Submitted:
13 August 2025
Posted:
14 August 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Pre-Formulative Study
2.1.1. Optimization and Selection of the Buffer Solution
| Buffer | Na₂HPO₄. 7H₂O (mM) |
Citric acid (mM) |
NaCl (mM) |
CaCl₂ (mM) |
KCl (mM) |
Mannitol (mM) |
Monovalent cations (mM) |
Ca²⁺ (mM) |
Total cations (mM) |
|---|---|---|---|---|---|---|---|---|---|
| BS1 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 40.49 | 0.76 | 41.25 |
| BS2 | 10.00 | 0.73 | 1.71 | 2.00 | 18.78 | 217.38 | 40.49 | 2.00 | 42.49 |
| BS3 | 10.00 | 0.73 | 1.71 | - | 18.78 | 217.38 | 40.49 | - | 40.49 |
| Formulations | Cations (mM) |
Mannitol (mM) |
pH | Osmolality (mOsmol/kg) | Viscosity (mPa . s) |
|
|---|---|---|---|---|---|---|
| Before ATF dilution | After ATF dilution | |||||
| GG(0.1)/BS1 | 41.25 | 217.38 | 6.45±0.02 | 299±0.58 | 28.62±0.66 | 31.27±0.64* |
| GG(0.1/BS2 | 42.49 | 217.38 | 6.97±0.01 | 297±0.58 | 28.54±2.11 | 26.48± 1.09 |
| GG(0.1)/BS3 | 40.49 | 217.38 | 6.73±0.01 | 302±1.00 | 2.18±1.02 | 8.97±0.99 |
2.1.2. Characterization of GG-Based Formulations and Their Mixtures with AG
| Formulation | GG (% w/w) |
AG (% w/w) |
Na₂HPO₄ . 7H₂O (mM) |
Citric acid (mM) |
NaCl (mM) | CaCl₂ (mM) |
KCl (mM) |
Mannitol (mM) |
EDTA (% w/w) |
BAK (% w/w) |
|---|---|---|---|---|---|---|---|---|---|---|
| GG(0.05) | 0.05 | - | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.05)/AG(0.2) | 0.05 | 0.2 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.05)/AG(0.3) | 0.05 | 0.3 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.1) | 0.1 | - | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.1)/AG(0.2) | 0.1 | 0.2 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.1)/AG(0.3) | 0.1 | 0.3 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.2) | 0.2 | - | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.2)/AG(0.2) | 0.2 | 0.2 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| GG(0.2)/AG(0.3) | 0.2 | 0.3 | 10.00 | 0.73 | 1.71 | 0.76 | 18.78 | 217.38 | 0.05 | 0.005 |
| Formulation | pH (±SE) |
Osmolality (mOsmol/kg ±SE) | Wettability (θ, ° ±SE) Before After ATF dilution ATF dilution |
Viscosity (mPa . s ±SE) Before After ATF dilution ATF dilution |
Increase factor (IF) |
IF mean |
||
|---|---|---|---|---|---|---|---|---|
| GG(0.05) | 6.18±0.03 | 302.0±1.00 | 56.10±2.96 | 54.50±1.01 | 9.05±1.12 | 14.27±0.83 | 1.58 | |
| GG(0.05)/AG(0.2) | 6.12±0.02 | 297.3±1.20 | 57.60±1.64 | 59.10±2.32 | 8.12±0.86 | 16.52±1.59 | 2.03 | |
| GG(0.05)/AG(0.3) | 6.28±0.05 | 299.0±0.58 | 54.90±3.10 | 56.20±3.00 | 12.04±0.35 | 25.87±1.31 | 2.15 | |
| 1.92 | ||||||||
| GG(0.1) | 6.42±0.03 | 291.0±1.00 | 49.90±1.61 | 54.70±1.74 | 14.07±0.43 | 34.82±1.02 | 2.47 | |
| GG(0.1)/AG(0.2) | 6.08±0.02 | 301.3±0.88 | 52.50±1.12 | 51.90±2.35 | 17.83±1.96 | 41.66±8.12 | 2.34 | |
| GG(0.1)/AG(0.3) | 5.96±0.06 | 305.7±0.67 | 51.30±1.13 | 49.60±1.38 | 19.30±2.96 | 49.22±2.59 | 2.55 | |
| 2.45 | ||||||||
| GG(0.2) | 6.12±0.04 | 300.0±0.58 | 51.70±3.06 | 53.20±1.46 | 106.58±4.35 | 382.58±6.28 | 3.59 | |
| GG(0.2)/AG(0.2) | 6.20±0.01 | 298.0±1.00 | 48.50±1.94 | 49.40±5.06 | 95.30±3.86 | 302.52±2.76 | 3.17 | |
| GG(0.2)/AG(0.3) | 6.32±0.00 | 309.3±0.67 | 51.70±1.50 | 56.50±1.90 | 105.41±1.39 | 333.03±4.21 | 3.16 | |
| 3.31 | ||||||||
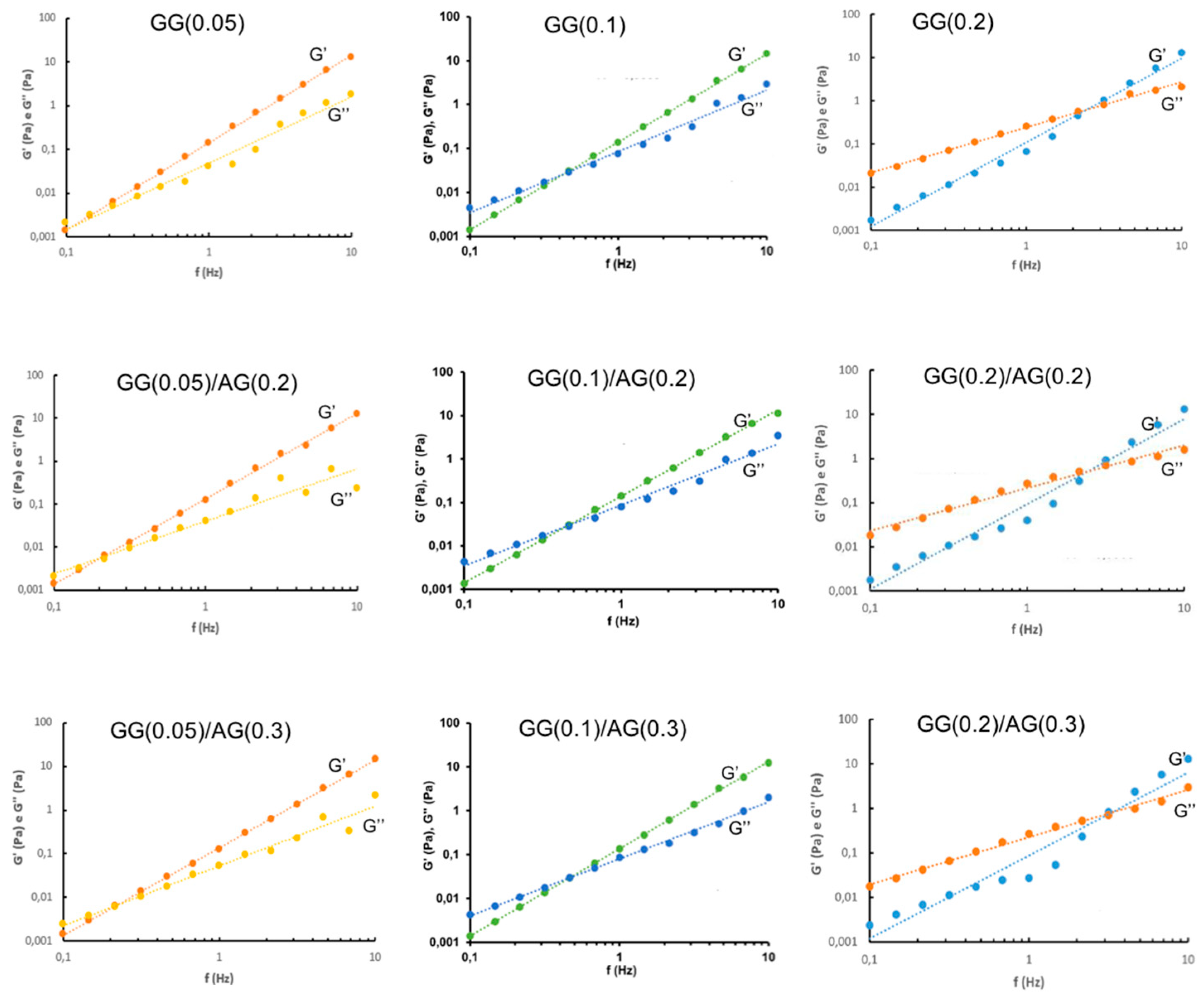
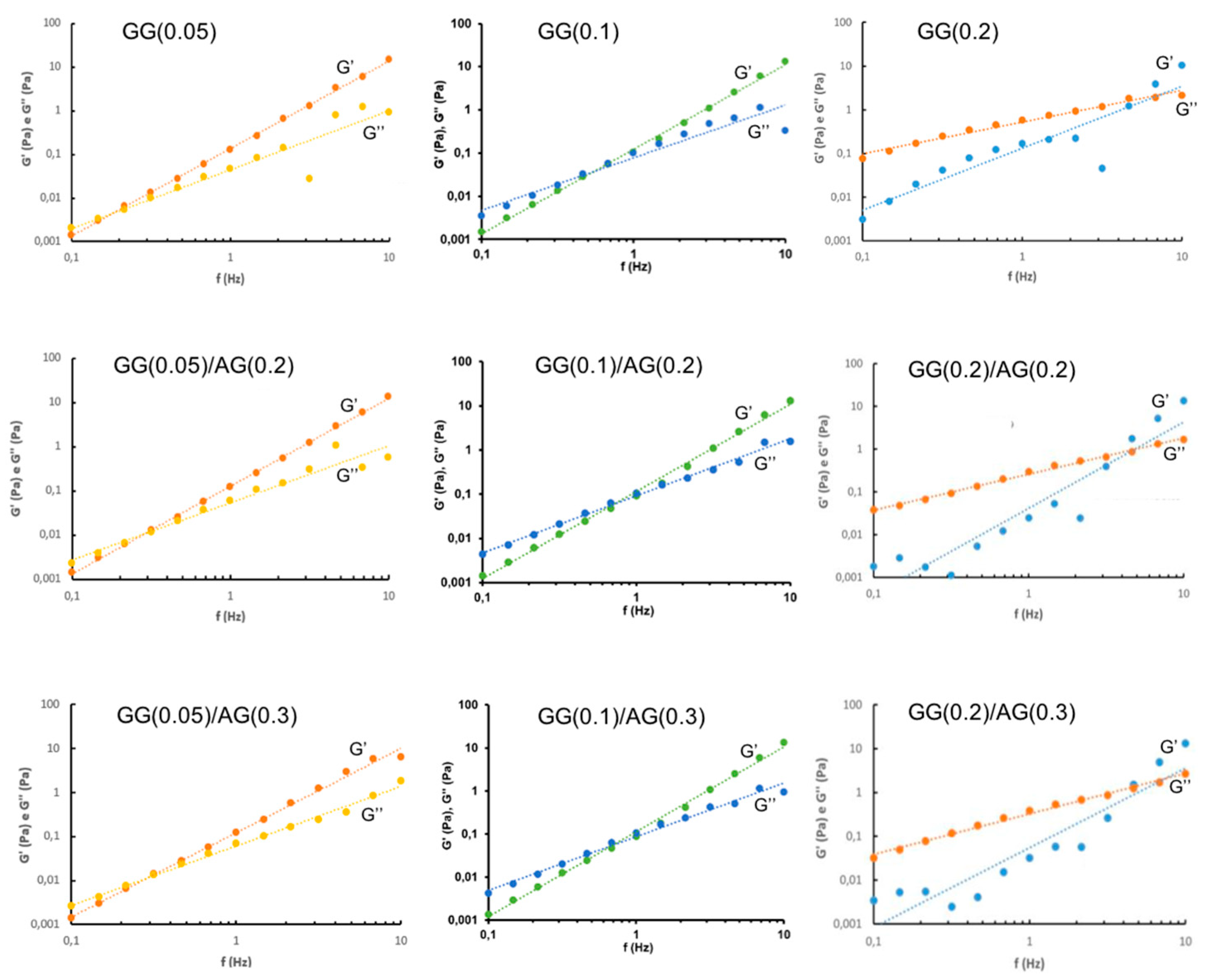
2.1.3. Design of Experiment (DoE) Optimization Study
| Formulation | Independent variables levels | Dependent variables values – before ATF dilution |
Dependent variables values – after ATF dilution |
|||||
|---|---|---|---|---|---|---|---|---|
| X1 (AG) | X2 (GG) | Viscosity (mPa . s) |
Elastic modulus (G’, Pa) |
Viscous modulus (G’’, Pa) |
Viscosity (mPa . s) |
Elastic modulus (G’, Pa) |
Viscous modulus (G’’, Pa) |
|
| GG(0.05) | -1 | -1 | 9.05 | 0.144 | 0.042 | 14.27 | 0.129 | 0.047 |
| GG(0.05)/AG(0.2) | 0 | -1 | 8.12 | 0.127 | 0.042 | 16.52 | 0.121 | 0.061 |
| GG(0.05)/AG(0.3) | +1 | -1 | 12.04 | 0.125 | 0.052 | 25.87 | 0.121 | 0.069 |
| GG(0.1) | -1 | 0 | 14.07 | 0.137 | 0.101 | 34.82 | 0.105 | 0.101 |
| GG(0.1)/AG(0.2) | 0 | 0 | 17.83 | 0.141 | 0.106 | 41.66 | 0.092 | 0.106 |
| GG(0.1)/AG(0.3) | +1 | 0 | 19.30 | 0.136 | 0.107 | 49.22 | 0.088 | 0.168 |
| GG(0.2) | -1 | +1 | 106.58 | 0.067 | 0.258 | 382.58 | 0.167 | 0.300 |
| GG(0.2)/AG(0.2) | 0 | +1 | 95.30 | 0.039 | 0.273 | 302.52 | 0.025 | 0.298 |
| GG(0.2)/AG(0.3) | +1 | +1 | 105.41 | 0.027 | 0.268 | 333.03 | 0.032 | 0.384 |
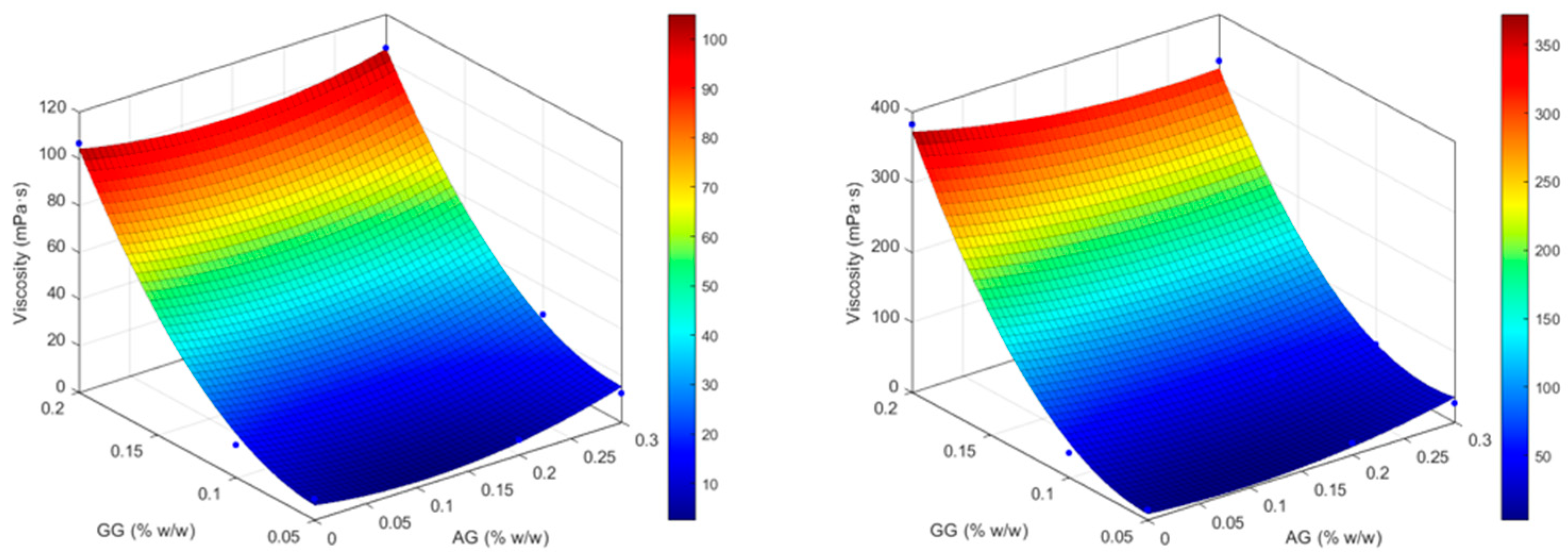
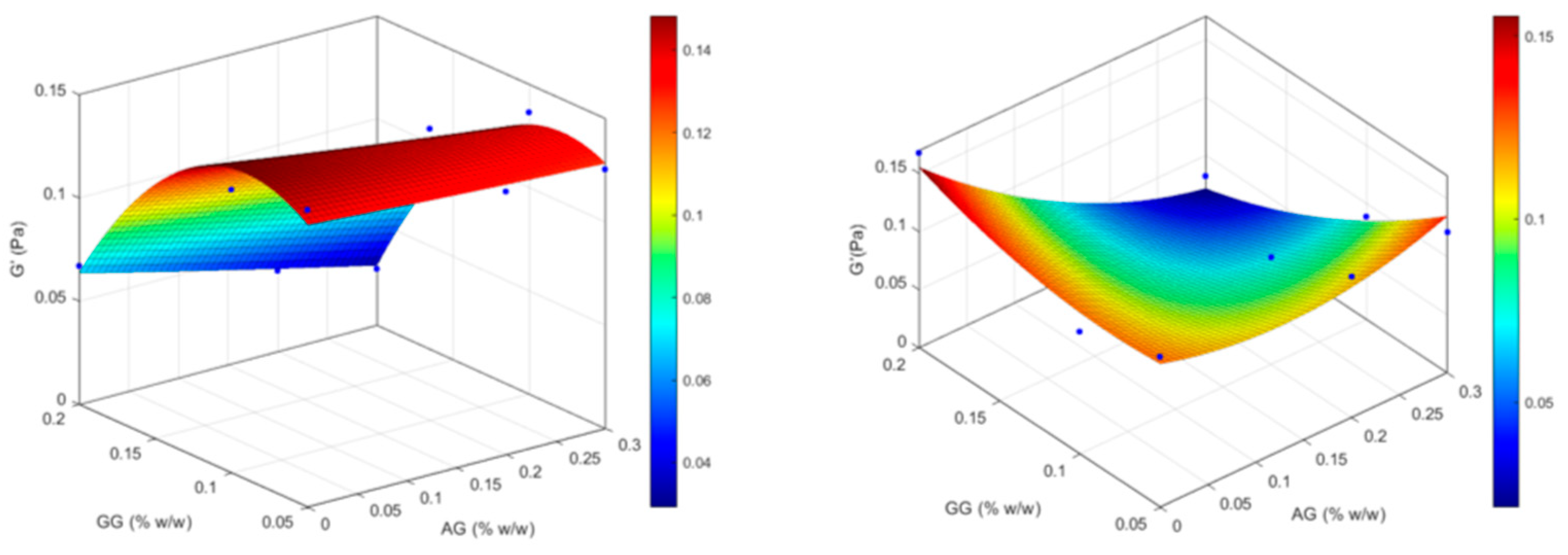
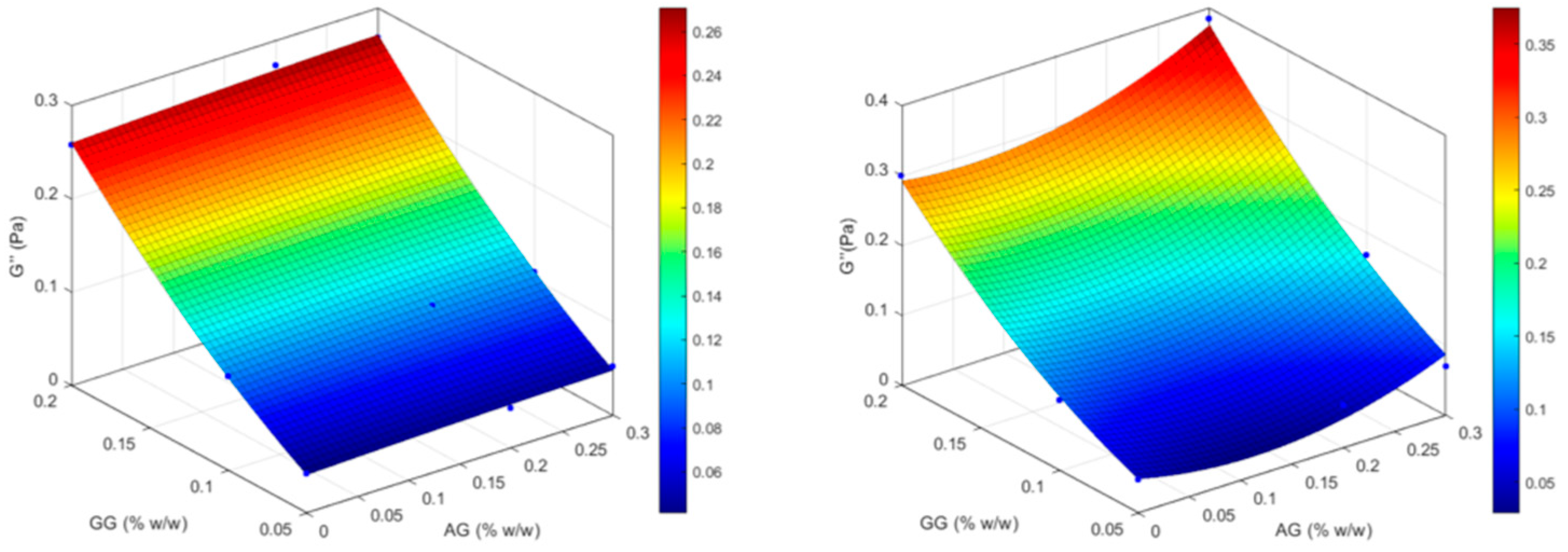
2.2. Biopharmaceutical Evaluation of the Selected Formulation
2.2.1. Ferning Test
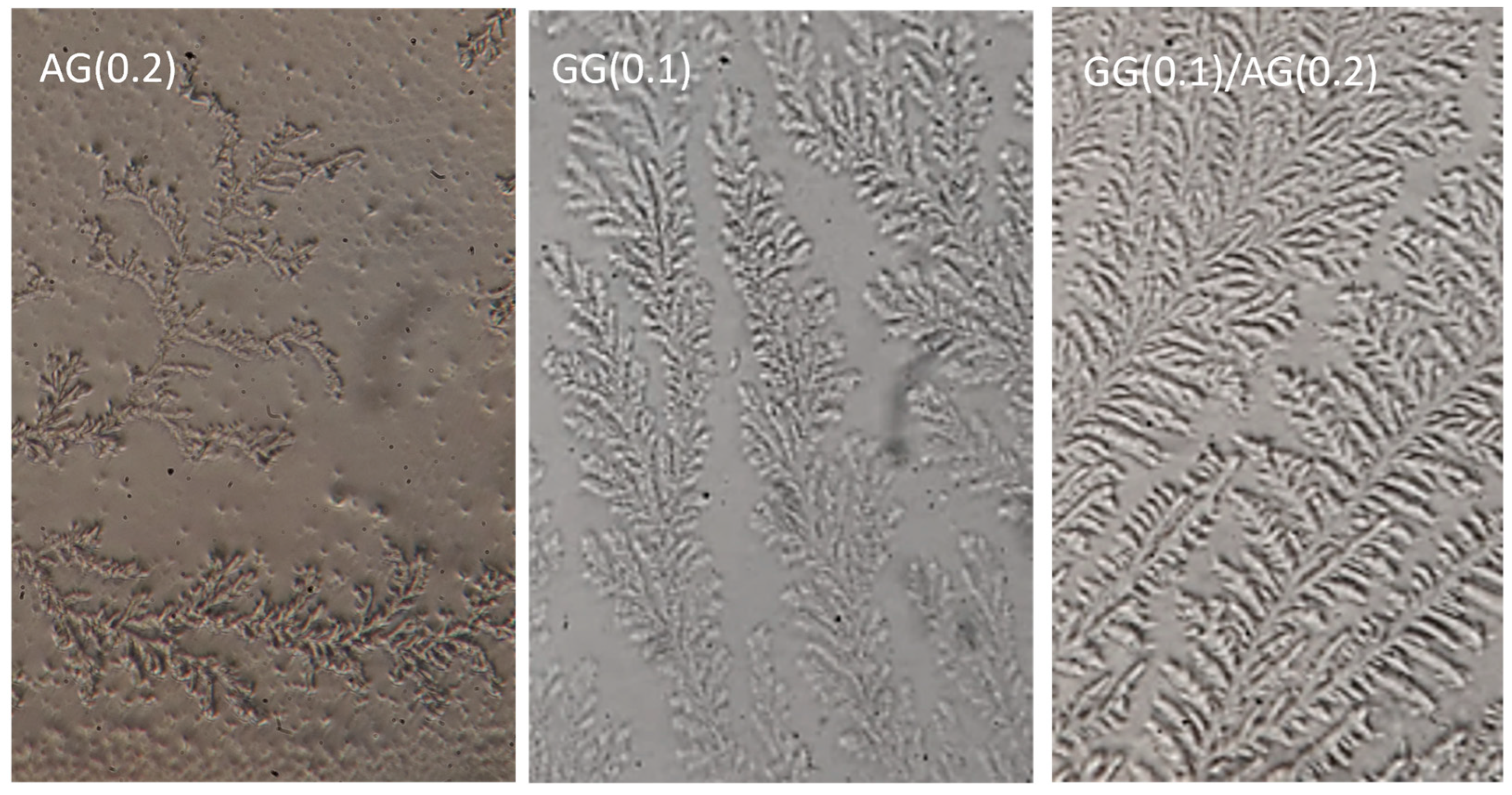
2.2.2. Mucoadhesion
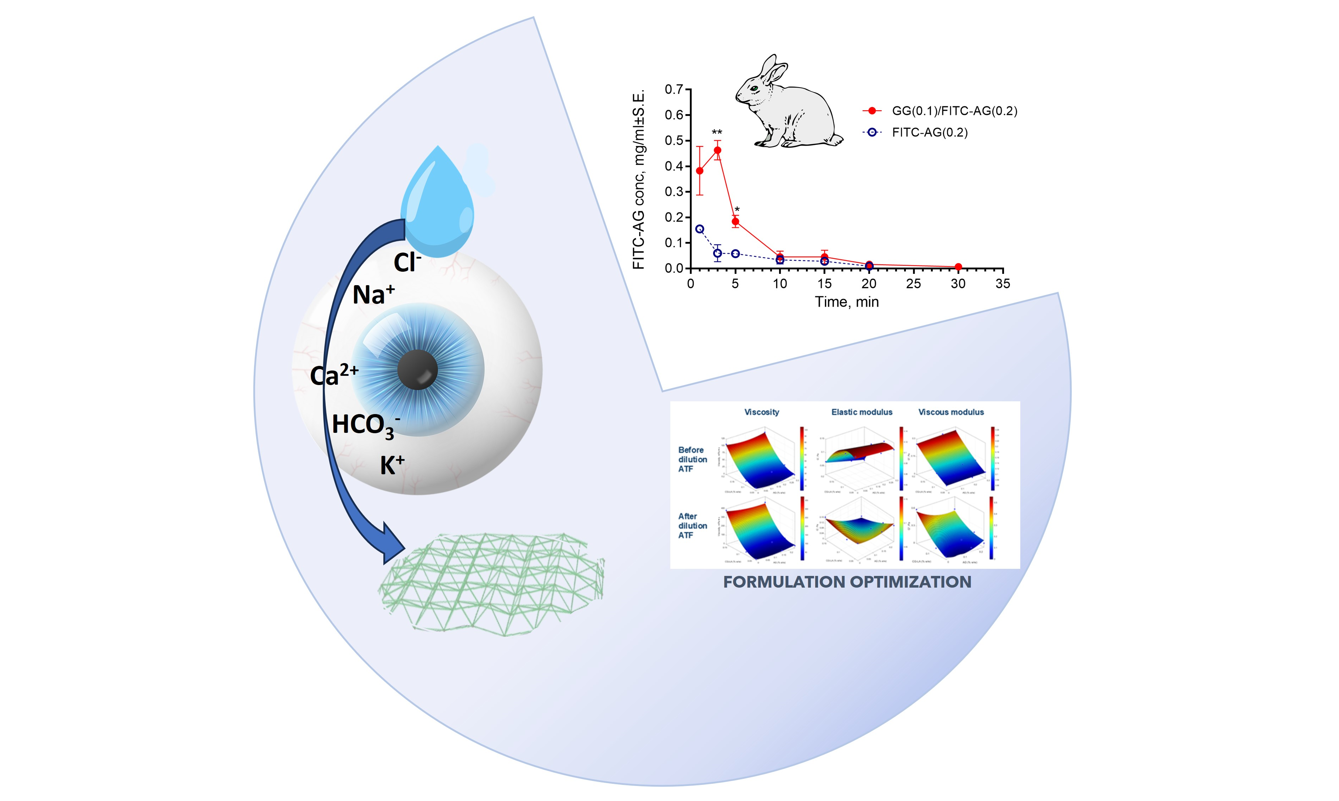
2.2.3. Evaluation of the Time of Residence of the Formulation in the Rabbit Eyes
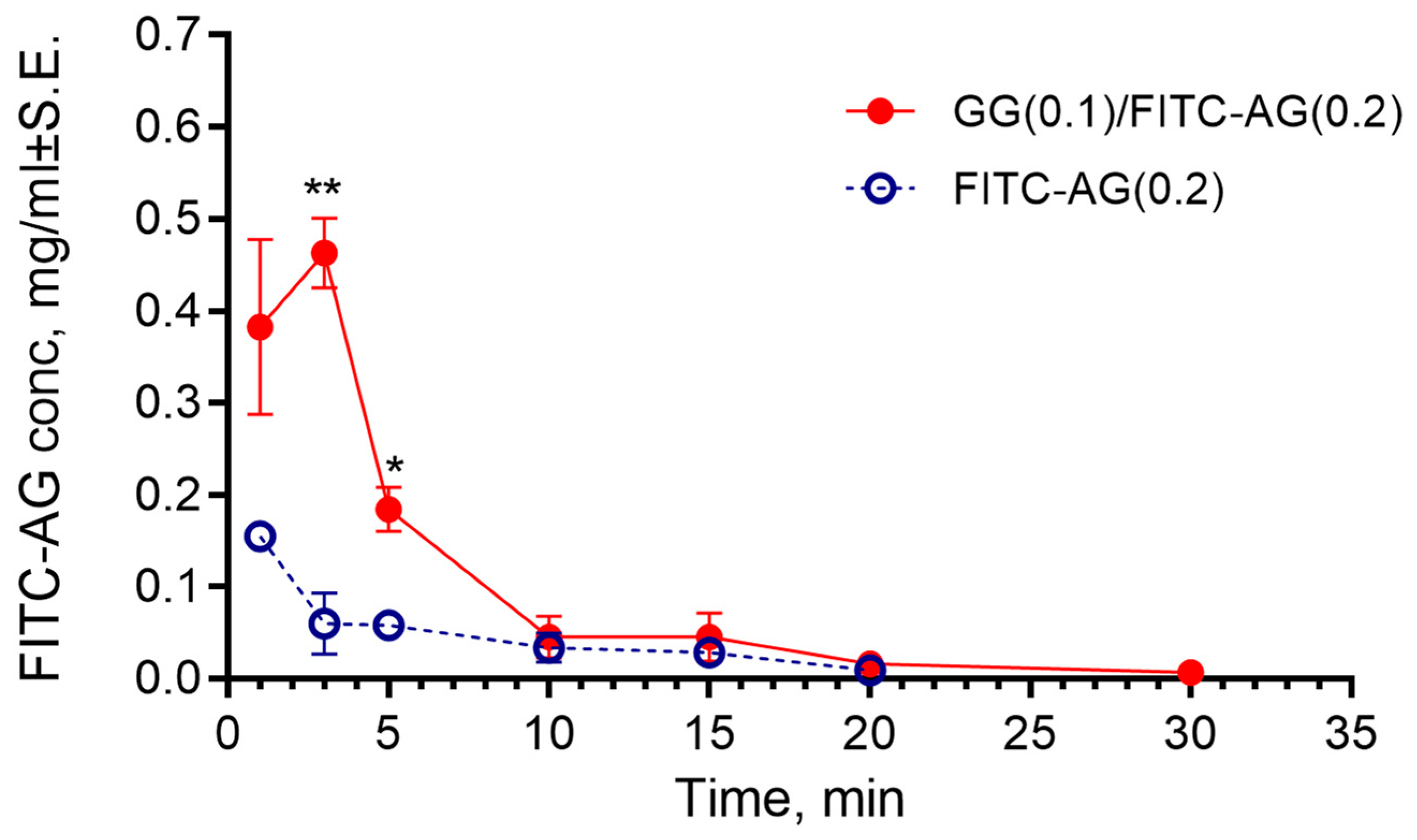
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Osmolality and pH Measurements
4.2.2. Wettability Assessment
4.2.3. Rheological Analysis
- Stress Sweep analysis, conducted at 32.0 °C ± 2.0 °C, with shear stress (τ) increasing from 0.1 to 100 Pa while the frequency was kept at 5 Hz, to determine the linear viscoelastic region (LVR).
- Frequency Sweep analysis: conducted at 32.0 ± 2.0 °C, with a frequency range of 0.1–10 Hz and a constant oscillatory stress of 1 Pa, to determine the elastic modulus (G'), viscous modulus (G''), and phase angle (δ) as a function of the frequency.
4.2.4. Preparation of Artificial Tear Fluid (ATF)
4.2.5. Design of Experiments (DOE): Optimization Study for the Selection of the Most Performant In Situ Gelling Formulation
| Independent Variables | Levels | ||
|---|---|---|---|
| +1 | 0 | -1 | |
| X1 = AG % w/w | 0.3 | 0.2 | 0 |
| X2 = GG % w/w | 0.2 | 0.1 | 0.05 |
4.2.6. Ferning Test
4.2.7. Mucoadhesion Test
4.2.8. Evaluation of the Time of Residence of the Formulation in the Rabbit Eyes
Fluorescein Isothiocyanate AG Synthesis
Animal Testing
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lallemand, F.; Schmitt, M.; Bourges, J.L.; Gurny, R.; Benita, S.; Garrigue, J.S. Cyclosporine A delivery to the eye: A comprehensive review of academic and industrial efforts. Eur J Pharm Biopharms 2017, 117, 14–28. [Google Scholar] [CrossRef]
- Ahmed, B.; Jaiswal, S.; Naryal, S.; Shah, R.M.; Alany, R.G.; Kaur, I.P. In situ gelling systems for ocular drug delivery. J Controll Rel 2024, 371, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.K.; Saxena, P.; Rai, A. Stimuli sensitive hydrogels for ophthalmic drug delivery: A review. Intl J Pharm Investig 2012, 2, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, R.; Janaswamy, S. Morphology of Western larch arabinogalactan. Carbohydr Res 2002, 337, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Dion, C.; Chappuis, E.; Ripoll, C. Does larch arabinogalactan enhance immune function? A review of mechanistic and clinical trials. Nutr Metabol 2016, 13, 28. [Google Scholar] [CrossRef]
- Silvani, L.; Bedei, A.; De Grazia, G.; Remiddi, S. Arabinogalactan and hyaluronic acid in ophthalmic solution: Experimental effect on xanthine oxidoreductase complex as key player in ocular inflammation (in vitro study). Exp Eye Res 2020, 196, 108058–108066. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, A.; Summa, F.F.; Oliva, P.; Lelj, F.; Remiddi, S.; Silvani, L.; Massa, A. Synergistic properties of Arabinogalactan (AG) and Hyaluronic Acid (HA) sodium salt mixtures. Molecules 2021, 26, 7246. [Google Scholar] [CrossRef]
- Bedei, A.; Rocha Cabrera, P.; Oliveira, L.; Castellini, L.; De Grazia, G.; Remiddi, S. Real-world treatment outcomes of an artificial tear containing arabinogalactan, hyaluronic acid and trehalose among subjects with dry eye. Clin Ophthalmol 2025, 19, 83–91. [Google Scholar] [CrossRef]
- Burgalassi, S.; Nicosia, N.; Monti, D.; Falcone, G.; Boldrini, E.; Chetoni, P. Larch arabinogalactan for dry eye protection and treatment of corneal lesions: Investigations in rabbits. J Ocular Pharmacol Therap 2007, 23, 541–550. [Google Scholar] [CrossRef]
- Burgalassi S, Nicosia N, Monti D, Falcone G, Boldrini E, Fabiani O, Lenzi, C; Pirone, A.; Chetoni, P. Arabinogalactan as active compound in the management of corneal wounds: In vitro toxicity and in vivo investigations on rabbits. Curr Eye Res 2011, 36, 21–28. [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan gum. Crit Rev Biotechnol 2000, 20, 177–211. [Google Scholar] [CrossRef]
- Kang, D.; Zhang, H.B.; Nitta, Y.; Fang, Y.P.; Nishinari, K. Gellan. In Polysaccharides; Ramawat, K., Mérillon, JM., Eds.; Springer: Cham: Switzerland. 2015; pp. 1–48. [CrossRef]
- Li, M.; Du, C.; Zhu, D.; Shen, M.; Cui, L.; Wang, J. Daytime variations of tear osmolarity and tear meniscus volume. Eye Contact Lens 2012, 38, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Stahl, U.; Willcox, M.; Stapleton, F. Osmolality and tear film dynamics. Clinical and Experimental Optometry 2012, 95, 3–11. [Google Scholar] [CrossRef]
- Aragona, P.; Di Stefano, G.; Ferreri, F.; Spinella, R.; Stilo, A. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjögren’s syndrome patients. Br J Ophthalmol 2002, 86, 879–884. [Google Scholar] [CrossRef]
- Troiano, P.; Monaco, G. Effect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: A cross-over study. Cornea 2008, 27, 1126–1130. [Google Scholar] [CrossRef]
- Baeyens, V.; Bron, A.; Baudouin, C. Vismed/Hylovis Study Group. Efficacy of 0.18% hypotonic sodium hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye disease. J Fr Ophtalmol 2012, 35, 412–419. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E. M. , Tomlinson, A., Calonge, M., Boboridis, K. G., Akova, Y. A., Geerling, G., Labetoulle, M., Rolando, M. Role of Hyperosmolarity in the Pathogenesis and Management of Dry Eye Disease: Proceedings of the OCEAN Group Meeting. The Ocular Surface 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Holly, F.J.; Lamberts, D.W. Effect of nonisotonic solutions on tear film osmolality. Invest Ophthalmol Vis Sci 1981, 20, 236–245. [Google Scholar] [PubMed]
- Kotreka, V.L.; Davis, M.C.; Adeyeye, A. Development of topical ophthalmic in situ gel-forming estradiol delivery system intended for the prevention of age-related cataracts. PLoS ONE 2017, 12, e0170720. [Google Scholar] [CrossRef]
- Pérez-Campos, S.J.; Chavarría-Hernández, N.; Tecante, A.; Ramírez-Gilly, M.; Rodríguez-Hernández, A.I. Gelation and microstructure of dilute gellan solutions with calcium ions. Food Hydrocolloids 2012, 28, 291–300. [Google Scholar] [CrossRef]
- Burgalassi, S.; Monti, D.; Tampucci, S.; Chetoni, P. In vitro evaluation of some parameters involved in mucoadhesion of aqueous polymeric dispersions. Pharm Dev Technol 2015, 20, 927–934. [Google Scholar] [CrossRef]
- Yang, C.; Leong, K.C. Influences of substrate wettability and liquid viscosity on isothermal spreading of liquid droplets on solid surfaces. Exp Fluids 2002, 33, 728–731. [Google Scholar] [CrossRef]
- Tiffany, J.M. The viscosity of human tears. Int Ophthalmol 1991, 15, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Matar, O.K.; Craster, R.V. Analysis of tear film rupture: Effect of non-Newtonian rheology. J Colloid Interface Sci 2003, 262, 130–148. [Google Scholar] [CrossRef]
- Chrai, S.S.; Robinson, J.R. Ocular evaluation of methylcellulose vehicle in albino rabbits. J Pharmaceut Sci 1974, 63, 1218–1223. [Google Scholar] [CrossRef]
- Winfield, A.J.; Jessiman, D.; Williams, A.; Esakowitz, L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol 1990, 74, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Recchioni, A.; Mocciardini, E. Viscoelastic properties of the human tear film. Exp Eye Res 2022, 219, 109083. [Google Scholar] [CrossRef]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological considerations of pharmaceutical formulations: Focus on viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.H.; Roberts, C.J.; Litsky, A.S.; Weber, P.A. A viscoelastic biomechanical model of the cornea describing the effect of viscosity and elasticity on hysteresis. Invest Ophthalmol Visual Sci 2008, 49, 3919–3926. [Google Scholar] [CrossRef] [PubMed]
- Masmali, A.M.; Purslow, C.; Murphy, P.J. The tear ferning test: A simple clinical technique to evaluate the ocular tear film. Clin Exp Optom 2014, 97, 399–406. [Google Scholar] [CrossRef]
- Moschini, R.; Gini, F.; Cappiello, M.; Balestri, F.; Falcone, G.; Boldrini, E.; Mura, U.; Del Corso, A. Interaction of arabinogalactan with mucins. Int J Biol Macromol 2014, 67, 446–451. [Google Scholar] [CrossRef]
- Tatykhanova, G.; Aseyev, V.; Kudaibergenov, S.E. Mucoadhesive properties of gellan and its modified derivatives. Rev and adv in chem 2020, 10, 140–157. [Google Scholar] [CrossRef]
- Snibson, G.R.; Greaves, J.L.; Soper, N.D.; Tiffany, J.M.; Wilson, C.G.; Bron, A.J. Ocular surface residence times of artificial tear solutions. Cornea 1992, 11, 288–293. [Google Scholar] [CrossRef]
- Rupenthal, I.D.; Colin, R.; Green, C.R.; Alany, R.G. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: Physicochemical characterisation and in vitro release. Int J Pharm 2011, 411, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Rupenthal, I.D.; Green, C.R.; Alany, R.G. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 2: Precorneal retention and in vivo pharmacodynamic study. Int J Pharm 2011, 411, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Parmanik, A.; Das, D.; Sahoo, R.N.; Nayak, A.K. Gellan gum-based in-situ gel formulations for ocular drug delivery: A practical approach. Int J Biol Macromol 2025, 290, 138979. [Google Scholar] [CrossRef]
- Zaharia, A.C.; Dumitrescu, O.M.; Radu, M.; Rogoz, R.E. Adherence to Therapy in Glaucoma Treatment-A Review. J Pers Med 2022, 12, 514. [Google Scholar] [CrossRef]
- Challener, C.A. Aiming for Improved Efficacy and Patient Compliance for Topical Ophthalmics. Pharm Tech 2023, 47, 18–19. [Google Scholar]
- Muñoz-Villegas, P.; Martínez-Bautista, H.; Olvera-Montaño, O. Determinants of adherence to treatment in patients with ophthalmic conditions. Expert Rev Clin Pharmacol 2023, 16, 1249–1259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





