1. Introduction
According to the World Health Organization’s International Agency for Research on Cancer, rectal cancer is the tenth most common cause of cancer-related death and ranks eighth in terms of cancer incidence worldwide; in Europe and the USA, it ranks respectively third and fourth. [
1] The survival rate for rectal cancer patients has significantly improved in recent years. [
2] In Israel, the survival rate for male rectal cancer patients is approximately 70%. [
3] Although the guidelines for rectal cancer treatment have changed over the years, the morbidity caused by surgery and chemoradiation remains a significant issue. [
4,
5] In addition to experiencing low anterior resection syndrome (fecal incontinence, frequency, urgency, or feelings of incomplete emptying), male patients also suffer from erectile dysfunction. [
6,
7,
8]
Over the past few decades, phosphodiesterase-5 (PDE5) inhibitors have revolutionized the treatment of erectile dysfunction, which is observed in about 52% of males between the ages of 40 and 70. [
9] PDE5, a metallohydrolase, is present in various tissues and is responsible for hydrolysis of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), regulating their levels by breaking them down into inactive derivatives. [
10,
11] PDE5 is the primary enzyme responsible for breaking down cGMP in the corpus cavernosum (erectile tissue of the penis). PDE5 inhibitors block the degradative action of PDE5, which prevents the breakdown of cGMP, prolonging the effect of cGMP, enhancing and sustaining erection in response to sexual stimulation. [
12] PDE5 plays a crucial role in maintaining intracellular homeostasis and the PDE5 enzyme family is involved in inflammatory processes, immune responses, and tumor cell biology. [
13,
14,
15,
16]
While PDE5Is were approved by the FDA decades ago for the treatment of erectile dysfunction, there is accumulating evidence of their anti-cancer/anti-tumoral activity. Colorectal tumorigenesis starts with the development of benign polyps. In vivo studies have shown that PDE5 inhibitors can reduce the rate of polyp formation by 50%. This effect may be linked to changes in cGMP levels, which improve the epithelial barrier function and suppress tumorigenesis, or it may involve a reduction in the activity of myeloid-derived suppressor cells. [
17,
18] PDE5 inhibitors have been shown to induce cell cycle arrest in rectal tumor cells in vitro, ultimately leading to apoptosis. A possible mechanism for this effect is an increase in levels of reactive oxidative species, which in turn impairs the function of CDK and PARP proteins. [
19] Moreover, high expression of PDE5 itself in tumors often correlates with worse prognosis. [
20] However, another aspect of their anticancer activity is seen when they are used in conjunction with chemotherapeutic agents. Enhanced permeation retention (EPR) is the phenomenon whereby macromolecules, nanoparticles, or liposomes tend to accumulate more in tumor tissue than in normal tissues due to an increase in both permeability and retention. Tumors induce angiogenesis to support their rapid growth and expansion. The blood vessel walls are often defective or “leaky,” allowing large molecules and particles (like drug-loaded nanoparticles) to pass easily from the bloodstream into the tumor tissue. Additionally, tumors often exhibit poor lymphatic drainage. Once these large particles enter the tumor, they are not efficiently removed, leading to prolonged retention. PDE5 inhibitors decrease efflux pump activity, thereby enhancing the EPR effect and increasing the concentration of the anticancer drug in tumor tissues. [
21]
In Israel, four primary healthcare providers cover a significant portion of the Israeli population. The largest of these, in terms of insured members, is Clalit Health Services (CHS). CHS data, managed via MDClone, integrates clinical information from community clinics, hospitals, and emergency departments, providing a comprehensive national dataset. [
22] According to the National Insurance Agency, in 2024, CHS provided health care to over 52% of Israel’s 9.67 million citizens. [
23] The CHS offers researchers access to a scientific platform called the "Research Room," which serves as a data source for the current study. This platform contains comprehensive patient data, including information on ethnicity, socioeconomic status, medications purchased or prescribed, diagnoses, and treatments administered with an optional anonymizing protocol. Access to this data enabled us to conduct a retrospective study to evaluate the potential correlations between the use of PDE5 inhibitors and survival improvements after completion of rectal cancer treatment.
2. Materials and Methods
2.1. Study Sample
The data for this study were obtained from CHS, Israel’s largest HMO, via its secure "research room" platform. The data had been pre-filtered according to predefined inclusion and exclusion criteria. The final sample was comprised of 1,552 patients, of whom 256 had purchased PDE5 inhibitors (PDE5I) as sexual performance enhancement drugs at some point. Among participants, 83.4% (n = 1295) were Jewish, 13.6% (n = 211) were Arab, and the remaining 3.0% (n = 46) belonged to other ethnic minority groups. The patients' ages at diagnosis ranged from 26 to 95 years (mean = 65.12, SD = 12.80).
2.2. Data Collection and Processing
The anonymizing level chosen for the current study required the removal of detailed descriptions, including the type and exact date of surgery, the place and date of radiotherapy, and the precise chemotherapy protocol, as well as the stage of disease at the time of diagnosis, from the database.
The dataset included both demographic and clinical features. Most variables were either binary or categorical, with a few exceptions that were numeric in nature. Demographic variables include socio-economic status and ethnicity. Examples of clinical covariates include diagnosis age, surgery year, PDE5I use (binary indicator based on purchase history), age of PDE5I use onset (set to 0 for non-users), Charlson Comorbidity Index (CCI) score, and individual (CCI) conditions. Variables with no variation or many missing values were removed. For example, the AIDS indicator, which had only zeros, was removed. An indicator for connective-tissue disease was also removed due to missing data in over 50% of patients. The final dataset consisted of a total of 26 covariates, a survival time variable (in years), and an event indicator (death = 1, censored = 0).
2.3. Patient Selection
In this retrospective study, we included individuals assigned male at birth who were diagnosed with non-metastatic rectal adenocarcinoma between January 2009 and October 2024. The treatment guidelines in Israel are similar to those accepted in the USA and European Community, which include 5-FU-based chemotherapy and radiotherapy in both neo-adjuvant and adjuvant, depending on the clinical and/or pathological status. Patients who were managed with a total neoadjuvant approach and did not undergo surgery were not enrolled due to the short follow-up time. The date of surgery was used as the initial time point for calculating PDE5I exposure time.
Patients were distributed into two groups: users and non-users of PDE5 inhibitors at the time of surgery. Patients who took the drug before surgery but did not continue after were combined with non-users, since we were assessing the effect of PDE5I on survival after surgery.
2.4. Ethnicity
In Israel, the population consists of Jews, Arabs, and other minority ethnic groups. Within the Jewish population, there are several subpopulations characterized or defined by their home languages, religious communities, and countries of origin. All these traits are stored in the database, but for this study’s purposes, they were combined under the single rubric “Jewish”.
2.5. PDE5I Use
The use of PDE5Is was based on data on purchases and dosages obtained from the CHS database. Since various drugs with similar bioeffects are available on the Israeli market, the minimal effective dose of sildenafil (50 mg) was used as the "active unit" for comparing the consumption rates of similar medications in this class.
2.6. Statistical Methods
Differences between users and non-users were examined with demographic and clinical attributes. These analyses were performed using non-parametric statistical tests: chi-squared tests for categorical variables and Wilcoxon rank-sum tests for scale variables. Normality of scale variables within PDE5I use levels was assessed graphically using histograms, Kernel Density Estimates (KDEs), and Shapiro-Wilks normality tests.
2.7. Initial Survival Analysis
We began our analysis with a log-rank test comparing survival distributions between PDE5I users and non-users. Next, we fit a Cox proportional hazards (PH) model including all available covariates to assess:
Which variables significantly influence survival.
Whether the effect of PDE5I use remains significant after adjusting for other covariates.
Addressing Confounding with Propensity Score Matching
To mitigate the potential confounding impact, we employed Propensity Score Matching (PSM). The covariates to be balanced were selected as follows:
We standardized all numerical covariates (0 mean and unit variance) and refit the Cox PH model.
We also fit a Random Survival Forest (RSF) model to the data, using 10,000 trees, log-rank splitting criteria, and a minimum terminal node size of 10. Bootstrap sampling was used for observations, and model performance was assessed using out-of-bag (OOB) data at each RSF iteration. Variables were subsampled at each split by the default method (number of covariates divided by three).
Variable importance was extracted from both models. For the RSF, we used the built-in variable importance measure “random”, which compares performance with splits over a specific variable with random splits over its values. For the Cox PH model, standardized coefficients were normalized to sum to one.
We selected the top six variables from both models (accounting for a total of seven variables, due to overlap), and used them for PSM, treating PDE5I use as the treatment indicator. We also incorporated an additional variable, as its levels differed significantly in the Cox PH model.
We tested three matching ratios of users to non-users across four caliper values and selected the one yielding optimal covariate balance, as evaluated by comparing mean standardized differences (for numeric variables) and differences in proportions (for categorical variables). Matching was performed using the nearest neighbor approach without replacement. The final matching algorithm used the best-performing ratio and caliper combination.
To assess whether the effect of PDE5I use on survival persisted after covariate balancing, we re-ran the log-rank test on the matched dataset.
2.8. Additional Analyses
We conducted stratified log-rank tests by dividing the whole dataset into two subgroups based on the levels of the two most informative categorical variables, creating two binary subgroups per variable.
P-values are reported for all statistical tests. Key findings are presented graphically. All analyses were conducted in R (version 4.2.3). Throughout the text, we denote degrees of freedom by df.
3. Results
The CHS database contained information on 1,552 patients diagnosed with non-metastatic rectal cancer at the time of their diagnosis. The characteristics of these patients are presented in Table 1.
The initial analysis focused on survival rates between users and non-users of a particular treatment. Results indicated that users performed significantly better, achieving statistical significance in the Log Rank, Breslow, and Tarone-Ware tests with a p-value < 0.0001 (see
Figure 2).
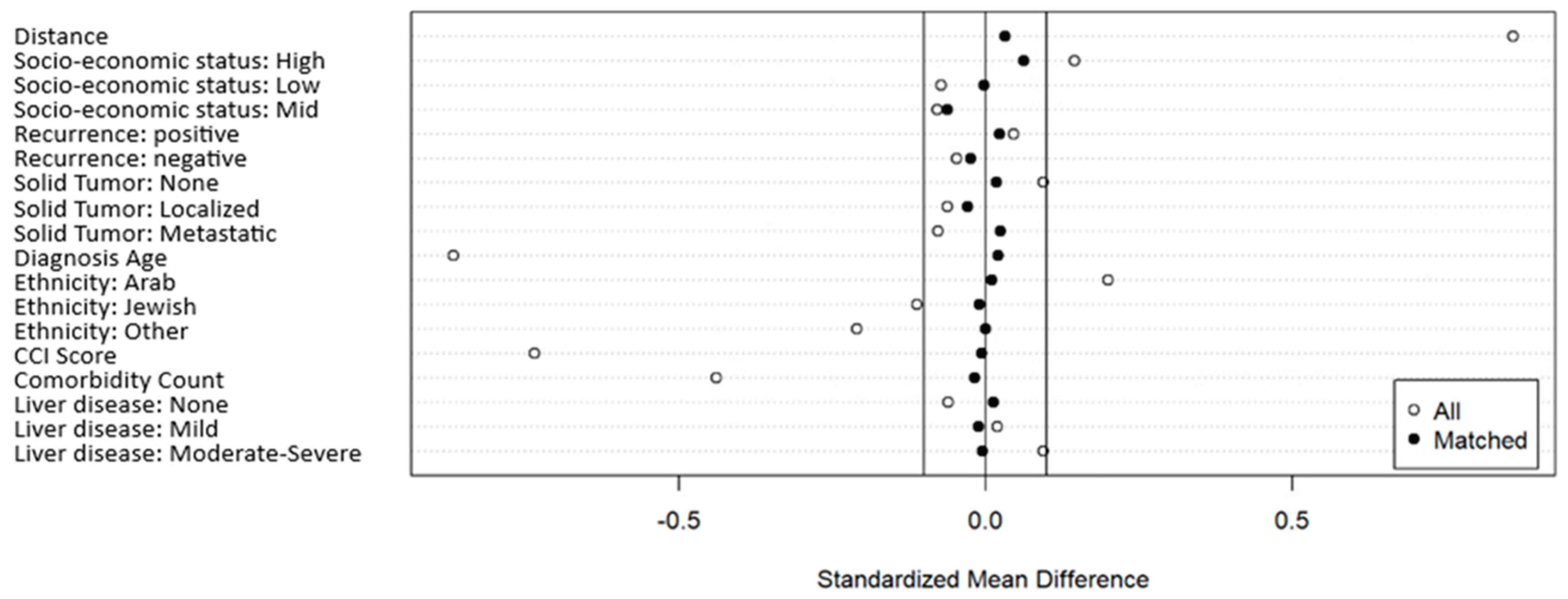
Figure 1.
Standardized Mean Differences pre- and post-PSM, with 1:3 User to Non-User Ratio and 0.2 Caliper.
Figure 1.
Standardized Mean Differences pre- and post-PSM, with 1:3 User to Non-User Ratio and 0.2 Caliper.
Figure 1: Standardized mean differences pre- and post-PSM, using our final PSM algorithm. The solid lines represent the 0.1 threshold. As can be seen, all matched standardized mean differences are below the 0.1 threshold (in absolute value).
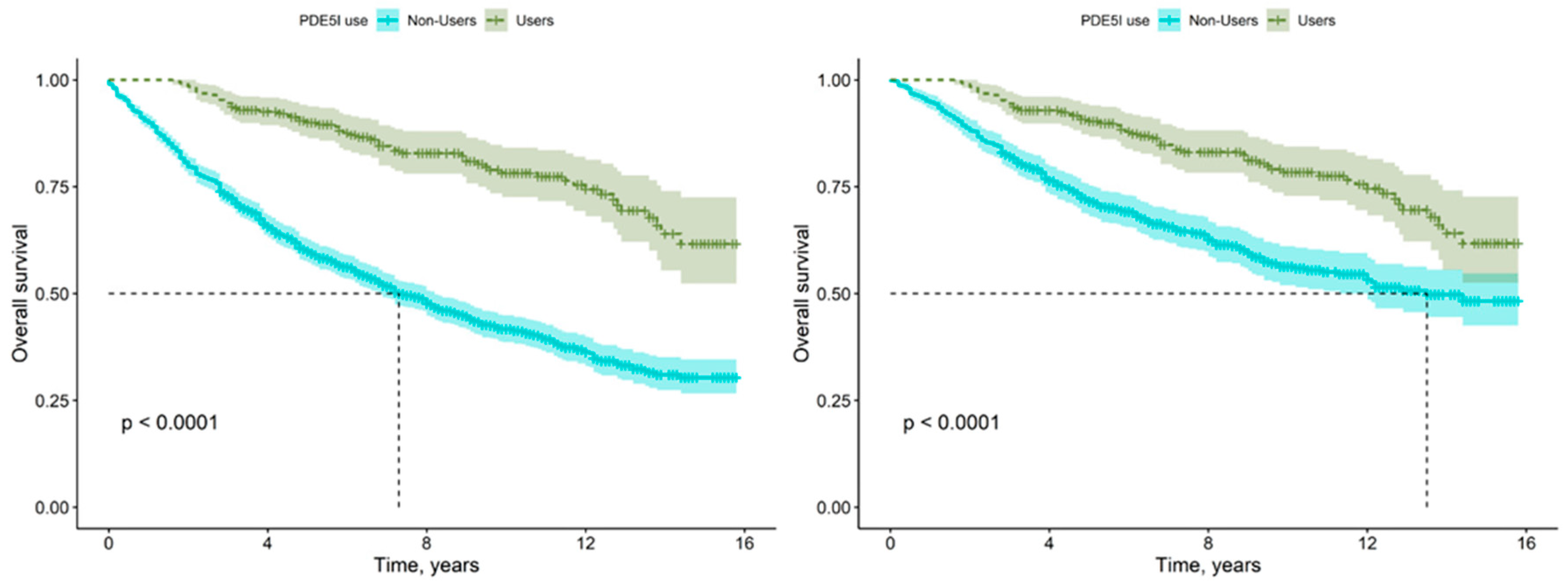
Figure 2.
KM Survival Estimates for PDE5I Users Versus Non-Users, pre- (left) and post-PSM (right).
Figure 2.
KM Survival Estimates for PDE5I Users Versus Non-Users, pre- (left) and post-PSM (right).
Figure 2 presents the KM survival estimates for PDE5I users and non-users pre-(left) and post-PSM (right). In both plots, green represents users, while blue represents non-users. The plots also display the log-rank test p-value, which is significant given any reasonable significance level, both before and after performing Propensity Score Matching. As can be seen (and is verified by the Log-Rank tests), although effect was reduced after matching, a clear and significant difference remains. The vertical and horizontal dashed lines in the graph represent the median survival for non-users. Median survival was not reached for PDE5I users.
Our non-parametric analyses revealed significant associations between PDE5I use and ethnicity (Χ² = 19.778, 2 df, p < 0.001) and age at diagnosis (Wilcoxon rank-sum test: W = 239625, p < 0.001, effect size = 0.286). Users and non-users did not significantly differ in terms of socioeconomic status. Among the PDE5I users (n=256), 204 (79.7%) were Jewish, while 54 (20.3%) were Arabs. This compared to 1091 Jews (84.2%), 159 Arabs (12.3%), and 46 (3.5%) belonging to some other ethnic minority, among non-PDE5I users. Users and non-users also significantly differed in terms of age at diagnosis, with users (mean = 57.42, SD = 10.63) tending to be younger than non-users (mean = 66.65, SD = 12.65).
Regarding clinical attributes, we observed a significant effect for radiotherapy (RT) (Chi-squared = 10.6, 1 df, p < 0.01), whereby 592 non-users (45.7%) received RT, compared to 146 (57%) of users. Users and non-users also differed significantly in terms of CCI scores. Non-users had a mean score of 5.11 (SD = 2.97), as opposed to 3.32 (SD = 2.42) among users (Wilcoxon rank-sum test: W = 228724, p < 0.001, effect size = 0.245). Users and non-users did not significantly differ in terms of recurrence rate, or tumor location. Full statistical summaries of the comparison of patients’ demographic and clinical attributes are provided in Table 1, as well as Shapiro-Wilks normality assessments of scale attributes, are provided in
Table S2. Distributions of patients by age at diagnosis are shown in
Figure S1
3.1. Initial Survival Analysis
The initial log-rank test comparing survival between PDE5I users and non-users revealed significantly better survival for PDE5I users (22.66% deaths versus 53.94% among non-users). We obtained a Chi-squared test statistic value of 94.75048, with p-value<0.001. The Observed-Expected table is presented below (Table 3A). A complete Cox PH model was fitted with all covariates. We obtained a Likelihood Ratio Test of 713.8 (32 df, n = 1552), with a p-value of <0.001. The total number of deaths was 757. Several covariates, including the use of PDE5Is, had statistically significant effects. The Hazard Ratio for PDE5I use versus non-use based on the Cox model was HR = 0.455, p-value<0.001. Full model results are provided in the supplementary data (
Table S1).
3.2. Variable Importance and PSM
To assess variable importance, we fit a standardized Cox PH model and an RSF model. The RSF achieved a mean OOB C-index of 0.754 with SD = 0.0015. The ordered importance of the top 6 variables/coefficients from each model is shown in
Table S3. As previously mentioned, we also added socio-economic status to the PSM algorithm, as it exhibited significance in the Cox PH model. The covariates used for matching were as follows: ethnicity, recurrence (inferred from medical records), malignancy (solid tumor from the CCI), age at diagnosis, CCI score, comorbidity count, liver disease (as indicated in the CCI), and socioeconomic status (classified into three levels).
We evaluated three matching ratios: 1:1, 1:2, and 1:3. The optimal balance was achieved with a 1:3 user-to-non-user ratio, and the caliper value was 0.2 (values tested included 0.05, 0.1, 0.15, and 0.2). The final matched dataset included 253 PDE5I users (3 were unmatched) and 630 non-users. All seven covariates used for matching achieved mean standardized differences < 0.1. Pre- and post-matching balance diagnostics are displayed in
Figure 2 via a Love plot.
3.3. Post Matching Analysis
To assess whether the effect of PDE5I use on patient survival remained significant after balancing the data concerning the most informative variables, we refit the log-rank test to the matched data. Indeed, this confirmed that PDE5I users continued to show significantly prolonged survival: χ² = 31.08973, p-value < 0.001. The Observed-Expected Table is presented below (Table 3B). The Hazard Ratio was again extracted from a Cox PH model, this time fitted post-PSM. We obtained HR = 0.361, p<0.001. Users’ and non-users’ survival curves, based on Kaplan-Meier estimators, are visually compared between pre- and post-PSM in
Figure 2. The median survival time for non-users, using the original data, was 7.3 years, whereas after balancing the data, it increased to 13.5 years. For users, the median survival time was not reached in either analysis.
3.4. Additional Analyses
All stratified log-rank tests conducted on subgroups, defined by ethnicity (Jewish vs. Arab) and disease recurrence status (recurrence vs. no recurrence), showed significant differences between users and non-users, with better survival rates for PDE5I users. Among patients with recurrence, we observed 259 events in non-users (79.9%) compared to 32 events in users (54.2%) (Chi-squared = 30.148, p < 0.001). Similarly, among patients without recurrence, the observed number of events was 440 (45.3%) for non-users and 26 (13.2%) among users (Χ² = 73.576, p < 0.001). For Jews, we identified 1091 events among non-users (54.4%) and 47 events (23%) among users (Chi-squared = 80.756, p < 0.001). Among the examined subsamples, the least significant effect was observed among Arab patients, with 59 (37.1%) and 11 (21.1%) events for non-users and users, respectively (Χ² = 4.869, p = 0.027). The full results for these subsamples are summarized in Table 2.
4. Discussion
Our retrospective study used the CHS database in Israel to examine the outcomes of 1,552 patients with rectal cancer, 256 of whom had purchased PDE5Is as sexual performance enhancement drugs. Non-parametric analyses revealed significant associations between PDE5I use and ethnicity (p < 0.001) and age at diagnosis (p < 0.001). Clinical attributes also differed significantly between users and non-users, including radiotherapy (p<0.01) and Charlson Comorbidity Index (CCI) scores (p<0.001). Initial survival analysis revealed significantly better survival rates for PDE5I users (p < 0.001), with a Hazard Ratio (HR) of 0.455 in the Cox model (p < 0.001). Variable importance was assessed using a standardized Cox PH model and an RSF model, with the RSF achieving a mean OOB C-index of 0.754. Covariates for matching included ethnicity, recurrence, malignancy, age at diagnosis, CCI score, comorbidity count, liver disease, and socio-economic status. Optimal balance in PSM was achieved with a 1:3 user-to-non-user ratio and a caliper of 0.2. Following PSM, the log-rank test confirmed significantly prolonged survival for PDE5I users (p < 0.001). The Hazard Ratio after PSM was HR = 0.361 (p<0.001). The median survival time for non-users increased from 7.3 years to 13.5 years after the data were balanced. Stratified log-rank tests on subgroups defined by ethnicity and recurrence status consistently showed better survival rates for PDE5I users (p < 0.001), except among Arab patients, where the effect was less significant (p = 0.027). Among patients with recurrence, non-users had more events (79.9%) compared to users (54.2%) (p<0.001). Similarly, among patients without recurrence, non-users had more events (45.3%) compared to users (13.2%) (p<0.001).
Our findings support those of several other studies into the possible anticancer effects of PDE5Is. An epidemiological analysis of a Swedish cohort clearly showed that PDE5I may reduce the incidence of colorectal cancer in men. [
24] A retrospective, matched cohort study analyzing data from 5,545 prostate cancer patients who underwent prostatectomy found that PDE5I administration was associated with improved overall survival and reduced risk of death, suggesting routine use of PDE5I after prostatectomy may improve survival in prostate cancer patients.[
25] Another nationwide Swedish cohort study found that gastric cancer patients who used PDE5I had lower cancer-specific mortality [
26]
Several potential mechanisms have been identified for the survival benefit observed with the use of PDE5Is. Emerging evidence suggests a duality in the effects of PDE5Is on tumorigenesis and oncoprotection in various sites and organs, as outlined in a recent review. [
13] Zhang’s study mentioned above combined their population-based evidence with in vivo and in vitro experiments to investigate the mechanisms by which the PDE5I sildenafil suppresses gastric cancer growth and found that it directly activates protein kinase G through PDE5 inhibition, regulating c-MYC expression and IL-6 transcription.
Overall, the hypotheses regarding the contribution of PDE5I to the survival of cancer patients can be divided into two approaches. The first is that the same mechanism that leads to improved erectile function is responsible for the positive effect on survival; increasing blood flow to the pelvis reduces inflammation and improves healing after oncological surgeries. This has been suggested as the cause of the anti-inflammatory effects observed in prostate tissue. [
14] Increased blood flow to the tumor area also improves accessibility of anti-tumor drugs to their target, thereby improving the survival of those patients receiving chemotherapy. [
17] Moreover, improved blood flow increases tumor oxygenation, which is crucial for radiation therapy. [
27] The second approach explains the clinical findings through more direct anti-cancer effects via molecular pathways involving cGMP or other molecular mechanisms - such as PDL1 upregulation, cell cycle arrest, and induction of apoptosis. [
28,
29]
It is worth noting that some evidence suggests patients taking PDE5 inhibitors may have a higher risk of developing melanoma, which is linked to the activation of the RAS/RAF/ERK signaling pathway in melanoma cells. [
30]
Overall, the findings of our study support the potential role of PDE5 inhibitors as adjunctive therapeutic agents in the management of rectal cancer. This option has been explored in silico with a novel, more potent PDE5 inhibitor, which had greater apoptotic effects and higher efficacy as an anti-tumor agent. [
31] There is still a lack of consistent and extensive basic research to explain the clinical findings and provide a precise mechanism for the improved survival seen in cancer patients.
This study has several limitations, including potential residual confounding despite the use of propensity score matching, which may not fully account for unmeasured variables influencing survival outcomes. The observational design precludes establishing causality between PDE5I use and improved survival, and there may be selection bias related to patients who purchase these medications, such as differences in health behavior or access to healthcare. Those patients who seek medication for erectile dysfunction may be healthier than those who do not. Additionally, the data relies on medication purchase history, which does not confirm actual usage or adherence to PDE5I therapy. The heterogeneity of the patient population, particularly in terms of ethnicity and comorbidities, may also impact generalizability. Lastly, missing data on certain clinical variables, such as tumor stage, limits the ability to comprehensively adjust for all relevant prognostic factors.
5. Conclusion
Here, we present evidence that the use of PDE5 inhibitors is associated with improved survival outcomes in patients with rectal cancer. Initial unadjusted analyses revealed significant differences in survival distributions, favoring PDE5I users, with a substantial reduction in hazard risk. To account for potential confounding factors such as ethnicity, age at diagnosis, comorbidities, and treatment variables, propensity score matching was employed, further reinforcing these findings. Post-matching analyses demonstrated that PDE5I users continued to experience significantly prolonged survival, with median survival times markedly increased compared to non-users. Stratified analyses across subgroups, including ethnicity and recurrence status, consistently indicated a survival benefit associated with PDE5I use, underscoring its potential role as an adjunctive therapeutic agent in rectal cancer management.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Acknowledgments
We want to thank Mrs. Snait Ayalon, data administration manager of Emek Medical Center, for her assistance in providing the essential data from the "Research room" and Dr. Gillian Kay for her language writing assistance.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Davies, M.L.; Harris, D.; Davies, M.; Lucas, M.; Drew, P.; Beynon, J. Selection criteria for the radical treatment of locally advanced rectal cancer. International journal of surgical oncology 2011, 2011, 678506. [Google Scholar] [CrossRef] [PubMed]
- Cancer Occurrence and Mortality Report for the Year 2020 Ministry of Health. Available online: https://www.gov.il/en/pages/05022023-03 (accessed on Feb 12, 2025).
- Wiltink, L.M.; Chen, T.Y.; Nout, R.A.; Meershoek-Klein Kranenbarg, E.; Fiocco, M.; Laurberg, S.; van de Velde, C.J.; Marijnen, C.A. Health-related quality of life 14 years after preoperative short-term radiotherapy and total mesorectal excision for rectal cancer: Report of a multicenter randomised trial. European Cancer Congress European Journal of Cancer 2013, 50, 2390–2398. [Google Scholar] [CrossRef]
- Wiltink, L.M.; Nout, R.A.; van der Voort van Zyp, J.R.N.; Ceha, H.M.; Fiocco, M.; Meershoek-Klein Kranenbarg, E.; Marinelli, A.W.K.S.; van de Velde, C.J.H.; Marijnen, C.A.M. Long-Term Health-Related Quality of Life in Patients With Rectal Cancer After Preoperative Short-Course and Long-Course (Chemo) Radiotherapy. Clinical Colorectal Cancer 2016, 15. [Google Scholar] [CrossRef]
- Ridolfi, T.J.; Berger, N.; Ludwig, K.A. Low Anterior Resection Syndrome: Current Management and Future Directions. Clinics in Colon and Rectal Surgery 2016, 29, 239. [Google Scholar] [CrossRef]
- Sclafani, F.; Peckitt, C.; Cunningham, D.; Tait, D.; Giralt, J.; Glimelius, B.; Keränen, S.R.; Bateman, A.; Hickish, T.; Tabernero, J.; et al. Short- and Long-Term Quality of Life and Bowel Function in Patients With MRI-Defined, High-Risk, Locally Advanced Rectal Cancer Treated With an Intensified Neoadjuvant Strategy in the Randomized Phase 2 EXPERT-C Trial. International Journal of Radiation Oncology*Biology*Physics 2015, 93, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.B.; Oggesen, B.T.; Fonnes, S.; Rosenberg, J. Erectile Dysfunction Is Common after Rectal Cancer Surgery: A Cohort Study. Current Oncology 2023, 30, 9317. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.A.; Lie, J.D. Phosphodiesterase-5 (PDE5) Inhibitors In the Management of Erectile Dysfunction. Pharmacy and Therapeutics 2013, 38, 407. [Google Scholar] [PubMed]
- Barone, I.; Giordano, C.; Bonofiglio, D.; Andò, S.; Catalano, S. Phosphodiesterase type 5 and cancers: progress and challenges. Oncotarget 2017, 8, 99179. [Google Scholar] [CrossRef]
- Catalano, S.; Panza, S.; Augimeri, G.; Giordano, C.; Malivindi, R.; Gelsomino, L.; Marsico, S.; Giordano, F.; Győrffy, B.; Bonofiglio, D.; et al. Phosphodiesterase 5 (PDE5) Is Highly Expressed in Cancer-Associated Fibroblasts and Enhances Breast Tumor Progression. Cancers 2019, 11, 1740. [Google Scholar] [CrossRef]
- Corbin, J.D. Mechanisms of action of PDE5 inhibition in erectile dysfunction. International journal of impotence research 2004, 16 Suppl 1. [Google Scholar] [CrossRef]
- Paronetto, M.P.; Crescioli, C. Rethinking of phosphodiesterase 5 inhibition: the old, the new and the perspective in human health. Frontiers in Endocrinology 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Peixoto, C.A.; Gomes, F.O.D.S. The role of phosphodiesterase-5 inhibitors in prostatic inflammation: A review. Journal of Inflammation (United Kingdom) 2015, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benjamins, J.A.; Nedelkoska, L. Cyclic GMP-dependent pathways protect differentiated oligodendrocytes from multiple types of injury. Neurochemical Research 2007, 32, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Khoshakhlagh, P.; Bahrololoumi-Shapourabadi, M.; Mohammadirad, A.; Ashtaral-Nakhai, L.; Minaie, B.; Abdollahi, M. Beneficial Effect of Phosphodiesterase-5 Inhibitor in Experimental Inflammatory Bowel Disease; Molecular Evidence for Involvement of Oxidative Stress. Toxicology Mechanisms and Methods 2007, 17, 281–288. [Google Scholar] [CrossRef]
- Cruz-Burgos, M.; Losada-Garcia, A.; Cruz-Hernández, C.D.; Cortés-Ramírez, S.A.; Camacho-Arroyo, I.; Gonzalez-Covarrubias, V.; Morales-Pacheco, M.; Trujillo-Bornios, S.I.; Rodríguez-Dorantes, M. New Approaches in Oncology for Repositioning Drugs: The Case of PDE5 Inhibitor Sildenafil. Frontiers in Oncology 2021, 11, 627229. [Google Scholar] [CrossRef]
- Pantziarka, P.; Sukhatme, V.; Crispino, S.; Bouche, G.; Meheus, L.; Sukhatme, P. Repurposing drugs in oncology (ReDO)—selective PDE5 inhibitors as anti-cancer agents. ecancermedicalscience 2018, 12. [Google Scholar] [CrossRef]
- Mei, X.L.; Yang, Y.; Zhang, Y.J.; Li, Y.; Zhao, J.M.; Qiu, J.G.; Zhang, W.J.; Jiang, Q.W.; Xue, Y.Q.; Zheng, D.W.; et al. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. American Journal of Cancer Research 2015, 5, 3311. [Google Scholar] [PubMed]
- Huang, W.; Sundquist, J.; Sundquist, K.; Ji, J. Phosphodiesterase-5 inhibitors use and risk for mortality and metastases among male patients with colorectal cancer. Nature Communications 2020 11:1 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Haider, M.; Elsherbeny, A.; Pittalà, V.; Fallica, A.N.; Alghamdi, M.A.; Greish, K. Personalized Medicine The Potential Role of Sildenafil in Cancer Management through EPR Augmentation. 2021. [Google Scholar] [CrossRef]
- Clalit At A Glance - Mor. Available online: https://mor-research.com/clalit-at-a-glance/ (accessed on Mar 12, 2025).
- https://www.btl.gov.il/Mediniyut/Situation/haveruth1/2025/Documents/capitatia_012025.docx - Пoиск в Google. Available online: https://www.google.com/search?client=firefox-b-d&q=https%3A%2F%2Fwww.btl.gov.il%2FMediniyut%2FSituation%2Fhaveruth1%2F2025%2FDocuments%2Fcapitatia_012025.docx (accessed on Feb 14, 2025).
- Huang, W.; Sundquist, J.; Sundquist, K.; Ji, J. Use of Phosphodiesterase 5 Inhibitors Is Associated With Lower Risk of Colorectal Cancer in Men With Benign Colorectal Neoplasms. Gastroenterology 2019, 157, 672–681.e4. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.R.; Heo, J.E.; Jang, W.S.; Lee, K.S.; Kang, S.K.; Han, H.; Choi, Y.D. Phosphodiesterase-5 Inhibitor Use in Robot Assisted Radical Prostatectomy Patients Is Associated with Reduced Risk of Death: A Propensity Score Matched Analysis of 1,058 Patients. World Journal of Men’s Health 2023, 41, 892–899. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, W.; Huang, D.; Xu, Z.; Xie, Q.; Tan, X.; He, W.; Yang, W.; Li, G.; Ji, J.; et al. Repurposing of phosphodiesterase-5 inhibitor sildenafil as a therapeutic agent to prevent gastric cancer growth through suppressing c-MYC stability for IL-6 transcription. Communications biology 2025, 8, 85. [Google Scholar] [CrossRef]
- Tubin, S.; Popper, H.H.; Brcic, L. Novel stereotactic body radiation therapy (SBRT)-based partial tumor irradiation targeting hypoxic segment of bulky tumors (SBRT-PATHY): Improvement of the radiotherapy outcome by exploiting the bystander and abscopal effects. Radiation Oncology 2019, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.-M.; Wang, K.; Huang, J.-R.; Mei, X.-L.; Shi, Z. Sildenafil Induces Cell Cycle Arrest and Apoptosis in Human Colorectal Cancer HT-29 Cells. Journal of Cancer Research Updates 2018, 7, 59–63. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, D.; Feng, J.; Li, W.; Wang, Z.; Lu, M.; Luo, Y.; Yang, W.; Xu, Z.; Xie, Q.; et al. PDE5 inhibitors against cancer via mediating immune cells in tumor microenvironment: AI-based approach for future drug repurposing exploration. Interdisciplinary Medicine 2024, 2, e20230062. [Google Scholar] [CrossRef]
- Li, W.Q.; Qureshi, A.A.; Robinson, K.C.; Han, J. Sildenafil use and increased risk of incident melanoma in US men: a prospective cohort study. JAMA internal medicine 2014, 174, 964. [Google Scholar] [CrossRef]
- Oladeji, S.M.; Conteh, D.N.; Bello, L.A.; Adegboyega, A.E.; Shokunbi, O.S. Rational Design and Optimization of Novel PDE5 Inhibitors for Targeted Colorectal Cancer Therapy: An In Silico Approach. International Journal of Molecular Sciences 2025, 26, 1–16. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).