Submitted:
11 August 2025
Posted:
18 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Part
3. Results and Discussion
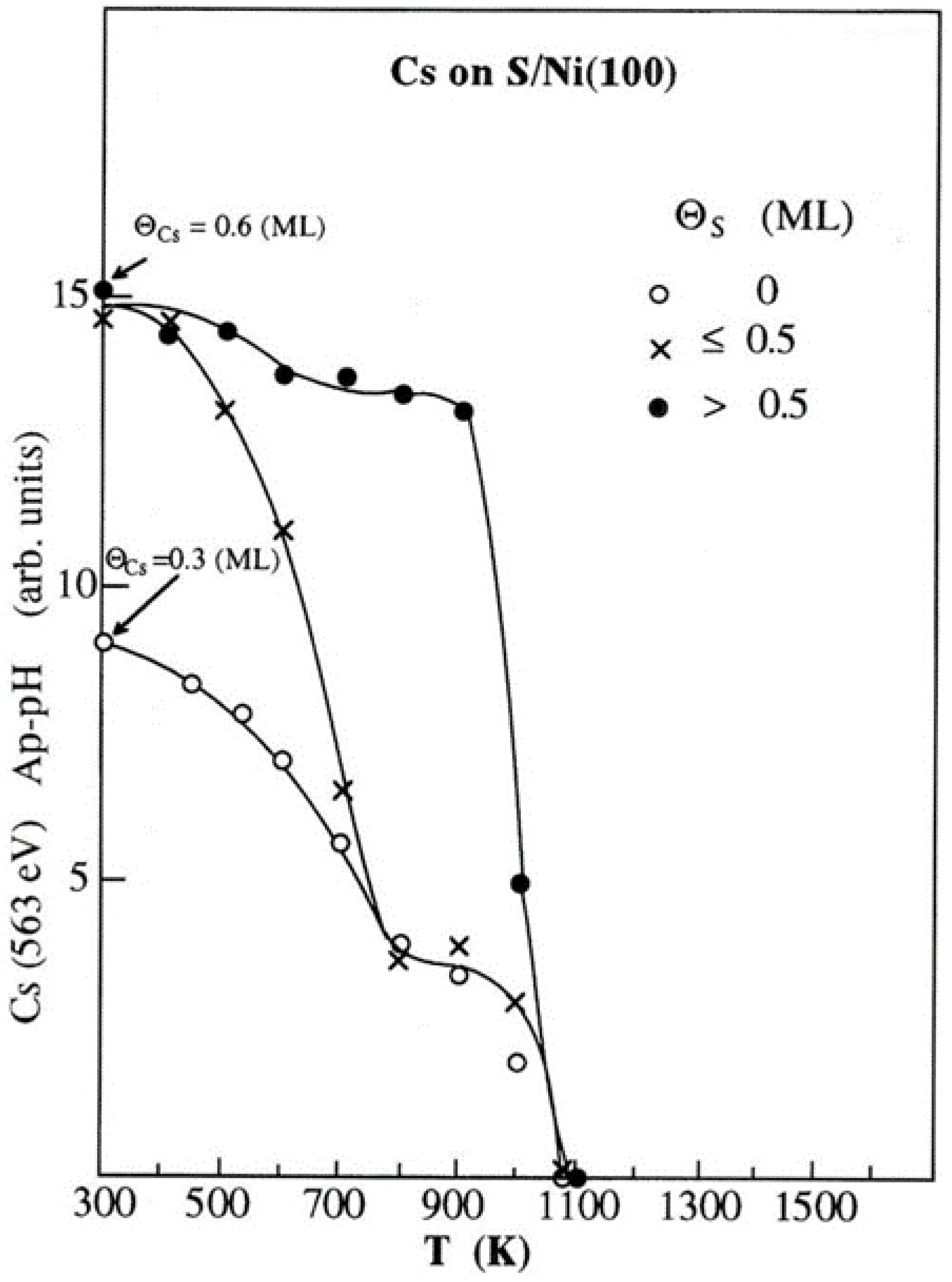
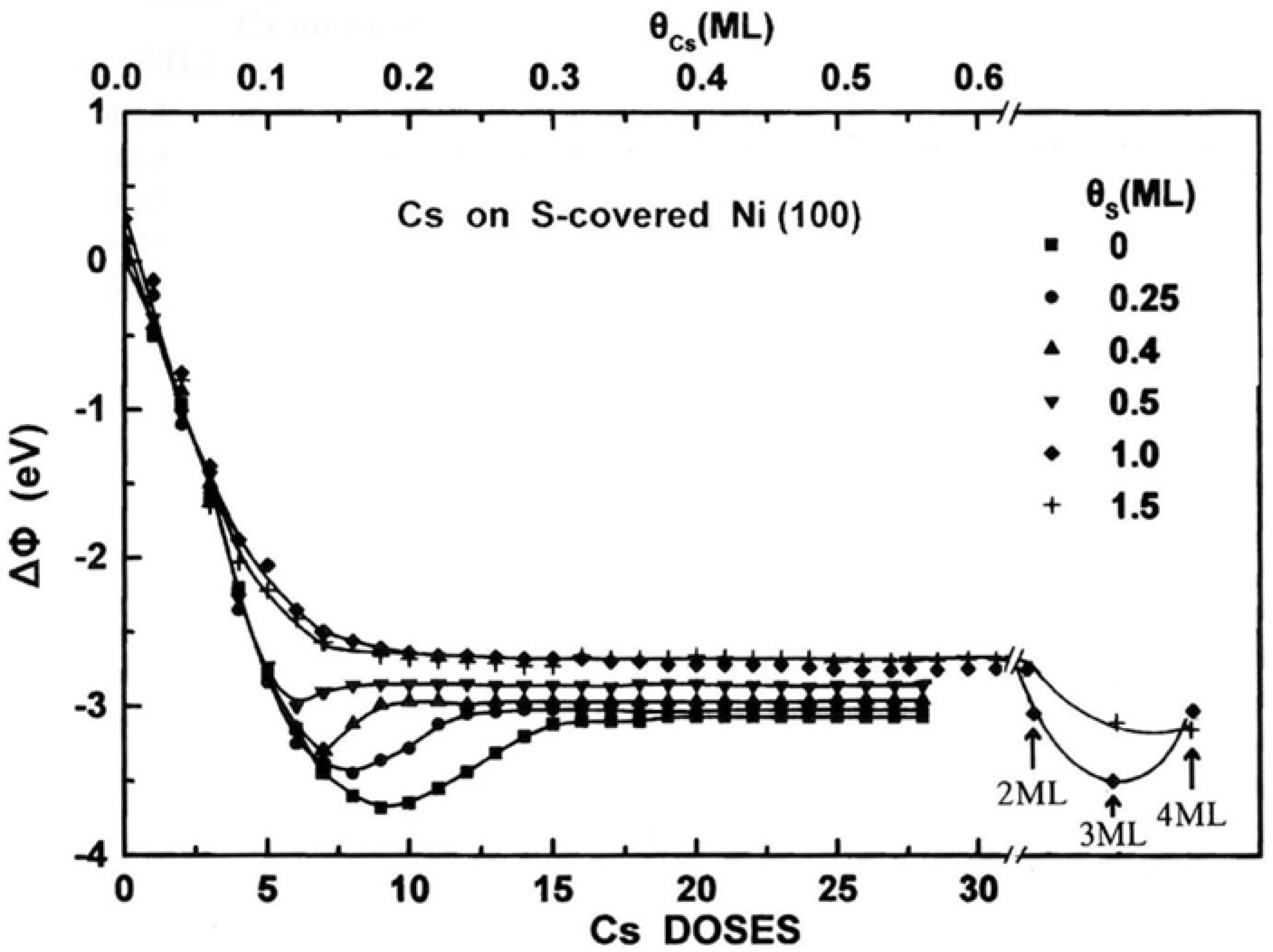
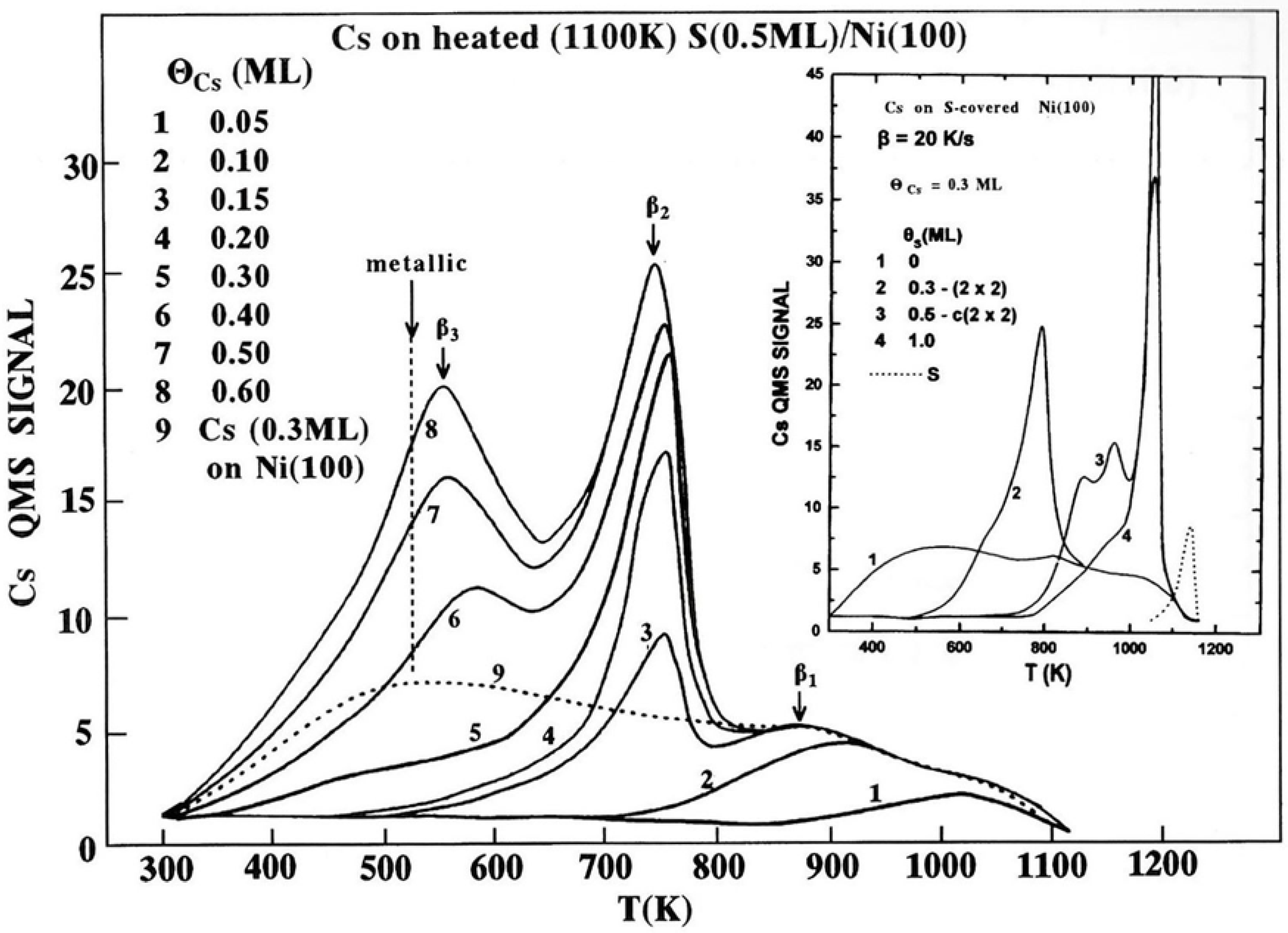
3.1. Cesium on S-Covered Ni(100)
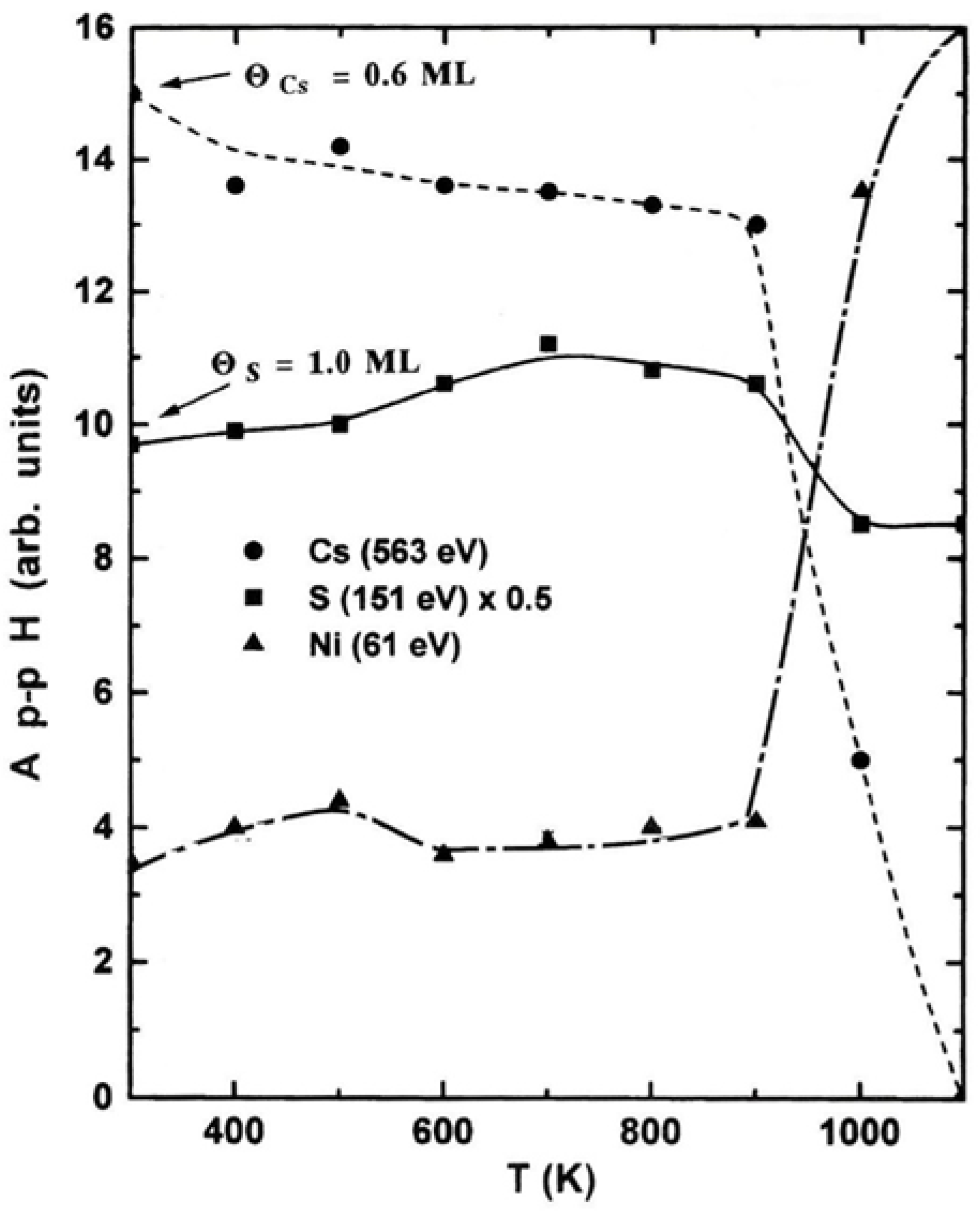
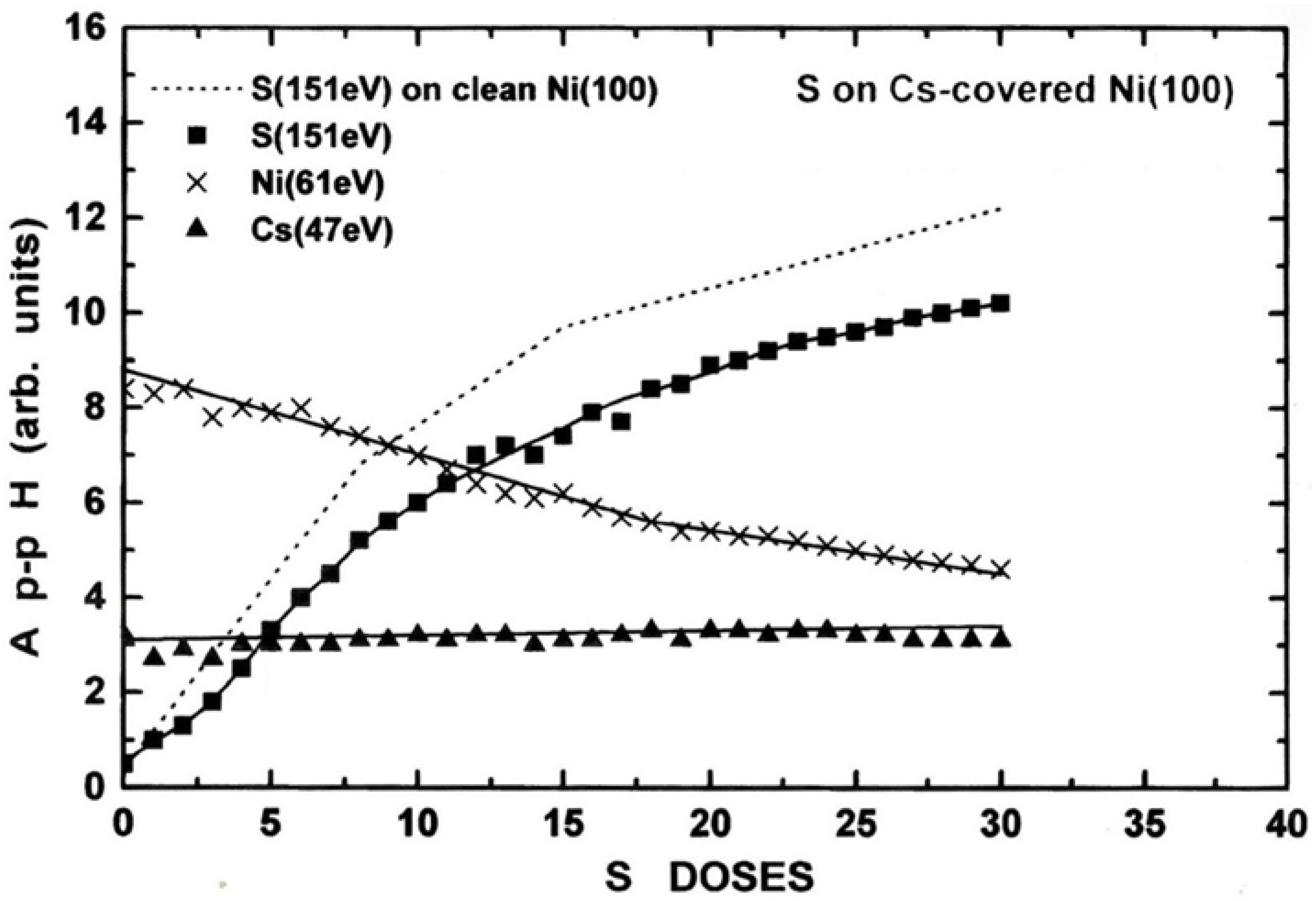
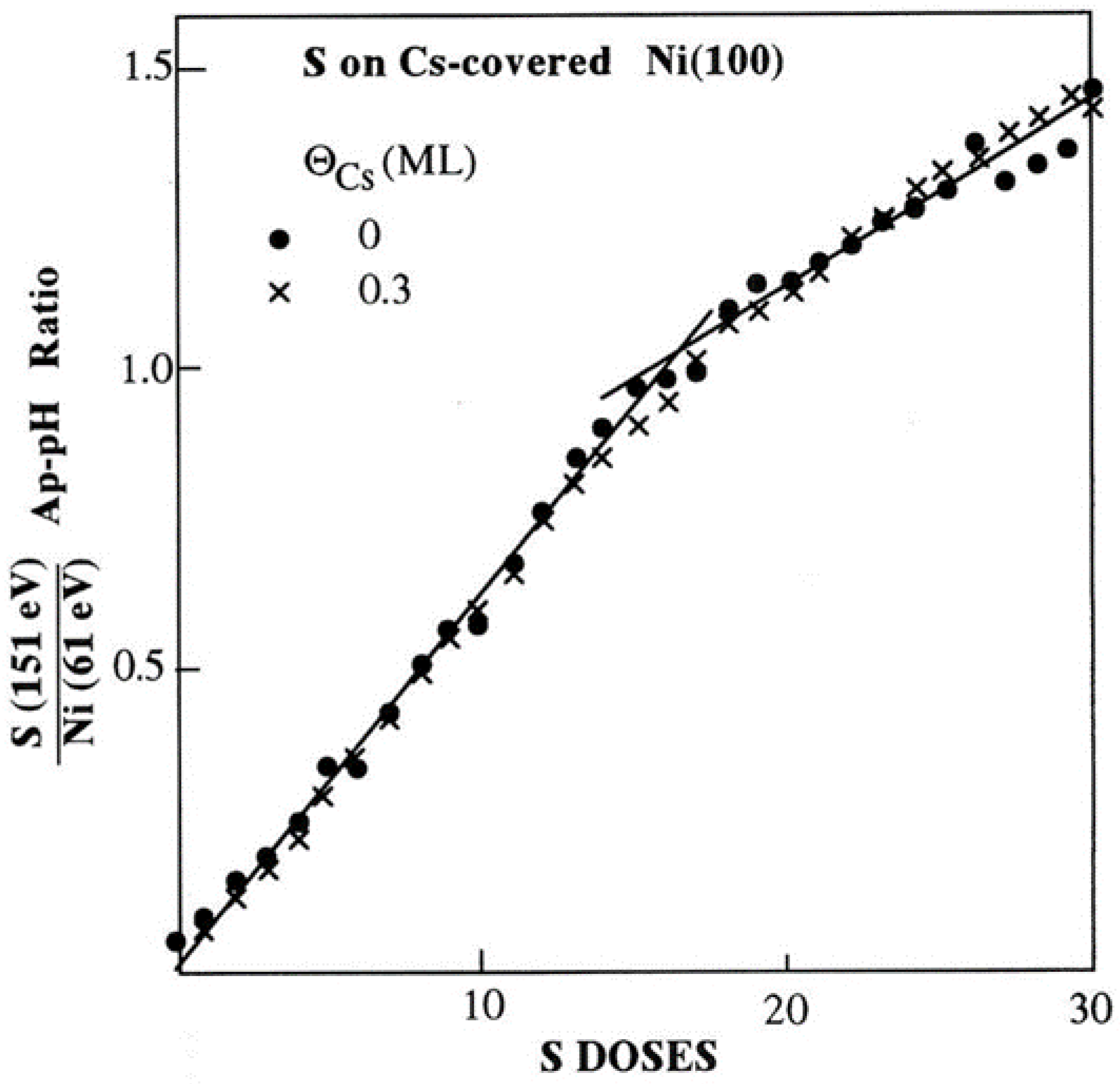
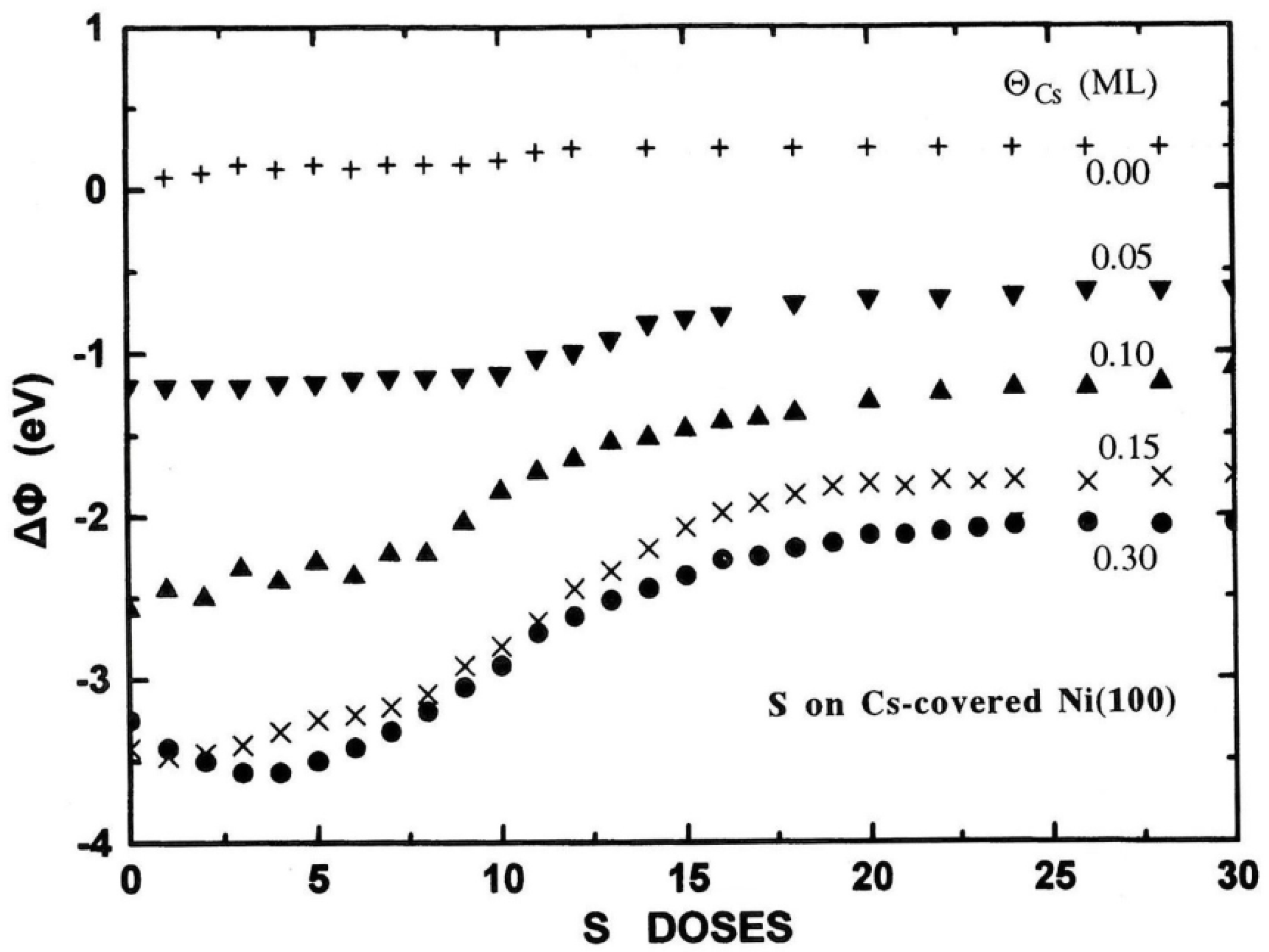
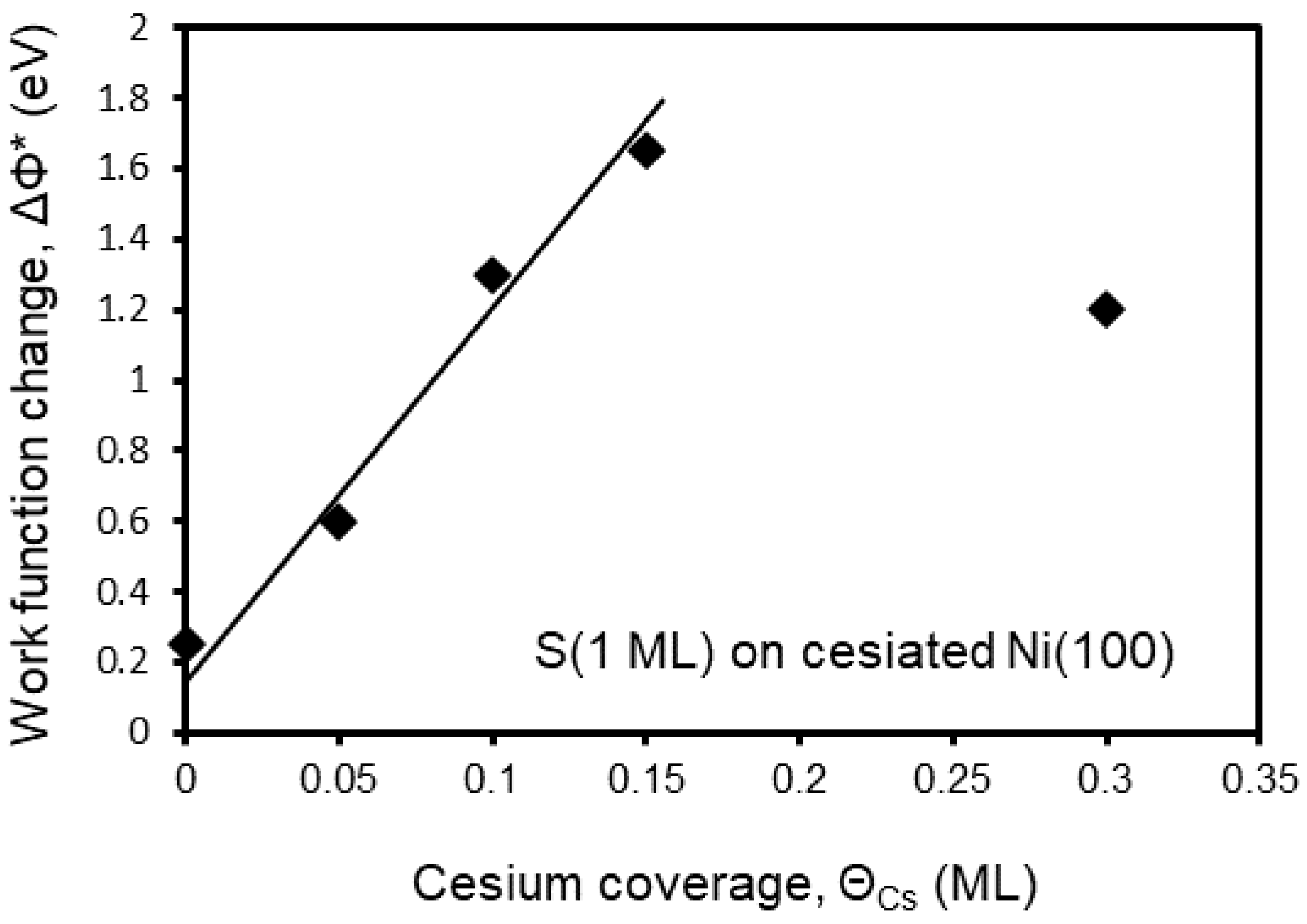
3.2. Sulfur on Cs-Covered Ni(100) Surface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cantoni, C.; Christen, D.K.; Feenstra, R.; Norton, D.P.; Goyal, A.; Ownby, G.W.; Zehner, D.M.; Norton, D.P. Reflection high-energy electron diffraction studies of epitaxial oxide seed-layer growth on rolling-assisted biaxially textured substrate Ni(001): The role of surface structure and chemistry. Appl. Phys. Lett. 2001, 79, 3077–3079. [Google Scholar] [CrossRef]
- Cantoni, C.; Christen, D.K.; Heatherly, L.; Kowalewski, M.M.; List, F.A.; Goyal, A.; Ownby, G.W.; Zehner, D.M.; Kang, B.W.; Kroeger, D.M. Quantification and control of the sulfur c(2 × 2) superstructure on {100}〈100〉 Ni for optimization of YSZ, CeO2, and SrTiO3 seed layer texture. J. Mater. Res. 2002, 17, 2549–2554. [Google Scholar] [CrossRef]
- Li, C.S.; Yu, Z.M.; Odier, P.; Wang, Y.; Jina, L.H.; Lu, Y.F. Deposition of La2Zr2O7 (LZO) films on NiW substrates by chemical solution deposition process. Physica C Superconductivity and its Applications 2011, 471, 974–977. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Fitzharris, W.D.; Katzer, J.R. Sulfur Poisoning and Carbon Deactivation of Alumina-Supported Ni, Co, Fe and Ru Catalysts in CO Hydrogenation. Stud. Surf. Sci. Catal. 1980, 6, 179–200. [Google Scholar]
- Stenger, H.G.; Satterfield, C.N. Effects of sulfur poisoning of a reduced fused magnetite catalyst in the Fischer-Tropsch synthesis. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 415–420. [Google Scholar] [CrossRef]
- Liu, Z.T.; Li, Y.W.; Zhou, J.L.; Zhang, Z.X.; Zhang, B.J. Deactivation model of Fischer-Tropsch synthesis over an FeCuK commercial catalyst. Applied Catalysis A General 1997, 161, 137–151. [Google Scholar] [CrossRef]
- Visconti, C.G.; Lietti, L.; Forzatti, P.; Zennaro, R. Fischer–Tropsch synthesis on sulphur poisoned Co/Al2O3 catalyst. Applied Catalysis A General 2007, 330, 49–56. [Google Scholar] [CrossRef]
- Curtis, V.; Nicolaides, C.P.; Coville, N.J.; Hildebrandt, D.; Glasser, D. The effect of sulfur on supported cobalt Fischer–Tropsch catalysts. Catalysis Today 1999, 49, 33–40. [CrossRef]
- McCue, A.J.; Anderson, J.A. Sulfur as a catalyst promoter or selectivity modifier in heterogeneous catalysis. Catal. Sci. Technol. 2014, 4, 272–294. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Y.; Yu, Y.; Du, Y.; Zhang, B. Engineering sulfur defects, atomic thickness, and porous structures into cobalt sulfide nanosheets for efficient electrocatalytic alkaline hydrogen evolution. ACS Catal. 2018, 8, 8077–8083. [Google Scholar] [CrossRef]
- Gu, C.; Zhou, G.; Yang, J.; Pang, H.; Zhang, M.; Zhao, Q.; Gu, X.; Tian, S.; Zhang, J.; Xu, L.; Tang, Y. NiS/MoS2 Mott-Schottky heterojunction-induced local charge redistribution for high-efficiency urea-assisted energy-saving hydrogen production. Chemical Engineering Journal 2022, 443, 136321. [Google Scholar] [CrossRef]
- Zhou, L.; Bo, B.; Yan, X.; Wang, C.; Chi, Y.; Yang, X. Brief review of surface passivation on III-V semiconductor. Crystals 2018, 8, 226. [Google Scholar] [CrossRef]
- Guo, S.; Yang, D.; Wang, D.; Fang, X.; Fang, D.; Chu, X.; Yang, X.; Tang, J.; Liao, L.; Wei, Z. Response improvement of GaAs two-dimensional non-layered sheet photodetector through sulfur passivation and plasma treatment. Vacuum 2022, 197, 110792. [Google Scholar] [CrossRef]
- Tajik, N.; Peng, Z.; Kuyanov, P.; LaPierre, R.R. Sulfur passivation and contact methods for GaAs nanowire solar cells. Nanotechnology 2011, 22, 225402. [Google Scholar] [CrossRef]
- Vayenas, C.G.; Bebelis, S.; Ladas, S. Dependence of catalytic rates on catalyst work function. Nature 1990, 343, 625–627. [Google Scholar] [CrossRef]
- Wang, L.; Ge, J.; Wang, A.; Deng, M.; Wang, X.; Bai, S.; Li, R.; Jiang, J.; Zhang, Q.; Luo, Y.; Xiong, Y. Designing p-type semiconductor–metal hybrid structures for improved photocatalysis. Angew. Chem. 2014, 126, 5207–5211. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Lia, X.; Liu, Y.; Cao, Y.; Wang, H.; Yu, H.; Peng, F.; Zhang, L.; Zhang, B.; Lv, K. High efficiency photocatalytic hydrogen production over ternary Cu/TiO2@Ti3C2Tx enabled by low-work-function 2D titanium carbide. Nano Energy 2018, 53, 97–107. [Google Scholar] [CrossRef]
- Kant, R.; Kaur, J.; Mishra, G.K. Theory for Influence of the Metal Electrolyte Interface on Heterogeneous Electron Transfer Rate Constant: Fractional Electron Transferred Transition State Approach. J. Phys. Chem. C 2020, 124, 2273–2288. [Google Scholar] [CrossRef]
- Kawano, H. Effective work functions for ionic and electronic emissions from mono-and polycrystalline surfaces. Progress in Surface Science 2008, 83, 1–165. [Google Scholar] [CrossRef]
- Peng, X.; Hu, L.; Qin, F.; Zhou, Y.; Chu, P.K. Low Work Function Surface Modifiers for Solution-Processed Electronics: A Review. Adv. Mater. Interfaces 2018, 5, 1701404. [Google Scholar] [CrossRef]
- Yamamoto, S. Fundamental physics of vacuum electron sources. Rep. Prog. Phys. 2006, 69, 181–232. [Google Scholar] [CrossRef]
- Kawano, H. Effective Work Functions of the Elements: Database, Most probable value, Previously recommended value, Polycrystalline thermionic contrast, Change at temperature, Anisotropic dependence sequence, Particle size dependence. Progress in Surface Science 2022, 97, 100583. [Google Scholar] [CrossRef]
- Papageorgopoulos, C.A.; Chen, J.M. Coadsorption of electropositive and electronegative elements: I. Cs and H2 on W(100). Surf. Sci. 1973, 39, 283–312. [Google Scholar] [CrossRef]
- Papageorgopoulos, C.A.; Chen, J.M. Coadsorption of Cesium and Oxygen on Ni(100). I. Cesium probing of Ni-O bonding. Surf. Sci. 1975, 52, 40–52. [Google Scholar] [CrossRef]
- Papageorgopoulos, C.A.; Desplat, J.L. Interaction of Cesium and Oxygen on W(110). I. Cesium adsorption on oxygenated and oxidized W(110). Surf. Sci. 1980, 92, 97–118. [Google Scholar] [CrossRef]
- Oudar, J. Sulfur adsorption and poisoning of metallic catalysts. Catalysis Reviews 1980, 22, 171–195. [Google Scholar] [CrossRef]
- MacLaren, J.M.; Pendry, J.B.; Vvedensky, D.D.; Joyner, R.W. Influence of poisons and promoters on local bonding of CO to Ni(100). Surf. Sci. 1985, 162, 322–328. [Google Scholar] [CrossRef]
- Kiskinova, M.P. Electronegative additives and poisoning in catalysis. Surf. Sci. Rep. 1988, 8, 359–402. [Google Scholar] [CrossRef]
- Joyner, R.W.; Meehan, P.; MacLaren, J.M.; Pendry, J.B. Poisoning of the methanation reaction on the Ni(100) surface; theoretical calculations compared with the results of goodman et al. Applied Catalysis 1986, 25, 9–17. [Google Scholar] [CrossRef]
- Zha, S.; Cheng, Z.; Liu, M. Sulfur Poisoning and Regeneration of Ni-Based Anodes in Solid Oxide Fuel Cells. J. Electrochem. Soc. 2007, 154, B201. [Google Scholar] [CrossRef]
- Malyi, O.I.; Chen, Z.; Kulish, V.V.; Bai, K.; Wu, P. Density functional theory study of the effects of alloying additions on sulfur adsorption on nickel surfaces Appl. Surf. Sci. 2013, 264, 320–328. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Yang, Z. Resistance to sulfur poisoning of Ni-based alloy with coinage (IB) metals. Appl. Surf. Sci. 2015, 357, 1785–1791. [Google Scholar] [CrossRef]
- Moud, P.H.; Andersson, K.J.; Lanza, R.; Pettersson, J.B.C.; Engvall, K. Effect of gas phase alkali species on tar reforming catalyst performance: Initial characterization and method development. Fuel 2015, 154, 95–106. [Google Scholar] [CrossRef]
- Hernandez, A.; Andersson, K.J.; Engvall, K.; Kantarelis, E. Gas-Phase Potassium Effects and the Role of the Support on the Tar Reforming of Biomass-Derived Producer Gas Over Sulfur-Equilibrated Ni/MgAl2O4. Energy & Fuels 2020, 34, 11103–11111. [Google Scholar]
- Hardegree, E.L.; Ho, P.; White, J.M. Sulfur adsorption on Ni (100) and its effect on CO chemisorption: I. TDS, AES and work function results. Surf. Sci. 1986, 165, 488–506. [Google Scholar] [CrossRef]
- Starke, U.; Bothe, F.; Oed, W.; Heinz, K. LEED analysis of the adsorption structure of c (2× 2) SNi (100) including substrate modifications. Surf. Sci. 1990, 232, 56–62. [Google Scholar] [CrossRef]
- Oed, W.; Starke, U.; Bothe, F.; Heinz, K. LEED structure analysis of p (2× 2) S/Ni (100). Surf. Sci. 1990, 234, 72–78. [Google Scholar] [CrossRef]
- Mercer, J.R.; Scarel, G.; Santoni, A.; Cowie, B.C.C.; Lewis, D.; Robinson, A.W.; McGrath, R.; Dhanak, V.R. Structural study of Rh (100) - c (2×2)-S using the normal-incidence standing X-ray wavefield method. Surf. Sci. 1996, 369, 36–44. [Google Scholar] [CrossRef]
- Gall, N.R.; Rut’kov, E.V.; Tontegode, A.Y.; Usufov, M.M. Surface sulfide on (100) W: formation, stability, absolute concentration of sulfur. Appl. Surf. Sci. 1996, 93, 353–358. [Google Scholar] [CrossRef]
- Papageorgopoulos, C.A.; Kamaratos, M.; Papageorgopoulos, A. Coadsorption of Na and elemental S on Ni (100). Surf. Sci. 1998, 402–404, 120–124. [Google Scholar] [CrossRef]
- Papageorgopoulos, C.A.; Kamaratos, M.; Papageorgopoulos, A.C. Coadsorption of cesium and elemental sulfur on Ni (100) surfaces. Surf. Sci. 1999, 433–435, 806–810. [Google Scholar] [CrossRef]
- Papageorgopoulos, A.C.; Kamaratos, M. K and S coadsorption on Ni(100) surfaces. J. Phys. Condens. Matter 2000, 12, 9281–9291. [Google Scholar] [CrossRef]
- Papageorgopoulos, A.C.; Kamaratos, M.; Papageorgopoulos, C.A. Li on S covered Ni(100) surfaces. Surf. Sci. 2001, 481, 143–149. [Google Scholar] [CrossRef]
- Demuth, J.E.; Jepsen, D.W.; Marcus, P.M. Crystallographic dependence of chemisorption bonding for sulfur on (001),(110), and (111) nickel. Phys. Rev. Lett. 1974, 32, 1182–1185. [Google Scholar] [CrossRef]
- Partridge, A.; Tatlock, G.J.; Leibsle, F.M.; Flipse, C.F.J.; Hormandinger, G.; Pendry, J.B. Scanning-tunneling-microscopy investigation of the p(2×2) and c(2×2) overlayers of S on Ni(100). Phys. Rev. B 1993, 48, 8267–8276. [Google Scholar] [CrossRef]
- Papageorgopoulos, C.A.; Kamaratos, M. Adsorption of elemental S on Ni(100) surfaces. Surf. Sci. 1995, 338, 77–82. [Google Scholar] [CrossRef]
- Demuth, J.E.; Jepsen, D.W.; Marcus, P.M. Chemisorption Bonding of c(2x2) Chalcogen Qverlayers on Ni(001). Phys. Rev. Lett. 1973, 31, 540–542. [Google Scholar] [CrossRef]
- Plummer, E.W.; Tonner, B.; Holzwarth, N.; Liebsch, A. Electronic structure of ordered sulfur overlayers on Ni (001). Phys. Rev. B 1980, 21, 4306–4321. [Google Scholar] [CrossRef]
- McGrath, R.; MacDowell, A.A.; Hashizume, T.; Sette, F.; Citrin, P.H. Low-temperature adsorption of H2S on Ni(001) studied by near-edge and surface-extended - x-ray-absorption fine structure. Phys. Rev. B 1989, 40, 9457–9463. [Google Scholar] [CrossRef] [PubMed]
- Blaszcsyszyn, M.; Blaszcsyszyn, R.; Meclewski, R.; Melmed, A.J.; Madey, T.E. Interactions of sulfur with nickel surfaces: adsorption, diffusion and desorption. Surf. Sci. 1983, 131, 433–447. [Google Scholar] [CrossRef]
- Papageorgopoulos, C.A.; Foulias, S.; Kennou, S.; Kamaratos, M. The effect of Cs on the oxidation of Si (111) surfaces. Surf. Sci. 1989, 211–212, 991–1000. [Google Scholar] [CrossRef]
- Wu, R.Q.; Wang, D.S. Effect of the surface states of different transition-metal substrates on a Cs overlayer. Phys. Rev. B 1990, 41, 12541–12552. [Google Scholar] [CrossRef]
- Vlachos, D.; Giotopoulou, E.; Foulias, S.D.; Kamaratos, M. Cesium growth on the SrTiO3 (100) surface. Mater. Res. Express 2015, 2, 116501. [Google Scholar] [CrossRef]
- Rasor, N.S.; Warner, C. Correlation of Emission Processes for Adsorbed Alkali Films on Metal Surfaces. J. Appl. Phys. 1964, 35, 2589–2600. [Google Scholar] [CrossRef]
- Gasser, R.P.H. Introduction to Chemisorption and Catalysis by Metals Oxford University Press 1985.
- Woodruff, D.P.; Delchar, T.A. Modern Techniques of Surface Science Cambridge University Press 1986.
- Raaen, S. Adsorption of carbon dioxide on mono-layer thick oxidized samarium films on Ni(100). Nanomaterials 2021, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Papageorgopoulos, C.A. Adsorption of Cs on H-precovered W(110) surfaces. Phys. Rev. B 1989, 40, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Papageorgopoulos, C.A.; Kamaratos, M. Adsorption of K on clean and hydrogenated Si(100) 2×1. Vacuum 1990, 41, 567–570. [Google Scholar] [CrossRef]
- Wimmer, E.; Freeman, A.J.; Hishes, J.R.; Karo, A.M. All-electron local-density theory of alkali-metal bonding on transition-metal surfaces: Cs on W(001). Phys. Rev. B 1983, 28, 3074–3091. [Google Scholar] [CrossRef]
- Muscat, J.P.; Batra, I. Coverage dependence of the work function of metals upon alkali-metal adsorption. Phys. Rev. B 1986, 34, 2889–2892. [Google Scholar] [CrossRef]
- Kennou, S.; Kamaratos, M.; Papageorgopoulos, C.A. Potassium adsorption on NiO(100). Vacuum 1990, 41, 22–24. [Google Scholar] [CrossRef]
- Kennou, S.; Kamaratos, M.; Papageorgopoulos, C.A. Cesium and oxygen adsorption on NiO(100). Surf. Sci. 1991, 256, 312–316. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef]
- Haynes, W.M. Editor in Chief CRC Handbook of Chemistry and Physics, 95th Edition, CRC Press Taylor & Francis Group (2014-2015).
- Greene, E.F.; Kelley, J.T.; Pickering, M.A.; Stewart, D.K. Silicon (111) and (100) surfaces and their interactions with Cs, K, Na and Li; phase changes and kinetics of desorption studied by surface ionization. Surf. Sci. 1984, 139, 185–207. [Google Scholar] [CrossRef]
- Cousty, J.; Papageorgopoulos, C.A.; Riwan, R. Electronic properties of Cs layers adsorbed on Pt (111). Surf. Sci. 1989, 223, 479–492. [Google Scholar] [CrossRef]
- Bhaskar, N.D.; Kahla, C.M.; Martin, L.R. Absorption of cesium by polycrystalline graphite-sticking coefficient studies. Carbon 1990, 28, 71–78. [Google Scholar] [CrossRef]
- Papageorgopoulos, A.C.; Kamaratos, M. K and S coadsorption on Ni(100) surfaces. J. Phys. Condens. Matter 2000, 12, 9281–9291. [Google Scholar] [CrossRef]
- Redhead, P.A. Thermal desorption of gases. Vacuum 1962, 12, 203–211. [Google Scholar] [CrossRef]
- Seok, T.J.; Cho, Y.J.; Jin, H.S.; Kim, D.H.; Kim, D.W.; Lee, S.M.; Park, J.B.; Won, J.Y.; Kim, S.K.; Hwang, C.S.; Park, T.J. High quality interfacial sulfur passivation via H2S pre-deposition annealing for an atomic-layer-deposited HfO2 film on a Ge substrate. J. Mater. Chem. C 2016, 4, 850–856. [Google Scholar] [CrossRef]
- Kunitsyna, E.V.; L’vova, T.V.; Dunaevskii, M.S.; Terent’ev, Ya.V.; Semenov, A.N.; Solov’ev, V.A.; Meltser, B.Ya.; Ivanov, S.V.; Yakovlev, Yu.P. Wet sulfur passivation of GaSb (1 0 0) surface for optoelectronic applications. Appl. Surf. Sci. 2010, 256, 5644–5649. [Google Scholar] [CrossRef]
- Petrovykh, D.Y.; Yang, M.J.; Whitman, L.J. Chemical and electronic properties of sulfur-passivated InAs surfaces. Surf. Sci. 2003, 523, 231–240. [Google Scholar] [CrossRef]
 |
| ΘCs (ML) (±0.004 ML) |
ΘS (ML) | Φmin (±0.02 eV) |
Φfinal (±0.02 eV) |
Φfinal – Φmin (±0.04 eV) |
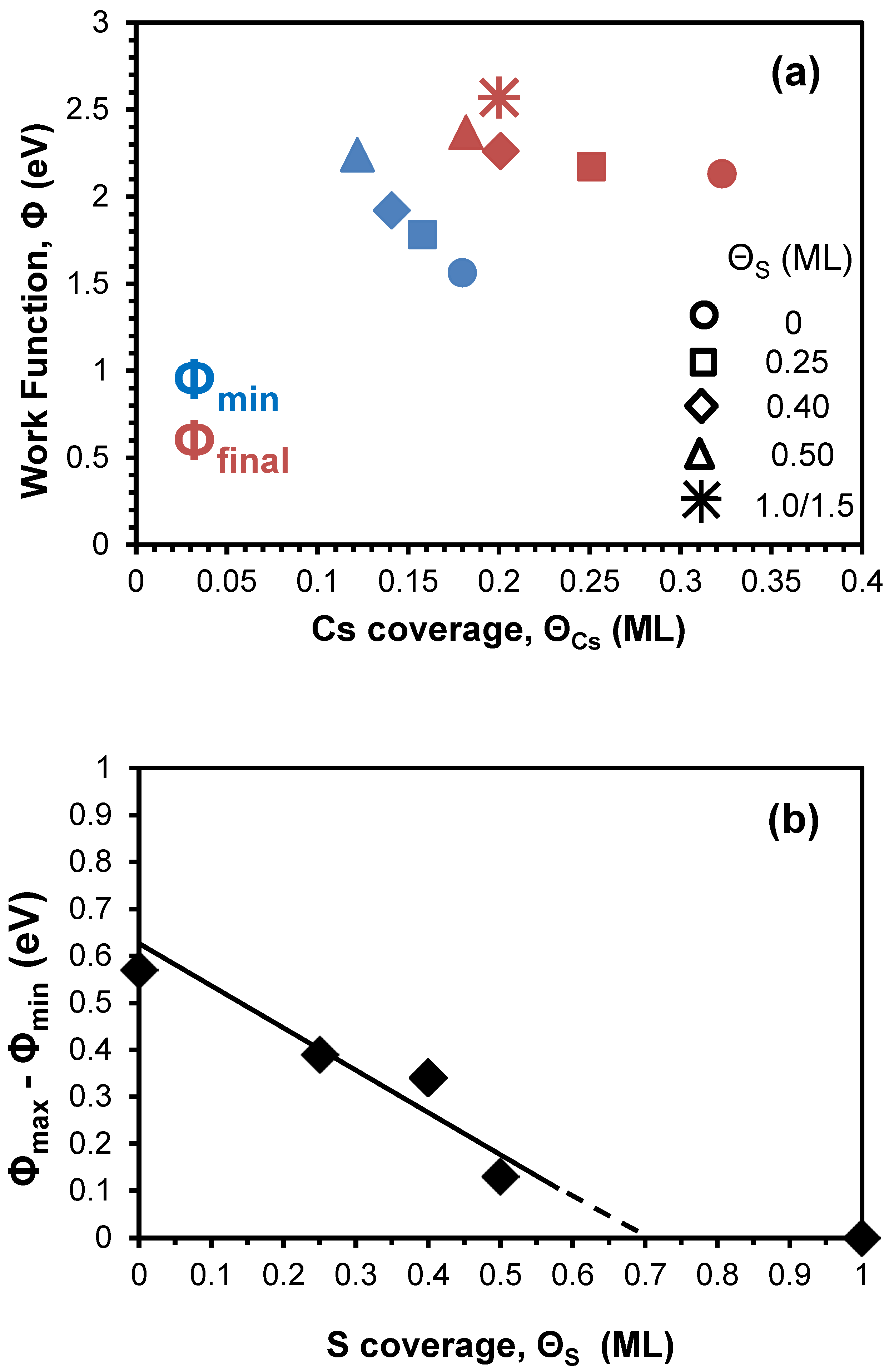
| 0.180 | 0 | 1.56 | - | 0.57 |
| 0.323 | - | 2.13 | ||
| 0.158 | 0.25 | 1.78 | - | 0.39 |
| 0.251 | - | 2.17 | ||
| 0.141 | 0.40 | 1.92 | - | 0.34 |
| 0.201 | - | 2.26 | ||
| 0.122 | 0.50 | 2.24 | - | 0.13 |
| 0.182 | - | 2.37 | ||
| 0.200 | 1.00 | - | 2.57 | - |
| 0.200 | 1.50 | - | 2.57 | - |
| 2.000 | 1.00 | - | 2.19 | - |
| 3.000 | 1.70 | - | - | |
| 4.000 | - | 2.21 | - | |
| 2.000 | 1.50 | - | 2.48 | - |
| 3.000 | - | 2.10 | - | |
| 4.000 | - | 2.06 | - |
| Cs TDS peak | Cesium coverage ΘCs (ML) | Temperature (K) (±10 K) |
Desorption energy (eV∙atom-1) (±0.03 eV) |
| β1 | 0.05 | 1020 | 2.66 |
| 0.10 | 910 | 2.36 | |
| 0.15 | 875 | 2.27 | |
| β2 | 0.15-0.50 | 755 | 1.95 |
| 0.60 | 745 | 1.92 | |
| β3 | 0.40 | 585 | 1.50 |
| 0.50-0.60 | 555 | 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




