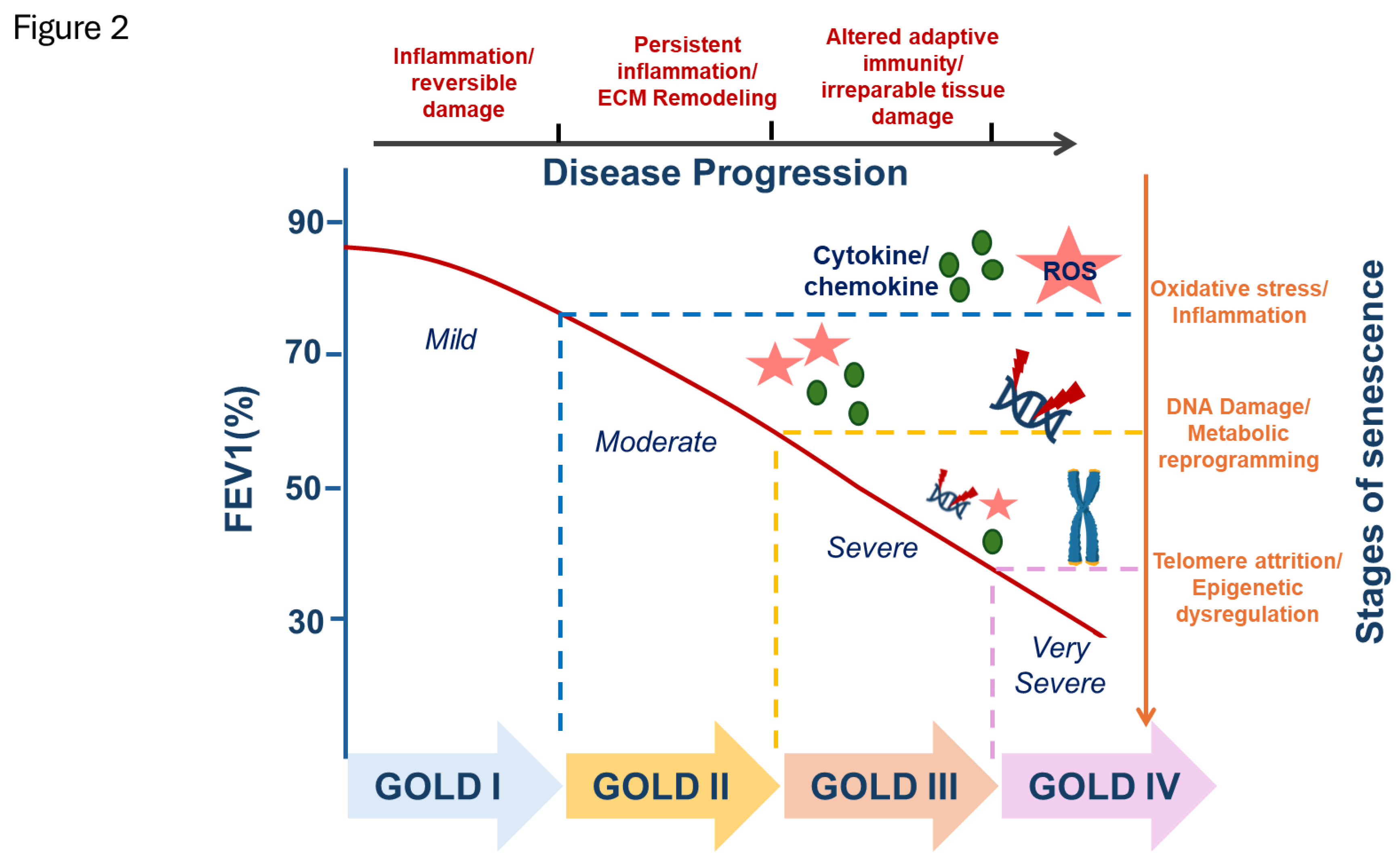
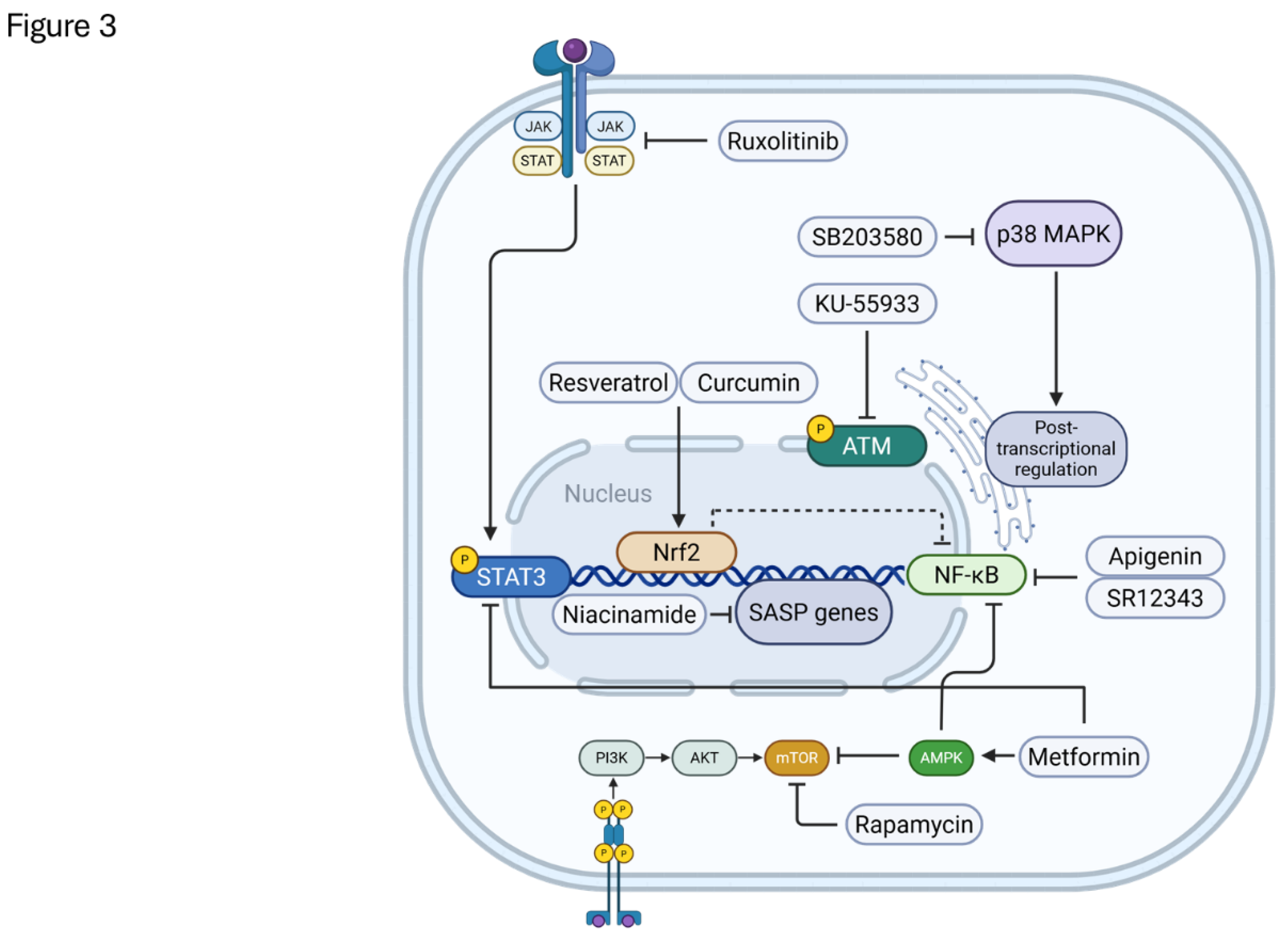
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




