Submitted:
12 August 2025
Posted:
14 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Waning Immunity and the “Breakthrough Infections” Phenomenon
3. Negative Efficacy of the mRNA Products, Adverse Sequelae of the Infections
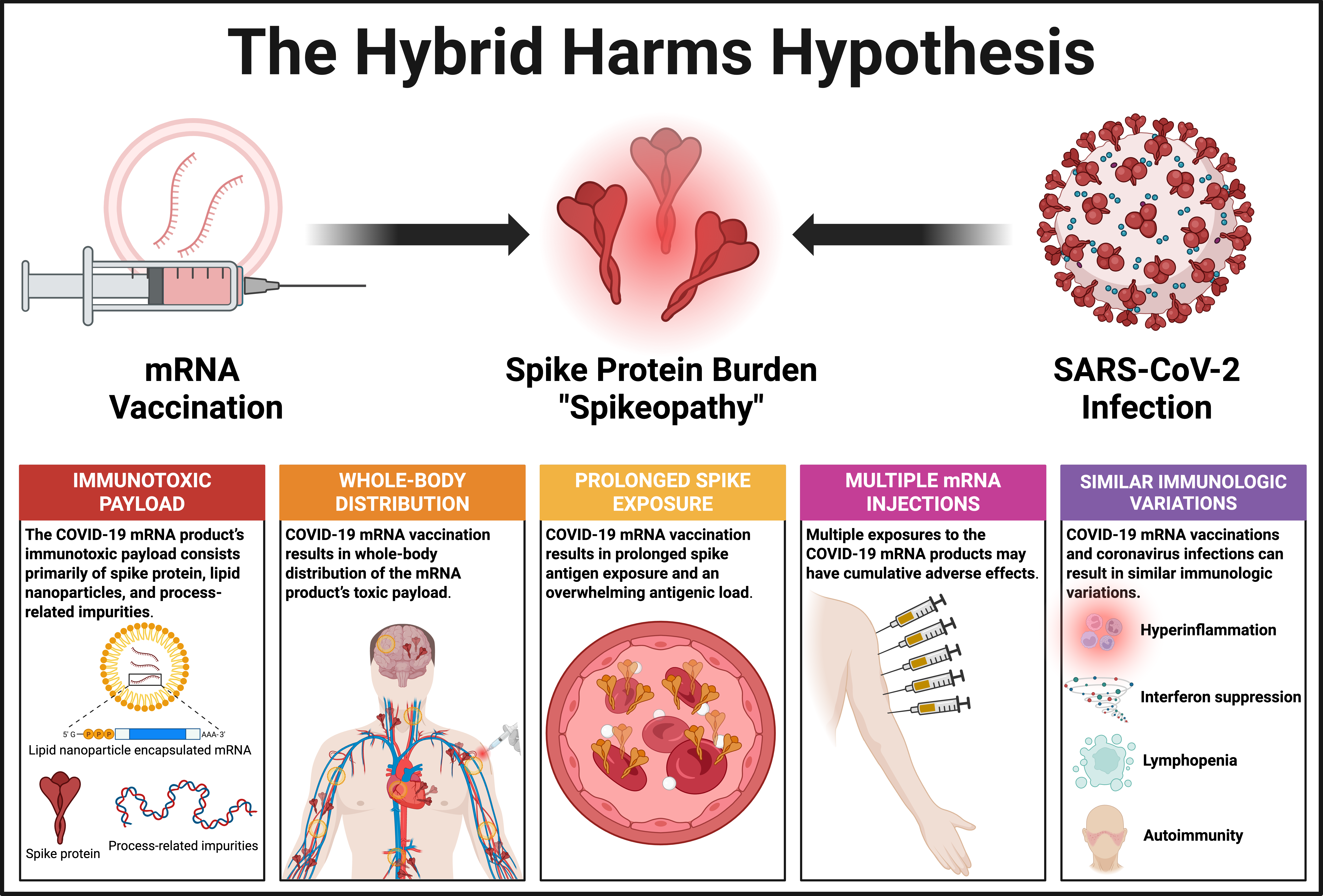
4. The Hybrid Harms Hypothesis
- Both COVID-19 mRNA vaccinations and coronavirus infections contribute to the total toxic burden of spike protein, either additively or synergistically.
- Direct toxic effects in the context of these “hybrid harms” are focused on disrupting endothelial function and triggering inflammation, potentially contributing to complications like myocarditis or thrombosis.
- Indirect effects occur via the induction of autoimmunity or chronic inflammation, particularly in the context of Long COVID, whereby persistent spike protein and/or immune complexes may drive symptoms and pathogenesis.
- Many cardiac, vascular, hematologic, autoimmune, neurological, and reproductive problems can be triggered by either the modified mRNA inoculations or coronavirus infections, or both. This hypothesis focuses on the third possibility.
- COVID-19 mRNA-inoculated individuals with either prior or subsequent exposure to SARS-CoV-2 appear to face a greater risk of thromboembolism and other vascular pathologies compared to SARS-CoV-2-naïve mRNA vaccine recipients.
- D-dimer elevation may occur, reflecting possible clot formation or immune-driven coagulopathy. Elevated D-dimer levels are commonly observed in hospitalized COVID-19 patients and correlate with thrombotic complications and worse outcomes.
- C-reactive protein (CRP) elevations are also common in pathologies resulting from the interaction between mRNA inoculations and coronavirus infections.
- Antibody testing for anti-spike antibodies provides a scientifically valid, albeit indirect measure of the body’s overall spike protein exposure from previous SARS-CoV-2 infection and/or the COVID-19 mRNA vaccinations.
5. Illusions of Protection Against Severe Disease
Hybrid Immunity Versus Hybrid Harms
6. Post Vaccine Syndrome Often Subsumes Post COVID Syndrome
7. Support for the Hybrid Harms Hypothesis
7.1. Overlapping “Spikeopathies” from mRNA Vaccinations and Coronavirus Infections
7.2. Hybrid Harms Due to Co-Amplification of Cardiovascular and Hematologic AEs
- A 26-year-old previously healthy male was admitted to the emergency room by his family physician after one month of worsening fatigue, palpitations, and dyspnea [325]. He had tested positive for SARS-CoV-2 eight weeks earlier, and he received a second dose of the COVID-19 mRNA vaccine four months prior to the infection. Echocardiography and cardiac MRI showed severely reduced left ventricular function and strong midmyocardial late gadolinium enhancement. Endomyocardial biopsy confirmed acute lymphocytic myocarditis.
- A 42-year-old male tested positive for Omicron and was admitted to the ICU in January 2022 with chest pain and ST-segment elevation in the inferior leads [326]. He had a history of peri-myocarditis in 2008 without recurrence or autoimmune disorder diagnoses. Four months earlier (22 August 2021), the patient had received his third dose of the Pfizer mRNA vaccine. Cardiac MRI confirmed myocarditis, with late gadolinium enhancement showing 22% left ventricular mass involvement.
- A 60-year-old male presented to the emergency department on 11 January 2022 with syncope and palpitations, testing positive for Omicron via RT-PCR [326]. He had received his third Pfizer mRNA vaccine dose four months prior, on 30 August 2021. He experienced ventricular tachycardia (250 beats/minute) requiring urgent cardioversion. No further malignant arrhythmias occurred during hospitalization. Coronary angiography showed non-obstructive disease, and cardiac MRI indicated acute myocarditis with 19% left ventricular mass involvement.
7.3. Epidemiological Studies from the Omicron Era
8. Discussion
9. Conclusions
Acknowledgments
Conflicts of Interest
Appendix A. Literature Sources for Table 2
References
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Heal. 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Zuliani, G.; Rigatelli, A.; Mazza, A.; Roncon, L. Arterial hypertension and risk of death in patients with COVID-19 infection: Systematic review and meta-analysis. J. Infect. 2020, 81, e84–e86. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; for the CORONADO investigators; Hadjadj, S. ; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef]
- Lazcano, U.; Cuadrado-Godia, E.; Grau, M.; Subirana, I.; Martínez-Carbonell, E.; Boher-Massaguer, M.; Rodríguez-Campello, A.; Giralt-Steinhauer, E.; Fernández-Pérez, I.; Jiménez-Conde, J.; et al. Increased COVID-19 Mortality in People With Previous Cerebrovascular Disease: A Population-Based Cohort Study. Stroke 2022, 53, 1276–1284. [Google Scholar] [CrossRef]
- Ge, E.; Li, Y.; Wu, S.; Candido, E.; Wei, X.; Augusto, O. Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study. PLOS ONE 2021, 16, e0258154. [Google Scholar] [CrossRef]
- Phelps, M.; Christensen, D.M.; Gerds, T.; Fosbøl, E.; Torp-Pedersen, C.; Schou, M.; Køber, L.; Kragholm, K.; Andersson, C.; Biering-Sørensen, T.; et al. Cardiovascular comorbidities as predictors for severe COVID-19 infection or death. Eur. Hear. J. - Qual. Care Clin. Outcomes 2020, 7, 172–180. [Google Scholar] [CrossRef]
- Suleyman, G.; Fadel, R.A.; Malette, K.M.; Hammond, C.; Abdulla, H.; Entz, A.; Demertzis, Z.; Hanna, Z.; Failla, A.; Dagher, C.; et al. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw. Open 2020, 3, e2012270. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Locke, E.; Green, P.; Berry, K.; O’hAre, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Dominitz, J.A.; Fan, V.S. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans With SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022310–e2022310. [Google Scholar] [CrossRef]
- Witkowski, J.M.; Fulop, T.; Bryl, E. Immunosenescence and COVID-19. Mech. Ageing Dev. 2022, 204, 111672–111672. [Google Scholar] [CrossRef]
- Fang, X.; Wang, J.; Liu, Y.; Zhang, X.; Wang, T.; Zhang, H.; Liang, Z.; Luo, F.; Li, W.; Liu, D.; et al. Combined and interactive effects of alcohol drinking and cigarette smoking on the risk of severe illness and poor clinical outcomes in patients with COVID-19: a multicentre retrospective cohort study. Public Heal. 2022, 205, 6–13. [Google Scholar] [CrossRef]
- Prodromos, C.; Rumschlag, T. Hydroxychloroquine is effective, and consistently so when provided early, for COVID-19: a systematic review. New Microbes New Infect. 2020, 38, 100776–100776. [Google Scholar] [CrossRef] [PubMed]
- Kory P, McCarthy J. War on Ivermectin: The Medicine that Saved Millions and Could Have Ended the Pandemic. Skyhorse Publishing, NY. 2023. https://www.amazon.com/War-Ivermectin-Medicine-Millions-Pandemic/dp/151077386X.
- Malhotra, A. Curing the pandemic of misinformation on COVID-19 mRNA vaccines through real evidence-based medicine - Part 1. J. Metab. Heal. 2022, 5. [Google Scholar] [CrossRef]
- Quinn, G.A.; Connolly, R.; Óhaiseadha, C.; Hynds, P.; Bagus, P.; Brown, R.B.; Cáceres, C.F.; Craig, C.; Connolly, M.; Domingo, J.L.; et al. What Lessons can Be Learned From the Management of the COVID-19 Pandemic? Int. J. Public Heal. 2025, 70, 1607727. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A. Curing the pandemic of misinformation on COVID-19 mRNA vaccines through real evidence-based medicine - Part 2. J. Metab. Heal. 2022, 5, 10. [Google Scholar] [CrossRef]
- Santin, A.; Scheim, D.; McCullough, P.; Yagisawa, M.; Borody, T. Ivermectin: a multifaceted drug of Nobel prize-honoured distinction with indicated efficacy against a new global scourge, COVID-19. New Microbes New Infect. 2021, 43, 100924. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. New Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef]
- Julian Gillespie, a prominent Australian barrister leading legal challenges against the regulatory approval of COVID-19 mRNA “vaccines”, argues that they should be classified as gene therapies and also genetically modified organisms (GMOs) under Australian law, specifically the Gene Technology Act 2000, which was bypassed during the EUA process. The Gene Technology Act 2000 requires specific gene-based risk assessments and licensing. Similar U.S. efforts to nullify the Biologics License Application contend the FDA’s waiver of National Environmental Policy Act assessments concealed their gene therapy status, evading proper scrutiny. These gene transfer technologies demand a distinct regulatory approval process; without adherence, their sale is illegal.
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. npj Vaccines 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Oldfield PR, Gutschi M, McCullough PA, Speicher DJ. BioNTech’s COVID-19 modRNA Vaccines: Dangerous genetic mechanism of action released before sufficient preclinical testing. Journal of American Physicians and Surgeons. 2024;29(4):118-126.
- Cosentino, M.; Marino, F. Understanding the Pharmacology of COVID-19 mRNA Vaccines: Playing Dice with the Spike? Int. J. Mol. Sci. 2022, 23, 10881. [Google Scholar] [CrossRef]
- Chaudhary, J.K.; Yadav, R.; Chaudhary, P.K.; Maurya, A.; Kant, N.; Al Rugaie, O.; Haokip, H.R.; Yadav, D.; Roshan, R.; Prasad, R.; et al. Insights into COVID-19 Vaccine Development Based on Immunogenic Structural Proteins of SARS-CoV-2, Host Immune Responses, and Herd Immunity. Cells 2021, 10, 2949. [Google Scholar] [CrossRef]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef]
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H.; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e7. [Google Scholar] [CrossRef]
- Rustagi, V.; Gupta, S.R.R.; Talwar, C.; Singh, A.; Xiao, Z.-Z.; Jamwal, R.; Bala, K.; Bhaskar, A.K.; Nagar, S.; Singh, I.K. SARS-CoV-2 pathophysiology and post-vaccination severity: a systematic review. Immunol. Res. 2024, 73, 17. [Google Scholar] [CrossRef]
- Du, J.; Lang, H.-M.; Ma, Y.; Chen, A.-W.; Qin, Y.-Y.; Zhang, X.-P.; Huang, C.-Q.; Du, \. Global trends in COVID-19 incidence and case fatality rates (2019–2023): a retrospective analysis. Front. Public Heal. 2024, 12, 1355097. [Google Scholar] [CrossRef] [PubMed]
- Binnicker, M.J.; Kraft, C.S. Challenges and Controversies to Testing for COVID-19. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Pujadas, E.; Chaudhry, F.; McBride, R.; Richter, F.; Zhao, S.; Wajnberg, A.; Nadkarni, G.; Glicksberg, B.S.; Houldsworth, J.; Cordon-Cardo, C. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir. Med. 2020, 8, e70. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; A Spencer, E.; Brassey, J.; Heneghan, C. Viral Cultures for Coronavirus Disease 2019 Infectivity Assessment: A Systematic Review. Clin. Infect. Dis. 2020, 73, e3884–e3899. [Google Scholar] [CrossRef]
- Mallett, S.; Allen, A.J.; Graziadio, S.; Taylor, S.A.; Sakai, N.S.; Green, K.; Suklan, J.; Hyde, C.; Shinkins, B.; Zhelev, Z.; et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020, 18, 1–17. [Google Scholar] [CrossRef]
- Kulkarni, D.; Lee, B.; Ismail, N.F.; Rahman, A.E.; Spinardi, J.; Kyaw, M.H.; Nair, H. Incidence, severity, risk factors and outcomes of SARS-CoV-2 reinfections during the Omicron period: a systematic review and meta-analysis. J. Glob. Heal. 2025, 15, 04032. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Sarangi, A.K.; Kandi, V.; Azam, M.; Tiwari, R.; Dhama, K. Omicron (B.1.1.529 variant of SARS-CoV-2); an emerging threat: Current global scenario. J. Med Virol. 2022, 94, 1780–1783. [Google Scholar] [CrossRef]
- Karyakarte, R.P.; Das, R.; Dudhate, S.; Agarasen, J.; Pillai, P.; Chandankhede, P.M.; Labhshetwar, R.S.; Gadiyal, Y.; Rajmane, M.V.; Kulkarni, P.P.; et al. Clinical Characteristics and Outcomes of Laboratory-Confirmed SARS-CoV-2 Cases Infected With Omicron Subvariants and the XBB Recombinant Variant. Cureus 2023, 15, e35261. [Google Scholar] [CrossRef] [PubMed]
- Tureček, P.; Kleisner, K. Symptomic Mimicry Between SARS-CoV-2 and the Common Cold Complex. Biosemiotics 2022, 15, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Joung, S.Y.; Ebinger, J.E.; Sun, N.; Liu, Y.; Wu, M.; Tang, A.B.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Sobhani, K.; et al. Awareness of SARS-CoV-2 Omicron Variant Infection Among Adults With Recent COVID-19 Seropositivity. JAMA Netw. Open 2022, 5, e2227241–e2227241. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.T.M.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef]
- Lorenzo-Redondo, R.; A Ozer, E.; Hultquist, J.F. Covid-19: is omicron less lethal than delta? BMJ 2022, 378, o1806. [Google Scholar] [CrossRef]
- Christie, B. Covid-19: Early studies give hope omicron is milder than other variants. BMJ 2021, 375, n3144. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, L.; Peng, Z.; Chen, L.-L.; Meng, X.; Zhang, C.; Ip, J.D.; Chan, W.-M.; Chu, A.W.-H.; Chan, K.-H.; et al. SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells. Emerg. Microbes Infect. 2022, 11, 277–283. [Google Scholar] [CrossRef]
- Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, Macdonald B, et al. Coronavirus Pandemic (COVID-19). 2020. Available: https://ourworldindata.org/coronavirus. Accessed 30 April 2024.
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; de Villiers, T.; Van der Walt, Z.; et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 2021, 116, 38–42. [Google Scholar] [CrossRef]
- Maslo, C.; Friedland, R.; Toubkin, M.; Laubscher, A.; Akaloo, T.; Kama, B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared With Previous Waves. JAMA 2021, 327, 583–584. [Google Scholar] [CrossRef]
- Dyer, O. COVID-19: South Africa’s surge in cases deepens alarm over omicron variant. BMJ. 2021;375:n3013.
- Jassat, W.; Karim, S.S.A.; Ozougwu, L.; Welch, R.; Mudara, C.; Masha, M.; Rousseau, P.; Wolmarans, M.; Selikow, A.; Govender, N.; et al. Trends in Cases, Hospitalizations, and Mortality Related to the Omicron BA.4/BA.5 Subvariants in South Africa. Clin. Infect. Dis. 2022, 76, 1468–1475. [Google Scholar] [CrossRef]
- Adjei, S.; Hong, K.; Molinari, N.-A.M.; Bull-Otterson, L.; Ajani, U.A.; Gundlapalli, A.V.; Harris, A.M.; Hsu, J.; Kadri, S.S.; Starnes, J.; et al. Mortality Risk Among Patients Hospitalized Primarily for COVID-19 During the Omicron and Delta Variant Pandemic Periods — United States, April 2020–June 2022. Mmwr-Morbidity Mortal. Wkly. Rep. 2022, 71, 1182–1189. [Google Scholar] [CrossRef]
- Pather, S.; Madhi, S.A.; Cowling, B.J.; Moss, P.; Kamil, J.P.; Ciesek, S.; Muik, A.; Türeci, Ö. SARS-CoV-2 Omicron variants: burden of disease, impact on vaccine effectiveness and need for variant-adapted vaccines. Front. Immunol. 2023, 14, 1130539. [Google Scholar] [CrossRef]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int. J. Infect. Dis. 2021, 114, 252–260. [Google Scholar] [CrossRef]
- Excess Mortality Project. Excess mortality calculations for different countries. Phinance Technologies. Accessed 7/18/2025. URL: https://phinancetechnologies.com/HumanityProjects/Projects.htm.
- Kuhbandner, C.; Reitzner, M. Estimation of Excess Mortality in Germany During 2020-2022. Cureus 2023, 15, e39371. [Google Scholar] [CrossRef]
- Aarstad J, Kvitastein OA. Is there a link between the 2021 COVID-19 vaccination uptake in Europe and 2022 excess all-cause mortality? Asian Pac. J. Health Sci. 2023;10(1):25-31. https://www.apjhs.com/index.php/apjhs/article/view/3017/1610.
- Economidou, E.C.; Soteriades, E.S. Excess mortality in Cyprus during the COVID-19 vaccination campaign. Vaccine 2023, 42, 3375–3376. [Google Scholar] [CrossRef]
- Raknes, G.; Fagerås, S.J.; Sveen, K.A.; Júlíusson, P.B.; Strøm, M.S. Excess non-COVID-19 mortality in Norway 2020–2022. BMC Public Heal. 2024, 24, 1–13. [Google Scholar] [CrossRef]
- Mostert, S.; Hoogland, M.; Huibers, M.; Kaspers, G. Excess mortality across countries in the Western World since the COVID-19 pandemic: ‘Our World in Data’ estimates of January 2020 to December 2022. BMJ Public Heal. 2024, 2, e000282. [Google Scholar] [CrossRef]
- Shir-Raz, Y.; Elisha, E.; Martin, B.; Ronel, N.; Guetzkow, J. Censorship and Suppression of Covid-19 Heterodoxy: Tactics and Counter-Tactics. Minerva 2022, 61, 407–433. [Google Scholar] [CrossRef]
- This efficacy was based solely on relative risk reductions. The absolute risk reduction was only about 1%. Specifically, the absolute risk reductions for BNT162b2 and mRNA-1273 were 0.7% and 1.1%, respectively.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. New Engl. J. Med. 2021, 385, E84–E84. [Google Scholar] [CrossRef]
- Haq MA, Roy AK, Ahmed R, Kuddusi RU, Sinha M, Hossain MS, Vandenent M, et al. Antibody longevity and waning following COVID-19 vaccination in a 1-year longitudinal cohort in Bangladesh. Sci Rep. 2024;14(1):11467. [CrossRef]
- Srivastava, K.; Carreño, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e4. [Google Scholar] [CrossRef]
- Menegale, F.; Manica, M.; Zardini, A.; Guzzetta, G.; Marziano, V.; D'ANdrea, V.; Trentini, F.; Ajelli, M.; Poletti, P.; Merler, S. Evaluation of Waning of SARS-CoV-2 Vaccine–Induced Immunity: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2310650–e2310650. [Google Scholar] [CrossRef]
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O'Connell AM, Simons D, Blomquist PB, Zaidi A, Nash S, Iwani Binti Abdul Aziz N, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022 Apr 21;386(16):1532-1546. [CrossRef]
- Mead, M.N.; Seneff, S.; Wolfinger, R.; Rose, J.; Denhaerynck, K.; Kirsch, S.; A McCullough, P. COVID-19 Modified mRNA “Vaccines”: Lessons Learned from Clinical Trials, Mass Vaccination, and the Bio-Pharmaceutical Complex, Part 1. Int. J. Vaccine Theory, Pr. Res. 2024, 3, 1112–1178. [Google Scholar] [CrossRef]
- Abou-Saleh, H.; Abo-Halawa, B.Y.; Younes, S.; Younes, N.; Al-Sadeq, D.W.; Shurrab, F.M.; Liu, N.; Qotba, H.; Al-Dewik, N.; Ismail, A.; et al. Neutralizing antibodies against SARS-CoV-2 are higher but decline faster in mRNA vaccinees compared to individuals with natural infection. J. Travel Med. 2022, 29. [Google Scholar] [CrossRef] [PubMed]
- Tamandjou, C.; Auvigne, V.; Schaeffer, J.; Vaux, S.; du Châtelet, I.P. Effectiveness of second booster compared to first booster and protection conferred by previous SARS-CoV-2 infection against symptomatic Omicron BA.2 and BA.4/5 in France. Vaccine 2023, 41, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W. Original antigen sin and COVID-19: implications for seasonal vaccination. Expert Opin. Biol. Ther. 2022, 22, 1353–1358. [Google Scholar] [CrossRef]
- Noori, M.; Nejadghaderi, S.A.; Rezaei, N. “Original antigenic sin”: A potential threat beyond the development of booster vaccination against novel SARS-CoV-2 variants. Infect. Control. Hosp. Epidemiology 2021, 43, 1091–1092. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef]
- Samanovic, M.I.; Cornelius, A.R.; Gray-Gaillard, S.L.; Allen, J.R.; Karmacharya, T.; Wilson, J.P.; Hyman, S.W.; Tuen, M.; Koralov, S.B.; Mulligan, M.J.; et al. Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2–experienced individuals. Sci. Transl. Med. 2022, 14, eabi8961. [Google Scholar] [CrossRef]
- Offit, P.A. Bivalent Covid-19 Vaccines — A Cautionary Tale. New Engl. J. Med. 2023, 388, 481–483. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, L.; Zhang, H.; Gao, J.; Mao, C.; Landesman-Bollag, E.; Mostoslavsky, G.; Lunderberg, J.M.; Zheng, W.; Hao, S.; et al. Longitudinal waning of mRNA vaccine-induced neutralizing antibodies against SARS-CoV-2 detected by an LFIA rapid test. Antib. Ther. 2022, 5, 55–62. [Google Scholar] [CrossRef]
- Afshar, Z.M.; Barary, M.; Hosseinzadeh, R.; Alijanpour, A.; Hosseinzadeh, D.; Ebrahimpour, S.; Nazary, K.; Sio, T.T.; Sullman, M.J.M.; Carson-Chahhoud, K.; et al. Breakthrough SARS-CoV-2 infections after vaccination: a critical review. Hum. Vaccines Immunother. 2022, 18, 2051412. [Google Scholar] [CrossRef]
- Moore, M.; Anderson, L.; Schiffer, J.T.; Matrajt, L.; Dimitrov, D. Durability of COVID-19 vaccine and infection induced immunity: A systematic review and meta-regression analysis. Vaccine 2025, 54, 126966. [Google Scholar] [CrossRef]
- Gopinath, S.; Ishak, A.; Dhawan, N.; Poudel, S.; Shrestha, P.S.; Singh, P.; Xie, E.; Tahir, P.; Marzaban, S.; Michel, J.; et al. Characteristics of COVID-19 Breakthrough Infections among Vaccinated Individuals and Associated Risk Factors: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 81. [Google Scholar] [CrossRef]
- Zilla, M.L.; Keetch, C.; Mitchell, G.; McBreen, J.; Shurin, M.R.; E Wheeler, S. SARS-CoV-2 Serologic Immune Response in Exogenously Immunosuppressed Patients. J. Appl. Lab. Med. 2020, 6, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Ghadimi-Moghadam, A.; Mortazavi, S.M.J.; Haghani, M.; Sihver, L. Breakthrough Infection and Death after COVID-19 Vaccination: A Physics Perspective. J. Biomed. Phys. Eng. 2023, online, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Miyamoto, S.; Suzuki, T. Infection-mediated immune response in SARS-CoV-2 breakthrough infection and implications for next-generation COVID-19 vaccine development. Vaccine 2024, 42, 1401–1406. [Google Scholar] [CrossRef]
- Huang, W.; Gao, C.X.; Luo, D.; Wang, Y.; Zheng, X.; Liu, C.; Wang, Y.; Li, Y.; Qian, H. Risk evaluation of venue types and human behaviors of COVID-19 outbreaks in public indoor environments: A systematic review and meta-analysis. Environ. Pollut. 2023, 341, 122970. [Google Scholar] [CrossRef] [PubMed]
- Jamous, Y.F.; Alnakhli, M.; Alshaibi, A.; Alhawsawi, M.; Binsalman, A.; Uduman, M.S.T.S. The Incidence and Severity of COVID-19 Infection Post Vaccination in Saudi Arabia. Cureus 2023, 15, e39766. [Google Scholar] [CrossRef]
- Shahid, I.; Alzahrani, A.R.; Jabeen, Q.; Al-Ghamdi, S.S.; Shahzad, N.; Rehman, S.; Algarni, A.S.; Bamagous, G.A.; AlanazI, I.M.M.; Ibrahim, I.A.A. SARS-CoV-2 Detection and COVID-19 Diagnosis: A Bird’s Eye View. Rev. Recent Clin. Trials 2023, 18, 1–25. [Google Scholar] [CrossRef]
- Martignoni, M.M.; Mohammadi, Z.; Loredo-Osti, J.C.; Hurford, A. Extensive SARS-CoV-2 testing reveals BA.1/BA.2 asymptomatic rates and underreporting in school children. Can. Commun. Dis. Rep. 2023, 49, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings — Barnstable County, Massachusetts, July 2021. Mmwr-Morbidity Mortal. Wkly. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef]
- Ben Fredj, S.; Ghammem, R.; Zammit, N.; Maatouk, A.; Haddad, N.; Haddad, N.; Kachroudi, M.; Rebai, S.; Laadhari, H.; Ghodhbani, M.M.; et al. Risk factors for severe Covid-19 breakthrough infections: an observational longitudinal study. BMC Infect. Dis. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Moreno-Perez, O.; Ribes, I.; Boix, V.; Martinez-García, M.Á.; Otero-Rodriguez, S.; Reus, S.; Sánchez-Martínez, R.; Ramos, J.M.; Chico-Sánchez, P.; Merino, E. Hospitalized patients with breakthrough COVID-19: Clinical features and poor outcome predictors. Int. J. Infect. Dis. 2022, 118, 89–94. [Google Scholar] [CrossRef]
- Christensen, P.A.; Olsen, R.J.; Long, S.W.; Snehal, R.; Davis, J.J.; Saavedra, M.O.; Reppond, K.; Shyer, M.N.; Cambric, J.; Gadd, R.; et al. Signals of Significantly Increased Vaccine Breakthrough, Decreased Hospitalization Rates, and Less Severe Disease in Patients with Coronavirus Disease 2019 Caused by the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas. Am. J. Pathol. 2022, 192, 642–652. [Google Scholar] [CrossRef]
- Albtoosh, A.S.; Farah, R.; Al Oweidat, K.; Hussein, O.M.; Obeid, A.A.; Hamila, H.M.; Radwan, M.N.M.; Ahmad, R.F.; Masadeh, H.M.; Hammad, A.I.; et al. Presenting clinical symptoms of post-COVID-19 breakthrough infection: Predictors of mortality in a Middle Eastern population. Vaccine: X 2024, 18, 100495. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Infection fatality rate of COVID-19 inferred from seroprevalence data. Bull. World Health Organ. 2021, 99, 19–33F. [Google Scholar] [CrossRef]
- Pezzullo, A.M.; Axfors, C.; Contopoulos-Ioannidis, D.G.; Apostolatos, A.; Ioannidis, J.P. Age-stratified infection fatality rate of COVID-19 in the non-elderly population. Environ. Res. 2022, 216, 114655–114655. [Google Scholar] [CrossRef]
- Lipsitch, M.; Krammer, F.; Regev-Yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat. Rev. Immunol. 2021, 22, 57–65. [Google Scholar] [CrossRef]
- Robinson, J. Are these the numbers scaring Boris? Study shows 29% of the 42 people who have died after catching the new strain had BOTH vaccinations as cases soar another 40%. Daily Mail News. Published June 13, 2021. Accessed June 20, 2025. https://www.dailymail.co.uk/news/article-9681613/Study-shows-29-people-died-catching-new-strain-vaccinations.html.
- UK Government. Public Health England. Research and analysis. Investigation of SARS-CoV-2 variants of concern: technical briefings. Published 21 December 2020. Last updated 17 September 2021. URL: https://www.gov.uk/government/publications/investigation-of-novel-sars-cov-2-variant-variant-ofconcern-20201201.
- Anonymous. 151 Dead, 563 Hospitalized in Illinois Breakthrough COVID Cases. NBC News Chicago. Published July 16, 2021. Accessed June 20, 2025. https://www.nbcchicago.com/news/coronavirus/151-dead-563-hospitalized-in-illinois-breakthrough-covid-cases/2556408/.
- Ong, D. 277 Fully Vaccinated Indiana Residents Have Died Of COVID-19 In 3 Weeks. International Business Times. Published January 23, 2022. Accessed June 20, 2025. https://www.ibtimes.com/277-fully-vaccinatedindiana-residents-have-died-covid-19-3-weeks-3381687.
- Anonymous. COVID-19 Cases in Fully Vaccinated Individuals. Massachusetts Department of Public Health. Published January 4, 2021. Accessed June 20, 2025. https://www.mass.gov/doc/weekly-reportcovid-19-cases-in-vaccinated-individuals-january-4-2022/download.
- Ong, D. 290 Fully Vaccinated Massachusetts Residents Died Of COVID-19 Over 1 Week. International Business Times. Published February 2, 2022. Accessed June 20, 2025. https://www.ibtimes.com/290-fullyvaccinated-massachusetts-residents-died-covid-19-over-1-week-3389234.
- Ong, D. 2,222 Fully Vaccinated Massachusetts Residents Have Died Of COVID-19. International Business Times. Published February 16, 2022. Accessed June 20, 2025. https://www.ibtimes.com/2222-fullyvaccinated-massachusetts-residents-have-died-covid-19-3403439.
- Attwell, K.; Hannah, A. Convergence on Coercion: Functional and Political Pressures as Drivers of Global Childhood Vaccine Mandates. Int. J. Heal. Policy Manag. 2022, 11, 2660–2671. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.K.; Alleaume, C.; Peretti-Watel, P.; theCOCONEL Group. The French public's attitudes to a future COVID-19 vaccine: The politicization of a public health issue. Soc. Sci. Med. 2020, 265, 113414. [Google Scholar] [CrossRef] [PubMed]
- Fenton N, Neil M. The Very Best Cheap Trick. In: Fighting Goliath: Exposing the flawed science and statistics behind the COVID-19 event. Sovereign Rights Publishing, United Kingdom. 2024. pp. 202-212.
- Basoulis, D.; Logioti, K.; Papaodyssea, I.; Chatzopoulos, M.; Alexopoulou, P.; Mavroudis, P.; Rapti, V.; Poulia, V.; Samara, S.; Georgakopoulou, V.E.; et al. Deaths “due to” COVID-19 and deaths “with” COVID-19 during the Omicron variant surge, among hospitalized patients in seven tertiary-care hospitals, Athens, Greece. Sci. Rep. 2025, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. New Engl. J. Med. 2021, 385, E83–E83. [Google Scholar] [CrossRef]
- Chitwood, M.H.; Russi, M.; Gunasekera, K.; Havumaki, J.; Klaassen, F.; Pitzer, V.E.; Salomon, J.A.; Swartwood, N.A.; Warren, J.L.; Weinberger, D.M.; et al. Reconstructing the course of the COVID-19 epidemic over 2020 for US states and counties: Results of a Bayesian evidence synthesis model. PLOS Comput. Biol. 2022, 18, e1010465. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Interim public health considerations for the provision of additional COVID-19 vaccine doses. https://www.ecdc.europa.eu/en/publications-data/covid-19-public-health-considerations-additional-vaccine-doses Accessed 22 June 2025.
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Burke, P.C.; Nowacki, A.S.; Simon, J.F.; Hagen, A.; Gordon, S.M. Effectiveness of the Coronavirus Disease 2019 Bivalent Vaccine. Open Forum Infect. Dis. 2023, 10. [Google Scholar] [CrossRef]
- Nakatani, E.; Morioka, H.; Kikuchi, T.; Fukushima, M. Behavioral and Health Outcomes of mRNA COVID-19 Vaccination: A Case-Control Study in Japanese Small and Medium-Sized Enterprises. Cureus 2024, 16, e75652. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- UK Health Security Agency. COVID-19 vaccine surveillance report, Week 8 Feb. 24, 2022. https://assets.publishing.service.gov.uk/media/621c91c0d3bf7f4f04b2b648/Vaccine_surveillance_report_-_week-8.pdf.
- Eythorsson, E.; Runolfsdottir, H.L.; Ingvarsson, R.F.; Sigurdsson, M.I.; Palsson, R. Rate of SARS-CoV-2 Reinfection During an Omicron Wave in Iceland. JAMA Netw. Open 2022, 5, e2225320–e2225320. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Ruffin, J.; Wiegand, R.; Grant, L.; Babu, T.M.; Briggs-Hagen, M.; Burgess, J.L.; Caban-Martinez, A.J.; Chu, H.Y.; Ellingson, K.D.; et al. Protection From COVID-19 Vaccination and Prior SARS-CoV-2 Infection Among Children Aged 6 Months–4 Years, United States, September 2022–April 2023. J. Pediatr. Infect. Dis. Soc. 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Berry, K.; Rajeevan, N.; Li, Y.; Yan, L.; Huang, Y.; Lin, H.-M.; Bui, D.; Hynes, D.M.; Rowneki, M.; et al. Effectiveness of the 2023-to-2024 XBB.1.5 COVID-19 Vaccines Over Long-Term Follow-up. Ann. Intern. Med. 2025, 178, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.K.; Burke, P.C.; Nowacki, A.S.; Gordon, S.M.; Martellucci, C.A. Risk of Coronavirus Disease 2019 (COVID-19) among those up-to-date and not up-to-date on COVID-19 vaccination by US CDC criteria. PLOS ONE 2023, 18, e0293449. [Google Scholar] [CrossRef]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Cohen, D.; Muhsen, K.; Chodick, G.; Patalon, T. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: Reinfections versus breakthrough infections. MedRxiv 2021. [Google Scholar] [CrossRef]
- Rose, J. Breakthrough Infection Signal in VAERS Corroborates IgG4-Mediated Increased Susceptibility to SARS-CoV-2. Sci Public Health Policy Law. 2025;6:2019-2025. Available from: https://publichealthpolicyjournal.com.
- Beattie, KA. Worldwide Bayesian causal impact analysis of vaccine administration on deaths and dases associated with COVID-19: A BigData analysis of 145 countries. Department of Political Science University of Alberta, Alberta, Canada. 2021. [CrossRef]
- Johns Hopkins University School of Medicine, Coronavirus Resource Center. Accessed on March 15, 2025. https://coronavirus.jhu.edu/.
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef]
- Montano, D. Frequency and Associations of Adverse Reactions of COVID-19 Vaccines Reported to Pharmacovigilance Systems in the European Union and the United States. Front. Public Heal. 2022, 9, 756633. [Google Scholar] [CrossRef]
- Yan, M.-M.; Zhao, H.; Li, Z.-R.; Chow, J.-W.; Zhang, Q.; Qi, Y.-P.; Wu, S.-S.; Zhong, M.-K.; Qiu, X.-Y. Serious adverse reaction associated with the COVID-19 vaccines of BNT162b2, Ad26.COV2.S, and mRNA-1273: Gaining insight through the VAERS. Front. Pharmacol. 2022, 13, 921760. [Google Scholar] [CrossRef]
- Classen, B. US COVID-19 vaccines proven to cause more harm than good based on pivotal clinical trial data analyzed using the proper scientific endpoint, “all cause severe morbidity”. Trends in Internal Medicine 2021; 1:1-6. URL: https://scivisionpub.com/pdfs/us-covid19-vaccines-proven-to-cause-more-harm-than-good-based-on-pivotal-clinical-trial-data-analyzed-using-the-proper-scientific--1811.pdf.
- Acevedo-Whitehouse, K.; Bruno, R. Potential health risks of mRNA-based vaccine therapy: A hypothesis. Med Hypotheses 2023, 171, 111015. [Google Scholar] [CrossRef]
- A Abdulkader, M.; A Merza, M. Immediate and Long-Term Adverse Events of COVID-19 Vaccines: A One-Year Follow-Up Study From the Kurdistan Region of Iraq. Cureus 2023, 15, e47670. [Google Scholar] [CrossRef]
- Doshi, P. Covid-19 vaccines: In the rush for regulatory approval, do we need more data? BMJ 2021, 373, n1244. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature 2020, 579, 321–321. [Google Scholar] [CrossRef] [PubMed]
- Gøtzsche PC, Demasi M. Serious harms of the COVID-19 vaccines: a systematic review. medRxiv Preprint. 2022.
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef] [PubMed]
- U.S. Centers for Disease Control. Historical Vaccine Safety Concerns. Accessed 7/10/2023. https://www.cdc.gov/vaccinesafety/concerns/concerns-history.html.
- Michels, C.; Perrier, D.; Kunadhasan, J.; Clark, E.; Gehrett, J.; Gehrett, B.; Kwiatek, K.; Adams, S.; Chandler, R.; Stagno, L.; et al. Forensic analysis of the 38 subject deaths in the 6-Month Interim Report of the Pfizer/BioNTech BNT162b2 mRNA Vaccine Clinical Trial. Int. J. Vaccine Theory, Pr. Res. 2023, 3, 973–1008. [Google Scholar] [CrossRef]
- Pfizer, Inc. Appendix 2.2 Cumulative and Interval Summary Tabulation of Serious and Non-serious Adverse Reactions From Post-marketing Data Sources (BNT162B2). New York, NY. 2022. Accessed: December 9, 2024: https://www.globalresearch.ca/wp-content/uploads/2023/05/pfizer-report.pdf.
- Pfizer, Inc. Periodic safety update report #3 for active substance: COVID-19 mRNA vaccine, BNT162b2. BioNTech Manufacturing GmbH, Mainz, Germany. 2022. Accessed: December 9, 2024: https://tkp.at/wp-content/uploads/2023/03/3.PSUR-1.pdf.
- Horowitz, D. Confidential Pfizer document shows the company observed 1.6 million adverse events covering nearly every organ system. 2023. Accessed: December 9, 2024: https://www.conservativereview.com/horowitz-confidential-pfizer-document-shows-the-company-observed-1-6-million-adverse-events-covering-nearly-every-organ-system-2661316948.html.
- Hulscher, N.; Hodkinson, R.; Makis, W.; McCullough, P.A. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Hear. Fail. 2024. [Google Scholar] [CrossRef]
- Hulscher N, Alexander PE, Amerling R, Gessling H, Hodkinson R, Makis W, Risch HA, et al. A systematic review of autopsy findings in deaths after COVID-19 vaccination. Science, Public Health Policy and the Law. 17 November 2024. v5.2019-2024. https://publichealthpolicyjournal.com/a-systematic-review-ofautopsy-findings-in-deaths-after-covid-19-vaccination/.
- Food and Drug Administration. Initial Results of Near Real-Time Safety Monitoring COVID-199 Vaccines in Persons Age 65 Years and Older [Internet] 2021. Accessed 11 May 2025. https://www.fda.gov/vaccinesblood-biologics/safety-availability-biologics/initial-results-near-real-time-safety-monitoring-covid-19-vaccines-persons-aged-65-years-and-older.
- Lataster, R. Reply to Fung et al. on COVID-19 vaccine case-counting window biases overstating vaccine effectiveness. J. Evaluation Clin. Pr. 2023, 30, 82–85. [Google Scholar] [CrossRef]
- HHJ News, Obituaries: Shawn Thomas Kuhn. Houston Home Journal. 10-16-2021. https://hhjonline.com/shawn-thomas-kuhn/.
- Gilbertson, D. ‘I’m going to miss my friend’: Southwest Airlines flight attendant, 36, dies from COVID-19. USA Today. Airline News. 8-12-2021. https://www.usatoday.com/story/travel/airlinenews/ 2021/08/11/southwest-airlines-flight-attendant-covid-maurice-reggie-shepperson/8100532002/.
- Suleyman, A. Fully Vaccinated New Orleans Woman Dies of COVID Aged 33 in Rare Breakthrough Case. Newsweek. 7-27-2021. https://www.newsweek.com/fully-vaccinated-new-orleans-woman-dies-covidaged- 33-rare-breakthrough-case-1613379.
- Parry, P.I.; Lefringhausen, A.; Turni, C.; Neil, C.J.; Cosford, R.; Hudson, N.J.; Gillespie, J. ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines 2023, 11, 2287. [Google Scholar] [CrossRef]
- Schwartz, L.; Aparicio-Alonso, M.; Henry, M.; Radman, M.; Attal, R.; Bakkar, A. Toxicity of the spike protein of COVID-19 is a redox shift phenomenon: A novel therapeutic approach. Free. Radic. Biol. Med. 2023, 206, 106–110. [Google Scholar] [CrossRef]
- Theoharides, T. Be aware of SARS-CoV-2 spike protein: There is more than meets the eye. J. Biol. Regul. Homeost. AGENTS 2021, 35, 833–838. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-P.; Wen, Y.-H.; Lin, W.-T.; Hsu, F.-P. Association between the side effect induced by COVID-19 vaccines and the immune regulatory gene polymorphism. Front. Immunol. 2022, 13, 941497. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Di Gioacchino M, Petrarca C, Lazzarin F, Di Giampaolo L, Sabbioni E, Boscolo P, Mariani-Costantini R, et al. Immunotoxicity of nanoparticles. Int J Immunopathol Pharmacol. 2011;24(1 Suppl):65S-71S.
- Cui, S.; Wang, Y.; Gong, Y.; Lin, X.; Zhao, Y.; Zhi, D.; Zhou, Q.; Zhang, S. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol. Res. 2018, 7, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Bouteau, A.; Herbst, C.; Igyártó, B.Z.; Klein, S.L. Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion. PLOS Pathog. 2022, 18, e1010830. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.-M. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine: Nanotechnology, Biol. Med. 2014, 10, 775–782. [Google Scholar] [CrossRef]
- Kedmi, R.; Ben-Arie, N.; Peer, D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials 2010, 31, 6867–6875. [Google Scholar] [CrossRef]
- Kashani, B.; Zandi, Z.; Pourbagheri-Sigaroodi, A.; Bashash, D.; Ghaffari, S.H. The role of toll-like receptor 4 (TLR4) in cancer progression: A possible therapeutic target? J. Cell. Physiol. 2020, 236, 4121–4137. [Google Scholar] [CrossRef]
- Parhiz, H.; Brenner, J.S.; Patel, P.N.; Papp, T.E.; Shahnawaz, H.; Li, Q.; Shi, R.; Zamora, M.E.; Yadegari, A.; Marcos-Contreras, O.A.; et al. Added to pre-existing inflammation, mRNA-lipid nanoparticles induce inflammation exacerbation (IE). J. Control. Release 2021, 344, 50–61. [Google Scholar] [CrossRef]
- Soegiarto, G.; Purnomosari, D. Challenges in the Vaccination of the Elderly and Strategies for Improvement. Pathophysiology 2023, 30, 155–173. [Google Scholar] [CrossRef]
- McKernan K, Helbert Y, Kane LT, McLaughlin S. Sequencing of bivalent Moderna and Pfizer mRNA vaccines reveals nanogram to microgram quantities of expression vector dsDNA per dose. OSF Prepr. 2023. [CrossRef]
- Speicher DJ, Rose J, Gutschi LM, McKernan K. DNA Fragments detected in monovalent and bivalent Pfizer/BioNTech and Moderna modRNA COVID-19 vaccines from Ontario, Canada: Exploratory dose response relationship with SERIOUS adverse events. OSF Preprints. Oct 19, 2023. [(accessed on 26 February 2024)]. Available online: https://osf.io/preprints/osf/mjc97.
- König, B.; Kirchner, J.O. Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 mRNA Vaccine Comirnaty®. Methods Protoc. 2024, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Tona, F.; Bottaro, S.; Vinci, A.; Dequal, G.; Daliento, L.; Thiene, G.; Iliceto, S. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity 2008, 41, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Levi R, Mansuri F, Jordan MM, Ladapo JA. Twelve-Month All-Cause Mortality after Initial COVID-19 Vaccination with Pfizer-BioNTech or mRNA-1273 among Adults Living in Florida. medRxiv [preprint]. Posted 29 April 2025. [CrossRef]
- Weiler, JL. Florida Study Reveals Elevated Mortality Risk Following Pfizer COVID-19 Vaccine Compared to Moderna. Popular Rationalism [Substack]. 6 May 2025. https://popularrationalism.substack.com/p/florida-study-reveals-elevatedmortality?utm_source=substack&utm_campaign=post_embed&utm_medium=web.
- Siebner, A.S.; Griesbaum, J.; E Huus, K.; Flügge, J.; Hopfensperger, K.; Michel, T.; Schneiderhan-Marra, N.; Sauter, D.; Kremsner, P.G.; E Ley, R.; et al. Class switch towards IgG2 and IgG4 is more pronounced in BNT162b2 compared to mRNA-1273 COVID-19 vaccinees. . 2025, 107990. [Google Scholar] [CrossRef]
- Sass, E. COVID-19 mRNA “vaccine” harms research collection. In Toxic Shot: Facing the Dangers of the COVID “Vaccines”. Zenodo. 2 July. [CrossRef]
- Japanese Pharmaceuticals and Medical Devices Agency (PMDA) SARS-CoV-2 mRNA Vaccine (BNT162, PF-07302048) 2021. [(accessed on 7 April 2023)]. Available online: https://www.pmda.go.jp/drugs/2021/P20210212001/672212000_30300AMX00231_I100_1.pdf.
- Judicial Watch Pfizer/BioNTech Study Found Lipid Nanoparticles Materials Outside Injection Site in Test Animals. judicialwatch.org. 2022. [(accessed on 12 July 2023)]. Available online: https://www.judicialwatch.org/nanoparticles-materials-outside-injection-site/.
- Di, J.; Du, Z.; Wu, K.; Jin, S.; Wang, X.; Li, T.; Xu, Y. Biodistribution and Non-linear Gene Expression of mRNA LNPs Affected by Delivery Route and Particle Size. Pharm. Res. 2022, 39, 105–114. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef]
- Baumeier, C.; Aleshcheva, G.; Harms, D.; Gross, U.; Hamm, C.; Assmus, B.; Westenfeld, R.; Kelm, M.; Rammos, S.; Wenzel, P.; et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int. J. Mol. Sci. 2022, 23, 6940. [Google Scholar] [CrossRef]
- Sriwastava, S.; Sharma, K.; Khalid, S.H.; Bhansali, S.; Shrestha, A.K.; Elkhooly, M.; Srivastava, S.; Khan, E.; Jaiswal, S.; Wen, S. COVID-19 Vaccination and Neurological Manifestations: A Review of Case Reports and Case Series. Brain Sci. 2022, 12, 407. [Google Scholar] [CrossRef]
- Vogrig, A.; Tartaglia, S.; Dentoni, M.; Fabris, M.; Bax, F.; Belluzzo, M.; Verriello, L.; Bagatto, D.; Gastaldi, M.; Tocco, P.; et al. Central nervous system immune-related disorders after SARS-CoV-2 vaccination: a multicenter study. Front. Immunol. 2024, 15, 1344184. [Google Scholar] [CrossRef] [PubMed]
- Schinas, G.; Polyzou, E.; Dimakopoulou, V.; Tsoupra, S.; Gogos, C.; Akinosoglou, K. Immune-mediated liver injury following COVID-19 vaccination. World J. Virol. 2023, 12, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 2020, 146, 105131–105131. [Google Scholar] [CrossRef]
- Posa, A. Spike protein-related proteinopathies: A focus on the neurological side of spikeopathies. Ann. Anat. - Anat. Anz. 2025, 260, 152662. [Google Scholar] [CrossRef]
- Mulroney, T.E.; Pöyry, T.; Yam-Puc, J.C.; Rust, M.; Harvey, R.F.; Kalmar, L.; Horner, E.; Booth, L.; Ferreira, A.P.; Stoneley, M.; et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 2023, 625, 189–194. [Google Scholar] [CrossRef]
- Seneff, S.; Kyriakopoulos, A.M.; Nigh, G.; A McCullough, P.; McCullough, P.A. A Potential Role of the Spike Protein in Neurodegenerative Diseases: A Narrative Review. Cureus 2023, 15, e34872. [Google Scholar] [CrossRef]
- Perez, J.-C.; Moret-Chalmin, C.; Montagnier, L. Emergence of a New Creutzfeldt-Jakob Disease: 26 Cases of the Human Version of Mad-Cow Disease, Days After a COVID-19 Injection. Int. J. Vaccine Theory, Pr. Res. 2023, 3, 727–770. [Google Scholar] [CrossRef]
- Nance, K.D.; Meier, J.L. Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines. ACS Central Sci. 2021, 7, 748–756. [Google Scholar] [CrossRef]
- Morais, P.; Adachi, H.; Yu, Y.-T. The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines. Front. Cell Dev. Biol. 2021, 9, 789427. [Google Scholar] [CrossRef]
- Kim, K.Q.; Burgute, B.D.; Tzeng, S.-C.; Jing, C.; Jungers, C.; Zhang, J.; Yan, L.L.; Vierstra, R.D.; Djuranovic, S.; Evans, B.S.; et al. N1-methylpseudouridine found within COVID-19 mRNA vaccines produces faithful protein products. Cell Rep. 2022, 40, 111300–111300. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Gychka, S.G. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines. Vaccines 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Krauson, A.J.; Casimero, F.V.C.; Siddiquee, Z.; Stone, J.R. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. npj Vaccines 2023, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.F.; Cheng, C.-A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clin. Infect. Dis. 2021, 74, 715–718. [Google Scholar] [CrossRef]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef]
- Boros, L.G.; Kyriakopoulos, A.M.; Brogna, C.; Piscopo, M.; McCullough, P.A.; Seneff, S. Long-lasting, biochemically modified mRNA, and its frameshifted recombinant spike proteins in human tissues and circulation after COVID-19 vaccination. Pharmacol. Res. Perspect. 2024, 12, e1218. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S.; Marino, G.; Montano, L.; Viduto, V.; Fabrowski, M.; Lettieri, G.; Piscopo, M. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. Proteom. – Clin. Appl. 2023, 17, e2300048. [Google Scholar] [CrossRef]
- Patterson, B.K.; Yogendra, R.; Francisco, E.B.; Guevara-Coto, J.; Long, E.; Pise, A.; Osgood, E.; Bream, J.; Kreimer, M.; Jeffers, D.; et al. Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals. Hum. Vaccines Immunother. 2025, 21, 2494934. [Google Scholar] [CrossRef]
- Ota, N.; Itani, M.; Aoki, T.; Sakurai, A.; Fujisawa, T.; Okada, Y.; Noda, K.; Arakawa, Y.; Tokuda, S.; Tanikawa, R. Expression of SARS-CoV-2 spike protein in cerebral Arteries: Implications for hemorrhagic stroke Post-mRNA vaccination. J. Clin. Neurosci. 2025, 136, 111223. [Google Scholar] [CrossRef]
- Bhattacharjee B, Lu P, Monteiro VS, Tabachnikova A, Wang K, Hooper WB, Bastos V, et al. Immunological and Antigenic Signatures Associated with Chronic Illnesses after COVID-19 Vaccination. medRxiv preprint 2025. [CrossRef]
- Personal communications with epidemiologist Nicolas Hulscher, McCullough Foundation. Article in press. 25 July 2025.
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008–113008. [Google Scholar] [CrossRef]
- Klingel, H.; Krüttgen, A.; Imöhl, M.; Kleines, M. Humoral immune response to SARS-CoV-2 mRNA vaccines is associated with choice of vaccine and systemic adverse reactions DMD TNR. Clin. Exp. Vaccine Res. 2023, 12, 60–69. [Google Scholar] [CrossRef]
- Ouranidis, A.; Vavilis, T.; Mandala, E.; Davidopoulou, C.; Stamoula, E.; Markopoulou, C.K.; Karagianni, A.; Kachrimanis, K. mRNA Therapeutic Modalities Design, Formulation and Manufacturing under Pharma 4.0 Principles. Biomedicines 2021, 10, 50. [Google Scholar] [CrossRef]
- Milano, G.; Gal, J.; Creisson, A.; Chamorey, E. Myocarditis and COVID-19 Mrna Vaccines: A Mechanistic Hypothesis Involving dsRNA. Futur. Virol. 2021, 17, 191–196. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2021, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kobayashi, S.; Hayashi, T.; Tachibana, M.; Saito, T.; Ogura, K.; Miyakoshi, S. Immune thrombocytopenic purpura in an elderly patient with cerebral hemorrhage after the fourth mRNA-1273 COVID-19 vaccination. Geriatr. Gerontol. Int. 2023, 23, 969–970. [Google Scholar] [CrossRef]
- Lee, E.-J.; Beltrami-Moreira, M.; Al-Samkari, H.; Cuker, A.; DiRaimo, J.; Gernsheimer, T.; Kruse, A.; Kessler, C.; Kruse, C.; Leavitt, A.D.; et al. SARS-CoV-2 vaccination and ITP in patients with de novo or preexisting ITP. Blood 2021, 139, 1564–1574. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, J.; Xia, H.; Wang, Y.; Zhang, C.; Chen, W.; Zhang, H.; Liu, Q.; Zhu, R.; et al. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell Discov. 2021, 7, 1–15. [Google Scholar] [CrossRef]
- Chevaisrakul, P.; Lumjiaktase, P.; Kietdumrongwong, P.; Chuatrisorn, I.; Chatsangjaroen, P.; Phanuphak, N. Hybrid and herd immunity 6 months after SARS-CoV-2 exposure among individuals from a community treatment program. Sci. Rep. 2023, 13, 1–9. [Google Scholar] [CrossRef]
- Espino, A.M.; Armina-Rodriguez, A.; Alvarez, L.; Ocasio-Malavé, C.; Ramos-Nieves, R.; Martinó, E.I.R.; López-Marte, P.; Torres, E.A.; Sariol, C.A. The Anti-SARS-CoV-2 IgG1 and IgG3 Antibody Isotypes with Limited Neutralizing Capacity against Omicron Elicited in a Latin Population a Switch toward IgG4 after Multiple Doses with the mRNA Pfizer–BioNTech Vaccine. Viruses 2024, 16, 187. [Google Scholar] [CrossRef] [PubMed]
- Motta, R.V.; Culver, E.L. IgG4 autoantibodies and autoantigens in the context of IgG4-autoimmune disease and IgG4-related disease. Front. Immunol. 2024, 15, 1272084. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.L.; Weiss, S.A.; Pauken, K.E.; Sen, D.R.; Sharpe, A.H. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 2021, 22, 809–819. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Burke, P.C.; Nowacki, A.S.; Gordon, S.M. Effectiveness of the 2023–2024 Formulation of the COVID-19 Messenger RNA Vaccine. Clin. Infect. Dis. 2024, 79, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Kiszel, P.; Sík, P.; Miklós, J.; Kajdácsi, E.; Sinkovits, G.; Cervenak, L.; Prohászka, Z. Class switch towards spike protein-specific IgG4 antibodies after SARS-CoV-2 mRNA vaccination depends on prior infection history. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Pérez, C.M.; Ruiz-Rius, S.; Ramírez-Morros, A.; Vidal, M.; Opi, D.H.; Santamaria, P.; Blanco, J.; Vidal-Alaball, J.; Beeson, J.G.; Molinos-Albert, L.M.; et al. Post-vaccination IgG4 and IgG2 class switch associates with increased risk of SARS-CoV-2 infections. J. Infect. 2025, 90, 106473. [Google Scholar] [CrossRef]
- Awaya, T.; Moroi, M.; Enomoto, Y.; Kunimasa, T.; Nakamura, M. What Should We Do after the COVID-19 Vaccination? Vaccine-Associated Diseases and Precautionary Measures against Adverse Reactions. Vaccines 2022, 10, 866. [Google Scholar] [CrossRef]
- Zagorec, N.; Horvatić, I.; Šenjug, P.; Horaček, M.; Ljubanović, D.G.; Galešić, K. Immune-mediated diseases after coronavirus disease 2019 vaccination: rare but important complication. Croat. Med J. 2022, 63, 389–393. [Google Scholar] [CrossRef]
- Franchini, M.; Liumbruno, G.M.; Pezzo, M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. Eur. J. Haematol. 2021, 107, 173–180. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Safary, A.; Esalatmanesh, K.; Eftekharsadat, A.T.; Nakjavani, M.-R.J.; Khabbazi, A. Autoimmune inflammatory rheumatic diseases post-COVID-19 vaccination. Int. Immunopharmacol. 2022, 110, 109061–109061. [Google Scholar] [CrossRef]
- Keijzer, S.; Oskam, N.; Heer, P.O.-D.; Steenhuis, M.; Keijser, J.B.; Wieske, L.; van Dam, K.P.; Stalman, E.W.; Kummer, L.Y.; Boekel, L.; et al. Longitudinal rheumatoid factor autoantibody responses after SARS-CoV-2 vaccination or infection. Front. Immunol. 2024, 15, 1314507. [Google Scholar] [CrossRef]
- Hileman, C.O.; Malakooti, S.K.; Patil, N.; Singer, N.G.; McComsey, G.A. New-onset autoimmune disease after COVID-19. Front. Immunol. 2024, 15, 1337406. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.; Shuai, Z.; Ye, D.; Pan, H. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, H.; Xia, S.-L. New-Onset Arthritis Following COVID-19 Vaccination: A Systematic Review of Case Reports. Vaccines 2023, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Sagy, I.; Zeller, L.; Raviv, Y.; Porges, T.; Bieber, A.; Abu-Shakra, M. New-onset systemic lupus erythematosus following BNT162b2 mRNA COVID-19 vaccine: a case series and literature review. Rheumatol. Int. 2022, 42, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, X.; Chen, X.; Li, Q. Insights into new-onset autoimmune diseases after COVID-19 vaccination. Autoimmun. Rev. 2023, 22, 103340–103340. [Google Scholar] [CrossRef]
- Mehta, P.; Fajgenbaum, D.C. Is severe COVID-19 a cytokine storm syndrome: a hyperinflammatory debate. Curr. Opin. Rheumatol. 2021, 33, 419–430. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’aNdrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, 1209-+. [Google Scholar] [CrossRef]
- DiPiazza, A.T.; Graham, B.S.; Ruckwardt, T.J. T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochem. Biophys. Res. Commun. 2021, 538, 211–217. [Google Scholar] [CrossRef]
- Olszewska, B.; Zaryczańska, A.; Nowicki, R.J.; Sokołowska-Wojdyło, M. Rare COVID-19 vaccine side effects got lost in the shuffle. Primary cutaneous lymphomas following COVID-19 vaccination: a systematic review. Front. Med. 2024, 11, 1325478. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Puel, A.; Bastard, P.; Bustamante, J.; Casanova, J.-L. Human autoantibodies underlying infectious diseases. J. Exp. Med. 2022, 219. [Google Scholar] [CrossRef]
- Combes, A.J.; Courau, T.; Kuhn, N.F.; Hu, K.H.; Ray, A.; Chen, W.S.; Chew, N.W.; Cleary, S.J.; Kushnoor, D.; Reeder, G.C.; et al. Global absence and targeting of protective immune states in severe COVID-19. Nature 2021, 591, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Xu, W.; Cong, X.; Fan, H.; Gilkeson, G.; Wu, X.; Hughes, H.; Jiang, W. COVID-19 mRNA vaccine BNT162b2 induces autoantibodies against type I interferons in a healthy woman. J. Autoimmun. 2022, 132, 102896–102896. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wen, X.; Cong, X.; Jiang, W. COVID-19 mRNA vaccine, but not a viral vector-based vaccine, promotes neutralizing anti-type I interferon autoantibody production in a small group of healthy individuals. J. Med Virol. 2023, 95, e29137. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, H.-S.; Hwang, N.; Huh, Y.; Bu, S.; Seo, K.J.; Kwon, S.H.; Lee, H.-K.; Kim, J.-W.; Yoon, B.K.; et al. Epigenomic landscape exhibits interferon signaling suppression in the patient of myocarditis after BNT162b2 vaccination. Sci. Rep. 2023, 13, 1–11. [Google Scholar] [CrossRef]
- Wahl, I.; Wardemann, H. Sterilizing immunity: Understanding COVID-19. Immunity 2022, 55, 2231–2235. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. New Engl. J. Med. 2022, 386, 1712–1720. [Google Scholar] [CrossRef]
- Ophir Y, Shira-Raz Y, Zakov S, McCullough PA. The efficacy of COVID-19 vaccine boosters against severe illness and deaths scientific fact or wishful myth? J Am Phys Surg. 2023; 28: 20-7. https://www.jpands.org/search-results.htm.
- Ioannidis, J.P.A. Estimating conditional vaccine effectiveness. Eur. J. Epidemiology 2022, 37, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Neil M, Fenton NE, McLachlan S. The extent and impact of vaccine status miscategorisation on covid-19 vaccine efficacy studies. MedRxiv Preprint. 2024. [CrossRef]
- Fung, K.; Jones, M.; Doshi, P. Sources of bias in observational studies of covid-19 vaccine effectiveness. J. Evaluation Clin. Pr. 2023, 30, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Doshi, P.; Fung, K. How the case counting window affected vaccine efficacy calculations in randomized trials of COVID-19 vaccines. J. Evaluation Clin. Pr. 2023, 30, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Lataster, R. How the adverse effect counting window affected vaccine safety calculations in randomised trials of COVID-19 vaccines. J. Evaluation Clin. Pr. 2024, 30, 453–458. [Google Scholar] [CrossRef]
- Lataster, R. Science summary: COVID-19 vaccines’ effectiveness and safety exaggerated in clinical trials & observational studies, academics find. Okay Then News Substack. 28 Feb. 2024. URL: https://okaythennews.substack.com/p/science-summary-covid-19-vaccines.
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, 75, e545–e551. [Google Scholar] [CrossRef]
- Rzymski, P.; Pazgan-Simon, M.; Simon, K.; Łapiński, T.; Zarębska-Michaluk, D.; Szczepańska, B.; Chojnicki, M.; Mozer-Lisewska, I.; Flisiak, R. Clinical Characteristics of Hospitalized COVID-19 Patients Who Received at Least One Dose of COVID-19 Vaccine. Vaccines 2021, 9, 781. [Google Scholar] [CrossRef]
- Adhikari, B.; Bednash, J.S.; Horowitz, J.C.; Rubinstein, M.P.; Vlasova, A.N. Brief research report: impact of vaccination on antibody responses and mortality from severe COVID-19. Front. Immunol. 2024, 15, 1325243. [Google Scholar] [CrossRef]
- Heymans, S.; Dawson, D.; Fuster, V.; Metra, M.; Tocchetti, C.G. Myocarditis Following SARS-CoV2 mRNA Vaccination Against COVID-19. Circ. 2022, 80, 1363–1365. [Google Scholar] [CrossRef]
- Block, J.P. Cardiac Complications After SARS-CoV-2 Infection and mRNA COVID-19 Vaccination — PCORnet, United States, January 2021–January 2022. Mmwr-Morbidity Mortal. Wkly. Rep. 2022, 71, 517–523. [Google Scholar] [CrossRef]
- Holland, D.J.; Blazak, P.L.; Martin, J.; Broom, J.; Poulter, R.S.; Stanton, T. Myocarditis and Cardiac Complications Associated With COVID-19 and mRNA Vaccination: A Pragmatic Narrative Review to Guide Clinical Practice. Hear. Lung Circ. 2022, 31, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Mead, M.N. Myocarditis after SARS-CoV-2 infection and COVID-19 vaccination: Epidemiology, outcomes, and new perspectives. Int J Cardiovasc Res Innov. 2025, 3, 1–43. [Google Scholar] [CrossRef]
- Flaxman, S.; Whittaker, C.; Semenova, E.; Rashid, T.; Parks, R.M.; Blenkinsop, A.; Unwin, H.J.T.; Mishra, S.; Bhatt, S.; Gurdasani, D.; et al. Assessment of COVID-19 as the Underlying Cause of Death Among Children and Young People Aged 0 to 19 Years in the US. JAMA Netw. Open 2023, 6, e2253590–e2253590. [Google Scholar] [CrossRef] [PubMed]
- Thornley S, Morris AJ, Sundborn G, S Bailey. How fatal is COVID-19 compared with seasonal influenza? The devil is in the detail [Rapid Response]. BMJ 2020; 371: m3883. [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911–112911. [Google Scholar] [CrossRef]
- Spinardi, J.R.; Srivastava, A. Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination—Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines 2023, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Epsi, N.J.; A Richard, S.; A Lindholm, D.; Mende, K.; Ganesan, A.; Huprikar, N.; Lalani, T.; Fries, A.C.; Maves, R.C.; E Colombo, R.; et al. Understanding “Hybrid Immunity”: Comparison and Predictors of Humoral Immune Responses to Severe Acute Respiratory Syndrome Coronavirus 2 Infection (SARS-CoV-2) and Coronavirus Disease 2019 (COVID-19) Vaccines. Clin. Infect. Dis. 2022, 76, e439–e449. [Google Scholar] [CrossRef]
- Klee, B.; Diexer, S.; Xu, C.; Gottschick, C.; Hartmann, C.; Meyer-Schlinkmann, K.M.; Kuhlmann, A.; Rosendahl, J.; Binder, M.; Gekle, M.; et al. Household transmission of Omicron variant of SARS-CoV-2 under conditions of hybrid immunity—a prospective study in Germany. Infection 2024, 53, 221–230. [Google Scholar] [CrossRef]
- Bigay, J.; Le Grand, R.; Martinon, F.; Maisonnasse, P. Vaccine-associated enhanced disease in humans and animal models: Lessons and challenges for vaccine development. Front. Microbiol. 2022, 13, 932408. [Google Scholar] [CrossRef]
- Gartlan, C.; Tipton, T.; Salguero, F.J.; Sattentau, Q.; Gorringe, A.; Carroll, M.W. Vaccine-Associated Enhanced Disease and Pathogenic Human Coronaviruses. Front. Immunol. 2022, 13, 882972. [Google Scholar] [CrossRef]
- Ebenig, A.; Muraleedharan, S.; Kazmierski, J.; Todt, D.; Auste, A.; Anzaghe, M.; Gömer, A.; Postmus, D.; Gogesch, P.; Niles, M.; et al. Vaccine-associated enhanced respiratory pathology in COVID-19 hamsters after TH2-biased immunization. Cell Rep. 2022, 40, 111214–111214. [Google Scholar] [CrossRef]
- Tunjungputri, R.N.; Tetrasiwi, E.N.; Veronica, M.; Pandelaki, J.; Ibrahim, F.; Nelwan, E.J.; Supinski, G.S. Vaccine-Associated Disease Enhancement (VADE): Considerations in Postvaccination COVID-19. Case Rep. Med. 2021, 2021, 1–5. [Google Scholar] [CrossRef]
- Boyce, T.G.; McClure, D.L.; Hanson, K.E.; Daley, M.F.; DeSilva, M.B.; Irving, S.A.; Jackson, L.A.; Klein, N.P.; Lewin, B.; Williams, J.T.B.; et al. Lack of Evidence for Vaccine-Associated Enhanced Disease From COVID-19 Vaccines Among Adults in the Vaccine Safety Datalink. Pharmacoepidemiol. Drug Saf. 2024, 33, e5863. [Google Scholar] [CrossRef]
- Parameswaran, A.; Apsingi, S.; Eachempati, K.K.; Dannana, C.S.; Jagathkar, G.; Iyer, M.; Aribandi, H. Incidence and severity of COVID-19 infection post-vaccination: a survey among Indian doctors. Infection 2022, 50, 889–895. [Google Scholar] [CrossRef]
- Grasselli, G.; Zanella, A.; Carlesso, E.; Florio, G.; Canakoglu, A.; Bellani, G.; Bottino, N.; Cabrini, L.; Castelli, G.P.; Catena, E.; et al. Association of COVID-19 Vaccinations With Intensive Care Unit Admissions and Outcome of Critically Ill Patients With COVID-19 Pneumonia in Lombardy, Italy. JAMA Netw. Open 2022, 5, e2238871–e2238871. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nalla, L.V.; Sharma, M.; Sharma, N.; Singh, A.A.; Malim, F.M.; Ghatage, M.; Mukarram, M.; Pawar, A.; Parihar, N.; et al. Association of COVID-19 with Comorbidities: An Update. ACS Pharmacol. Transl. Sci. 2023, 6, 334–354. [Google Scholar] [CrossRef]
- Cao, C.; Cai, Z.; Xiao, X.; Rao, J.; Chen, J.; Hu, N.; Yang, M.; Xing, X.; Wang, Y.; Li, M.; et al. The architecture of the SARS-CoV-2 RNA genome inside virion. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, K.R.; Loftus, G.; Traylor, L.; Goodwin, R.; Arce, S. Comparison of COVID-19 Vaccine-Associated Myocarditis and Viral Myocarditis Pathology. Vaccines 2023, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.; Rojas, M.; Beltrán, S.; Polo, F.; Camacho-Domínguez, L.; Morales, S.D.; Gershwin, M.E.; Anaya, J.-M. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J. Autoimmun. 2022, 132, 102898–102898. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Herrán, M.; Ramírez-Santana, C.; Leung, P.S.; Anaya, J.-M.; Ridgway, W.M.; Gershwin, M.E. Molecular mimicry and autoimmunity in the time of COVID-19. J. Autoimmun. 2023, 139, 103070–103070. [Google Scholar] [CrossRef]
- Talotta, R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin. Immunol. 2021, 224, 108665–108665. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Tzivaki, I.; Marangos, M. Covid-19 vaccine and autoimmunity: Awakening the sleeping dragon. Clin Immunol. 2021, 226, 108721–108721. [Google Scholar] [CrossRef]
- Polykretis, P.; Donzelli, A.; Lindsay, J.C.; Wiseman, D.; Kyriakopoulos, A.M.; Mörz, M.; Bellavite, P.; Fukushima, M.; Seneff, S.; McCullough, P.A. Autoimmune inflammatory reactions triggered by the COVID-19 genetic vaccines in terminally differentiated tissues. Autoimmunity 2023, 56, 2259123. [Google Scholar] [CrossRef]
- Wang, L.; Davis, P.B.; Kaelber, D.C.; Volkow, N.D.; Xu, R. Comparison of mRNA-1273 and BNT162b2 Vaccines on Breakthrough SARS-CoV-2 Infections, Hospitalizations, and Death During the Delta-Predominant Period. JAMA 2022, 327, 678–680. [Google Scholar] [CrossRef]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw. Open 2021, 4, e2140364–e2140364. [Google Scholar] [CrossRef]
- Kitagawa, H.; Kaiki, Y.; Sugiyama, A.; Nagashima, S.; Kurisu, A.; Nomura, T.; Omori, K.; Akita, T.; Shigemoto, N.; Tanaka, J.; et al. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J. Infect. Chemother. 2022, 28, 576–581. [Google Scholar] [CrossRef]
- Valera-Rubio, M. (.; Sierra-Torres, M.I.(.; García, R.(.C.; Cordero-Ramos, J.(.; López-Márquez, M.R.(.; Cruz-Salgado, Ó.(.; Calleja-Hernández, M.Á.(. Adverse events reported after administration of BNT162b2 and mRNA-1273 COVID-19 vaccines among hospital workers: a cross-sectional survey-based study in a Spanish hospital. Expert Rev. Vaccines 2022, 21, 533–540. [Google Scholar] [CrossRef]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA 2021, 325, 2201–2202. [Google Scholar] [CrossRef]
- Chapin-Bardales, J.; Myers, T.; Gee, J.; Shay, D.K.; Marquez, P.; Baggs, J.; Zhang, B.; Licata, C.; Shimabukuro, T.T. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: Findings from the CDC v-safe surveillance system. Vaccine 2021, 39, 7066–7073. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, T.-A.; Chiang, P.-H.; Hsieh, A.-R.; Wu, B.-J.; Chen, P.-Y.; Lin, K.-C.; Tsai, Z.-S.; Lin, M.-H.; Chen, T.-J.; et al. Incidence and Nature of Short-Term Adverse Events following COVID-19 Second Boosters: Insights from Taiwan’s Universal Vaccination Strategy. Vaccines 2024, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Urdaneta, V.; Esposito, D.B.; Dharia, P.; Moraga, M.S.; Anteyi, K.; Oduyebo-Omotosho, T.; Rossi, M.; Burton, P.; Vega, J.M.; Dawson, R.; et al. Global Safety Assessment of Adverse Events of Special Interest Following 2 Years of Use and 772 Million Administered Doses of mRNA-1273. Open Forum Infect. Dis. 2024, 11, ofae067. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. More antibodies are not always better: Fc effector functions play a critical role in SARS-CoV-2 infection and protection. Prog Mol Biol Transl Sci. 2025;213:413-447. [CrossRef]
- Brisotto, G.; Montico, M.; Turetta, M.; Zanussi, S.; Cozzi, M.R.; Vettori, R.; Boschin, R.B.; Vinante, L.; Matrone, F.; Revelant, A.; et al. Integration of Cellular and Humoral Immune Responses as an Immunomonitoring Tool for SARS-CoV-2 Vaccination in Healthy and Fragile Subjects. Viruses 2023, 15, 1276. [Google Scholar] [CrossRef]
- Debes, A.K.; Xiao, S.; Colantuoni, E.; Egbert, E.R.; Caturegli, P.; Gadala, A.; Milstone, A.M. Association of Vaccine Type and Prior SARS-CoV-2 Infection With Symptoms and Antibody Measurements Following Vaccination Among Health Care Workers. JAMA Intern. Med. 2021, 181, 1660–1662. [Google Scholar] [CrossRef]
- Kobashi, Y.; Shimazu, Y.; Kawamura, T.; Nishikawa, Y.; Omata, F.; Kaneko, Y.; Kodama, T.; Tsubokura, M.; Yunihastuti, E. Factors associated with anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein antibody titer and neutralizing activity among healthcare workers following vaccination with the BNT162b2 vaccine. PLOS ONE 2022, 17, e0269917. [Google Scholar] [CrossRef]
- Levy, I.; Levin, E.G.; Olmer, L.; Regev-Yochay, G.; Agmon-Levin, N.; Wieder-Finesod, A.; Indenbaum, V.; Herzog, K.; Doolman, R.; Asraf, K.; et al. Correlation between Adverse Events and Antibody Titers among Healthcare Workers Vaccinated with BNT162b2 mRNA COVID-19 Vaccine. Vaccines 2022, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg. Health-Eur. 2021, 10, 100208. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, V.; Weber, B.; Cheng, S.; Ebinger, J.E. Review of Immunologic Manifestations of COVID-19 Infection and Vaccination. Hear. Fail. Clin. 2023, 19, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Rechavi, Y.; Shashar, M.; Lellouche, J.; Yana, M.; Yakubovich, D.; Sharon, N. Occurrence of BNT162b2 Vaccine Adverse Reactions Is Associated with Enhanced SARS-CoV-2 IgG Antibody Response. Vaccines 2021, 9, 977. [Google Scholar] [CrossRef]
- Takeuchi, M.; Higa, Y.; Esaki, A.; Nabeshima, Y.; Nakazono, A.; Moreland, N.J. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? PLOS ONE 2021, 16, e0257668. [Google Scholar] [CrossRef]
- Uwamino, Y.; Kurafuji, T.; Sato, Y.; Tomita, Y.; Shibata, A.; Tanabe, A.; Yatabe, Y.; Noguchi, M.; Arai, T.; Ohno, A.; et al. Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: An observational study of 646 Japanese healthcare workers and university staff. Vaccine 2022, 40, 1019–1025. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Yong, S.J.; Liu, S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev. Med Virol. 2021, 32, e2315. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Lam, I.C.H.; Wong, C.K.H.; Zhang, R.; Chui, C.S.L.; Lai, F.T.T.; Li, X.; Chan, E.W.Y.; Luo, H.; Zhang, Q.; Man, K.K.C.; et al. Long-term post-acute sequelae of COVID-19 infection: a retrospective, multi-database cohort study in Hong Kong and the UK. eClinicalMedicine 2023, 60, 102000. [Google Scholar] [CrossRef] [PubMed]
- Arjun, M.C.; Singh, A.K.; Pal, D.; Das, K.; G. , A.; Venkateshan, M.; Mishra, B.; Patro, B.K.; Mohapatra, P.R.; Subba, S.H.; et al. Characteristics and predictors of Long COVID among diagnosed cases of COVID-19. PLOS ONE 2022, 17, e0278825. [Google Scholar] [CrossRef] [PubMed]
- Bocquet-Garçon, A. Impacts of the SARS-CoV-2 Spike Protein on the Innate Immune System: A Review. Cureus 2024, 16, e57008. [Google Scholar] [CrossRef]
- Craddock, V.; Mahajan, A.; Spikes, L.; Krishnamachary, B.; Ram, A.K.; Kumar, A.; Chen, L.; Chalise, P.; Dhillon, N.K. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. J. Med Virol. 2023, 95, e28568–e28568. [Google Scholar] [CrossRef]
- Dhuli, K.; Medori, M.C.; Micheletti, C.; Donato, K.; Fioretti, F.; Calzoni, A.; Praderio, A.; De Angelis, M.G.; Arabia, G.; Cristoni, S.; et al. Presence of viral spike protein and vaccinal spike protein in the blood serum of patients with long-COVID syndrome (Retracted Article). 2023, 27, 13–19. [CrossRef]
- Vogel G, Couzin-Frankel J. Rare link between coronavirus vaccines and Long Covid-like illness starts to gain acceptance. Science 2023; 381: 6653. [CrossRef]
- Hulscher, N.; Procter, B.C.; Wynn, C.; A McCullough, P. Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination. Cureus 2023, 15, e49204. [Google Scholar] [CrossRef]
- White JR, Abraham RL, Coleman WT, Pitre E, Stevenson MM, Kaplan JL, Goldberg AG, et al. SARS-CoV-2 Semi-Quantitative Total Antibody Correlates with Symptoms of Long COVID in Both Vaccinated and Unvaccinated Subjects. Preprints.org. Posted:. 16 July 2025. [CrossRef]
- Diexer, S.; Klee, B.; Gottschick, C.; Xu, C.; Broda, A.; Purschke, O.; Binder, M.; Frese, T.; Girndt, M.; Hoell, J.I.; et al. Association between virus variants, vaccination, previous infections, and post-COVID-19 risk. Int. J. Infect. Dis. 2023, 136, 14–21. [Google Scholar] [CrossRef]
- Bhargava A, Inslicht, S. Postacute sequelae of SARS-CoV-2 in the population: Risk factors and vaccines. Preprint. [CrossRef]
- . [CrossRef]
- Scholkmann, F.; May, C.-A. COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVIDvac-syndrome”): Similarities and differences. Pathol. - Res. Pr. 2023, 246, 154497–154497. [Google Scholar] [CrossRef]
- See, I.; Lale, A.; Marquez, P.; Streiff, M.B.; Wheeler, A.P.; Tepper, N.K.; Woo, E.J.; Broder, K.R.; Edwards, K.M.; Gallego, R.; et al. Case Series of Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination—United States, December 2020 to August 2021. Ann. Intern. Med. 2022, 175, 513–522. [Google Scholar] [CrossRef]
- Sangli, S.; Virani, A.; Cheronis, N.; Vannatter, B.; Minich, C.; Noronha, S.; Bhagavatula, R.; Speredelozzi, D.; Sareen, M.; Kaplan, R.B. Thrombosis With Thrombocytopenia After the Messenger RNA–1273 Vaccine. Ann. Intern. Med. 2021, 174, 1480–1482. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2021, 327, 80–82. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef]
- Liu, R.; Pan, J.; Zhang, C.; Sun, X. Cardiovascular Complications of COVID-19 Vaccines. Front. Cardiovasc. Med. 2022, 9, 840929. [Google Scholar] [CrossRef]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef]
- Hoteit, L.; Deeb, A.-P.; Andraska, E.A.; Kaltenmeier, C.; Yazdani, H.O.; Tohme, S.; Neal, M.D.; Mota, R.I. The Pathobiological Basis for Thrombotic Complications in COVID-19: a Review of the Literature. Curr. Pathobiol. Rep. 2021, 9, 107–117. [Google Scholar] [CrossRef]
- Fan, B.E.; Shen, J.Y.; Lim, X.R.; Tu, T.M.; Chang, C.C.R.; Khin, H.S.W.; Koh, J.S.; Rao, J.P.; Lau, S.L.; Tan, G.B.; et al. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV -2 vaccination: A black swan event. Am. J. Hematol. 2021, 96, E357–E361. [Google Scholar] [CrossRef]
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.F.; Uzzo, M.L.; Argo, A.; Zerbo, S. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front. Immunol. 2021, 12, 729251. [Google Scholar] [CrossRef]
- Padilla-Flores, T.; Sampieri, A.; Vaca, L. Incidence and management of the main serious adverse events reported after COVID-19 vaccination. Pharmacol. Res. Perspect. 2024, 12, e1224. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H. The roles of platelets in COVID-19-associated coagulopathy and vaccine-induced immune thrombotic thrombocytopenia. Trends Cardiovasc. Med. 2021, 32, 1–9. [Google Scholar] [CrossRef]
- Schreckenberg, R.; Woitasky, N.; Itani, N.; Czech, L.; Ferdinandy, P.; Schulz, R. Cardiac side effects of RNA-based SARS-CoV-2 vaccines: Hidden cardiotoxic effects of mRNA-1273 and BNT162b2 on ventricular myocyte function and structure. Br. J. Pharmacol. 2023, 181, 345–361. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 VaST Work Group Report – May 17, 2021. 2021. [cited 2023 January 5]; Available from: https://www.cdc.gov/vaccines/acip/work-groups-vast/report-2021-05-17.html. 17 May.
- Anonymous. ACC Underscores Safety of COVID-19 Vaccine. ACC News Story.
- Oct 14, 2022. https://www.acc.org/Latest-in-Cardiology/Articles/2022/10/14/15/13/ACC-Underscores-Safety-of-COVID-19-Vaccine. 2022.
- Rose, J.; Hulscher, N.; McCullough, P.A. Determinants of COVID-19 vaccine-induced myocarditis. Ther. Adv. Drug Saf. 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.-P.; et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Buergin, N.; Lopez-Ayala, P.; Hirsiger, J.R.; Mueller, P.; Median, D.; Glarner, N.; Rumora, K.; Herrmann, T.; Koechlin, L.; Haaf, P.; et al. Sex-specific differences in myocardial injury incidence after COVID-19 mRNA-1273 booster vaccination. Eur. J. Hear. Fail. 2023, 25, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Mansanguan, S.; Charunwatthana, P.; Piyaphanee, W.; Dechkhajorn, W.; Poolcharoen, A.; Mansanguan, C. Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Trop. Med. Infect. Dis. 2022, 7, 196. [Google Scholar] [CrossRef]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes With Recent SARS-CoV-2 Infection. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef]
- Foltran, D.; Delmas, C.; Flumian, C.; De Paoli, P.; Salvo, F.; Gautier, S.; Drici, M.-D.; Karsenty, C.; Montastruc, F. Myocarditis and pericarditis in adolescents after first and second doses of mRNA COVID-19 vaccines. Eur. Hear. J. - Qual. Care Clin. Outcomes 2021, 8, 99–103. [Google Scholar] [CrossRef]
- Karlstad, Ø.; Hovi, P.; Husby, A.; Härkänen, T.; Selmer, R.M.; Pihlström, N.; Hansen, J.V.; Nohynek, H.; Gunnes, N.; Sundström, A.; et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022, 7, 600–612. [Google Scholar] [CrossRef]
- Buchan, S.A.; Seo, C.Y.; Johnson, C.; Alley, S.; Kwong, J.C.; Nasreen, S.; Calzavara, A.; Lu, D.; Harris, T.M.; Yu, K.; et al. Epidemiology of Myocarditis and Pericarditis Following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval Among Adolescents and Adults in Ontario, Canada. JAMA Netw. Open 2022, 5, e2218505–e2218505. [Google Scholar] [CrossRef]
- Gao, J.; Feng, L.; Li, Y.; Lowe, S.; Guo, Z.; Bentley, R.; Xie, C.; Wu, B.; Xie, P.; Xia, W.; et al. A Systematic Review and Meta-analysis of the Association Between SARS-CoV-2 Vaccination and Myocarditis or Pericarditis. Am. J. Prev. Med. 2022, 64, 275–284. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Feng, S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect. Dis. 2022, 22, 1131–1141. [Google Scholar] [CrossRef]
- Bohné, M.; Bohnen, S.; Willems, S.; Klingel, K.; Kivelitz, D.; Bahlmann, E.; Shirotani, M. Acute Lymphocytic Myocarditis in a Young Male Post-COVID-19. Case Rep. Cardiol. 2023, 2023, 1–7. [Google Scholar] [CrossRef]
- Fishman, B.; Goitein, O.; Berkovitch, A.; Rahav, G.; Matetzky, S.; Vandenbriele, C.; Abela, M.; Alvarez, C.; Liu, Z.; Vervaat, F. First report of myocarditis in two patients with COVID-19 Omicron variant: case report. Eur. Hear. J. - Case Rep. 2022, 6, ytac407. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, A.; Schicchi, N.; Calcagnoli, F.; Falchetti, E.; Ciampani, N.; Argalia, G.; Mariani, A. Acute myocarditis following Comirnaty vaccination in a healthy man with previous SARS-CoV-2 infection. Radiol. Case Rep. 2021, 16, 3321–3325. [Google Scholar] [CrossRef] [PubMed]
- Shime, M.; Nozaki, Y.; Morita, A.; Ishiodori, T.; Murakami, T.; Yamasaki, H.; Yamamoto, M.; Takada, H. Life-threatening severe acute respiratory syndrome coronavirus-2 mRNA vaccine-associated myocarditis after COVID-19 myocarditis. J. Paediatr. Child Heal. 2023, 59, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Etuk, A.S.; Jackson, I.N.; Panayiotou, H. A Rare Case of Myocarditis After the First Dose of Moderna Vaccine in a Patient With Two Previous COVID-19 Infections. Cureus 2022, 14, e24802. [Google Scholar] [CrossRef]
- Zaveri, S.; Tagliaferri, A.R.; Woldemariam, S.; Aron, P.; Palacios, C.; Melki, G.; Michael, P. A Case of Multifactorial Viral Myocarditis. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Gholipour, M.; Samidoost, P.; Moayerifar, M.; Ghasemzadeh, G. A case report of QTc prolongation: Drug induced or myocarditis in Severe Acute Respiratory Syndrome Coronavirus 2. SAGE Open Med Case Rep. 2024, 12. [Google Scholar] [CrossRef]
- Al Harbi, S.; AlFaifi, M.; Al-Dorzi, H.M.; Aljuhani, O.; Alenazi, A.A.; Alalawi, M.; Al Sulaiman, K. A case report on the association between QTc prolongation and remdesivir therapy in a critically ill patient. IDCases 2022, 29, e01572. [Google Scholar] [CrossRef]
- Nabati, M.; Parsaee, H. Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A Literature Review. Cardiovasc. Toxicol. 2021, 22, 268–272. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.; et al. Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation 2022, 146, 743–754. [Google Scholar] [CrossRef]
- Bourdon, P.S.; Pantazatos, S.P. Why a major study on myocarditis risk following COVID vaccination should not influence public-health policy. Front. Med. 2023, 10, 1126945. [Google Scholar] [CrossRef] [PubMed]
- Stowe, J.; Miller, E.; Andrews, N.; Whitaker, H.J. Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A self-controlled case series analysis in England. PLOS Med. 2023, 20, e1004245. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A.; Royuela, A.; García-Gómez, S.; Gómez-Lozano, N.; Sánchez-Arjona, A.; de la Fuente, J.; Anel, J.; Sánchez-Galarraga, I.; Pérez-Redondo, M.; González, E.; et al. Association of SARS-CoV-2 immunoserology and vaccination status with myocardial infarction severity and outcome. Vaccine 2024, 42, 126305. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Lee, Y.; Heo, S.-J.; Kim, N.; Jung, I. The impact of COVID-19 status and vaccine type following the first dose on acute heart disease: A nationwide retrospective cohort study in South Korea. Epidemiology Infect. 2024, 152, e134. [Google Scholar] [CrossRef]
- Cianci, R.; Caldarelli, M.; Rio, P.; Pignataro, G.; Fernandez, M.S.; Ocarino, F.; Della Polla, D.A.; Franceschi, F.; Gasbarrini, A.; Gambassi, G.; et al. Outcomes of Patients with Heart Failure Hospitalized for COVID-19—A Study in a Tertiary Italian Center. Diseases 2024, 12, 337. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Our World In Data (OWID). Accessed 8 May 2025. https://ourworldindata.org/grapher/covid-vaccinationdoses-per-capita.
- Ndugga N, Hill L, Artiga S. COVID-19 Cases and Deaths, Vaccinations, and Treatments by Race/Ethnicity as of Fall 2022. KFF. 17 November 2022.
- https://www.kff.org/racial-equity-and-health-policy/issue-brief/covid-19-cases-and-deaths-vaccinationsand-treatments-by-race-ethnicity-as-of-fall-2022/.
- Goldstein, E. Mortality associated with Omicron and influenza infections in France before and during the COVID-19 pandemic. Epidemiology Infect. 2023, 151, 1–22. [Google Scholar] [CrossRef]
- Müller, S.; Schmetz, A.; Knaul, J.K.; Wilke, T.; Yang, J.; Dornig, S.; Lehmann, C.; Spinner, C.D. COVID-19 Disease Burden in the Omicron Variant-Dominated Endemic Phase: Insights from the ROUTINE-COV19 Study Using Real-World German Statutory Health Insurance Data. Viruses 2025, 17, 424. [Google Scholar] [CrossRef]
- Forthun, I.; Madsen, C.; Emilsson, L.; Nilsson, A.; Kepp, K.P.; Björk, J.; Vollset, S.E.; Lallukka, T.; Knudsen, A.K.S. Excess mortality in Denmark, Finland, Norway and Sweden during the COVID-19 pandemic 2020–2022. Eur. J. Public Heal. 2024, 34, 737–743. [Google Scholar] [CrossRef]
- Jang, M.; Kim, S.; Choi, S.; Ryu, B.; Choi, S.Y.; Choi, S.; An, M.; Kim, S.-S. Estimating Excess Mortality During the COVID-19 Pandemic Between 2020–2022 in Korea. J. Korean Med Sci. 2024, 39, e267. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Jang, H.; Oh, J. Excess mortality during the Coronavirus disease pandemic in Korea. BMC Public Heal. 2023, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, Y.; Zi, Y.; Zhu, Y. The shift of percent excess mortality from zero-COVID policy to living-with-COVID policy in Singapore, South Korea, Australia, New Zealand and Hong Kong SAR. Front. Public Heal. 2023, 11, 1085451. [Google Scholar] [CrossRef]
- Scherb, H.; Hayashi, K.; Nat. , 1.D.-M.D.R.; (Md), H.C.C.2.M.D. Annual All-Cause Mortality Rate in Germany and Japan (2005 to 2022) With Focus on The Covid-19 Pandemic: Hypotheses And Trend Analyses. Med. Clin. Sci. 2023, 5. [Google Scholar] [CrossRef]
- Durante, A.C.D.P.; Lacaza, R.; Lapitan, P.; Kochhar, N.; Tan, E.S.; Thomas, M. Mixed effects modelling of excess mortality and COVID-19 lockdowns in Thailand. Sci. Rep. 2024, 14, 1–15. [Google Scholar] [CrossRef]
- Okoro, E.; A Ikoba, N.; E Okoro, B.; Akpila, A.S.; O Salihu, M. Paradoxical increase in global COVID-19 deaths with vaccination coverage: World Health Organization estimates (2020–2023). Int. J. Risk Saf. Med. 2025. [Google Scholar] [CrossRef]
- Röltgen, K.; Boyd, S.D. Antibody and B Cell Responses to SARS-CoV-2 Infection and Vaccination: The End of the Beginning. Annu. Rev. Pathol. Mech. Dis. 2024, 19, 69–97. [Google Scholar] [CrossRef]
- Kelleni, M.T. Evolution of SARS CoV-2 Omicron subvariants BF.7 and XBB.1.5: Time to follow Africa and abort all COVID restrictions. J. Infect. 2023, 86, 405–405. [Google Scholar] [CrossRef]
- Kelleni, M.T. COVID-19 mortality paradox (United States vs Africa): Mass vaccination vs early treatment. World J. Exp. Med. 2024, 14, 88674. [Google Scholar] [CrossRef]
- Yan, V.K.C.; Zhang, Y.; Yang, D.; Li, X.; Mak, L.Y.; Lai, F.T.T.; Chui, C.S.L.; Wan, E.Y.F.; Wong, C.; Chan, S.C.W.; et al. Post-acute sequelae of hospitalised COVID-19 re-infection compared with hospitalised first-time infection: a population-based retrospective cohort study in Hong Kong. BMJ Public Heal. 2025, 3, e000833. [Google Scholar] [CrossRef]
- DeGrasse, D.C.; Black, S.D. The Rise of SARS-CoV-2 (COVID-19) Omicron Subvariant Pathogenicity. Cureus 2023, 15, e40148. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Sa, S.; Hong, M.; Kim, J.; Shim, S.R.; Han, H.W. Adverse Events and Safety Profile of the COVID-19 Vaccines in Adolescents: Safety Monitoring for Adverse Events Using Real-World Data. Vaccines 2022, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Rella, S.A.; Kulikova, Y.A.; Dermitzakis, E.T.; Kondrashov, F.A. Rates of SARS-CoV-2 transmission and vaccination impact the fate of vaccine-resistant strains. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chau, N.V.V.; Ngoc, N.M.; Nguyet, L.A.; Quang, V.M.; Ny, N.T.H.; Khoa, D.B.; Phong, N.T.; Toan, L.M.; Hong, N.T.T.; Tuyen, N.T.K.; et al. An observational study of breakthrough SARS-CoV-2 Delta variant infections among vaccinated healthcare workers in Vietnam. eClinicalMedicine 2021, 41, 101143. [Google Scholar] [CrossRef]
- Yamamoto, K. Adverse effects of COVID-19 vaccines and measures to prevent them. Virol. J. 2022, 19, 1–3. [Google Scholar] [CrossRef]
- Lamprinou, M.; Sachinidis, A.; Stamoula, E.; Vavilis, T.; Papazisis, G. COVID-19 vaccines adverse events: potential molecular mechanisms. Immunol. Res. 2023, 71, 356–372. [Google Scholar] [CrossRef]
- Lyons-Weiler, J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J. Transl. Autoimmun. 2020, 3. [Google Scholar] [CrossRef]
- Kim, S.-J.; Rhee, T.G.; Shim, S.R. Autoimmune and auto-inflammatory adverse events after COVID-19 vaccination in the United States. Clin. Immunol. 2023, 259, 109882. [Google Scholar] [CrossRef]
- Xu, L.; Ma, Z.; Li, Y.; Pang, Z.; Xiao, S. Antibody dependent enhancement: Unavoidable problems in vaccine development. Adv Immunol. 2021;151:99-133. [CrossRef]
- Ajmeriya, S.; Kumar, A.; Karmakar, S.; Rana, S.; Singh, H. Neutralizing Antibodies and Antibody-Dependent Enhancement in COVID-19: A Perspective. J. Indian Inst. Sci. 2022, 102, 671–687. [Google Scholar] [CrossRef]
- Plūme, J.; Galvanovskis, A.; Šmite, S.; Romanchikova, N.; Zayakin, P.; Linē, A. Early and strong antibody responses to SARS-CoV-2 predict disease severity in COVID-19 patients. J. Transl. Med. 2022, 20, 1–12. [Google Scholar] [CrossRef]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef]
- Sumantri, S.; Rengganis, I. Immunological dysfunction and mast cell activation syndrome in long COVID. Asia Pac. Allergy 2023, 13, 50–53. [Google Scholar] [CrossRef]
- Gorman, R.d.S.; Syed, I.U. Connecting the Dots in Emerging Mast Cell Research: Do Factors Affecting Mast Cell Activation Provide a Missing Link between Adverse COVID-19 Outcomes and the Social Determinants of Health? Med Sci. 2022, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Bossche, GV. The inescapable immune escape pandemic. Pierucci Publishing, Aspen, CO. 2023. https://www.boswellbooks.com/book/9781956257809.
- Mead, M.N.; Seneff, S.; Rose, J.; Wolfinger, R.; Hulscher, N.; McCullough, P.A. COVID-19 Modified mRNA “Vaccines”: Lessons Learned from Clinical Trials, Mass Vaccination, and the Bio-Pharmaceutical Complex, Part 2. Int. J. Vaccine Theory, Pr. Res. 2024, 3, 1275–1344. [Google Scholar] [CrossRef]
- Kanduc, D.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol. Res. 2020, 68, 310–313. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases. Front. Immunol. 2021, 11, 617089. [Google Scholar] [CrossRef]
- Güven, O.; Karakurt, G.; Naser, A.; Selçuk, H.; Keleş, D.V.; Gedik, E.; Avsever, M.; Köse, F.F. The Impact of COVID-19 Infection on the Development of Stroke, Pulmonary Embolism, and Myocardial Infarction: A Retrospective Study. Cureus 2025, 17. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Makary, M.A. An Evidence-Based Approach to Covid-19 Vaccination. New Engl. J. Med. 2025, 392, 2484–2486. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration; Health & Human Services. Guidance for Industry. E 10 Choice of Control Group and Related Issues in Clinical Trials. May 2001. URL: https://www.fda.gov/media/71349/download.
- Taccetta, C. The “Noise” of mRNA. The Daily Clout. 9 July 2025. URL: https://dailyclout.io/the-noise-ofmrna/.
- Le, J. Vivian EM. Overview of Pharmacokinetics. Merck Manual, Professional Version. Reviewed/Revised Nov 2024. URL: https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/overview-of-pharmacokinetics.
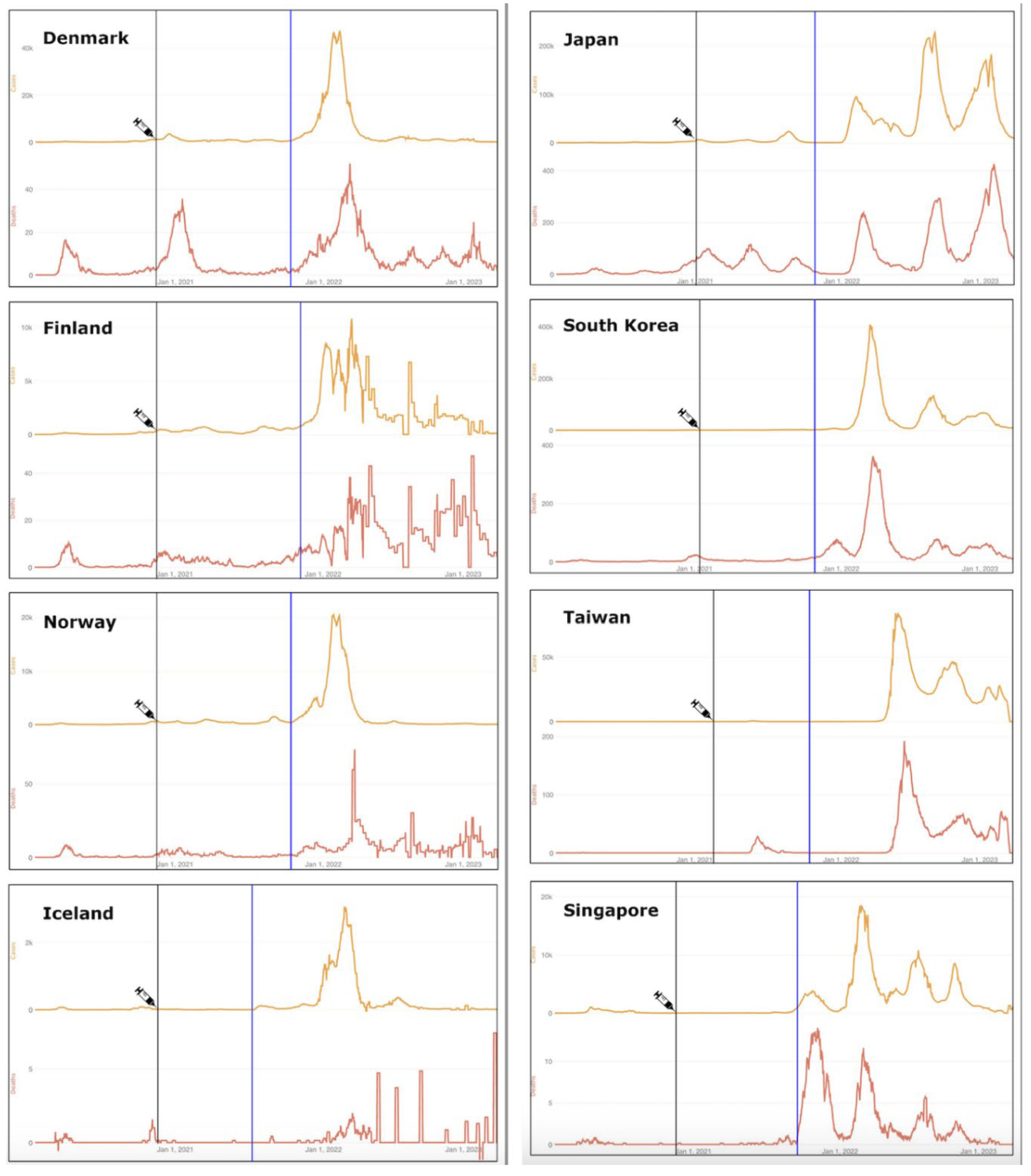
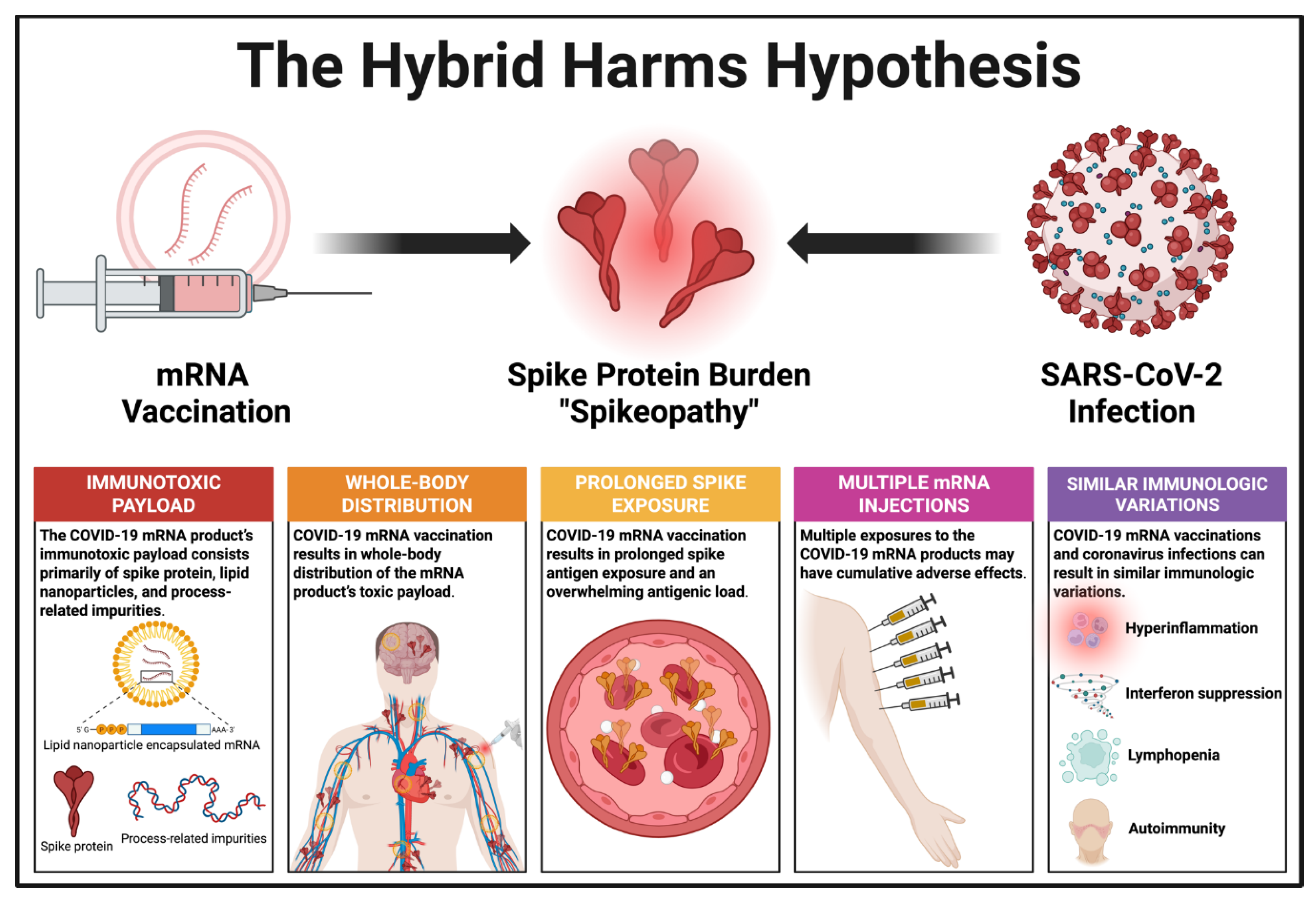
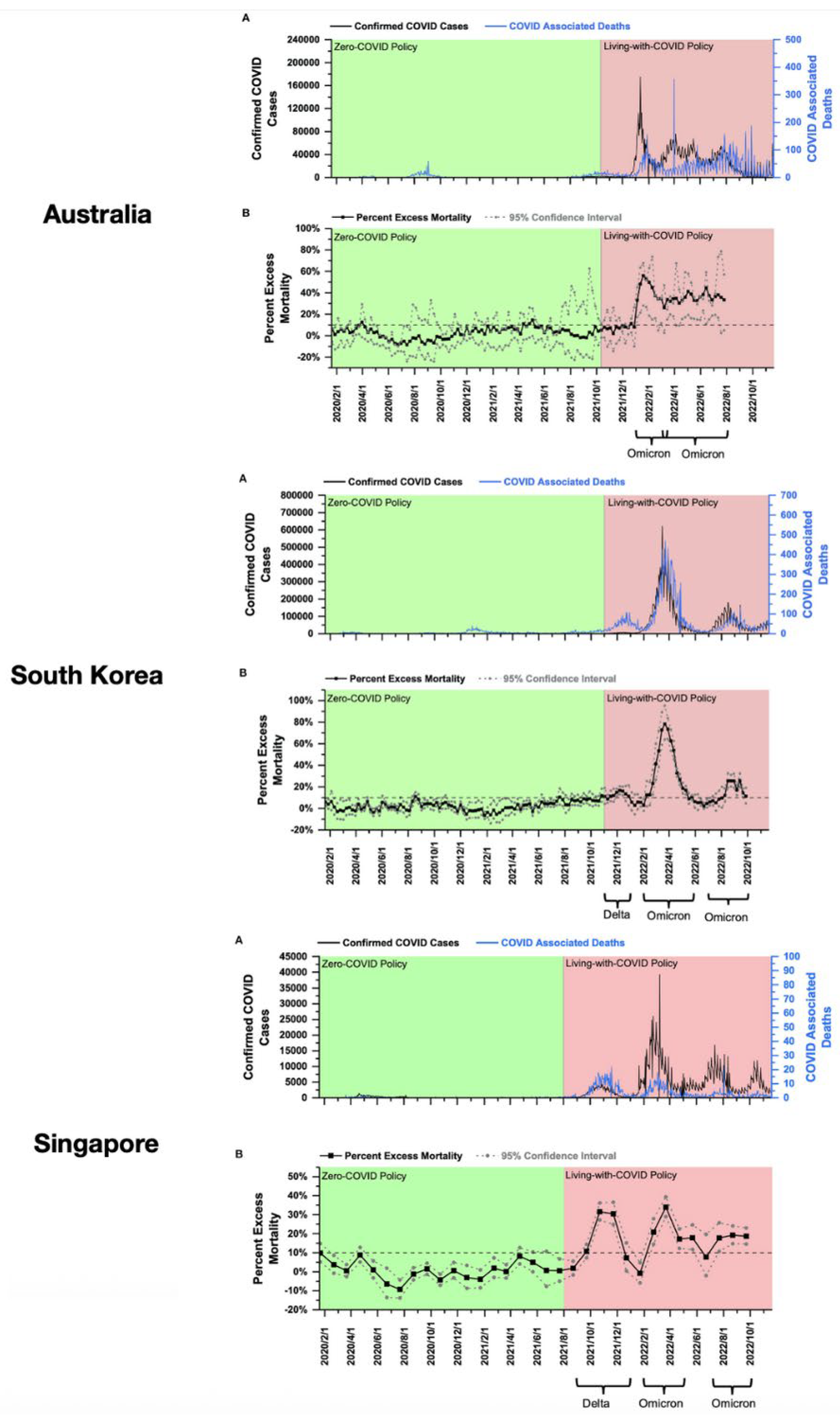
| Organ system | PASC/PCVS Combined | Organ system | PASC/PCVS Combined |
|---|---|---|---|
| Cardiovascular |
|
Gastrointestinal |
|
| Coagulation/ Hematological |
|
Gynecological |
|
| Dermatological |
|
Mental Health |
|
| Endocrine |
|
Musculoskeletal |
|
| Immunological/ Autoimmune |
|
Neurological |
|
| Otolaryngological |
|
Pulmonary |
|
| Renal |
|
||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





