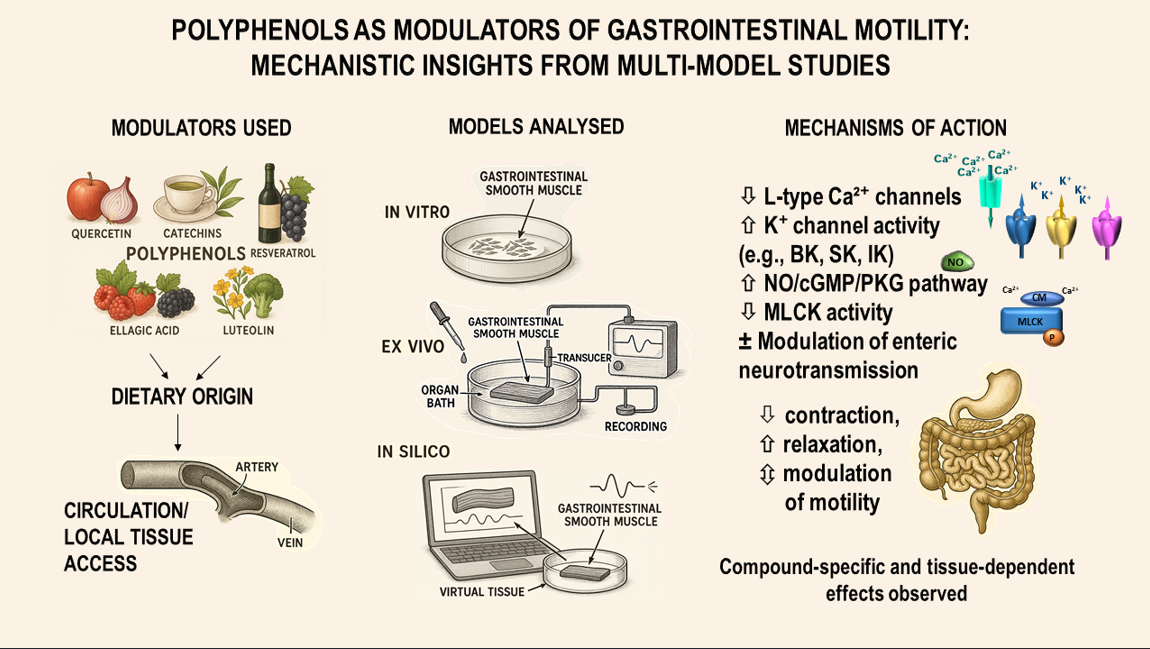
1. Introduction
Gastrointestinal (GI) motility, orchestrated by the coordinated activity of interstitial Cajal cells, enteric neurons, and GI smooth muscle cells, plays a fundamental role in processes such as digestion, nutrient absorption, and waste elimination. Dysregulation of this complex system can lead to a diverse array of clinical manifestations, including functional GI disorders like irritable bowel syndrome (IBS), functional dyspepsia, or postoperative ileus [
1]. However, existing pharmacological treatments for motility disorders frequently exhibit limited effectiveness and are often associated with adverse effects, underscoring the critical imperative for the development of novel, more precise, and safer therapeutic strategies [
2,
3].
Polyphenols, a chemically diverse class of secondary plant metabolites, have attracted significant attention owing to their wide-ranging biological properties, including antioxidant, anti-inflammatory, and neuromodulatory effects [
4,
5,
6]. Within this class, flavonoids represent the most prevalent and structurally versatile subclass, commonly found in fruits, vegetables, herbs, and various traditional medicinal preparations. Growing evidence indicates that polyphenols may influence GI motility by modulating key elements such as ion channels, neurotransmitter systems, and smooth muscle contraction mechanisms.
Despite a continuously expanding interest in the therapeutic potential of polyphenols for GI health, their precise effects on gut motility remain incompletely characterized. While certain polyphenols exhibit clear spasmolytic properties, others might exert prokinetic effects, depending on specific chemical structure, applied concentration, and the prevailing physiological or pathological condition of the GI tract.
This review offers a comprehensive and analytical synthesis of the contemporary experimental evidence concerning the effects of polyphenols (with a specific focus on flavonoids) on GI smooth muscle contractility. This organization, categorized by flavonoid subclass, aims to elucidate structure-activity relationships, delineate common mechanistic pathways, and identify lead candidates with potential for subsequent clinical translation.
2. Materials and Methods
This review was prepared through a structured and critical synthesis of the existing literature. A comprehensive search was executed across major electronic databases, specifically PubMed, Scopus, and Web of Science, encompassing articles published from January 1990 through January 2025.
The search strategy utilized various combinations of the following keywords: “polyphenols”, “flavonoids,” “gastrointestinal motility,” “smooth muscle”, “contraction”, “relaxation,” and specific compound names such as luteolin, quercetin, and catechin.
Inclusion criteria for selected articles were defined as follows:
Original research encompassing in vitro, ex vivo, and in vivo studies, peer-reviewed only.
Focus on the modulatory effects of individual polyphenols or polyphenol-rich extracts on gastrointestinal smooth muscle contractility.
Dose-response data were not required for inclusion.
Publication in the English language.
Conversely, studies were excluded if they met any of the following criteria:
Review articles, conference abstracts, opinion papers, or studies presenting no primary experimental data on contractility.
Articles primarily investigating antioxidant or anticancer effects without explicit relevance to GI smooth muscle function.
Following the initial search, titles and abstracts were independently screened by two researchers. Full texts of potentially relevant articles were then retrieved and precisely reviewed for data extraction. Furthermore, the reference lists of pivotal articles were cross-referenced to identify additional relevant studies (snowballing).
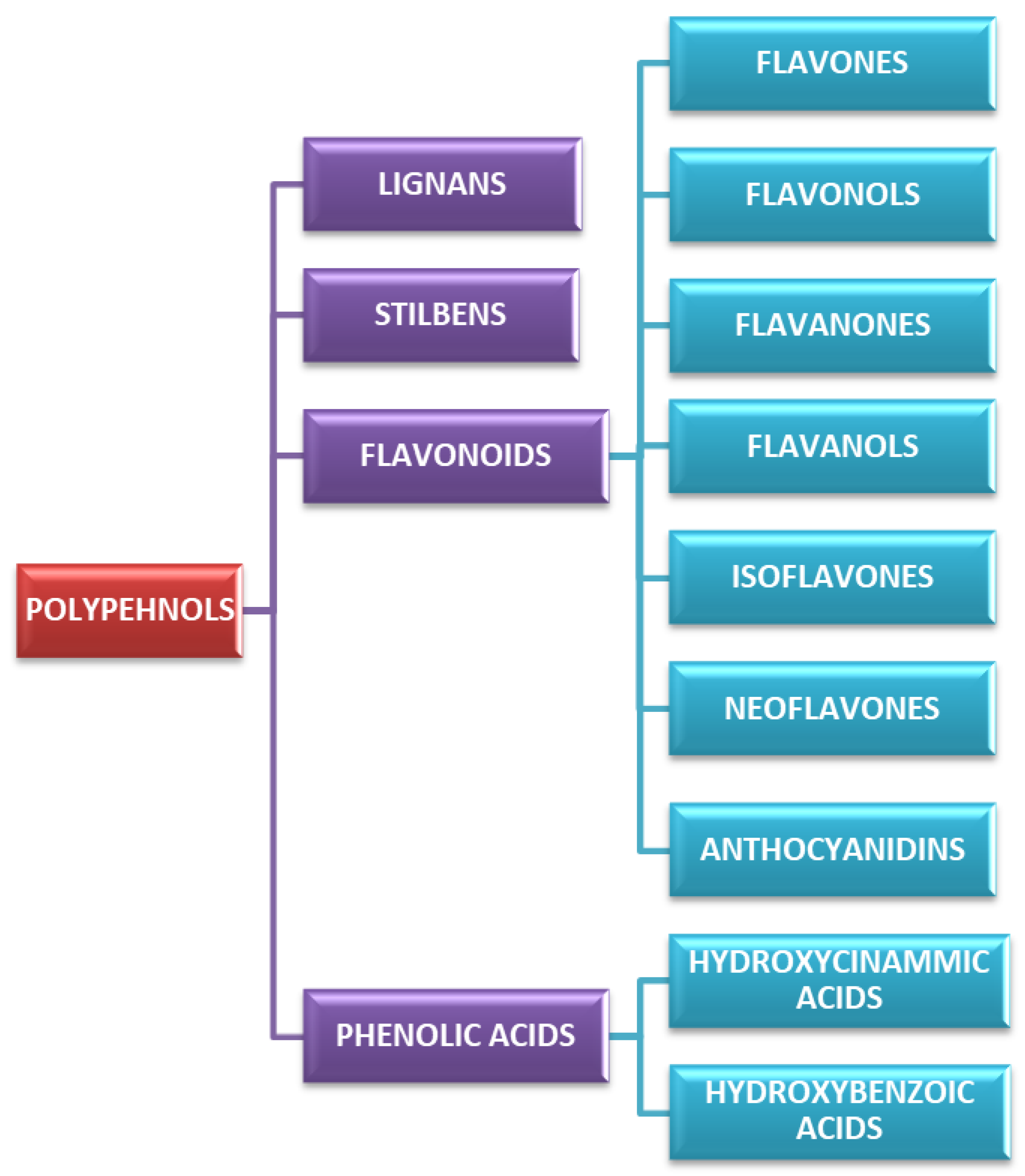
3. Classification and Chemistry of Polyphenols
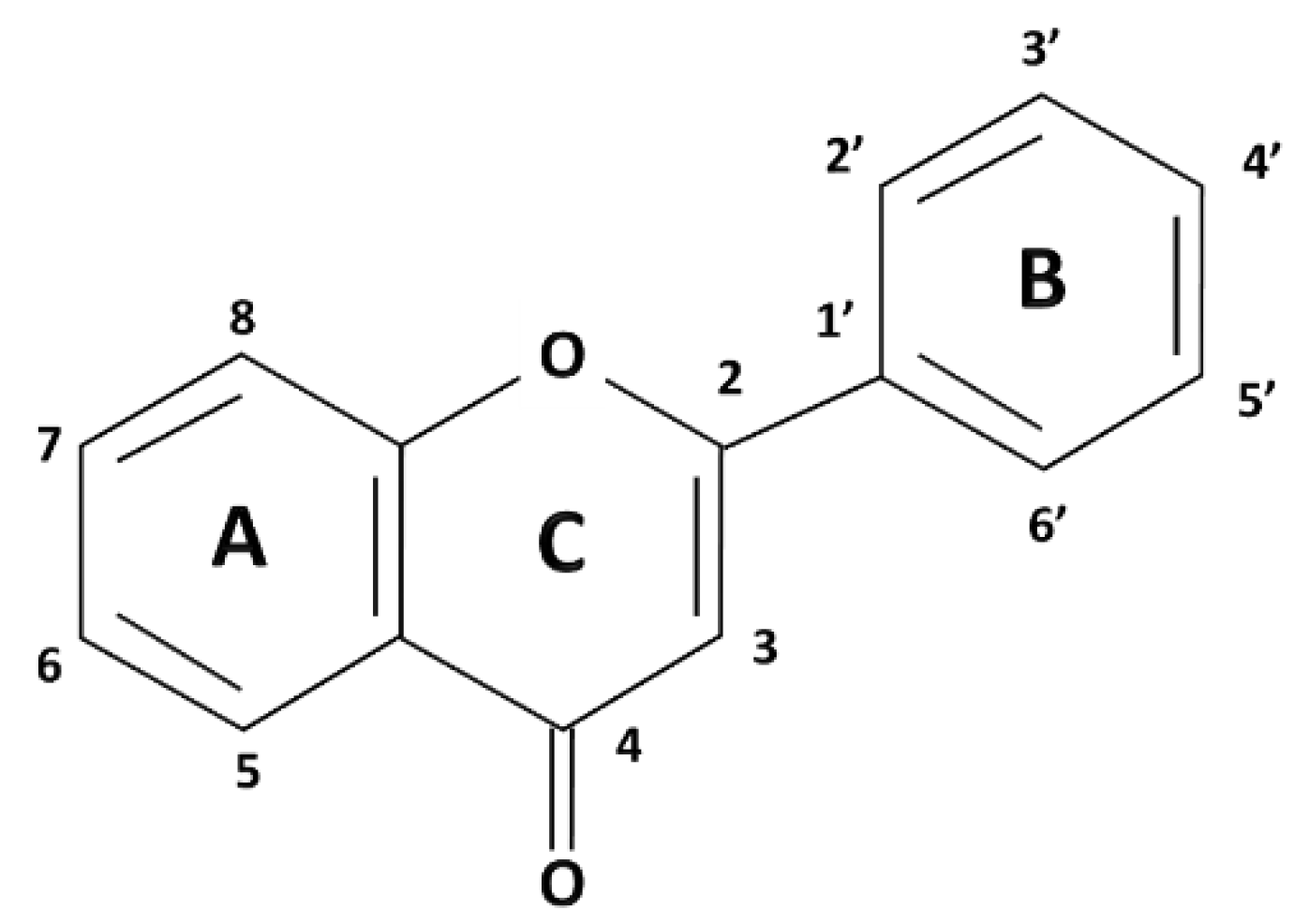
Polyphenols are a broad class of plant-derived compounds characterized by a chemical structure containing one or more hydroxyl groups directly bonded to at least one aromatic (phenyl) ring. Structurally, they are primarily categorized into two major groups: flavonoids, defined by their characteristic C6-C3-C6 carbon skeleton, and non-flavonoids (
Figure 1 and
Figure 2) [
7,
8].
Among polyphenols, flavonoids represent a highly prevalent and structurally diverse class, widely distributed across various dietary sources, including fruits, vegetables, herbs, and beverages such as tea and wine. Their defining structural characteristic is a common diphenylpropane (C6-C3-C6) backbone, consisting of two aromatic rings (A and B) linked by a three-carbon chain that forms a heterocyclic C-ring (
Figure 1). Further classification of flavonoids into six primary subclasses, namely flavones, flavonols, flavanones, flavanols (catechins), isoflavones, neoflavones, and anthocyanidins, is determined by the oxidation state and substitution pattern of this C-ring (
Figure 2).
Flavones, exemplified by compounds such as apigenin and luteolin, are structurally defined by a C2-C3 double bond and a C4 ketone group within their heterocyclic C-ring. Commonly, they feature hydroxylation at positions 5 and 7 of the A-ring and position 4 of the B-ring (
Figure 1) [
9,
10].
Flavonols, including well-known examples such as quercetin, kaempferol, and myricetin, are distinguished from flavones by the presence of a hydroxyl group at the C3 position of their heterocyclic C-ring
(Figure 1). This additional -OH not only enhances their antioxidant capacities but also significantly influences their interactions with various biological targets, including ion channels and enzymes [
11,
12,
13].
Flavanones such as naringenin and hesperidin are characterized by the absence of a C2=C3 double bond in their C-ring, which typically confers greater structural flexibility. These compounds are highly prevalent in citrus fruits and frequently occur in their glycosylated forms [
14,
15].
Flavanols, also known as catechins, with representatives including catechin, epicatechin, are distinct saturated flavonoids lacking both the C2=C3 double bond and the 4-keto group. These compounds are particularly abundant in tea, cocoa, and various fruits. Their galloylated derivatives often exhibit enhanced bioactivity [
16,
17].
Isoflavones, with representative compounds such as genistein and daidzein, are uniquely characterized by the attachment of their B-ring at the C3 position of the C-ring and are primarily found in soy products. Their structural similarity to estrogen allows them to act as phytoestrogens. Still, their effects on GI smooth muscle are distinct and varied, often involving direct modulation of ion channels and second messenger systems [
18,
19].
Neoflavones, with examples like dalbergin and coutareagenin, are a minor subclass of flavonoids characterised by attachment of the B-ring at the C4 position of the C-ring. They have diverse biological activities with recent research suggesting their chemoprotective capabilities [
20,
21].
Anthocyanidins, including examples like malvidin and petunidin, are positively charged chromophores responsible for imparting red, purple, and blue hues to numerous fruits and flowers. Given their inherent structural instability at neutral pH, they are predominantly found in nature as their more stable glycosylated forms, known as anthocyanins [
22]. Although known for their potent antioxidant properties, their direct effects on GI smooth muscle are less well-defined.
Post-synthetic modifications, including hydroxylation, methylation, glycosylation, and acylation of these core flavonoid structures, substantially augment their chemical diversity. These modifications critically impact their bioavailability, metabolic fate, and overall biological activity. For example, the conjugated C2=C3 double bond and 4-keto group present in flavones and flavonols are typically linked to more potent myorelaxant effects in gastrointestinal smooth muscle, a phenomenon potentially mediated by enhanced interaction with L-type calcium channels [
8].
Beyond the expansive class of flavonoids, several other major polyphenol categories (non-flavonoids), notably phenolic acids, stilbenes, and lignans, also hold significant relevance for GI function.
Phenolic acids are classified into hydroxybenzoic acids and hydroxycinnamic acids. They comprise derivatives of both benzoic acid (gallic acid) and cinnamic acid (caffeic acid, ferulic acid, rosmarinic acid). Structurally, they are characterized by a single aromatic ring featuring various hydroxyl and/or methoxy substitutions, often extended by carboxylic acid or other polar functional groups [
23]. These compounds are ubiquitous in plant-derived foods, with high concentrations particularly noted in herbs, coffee, and whole grains. Their comparatively small molecular size contributes to efficient absorption in the upper GI tract, and many demonstrate potent local anti-inflammatory and antioxidant activities [
24].
Stilbenes, such as resveratrol and pterostilbene, are characterized by a C6-C2-C6 carbon skeleton, comprising two aromatic rings connected by an ethylene bridge
(Figure 1). Although less ubiquitously distributed in nature compared to other polyphenol classes, stilbenes are present in notable quantities in grapes, berries, and peanuts. They are recognized for their potent antioxidant and anti-inflammatory attributes [
25,
26].
Lignans are structurally formed via the oxidative dimerization of two phenylpropanoid (C6-C3) units
(Figure 1). These compounds are primarily encountered in seeds, whole grains, and certain vegetables. Key representative compounds include secoisolariciresinol and matairesinol. Crucially, within the human gut, lignans undergo biotransformation by the resident microbiota into enterolignans, such as enterodiol and enterolactone, which possess mild estrogenic and anti-inflammatory activities [
8].
4. Mechanisms of Action of Polyphenols on Gastrointestinal Smooth Muscle
Smooth muscle contraction and relaxation in the GI tract are precisely regulated processes, dependent on interplay among electrical excitability, intracellular calcium dynamics, contractile protein activation, and extensive neuromodulation by the enteric nervous system. Dietary polyphenols actively modulate these diverse regulatory levels, thereby influencing GI motility in both physiological states and pathological contexts (
Figure 3).
4.1. Excitation-Contraction Coupling and the Role of Calcium
Smooth muscle contraction is fundamentally initiated by membrane depolarization, which leads to the opening of sarcolemmal voltage-gated L-type calcium channels (VGCCs). The subsequent influx of extracellular calcium elevates cytosolic calcium concentrations, prompting the formation of a calcium-calmodulin complex. This complex, in turn, activates myosin light chain kinase (MLCK), which phosphorylates the 20 kDa regulatory light chains (MLC20) of myosin II. This phosphorylation facilitates actin-myosin cross-bridge formation, thereby generating contractile force [
27].
In GI smooth muscle, calcium influx can also be augmented by activation of ligand-gated channels or via store-operated calcium entry mechanisms. The force and temporal characteristics of contraction are determined by both calcium availability and the dynamic equilibrium between MLCK and myosin light chain phosphatase (MLCP) activities [
28].
4.2. Relaxation via Potassium Channels and Membrane Hyperpolarization
Smooth muscle relaxation typically ensues from repolarization or hyperpolarization of the membrane potential, thereby diminishing calcium influx through voltage-dependent channels. This phenomenon is principally mediated by the activation of various potassium channels, notably:
Large-conductance calcium-activated potassium (BK) channels: Sensitive to both membrane depolarization and elevations in intracellular calcium.
ATP-sensitive potassium (KATP) channels: Functionally link cellular metabolic status to membrane potential.
Voltage-gated and inward rectifier potassium channels: Contribute significantly to the maintenance of basal tone and electrical excitability.
The efflux of K⁺ ions resulting from the opening of these channels drives the membrane potential toward more hyperpolarized states, consequently limiting excitatory calcium currents [
29,
30].
4.3. Regulation by Nitric Oxide and the cGMP Pathway
Nitric oxide (NO) exerts a fundamental inhibitory role in GI motility, primarily via its action on non-adrenergic, non-cholinergic inhibitory neurons within the enteric nervous system. In smooth muscle cells, NO activates soluble guanylate cyclase (sGC), leading to elevated intracellular cyclic guanosine monophosphate (cGMP) levels. cGMP subsequently activates protein kinase G (PKG), which in turn reduces intracellular calcium concentrations and inhibits the phosphorylation of contractile proteins, thereby promoting relaxation. Furthermore, evidence suggests that NO directly modulates specific potassium channels, including BK, small-conductance (SK), and intermediate-conductance (IK) calcium-activated potassium channels. These direct effects also contribute to membrane potential regulation and, consequently, to GI tract contractility [
31,
32].
This pathway is crucial for processes such as descending relaxation during peristalsis, modulation of lower esophageal sphincter tone, and adaptive gastric relaxation. Its regulation can occur at multiple levels, encompassing NO synthesis by nitric oxide synthase (NOS), sGC activation, and cGMP degradation by phosphodiesterases [
33].
4.4. Modulation of Contractile Machinery: MLCK and MLCP
Independent of direct calcium signaling, the magnitude of contraction is regulated by the balanced activities of MLCK and MLCP. While MLCK drives contraction through MLC20 phosphorylation, MLCP counteracts this process by dephosphorylating MLC20, thereby promoting relaxation. Notably, MLCP activity is itself subject to regulation by Rho-associated protein kinase (ROCK) and protein kinase C (PKC). These kinases can inhibit MLCP, consequently prolonging contraction and contributing to calcium sensitization.
Therefore, compounds that inhibit MLCK, activate MLCP, or interfere with the RhoA/ROCK signaling pathway can effectively reduce contractility, even in situations where intracellular calcium levels are elevated [
27,
28].
4.5. Neurotransmitter Receptor-Mediated Modulation
GI smooth muscle function is extensively modulated by the enteric nervous system, which integrates diverse inputs from various neurotransmitters and hormones. Key receptors mediating motility regulation include:
Serotonin receptors: These receptors critically modulate smooth muscle contraction and relaxation. The precise balance between activating excitatory subtypes (e.g., 5-HT
3, 5-HT
4, 5-HT
2) and inhibitory subtypes (e.g., 5-HT
7) enables motility adapted to specific digestive requirements [
34,
35].
Adrenergic receptors (ADRA): These receptors can elicit either relaxation or contraction depending on their specific distribution and subtype. ADRA1 receptors typically promote contraction and sphincter tone. In contrast, ADRA2 receptors inhibit neurotransmitter release, thereby diminishing contraction, while ADRB2 receptors mediate smooth muscle relaxation. Collectively, these subtypes regulate gut motility and tone in response to sympathetic innervation [
36].
Muscarinic acetylcholine receptors (mAChRs): The M2 and M3 subtypes are predominantly involved. M3 receptors serve as the principal mediators of smooth muscle contraction. Their activation, coupled to G
q/11 proteins, stimulates phosphoinositide hydrolysis, leading to the production of inositol trisphosphate (IP
3). IP
3 then triggers Ca²⁺ release from the sarcoplasmic reticulum (SR), elevating intracellular Ca²⁺ concentration. This Ca²⁺ increase activates calmodulin and, consequently, MLCK, thereby promoting MLC20 phosphorylation and initiating contraction [
34]. M2 receptors constitute approximately 80% of muscarinic receptors in GI smooth muscle. Coupled to G
i/o proteins, they primarily inhibit adenylate cyclase (AC), resulting in reduced cyclic AMP levels. Although M2 receptors do not directly induce contraction, they enhance the contractile response by increasing the Ca²⁺ sensitivity of the contractile apparatus and modulating ion channel activity, specifically by inhibiting K⁺ currents and modulating voltage-dependent Ca²⁺ channels. The synergistic activation of M2 and M3 receptors leads to membrane depolarization via the generation of non-selective cationic and chloride currents, further facilitating Ca²⁺ influx and contraction [
34]. Significantly, the contractile contribution of M2 receptors is conditional on M3 receptor activation; antagonism of M3 receptors typically abolishes the contractile response, indicating that M3 receptors are indispensable for initiating contraction, whereas M2 receptors serve to modulate and sustain it [
35].
Opioid receptors (μ, δ, κ) play an important role in modulating smooth muscle contraction and GI motility, primarily leading to the inhibition of both motility and secretion [
37].
Compounds capable of modulating these complex neurotransmitter receptor systems can indirectly influence smooth muscle tone by altering neuronal input, rather than directly acting on the muscle cells themselves [
38].
4.6. Inflammatory and Oxidative Stress Modulation
Under various pathophysiological conditions, including postoperative ileus, irritable bowel syndrome, or inflammatory bowel disease, gastrointestinal contractile function can become compromised. This impairment often stems from cytokine-induced disruption of signaling pathways, oxidative damage, and underlying neuromuscular dysfunction. In this context, polyphenols capable of attenuating the expression of inflammatory mediators (e.g., iNOS, COX-2) or scavenging reactive oxygen species can play a crucial role in preserving or restoring contractile responsiveness. Thus, their anti-inflammatory and antioxidant activities represent significant indirect mechanisms of action [
39].
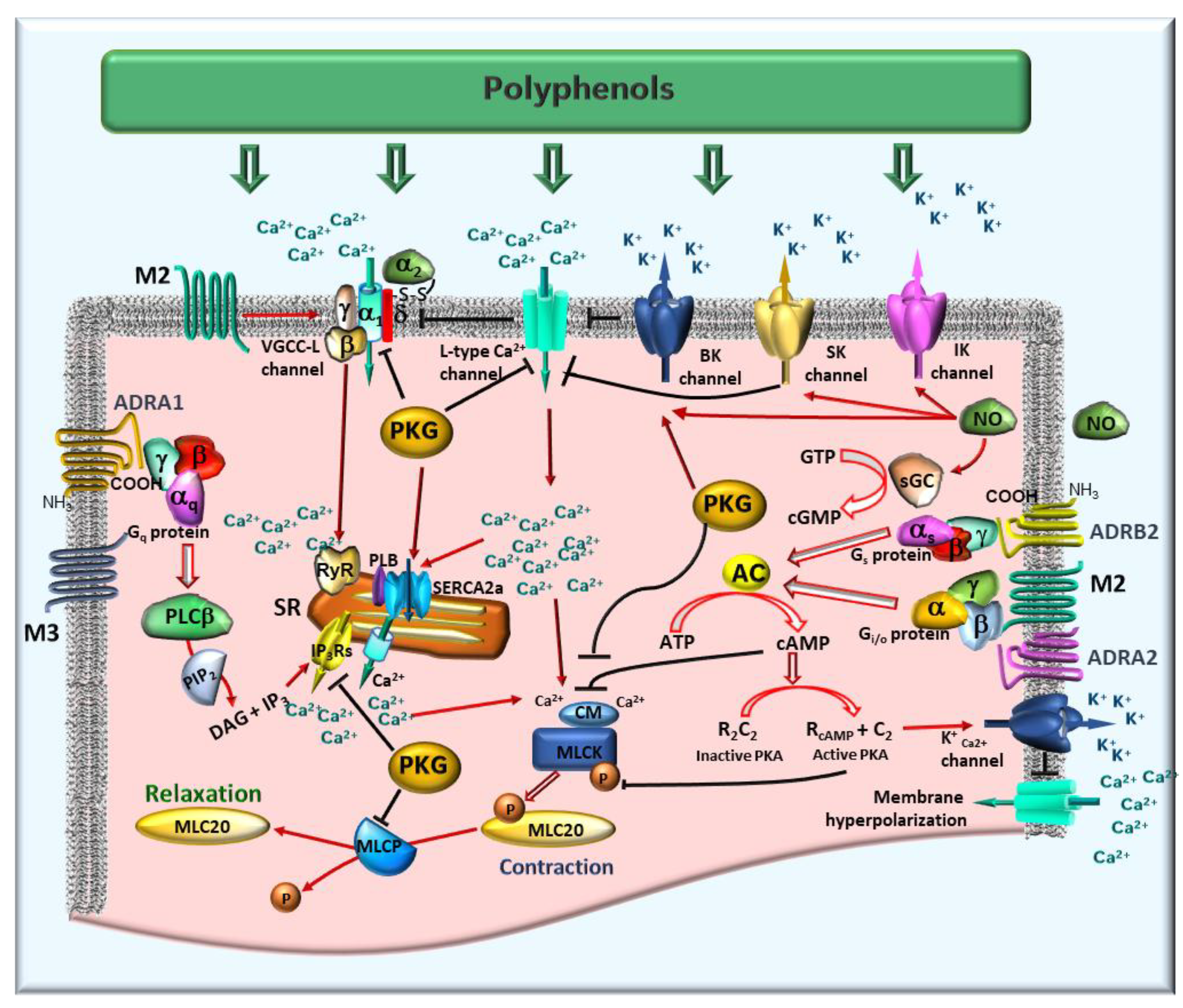
Figure 3.
Proposed mechanisms of polyphenol action on gastrointestinal (GI) smooth muscle contractility. Polyphenols primarily modulate calcium (Ca²⁺) levels, both by inhibiting voltage-gated calcium channels (VGCCs) to reduce extracellular Ca²⁺ influx [
40], and by regulating Ca²⁺ release from the sarcoplasmic reticulum (SR) via modulation of inositol trisphosphate receptors (IP₃Rs) and ryanodine receptors (RyRs). They also engage with key receptor systems, notably the muscarinic M3 (M3) and alpha-1 adrenergic (ADRB1) receptors, which are typically coupled to Gq-proteins. This interaction activates phospholipase C beta (PLCβ), initiating the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP₂) to generate diacylglycerol (DAG) and inositol trisphosphate (IP₃). IP₃ subsequently stimulates IP₃Rs on the SR, promoting Ca²⁺ release and triggering contraction. Simultaneously, polyphenols modulate signaling through muscarinic M2 (M2) receptors (G
i/o-coupled) and beta-2 adrenergic (ADRB2) receptors (G
s-coupled proteins [
36]. These interactions influence adenylate cyclase (AC) activity, resulting in altered cyclic adenosine monophosphate (cAMP) levels. Elevated cAMP, alongside cyclic guanosine monophosphate (cGMP) (often stimulated by nitric oxide, NO, as described below), activates protein kinase A (PKA) and protein kinase G (PKG), respectively. PKA and PKG facilitate smooth muscle relaxation by stimulating myosin light chain phosphatase (MLCP). MLCP dephosphorylates myosin light chain 20 (MLC20), thereby reducing the contractile force. Conversely, smooth muscle contraction is predominantly initiated by MLC20 phosphorylation, catalyzed by myosin light chain kinase (MLCK) [
40]. Additionally, polyphenols influence NO production, which activates soluble guanylate cyclase (sGC), thereby enhancing cGMP-mediated signaling and ultimately promoting relaxation [
33]. Direct modulation of potassium channels (including large-conductance calcium-activated potassium (BK), small-conductance calcium-activated potassium (SK), and intermediate-conductance calcium-activated potassium (IK) channels) further contributes to membrane potential regulation and thus contractility [
29,
30]. Lastly, polyphenols can impact Ca²⁺ reuptake into the SR through sarco/endoplasmic reticulum Ca²⁺-ATPase 2a (SERCA2a), an enzyme regulated by phospholamban (PLB), thereby regulating intracellular Ca²⁺ dynamics [
28]. Arrows denote stimulatory effects; black lines represent inhibitory effects.
Figure 3.
Proposed mechanisms of polyphenol action on gastrointestinal (GI) smooth muscle contractility. Polyphenols primarily modulate calcium (Ca²⁺) levels, both by inhibiting voltage-gated calcium channels (VGCCs) to reduce extracellular Ca²⁺ influx [
40], and by regulating Ca²⁺ release from the sarcoplasmic reticulum (SR) via modulation of inositol trisphosphate receptors (IP₃Rs) and ryanodine receptors (RyRs). They also engage with key receptor systems, notably the muscarinic M3 (M3) and alpha-1 adrenergic (ADRB1) receptors, which are typically coupled to Gq-proteins. This interaction activates phospholipase C beta (PLCβ), initiating the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP₂) to generate diacylglycerol (DAG) and inositol trisphosphate (IP₃). IP₃ subsequently stimulates IP₃Rs on the SR, promoting Ca²⁺ release and triggering contraction. Simultaneously, polyphenols modulate signaling through muscarinic M2 (M2) receptors (G
i/o-coupled) and beta-2 adrenergic (ADRB2) receptors (G
s-coupled proteins [
36]. These interactions influence adenylate cyclase (AC) activity, resulting in altered cyclic adenosine monophosphate (cAMP) levels. Elevated cAMP, alongside cyclic guanosine monophosphate (cGMP) (often stimulated by nitric oxide, NO, as described below), activates protein kinase A (PKA) and protein kinase G (PKG), respectively. PKA and PKG facilitate smooth muscle relaxation by stimulating myosin light chain phosphatase (MLCP). MLCP dephosphorylates myosin light chain 20 (MLC20), thereby reducing the contractile force. Conversely, smooth muscle contraction is predominantly initiated by MLC20 phosphorylation, catalyzed by myosin light chain kinase (MLCK) [
40]. Additionally, polyphenols influence NO production, which activates soluble guanylate cyclase (sGC), thereby enhancing cGMP-mediated signaling and ultimately promoting relaxation [
33]. Direct modulation of potassium channels (including large-conductance calcium-activated potassium (BK), small-conductance calcium-activated potassium (SK), and intermediate-conductance calcium-activated potassium (IK) channels) further contributes to membrane potential regulation and thus contractility [
29,
30]. Lastly, polyphenols can impact Ca²⁺ reuptake into the SR through sarco/endoplasmic reticulum Ca²⁺-ATPase 2a (SERCA2a), an enzyme regulated by phospholamban (PLB), thereby regulating intracellular Ca²⁺ dynamics [
28]. Arrows denote stimulatory effects; black lines represent inhibitory effects.
5. Effects by Flavonoid Subclass: Unraveling Structure-Activity Relationships in GI Motility Modulation
This chapter explores the specific effects of various flavonoid subclasses on GI smooth muscle contractility. By examining individual compounds and their mechanisms, we aim to delineate the structure-activity relationships that govern their influence on GI motility, providing crucial insights for potential therapeutic applications (
Table 1).
5.1. Flavones: Potent Modulators of GI Smooth Muscle Activity
5.1.1. Luteolin
Luteolin, a prevalent flavone abundant in numerous herbs and vegetables, has demonstrated a significant inhibitory impact on GI smooth muscle contractility. In murine models, luteolin dose-dependently attenuated both colonic smooth muscle motility and enteric neural activity, specifically, influencing colonic motor complexes. The central mechanism underpinning this effect involves the inhibition of L-type calcium channels, as evidenced by its partial reversal with the known channel agonist, BayK8644. Crucially, luteolin’s relaxant activity remained insensitive to pharmacological blockade of various potassium channels (TEA, apamin, glibenclamide), voltage-gated sodium channels (TTX), NO synthase (L-NAME), NO-sensitive guanylyl cyclase (ODQ), and ANO1 channels [
10].
5.1.2. Apigenin
Apigenin, a prominent flavone widely present in botanical sources such as chamomile, parsley, and celery, demonstrates a broad range of myorelaxant effects throughout the GI tract. Ex vivo investigations utilizing isolated gut tissues have established apigenin as one of the most potent flavonoids in promoting smooth muscle relaxation, notably surpassing genistein, quercetin, and naringenin in efficacy. Its relaxant action largely proceeds independently of classical neural or systemic biochemical pathways, as evidenced by its persistence even in the presence of inhibitors of sodium channels (tetrodotoxin, TTX), NO synthase (L-NAME), cyclooxygenase (indomethacin), and various potassium channels (TEA). Structural elucidation has underscored the critical contribution of the C2=C3 double bond and specific hydroxyl substitutions to apigenin's exceptional activity, aligning well with broader structure-activity relationship patterns identified within the flavonoid class [
9].
Furthermore, apigenin has been repeatedly isolated and identified as a major active principle within numerous plant extracts celebrated for their antispasmodic properties. For instance, studies on
Achillea millefolium and
Baccharis conferta have successfully isolated apigenin and its derivatives from fractions displaying pronounced inhibitory effects on smooth muscle contraction. In the case of
B. conferta, apigenin significantly contributed to the extract's overall dose-dependent spasmolytic activity [
41,
42].
5.2. Flavanones: Diverse Influences on GI Motility
5.2.1. Naringenin
Naringenin is a citrus-derived flavanone that consistently exerts a concentration-dependent relaxant effect on gastrointestinal smooth muscle. In isolated rat colon preparations, naringenin effectively inhibited spontaneous contractions and suppressed calcium-induced contractile responses at a concentration of 100 µM. Patch-clamp electrophysiology confirmed that this relaxant effect critically involves the activation of BK channels, as its effect was abolished by both the non-selective potassium channel blocker TEA and the specific BK channel inhibitor iberiotoxin. Furthermore, naringenin was observed to induce hyperpolarization of colonic smooth muscle cells, providing additional evidence for its role in modulating membrane potential [
14].
In vivo investigations have further proved naringenin's influence, demonstrating its efficacy in slowing neostigmine-enhanced colonic transit in rats. This finding highlights its promising therapeutic potential in conditions characterized by gastrointestinal hypermotility. Additionally, plant extracts such as those from
Varronia dardani, where naringenin is a primary constituent, have exhibited non-selective spasmolytic activity in the rat uterus, suggesting that naringenin significantly contributes to the broader antispasmodic effects of various flavonoid-rich botanicals [
43].
Intriguingly, when comparatively assessed with other flavonoids in guinea pig intestinal peristalsis models, naringenin elicited a moderate inhibition of distension sensitivity, yet demonstrated less pronounced effects on peristaltic propulsion than apigenin or genistein [
19].
5.2.2. Hesperidin
Hesperidin has been extensively investigated for its capacity to improve gastrointestinal motility via diverse mechanisms. In a rat model of postoperative ileus, hesperidin significantly enhanced both gastric emptying and intestinal transit. Concurrently, in isolated ileum and cecum tissues, it increased the amplitude, but notably not the frequency, of spontaneous contractions. These pro-contractile effects were effectively antagonized by the MLCK inhibitor ML-7 and the calcium channel blocker verapamil, unequivocally indicating mediation via intracellular calcium signaling and MLCK activation. Furthermore, hesperidin was observed to augment MLC phosphorylation and simultaneously attenuate the expression of inflammatory markers, including iNOS and COX-2, within the gut wall [
15].
Complementary evidence from a loperamide-induced constipation model revealed that hesperidin improved stool consistency and accelerated colonic transit without influencing food intake. Mechanistically, hesperidin upregulated the expression of 5-HT
4 receptors and downstream cAMP/PKA/CREB signaling cascade in smooth muscle cells, thereby contributing to both enhanced motility and reduced inflammation [
44].
5.2.3. Hesperetin
Hesperetin, the aglycone of hesperidin, demonstrates potent antispasmodic properties in isolated rat mesenteric jejunum tissues. It effectively inhibited both acetylcholine- and KCl-induced contractions in a dose-dependent and reversible manner. The spasmolytic activity of hesperetin was significantly attenuated by 4-aminopyridine, glibenclamide, and L-NAME, thereby implicating VGCCs and K
ATP channels, and the NO signaling pathway in its mechanism of action. Intriguingly, its activity remained unaffected by inhibitors such as apamin and TEA, suggesting a selective rather than broad action on potassium channels. Furthermore, indomethacin augmented hesperetin's relaxant effect, indicating a potential interplay with prostaglandin pathways [
45].
5.3. Flavonols: Prevalent Modulators of GI Motility
5.3.1. Quercetin
Quercetin, arguably one of the most thoroughly investigated flavonols, exerts modulatory effects on gastrointestinal motility, with compelling evidence indicating both direct smooth muscle relaxation and significant enteric neural involvement. In guinea pig intestinal models, quercetin dose-dependently inhibited peristaltic activity. This effect was primarily characterized by a notable reduction in distension sensitivity, while having a limited impact on active peristaltic propulsion. The observed antiperistaltic effect was partially reversed by apamin, L-NAME, and naloxone (an opioid receptor antagonist), collectively suggesting the involvement of SK channels, endogenous NO synthesis, and opioid receptor pathways [
19].
In mouse stomach models, quercetin induced a reversible, dose-dependent relaxation of smooth muscle. Structural comparisons have elucidated that its efficacy diminishes upon glycosylation or saturation of the C2-C3 double bond, underscoring the critical role of specific structural motifs in its bioactivity. In vitro investigations utilizing
A. millefolium extracts further confirmed quercetin’s potent spasmolytic effect (IC₅₀ ≈ 7.8 μM), with the underlying mechanism strongly implicating calcium channel blockade [
9].
Notably, quercetin has been consistently identified as a major bioactive constituent in numerous polyphenol-rich extracts, including those from
Catha edulis,
Citrullus lanatus, and
Cucumis melo, all of which demonstrated significant inhibitory effects on smooth muscle contractility. These effects frequently mimicked the pharmacological profile of established calcium channel blockers, such as verapamil [
12].
A recent study in our lab sought to clarify the mechanistic basis of quercetin's relaxant effects, utilizing a more clinically relevant model of human gastric smooth muscle tissue. Our research was the first to definitively establish that quercetin-induced relaxation of human gastric smooth muscle is mediated directly through K
ATP channels. We demonstrated this using glibenclamide, a specific K
ATP channel blocker, which significantly inhibited the relaxant effect. We provided crucial evidence that this relaxing effect is independent of the NO pathway, as neither NOS nor guanylyl cyclase blockers had any inhibitory effect. This distinguishes quercetin’s mechanism from many other vasodilators and GI relaxants. We identified that other potassium channels, specifically BK and SK channels, also modulate quercetin's effects, as their blockade extended the relaxation response. Tamoxifen also increased muscle relaxation, suggesting a role for estrogen-related receptors. We connected our findings to a direct clinical application, positioning quercetin as a promising nutraceutical for the treatment of functional dyspepsia and other gastric motility disturbances. This provides a clear, translational link from our basic science research to potential therapeutic use [
13].
5.3.2. Kaempferol
Kaempferol, another ubiquitously distributed flavonol, has consistently demonstrated robust smooth muscle relaxant properties across diverse in vitro and in vivo experimental models. It has been identified as a key active compound within extracts from
Tamarix dioica,
C. lanatus, and
C. melo, significantly contributing to their observed spasmolytic effects. These actions predominantly appear to be mediated through direct calcium channel antagonism. Specifically, these extracts consistently inhibited high K⁺-induced contractions and induced rightward shifts in calcium dose-response curves in isolated jejunum, trachea, and bladder tissues, strongly indicating a shared underlying mechanism involving VGCCs [
11].
Advanced investigations, including gene network analyses and molecular docking studies, have further implicated kaempferol in modulating pathways crucial for intracellular calcium homeostasis and inflammation. Its consistent presence in polyphenol-rich botanical mixtures, which are associated with antidiarrheal, antiperistaltic, and antisecretory properties, underscores its significant therapeutic potential in the clinical management of functional gastrointestinal disorders. Furthermore, within
C. edulis extracts, kaempferol was definitively confirmed via NMR spectroscopy as one of the principal active constituents responsible for blocking calcium-dependent contractions in rat intestine, once again mirroring the established pharmacological action of verapamil [
17].
5.3.3. Myricetin
Myricetin, while less comprehensively studied in isolation compared to other flavonols, was consistently identified as a prominent bioactive constituent, alongside quercetin and kaempferol, in
C. edulis extracts. This extract notably reduced spontaneous contractions in the isolated rat colon and ileum. The extract’s effect remained unaltered by TTX, a sodium channel blocker, thereby suggesting a direct action on smooth muscle cells rather than neurogenic mediation. Analogous to its structural counterparts, myricetin is posited to exert its effects through calcium channel inhibition, although detailed specific mechanistic data remain scarce [
12].
Myricetin was also detected in
T. dioica extracts, where its involvement in K
ATP channel activation was hypothesized as a component of a broader spasmolytic mechanism. Nevertheless, due to the inherent complexity of such crude extracts, the precise individual contribution of myricetin to these observed effects warrants further studying [
11].
5.3.4. Isorhamnetin
Isorhamnetin, a methylated metabolite of quercetin, has been identified within the polyphenolic fraction of
Berberis lycium. Comprehensive investigations, spanning in silico predictions, in vitro assays, and in vivo models, have demonstrated that this flavonol significantly contributes to the extract’s overall antispasmodic and antidiarrheal effects. Experimental data specifically revealed its capacity to inhibit jejunal and bladder contractions, exert calcium channel antagonism, and modulate muscarinic signaling pathways. Furthermore, the extract’s ability to downregulate pro-inflammatory markers such as IL-1β and TNF-α collectively reinforces the substantial therapeutic potential of isorhamnetin-containing preparations for managing various gastrointestinal motility disorders [
46].
5.4. Flavanols: Key Calcium Antagonists in GI Smooth Muscle
5.4.1. Catechin
Catechin, a principal dietary flavanol found in tea, cocoa, and various fruits, consistently induced smooth muscle relaxation across a range of GI models. In isolated rabbit jejunum, catechin dose-dependently inhibited both spontaneous and high potassium K
+-induced contractions, with a preferential effect against the latter. This was reflected in a rightward shift of the calcium concentration-response curve, closely mimicking the action of verapamil, a classical calcium channel blocker. These effects were reproduced in other smooth muscle tissues, including rat stomach fundus and guinea pig ileum, supporting catechin’s consistent myorelaxant action via calcium channel antagonism [
16].
Mechanistic studies indicated that catechin’s spasmolytic effect is not mediated by neural pathways or classic relaxant mediators. Its activity was unaffected by TTX, L-NAME (NO synthase inhibitor), indomethacin (COX inhibitor), or tetraethylammonium (non-selective K⁺ channel blocker), suggesting a direct action on smooth muscle calcium dynamics [
9].
Additionally, studies simulating gastric conditions demonstrated that catechin, in the presence of nitrite, facilitates NO production, leading to gastric muscle relaxation [
47].
5.4.2. Epicatechin
Epicatechin shares structural similarity with catechin and demonstrates comparable spasmolytic properties. In studies using C. lanatus seed extracts, which contain epicatechin among other flavonoids, a dose-dependent relaxation of K⁺-induced contractions was observed in isolated jejunum and tracheal tissues. Like catechin, epicatechin shifted calcium dose-response curves and suppressed calcium-dependent contractions, indicating calcium channel antagonism.
Moreover,
in silico analyses revealed strong binding affinity of epicatechin to several calcium-regulatory targets, including voltage-gated calcium channels and MLCK. These interactions provide a molecular basis for its relaxant effects. The extract also demonstrated in vivo efficacy by reducing intestinal motility and fluid secretion, consistent with the observed in vitro inhibition of smooth muscle activity [
17].
5.5. Isoflavones - Unique Structures with Diverse Pharmacological Profiles
5.5.1. Daidzein
Daidzein, a prominent isoflavone sourced from soy, consistently inhibits intestinal motility in both physiological and hypercontractile states. In isolated jejunal smooth muscle preparations, daidzein reduced spontaneous contractility and suppressed contractions induced by various agonists, including acetylcholine, histamine, erythromycin, and high extracellular Ca
2+ concentrations. The relaxant effect was dependent on extracellular calcium, as it was abolished in Ca
2+-free media and its pharmacological profile closely mimicked that of the L-type Ca
2+ channel blocker, verapamil. Furthermore, a role for adrenergic receptors was indicated, as the effect was partially attenuated by both the α-adrenergic antagonist phentolamine and the β-adrenergic antagonist propranolol [
18].
Complementary patch-clamp studies on guinea pig gastric myocytes revealed that daidzein dose-dependently suppresses voltage-dependent Ba²⁺ currents. This finding supports the hypothesis that daidzein's action involves tyrosine kinase inhibition, which can, in turn, reduce calcium influx via L-type calcium channels. Importantly, daidzein's effect remained unaltered by inhibition of nitric oxide synthase, underscoring that its primary mechanism is largely independent of nitric oxide-mediated signaling pathways [
48].
5.5.2. Genistein
Genistein, a structural analog of daidzein, has been shown to exert potent antiperistaltic effects in isolated guinea pig intestine. In comparative analyses with other flavonoids, genistein displayed a unique pharmacological profile, characterized by the inhibition of both distension sensitivity and peristaltic propulsion which is a pattern shared exclusively with apigenin. In contrast to the effects of quercetin, genistein’s actions were not attenuated by apamin, naloxone, or L-NAME, thereby suggesting a minimal role for neural inhibitory pathways or nitric oxide signaling [
19].
The observation that neostigmine successfully restored genistein-inhibited peristalsis, yet failed to reverse the effects of quercetin, underscores a distinct mechanistic difference likely localized to the excitation-contraction coupling process within the smooth muscle cell itself. Furthermore, studies on the gastric muscle have revealed that genistein produces a strong relaxant effect that is independent of neural blockade, providing additional evidence for its direct action on smooth muscle cells [
9].
5.5.3. Formononetin
Formononetin, a naturally occurring isoflavone, induces vascular smooth muscle relaxation through a dual mechanism involving both endothelium-dependent and -independent pathways. Although the bulk of the evidence is derived from aortic tissue, its relevance to gastrointestinal smooth muscle is underscored by the shared molecular targets, particularly KATP and BK channels.
In rat aortic rings, formononetin-induced vasodilation was attenuated by L-NAME and methylene blue, thereby implicating endothelial nitric oxide synthase (eNOS) and the NO-cGMP signaling pathway. In contrast, in endothelium-denuded tissues, the relaxant effect persisted but was abrogated by K
ATP channel blocker glibenclamide and the specific BK channel inhibitor iberiotoxin. Subsequent cellular assays definitively confirmed that formononetin directly activates these potassium channels in smooth muscle cells [
49].
5.6. Anthocyanins - Novel Modulators with Endothelium-Dependent Actions
5.6.1. Pelargonidin
Pelargonidin, a red-pigmented anthocyanin found in foods such as strawberries and red radishes, has been implicated in the modulation of vascular tone through NO-dependent pathways. While direct evidence of its effect on gastrointestinal smooth muscle remains sparse, valuable insights can be extrapolated from studies on vascular endothelial cells.
In an investigation of red wine polyphenols, pelargonidin was identified as a potent stimulator of endothelial NO production. Using electron paramagnetic resonance spectroscopy, researchers confirmed that pelargonidin promoted a measurable intracellular calcium influx, which in turn increased NO synthesis in endothelial cells, a response that was blocked by inhibition of NO synthase.
Crucially, this pro-NO effect was not observed in isolated smooth muscle cells, which strongly suggests that pelargonidin's primary mechanism involves an endothelium-mediated signaling cascade [
50].
Although these findings do not provide direct confirmation of an effect on GI smooth muscle, they compellingly suggest that pelargonidin may contribute to smooth muscle relaxation through an endothelium-derived NO signaling pathway. This mechanism could be particularly relevant in the highly vascularized regions of the GI tract. Further, targeted investigations are warranted to determine whether a similar mechanism is operational in the regulation of enteric smooth muscle.
6. Effects of Stilbenes: Resveratrol as a Multi-Targeted Spasmolytic Agent
Resveratrol, a key representative of stilbene polyphenol, found in red wine and various plant sources, is studied for its wide-ranging cardiovascular, anti-inflammatory, and antioxidant properties. Within the context of gastrointestinal physiology, resveratrol demonstrates potent spasmolytic effects on smooth muscle tissue, mediated by distinct mechanisms that vary depending on the experimental model.
In rat models, resveratrol additionally decreased basal tone and reduced the contractile amplitude of GI smooth muscle. These effects were partially reversed by antagonists of α-adrenergic receptors (phentolamine), NO synthase inhibitors, and K
ATP channel blockers (glibenclamide), which suggests a broader mechanistic profile involving L-type Ca²⁺ channels and cAMP signaling pathways. Consistent with these findings, a rightward shift in calcium dose-response curves provided further confirmation of its inhibitory effect on calcium influx [
26].
Furthermore, in models of intestinal ischemia/reperfusion injury, resveratrol successfully restored impaired contractility. This effect was coupled with a reduction in oxidative stress and proinflammatory cytokines (IL-1β, TNF, MPO), while concurrently replenishing glutathione levels [
51].
To clarify the mechanistic basis of resveratrol's relaxant effects, our study utilized a clinically relevant human gastric smooth muscle model. We were the first to investigate the direct relaxant effects of resveratrol. This provides crucial translational data and a more clinically relevant perspective compared to studies using animal models. We established that resveratrol's relaxing effects are primarily mediated by the activation of BK channels. This was confirmed by the fact that BK channel blockers like TEA, iberiotoxin, and charybdotoxin significantly inhibited the relaxation response. We ruled out the involvement of other common relaxant pathways, including the NO signaling cascade and other potassium channels (K
ATP and K
V), by showing that their respective blockers (L-NAME, L-NNA, ODQ, glibenclamide, and 4-AP) did not affect resveratrol's action. The relaxant effect was also independent of tamoxifen-sensitive receptors [
25].
7. Effects of Phenolic Acids: Diverse Mechanisms of Motility Modulation
7.1. Hydroxycinnamic Acids: Modulators of Contractility and Inflammation
7.1.1. Caffeic acid
Caffeic acid, a hydroxycinnamic acid derivative widely present in coffee, pears, and apples, demonstrates significant relaxant activity on smooth muscle across multiple organ systems. In an organ bath study comparing the aorta, uterus, and ileum, caffeic acid induced dose-dependent relaxation in preparations precontracted with diverse agonists, including high K⁺ concentration, phenylephrine, oxytocin, and carbachol. Notably, the ileum exhibited the highest sensitivity to caffeic acid, with an EC₅₀ of approximately 2.0 mM. These result suggest that caffeic acid exerts complex antispasmodic effects with various mechanisms involved [
23].
7.1.2. Rosmarinic Acid
Rosmarinic acid, a major phenolic constituent of
Salvia sclarea and
Melissa officinalis, has demonstrated promising therapeutic effects in models of inflammatory bowel disease. In vivo studies have shown that it effectively attenuates intestinal inflammation, restores epithelial barrier function, and modulates the composition of the gut microbiota. Molecular analyses confirmed that rosmarinic acid downregulates the expression of key contractility-associated genes, including ROCK, MLCK, as well as major inflammatory mediators (TNF-α, IL-1β, and IL-6) [
24].
8. Effects of Polyphenol-Rich Extracts and Mixed Compositions: Diverse Actions on GI Motility
Polyphenol-rich botanical extracts and complex natural compositions are widely used for their medicinal properties, including their impact on GI motility. These extracts often contain a synergistic blend of compounds, such as flavonoids, gingerols, and curcuminoids, which exert multifaceted effects on GI smooth muscle. Their mechanisms range from direct modulation of ion channels and receptor antagonism to indirect anti-inflammatory actions (
Table 2). This section explores the effects of two prominent plant extracts and the active compounds that contribute to their complex pharmacological profiles.
8.1. Zingiber officinale (ginger)Extract
Gingerols, particularly 6-gingerol, are identified as the primary active compounds in
Zingiber officinale (ginger) extract, which is traditionally used to alleviate nausea and GI discomfort. Studies on mouse ileum and colon have shown that the extract, through the action of its gingerol and shogaol components, potently inhibits smooth muscle contractions via a non-competitive antagonism of muscarinic M3 and serotonin 5-HT₃ receptors. Furthermore, the extract has been observed to increase tone in the lower esophageal sphincter (LES), suggesting its potential as a therapeutic agent for gastroesophageal reflux disease (GERD) [
52].
Patch-clamp studies in rat colon have provided a molecular basis for these effects, demonstrating that gingerol directly inhibits L-type Ca²⁺ channel currents in smooth muscle cells. This action was attenuated by the calcium channel blocker nifedipine, confirming a direct calcium-channel-related mechanism. The compound was found to shift the voltage-dependence of channel activation, further supporting its role as a direct modulator of these ion channels [
53].
Complementary functional studies on 5-HT₃ receptor binding and function have shown that gingerols and shogaols inhibit serotonin-induced cation flux and ileal contraction. This is likely via interaction with modulatory rather than orthosteric binding sites on the receptor complex [
54,
55].
Evidence from clinical trials demonstrates that ginger extract significantly accelerates gastric emptying and stimulates antral contractions. This prokinetic effect, particularly relevant to conditions like nausea and vomiting in pregnancy, positions ginger as a harmless and effective alternative therapy for managing these symptoms [
56,
57,
58]. These clinical outcomes are consistent with ginger's known mechanisms, including the targeting of signaling molecules and receptors that regulate GI smooth muscle activity [
59,
60,
61].
8.2. Curcuma longa (turmeric)Extract
Curcuminoids, the primary active polyphenolic pigments extracted from
Curcuma longa (turmeric), are responsible for its strong antispasmodic activity observed in both guinea pig ileum and rat uterus preparations. The extract, rich in these compounds, dose-dependently induces marked relaxation of gastrointestinal smooth muscle in mice, acting through non-competitive antagonism of cholinergic (carbachol), histaminergic, and serotonergic pathways. Curcumonoids also inhibit K⁺-induced contractions by blocking L-type Ca²⁺ channels, producing potent spasmolysis in mouse ileum and colon [
62]. In a murine colitis model, Curcuma extract suppressed both spontaneous and carbachol-induced colonic contractions. These reversible effects in inflamed tissue were accompanied by a partial recovery of contractile activity independent of the extract's anti-inflammatory properties [
63].
Pharmacological evaluations in isolated rabbit jejunum and other smooth muscle tissues [
64] showed that turmeric extract and curcumin inhibited both spontaneous and depolarization-induced contractions, a pattern consistent with calcium-channel blockade.
Structure-activity studies in guinea-pig ileum confirmed that bisdemethoxycurcumin is the most potent spasmolytic, followed by curcumin and demethoxycurcumin, whereas tetrahydrocurcumin and hydrolysis products were largely inactive [
65].
Review articles further summarize curcumin’s capacity to reduce hypercontractility, normalize disordered motility, and protect neuromuscular transmission in inflammatory states. These effects likely result from both direct smooth-muscle relaxation and indirect modulation of oxidative and inflammatory processes, as noted across clinical and experimental reports [
64,
66].
8.3. Bidens tripartita
Extracts of
Bidens tripartita, a plant traditionally used for digestive ailments, have demonstrated potent prokinetic effects in ex vivo models. From the plant's aerial parts, researchers isolated several flavonoids, including luteolin, cymaroside, and flavanomarein, and prepared six different extracts and fractions. The flavonoid-rich extracts enhanced both spontaneous and ACh-induced contractions of porcine isolated jejunal smooth muscle. Notably, individual flavonoid constituents, particularly cymaroside, amplified the ACh response by up to 250%, suggesting a substantial synergistic or potentiating effect [
67]. Taken together these findings indicate its potential as a prokinetic agent for managing hypomotility conditions such as constipation-dominant IBS or functional dyspepsia.
8.4. Roman Chamomile (Chamaemelum nobile)
Roman Chamomile, a low perennial plant with a history of use in traditional medicine since the Middle Ages, is valued for its ability to treat spasms of the GI system. The limited experimental data available support its relaxant properties, with a single study demonstrating biphasic effects on GI smooth muscle in isolated organ bath experiments using guinea pig, rat, and human preparations [
68].
However, the plant extract's primary effect was a sustained relaxant action on precontracted smooth muscle. This effect was found to be flavonoid-dependent and persisted even in the presence of β-adrenergic blockers. The relaxant properties of four flavonoids isolated from the plant material (hispidulin, luteolin, eupafolin, and apigenin) were observed across species, supporting the plant's broad pharmacological relevance [
68].
Further studies with chamomile essential oil showed only relaxant effects, indicating that its volatile constituents act through a mechanism distinct from the polyphenolic fraction.
8.5. Catha edulis
Crude extracts of
Catha edulis, which are rich in flavonoids such as quercetin, kaempferol, and myricetin, significantly inhibited spontaneous contractions in rat colon and ileum. These effects were comparable to those induced by the calcium channel blocker verapamil and were unaffected by TTX, definitively indicating a direct action on smooth muscle rather than neural mediation. The extract reduced both K
+-induced and Ca
2+-stimulated contractions, suggesting that calcium channel blockade is the primary mechanism. The identity of the flavonoids responsible for this activity was confirmed by NMR spectroscopy[
12].
8.6. Tamarix dioica
Tamarix dioica is traditionally used to manage various disorders related to smooth muscle in the gastrointestinal, respiratory, and cardiovascular systems. It is useful in the treatment of diarrhea, dysentery, and inflammation. The methanolic extract of
T. dioica has demonstrated a broad spectrum of pharmacological activities, including spasmolytic, bronchodilatory, vasorelaxant, and antidiarrheal effects. Phytochemical analysis revealed a high flavonoid content, with rutin, catechin, kaempferol, myricetin, and apigenin identified as principal constituents. Mechanistically, the extract’s relaxant effects appear to involve the activation of K
ATP channels, as its actions were inhibited by glibenclamide in ex vivo rat and rabbit jejunum models. In vivo experiments further validated its use in treating diarrheal syndromes, aligning with its ethnomedicinal application [
11].
8.7. Citrullus lanatus (watermelon seeds)
Hydroethanolic extracts from watermelon seeds (
Citrullus lanatus) are rich in polyphenolic compounds, including catechin, epicatechin, quercetin, and kaempferol. These extracts exhibit potent spasmolytic effects across various smooth muscle tissues. They effectively reduced K
+-induced contractions and shifted calcium dose-response curves in rabbit isolated jejunum and tracheal preparations, providing clear evidence for calcium antagonism [
69].
In vivo studies demonstrated that these extracts produce antiperistaltic, antidiarrheal, and antisecretory effects. Complementary in silico analyses have confirmed that these compounds target key calcium-related signaling proteins, thereby providing a molecular basis for their action as calcium channel antagonists [
17].
8.8. Cucumis melo (melon seeds)
Cucumis melo is a nutritious and therapeutic fruit which has been used in traditional medicine for centuries in Pakistan, Iran, India, and China for gastrointestinal, circulatory, neurological, and urogenital problems. A polyphenol-rich extract (rutin, kaempferol, quercetin, apigenin, and luteolin) derived from
C. melo seeds demonstrated strong inhibitory effects on K
+-induced smooth muscle contractions, a finding consistent with calcium antagonism. Gene network analyses identified high-affinity interactions between the flavonoids in the extract and genes associated with inflammatory signaling and calcium regulation [
70].
8.9. Achillea millefolium (Yarrow)
Yarrow (
Achillea millefolium) extracts inhibited contractions of guinea pig ileum in a dose-dependent manner, with quercetin, luteolin, and apigenin identified as the main active aglycones. The very low micromolar range of the IC₅₀ values indicates their high potency. The primary mechanism of action is hypothesized to involve calcium channel blockade, with possible secondary modulation of inflammatory or receptor-mediated pathways [
41].
8.10. Baccharis conferta
Ethanolic extracts of
Baccharis conferta, particularly those enriched in flavonoids, exhibited potent spasmolytic activity in histamine-contracted intestinal tissues. The identified active constituents included apigenin, naringenin derivatives, and cirsimaritin [
42].
8.11. Berberis lycium
Berberis lycium is a member of the Berberidaceae family and is highly esteemed in traditional remedies worldwide with a long history of treating diarrhea and abdominal spasms. The flavonoid-rich hydromethanolic extract of
B. lycium showed antispasmodic, antidiarrheal, and bronchodilatory activities in both in vitro and in vivo models. Network pharmacology studies linked its action to the modulation of inflammatory genes and calcium-regulating proteins. Functional assays confirmed both calcium channel blockade and the inhibition of carbachol-induced contractions. The extract’s comprehensive pharmacological profile suggests its potential utility in managing disorders involving both hypercontractile and inflammatory components [
46].
8.12. Melissa officinalis (lemon balm)
Melissa officinalis, commonly known as lemon balm, is a traditional medicinal herb valued for its digestive, antispasmodic, and sedative properties. Its complex phytochemical profile includes mono-, sesqui-, and triterpenes, as well as a variety of phenolic compounds, with rosmarinic, chlorogenic, and lithospermic acids being the most abundant [
71].
Rosmarinic acid has been linked to vasorelaxant effects in the isolated rat thoracic aorta, mediated primarily by NO-dependent mechanisms, with a possible involvement of prostacyclin and endothelium-derived hyperpolarizing factor (EDHF) pathways [
72].
Recent investigations into intestinal smooth muscle have revealed a more complex, region-dependent action of
M. officinalis extract. In isolated chicken proximal and distal jejunum, the crude extract generally enhanced acetylcholine (ACh)-induced contractility and increased spontaneous motor activity in the distal segment. Conversely, spontaneous contractions were reduced by as much as ~67% of control values in the proximal jejunum. Furthermore, testing individual phenolic acids (rosmarinic, chlorogenic, and lithospermic) showed predominantly myorelaxant effects on ACh-induced contractions. This contrasts with the extract’s overall myocontractile action, suggesting complex synergistic or antagonistic interactions among constituents that cannot be attributed to a single compound [
73].
8.13. Salvia sclarea
Salvia sclarea (clary sage), traditionally valued for its antispasmodic and aromatic properties in managing digestive complaints, has also been applied in treating respiratory disorders and various inflammatory conditions. Phytochemical analyses of its methanolic extracts have identified several polyphenolic constituents, including rosmarinic and caffeic acids, apigenin, luteolin, and salvigenin, along with their respective glycosides, which likely contribute to its pharmacological profile [
74].
Experimental studies on isolated rat ileum demonstrated that
S. sclarea extracts reduced both spontaneous and induced contractions, including those triggered by potassium depolarization and acetylcholine. The observed inhibition of K⁺-induced contractions strongly suggests a mechanism involving the blockade of voltage-dependent L-type Ca
2+ channels and the activation of K
+ channels. Furthermore, the extracts relaxed tracheal smooth muscle, highlighting a notable bronchodilatory potential [
74]. Molecular docking studies on these extracts implicated active components, such as flavonoid glycosides (e.g., apigenin-7-O-glucoside and luteolin-7-O-glucoside), as primary compounds responsible for targeting calcium channels.
9. Compounds Lacking Direct Experimental Data
Several polyphenolic compounds, including tangeretin, nobiletin, biochanin A, glycitein, malvidin, and petunidin, have been identified in the literature as structurally relevant or biologically active in other contexts. However, a critical gap exists in the current research: no original experimental studies were found that specifically investigated their effects on GI smooth muscle contractility. Consequently, these compounds were excluded from detailed analysis within this review. This identified gap represents a significant opportunity for future research to explore their potential roles in modulating GI motility.
10. Classification of Experimental Models
For clarity within this review, the experimental models discussed and presented in
Table 1 and
Table 2 adhere to standard terminology:
In silico: Refers to computer-based simulations or computational analyses.
In vivo: Denotes experiments conducted in living organisms.
Ex vivo: Pertains to studies performed on tissues or organs freshly isolated from living animals and maintained under physiological conditions. While some authors may refer to such preparations as in vitro (in glass), this review adopts the term ex vivo to underscore the preservation of native tissue architecture and function, which is critical for assessing physiological relevance. The term in vitro refers to experiments conducted with isolated cells, subcellular components, or purified molecules in a controlled laboratory setting, like a test tube or petri dish.
11. Conclusions
The evidence reviewed here demonstrates that dietary polyphenols, especially flavonoids, modulate gastrointestinal smooth muscle contractility through various mechanisms. These effects range from prokinetic stimulation to potent spasmolytic activity, with the response often depending on chemical structure, concentration, and experimental context.
The most frequently implicated mechanistic targets include voltage-dependent L-type calcium channels, ATP-sensitive and large-conductance calcium-activated potassium channels, nitric oxide signaling, and cAMP/PKA or MLCK-dependent phosphorylation cascades. Intriguingly, many polyphenols act directly on smooth muscle cells, bypassing neural input, while others modulate enteric neurotransmission or inflammatory pathways. The specific bioactivity of these compounds is significantly influenced by structural features, such as hydroxylation patterns, glycosylation, and the saturation of the C2-C3 double bond.
12. Future Directions
The traditional use of polyphenol-rich plant extracts for gastrointestinal disorders is supported by evidence of their synergistic action. However, the complexity of these mixtures poses a challenge for precisely attributing their mechanisms. This underscores the need for future structure-activity studies and bioassay-guided fractionation to isolate and characterize individual contributions.
This comprehensive review highlights that polyphenols are multi-target modulators of gastrointestinal motility, offering therapeutic potential for functional bowel disorders, postoperative ileus, and other related dysmotilities. Advancing this potential into clinical practice requires future studies to resolve key gaps in their in vivo bioactivity, site-specific pharmacokinetics, and potential interactions with conventional therapies.
Author Contributions
Conceptualization, A.C., T.K., and B.M.; methodology, T.K. and B.M.; formal analysis and data curation, A.C., T.K., K.D., and M.B.; writing—original draft preparation, A.C.; writing—review and editing, K.D., J.K., A.R., M.B., and P.Z.; visualization, A.C. and B.M.; supervision, M.T., T.K., H.R.H., and B.M.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript..
Funding
This work was supported by the Medical University of Białystok. Grant B.SUB.23.154 and B.SUB.24.152.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-HT |
Serotonin receptors |
| ACh |
Acetylcholine |
| ADRA |
Adrenergic receptors |
| BK |
Large-conductance calcium-activated potassium channels |
| cGMP |
Cyclic guanosine monophosphate |
| COX-2 |
Cyclooxygenase-2 |
| CREB |
cAMP response element-binding protein |
| eNOS |
Endothelial nitric oxide synthase |
| GI |
Gastrointestinal |
| IBS |
Irritable bowel syndrome |
| IK |
Intermediate-conductance calcium-activated potassium channels |
| IL |
Interleukin |
| iNOS |
Inducible nitric oxide synthase |
| IP3
|
|
Inositol trisphosphate |
|
| IP₃R |
|
Inositol trisphosphate potassium receptor |
|
| KATP
|
ATP-sensitive potassium channels |
| LES |
Lower esophageal sphincter |
| L-NAME |
N(G)-nitro-L-arginine methyl ester |
| L-NOARG |
Nitroarginine |
| M2, M3 |
Muscarinic acetylcholine receptors |
| MLC20 |
20 kDa regulatory light chains |
| MLCK |
Myosin light chain kinase |
| MLCP |
Myosin light chain phosphatase |
| MPO |
Myeloperoxidase |
| NMR |
Nuclear magnetic resonance spectroscopy |
| NO |
Nitric oxide |
| NOS |
Nitric oxide synthase |
| ODQ |
NO-sensitive guanylyl cyclase |
| PKA |
Protein kinase A |
| PKC |
Protein kinase C |
| PKG |
Protein kinase G |
| PLCβ |
|
| ROCK |
Rho-associated protein kinase |
| RyR |
Ryanodine receptor |
| sGC |
Soluble guanylate cyclase |
| SK |
Small-conductance calcium-activated potassium channels |
| SR |
Sarcoplasmic reticulum |
| TEA |
Triethylamine |
| TNF |
Tumor necrosis factor |
| TTX |
Tetrodotoxin |
| VGCC |
Voltage gated potassium channel |
| |
|
| |
|
| |
|
| |
|
References
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable Bowel Syndrome. The Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zogg, H.; Ghoshal, U.C.; Ro, S. Current Treatment Options and Therapeutic Insights for Gastrointestinal Dysmotility and Functional Gastrointestinal Disorders. Front Pharmacol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Nita, A.F.; Chanpong, A.; Nikaki, K.; Rybak, A.; Thapar, N.; Borrelli, O. Recent Advances in the Treatment of Gastrointestinal Motility Disorders in Children. Expert Rev Gastroenterol Hepatol 2023, 17, 1285–1300. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Arias-Sánchez, R.A.; Torner, L.; Fenton Navarro, B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules 2023, 28, 5415. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J AOAC Int 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Amira, S.; Rotondo, A.; Mulè, F. Relaxant Effects of Flavonoids on the Mouse Isolated Stomach: Structure-Activity Relationships. Eur J Pharmacol 2008, 599, 126–130. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, Y.; Wan, L.; Ye, J.; Lu, H.-L.; Huang, X.; Xu, W.-X. Luteolin Suppresses Colonic Smooth Muscle Motility via Inhibiting L-Type Calcium Channel Currents in Mice. Gen Physiol Biophys 2020, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, S.; Aleem, A.; Saqib, F.; Ormenisan, A.; Elena Neculau, A.; Anastasiu, C. The Potential Involvement of an ATP-Dependent Potassium Channel-Opening Mechanism in the Smooth Muscle Relaxant Properties of Tamarix Dioica Roxb. Biomolecules 2019, 9, 722. [Google Scholar] [CrossRef]
- Nigusse, T.; Zhang, L.; Wang, R.; Wang, X.; Li, J.; Liu, C. Flavonoids in a Crude Extract of Catha Edulis Inhibit Rat Intestinal Contraction via Blocking Ca 2+ Channels. Neurogastroenterology & Motility 2019, 31. [Google Scholar] [CrossRef]
- Modzelewska, B.; Drygalski, K.; Kleszczewski, T.; Chomentowski, A.; Koryciński, K.; Kiełczewska, A.; Pawłuszewicz, P.; Razak Hady, H. Quercetin Relaxes Human Gastric Smooth Muscles Directly through ATP-sensitive Potassium Channels and Not Depending on the Nitric Oxide Pathway. Neurogastroenterology & Motility 2021, 33, e14093. [Google Scholar] [CrossRef]
- Yang, Z.; Pan, A.; Zuo, W.; Guo, J.; Zhou, W. Relaxant Effect of Flavonoid Naringenin on Contractile Activity of Rat Colonic Smooth Muscle. J Ethnopharmacol 2014, 155, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chu, H.; Lin, Y.; Han, F.; Li, Y.; Wang, A.; Wang, F.; Chen, D.; Wang, J. Hesperidin Alleviates Rat Postoperative Ileus through Anti-Inflammation and Stimulation of Ca2+-Dependent Myosin Phosphorylation. Acta Pharmacol Sin 2016, 37, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ghayur, M.N.; Khan, H.; Gilani, A.H. Antispasmodic, Bronchodilator and Vasodilator Activities of (+)-Catechin, a Naturally Occurring Flavonoid. Arch Pharm Res 2007, 30, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Saqib, F.; Qamar, M.; Ziora, Z.M. Antispasmodic Activity of the Ethanol Extract of Citrullus Lanatus Seeds: Justifying Ethnomedicinal Use in Pakistan to Treat Asthma and Diarrhea. J Ethnopharmacol 2022, 295, 115314. [Google Scholar] [CrossRef]
- Chen, D.; Xiong, Y.; Tang, Z.; Lv, B.; Lin, Y. Inhibitory Effects of Daidzein on Intestinal Motility in Normal and High Contractile States. Pharm Biol 2012, 50, 1561–1566. [Google Scholar] [CrossRef]
- Gharzouli, K.; Holzer, P. Inhibition of Guinea Pig Intestinal Peristalsis by the Flavonoids Quercetin, Naringenin, Apigenin and Genistein. Pharmacology 2004, 70, 5–14. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, N.J.; Yun, H.; Han, Y.T. Recent Advances in Synthesis of 4-Arylcoumarins. Molecules 2018, Vol. 23, Page 2417 2018, 23, 2417. [Google Scholar] [CrossRef]
- Luqman, S.; Meena, A.; Singh, P.; Kondratyuk, T.P.; Marler, L.E.; Pezzuto, J.M.; Negi, A.S. Neoflavonoids and Tetrahydroquinolones as Possible Cancer Chemopreventive Agents. Chem Biol Drug Des 2012, 80, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Vos, R.; Tack, J. Effects of Capsaicin on the Sensorimotor Function of the Proximal Stomach in Humans. Aliment Pharmacol Ther 2004, 19, 415–425. [Google Scholar] [CrossRef]
- de Alencar Silva, A.; Pereira-de-Morais, L.; Rodrigues da Silva, R.E.; de Menezes Dantas, D.; Brito Milfont, C.G.; Gomes, M.F.; Araújo, I.M.; Kerntopf, M.R.; Alencar de Menezes, I.R.; Barbosa, R. Pharmacological Screening of the Phenolic Compound Caffeic Acid Using Rat Aorta, Uterus and Ileum Smooth Muscle. Chem Biol Interact 2020, 332, 109269. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.; Xu, S.; Li, X.; Zhang, Y.; Gao, X. Rosmarinic Acid Alleviates Intestinal Inflammatory Damage and Inhibits Endoplasmic Reticulum Stress and Smooth Muscle Contraction Abnormalities in Intestinal Tissues by Regulating Gut Microbiota. Microbiol Spectr 2023, 11. [Google Scholar] [CrossRef]
- Modzelewska, B.; Drygalski, K.; Hady, H.R.; Kiełczewska, A.; Chomentowski, A.; Koryciński, K.; Głuszyńska, P.; Kleszczewski, T. Resveratrol Relaxes Human Gastric Smooth Muscles Through High Conductance Calcium-Activated Potassium Channel in a Nitric Oxide-Independent Manner. Front Pharmacol 2022, 13. [Google Scholar] [CrossRef]
- Zhang, L.-X. Resveratrol and Genistein Inhibition of Rat Isolated Gastrointestinal Contractions and Related Mechanisms. World J Gastroenterol 2014, 20, 15335. [Google Scholar] [CrossRef] [PubMed]
- Kishi, H.; Ye, L.-H.; Nakamura, A.; Okagaki, T.; Iwata, A.; Tanaka, T.; Kohama, K. Structure and Function of Smooth Muscle Myosin Light Chain Kinase. In Advances in Experimental Medicine and Biology; Kluwer Academic/Plenum Publishers, 1998; Vol. 453, pp. 229–234. [Google Scholar]
- He, W.; Peng, Y.; Zhang, W.; Lv, N.; Tang, J.; Chen, C.; Zhang, C.; Gao, S.; Chen, H.; Zhi, G.; et al. Myosin Light Chain Kinase Is Central to Smooth Muscle Contraction and Required for Gastrointestinal Motility in Mice. Gastroenterology 2008, 135, 610–620.e2. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xin, F.; Liu, P.; Zhao, H.-Y.; Zhang, S.-T.; Han, P.; Huang, H.-X.; Wang, W. Role of BK Ca in Stretch-Induced Relaxation of Colonic Smooth Muscle. Biomed Res Int 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, D.H.; Son, S.M.; Choi, S.-Y.; You, R.Y.; Kim, C.H.; Choi, W.; Kim, H.S.; Lim, Y.J.; Han, J.Y.; et al. Physiological Function and Molecular Composition of ATP-Sensitive K+ Channels in Human Gastric Smooth Muscle. J Smooth Muscle Res 2020, 56, 29–45. [Google Scholar] [CrossRef]
- Lang, R.J.; Harvey, J.R.; McPhee, G.J.; Klemm, M.F. Nitric Oxide and Thiol Reagent Modulation of Ca2+-Activated K+ (BKCa) Channels in Myocytes of the Guinea-Pig Taenia Caeci. J Physiol 2000, 525 Pt 2, 363–376. [Google Scholar] [CrossRef]
- Modzelewska, B.; Sipowicz, M.A.; Saavedra, J.E.; Keefer, L.K.; Kostrzewska, A. Involvement of K+ATPChannels in Nitric Oxide-Induced Inhibition of Spontaneous Contractile Activity of the Nonpregnant Human Myometrium. Biochem Biophys Res Commun 1998, 253, 653–657. [Google Scholar] [CrossRef]
- Idrizaj, E.; Traini, C.; Vannucchi, M.G.; Baccari, M.C. Nitric Oxide: From Gastric Motility to Gastric Dysmotility. Int J Mol Sci 2021, 22, 9990. [Google Scholar] [CrossRef]
- Tanahashi, Y.; Komori, S.; Matsuyama, H.; Kitazawa, T.; Unno, T. Functions of Muscarinic Receptor Subtypes in Gastrointestinal Smooth Muscle: A Review of Studies with Receptor-Knockout Mice. Int J Mol Sci 2021, 22, 926. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, F.; Pak, K.; Griffin, M. Muscarinic Agonists and Antagonists: Effects on Gastrointestinal Function. Biology, Chemistry, and Environmental Sciences Faculty Books and Book Chapters 2012. [Google Scholar]
- Seiler, R.; Rickenbacher, A.; Shaw, S.; Balsiger, B.M. α- and β-Adrenergic Receptor Mechanisms in Spontaneous Contractile Activity of Rat Ileal Longitudinal Smooth Muscle. Journal of Gastrointestinal Surgery 2005, 9, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K. YFa and Analogs: Investigation of Opioid Receptors in Smooth Muscle Contraction. World J Gastroenterol 2011, 17, 4523. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.A.; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol Res Pract 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The Emerging Role of Oxidative Stress in Inflammatory Bowel Disease. Front Endocrinol (Lausanne) 2024, 15, 1390351. [Google Scholar] [CrossRef]
- Kishi, H.; Ye, L.H.; Nakamura, A.; Okagaki, T.; Iwata, A.; Tanaka, T.; Kohama, K. Structure and Function of Smooth Muscle Myosin Light Chain Kinase. Adv Exp Med Biol 1998, 453, 229–234. [Google Scholar] [CrossRef]
- Lemmens-Gruber, R.; Marchart, E.; Rawnduzi, P.; Engel, N.; Benedek, B.; Kopp, B. Investigation of the Spasmolytic Activity of the Flavonoid Fraction of Achillea Millefolium s.l. on Isolated Guinea-Pig Ilea. Arzneimittelforschung 2011, 56, 582–588. [Google Scholar] [CrossRef]
- Weimann, C.; Göransson, U.; Pongprayoon-Claeson, U.; Claeson, P.; Bohlin, L.; Rimpler, H.; Heinrich, M. Spasmolytic Effects of Baccharis Conferta and Some of Its Constituents. Journal of Pharmacy and Pharmacology 2002, 54, 99–104. [Google Scholar] [CrossRef]
- G Veloso, C.A.; Alencar D Figueiredo, I.; da Silva, G.R.; Miranda de Melo, J.I.; S da Silva, M.; Tavares, J.F.; de A Cavalcante, F.; de O Costa, V.C. Flavonoids from Varronia Dardani (Taroda) J.S. Mill (Cordiaceae) and the Evaluation of Spasmolytic Activity of Its Crude Ethanolic Extract. Nat Prod Res 2021, 35, 4197–4201. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Gu, Y. Hesperidin Improves Colonic Motility in Loeramide-Induced Constipation Rat Model via 5-Hydroxytryptamine 4R/CAMP Signaling Pathway. Digestion 2020, 101, 692–705. [Google Scholar] [CrossRef]
- Mendel, M.; Chłopecka, M.; Dziekan, N.; Karlik, W. Antispasmodic Effect of Selected Citrus Flavonoids on Rat Isolated Jejunum Specimens. Eur J Pharmacol 2016, 791, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Hussain Shah, S.A.; Aleem, A. Investigations of Plausible Pharmacodynamics Supporting the Antispasmodic, Bronchodilator, and Antidiarrheal Activities of Berberis Lycium Royle. Via in Silico, in Vitro, and in Vivo Studies. J Ethnopharmacol 2023, 305, 116115. [Google Scholar] [CrossRef]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Laranjinha, J. Dietary Polyphenols Generate Nitric Oxide from Nitrite in the Stomach and Induce Smooth Muscle Relaxation. Toxicology 2009, 265, 41–48. [Google Scholar] [CrossRef]
- Zhu, H.-L.; Ito, Y.; Teramoto, N. Effects of Tyrosine Kinase Inhibitors on Voltage-Dependent Ba(2+) Currents in the Guinea-Pig Gastric Antrum. J Physiol Pharmacol 2006, 57, 415–424. [Google Scholar] [PubMed]
- Wu, J.-H.; Li, Q.; Wu, M.-Y.; Guo, D.-J.; Chen, H.-L.; Chen, S.-L.; Seto, S.-W.; Au, A.L.S.; Poon, C.C.W.; Leung, G.P.H. Formononetin, an Isoflavone, Relaxes Rat Isolated Aorta through Endothelium-Dependent and Endothelium-Independent Pathways. J Nutr Biochem 2010, 21, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Stoclet, J.C.; Kleschyov, A.; Andriambeloson, E.; Diebolt, M.; Andriantsitohaina, R. Endothelial No Release Caused by Red Wine Polyphenols. J Physiol Pharmacol 1999, 50, 535–540. [Google Scholar]
- Parlar, A.; Arslan, S.O. Resveratrol Normalizes the Deterioration of Smooth Muscle Contractility after Intestinal Ischemia and Reperfusion in Rats Associated With an Antioxidative Effect and Modulating Tumor Necrosis Factor Alpha Activity. Ann Vasc Surg 2019, 61, 416–426. [Google Scholar] [CrossRef]
- Promdam, N.; Khuituan, P.; Panichayupakaranant, P. Effects of Standardized [6]-Gingerol Extracts and [6]-Gingerol on Isolated Ileum and Lower Esophageal Sphincter Contractions in Mice. Food Chem 2022, 378, 132077. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-X.; Tang, X.-D.; Wang, F.-Y.; Duan, Z.-J.; Li, Y.-C.; Qiu, J.-J.; Guo, H.-S. Effect of Gingerol on Colonic Motility via Inhibition of Calcium Channel Currents in Rats. World J Gastroenterol 2015, 21, 13466–13472. [Google Scholar] [CrossRef]
- Pertz, H.; Lehmann, J.; Roth-Ehrang, R.; Elz, S. Effects of Ginger Constituents on the Gastrointestinal Tract: Role of Cholinergic M 3 and Serotonergic 5-HT 3 and 5-HT 4 Receptors. Planta Med 2011, 77, 973–978. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.; Windeck, T.; Ploch, M.; Verspohl, E.J. Mode of Action of Gingerols and Shogaols on 5-HT3 Receptors: Binding Studies, Cation Uptake by the Receptor Channel and Contraction of Isolated Guinea-Pig Ileum. Eur J Pharmacol 2006, 530, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Lete, I.; Allué, J. The Effectiveness of Ginger in the Prevention of Nausea and Vomiting during Pregnancy and Chemotherapy. Integr Med Insights 2016, 11, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lutomski, J.; McCarthy, F.P.; Greene, R.A. Hyperemesis Gravidarum: Current Perspectives. Int J Womens Health 2014, 6, 719. [Google Scholar] [CrossRef] [PubMed]
- Palatty, P.L.; Haniadka, R.; Valder, B.; Arora, R.; Baliga, M.S. Ginger in the Prevention of Nausea and Vomiting: A Review. Crit Rev Food Sci Nutr 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Sun, X.; Nie, F.; Sun, J.; Zhang, J.; Wang, Y. Medicinal Plants for Chemotherapy-Induced Nausea and Vomiting: A Systematic Review of Antiemetic, Chemosensitizing, and Immunomodulatory Mechanisms. Ther Clin Risk Manag 2025, 21, 1187–1218. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in Gastrointestinal Disorders: A Systematic Review of Clinical Trials. Food Sci Nutr 2019, 7, 96–108. [Google Scholar] [CrossRef]
- Fahimi, F.; Khodadad, K.; Amini, S.; Naghibi, F.; Salamzadeh, J.; Baniasadi, S. Evaluating the Effect of Zingiber Officinalis on Nausea and Vomiting in Patients Receiving Cisplatin Based Regimens. Iran J Pharm Res 2011, 10, 379–384. [Google Scholar]
- Micucci, M.; Aldini, R.; Cevenini, M.; Colliva, C.; Spinozzi, S.; Roda, G.; Montagnani, M.; Camborata, C.; Camarda, L.; Chiarini, A.; et al. Curcuma Longa L. as a Therapeutic Agent in Intestinal Motility Disorders. 2: Safety Profile in Mouse. PLoS One 2013, 8, e80925. [Google Scholar] [CrossRef]
- Aldini, R.; Budriesi, R.; Roda, G.; Micucci, M.; Ioan, P.; D’Errico-Grigioni, A.; Sartini, A.; Guidetti, E.; Marocchi, M.; Cevenini, M.; et al. Curcuma Longa Extract Exerts a Myorelaxant Effect on the Ileum and Colon in a Mouse Experimental Colitis Model, Independent of the Anti-Inflammatory Effect. PLoS One 2012, 7, e44650. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Shah, A.J.; Ghayur, M.N.; Majeed, K. Pharmacological Basis for the Use of Turmeric in Gastrointestinal and Respiratory Disorders. Life Sci 2005, 76, 3089–3105. [Google Scholar] [CrossRef]
- Jamil, Q.U.A.; Iqbal, S.M.; Jaeger, W.; Studenik, C. Vasodilating, Spasmolytic, Inotropic and Chronotropic Activities of Curcuminoids from Curcuma Longa in Isolated Organ Preparations of Guinea Pigs. J Physiol Pharmacol 2018, 69. [Google Scholar] [CrossRef]
- Rahimi, R. Herbal Medicines for the Management of Irritable Bowel Syndrome: A Comprehensive Review. World J Gastroenterol 2012, 18, 589. [Google Scholar] [CrossRef]
- Mendel, M.; Chłopecka, M.; Latek, U.; Karlik, W.; Tomczykowa, M.; Strawa, J.; Tomczyk, M. Evaluation of the Effects of Bidens Tripartita Extracts and Their Main Constituents on Intestinal Motility – An Ex Vivo Study. J Ethnopharmacol 2020, 259, 112982. [Google Scholar] [CrossRef] [PubMed]
- Sándor, Z.; Mottaghipisheh, J.; Veres, K.; Hohmann, J.; Bencsik, T.; Horváth, A.; Kelemen, D.; Papp, R.; Barthó, L.; Csupor, D. Evidence Supports Tradition: The in Vitro Effects of Roman Chamomile on Smooth Muscles. Front Pharmacol 2018, 9. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F. Scientific Basis for Medicinal Use of Citrullus Lanatus (Thunb.) in Diarrhea and Asthma: In Vitro, in Vivo and in Silico Studies. Phytomedicine 2022, 98, 153978. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F.; Akhtar, S.; Ali, A.; Tallei, T.E.; Simal–Gandara, J. Mechanistic Insights of Cucumis Melo L. Seeds for Gastrointestinal Muscle Spasms through Calcium Signaling Pathway–Related Gene Regulation Networks in WGCNA and in Vitro, in Vivo Studies. Comput Biol Med 2023, 155, 106596. [Google Scholar] [CrossRef]
- Sadraei, H.; Ghannadi, A.; Malekshahi, K. Relaxant Effect of Essential Oil of Melissa Officinalis and Citral on Rat Ileum Contractions. Fitoterapia 2003, 74, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, S.; Orhan, I.; Turan, N.N.; Şahan, G.; Ark, M.; Tosun, F. Endothelium-Dependent Induction of Vasorelaxation by Melissa Officinalis L. Ssp. Officinalis in Rat Isolated Thoracic Aorta. Phytomedicine 2008, 15, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Posłuszny, M.A.; Chłopecka, M.; Suor-Cherer, S.; Cisse, S.; Benarbia, M. el A.; Mendel, M. Modulation of Chicken Gut Contractility by Melissa Officinalis—Ex Vivo Study. Poult Sci 2023, 102, 103045. [Google Scholar] [CrossRef] [PubMed]
- Randjelović, M.; Branković, S.; Jovanović, M.; Kitić, N.; Živanović, S.; Mihajilov-Krstev, T.; Miladinović, B.; Milutinović, M.; Kitić, D. An In Vitro and In Silico Characterization of Salvia Sclarea L. Methanolic Extracts as Spasmolytic Agents. Pharmaceutics 2023, 15, 1376. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).