Submitted:
11 August 2025
Posted:
12 August 2025
You are already at the latest version
Abstract
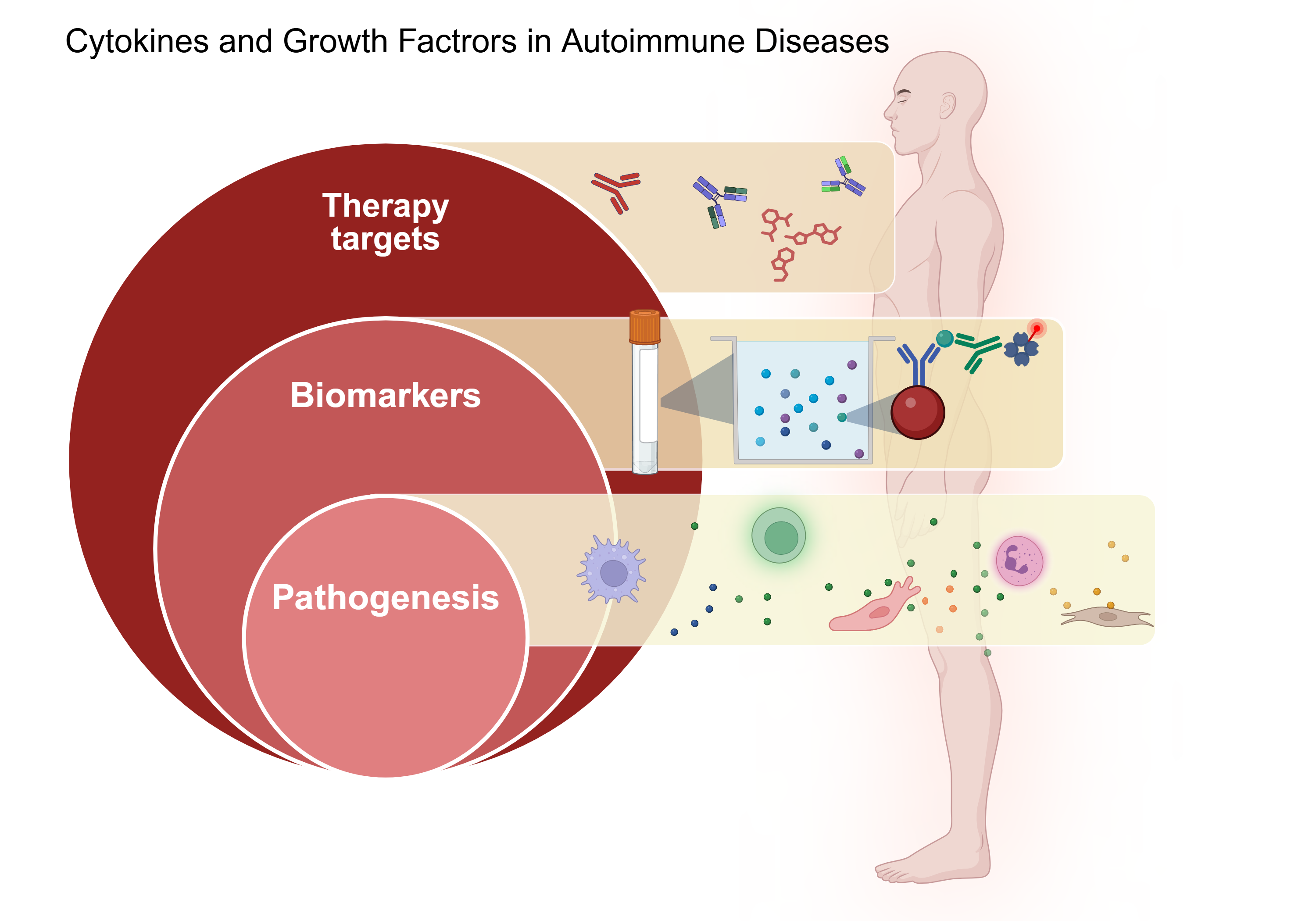
Keywords:
1. Introduction
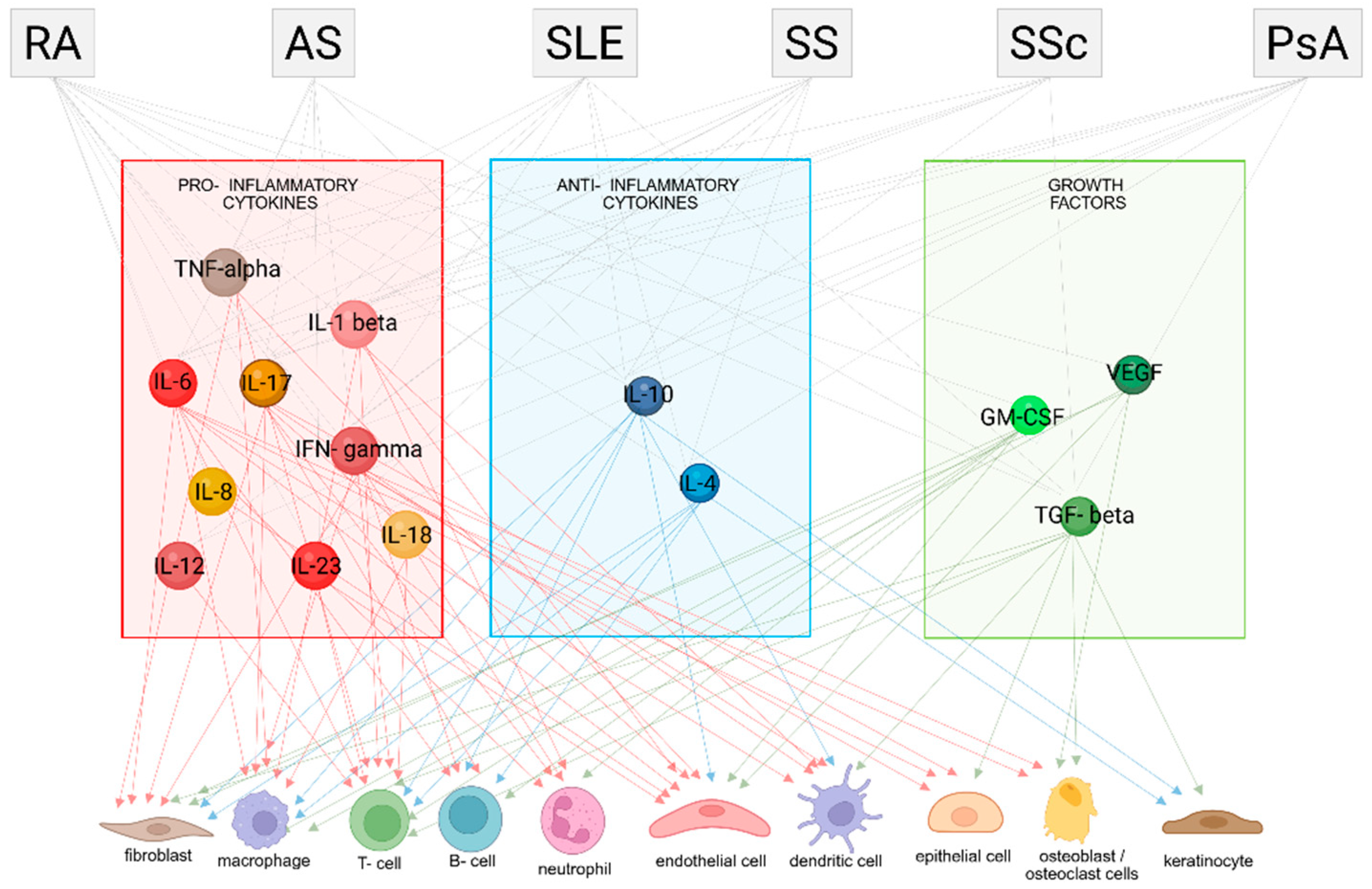
2. Pro-Inflammatory Cytokines in Autoimmune Diseases
2.1. Tumor Necrosis Factor Alpha (TNF-α)
2.1.1. TNF-α Protein and Physiological Role
2.1.2. TNF-α in Autoimmune Diseases
2.2. Interleukin-1β (IL-1β)
2.2.1. IL-1β Protein and Physiological Role
2.2.2. IL-1β in Autoimmune Diseases
2.3. Interleukin-6 (IL-6)
2.3.1. IL-6 protein and physiological role.
2.3.2. IL-6 in Autoimmune Diseases.
2.4. Interleukin-17 (IL-17)
2.4.1. IL-17 protein and physiological role
2.4.2. IL-17 in Autoimmune Diseases
2.5. Interferon-Gamma (IFN-γ)
2.5.1. IFN-γ Protein and Physiological Role
2.5.2. IFN-γ in Autoimmune Diseases
3. Anti-inflammatory cytokines as biomarkers in ADs
3.1. Interleukin 10 (IL-10)
3.1.1. IL-10 protein and physiological role
3.1.2. IL-10 in Autoimmune Diseases
3.2. Interleukin-4 (IL-4)
3.2.1. IL-4 Protein and Its Physiological Role
3.2.2. IL-4 in Autoimmune Diseases
4. Growth Factors as Biomarkers in ADs
4.1. Transforming Growth Factor Beta (TGF-β)
4.1.1. TGF-β Protein and Physiological Role
4.1.2. TGF-β in Autoimmune Diseases
4.2. Other Growth Factors Involved in Autoimmune Diseases
5. Challenges in Biomarkers Development
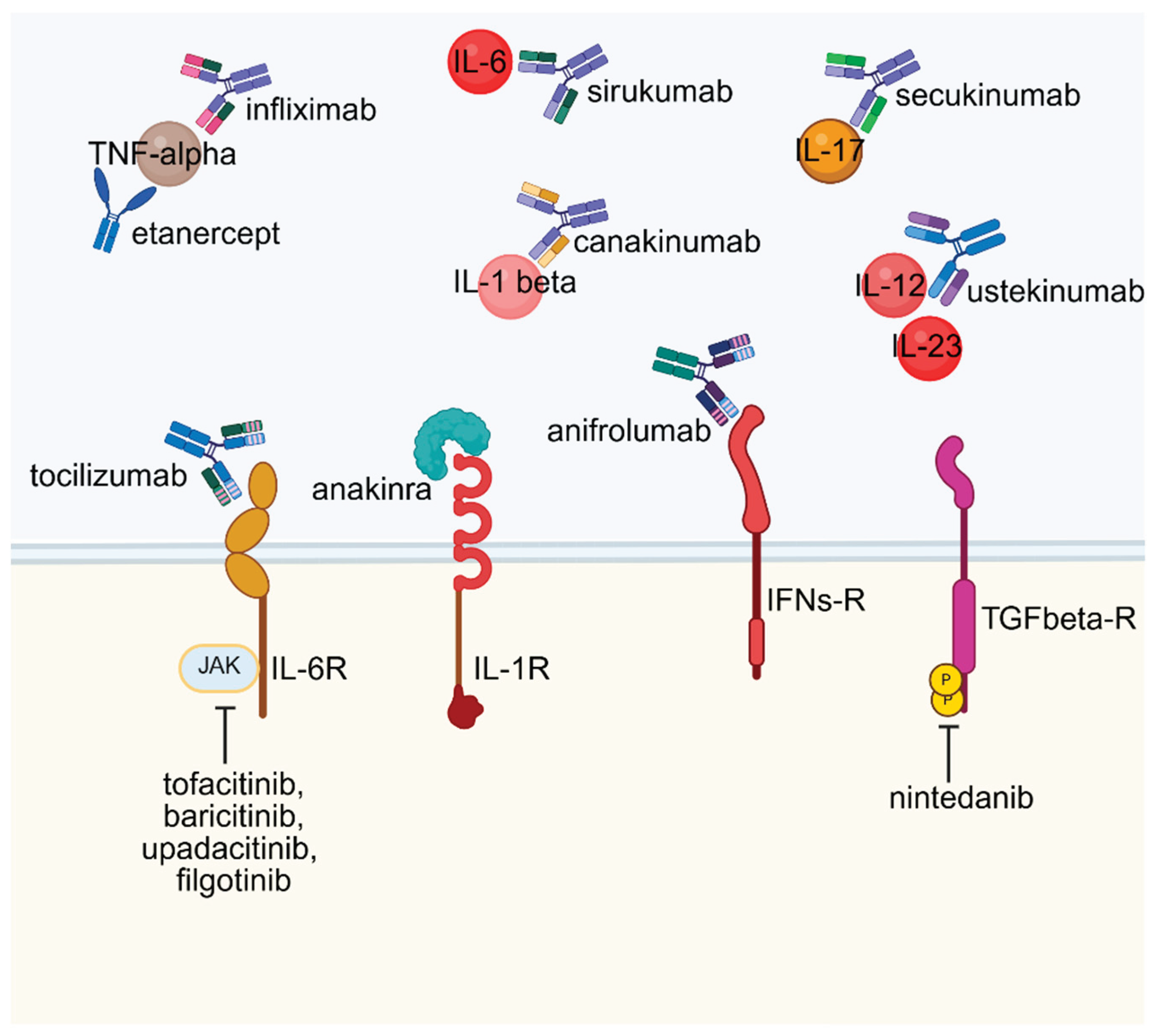
6. Therapeutic Implications
6.1. Targeting Secreted Immune Mediators in Autoimmune Therapy
6.2. Targeting Cell Surface Receptors in Autoimmune Therapy
6.3. Targeting Intracellular Signaling Pathways in Autoimmune Therapy
7. Conclusions
Author Contributions
Data Availability Statement
Conflicts of Interest
Abbreviations
| RA | Rheumatoid Arthritis |
| SLE | Systemic Lupus Erythematosus |
| PsA | Psoriatic Arthritis |
| SS | Sjögren’s Syndrome |
| AS | Ankylosing Spondylitis |
| SSc | Systemic Sclerosis |
References
- Messina, J.M.; Luo, M.; Hossan, M.S.; Gadelrab, H.A.; Yang, X.; John, A.; Wilmore, J.R.; Luo, J. Unveiling Cytokine Charge Disparity as a Potential Mechanism for Immune Regulation. Cytokine & Growth Factor Reviews 2024, 77, 1–14. [Google Scholar] [CrossRef]
- Yasmeen, F.; Pirzada, R.H.; Ahmad, B.; Choi, B.; Choi, S. Understanding Autoimmunity: Mechanisms, Predisposing Factors, and Cytokine Therapies. International Journal of Molecular Sciences 2024, 25, 7666. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.K.; Olsson, L.M. Recent Advances in the Genetics of Autoimmune Disease. Annual Review of Immunology 2009, 27, 363–391. [Google Scholar] [CrossRef]
- Gawda, A.; Majka, G.; Nowak, B.; Marcinkiewicz, J. Air Pollution, Oxidative Stress, and Exacerbation of Autoimmune Diseases. Central European Journal of Immunology 2017, 3, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. International Journal of Molecular Sciences 2021, 22, 2719. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Fan, Y.; Zhao, X. Systemic Lupus Erythematosus: Updated Insights on the Pathogenesis, Diagnosis, Prevention and Therapeutics. Signal Transduction and Targeted Therapy 2025, 10. [Google Scholar] [CrossRef] [PubMed]
- Karmacharya, P.; Chakradhar, R.; Ogdie, A. The Epidemiology of Psoriatic Arthritis: A Literature Review. Best Practice & Research Clinical Rheumatology 2021, 35, 101692. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. International Journal of Molecular Sciences 2023, 24, 4901. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Chatzis, L.G.; Fulvio, G.; La Rocca, G.; Pontarini, E.; Bombardieri, M. Pathogenesis of Sjögren’s Disease: One Year in Review 2024. Clinical and Experimental Rheumatology 2024. [Google Scholar] [CrossRef]
- Alexander, M. Ankylosing Spondylitis Pathogenesis and Pathophysiology. In Ankylosing Spondylitis - Recent Concepts; IntechOpen: 2023.
- Jimenez, S.A.; Mendoza, F.A.; Piera-Velazquez, S. A Review of Recent Studies on the Pathogenesis of Systemic Sclerosis: Focus on Fibrosis Pathways. Frontiers in Immunology 2025, 16. [Google Scholar] [CrossRef]
- Takahashi, T.; Asano, Y. The Evolving Landscape of Systemic Sclerosis Pathogenesis: From Foundational Mechanisms to Organ-Specific Modifiers; MDPI AG: 2025.
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. International Journal of Molecular Sciences 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Richter, P.; Macovei, L.A.; Mihai, I.R.; Cardoneanu, A.; Burlui, M.A.; Rezus, E. Cytokines in Systemic Lupus Erythematosus—Focus on TNF-α and IL-17. International Journal of Molecular Sciences 2023, 24, 14413. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. International Journal of Molecular Sciences 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Alshevskaya, A.; Zhukova, J.; Lopatnikova, J.; Vasilyev, F.; Khutornoy, I.; Golikova, E.; Kireev, F.; Sennikov, S. Nonlinear Dynamics of TNFR1 and TNFR2 Expression on Immune Cells: Genetic and Age-Related Aspects of Inflamm-Aging Mechanisms. Biomedicines 2025, 13. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H. TNF Receptors: Structure-Function Relationships and Therapeutic Targeting Strategies. Biochimica et Biophysica Acta (BBA) - Biomembranes 2025, 1867, 184394. [Google Scholar] [CrossRef]
- Kaye, A.D.; Perilloux, D.M.; Hawkins, A.M.; Wester, G.C.; Ragaland, A.R.; Hebert, S.V.; Kim, J.; Heisler, M.; Kelkar, R.A.; Chami, A.A.; et al. Tumor Necrosis Factor and Interleukin Modulators for Pathologic Pain States: A Narrative Review. Pain and Therapy 2024, 13, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. International Journal of Molecular Sciences 2021, 22, 10922. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. International Journal of Molecular Sciences 2021, 22, 2719. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF Biology, Pathogenic Mechanisms and Emerging Therapeutic Strategies. Nature Reviews Rheumatology 2015, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. New England Journal of Medicine 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Postal, M.; Appenzeller, S. The Role of Tumor Necrosis Factor-Alpha (TNF-α) in the Pathogenesis of Systemic Lupus Erythematosus. Cytokine 2011, 56, 537–543. [Google Scholar] [CrossRef]
- Ghorbaninezhad, F.; Leone, P.; Alemohammad, H.; Najafzadeh, B.; Nourbakhsh, N.; Prete, M.; Malerba, E.; Saeedi, H.; Tabrizi, N.; Racanelli, V.; et al. Tumor Necrosis Factor-α in Systemic Lupus Erythematosus: Structure, Function and Therapeutic Implications (Review). International Journal of Molecular Medicine 2022, 49. [Google Scholar] [CrossRef] [PubMed]
- Gómez, D.; Correa, P.A.; Gómez, L.M.; Cadena, J.; Molina, J.F.; Anaya, J.-M. Th1/Th2 Cytokines in Patients with Systemic Lupus Erythematosus: Is Tumor Necrosis Factor α Protective? Seminars in Arthritis and Rheumatism 2004, 33, 404–413. [Google Scholar] [CrossRef]
- Jinshan, Z.; Yong, Q.; Fangqi, C.; Juanmei, C.; Min, L.; Changzheng, H. The Role of TNF-α as a Potential Marker for Acute Cutaneous Lupus Erythematosus in Patients with Systemic Lupus Erythematosus. The Journal of Dermatology 2024, 51, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Zelová, H.; Hošek, J. TNF-α Signalling and Inflammation: Interactions between Old Acquaintances. Inflammation Research 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Ewert, P.; Aguilera, S.; Alliende, C.; Kwon, Y.; Albornoz, A.; Molina, C.; Urzúa, U.; Quest, A.F.G.; Olea, N.; Pérez, P.; et al. Disruption of Tight Junction Structure in Salivary Glands from Sjögren’s Syndrome Patients Is Linked to Proinflammatory Cytokine Exposure. Arthritis & Rheumatism 2010, 62, 1280–1289. [Google Scholar] [CrossRef]
- Chen, C.; Liang, Y.; Zhang, Z.; Zhang, Z.; Yang, Z. Relationships between Increased Circulating YKL-40, IL-6 and TNF-α Levels and Phenotypes and Disease Activity of Primary Sjögren’s Syndrome. International Immunopharmacology 2020, 88, 106878. [Google Scholar] [CrossRef] [PubMed]
- Limaye, A.; Hall, B.E.; Zhang, L.; Cho, A.; Prochazkova, M.; Zheng, C.; Walker, M.; Adewusi, F.; Burbelo, P.D.; Sun, Z.J.; et al. Targeted TNF-α Overexpression Drives Salivary Gland Inflammation. Journal of Dental Research 2019, 98, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.; Bridgewood, C.; Rowe, H.; Altaie, A.; Jones, E.; McGonagle, D. Cytokine “Fine Tuning” of Enthesis Tissue Homeostasis as a Pointer to Spondyloarthritis Pathogenesis with a Focus on Relevant TNF and IL-17 Targeted Therapies. Seminars in Immunopathology 2021, 43, 193–206. [Google Scholar] [CrossRef]
- Schett, G.; David, J.-P. The Multiple Faces of Autoimmune-Mediated Bone Loss. Nature Reviews Endocrinology 2010, 6, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Nam, B.; Lee, Y.L.; Park, H.; Weon, S.; Choi, S.-H.; Park, Y.-S.; Kim, T.-H. The TNF-NF-κB-DKK1 Axis Promoted Bone Formation in the Enthesis of Ankylosing Spondylitis. Journal of Rheumatic Diseases 2021, 28, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Frontiers in Immunology 2021, 12. [Google Scholar] [CrossRef]
- Kosałka-Węgiel, J.; Lichołai, S.; Pacholczak-Madej, R.; Dziedzina, S.; Milewski, M.; Kuszmiersz, P.; Korona, A.; Gąsior, J.; Matyja-Bednarczyk, A.; Kwiatkowska, H.; et al. Serum IL-17 and TNFα as Prognostic Biomarkers in Systemic Sclerosis Patients: A Prospective Study. Rheumatology International 2023, 44, 119–128. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S. O.; da Paz, A. S.; Farias, I. M. V. C.; Moreira, D. S.; Ribeiro, M. A. F.; Alves, T. S. G. N.; Lemos, A. C. M.; Santiago, M. B. Bronchoalveolar Lavage in Systemic Sclerosis Patients: A Systematic Review. Current rheumatology reviews 2021, 17, 176–183. [Google Scholar] [CrossRef]
- Wijdan, S.A.; Bokhari, S.M.N.A.; Alvares, J.; Latif, V. The Role of Interleukin-1 Beta in Inflammation and the Potential of Immune-Targeted Therapies. Pharmacological Research - Reports 2025, 3, 100027. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta—A Friend or Foe in Malignancies? International Journal of Molecular Sciences 2018, 19, 19–2155. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The Role of Interleukin-1 in General Pathology. Inflammation and Regeneration 2019, 39. [Google Scholar] [CrossRef]
- Pyrillou, K.; Burzynski, L.C.; Clarke, M.C.H. Alternative Pathways of IL-1 Activation, and Its Role in Health and Disease. Frontiers in Immunology 2020, 11. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome Activation and Regulation: Toward a Better Understanding of Complex Mechanisms. Cell Discovery 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, L.; Hernandez Santana, Y.E.; Walsh, P.T.; Fallon, P.G. The IL-1 Cytokine Family as Custodians of Barrier Immunity. Cytokine 2022, 154, 155890. [Google Scholar] [CrossRef]
- Bottini, N.; Firestein, G.S. Duality of Fibroblast-like Synoviocytes in RA: Passive Responders and Imprinted Aggressors. Nature Reviews Rheumatology 2012, 9, 24–33. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid Arthritis: Pathological Mechanisms and Modern Pharmacologic Therapies. Bone Research 2018, 6. [Google Scholar] [CrossRef]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. International Journal of Molecular Sciences 2021, 22, 10922. [Google Scholar] [CrossRef] [PubMed]
- Caielli, S.; Balasubramanian, P.; Rodriguez-Alcazar, J.; Balaji, U.; Robinson, L.; Wan, Z.; Baisch, J.; Smitherman, C.; Walters, L.; Sparagana, P.; et al. Type I IFN Drives Unconventional IL-1β Secretion in Lupus Monocytes. Immunity 2024, 57, 2497–2513.e12. [Google Scholar] [CrossRef] [PubMed]
- Rzeszotarska, E.; Sowinska, A.; Stypinska, B.; Lutkowska, A.; Felis-Giemza, A.; Olesinska, M.; Puszczewicz, M.; Majewski, D.; Jagodzinski, P.P.; Haładyj, E.; et al. IL-1β, IL-10 and TNF-α Polymorphisms May Affect Systemic Lupus Erythematosus Risk and Phenotype. Clinical and Experimental Rheumatology 2021. [Google Scholar] [CrossRef]
- Lai, J.-H.; Wu, D.-W.; Huang, C.-Y.; Hung, L.-F.; Wu, C.-H.; Ka, S.-M.; Chen, A.; Huang, J.-L.; Ho, L.-J. Induction of LY6E Regulates Interleukin-1β Production, Potentially Contributing to the Immunopathogenesis of Systemic Lupus Erythematosus. Cell Communication and Signaling 2025, 23. [Google Scholar] [CrossRef] [PubMed]
- Lovato, B.H.; Fogagnolo, L.; Souza, E.M. de; Silva, L.J.B. da; Velho, P.E.N.F.; Cintra, M.L.; Teixeira, F. IL-1β and IL-17 in Cutaneous Lupus Erythematous Skin Biopsies: Could Immunohistochemicals Indicate a Tendency towards Systemic Involvement? Anais Brasileiros de Dermatologia 2024, 99, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Blokland, S.L.M.; Flessa, C.-M.; van Roon, J.A.G.; Mavragani, C.P. Emerging Roles for Chemokines and Cytokines as Orchestrators of Immunopathology in Sjögren’s Syndrome. Rheumatology 2019, 60, 3072–3087. [Google Scholar] [CrossRef] [PubMed]
- Martín-Nares, E.; Hernández-Molina, G.; Lima, G.; Hernández-Ramírez, D.F.; Chan-Campos, I.; Saavedra-González, V.; Llorente, L. Tear Levels of IL-7, IL-1α, and IL-1β May Differentiate between IgG4-Related Disease and Sjögren’s Syndrome. Clinical Rheumatology 2023, 42, 1101–1105. [Google Scholar] [CrossRef]
- Baldini, C.; Rossi, C.; Ferro, F.; Santini, E.; Seccia, V.; Donati, V.; Solini, A. The P2X7 Receptor–Inflammasome Complex Has a Role in Modulating the Inflammatory Response in Primary Sjögren’s Syndrome. Journal of Internal Medicine 2013, 274, 480–489. [Google Scholar] [CrossRef]
- Conti, P.; Stellin, L.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Emidio, P.D.; Ronconi, G. Advances in Mast Cell Activation by IL-1 and IL-33 in Sjögren’s Syndrome: Promising Inhibitory Effect of IL-37. International Journal of Molecular Sciences 2020, 21. [Google Scholar] [CrossRef]
- Al-Hwas, Z.S.; Ali, N.H.; Al-Hamdi, K.I. Distinct Inflammasome IL-1β Gene Expression Profile in Patients with Psoriatic Arthritis in Basra City. International journal of health sciences 2022, 4570–4577. [Google Scholar] [CrossRef]
- Stoeckman, A.K.; Baechler, E.C.; Ortmann, W.A.; Behrens, T.W.; Michet, C.J.; Peterson, E.J. A Distinct Inflammatory Gene Expression Profile in Patients with Psoriatic Arthritis. Genes & Immunity 2006, 7, 583–591. [Google Scholar] [CrossRef]
- Dilek, G.; Kalcik Unan, M.; Nas, K. Immune Response and Cytokine Pathways in Psoriatic Arthritis: A Systematic Review. Archives of Rheumatology 2025, 40, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R.; Hassan, H.I.; Hofny, E.R.M.; Elkholy, M.; Fatehy, N.A.; Abd Elmoniem, A. e A.; Ezz El-Din, A.M.; Afifi, O.A.; Rashed, H.G. Alterations of Mononuclear Inflammatory Cells, CD4/CD8+ T Cells, Interleukin 1β, and Tumour Necrosis Factor α in the Bronchoalveolar Lavage Fluid, Peripheral Blood, and Skin of Patients with Systemic Sclerosis. Journal of Clinical Pathology 2005, 58, 178–184. [Google Scholar] [CrossRef]
- Maleszewska, M.; Moonen, J.-R.A.J.; Huijkman, N.; van de Sluis, B.; Krenning, G.; Harmsen, M.C. IL-1β and TGFβ2 Synergistically Induce Endothelial to Mesenchymal Transition in an NFκB-Dependent Manner. Immunobiology 2013, 218, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P. , Lapoirie, J., Leleu, D., Levionnois, E., Grenier, C., Jurado-Mestre, B., Lazaro, E., Duffau, P., Richez, C., Seneschal, J., et al. Interleukin-1β-Activated Microvascular Endothelial Cells Promote DC-SIGN-Positive Alternatively Activated Macrophages as a Mechanism of Skin Fibrosis in Systemic Sclerosis. Arthritis & rheumatology (Hoboken, N.J.) 2022, 74, 1013–1026. [Google Scholar] [CrossRef]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the Interface of Human Health and Disease. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 Trans-Signalling: Past, Present and Future Prospects. Nature Reviews Immunology 2023, 23, 666–681. [Google Scholar] [CrossRef]
- Alonzi, T.; Fattori, E.; Lazzaro, D.; Costa, P.; Probert, L.; Kollias, G.; De Benedetti, F.; Poli, V.; Ciliberto, G. Interleukin 6 Is Required for the Development of Collagen-Induced Arthritis. The Journal of Experimental Medicine 1998, 187, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Okuda, Y. IL-6-Deficient Mice Are Resistant to the Induction of Experimental Autoimmune Encephalomyelitis Provoked by Myelin Oligodendrocyte Glycoprotein. International Immunology 1998, 10, 703–708. [Google Scholar] [CrossRef]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. International Journal of Molecular Sciences 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harbor Perspectives in Biology 2014, 6, a016295–a016295. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.; Rizvanov, A.; Urbanowicz, R.A.; Khaiboullina, S. Inflammasome Contribution to the Activation of Th1, Th2, and Th17 Immune Responses. Frontiers in Microbiology 2022, 13. [Google Scholar] [CrossRef]
- Srirangan, S.; Choy, E.H. The Role of Interleukin 6 in the Pathophysiology of Rheumatoid Arthritis. Therapeutic Advances in Musculoskeletal Disease 2010, 2, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Woś, I.; Tabarkiewicz, J. Effect of Interleukin-6, -17, -21, -22, and -23 and STAT3 on Signal Transduction Pathways and Their Inhibition in Autoimmune Arthritis. Immunologic Research 2021, 69, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Li, X.; Deng, Q.; Shi, J.; Feng, Y.; Bai, L. Advances of the Small Molecule Drugs Regulating Fibroblast-like Synovial Proliferation for Rheumatoid Arthritis. Frontiers in Pharmacology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Carbone, G.; Wilson, A.; Diehl, S.A.; Bunn, J.; Cooper, S.M.; Rincon, M. Interleukin-6 Receptor Blockade Selectively Reduces IL-21 Production by CD4 T Cells and IgG4 Autoantibodies in Rheumatoid Arthritis. International Journal of Biological Sciences 2013, 9, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Dienz, O.; Rincon, M. The Effects of IL-6 on CD4 T Cell Responses. Clinical Immunology 2009, 130, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Favalli, E.G. Understanding the Role of Interleukin-6 (IL-6) in the Joint and Beyond: A Comprehensive Review of IL-6 Inhibition for the Management of Rheumatoid Arthritis. Rheumatology and Therapy 2020, 7, 473–516. [Google Scholar] [CrossRef]
- Idborg, H.; Oke, V. Cytokines as Biomarkers in Systemic Lupus Erythematosus: Value for Diagnosis and Drug Therapy. International Journal of Molecular Sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Parodis, I.; Lindblom, J.; Toro-Domínguez, D.; Beretta, L.; Borghi, M.O.; Castillo, J.; Carnero-Montoro, E.; Enman, Y.; Mohan, C.; Alarcón-Riquelme, M.E.; et al. Interferon and B-Cell Signatures Inform Precision Medicine in Lupus Nephritis. Kidney International Reports 2024, 9, 1817–1835. [Google Scholar] [CrossRef] [PubMed]
- Sawaf, M.; Dumortier, H.; Monneaux, F. Follicular Helper T Cells in Systemic Lupus Erythematosus: Why Should They Be Considered as Interesting Therapeutic Targets? Journal of Immunology Research 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- López-Villalobos, E.F.; Muñoz-Valle, J.F.; Palafox-Sánchez, C.A.; García-Arellano, S.; Martínez-Fernández, D.E.; Orozco-Barocio, G.; García-Espinoza, J.A.; Oregon-Romero, E. Cytokine Profiles and Clinical Characteristics in Primary Sjögren´s Syndrome Patient Groups. Journal of Clinical Laboratory Analysis 2020, 35. [Google Scholar] [CrossRef]
- Sisto, M.; Tamma, R.; Ribatti, D.; Lisi, S. IL-6 Contributes to the TGF-Β1-Mediated Epithelial to Mesenchymal Transition in Human Salivary Gland Epithelial Cells. Archivum Immunologiae et Therapiae Experimentalis 2020, 68. [Google Scholar] [CrossRef]
- Sarrand, J.; Soyfoo, M.S. Involvement of Epithelial-Mesenchymal Transition (EMT) in Autoimmune Diseases. International Journal of Molecular Sciences 2023, 24, 14481. [Google Scholar] [CrossRef]
- Ma, D.; Feng, Y.; Lin, X. Immune and Non-Immune Mediators in the Fibrosis Pathogenesis of Salivary Gland in Sjögren’s Syndrome. Frontiers in Immunology 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, J.; Wen, Z.; Wu, Z.; Li, Q.; Xia, Y.; Yang, Q.; Yang, C. Serum IL-6 and TNF-α Levels Are Correlated with Disease Severity in Patients with Ankylosing Spondylitis. Laboratory Medicine 2021, 53, 149–155. [Google Scholar] [CrossRef]
- Yokota, K.; Sato, K.; Miyazaki, T.; Aizaki, Y.; Tanaka, S.; Sekikawa, M.; Kozu, N.; Kadono, Y.; Oda, H.; Mimura, T. Characterization and Function of Tumor Necrosis Factor and Interleukin-6–Induced Osteoclasts in Rheumatoid Arthritis. Arthritis & Rheumatology 2021, 73, 1145–1154. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. International Journal of Molecular Sciences 2023, 24, 4901. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Hayami, Y.; Naniwa, T.; Ueda, R. The Th17/IL-23 Axis and Natural Immunity in Psoriatic Arthritis. International Journal of Rheumatology 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gravallese, E.M.; Schett, G. Effects of the IL-23–IL-17 Pathway on Bone in Spondyloarthritis. Nature Reviews Rheumatology 2018, 14, 631–640. [Google Scholar] [CrossRef]
- Ibrahim-Achi, Z.; de Vera-González, A.; González-Delgado, A.; López-Mejías, R.; González-Gay, M.Á.; Ferraz-Amaro, I. Interleukin-6 Serum Levels Are Associated with Disease Features and Cardiovascular Risk in Patients with Systemic Sclerosis. Clinical and Experimental Rheumatology 2023. [Google Scholar] [CrossRef]
- Cardoneanu, A.; Burlui, A.M.; Macovei, L.A.; Bratoiu, I.; Richter, P.; Rezus, E. Targeting Systemic Sclerosis from Pathogenic Mechanisms to Clinical Manifestations: Why IL-6? Biomedicines 2022, 10, 318. [Google Scholar] [CrossRef]
- Yoshizaki, K. Pathogenic Role of IL-6 Combined with TNF-α or IL-1 in the Induction of Acute Phase Proteins SAA and CRP in Chronic Inflammatory Diseases. In Advances in Experimental Medicine and Biology; Springer New York: New York, NY, 2010; pp. 141–150. [Google Scholar]
- Saito, F.; Tasaka, S.; Inoue, K.; Miyamoto, K.; Nakano, Y.; Ogawa, Y.; Yamada, W.; Shiraishi, Y.; Hasegawa, N.; Fujishima, S.; et al. Role of Interleukin-6 in Bleomycin-Induced Lung Inflammatory Changes in Mice. American Journal of Respiratory Cell and Molecular Biology 2008, 38, 566–571. [Google Scholar] [CrossRef]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S. The IL-17 Family in Diseases: From Bench to Bedside. Signal Transduction and Targeted Therapy 2023, 8. [Google Scholar] [CrossRef]
- Sieper, J.; Poddubnyy, D.; Miossec, P. The IL-23–IL-17 Pathway as a Therapeutic Target in Axial Spondyloarthritis. Nature Reviews Rheumatology 2019, 15, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, M. The Role of IL-17 in Systemic Autoinflammatory Diseases: Mechanisms and Therapeutic Perspectives. Clinical Reviews in Allergy & Immunology 2025, 68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ouyang, Y.; You, W.; Liu, W.; Cheng, Y.; Mai, X.; Shen, Z. Physiological Roles of Human Interleukin-17 Family. Experimental Dermatology 2023, 33. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Amatya, N.; Revu, S.; Jawale, C.V.; Wu, D.; Rittenhouse, N.; Menk, A.; Kupul, S.; Du, F.; Raphael, I.; et al. IL-17 Metabolically Reprograms Activated Fibroblastic Reticular Cells for Proliferation and Survival. Nature Immunology 2019, 20, 534–545. [Google Scholar] [CrossRef]
- Gaffen, S.L. The Role of Interleukin-17 in the Pathogenesis of Rheumatoid Arthritis. Current Rheumatology Reports 2009, 11, 365–370. [Google Scholar] [CrossRef]
- Yin, R.; Xu, R.; Ding, L.; Sui, W.; Niu, M.; Wang, M.; Xu, L.; Wang, H.; Srirat, C. Circulating IL-17 Level Is Positively Associated with Disease Activity in Patients with Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. BioMed Research International 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Zhang, J.; Lin, Y.; Chen, W.; Fan, X.; Zhang, D. Pathogenesis and Treatment of Sjogren’s Syndrome: Review and Update. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Lorusso, L.; Ingravallo, G.; Ribatti, D.; Lisi, S. TGFβ1-Smad Canonical and -Erk Noncanonical Pathways Participate in Interleukin-17-Induced Epithelial–Mesenchymal Transition in Sjögren’s Syndrome. Laboratory Investigation 2020, 100, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.Q.; Yin, H.; Lee, B.H.; Chiorini, J.A.; Peck, A.B. IL17: Potential Therapeutic Target in Sjögren’s Syndrome Using Adenovirus-Mediated Gene Transfer. Laboratory Investigation 2011, 91, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Feng, Y.; Lin, X. Immune and Non-Immune Mediators in the Fibrosis Pathogenesis of Salivary Gland in Sjögren’s Syndrome. Frontiers in Immunology 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Kollnberger, S.D.; Wedderburn, L.R.; Bowness, P. Expansion and Enhanced Survival of Natural Killer Cells Expressing the Killer Immunoglobulin-like Receptor KIR3DL2 in Spondylarthritis. Arthritis & Rheumatism 2005, 52, 3586–3595. [Google Scholar] [CrossRef]
- Harrison, S.R.; Marzo-Ortega, H. Have Therapeutics Enhanced Our Knowledge of Axial Spondyloarthritis? Current Rheumatology Reports 2023, 25, 56–67. [Google Scholar] [CrossRef]
- Colbert, R.A.; DeLay, M.L.; Klenk, E.I.; Layh-Schmitt, G. From HLA-B27 to Spondyloarthritis: A Journey through the ER. Immunological Reviews 2009, 233, 181–202. [Google Scholar] [CrossRef]
- Wilson, N.J.; Boniface, K.; Chan, J.R.; McKenzie, B.S.; Blumenschein, W.M.; Mattson, J.D.; Basham, B.; Smith, K.; Chen, T.; Morel, F.; et al. Development, Cytokine Profile and Function of Human Interleukin 17–Producing Helper T Cells. Nature Immunology 2007, 8, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Steel, K.J.A.; Srenathan, U.; Ridley, M.; Durham, L.E.; Wu, S.; Ryan, S.E.; Hughes, C.D.; Chan, E.; Kirkham, B.W.; Taams, L.S. Polyfunctional, Proinflammatory, Tissue-Resident Memory Phenotype and Function of Synovial Interleukin-17A+CD8+ T Cells in Psoriatic Arthritis. Arthritis & Rheumatology 2020, 72, 435–447. [Google Scholar] [CrossRef]
- Seki, N.; Tsujimoto, H.; Tanemura, S.; Ishigaki, S.; Takei, H.; Sugahara, K.; Yoshimoto, K.; Akiyama, M.; Kaneko, Y.; Chiba, K.; et al. Th17/IL-17A Axis Is Critical for Pulmonary Arterial Hypertension (PAH) in Systemic Sclerosis (SSc): SSc Patients with High Levels of Serum IL-17A Exhibit Reduced Lung Functions and Increased Prevalence of PAH. Cytokine 2024, 176, 156534. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Xing, X.; Wan, L.; Li, M. Increased Frequency of Th17 Cells in Systemic Sclerosis Is Related to Disease Activity and Collagen Overproduction. Arthritis Research & Therapy, 2014; 16. [Google Scholar] [CrossRef]
- Lin, C.M.A.; Isaacs, J.D.; Cooles, F.A.H. Role of IFN-α in Rheumatoid Arthritis. Current Rheumatology Reports 2023, 26, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kato, M. New Insights into IFN-γ in Rheumatoid Arthritis: Role in the Era of JAK Inhibitors. Immunological Medicine 2020, 43, 72–78. [Google Scholar] [CrossRef]
- Puigdevall, L.; Michiels, C.; Stewardson, C.; Dumoutier, L. JAK/STAT: Why Choose a Classical or an Alternative Pathway When You Can Have Both? Journal of Cellular and Molecular Medicine 2022, 26, 1865–1875. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNγ: Signalling, Epigenetics and Roles in Immunity, Metabolism, Disease and Cancer Immunotherapy. Nature Reviews Immunology 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Heo, S.; Kang, J.R.; Kweon, J.; Lee, Y.; Baek, J.-H. Rheumatoid Arthritis: A Complex Tale of Autoimmune Hypersensitivity. Exploration of Immunology 2024, 358–375. [Google Scholar] [CrossRef]
- Lee, K.; Min, H.K.; Koh, S.-H.; Lee, S.-H.; Kim, H.-R.; Ju, J.H.; Kim, H.-Y. Prognostic Signature of Interferon-γ and Interleurkin-17A in Early Rheumatoid Arthritis. Clinical and Experimental Rheumatology 2021. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J. ye; Kim, S.-Y.; Jung, K.; Cho, M.-L. Interferon-Gamma Regulates Inflammatory Cell Death by Targeting Necroptosis in Experimental Autoimmune Arthritis. Scientific Reports 2017, 7. [Google Scholar] [CrossRef]
- Ying Chen IFN-γ Promotes the Development of Systemic Lupus Erythematosus through the IFNGR1/2-PSTAT1-TBX21 Signaling Axis. Am J Transl Res.
- Lee, Y.H.; Song, G.G. Association between the Interferon-γ +874 T/A Polymorphism and Susceptibility to Systemic Lupus Erythematosus and Rheumatoid Arthritis: A Meta-analysis. International Journal of Immunogenetics 2022, 49, 365–371. [Google Scholar] [CrossRef]
- Sebastian, A.; Madej, M.; Gajdanowicz, P.; Sebastian, M.; Łuczak, A.; Zemelka-Wiącek, M.; Jutel, M.; Wiland, P. Interferon Gamma Targeted Therapy: Is It Justified in Primary Sjögren’s Syndrome? Journal of Clinical Medicine 2022, 11, 5405. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Liu, Z.; Li, X. Tear Cytokine Levels in Sjogren’s Syndrome-Related Dry Eye Disease Compared with Non-Sjogren’s Syndrome-Related Dry Eye Disease Patients: A Meta-Analysis. Medicine 2024, 103, e40669. [Google Scholar] [CrossRef]
- Cao, T.; Zhou, J.; Liu, Q.; Mao, T.; Chen, B.; Wu, Q.; Wang, L.; Pathak, J.L.; Watanabe, N.; Li, J. Interferon-γ Induces Salivary Gland Epithelial Cell Ferroptosis in Sjogren’s Syndrome via JAK/STAT1-Mediated Inhibition of System Xc-. Free Radical Biology and Medicine 2023, 205, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Pathak, J.L.; Cao, T.; Chen, B.; Wei, W.; Hu, S.; Mao, T.; Wu, X.; Watanabe, N.; Li, X.; et al. CD4 T Cell-Secreted IFN-γ in Sjögren’s Syndrome Induces Salivary Gland Epithelial Cell Ferroptosis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2024, 1870, 167121. [Google Scholar] [CrossRef] [PubMed]
- Finotti, G.; Tamassia, N.; Cassatella, M.A. Interferon-Λs and Plasmacytoid Dendritic Cells: A Close Relationship. Frontiers in Immunology 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lou, B.; Jiang, Z.; Yu, C. Predictive Values of Blood Type I and Type II Interferon Production for Disease Activity and Clinical Response to TNF-<I>A</I> Blocking Therapy in Patients with Ankylosing Spondylitis. The Tohoku Journal of Experimental Medicine 2023, 260, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, G.; Guan, Y.; Zhao, X.; Wang, Q.; Li, H.; Qi, J. Association of IFN-γ Polymorphisms with Ankylosing Spondylitis Risk. Journal of Cellular and Molecular Medicine 2020, 24, 10615–10620. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Adamopoulos, I.E. Psoriatic Arthritis under the Influence of IFNγ. Clinical Immunology 2020, 218, 108513. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Ntouros, P.A.; Nezos, A.; Vlachogiannis, N.I.; McInnes, I.B.; Tektonidou, M.G.; Skarlis, C.; Souliotis, V.L.; Mavragani, C.P.; Sfikakis, P.P. Type-I Interferon Pathway and DNA Damage Accumulation in Peripheral Blood of Patients with Psoriatic Arthritis. Frontiers in Immunology 2023, 14. [Google Scholar] [CrossRef]
- Terao, C.; Kawaguchi, T.; Dieude, P.; Varga, J.; Kuwana, M.; Hudson, M.; Kawaguchi, Y.; Matucci-Cerinic, M.; Ohmura, K.; Riemekasten, G.; et al. Transethnic Meta-Analysis Identifies GSDMA and PRDM1 as Susceptibility Genes to Systemic Sclerosis. Annals of the Rheumatic Diseases 2017, 76, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, O.; Martin, J.-E.; Rueda, B.; Koeleman, B.P.C.; Ying, J.; Teruel, M.; Diaz-Gallo, L.-M.; Broen, J.C.; Vonk, M.C.; Simeon, C.P.; et al. Identification of Novel Genetic Markers Associated with Clinical Phenotypes of Systemic Sclerosis through a Genome-Wide Association Strategy. PLoS Genetics 2011, 7, e1002178. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-Inflammatory Effect of IL-10 Mediated by Metabolic Reprogramming of Macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Bieber, K.; Manz, R.A. IL-10 Revisited in Systemic Lupus Erythematosus. Frontiers in Immunology 2022, 13. [Google Scholar] [CrossRef]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of Interleukin-10. Cytokine & Growth Factor Reviews 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Mittal, S.K.; Roche, P.A. Suppression of Antigen Presentation by IL-10. Current Opinion in Immunology 2015, 34, 22–27. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. International Journal of Molecular Sciences 2021, 22, 4972. [Google Scholar] [CrossRef] [PubMed]
- M1 and M2 Macrophages Polarization via mTORC1 Influences Innate Immunity and Outcome of Ehrlichia Infection. Journal of Cellular Immunology 2020, 2. [CrossRef]
- Paschalidi, P.; Gkouveris, I.; Soundia, A.; Kalfarentzos, E.; Vardas, E.; Georgaki, M.; Kostakis, G.; Erovic, B.M.; Tetradis, S.; Perisanidis, C.; et al. The Role of M1 and M2 Macrophage Polarization in Progression of Medication-Related Osteonecrosis of the Jaw. Clinical Oral Investigations 2020, 25, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Kunkel, S.L.; Chang, C.-H. Negative Regulation of MyD88-Dependent Signaling by IL-10 in Dendritic Cells. Proceedings of the National Academy of Sciences 2009, 106, 18327–18332. [Google Scholar] [CrossRef]
- Greenhill, C.J.; Jones, G.W.; Nowell, M.A.; Newton, Z.; Harvey, A.K.; Moideen, A.N.; Collins, F.L.; Bloom, A.C.; Coll, R.C.; Robertson, A.A.; et al. Interleukin-10 Regulates the Inflammasome-Driven Augmentation of Inflammatory Arthritis and Joint Destruction. Arthritis Research & Therapy 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Cush, J.J.; Splawski, J.B.; Thomas, R.; Mcfarlin, J.E.; Schulze-Koops, H.; Davis, L.S.; Fujita, K.; Lipsky, P.E. Elevated Interleukin-10 Levels in Patients with Rheumatoid Arthritis. Arthritis & Rheumatism 1995, 38, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.G.-K.; Sugiyama, E.; Shinoda, K.; Taki, H.; Hounoki, H.; Abdel-Aziz, H.O.; Maruyama, M.; Kobayashi, M.; Ogawa, H.; Miyahara, T. Interleukin-10 Inhibits RANKL-Mediated Expression of NFATc1 in Part via Suppression of c-Fos and c-Jun in RAW264.7 Cells and Mouse Bone Marrow Cells. Bone 2007, 41, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Torres, V.; Castejón, R.; Martínez-Urbistondo, M.; Gutiérrez-Rojas, Á.; Vázquez-Comendador, J.; Tutor, P.; Durán-del Campo, P.; Mellor-Pita, S.; Rosado, S.; Vargas-Núñez, J. Serum Cytokines to Predict Systemic Lupus Erythematosus Clinical and Serological Activity. Clinical and Translational Science 2022, 15, 1676–1686. [Google Scholar] [CrossRef]
- Richter, P.; Macovei, L.A.; Rezus, C.; Boiculese, V.L.; Buliga-Finis, O.N.; Rezus, E. IL-10 in Systemic Lupus Erythematosus: Balancing Immunoregulation and Autoimmunity. International Journal of Molecular Sciences 2025, 26, 3290. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, Z.; Tang, X.; Li, W.; Chen, W.; Yao, G. IL-27 Regulated CD4+IL-10+ T Cells in Experimental Sjögren Syndrome. Frontiers in Immunology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Ma, J.; Cui, J.; Gu, Y.; Shan, Y. Subpopulation Dynamics of T and B Lymphocytes in Sjögren’s Syndrome: Implications for Disease Activity and Treatment. Frontiers in Immunology 2024, 15. [Google Scholar] [CrossRef]
- Ma, D.; Feng, Y.; Lin, X. Immune and Non-Immune Mediators in the Fibrosis Pathogenesis of Salivary Gland in Sjögren’s Syndrome. Frontiers in Immunology 2024, 15. [Google Scholar] [CrossRef]
- Braga, M.; Lara-Armi, F.F.; Neves, J.S.F.; Rocha-Loures, M.A.; Terron-Monich, M. de S.; Bahls-Pinto, L.D.; de Lima Neto, Q.A.; Zacarias, J.M.V.; Sell, A.M.; Visentainer, J.E.L. Influence of IL10 (Rs1800896) Polymorphism and TNF-α, IL-10, IL-17A, and IL-17F Serum Levels in Ankylosing Spondylitis. Frontiers in Immunology 2021, 12. [Google Scholar] [CrossRef]
- Shehata, L.; Thouvenel, C.D.; Hondowicz, B.D.; Pew, L.A.; Pritchard, G.H.; Rawlings, D.J.; Choi, J.; Pepper, M. Interleukin-4 Downregulates Transcription Factor BCL6 to Promote Memory B Cell Selection in Germinal Centers. Immunity 2024, 57, 843–858.e5. [Google Scholar] [CrossRef]
- Duan, L.; Liu, D.; Chen, H.; Mintz, M.A.; Chou, M.Y.; Kotov, D.I.; Xu, Y.; An, J.; Laidlaw, B.J.; Cyster, J.G. Follicular Dendritic Cells Restrict Interleukin-4 Availability in Germinal Centers and Foster Memory B Cell Generation. Immunity 2021, 54, 2256–2272.e6. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E. IL-4 and IL-13: Regulators and Effectors of Wound Repair. Annual Review of Immunology 2023, 41, 229–254. [Google Scholar] [CrossRef]
- Pan, K.; Li, Q.; Guo, Z.; Li, Z. Healing Action of Interleukin-4 (IL-4) in Acute and Chronic Inflammatory Conditions: Mechanisms and Therapeutic Strategies. Pharmacology & Therapeutics 2025, 265, 108760. [Google Scholar] [CrossRef]
- Yang, W.-C.; Hwang, Y.-S.; Chen, Y.-Y.; Liu, C.-L.; Shen, C.-N.; Hong, W.-H.; Lo, S.-M.; Shen, C.-R. Interleukin-4 Supports the Suppressive Immune Responses Elicited by Regulatory T Cells. Frontiers in Immunology 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef]
- Panda, S.K.; Wigerblad, G.; Jiang, L.; Jiménez-Andrade, Y.; Iyer, V.S.; Shen, Y.; Boddul, S.V.; Guerreiro-Cacais, A.O.; Raposo, B.; Kasza, Z.; et al. IL-4 Controls Activated Neutrophil FcγR2b Expression and Migration into Inflamed Joints. Proceedings of the National Academy of Sciences 2020, 117, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-C.; Hwang, Y.-S.; Chen, Y.-Y.; Liu, C.-L.; Shen, C.-N.; Hong, W.-H.; Lo, S.-M.; Shen, C.-R. Interleukin-4 Supports the Suppressive Immune Responses Elicited by Regulatory T Cells. Frontiers in Immunology 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Liu, Z.; Li, X. Tear Cytokine Levels in Sjogren’s Syndrome-Related Dry Eye Disease Compared with Non-Sjogren’s Syndrome-Related Dry Eye Disease Patients: A Meta-Analysis. Medicine 2024, 103, e40669. [Google Scholar] [CrossRef]
- Wirth, T.; Balandraud, N.; Boyer, L.; Lafforgue, P.; Pham, T. Biomarkers in Psoriatic Arthritis: A Meta-Analysis and Systematic Review. Frontiers in Immunology 2022, 13. [Google Scholar] [CrossRef]
- Onderdijk, A.J.; Baerveldt, E.M.; Kurek, D.; Kant, M.; Florencia, E.F.; Debets, R.; Prens, E.P. IL-4 Downregulates IL-1β and IL-6 and Induces GATA3 in Psoriatic Epidermal Cells: Route of Action of a Th2 Cytokine. The Journal of Immunology 2015, 195, 1744–1752. [Google Scholar] [CrossRef]
- Guenova, E.; Skabytska, Y.; Hoetzenecker, W.; Weindl, G.; Sauer, K.; Tham, M.; Kim, K.-W.; Park, J.-H.; Seo, J.H.; Ignatova, D.; et al. IL-4 Abrogates T H 17 Cell-Mediated Inflammation by Selective Silencing of IL-23 in Antigen-Presenting Cells. Proceedings of the National Academy of Sciences 2015, 112, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harbor Perspectives in Biology 2017, 9, a022236. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease and Therapeutics. Signal Transduction and Targeted Therapy 2024, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qian, A.; Cheng, Y.; Li, M.; Huang, C. Comprehensive Systematic Review and Meta-Analysis of the TGF-Β1 T869C Gene Polymorphism and Autoimmune Disease Susceptibility. Frontiers in Genetics 2025, 16. [Google Scholar] [CrossRef] [PubMed]
- Susianti, H.; Handono, K.; Purnomo, B.B.; Widodo, N.; Gunawan, A.; Kalim, H. Changes to Signal Peptide and the Level of Transforming Growth Factor- Β1 Due to T869C Polymorphism of TGF Β1 Associated with Lupus Renal Fibrosis. SpringerPlus 2014, 3. [Google Scholar] [CrossRef]
- Kim, S.; Han, S.; Kim, G.; Lee, J.; Kang, Y. TGF-SS1 Polymorphism Determines the Progression of Joint Damage in Rheumatoid Arthritis. Scandinavian Journal of Rheumatology 2004, 33, 389–394. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, W.; Song, H.; Long, X.; Zhang, Z.; Tang, X.; Chen, H.; Lin, H.; Sun, L. Tofacitinib Ameliorates Lupus Through Suppression of T Cell Activation Mediated by TGF-Beta Type I Receptor. Frontiers in Immunology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Lauraine, M.; de Taffin de Tilques, M.; Melamed-Kadosh, D.; Cherqaoui, B.; Rincheval, V.; Prevost, E.; Rincheval-Arnold, A.; Cela, E.; Admon, A.; Guénal, I.; et al. TGFβ Signaling Pathway Is Altered by HLA-B27 Expression, Resulting in Pathogenic Consequences Relevant for Spondyloarthritis. Arthritis Research & Therapy 2024, 26. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.A.; Mendoza, F.A.; Piera-Velazquez, S. A Review of Recent Studies on the Pathogenesis of Systemic Sclerosis: Focus on Fibrosis Pathways. Frontiers in Immunology 2025, 16. [Google Scholar] [CrossRef]
- Leask, A. Targeting the TGFβ, Endothelin-1 and CCN2 Axis to Combat Fibrosis in Scleroderma. Cellular Signalling 2008, 20, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harbor Perspectives in Biology 2017, 9, a022236. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.T.; Gonçalves, S.M.C.; Almeida, A.R. de; Gonçalves, R.S.G.; Sampaio, M.C.P.D.; Vilar, K. de M.; Pereira, M.C.; Rêgo, M.J.B. de M.; Pitta, I. da R.; Marques, C.D.L.; et al. Reassessing the Role of the Active TGF-Β1 as a Biomarker in Systemic Sclerosis: Association of Serum Levels with Clinical Manifestations. Disease Markers 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fuentelsaz-Romero, S.; Cuervo, A.; Estrada-Capetillo, L.; Celis, R.; García-Campos, R.; Ramírez, J.; Sastre, S.; Samaniego, R.; Puig-Kröger, A.; Cañete, J.D. GM-CSF Expression and Macrophage Polarization in Joints of Undifferentiated Arthritis Patients Evolving to Rheumatoid Arthritis or Psoriatic Arthritis. Frontiers in Immunology 2021, 11. [Google Scholar] [CrossRef]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef]
- Cook, A.D.; Braine, E.L.; Campbell, I.K.; Rich, M.J.; Hamilton, J.A. Blockade of Collagen-Induced Arthritis Post-Onset by Antibody to Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF): Requirement for GM-CSF in the Effector Phase of Disease. Arthritis Research 2001, 3. [Google Scholar] [CrossRef]
- Kim, H.-R.; Kim, K.-W.; Kim, B.-M.; Cho, M.-L.; Lee, S.-H. The Effect of Vascular Endothelial Growth Factor on Osteoclastogenesis in Rheumatoid Arthritis. PLOS ONE 2015, 10, e0124909. [Google Scholar] [CrossRef]
- Yoo, S.-A.; Kwok, S.-K.; Kim, W.-U. Proinflammatory Role of Vascular Endothelial Growth Factor in the Pathogenesis of Rheumatoid Arthritis: Prospects for Therapeutic Intervention. Mediators of Inflammation 2008, 2008. [Google Scholar] [CrossRef]
- Bilgi, P.T.; Cetin, E.; Ozgonenel, L.; Aslan, A.; Aral, H.; Inal, B.B.; Guvenen, G. Elevated Levels of Serum Vascular Endothelial Growth Factor in Patients with Rheumatoid Arthritis. Clinical Biochemistry 2009, 42, 343. [Google Scholar] [CrossRef]
- Di Lorenzo, B.; Zoroddu, S.; Mangoni, A.A.; Paliogiannis, P.; Erre, G.L.; Satta, R.; Carru, C.; Zinellu, A. VEGF in Psoriatic Arthritis: Systematic Review and Meta-Analysis. Clinica Chimica Acta 2025, 567, 120084. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Xu, B.; Kumar, M. Biomarkers in Immunology: Their Impact on Immune Function and Response. Advances in Biomarker Sciences and Technology 2025, 7, 95–110. [Google Scholar] [CrossRef]
- Decker, M.-L.; Gotta, V.; Wellmann, S.; Ritz, N. Cytokine Profiling in Healthy Children Shows Association of Age with Cytokine Concentrations. Scientific Reports 2017, 7. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Yu, K.; Bagni, R.K.; Lan, Q.; Rothman, N.; Purdue, M.P. Intra-Individual Variability over Time in Serum Cytokine Levels among Participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cytokine 2011, 56, 145–148. [Google Scholar] [CrossRef]
- Kaushik, D.; Xu, B.; Kumar, M. Biomarkers in Immunology: Their Impact on Immune Function and Response. Advances in Biomarker Sciences and Technology 2025, 7, 95–110. [Google Scholar] [CrossRef]
- Tarrant, J.M. Blood Cytokines as Biomarkers of In Vivo Toxicity in Preclinical Safety Assessment: Considerations for Their Use. Toxicological Sciences 2010, 117, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Angrish, M.M.; Pleil, J.D.; Stiegel, M.A.; Madden, M.C.; Moser, V.C.; Herr, D.W. Taxonomic Applicability of Inflammatory Cytokines in Adverse Outcome Pathway (AOP) Development. Journal of Toxicology and Environmental Health, Part A 2016, 79, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Chetaille Nézondet, A.-L.; Poubelle, P.E.; Pelletier, M. The Evaluation of Cytokines to Help Establish Diagnosis and Guide Treatment of Autoinflammatory and Autoimmune Diseases. Journal of Leukocyte Biology 2020, 108, 647–657. [Google Scholar] [CrossRef]
- Wang, X.; Fan, D.; Yang, Y.; Gimple, R.C.; Zhou, S. Integrative Multi-Omics Approaches to Explore Immune Cell Functions: Challenges and Opportunities. iScience 2023, 26, 106359. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; Zhu, W. Unveiling Causal Pathways in Autoimmune Diseases: A Multi-Omics Approach. Autoimmunity 2025, 58. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.G.; Gartzonikas, I.K.; Pappa, T.K.; Markatseli, T.E.; Migkos, M.P.; Voulgari, P.V.; Drosos, A.A. Eight-Year Survival Study of First-Line Tumour Necrosis Factor α Inhibitors in Rheumatoid Arthritis: Real-World Data from a University Centre Registry. Rheumatology Advances in Practice 2019, 3. [Google Scholar] [CrossRef] [PubMed]
- Gossec, L.; Smolen, J.S.; Ramiro, S.; de Wit, M.; Cutolo, M.; Dougados, M.; Emery, P.; Landewé, R.; Oliver, S.; Aletaha, D.; et al. European League Against Rheumatism (EULAR) Recommendations for the Management of Psoriatic Arthritis with Pharmacological Therapies: 2015 Update. Annals of the Rheumatic Diseases 2016, 75, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montoya, L.; Emery, P. Disease Modification in Ankylosing Spondylitis with TNF Inhibitors: Spotlight on Early Phase Clinical Trials. Expert Opinion on Investigational Drugs 2021, 30, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Ravaud, P.; Steinfeld, S.; Baron, G.; Goetz, J.; Hachulla, E.; Combe, B.; Puéchal, X.; Pennec, Y.; Sauvezie, B.; et al. Inefficacy of Infliximab in Primary Sjögren’s Syndrome: Results of the Randomized, Controlled Trial of Remicade in Primary Sjögren’s Syndrome (TRIPSS). Arthritis & Rheumatism 2004, 50, 1270–1276. [Google Scholar] [CrossRef]
- Sankar, V.; Brennan, M.T.; Kok, M.R.; Leakan, R.A.; Smith, J.A.; Manny, J.; Baum, B.J.; Pillemer, S.R. Etanercept in Sjögren’s Syndrome: A Twelve-week Randomized, Double-blind, Placebo-controlled Pilot Clinical Trial. Arthritis & Rheumatism 2004, 50, 2240–2245. [Google Scholar] [CrossRef]
- Murdaca, G. , Spanò, F., Contatore, M., Guastalla, A., & Puppo, F. Potential use of TNF-α inhibitors in systemic sclerosis. Immunotherapy 2014, 6, 283–289. [Google Scholar] [CrossRef]
- Shovman, O.; Tamar, S.; Amital, H.; Watad, A.; Shoenfeld, Y. Diverse Patterns of Anti-TNF-α-Induced Lupus: Case Series and Review of the Literature. Clinical Rheumatology 2017, 37, 563–568. [Google Scholar] [CrossRef]
- Rovin, B.H.; van Vollenhoven, R.F.; Aranow, C.; Wagner, C.; Gordon, R.; Zhuang, Y.; Belkowski, S.; Hsu, B. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Treatment With Sirukumab (CNTO 136) in Patients With Active Lupus Nephritis. Arthritis & Rheumatology 2016, 68, 2174–2183. [Google Scholar] [CrossRef]
- Liu, N.; Su, D.; Liu, K.; Liu, B.; Wang, S.; Zhang, X. The Effects of IL-17/IL-17R Inhibitors on Atherosclerosis in Psoriasis and Psoriatic Arthritis. Medicine 2021, 100, e24549. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, S. , Nikiphorou, E., Sepriano, A., Ortolan, A., Webers, C., Baraliakos, X., Landewé, R. B. M., Van den Bosch, F. E., Boteva, B., Bremander, A., et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Annals of the rheumatic diseases 2023, 82, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Sambataro, D. , Sambataro, G., Dal Bosco, Y., & Polosa, R. Present and future of biologic drugs in primary Sjögren's syndrome. Expert opinion on biological therapy 2017, 17, 63–75. [Google Scholar] [CrossRef]
- Wu, D.; Hou, S.-Y.; Zhao, S.; Hou, L.-X.; Jiao, T.; Xu, N.-N.; Zhang, N. Meta-Analysis of IL-17 Inhibitors in Two Populations of Rheumatoid Arthritis Patients: Biologic-Naïve or Tumor Necrosis Factor Inhibitor Inadequate Responders. Clinical Rheumatology 2019, 38, 2747–2756. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Hahn, B.H.; Tsokos, G.C.; Wagner, C.L.; Lipsky, P.; Touma, Z.; Werth, V.P.; Gordon, R.M.; Zhou, B.; Hsu, B.; et al. Efficacy and Safety of Ustekinumab, an IL-12 and IL-23 Inhibitor, in Patients with Active Systemic Lupus Erythematosus: Results of a Multicentre, Double-Blind, Phase 2, Randomised, Controlled Study. The Lancet 2018, 392, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q. Biologic therapy in Sjögren's syndrome. Clinical rheumatology 2021, 40, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Danve, A. , & Deodhar, A. Treatment of axial spondyloarthritis: an update. Nature reviews. Rheumatology 2022, 18, 205–216. [Google Scholar] [CrossRef]
- Yavari, K.; Grisanti, J. Case Report on the Use of Canakinumab for Treatment of Recurrent Fevers and Proteinuria in Refractory Systemic Lupus Erythematosus. Therapeutic Advances in Rare Disease 2023, 4. [Google Scholar] [CrossRef]
- Arnold, D. D. , Yalamanoglu, A., & Boyman, O. Systematic Review of Safety and Efficacy of IL-1-Targeted Biologics in Treating Immune-Mediated Disorders. Frontiers in immunology 2022, 13, 888392. [Google Scholar] [PubMed]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harbor Perspectives in Biology 2017, 11, a028548. [Google Scholar] [CrossRef]
- DiDonato, M.; Simpson, C.T.; Vo, T.; Knuth, M.; Geierstanger, B.; Jamontt, J.; Jones, D.H.; Fathman, J.W.; DeLarosa, D.; Junt, T.; et al. A Novel Interleukin-10 Antibody Graft to Treat Inflammatory Bowel Disease. Structure 2025, 33, 475–488.e7. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Singh, S.P.; Egwuagu, C.E. IL-6/IL-12 Superfamily of Cytokines and Regulatory Lymphocytes Play Critical Roles in the Etiology and Suppression of CNS Autoimmune Diseases. Frontiers in Immunology 2025, 16. [Google Scholar] [CrossRef]
- Scott, L.J. Correction to: Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2018, 78, 285. [Google Scholar] [CrossRef]
- Kastrati, K. , Aletaha, D., Burmester, G. R., Chwala, E., Dejaco, C., Dougados, M., McInnes, I. B., Ravelli, A., Sattar, N., Stamm, T. A., et al. (2022). A systematic literature review informing the consensus statement on efficacy and safety of pharmacological treatment with interleukin-6 pathway inhibition with biological DMARDs in immune-mediated inflammatory diseases. RMD open 2022, 8, e002359. [Google Scholar] [CrossRef]
- Khanna, D. , Lin, C. J. F., Furst, D. E., Goldin, J., Kim, G., Kuwana, M., Allanore, Y., Matucci-Cerinic, M., Distler, O., Shima, Y., et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. Respiratory medicine 2020, 8, 963–974. [Google Scholar] [CrossRef]
- Del Galdo, F. , Lescoat, A., Conaghan, P. G., Bertoldo, E., Čolić, J., Santiago, T., Suliman, Y. A., Matucci-Cerinic, M., Gabrielli, A., Distler, O., et al. EULAR recommendations for the treatment of systemic sclerosis: 2023 update. Annals of the rheumatic diseases 2025, 84, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Felten, R.; Giannini, M.; Nespola, B.; Lannes, B.; Levy, D.; Seror, R.; Vittecoq, O.; Hachulla, E.; Perdriger, A.; Dieude, P.; et al. Refining Myositis Associated with Primary Sjögren’s Syndrome: Data from the Prospective Cohort ASSESS. Rheumatology 2020, 60, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Illei, G.G.; Shirota, Y.; Yarboro, C.H.; Daruwalla, J.; Tackey, E.; Takada, K.; Fleisher, T.; Balow, J.E.; Lipsky, P.E. Tocilizumab in Systemic Lupus Erythematosus: Data on Safety, Preliminary Efficacy, and Impact on Circulating Plasma Cells from an Open-label Phase I Dosage-escalation Study. Arthritis & Rheumatism 2010, 62, 542–552. [Google Scholar] [CrossRef]
- Marinho, A. , Delgado Alves, J., Fortuna, J., Faria, R., Almeida, I., Alves, G., Araújo Correia, J., Campar, A., Brandão, M., Crespo, J., et al. Biological therapy in systemic lupus erythematosus, antiphospholipid syndrome, and Sjögren's syndrome: evidence- and practice-based guidance. Frontiers in immunology 2023, 14, 1117699. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.M.; Schechtman, J.; Bennett, R.; Handel, M.L.; Burmester, G.; Tesser, J.; Modafferi, D.; Poulakos, J.; Sun, G. Anakinra, a Recombinant Human Interleukin-1 Receptor Antagonist (r-metHuIL-1ra), in Patients with Rheumatoid Arthritis: A Large, International, Multicenter, Placebo-controlled Trial. Arthritis & Rheumatism 2003, 48, 927–934. [Google Scholar] [CrossRef]
- Norheim, K. B. , Harboe, E., Gøransson, L. G., & Omdal, R. Interleukin-1 inhibition and fatigue in primary Sjögren's syndrome--a double blind, randomised clinical trial. PloS one 2012, 7, e30123. [Google Scholar] [CrossRef]
- Baker, T.; Sharifian, H.; Newcombe, P.J.; Gavin, P.G.; Lazarus, M.N.; Ramaswamy, M.; White, W.I.; Ferrari, N.; Muthas, D.; Tummala, R.; et al. Type I Interferon Blockade with Anifrolumab in Patients with Systemic Lupus Erythematosus Modulates Key Immunopathological Pathways in a Gene Expression and Proteomic Analysis of Two Phase 3 Trials. Annals of the Rheumatic Diseases 2024, 83, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A. , Kostopoulou, M., Andersen, J., Aringer, M., Arnaud, L., Bae, S. C., Boletis, J., Bruce, I. N., Cervera, R., Doria, A., et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Annals of the Rheumatic Diseases 2024, 83, 15–29. [Google Scholar] [CrossRef]
- Blagov, A. V. , Kashtalap, V. V., Lapshina, K. O., Karimova, A. E., Asoyan, A. Z., & Orekhov, A. New strategies for treating Sjogren's syndrome. Cellular and molecular biology (Noisy-le-Grand, France) 2025, 71, 111–119. [Google Scholar] [CrossRef]
- Kiełbowski, K.; Plewa, P.; Bratborska, A.W.; Bakinowska, E.; Pawlik, A. JAK Inhibitors in Rheumatoid Arthritis: Immunomodulatory Properties and Clinical Efficacy. International Journal of Molecular Sciences 2024, 25, 8327. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update. Annals of the Rheumatic Diseases 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. International Journal of Molecular Sciences 2023, 24, 4901. [Google Scholar] [CrossRef]
- Gossec, L.; Kerschbaumer, A.; Ferreira, R.J.O.; Aletaha, D.; Baraliakos, X.; Bertheussen, H.; Boehncke, W.-H.; Esbensen, B.A.; McInnes, I.B.; McGonagle, D.; et al. EULAR Recommendations for the Management of Psoriatic Arthritis with Pharmacological Therapies: 2023 Update. Annals of the Rheumatic Diseases 2024, 83, 706–719. [Google Scholar] [CrossRef]
- Ahmed, S.; Yesudian, R.; Ubaide, H.; Coates, L.C. Rationale and Concerns for Using JAK Inhibitors in Axial Spondyloarthritis. Rheumatology Advances in Practice 2024, 8. [Google Scholar] [CrossRef]
- Ramiro, S.; Nikiphorou, E.; Sepriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.M.; Van den Bosch, F.E.; Boteva, B.; Bremander, A.; et al. ASAS-EULAR Recommendations for the Management of Axial Spondyloarthritis: 2022 Update. Annals of the Rheumatic Diseases 2023, 82, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hasni, S.A.; Gupta, S.; Davis, M.; Poncio, E.; Temesgen-Oyelakin, Y.; Carlucci, P.M.; Wang, X.; Naqi, M.; Playford, M.P.; Goel, R.R.; et al. Phase 1 Double-Blind Randomized Safety Trial of the Janus Kinase Inhibitor Tofacitinib in Systemic Lupus Erythematosus. Nature Communications 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, L.; Duan, X.; Huo, Y.; Liu, S.; Zhao, C.; Wang, Q.; Tian, X.; Chen, Y.; Li, M. Tofacitinib Versus Methotrexate in Treating Mucocutaneous and Musculoskeletal Lesions of Systemic Lupus Erythematosus: Real-World Results From the CSTAR Cohort XXXII. International Journal of Rheumatic Diseases 2025, 28. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J.; Furie, R.A.; Tanaka, Y.; Kalunian, K.C.; Mosca, M.; Petri, M.A.; Dörner, T.; Cardiel, M.H.; Bruce, I.N.; Gomez, E.; et al. Baricitinib for Systemic Lupus Erythematosus: A Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial. The Lancet 2018, 392, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Ceobanu, G.; Edwards, C.J. JAK Inhibitors in Systemic Lupus Erythematosus: Translating Pathogenesis into Therapy. Lupus 2024, 33, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.T.; Tanaka, Y.; D’Cruz, D.; Vila-Rivera, K.; Siri, D.; Zeng, X.; Saxena, A.; Aringer, M.; D’Silva, K.M.; Cheng, L.; et al. Efficacy and Safety of Upadacitinib or Elsubrutinib Alone or in Combination for Patients With Systemic Lupus Erythematosus: A Phase 2 Randomized Controlled Trial. Arthritis & Rheumatology, 2024. [Google Scholar] [CrossRef]
- Baker, M.; Chaichian, Y.; Genovese, M.; Derebail, V.; Rao, P.; Chatham, W.; Bubb, M.; Lim, S.; Hajian, H.; Gurtovaya, O.; et al. Phase II, Randomised, Double-Blind, Multicentre Study Evaluating the Safety and Efficacy of Filgotinib and Lanraplenib in Patients with Lupus Membranous Nephropathy. RMD Open 2020, 6, e001490. [Google Scholar] [CrossRef]
- Liu, Q.; Zeng, Y.; Xing, X.; Huang, B.; Feng, R.; Wang, Y.; Wang, N.; Zhang, X.; Li, Y.; Su, L.; et al. Evaluating the Therapeutic Potential of Tofacitinib in Sjögren’s Disease: A Comprehensive Clinical and Immunological Assessment. Rheumatology 2025. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Liu, H.; Dou, L.; Yang, Y.; Leng, X.; Li, M.; Zhang, W.; Zhao, Y.; Zeng, X. Pilot Study of Baricitinib for Active Sjogren’s Syndrome. Annals of the Rheumatic Diseases 2022, 81, 1050–1052. [Google Scholar] [CrossRef]
- Price, E.; Bombardieri, M.; Kivitz, A.; Matzkies, F.; Gurtovaya, O.; Pechonkina, A.; Jiang, W.; Downie, B.; Mathur, A.; Mozaffarian, A.; et al. Safety and Efficacy of Filgotinib, Lanraplenib and Tirabrutinib in Sjögren’s Syndrome: A Randomized, Phase 2, Double-Blind, Placebo-Controlled Study. Rheumatology 2022, 61, 4797–4808. [Google Scholar] [CrossRef] [PubMed]
- Sener, S.; Sener, Y.Z.; Batu, E.D.; Sari, A.; Akdogan, A. A Systematic Literature Review of Janus Kinase Inhibitors for the Treatment of Systemic Sclerosis. Journal of Scleroderma and Related Disorders 2025. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. New England Journal of Medicine 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Salinas, C.A.; Louder, A.; Polinski, J.; Zhang, T.C.; Bower, H.; Phillips, S.; Song, Y.; Rashidi, E.; Bosan, R.; Chang, H.-C.; et al. Evaluation of VTE, MACE, and Serious Infections Among Patients with RA Treated with Baricitinib Compared to TNFi: A Multi-Database Study of Patients in Routine Care Using Disease Registries and Claims Databases. Rheumatology and Therapy 2022. [Google Scholar] [CrossRef] [PubMed]
- Weng, C. , Zhou, Y., Zhang, L., Wang, G., Ding, Z., Xue, L., & Liu, Z. Efficacy and safety of pharmacological treatments for autoimmune disease-associated interstitial lung disease: A systematic review and network meta-analysis. Seminars in arthritis and rheumatism 2024, 68, 152500. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K. R. , Wells, A. U., Cottin, V., Devaraj, A., Walsh, S. L. F., Inoue, Y., Richeldi, L., Kolb, M., Tetzlaff, K., Stowasser, S., et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. The New England journal of medicine 2019, 38, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Atienza-Mateo, B. , Serrano-Combarro, A., Loarce Martos, J., Vegas-Revenga, N., Martín López, M., Castañeda, S., Melero-González, R. B., Mena Vázquez, N., Carrasco-Cubero, C., Díez Morrondo, C., et al. Real-world evidence of the antifibrotic nintedanib in rheumatoid arthritis-interstitial lung disease. National multicenter study of 74 patients. Seminars in arthritis and rheumatism 2025, 72, 152710. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, M. , Lepri, G., Iannone, C., Cassione, E. B., Guggino, G., Lo Monaco, A., Foti, R., Fornaro, M., Chimenti, M. S., Fassio, A., et al. Nintedanib in Rheumatoid Arthritis-Related Interstitial Lung Disease: Real-World Safety Profile and Risk of Side Effects and Discontinuation. The Journal of rheumatology 2025, 52, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Denton, C. P. , Goh, N. S., Humphries, S. M., Maher, T. M., Spiera, R., Devaraj, A., Ho, L., Stock, C., Erhardt, E., Alves, M., & Wells, A. U. Extent of fibrosis and lung function decline in patients with systemic sclerosis and interstitial lung disease: data from the SENSCIS trial. Rheumatology (Oxford, England) 2023, 62, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Tekgoz, E. , Colak, S. Y., Gunes, E. C., Ocal, N., Cinar, M., & Yilmaz, S. Nintedanib and its combination with immunosuppressives in connective tissue disease-related interstitial lung diseases. Irish journal of medical science 2025, 194, 391–397. [Google Scholar] [CrossRef]
- Ma, J. , Li, G., Wang, H., & Mo, C. Comprehensive review of potential drugs with anti-pulmonary fibrosis properties. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2024, 173, 116282. [Google Scholar] [CrossRef]
- De Marco, M. , Armentaro, G., Falco, A., Minniti, A., Cammarota, A. L., Iannone, C., Basile, A., D'Ardia, A., Zeppa, P., Marzullo, L., et al. Overexpression of BAG3 (Bcl2-associated athanogene 3) in serum and skin of patients with systemic sclerosis. Clinical and experimental rheumatology 2024, 42, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Freedman, P. , De Marco, M., Rosati, A., Marzullo, L., Del Papa, N., Turco, M. C., & O'Reilly, S. Extracellular BAG3 is elevated in early diffuse systemic sclerosis. Military Medical Research 2025, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M. , Basile, A., Cammarota, A. L., Iannone, C., Falco, A., Marzullo, L., Rosati, A., Caporali, R., Turco, M. C., & Del Papa, N. Response to antifibrotic therapy and decrease of circulating BAG3 protein levels in systemic sclerosis patients with reduced forced vital capacity. Biomedicine & pharmacotherapy = Biomedicine & pharmacotherapie 2024, 174, 116578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




