Submitted:
18 August 2025
Posted:
18 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Patients
2.2. Examination
2.3. Treatment
2.4. HFs Counting Methods
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographics
3.2. Outcomes
4. Discussion
5. Conclusions
Authorship
Competing Interests
Author Contributions
Funding
Institutional Review Board Statement
References
- Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, Rothschild PR, Omri S, Gélizé E, Jonet L, Delaunay K, De Kozak Y, Berdugo M, Zhao M, Crisanti P, Behar-Cohen F. Mechanisms of macular edema: Beyond the surface. Progress in retinal and eye research 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Thomas RL, Dunstan FD, Luzio SD, Chowdhury SR, North RV, Hale SL, Gibbins RL, Owens DR Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. The British journal of ophthalmology 2015, 99, 64–68. [CrossRef]
- Das A, McGuire PG, Rangasamy S Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology 2015, 122, 1375–1394. [CrossRef]
- Elnahry AG, Noureldine AM, Abdel-Kader AA, Sorour OA, Ramsey DJ Optical Coherence Tomography Angiography Biomarkers Predict Anatomical Response to Bevacizumab in Diabetic Macular Edema. Diabetes, metabolic syndrome and obesity: targets and therapy 2022, 15, 395–405. [CrossRef]
- Tatsumi T Current Treatments for Diabetic Macular Edema. International journal of molecular sciences 2023, 24. [CrossRef]
- Huang H, Jansonius NM, Chen H, Los LI Hyperreflective Dots on OCT as a Predictor of Treatment Outcome in Diabetic Macular Edema: A Systematic Review. Ophthalmology Retina 2022, 6, 814–827. [CrossRef]
- Okuwobi IP, Ji Z, Fan W, Yuan S, Bekalo L, Chen Q Automated Quantification of Hyperreflective Foci in SD-OCT With Diabetic Retinopathy. IEEE journal of biomedical and health informatics 2020, 24, 1125–1136. [CrossRef]
- De Benedetto U, Sacconi R, Pierro L, Lattanzio R, Bandello F Optical coherence tomographic hyperreflective foci in early stages of diabetic retinopathy. Retina (Philadelphia, Pa) 2015, 35, 449–453. [CrossRef]
- Waldstein SM, Vogl WD, Bogunovic H, Sadeghipour A, Riedl S, Schmidt-Erfurth U Characterization of Drusen and Hyperreflective Foci as Biomarkers for Disease Progression in Age-Related Macular Degeneration Using Artificial Intelligence in Optical Coherence Tomography. JAMA ophthalmology 2020, 138, 740–747. [CrossRef]
- Duic C, Pfau K, Keenan TDL, Wiley H, Thavikulwat A, Chew EY, Cukras C Hyperreflective Foci in Age-Related Macular Degeneration are Associated with Disease Severity and Functional Impairment. Ophthalmology Retina 2023, 7, 307–317. [CrossRef]
- Lee SY, Yoon CK, Park UC, Park KH, Lee EK. Choroidal hyperreflective foci as biomarkers of severity in stargardt disease. Retina (Philadelphia, Pa) 2025, 45, 774–784. [Google Scholar] [CrossRef]
- Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology 2009, 116, 914–920. [CrossRef]
- Chen NN, Chen WD, Lai CH, Kuo CN, Chen CL, Huang JC, Wu PC, Wu PL, Chen CY Optical Coherence Tomographic Patterns as Predictors of Structural Outcome After Intravitreal Ranibizumab in Diabetic Macula Edema. Clinical ophthalmology 2020, 14, 4023–4030. [CrossRef]
- von Schulthess EL, Maunz A, Chakravarthy U, Holekamp N, Pauleikhoff D, Patel K, Bachmeier I, Yu S, Cohen Y, Scherb MP, Jones IL, Gibson K, Willis JR, Glittenberg C, Singh RP, Fauser S. Intraretinal Hyper-Reflective Foci Are Almost Universally Present and Co-Localize With Intraretinal Fluid in Diabetic Macular Edema. Investigative ophthalmology & visual science 2024, 65, 26. [Google Scholar] [CrossRef]
- Wu J, Zhang C, Yang Q, Xie H, Zhang J, Qiu Q, Liu K, Luo D, Liu F, Zhang J Imaging Hyperreflective Foci as an Inflammatory Biomarker after Anti-VEGF Treatment in Neovascular Age-Related Macular Degeneration Patients with Optical Coherence Tomography Angiography. BioMed research international 2021, 2021, 6648191. [CrossRef]
- Frizziero L, Midena G, Danieli L, Torresin T, Perfetto A, Parrozzani R, Pilotto E, Midena E Hyperreflective Retinal Foci (HRF): Definition and Role of an Invaluable OCT Sign. Journal of clinical medicine 2025, 14. [CrossRef]
- Vujosevic S, Bini S, Midena G, Berton M, Pilotto E, Midena E Hyperreflective intraretinal spots in diabetics without and with nonproliferative diabetic retinopathy: an in vivo study using spectral domain OCT. Journal of diabetes research 2013, 2013, 491835. [CrossRef]
- Uji A, Murakami T, Nishijima K, Akagi T, Horii T, Arakawa N, Muraoka Y, Ellabban AA, Yoshimura N Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. American journal of ophthalmology 2012, 153, 710–717. [CrossRef]
- Chung YR, Lee SY, Kim YH, Byeon HE, Kim JH, Lee K Hyperreflective foci in diabetic macular edema with serous retinal detachment: association with dyslipidemia. Acta diabetologica 2020, 57, 861–866. [CrossRef]
- Hatamnejad A, Orr S, Dadak R, Khanani A, Singh R, Choudhry N Anti-VEGF and steroid combination therapy relative to anti-VEGF mono therapy for the treatment of refractory DME: A systematic review of efficacy and meta-analysis of safety. Acta ophthalmologica 2024, 102, e204–e214. [CrossRef]
- Sharma D, Zachary I, Jia H Mechanisms of Acquired Resistance to Anti-VEGF Therapy for Neovascular Eye Diseases. Investigative ophthalmology & visual science 2023, 64, 28. [CrossRef]
- Agostini H, Abreu F, Baumal CR, Chang DS, K GC, Demetriades AM, Kodjikian L, Lim JI, Margaron P, Monés JM, Peto T, Ricci F, Rüth M, Singh RP, Stoilov I, Swaminathan B, Willis JR, Westenskow PD Faricimab for neovascular age-related macular degeneration and diabetic macular edema: from preclinical studies to phase 3 outcomes. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 2024, 262, 3437–3451. [CrossRef]
- Chatziralli I Ranibizumab for the treatment of diabetic retinopathy. Expert opinion on biological therapy 2021, 21, 991–997. [CrossRef]
- Tang L, Luo D, Qiu Q, Xu GT, Zhang J Hyperreflective Foci in Diabetic Macular Edema with Subretinal Fluid: Association with Visual Outcomes after Anti-VEGF Treatment. Ophthalmic research 2023, 66, 39–47. [CrossRef]
- Al-Latayfeh M, Abdel Rahman M, Shatnawi R Outcome of Single Dexamethasone Implant Injection in the Treatment of Persistent Diabetic Macular Edema After Anti-VEGF Treatment: Real-Life Data from a Tertiary Hospital in Jordan. Clinical ophthalmology 2021, 15, 1285–1291. [CrossRef]
- Vujosevic S, Lupidi M, Donati S, Astarita C, Gallinaro V, Pilotto E Role of inflammation in diabetic macular edema and neovascular age-related macular degeneration. Survey of ophthalmology 2024, 69, 870–881. [CrossRef]
- Yao X, Zhao Z, Zhang W, Liu R, Ni T, Cui B, Lei Y, Du J, Ai D, Jiang H, Lv H, Li X Specialized Retinal Endothelial Cells Modulate Blood-Retina Barrier in Diabetic Retinopathy. Diabetes 2024, 73, 225–236. [CrossRef]
- Utsumi T, Noma H, Yasuda K, Goto H, Shimura M Effects of ranibizumab on growth factors and mediators of inflammation in the aqueous humor of patients with diabetic macular edema. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 2021, 259, 2597–2603. [CrossRef]
- Taloni A, Coco G, Rastelli D, Buffon G, Scorcia V, Giannaccare G Safety and Efficacy of Dexamethasone Intravitreal Implant Given Either First-Line or Second-Line in Diabetic Macular Edema. Patient preference and adherence 2023, 17, 3307–3329. [CrossRef]
- Liu S, Wang D, Chen F, Zhang X Hyperreflective foci in OCT image as a biomarker of poor prognosis in diabetic macular edema patients treating with Conbercept in China. BMC ophthalmology 2019, 19, 157. [CrossRef]
- Chatziralli IP, Sergentanis TN, Sivaprasad S. Hyperreflective foci as an independent visual outcome predictor in macular edema due to retinal vascular diseases treated with intravitreal dexamethasone or ranibizumab. Retina (Philadelphia, Pa) 2016, 36, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
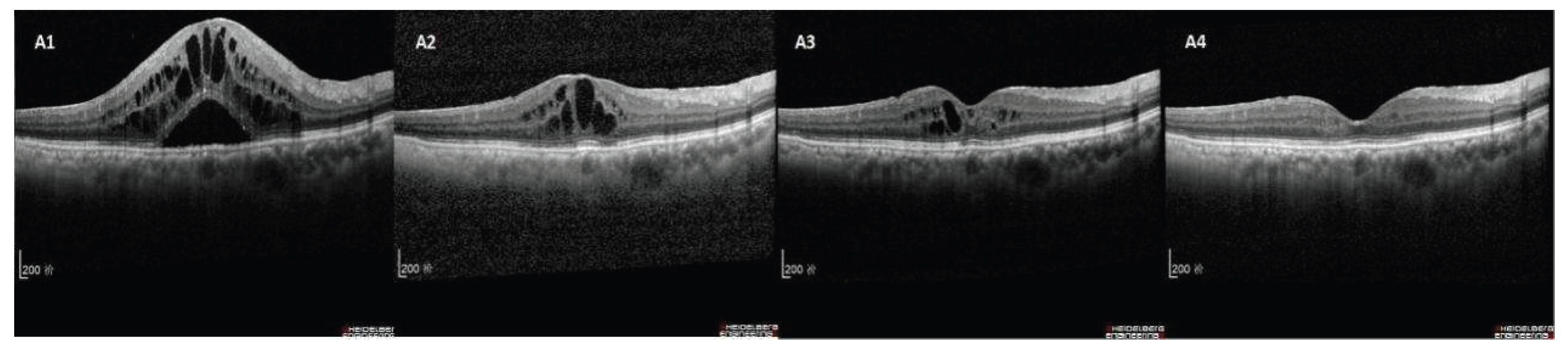
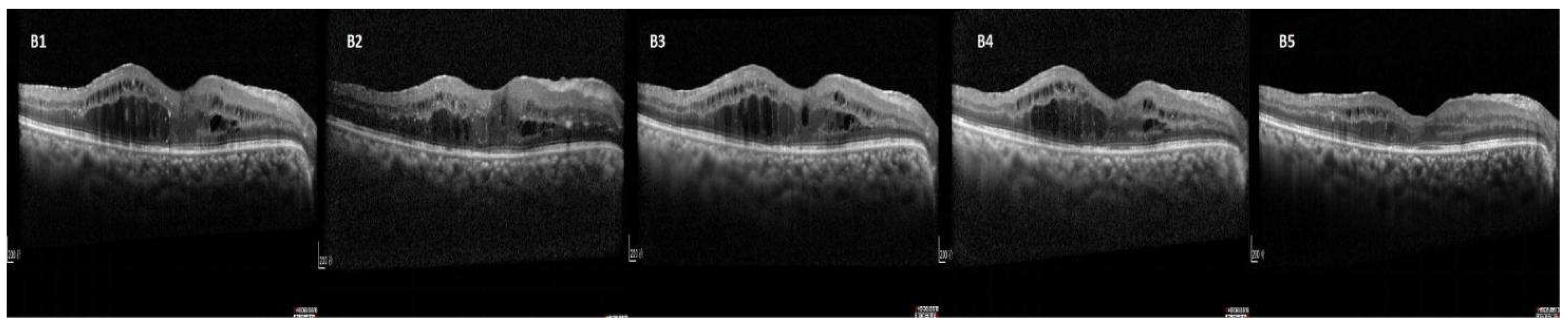
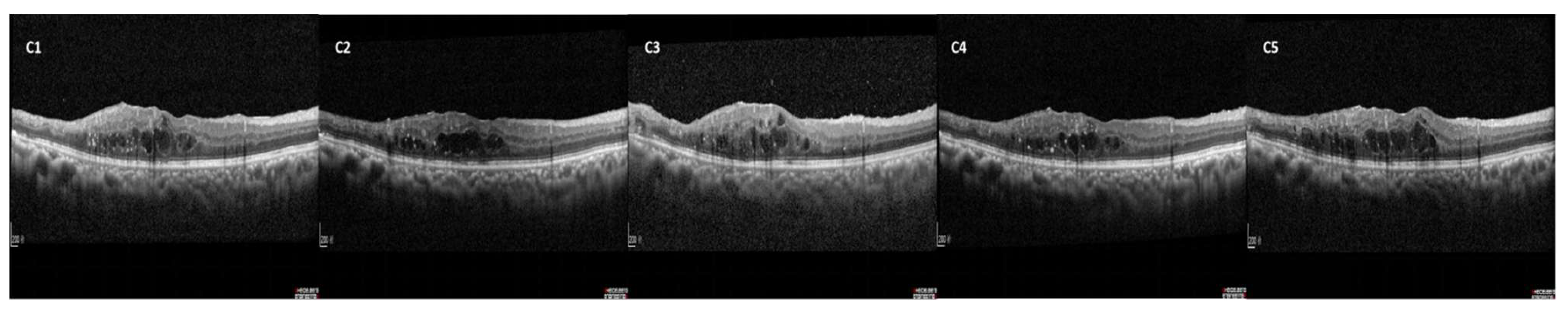
| Characteristics | responder group (n=73) |
no-responder group (n=39) | P value |
| Eyes/patients | 73/52 | 39/26 | - |
| Age (year), mean± SD | 59.27±7.16 | 55.63±7.73 | 0.776 |
| Male/female | 28/24 | 15/11 | 0.747 |
| BMI (kg/m2), mean± SD | 24.88±1.97 | 25.01±1.73 | 0.706 |
| Systemic profile: | |||
| HbA1c (%), mean± SD | 6.66±1.98 | 7.13±1.87 | 0.273 |
| Duration of DM (y), median (IQR) | 8.35(4.00,11.50) | 7.50(4.50,10.00) | 0.234 |
| Hypertension, n (%) | 20(38.5) | 11(42.3) | 0.744 |
| Ocular profile, n (%) | |||
| History of laser | 12 (16.4) | 7 (17.9) | 0.839 |
| pseudophakic | 9 (12.3) | 6 (15.4) | 0.651 |
| DRIL, n (%) | 2 (2.7) | 2 (5.1) | 0.516 |
| ELM/EZ disruption, n (%) | 3(4.1) | 2 (5.1) | 0.804 |
| LogMAR BCVA, mean± SD | 0.54±0.73 | 0.52±0.61 | 0.827 |
| IOP (mmHg), mean± SD | 15.30±3.47 | 14.83±3.55 | 0.709 |
| CMT (mm), mean± SD | 456.53±109.73 | 468.99±127.10 | 0.614 |
| Characteristics | responder group (n=26) | no-responder group (n=13) | P value |
| Eyes/patients | 26/17 | 13/9 | - |
| Age (year), mean± SD | 57.25±7.36 | 54.58±9.93 | 0.598 |
| Male/female | 10/7 | 5/4 | 0.873 |
| BMI (kg/m2), mean± SD | 24.99±2.73 | 25.11±1.63 | 0.698 |
| Systemic profile: | |||
| HbA1c (%), mean± SD | 7.08±1.17 | 7.19±1.56 | 0.673 |
| Duration of DM (y), median (IQR) | 7.35(4.25,10.50) | 7.80(4.35,11.00) | 0.304 |
| Hypertension, n (%) | 7(41.2) | 4(44.4) | 0.873 |
| Ocular profile, n (%) | |||
| History of laser | 5 (19.2) | 2 (15.4) | 0.768 |
| pseudophakic | 4 (15.4) | 2 (15.4) | 1.000 |
| DRIL, n (%) | 1 (3.8) | 1 (7.7) | 0.608 |
| ELM/EZ disruption, n (%) | 1 (3.8) | 1 (7.7) | 0.608 |
| LogMAR BCVA, mean± SD | 0.53±0.39 | 0.51±0.67 | 0.772 |
| IOP (mmHg), mean± SD | 14.60±4.71 | 15.03±2.59 | 0.609 |
| CMT (mm), mean± SD | 472.53±112.03 | 464.57±137.83 | 0.511 |
| BCVA (log MAR), mean ± SD | responder group (n=73) | no-responder group (n=39) | P value |
| Baseline | 0.54±0.73 | 0.52±0.61 | 0.827 |
| 1st injection | 0.44± 0.42 | 0.50± 0.50 | 0.044* |
| 2nd injection | 0.39± 0.37 | 0.49± 0.48 | 0.031* |
| 3rd injection | 0.35± 0.40 | 0.47± 0.38 | 0.027* |
| CMT (μm), mean ± SD | responder group (n=73) | no-responder group (n=39) | P value |
| Baseline | 456.53±109.73 | 468.99±127.10 | 0.614 |
| 1st injection | 337.77± 93.18 | 440.95± 99.01 | 0.027* |
| 2nd injection | 290.69± 66.38 | 433.18± 78.66 | 0.016* |
| 3rd injection | 235.47± 49.13 | 427.45± 52.91 | 0.008* |
| BCVA (log MAR), mean ± SD | responder group (n=73) |
no-responder group (n=39) | P value |
| Baseline | 0.53±0.39 | 0.51±0.67 | 0.772 |
| 1 month | 0.37± 0.41 | 0.43± 0.88 | 0.039* |
| CMT (μm), mean ± SD | responder group (n=73) | no-responder group (n=39) | P value |
| Baseline | 472.53±112.03 | 464.57±137.83 | 0.747 |
| 1 month | 255.99± 69.83 | 397.48± 82.77 | 0.014* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




