Submitted:
18 August 2025
Posted:
19 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Material Used
2.2. Preparation of H-ZSM-5 Zeolite Catalysts
2.3. Catalyst Preparation
2.4. Catalyst Characterization
2.5. Catalytic Tests
3. Results and Discussion
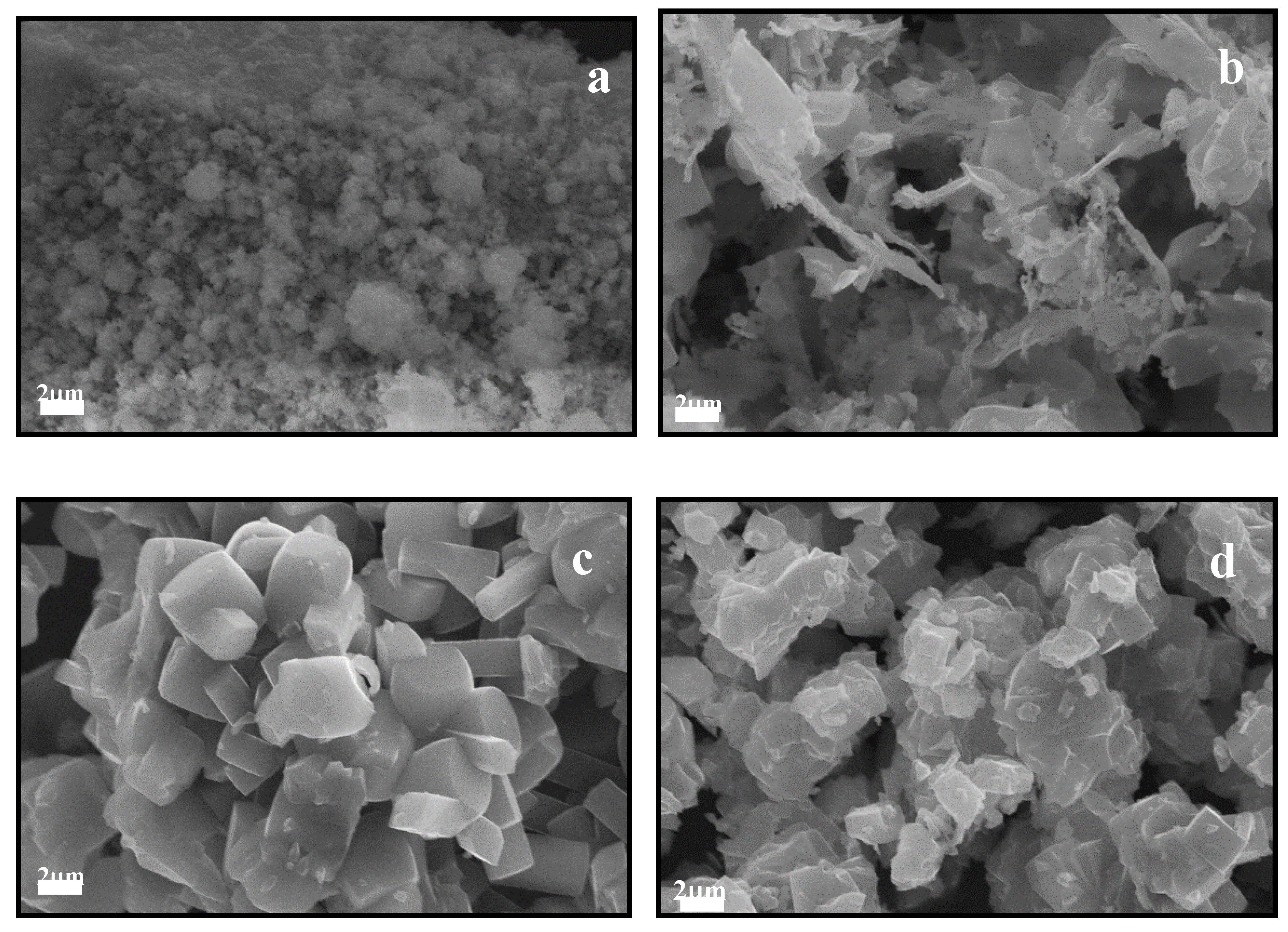
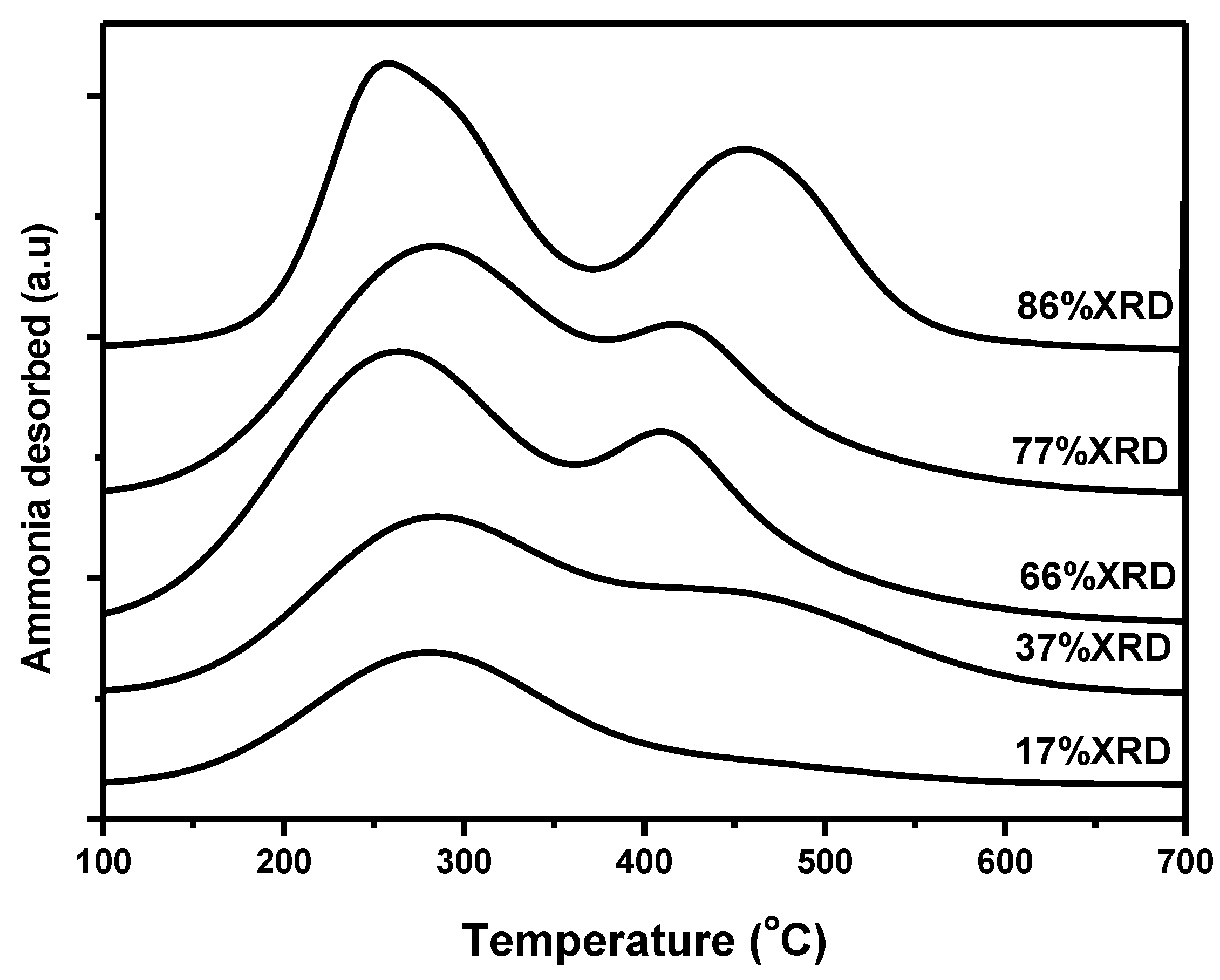
3.1. Catalyst Characterization
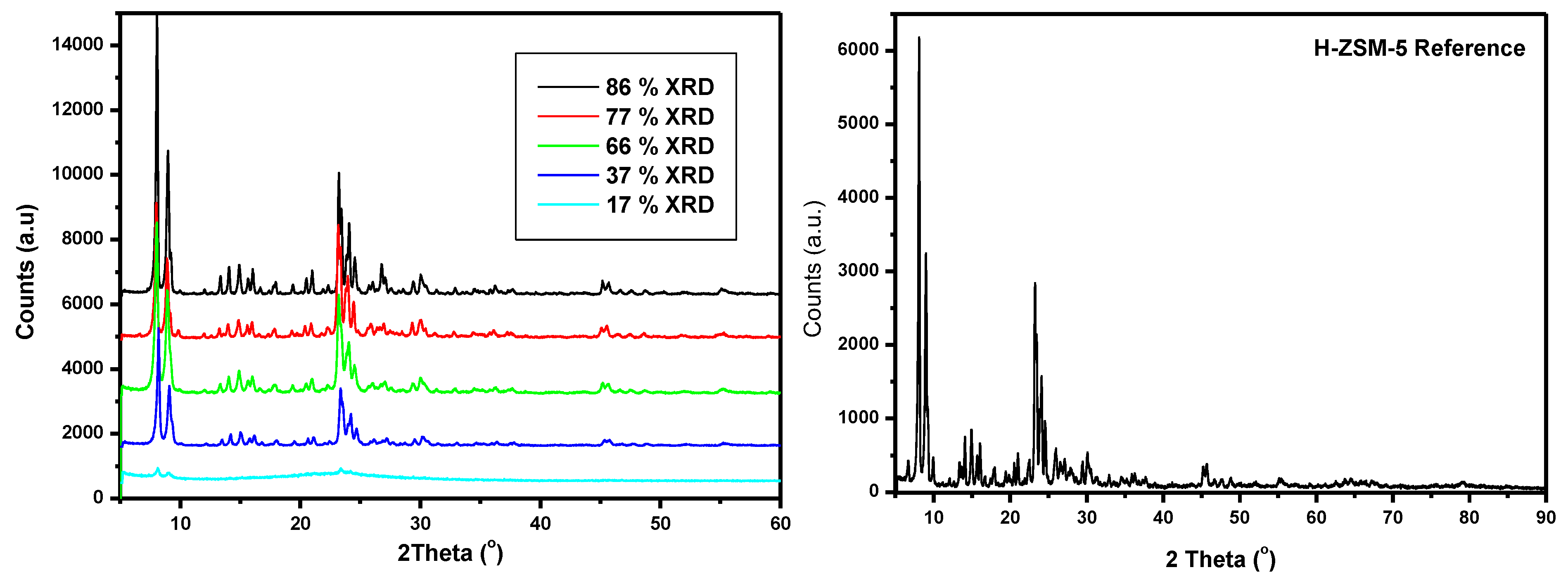
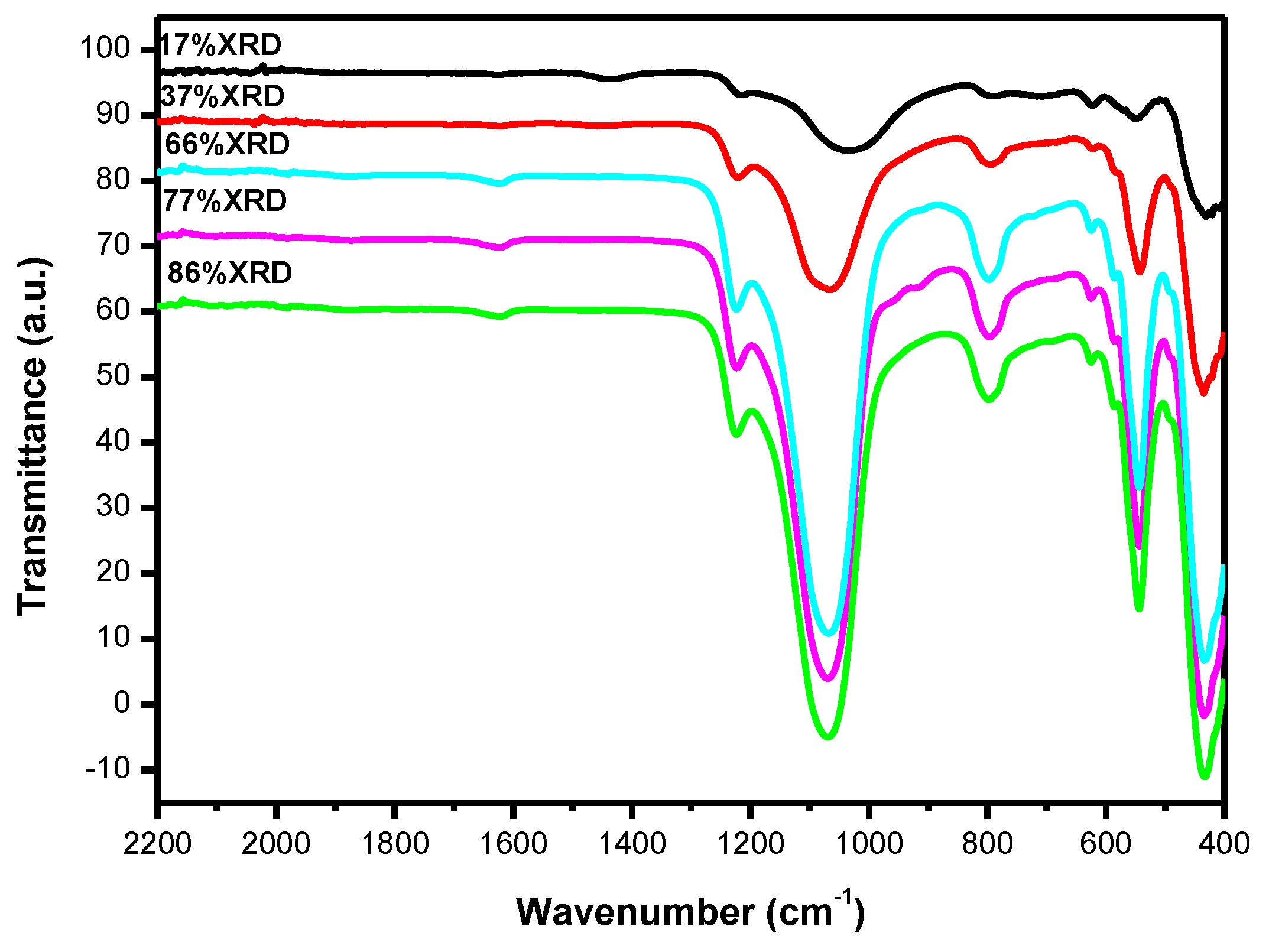
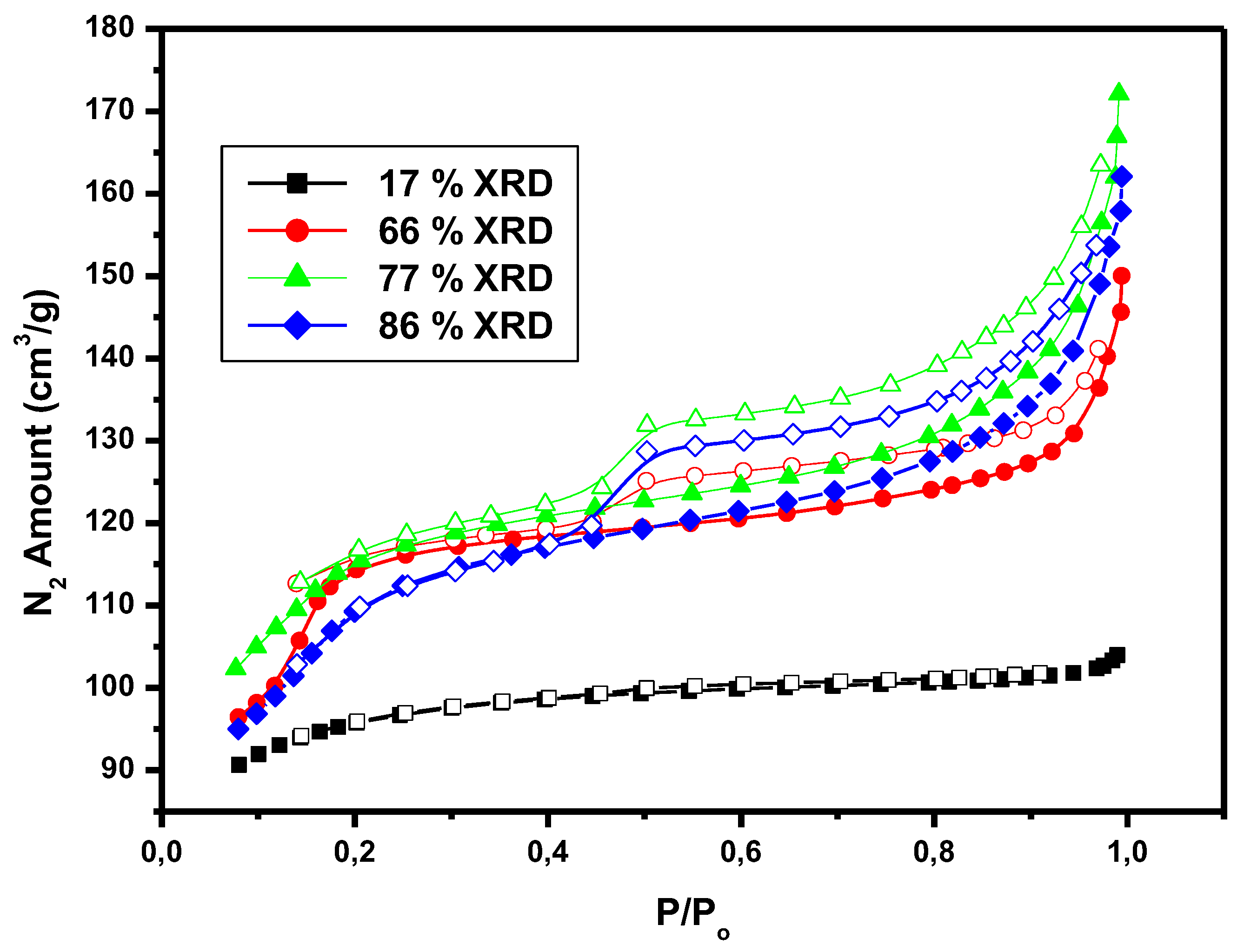
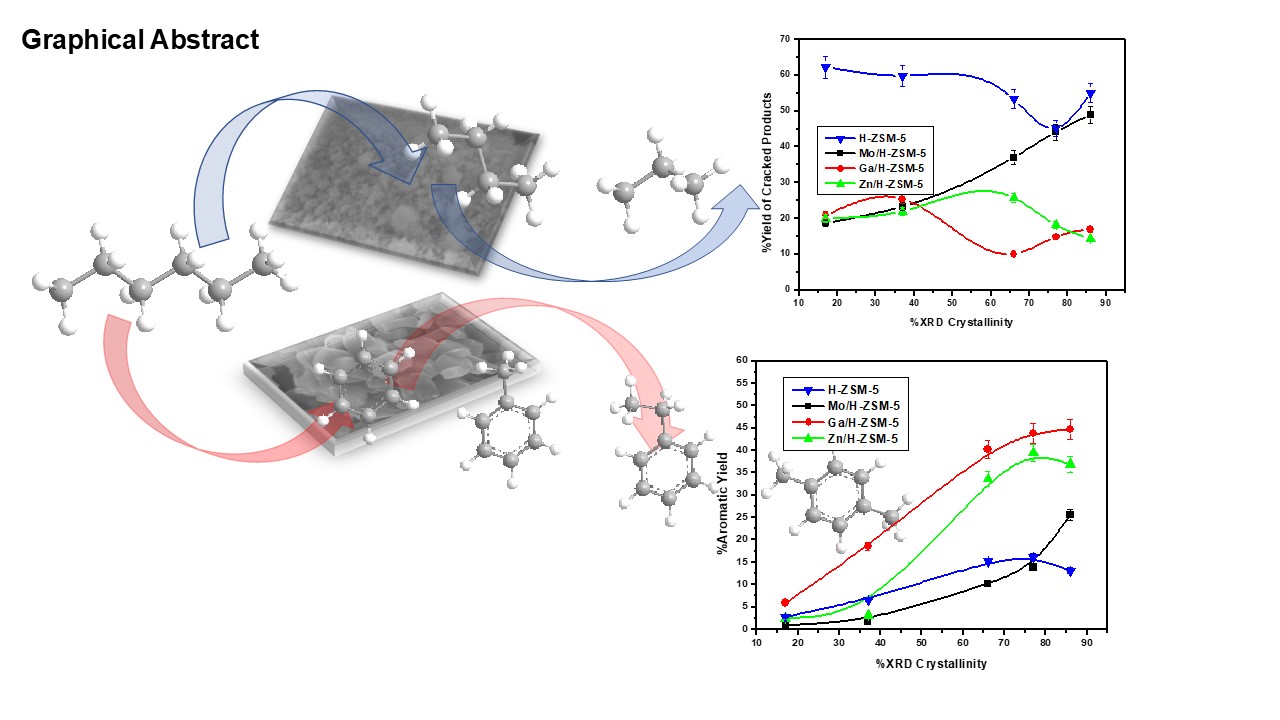
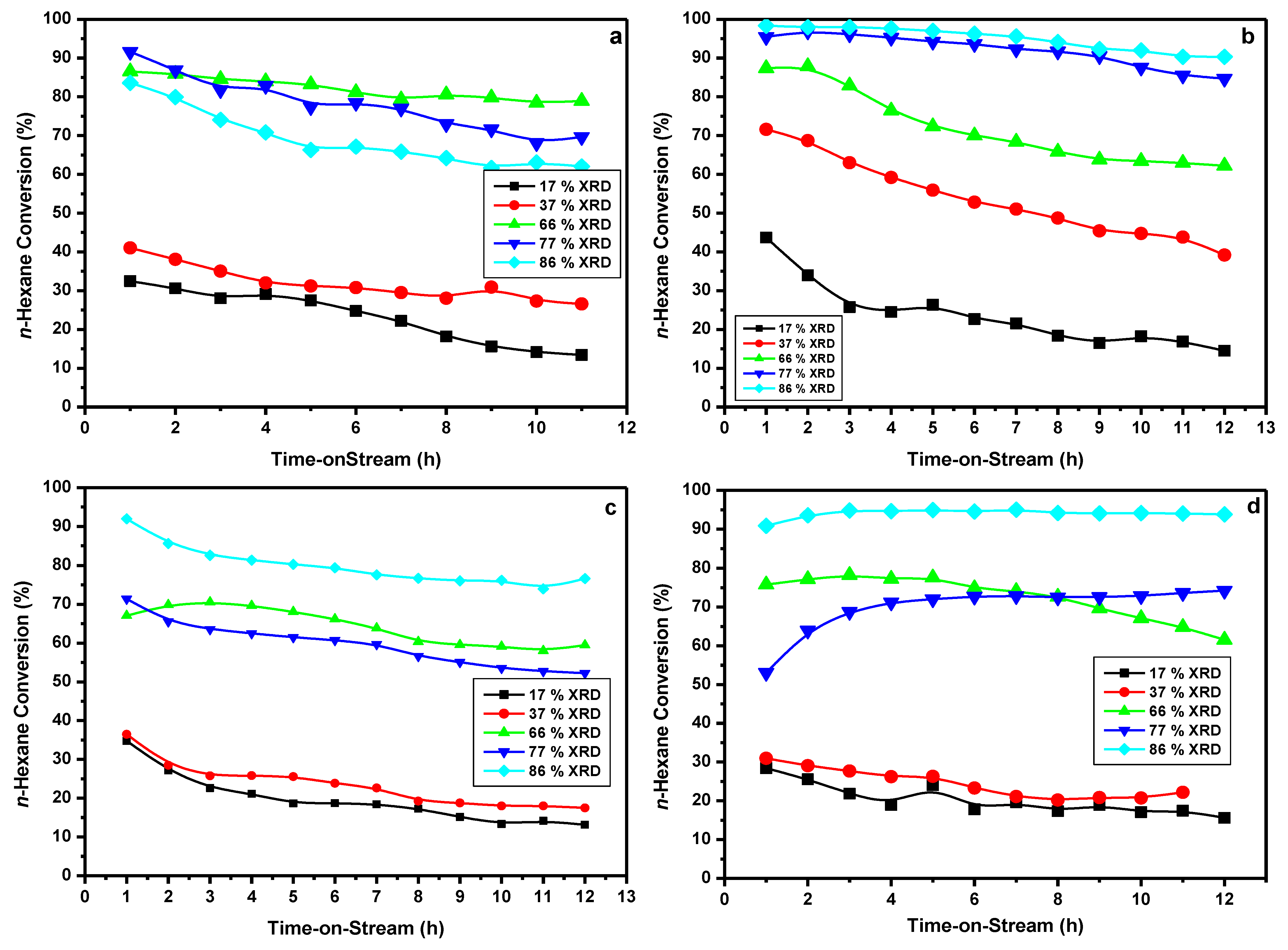
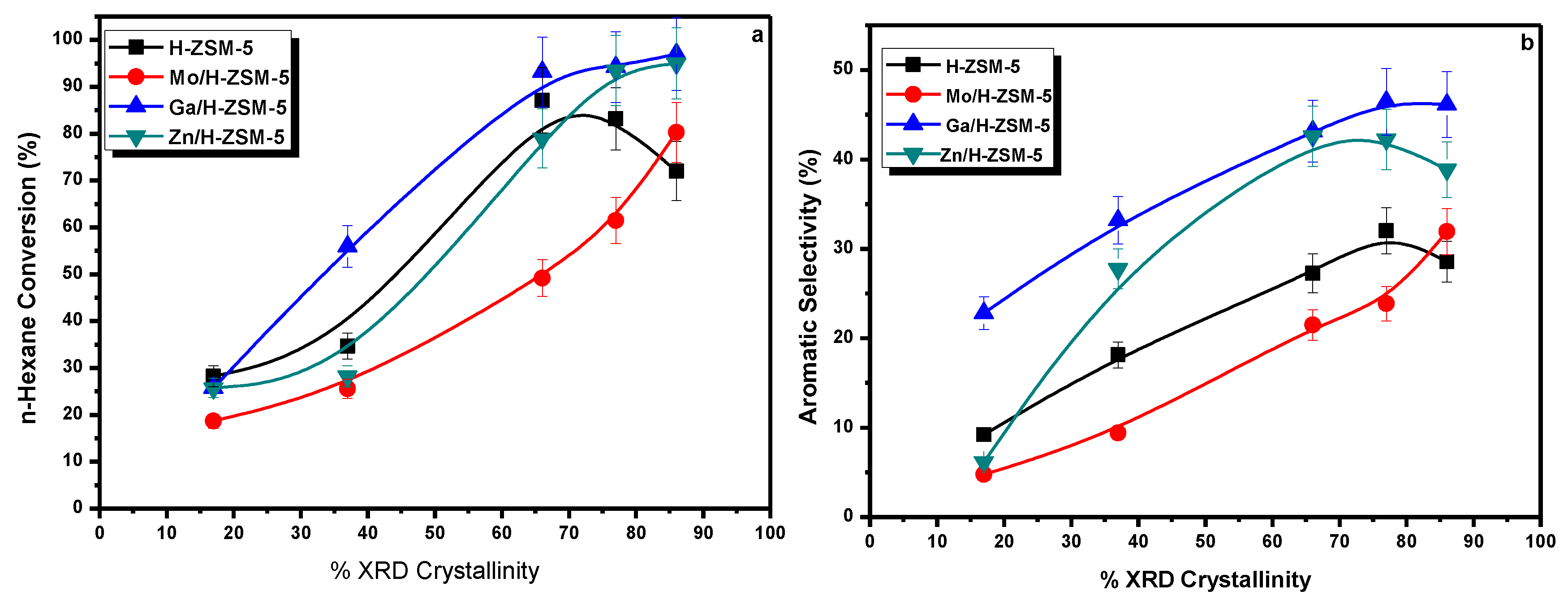
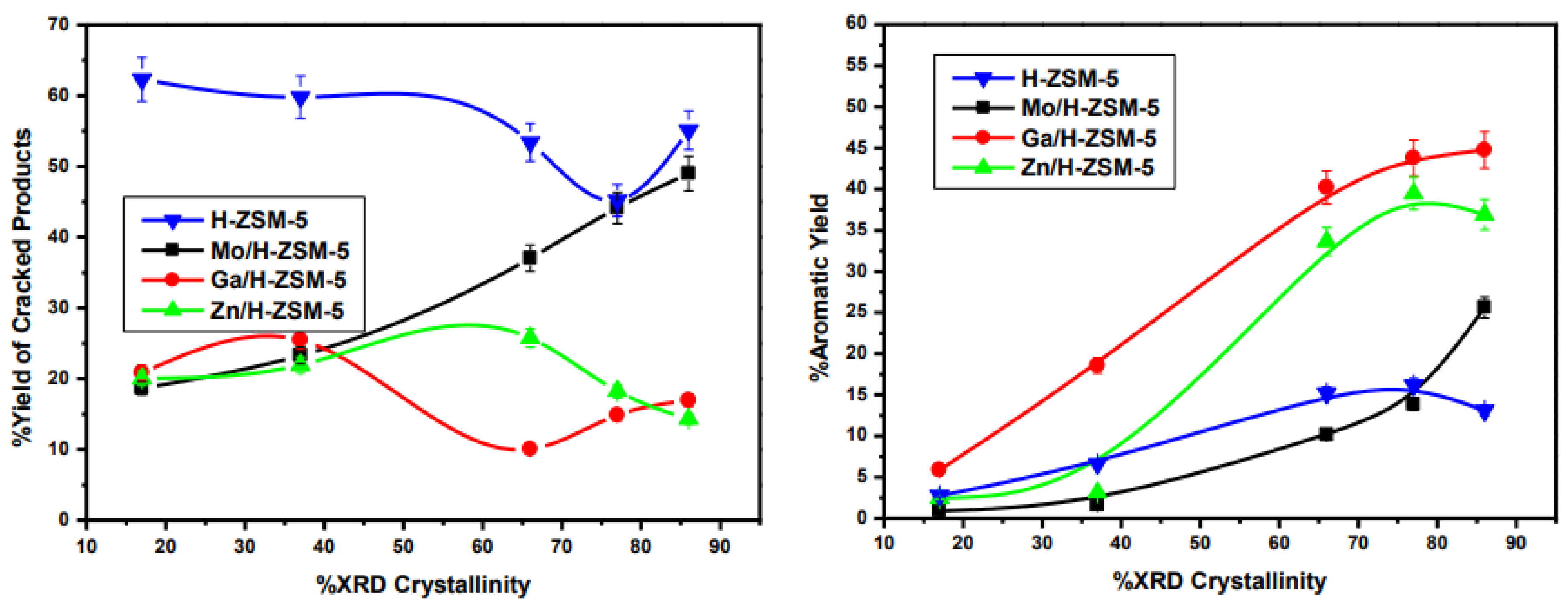
3.2. Catalytic Activity Results
4. Conclusions
Acknowledgments
References
- Smiešková, E. Rojasová, P. Hudec, L. Šabo, Applied Catalysis A: General 268 (2004) 235–240.
- Bhan, W. Nicholas Delgass, Catalysis Reviews 50 (2008) 19–151.
- J. Jae, G.A. Tompsett, A.J. Foster, K.D. Hammond, S.M. Auerbach, R.F. Lobo, G.W. Huber, Journal of Catalysis 279 (2011) 257–268.
- X. Jia, W. Khan, Z. Wu, J. Choi, A.C.K. Yip, Advanced Powder Technology 30 (2019) 467–484.
- S.M. Csicsery, Zeolites 4 (1984) 202–213.
- Y. Fang, F. Yang, X. He, X. Zhu, Frontiers of Chemical Science and Engineering 13 (2019) 543–553.
- I.B. Dauda, M. Yusuf, S. Gbadamasi, M. Bello, A.Y. Atta, B.O. Aderemi, B.Y. Jibril, ACS Omega 5 (2020) 2725–2733.
- M.W. Schreiber, C.P. Plaisance, M. Baumgärtl, K. Reuter, A. Jentys, R. Bermejo-Deval, J.A. Lercher, Journal of the American Chemical Society 140 (2018) 4849–4859.
- X. Zhao, Y. Chu, G. Qi, Q. Wang, W. Gao, X. Wang, S. Li, J. Xu, F. Deng, Chem. Commun. 56 (2020) 12029–12032.
- T.E. Tshabalala, Aromatization of N-Hexane over Metal Modified H-ZSM-5 Zeolite Catalysts, 2009.
- Q. Zhang, X. Liu, S. Hu, G. Ye, X. Zhou, W. Yuan, AIChE Journal n/a (2021) e17355.
- M. Xin, E. Xing, X. Gao, Y. Wang, Y. Ouyang, G. Xu, Y. Luo, X. Shu, Industrial & Engineering Chemistry Research 58 (2019) 6970–6981.
- T.E. Tshabalala, M.S. Scurrell, Catalysis Communications 72 (2015).
- C.P. Nicolaides, N.P. Sincadu, M.S. Scurrell, Catalysis Today 71 (2002) 429–435.
- C.P. Nicolaides, N.P. Sincadu, M.S. Scurrell, in: E. van Steen, M. Claeys, L.H.B.T.-S. in S.S. and C. Callanan (Eds.), Recent Advances in the Science and Technology of Zeolites and Related Materials, Elsevier, 2004, pp. 2347–2352.
- C.P. Nicolaides, N.P. Sincadu, M.S. Scurrell, in: E. Iglesia, J.J. Spivey, T.H.B.T.-S. in S.S. and C. Fleisch (Eds.), Natural Gas Conversion VI, Elsevier, 2001, pp. 333–338.
- C.S. Cundy, P.A. Cox, Microporous and Mesoporous Materials 82 (2005) 1–78.
- T. Horikawa, D.D. Do, D. Nicholson, Advances in Colloid and Interface Science 169 (2011) 40–58.
- Huang, C.H. Bartholomew, B.F. Woodfield, Microporous and Mesoporous Materials 184 (2014) 112–121.
- K.S. Triantafyllidis, L. Nalbandian, P.N. Trikalitis, A.K. Ladavos, T. Mavromoustakos, C.P. Nicolaides, Microporous and Mesoporous Materials 75 (2004) 89–100.
- X. Su, W. Zan, X. Bai, G. Wang, W. Wu, Catalysis Science & Technology 7 (2017) 1943–1952.
- N. Viswanadham, G. Muralidhar, T.S.R.P. Rao, Journal of Molecular Catalysis A: Chemical 223 (2004) 269–274.
- Q. Li, F. Zhang, J. Jarvis, P. He, M.M. Yung, A. Wang, K. Zhao, H. Song, Fuel 219 (2018) 331–339.
- M.S. Beheshti, M. Behzad, J. Ahmadpour, H. Arabi, Microporous and Mesoporous Materials 291 (2020) 109699.
- Wang, J. Xu, G. Qi, Y. Gong, W. Wang, P. Gao, Q. Wang, N. Feng, X. Liu, F. Deng, Journal of Catalysis 332 (2015) 127–137.
- S.K. Saxena, N. Viswanadham, Applied Materials Today 5 (2016) 25–32.
- J. Deischter, K. Schute, D.S. Neves, B.E. Ebert, L.M. Blank, R. Palkovits, Green Chemistry 21 (2019) 1710–1717.
- J. Wang, J. Jiang, X. Wang, S. Pang, Y. Sun, X. Meng, M. Li, R. Ruan, A.J. Ragauskas, Fuel 278 (2020) 118322.
| %XRD Crystallinity | Specific Surface Areas | Pore Volume | Total Pore Volume(cm3/g) | |||
|---|---|---|---|---|---|---|
| SBET (m2/g) |
SEXT (m2/g) |
VMicro (cm3/g) |
VMeso (cm3/g) |
|||
| 17 | 165 | 89 | 0.099 | 0.078 | 0.177 | |
| 37 | 224 | 105 | 0.110 | 0.049 | 0.158 | |
| 66 | 335 | 144 | 0.103 | 0.080 | 0.183 | |
| 77 | 382 | 152 | 0.166 | 0.055 | 0.211 | |
| 86 | 408 | 183 | 0.183 | 0.048 | 0.231 | |
| Catalysts | %XRD Crystallinity |
%Conversion |
Product Yield (%) | ||||
|---|---|---|---|---|---|---|---|
| Benzene | Toluene | Et-Benzene | o-Xylene | m,p-Xylene | |||
| H-ZSM-5 | 17 | 27.5 | 1.3 | 0.74 | 0.53 | 0.04 | 0.14 |
| 37 | 31.2 | 1.8 | 2.3 | 1.4 | 0.17 | 0.95 | |
| 66 | 83.8 | 4.1 | 3.4 | 2.7 | 1.6 | 1.3 | |
| 77 | 77.4 | 2.1 | 6.4 | 4.8 | 1.6 | 1.3 | |
| 86 | 66.3 | 1.6 | 4.8 | 4.2 | 1.8 | 0.70 | |
| Ga/H-ZSM-5 | 17 | 25.7 | 1.6 | 2.1 | 1.8 | 0.32 | 0.17 |
| 37 | 55.9 | 4.6 | 7.4 | 4.4 | 2.05 | 2.3 | |
| 66 | 93.2 | 8.2 | 10.0 | 7.6 | 5.9 | 8.5 | |
| 77 | 94.2 | 9.9 | 13.3 | 6.2 | 6.6 | 7.7 | |
| 86 | 97.0 | 8.2 | 15.5 | 9.0 | 6.7 | 5.4 | |
| Zn/H-ZSM-5 | 17 | 24.6 | 1.3 | 0.69 | 0.17 | 0.02 | 0,00 |
| 37 | 22.7 | 2.8 | 1.12 | 0.37 | 0.05 | 0.00 | |
| 66 | 79.0 | 9.5 | 13.3 | 6.6 | 3.3 | 0.9 | |
| 77 | 93.5 | 11.1 | 12.1 | 7.5 | 5.7 | 6.1 | |
| 86 | 95.0 | 11.7 | 9.8 | 5.5 | 3.8 | 3.0 | |
| Mo/H-ZSM-5 | 17 | 17.6 | 0.38 | 0.42 | 0.26 | 0.02 | 0.02 |
| 37 | 21.5 | 0.24 | 0.71 | 0.48 | 0.04 | 0.15 | |
| 66 | 49.2 | 1.5 | 4.1 | 2.5 | 1.2 | 1.0 | |
| 77 | 61.5 | 1.9 | 5.7 | 3.8 | 1.3 | 1.1 | |
| 86 | 80.3 | 5.3 | 10.6 | 6.0 | 2.0 | 1.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





