Submitted:
28 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design and participants
- 1)
- BBIP-CorV primed/ PastoCovac Plus boosted (BP)
- 2)
- BBIP-CorV primed / PastoCovac boosted (BPa)
- 3)
- BBIP-CorV primed / BBIP-CorV boosted (BB)
2.2. Antibodies response
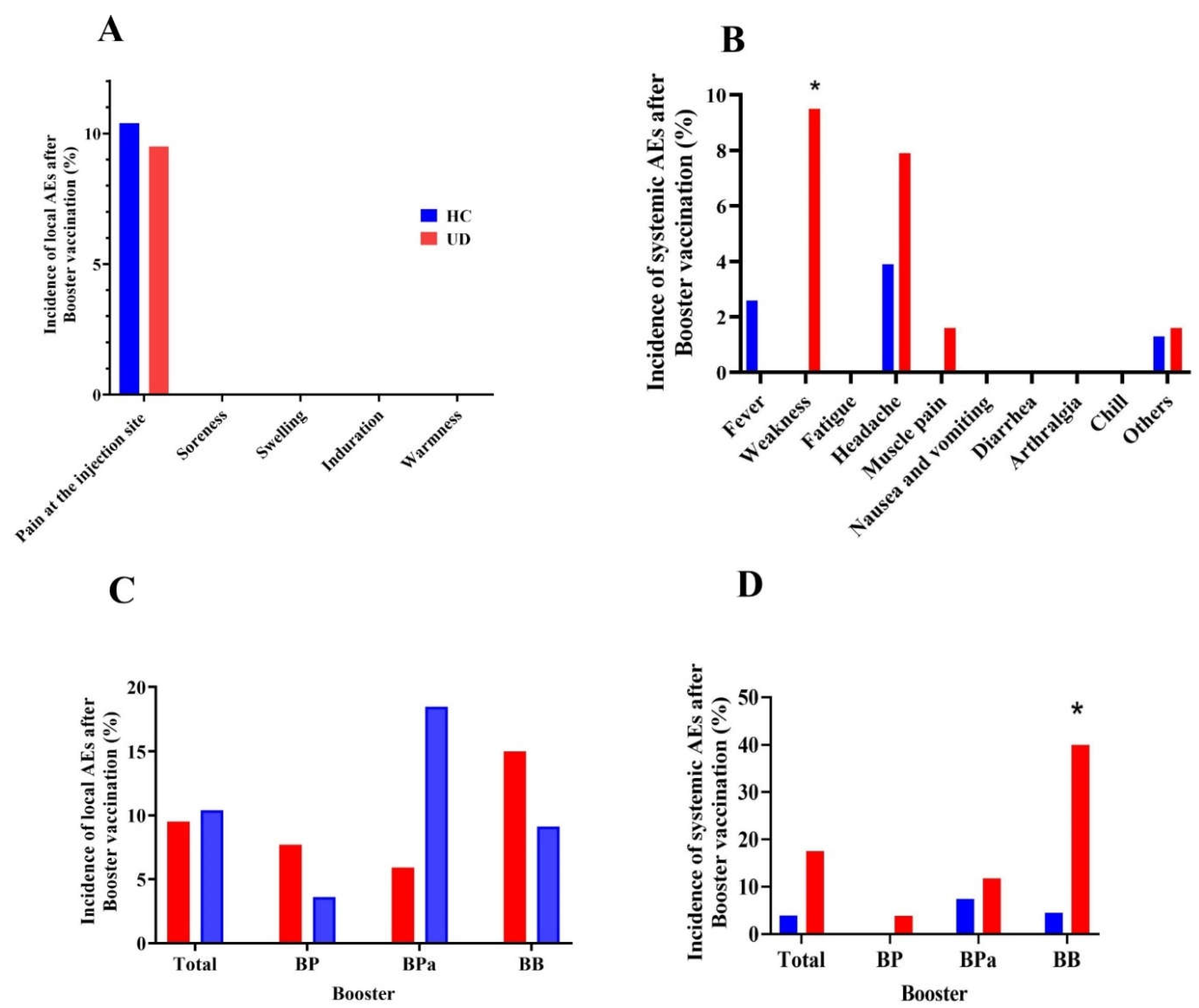
2.3. Safety Assessment
2.4. Statistical analysis
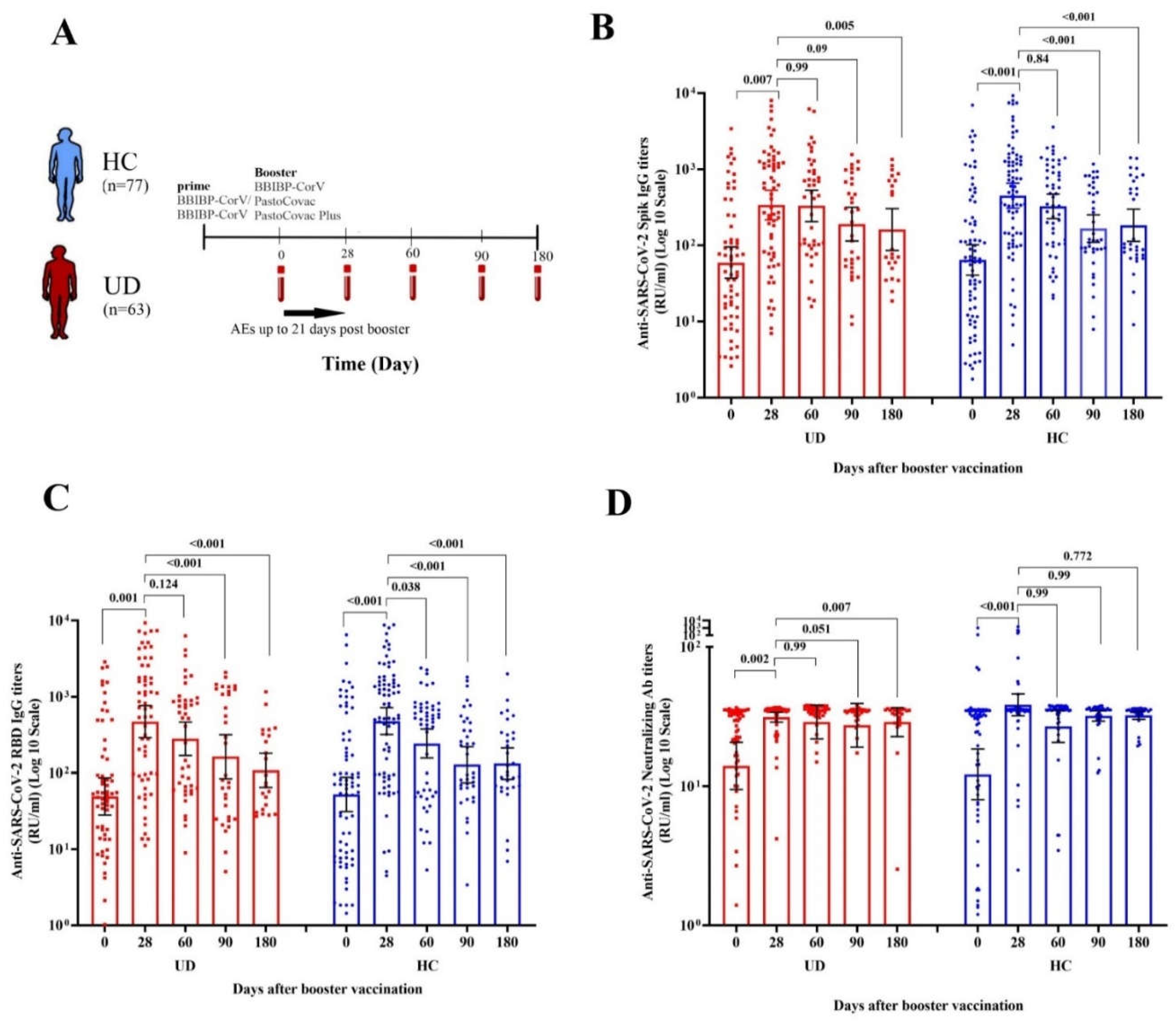
3. Results
3.1. Study population
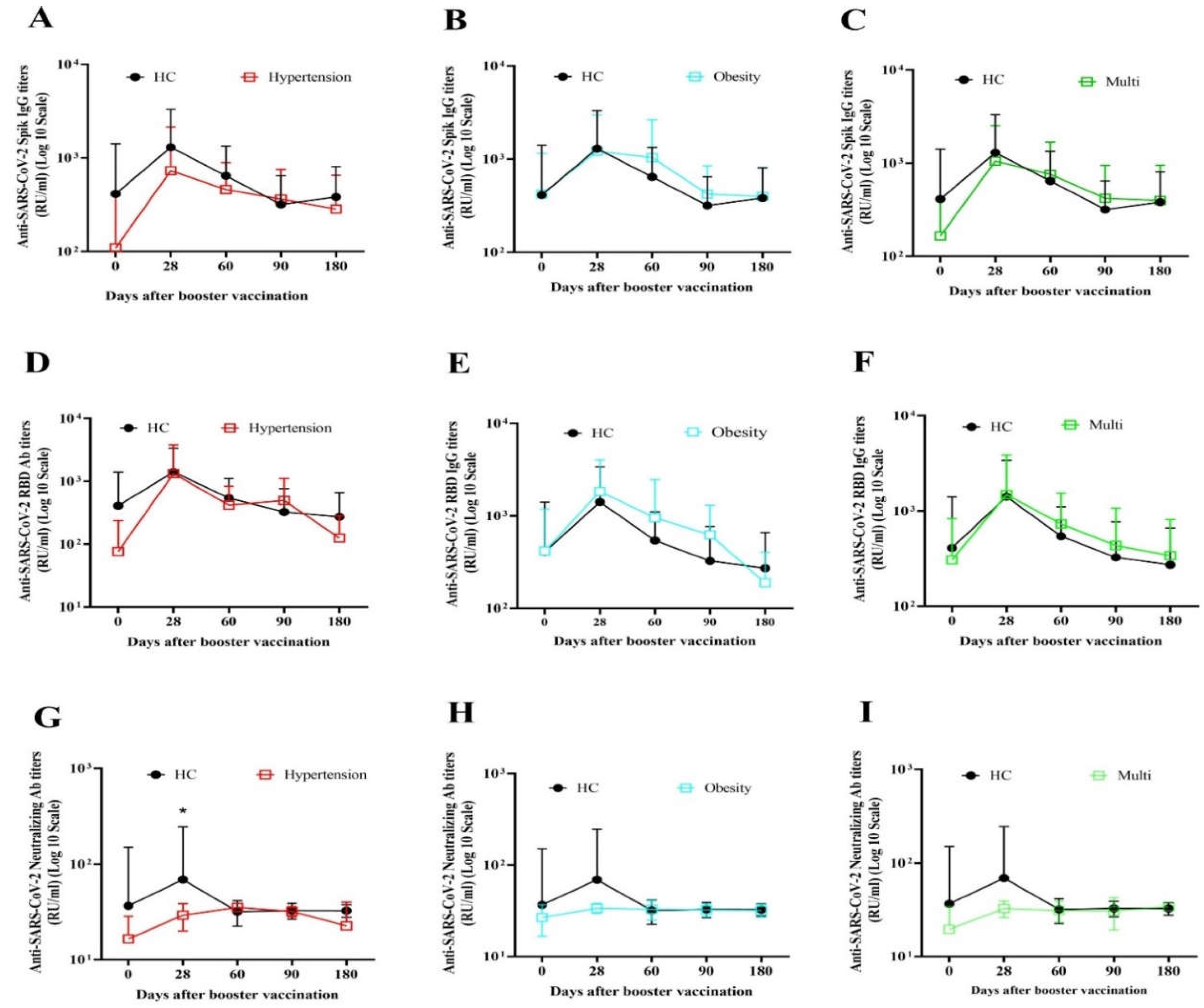
3.2.1. Immunogenicity and antibody persistency of boosters among people with underlying diseases
3.3. Comparative safety outcomes of COVID-19 boosters between UD and HC groups
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability Statement
Conflicts of interest
References
- WHO. Coronavirus (COVID-19) Dashboard. 2023:https://covid19.who.int/.
- WHO. WHO Health Emergency Dashboard.https://covid19.who.int/region/emro/country/ir.
- 2023.
- CDC. End of the Federal COVID-19 Public Health Emergency (PHE) Declaration [Internet]. 2023.
- Yin T, Li Y, Ying Y, Luo Z. Prevalence of comorbidity in Chinese patients with COVID-19: systematic review and meta-analysis of risk factors. BMC infectious diseases. 2021;21:1-13. [CrossRef]
- Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Archives of academic emergency medicine. 2020;8(1).
- Huynh G, Nguyen TV, Nguyen DD, Lam QM, Pham TN, Nguyen HTN. Knowledge about COVID-19, beliefs and vaccination acceptance against COVID-19 among high-risk people in Ho Chi Minh City, Vietnam. Infection and drug resistance. 2021:1773-80. [CrossRef]
- Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713-21. e9. [CrossRef]
- Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes/metabolism research and reviews. 2022;38(1):e3465.
- Zhu Q, Zhang Y, Kang J, Chen Z, Peng M, Chen M, et al. Weakened humoral and cellular immune response to the inactivated COVID-19 vaccines in Chinese individuals with obesity/overweight. Genes & Diseases. 2022. [CrossRef]
- Malavazos AE, Basilico S, Iacobellis G, Milani V, Cardani R, Boniardi F, et al. Antibody responses to BNT162b2 mRNA vaccine: infection-naïve individuals with abdominal obesity warrant attention. Obesity. 2022;30(3):606-13. [CrossRef]
- Dicker D, Golan R, Baker JL, Busetto L, Frühbeck G, Goossens GH, et al. Vaccinating people with obesity for COVID-19: EASO call for action. Obesity Facts. 2021;14(3):334. [CrossRef]
- Sauré D, O'Ryan M, Torres JP, Zuniga M, Santelices E, Basso LJ. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. The Lancet Infectious Diseases. 2022;22(1):56-63. [CrossRef]
- Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, et al. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39(44):6492-509. [CrossRef]
- Karamese M, Tutuncu EE. The effectiveness of inactivated SARS-CoV-2 vaccine (CoronaVac) on antibody response in participants aged 65 years and older. Journal of Medical Virology. 2022;94(1):173-7. [CrossRef]
- Ali H, Alterki A, Sindhu S, Alahmad B, Hammad M, Al-Sabah S, et al. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Frontiers in immunology. 2021:4909. [CrossRef]
- Soegiarto G, Wulandari L, Purnomosari D, Fahmita KD, Gautama HI, Hadmoko ST, et al. Hypertension is associated with antibody response and breakthrough infection in health care workers following vaccination with inactivated SARS-CoV-2. Vaccine. 2022;40(30):4046-56. [CrossRef]
- Pellini R, Venuti A, Pimpinelli F, Abril E, Blandino G, Campo F, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36:100928. [CrossRef]
- Ai J, Zhang H, Zhang Q, Zhang Y, Lin K, Fu Z, et al. Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and Variants of Concern. Cell research. 2022;32(1):103-6. [CrossRef]
- Cao Y, Hao X, Wang X, Wu Q, Song R, Zhao D, et al. Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine. Cell research. 2022;32(1):107-9. [CrossRef]
- Toledo-Romaní ME, García-Carmenate M, Valenzuela-Silva C, Baldoquín-Rodríguez W, Martínez-Pérez M, Rodríguez-González M, et al. Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of a heterologous three-dose combination with SOBERANA-Plus: a double-blind, randomised, placebo-controlled phase 3 clinical trial. The Lancet Regional Health–Americas. 2023;18. [CrossRef]
- Toledo-Romani ME, García-Carmenate M, Verdecia-Sánchez L, Pérez-Rodríguez S, Rodriguez-González M, Valenzuela-Silva C, et al. Safety and immunogenicity of anti-SARS CoV-2 conjugate vaccine SOBERANA 02 in a two-dose or three-dose heterologous scheme in adults: Phase IIb Clinical Trial. MedRxiv. 2022:2022.01. 01.21268271. [CrossRef]
- Sadat Larijani M, Sorouri R, Eybpoosh S, Doroud D, Moradi L, Ahmadinezhad M, et al. Assessment of long-term adverse events regarding different COVID-19 vaccine regimens within an 18-month follow-up study. Pathogens and Disease. 2023:ftad010. [CrossRef]
- Puga-Gómez R, Ricardo-Delgado Y, Rojas-Iriarte C, Céspedes-Henriquez L, Piedra-Bello M, Vega-Mendoza D, et al. Open label phase I/II clinical trial and predicted efficacy of SARS-CoV-2 RBD protein vaccines SOBERANA 02 and SOBERANA Plus in children. medRxiv. 2022:2022.03. 03.22271313. [CrossRef]
- Mafinezhad S, Bayani G, Ehteshammanesh H, Langari M, Shokrollahi N, Bozorgnia Y, et al. Evaluation of the side effects of Sinopharm and PastoCovac COVID-19 vaccines in children aged 5-12 years in Iran. International Journal of Pediatrics. 2023;11(4):17572-82.
- CDC. Diabetes Tests. 2023:https://www.cdc.gov/diabetes/basics/getting-tested.html#:~:text=A%20fasting%blood%sugar%level,higher%indicates%you%have%diabetes.
- CDC. High Blood Pressure Symptoms and Causes. 2021:https://www.cdc.gov/bloodpressure/about.htm.
- Muangnoicharoen S, Wiangcharoen R, Nanthapisal S, Kamolratakul S, Lawpoolsri Niyom S, Jongkaewwattana A, et al. Single Ad26. COV2. S Booster Dose Following Two Doses of BBIBP-CorV Vaccine Against SARS-CoV-2 Infection in Adults: Day 28 Results of a Phase 1/2 Open-Label Trial. 2023.
- Amellal H, Assaid N, Akarid K, Maaroufi A, Ezzikouri S, Sarih Mh. Mix-and-match COVID-19 vaccines trigger high antibody response after the third dose vaccine in Moroccan health care workers. Vaccine: X. 2023;14:100288. [CrossRef]
- Zhang Y, Chen H, Lv J, Huang T, Zhang R, Zhang D, et al. Evaluation of Immunogenicity and Safety of Vero Cell-Derived Inactivated COVID-19 Vaccine in Older Patients with Hypertension and Diabetes Mellitus. Vaccines. 2022;10(7):1020. [CrossRef]
- Li C, Bi H, Fu Z, Li A, Wan N, Hu J, et al. Retrospective study of the immunogenicity and safety of the CoronaVac SARS-CoV-2 vaccine in people with underlying medical conditions. Communications Medicine. 2022;2(1):151.
- Pellini R, Venuti A, Pimpinelli F, Abril E, Blandino G, Campo F, et al. Obesity may hamper SARS-CoV-2 vaccine immunogenicity. MedRXiv. 2021:2021.02. 24.21251664. [CrossRef]
- Rifai A, Wahono CS, Pratama MZ, Handono K, Susianti H, Iskandar A, et al. Association between the effectiveness and immunogenicity of inactivated SARS-CoV2 vaccine (CoronaVac) with the presence of hypertension among health care workers. Clinical and Experimental Hypertension. 2022;44(3):233-9. [CrossRef]
- Toledo-Romani ME, Garcia-Carmenate M, Silva CV, Baldoquin-Rodriguez W, Pérez MM, Gonzalez MR, et al. Efficacy and safety of SOBERANA 02, a COVID-19 conjugate vaccine in heterologous three-dose combination. MedRxiv. 2021. [CrossRef]
- Pellini R, Venuti A, Pimpinelli F, Abril E, Blandino G, Campo F, et al. Early onset of SARS-CoV-2 antibodies after first dose of BNT162b2: Correlation with age, gender and BMI. Vaccines. 2021;9(7):685. [CrossRef]
- Míguez HM, García IM, Gómez MO, Merino IMG, Cano EL, De La Torre A. Immunogenicity, effectiveness and safety of COVID-19 vaccine in older adults living in nursing homes: A real-life study. Revista Española de Geriatría y Gerontología. 2023. [CrossRef]
- Bruel T, Pinaud L, Tondeur L, Planas D, Staropoli I, Porrot F, et al. Neutralising antibody responses to SARS-CoV-2 omicron among elderly nursing home residents following a booster dose of BNT162b2 vaccine: A community-based, prospective, longitudinal cohort study. EClinicalMedicine. 2022;51:101576. [CrossRef]
- Shapiro JR, Sitaras I, Park HS, Aytenfisu TY, Caputo C, Li M, et al. Association of Frailty, Age, and Biological Sex With Severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA Vaccine–Induced Immunity in Older Adults. Clinical Infectious Diseases. 2022;75(Supplement_1):S61-S71. [CrossRef]
- Gaborit B, Fernandes S, Loubet P, Ninove L, Dutour A, Cariou B, et al. Early humoral response to COVID-19 vaccination in patients living with obesity and diabetes in France. The COVPOP OBEDIAB study with results from the ANRS0001S COV-POPART cohort. Metabolism. 2023;142:155412. [CrossRef]
- Fathi M, Vakili K, Sayehmiri F, Mohamadkhani A, Hajiesmaeili M, Rezaei-Tavirani M, et al. The prognostic value of comorbidity for the severity of COVID-19: A systematic review and meta-analysis study. PloS one. 2021;16(2):e0246190. [CrossRef]
- Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. The Journal of Immunology. 2017;198(10):4046-53. [CrossRef]
- Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. International Journal of Infectious Diseases. 2016;49:129-33. [CrossRef]
- Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, et al. Declined antibody responses to COVID-19 mRNA vaccine within first three months. medRxiv. 2021:2021.04. 19.21255714. [CrossRef]
- Bignucolo A, Scarabel L, Mezzalira S, Polesel J, Cecchin E, Toffoli G. Sex disparities in efficacy in COVID-19 vaccines: a systematic review and meta-analysis. Vaccines. 2021;9(8):825. [CrossRef]
- Erdem MG, Unlu O, Buber S, Demirci M, Kocazeybek BS. Could Prior COVID-19 Affect the Neutralizing Antibody after the Third BNT162b2 Booster Dose: A Longitudinal Study. Vaccines. 2023;11(3):560. [CrossRef]
- Salimian J, Ahmadi A, Amani J, Olad G, Halabian R, Saffaei A, et al. Safety and immunogenicity of a recombinant receptor-binding domain-based protein subunit vaccine (Noora vaccine™) against COVID-19 in adults: A randomized, double-blind, placebo-controlled, Phase 1 trial. Journal of Medical Virology. 2023;95(2). [CrossRef]
- Heidary M, Kaviar VH, Shirani M, Ghanavati R, Motahar M, Sholeh M, et al. A comprehensive review of the protein subunit vaccines against COVID-19. Frontiers in microbiology. 2022;13:927306. [CrossRef]
- Kaabi NA, Yang YK, Zhang J, Xu K, Liang Y, Kang Y, et al. Immunogenicity and safety of NVSI-06-07 as a heterologous booster after priming with BBIBP-CorV: a phase 2 trial. Signal Transduction and Targeted Therapy. 2022;7(1):172. [CrossRef]
- Ramezani A, Sorouri R, Haji Maghsoudi S, Dahmardeh S, Doroud D, Sadat Larijani M, et al. PastoCovac and PastoCovac Plus as protein subunit COVID-19 vaccines led to great humoral immune responses in BBIP-CorV immunized individuals. Scientific Reports. 2023;13(1):8065. [CrossRef]
- Li C, Li A, Bi H, Hu J, Yang F, Zhou T, et al. Immunogenicity and safety of the CoronaVac inactivated SARS-CoV-2 vaccine in people with underlying medical conditions: a retrospective study. medRxiv. 2022:2022.04. 28.22274402. [CrossRef]
| Total N(%) |
HC N(%) |
UD N(%) |
|
|---|---|---|---|
| Gender | |||
| Female | 74 (52.9%) | 41 (53.2%) | 33 (52.4%) |
| Male | 66 (47.1%) | 36(46.8%) | 30 (47.6%) |
| Age | |||
| ≤ 40 | 57 (40.7%) | 38 (49.4%) | 19 (30.2%) |
| > 40 | 83 (59.3%) | 39 (50.6%) | 44 (69.8%) |
| COVID-19 History | |||
| No | 113 (80.7) | 59 (76.9) | 54 (85.7) |
| Yes | 27 (19.3) | 18 (23.4) | 9 (14.3) |
| Booster | |||
| BP | 54 (38.6%) | 28 (36.4%) | 26 (41.3%) |
| BPa | 44 (31.4%) | 27 (35.1%) | 17 (27.0) |
| BB | 42 (30.0) | 22 (28.6%) | 20 (31.7%) |
| HC | UD | P value | |
|---|---|---|---|
| Anti-SARS-CoV-2 Spike antibody (Geometric mean, 95% CI) | |||
| Antibodies titers | |||
| Day 0 | 64.26 (40.53-101.88) | 59.22 (36.87-95.12) | 0.86 |
| Day 28 | 449.52 (301.75-656.59) | 339.09 (216.83-530.28) | 0.38 |
| Day 60 | 325.87 (224.88-472.21) | 330.00 (205.34-530.34) | 0.97 |
| Day 90 | 166.11 (109.63-251.69) | 189.99 (113.92-316.86) | 0.79 |
| Day 180 | 183.41 (112.74-298.35) | 161.22 (85.65-303.49) | 0.39 |
| Mean Fold rise | |||
| Day 28 | 7.00 (5.01-9.76) | 5.72 (3.92-8.37) | 0.31 |
| Day 60 | 3.88 (2.33-6.46) | 4.27 (2.35-7.77) | 0.75 |
| Day 90 | 1.67 (0.95-2.94) | 2.88 (1.29-6.45) | 0.25 |
| Day 180 | 1.54 (0.69-3.41) | 1.61 (0.56-4.65) | 0.98 |
| Neutralizing antibody (Geometric mean, 95% CI) | |||
| Antibodies titers | |||
| Day 0 | 12.16 (8.01-18.47) | 13.97 (9.47-20.62) | 0.78 |
| Day 28 | 38.31 (31.95-45.95) | 31.27 (28.79-33.96) | 0.054 |
| Day 60 | 26.84 (20.61-34.96) | 28.85 (21.82-38.15) | 0.90 |
| Day 90 | 31.91 (29.31-34.74) | 27.32 (19.04-39.22) | 0.10 |
| Day 180 | 32.22 (30.14-34.45) | 28.79 (22.66-36.57) | 0.34 |
| Mean Fold rise | |||
| Day 28 | 9.14 (5.98- 13.96) | 9.52 (5.24-19.30) | 0.55 |
| Day 60 | 3.51 (2.01-6.12) | 4.10 (2.04-8.23) | 0.95 |
| Day 90 | 1.78 (1.22-2.60) | 1.55 (0.87-2.78) | 0.32 |
| Day 180 | 1.52 (1.17-1.97) | 1.66 (0.80-3.45) | 0.83 |
| Anti-SARS-CoV-2 RBD antibody (Geometric mean, 95% CI) | |||
| Antibodies titers | |||
| Day 0 | 52.38 (31.24-87.83) | 49.12 (28.07-85.95) | 0.88 |
| Day 28 | 478.74 (319.48-717.38) | 470.89 (290.65-762.90) | 0.98 |
| Day 60 | 242.97 (156.99-376.03) | 279.99 (169.23-463.24) | 0.34 |
| Day 90 | 128.02 (74.30-220.57) | 163.21 (84.18-316.43) | 0.77 |
| Day 180 | 132.60 (82.72-212.55) | 107.96 (64.67-180.24) | 0.34 |
| Mean Fold rise | |||
| Day 28 | 3.15 (2.13-4.66) | 2.23 (1.52-3.27) | 0.27 |
| Day 60 | 1.61 (1.03-2.49) | 1.78 (1.19-2.66) | 0.54 |
| Day 90 | 1.31 (0.66-2.61) | 3.70 (1.27-10.78) | 0.39 |
| Day 180 | 1.05 (0.44-2.54) | 1.29 (0.37-4.45) | 0.11 |
| Day 28 | Day 60 | Day 90 | Day 180 | |||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | P | Median (IQR) | P | Median (IQR) | P | Median (IQR) | P | |
| Anti-SARS-CoV-2 Spike antibody median fold rise (IQR) | ||||||||
| Age | ||||||||
| ≤ 40 | 1.61 (6.07) | 0.02 | 2.73(9.32) | 0.55 | 1.37(4.77) | 0.22 | 0.92(3.30) | 0.36 |
| > 40 | 7.69 (14.26) | 4.12(25.18) | 3.90(14.19) | 2.77(17.39) | ||||
| Gender | ||||||||
| Female | 3.45(11.14) | 0.3 | 1.90(5.53) | 0.08 | 1.33(11.02) | 0.13 | 0.86(9.89) | 0.33 |
| Male | 5.80(18.23) | 6.84(30.94) | 5.02(11.19) | 3.49(14.48) | ||||
| COVID-19 History | ||||||||
| No | 4.58(12.96) | 0.62 | 4.04(17.82) | 0.19 | 3.86(11.04) | 0.63 | 1.86(14.40) | 0.66 |
| Yes | 4.30(11.63) | 1.14(25.51) | 2.37(12.60) | 0.75(18.53) | ||||
| Types of UD | ||||||||
| Hypertension | 9.39(34.58) | 0.21 | 4.12(19.25) | 0.91 | 5.26(86) | 0.54 | 19.53() | 0.25 |
| Obesity | 3.92(10.58) | 2.45(25.18) | 3.86(12.44) | 0.92(14.43) | ||||
| Multi | 4.48(14.03) | 3.56(6.34) | 1.55(4.73) | 0.98(2.28) | ||||
| Booster | ||||||||
| BP | 11.01 (36.75) | <0.001 | 6.07 (31.60) | 0.33 | 5.62 (110.79) | 0.53 | 1.43 (64.41) | 0.83 |
| BPa | 11.34 (20.25) | 6.05 (28.82) | 4.42 (11.83) | 3.50 (14.96) | ||||
| BB | 1.55 (1.19) | 2.18 (2.53) | 1.45 (2.86) | 1.14 (1.95) | ||||
| Anti-SARS-CoV-2 Neutralizing antibody median fold rise (IQR) | ||||||||
| Age | ||||||||
| ≤ 40 | 1.05(0.52) | 0.19 | 1.05(0.43) | 0.28 | 1(0.46) | 0.69 | 0.96(0.16) | 0.36 |
| > 40 | 1.18(1.81) | 1.11(0.85) | 1.12(0.69) | 1.03(0.67) | ||||
| Gender | ||||||||
| Female | 1.09(1.16) | 0.4 | 1.05 (0.13) | 0.03 | 1(0.19) | 0.17 | 0.99(0.20) | 0.92 |
| Male | 1.14(1.54) | 1.23 (1.39) | 1.46(0.68) | 1.01(11.86) | ||||
| COVID-19 History | ||||||||
| No | 1.12(1.38) | 0.11 | 1.10(0.96) | 0.61 | 1.10(0.71) | 0.30 | 0.98(0.58) | 0.84 |
| Yes | 1.02(0.15) | 1.08(0.16) | 0.99(0.15) | 1.10(0.23) | ||||
| Types of UD | ||||||||
| Hypertension | 1.60 (2.03) | 1.18(1.03) | 0.57 | 1.57(170.96) | 0.73 | 0.96() | 0.94 | |
| Obesity | 1.09 (0.39) | 0.04 | 1.08(0.19) | 1.10(0.54) | 1.01(0.37) | |||
| Multi | 1.50 (8.13) | 1.13(4.80) | 0.99(2.77) | 1.01(11.47) | ||||
| Booster | ||||||||
| BP | 1.61 (3.04) | 1.05(1.18) | 0.1 | 1.50(2.19) | 0.29 | 1.03(60.69) | 0.42 | |
| BPa | 1.11 (0.61) | 0.01 | 1.20(0.90) | 1.11(0.63) | 1.01(0.59) | |||
| BB | 1.03 (0.14) | 1.03(0.18) | 1(0.10) | 0.96(0.10) | ||||
| Anti-SARS-CoV-2 RBD antibody median fold rise (IQR) | ||||||||
| Age | ||||||||
| ≤ 40 | 2.28(32.18) | 0.73 | 1.36(11.32) | 0.35 | 0.35(9.53) | 0.05 | 0.35(0.17) | 0.12 |
| > 40 | 5.10(29.58) | 4.22(18.20) | 8.73(20.61) | 1.18(11.28) | ||||
| Gender | ||||||||
| Female | 2.29(30.07) | 0.07 | 1.28 (4.23) | 0.02 | 0.82(19.25) | 0.24 | 0.039(2.54) | 0.47 |
| Male | 10.75(36.62) | 9.70(46.30) | 9.39(21.23) | 0.72(20.92) | ||||
| COVID-19 History | ||||||||
| No | 4.69(34.15) | 0.78 | 3.19(17.45) | 0.35 | 1.43(22.66) | 0.53 | 0.52(4.61) | 0.96 |
| Yes | 6.38(27.27) | 0.98(15.66) | 1.10(19.35) | 0.66(5.26) | ||||
| Types of UD | ||||||||
| Hypertension | 6.38(38.46) | 0.66 | 2.17(21.07) | 0.92 | 11.56(192.97) | 0.32 | 37.53() | 0.18 |
| Obesity | 5.10(29.81) | 1.80(17.13) | 1.62(19.23) | 0.40(2.99) | ||||
| Multi | 4.63(17.70) | 1.95(9.35) | 0.65(13.25) | 0.38(2.58) | ||||
| Booster | ||||||||
| BP | 15.26 (121.92) | 4.74 (20.33) | 11.57 (151.55) | 0.54 (34.19) | 0.72 | |||
| BPa | 13.85 (23.06) | <0.001 | 9.83 (45.09) | 0.03 | 8.73 (19.05) | 0.03 | 0.95 (5.66) | |
| BB | 1.26 (0.57) | 1.05 (0.94) | 0.34 (0.67) | 0.39 (0.55) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





