1. Introduction
Musculoskeletal ultrasound (US) has become an indispensable tool for guiding interventional procedures due to its real-time visualization, absence of ionizing radiation, and cost-effectiveness [
1]. In addition to these advantages, US enables continuous monitoring of soft tissues and needle trajectory, facilitating accurate targeting while minimizing neurovascular risk [
1]. The clinical applications of US guidance have grown substantially, encompassing joint injections, nerve blocks, and specialized percutaneous tendon procedures. Notably, in cases of calcific shoulder tendinitis, US-guided barbotage yields good-to-excellent results in over 90% of patients, with less than 1% requiring surgery, underscoring the high success rates and safety profile of US-guided treatments [
2].
In the context of foreign body and implant management, US offers distinct advantages relevant to contraceptive devices. Its sensitivity allows the detection of non-palpable implants, which typically appear as echogenic rods with characteristic reverberation shadows, facilitating accurate localization and removal. Studies have demonstrated that impalpable Implanon
® rods can be successfully located and extracted with US guidance in nearly all cases [
3]. Real-time guidance is particularly valuable when implants migrate or are positioned near critical structures, as blind removal attempts pose substantial risks.
While contraceptive arm implants are generally inserted subdermally and removed by palpation, approximately 3–5% of cases present challenges when the device becomes deeply placed, fractured, or migrated, complicating retrieval [
3,
4,
5,
6,
7,
8]. Such cenarios can lead to serious complications including reported cases of implants impinging on neurovascular structures, such as ulnar and median nerve neuropathy [
4,
6,
7,
8,
9]. In these high-risk situations, meticulous imaging and specialized care are essential to prevent permanent neurological injury [
7,
8,
9]. We present a unique case of a 38-year-old woman with neuropathic arm pain caused by a retained Implanon directly abutting the ulnar nerve, which was successfully removed using a US-guided hydrodissection (HD) plus percutaneous needle stabilization techniques. This case underscores the value of high-resolution US in managing non-palpable implants while avoiding nerve damage and contributes to the evolving literature on minimally invasive solutions for complex implant removal.
Hydrodissection (HD) is an advanced ultrasound-guided technique that involves injecting fluid to separate nerves from compressive structures[
10], such as fascia[
11,
12], scar tissue[
13], or implants. Unlike conventional ultrasound guidance, which primarily visualizes anatomy, HD creates a dynamic protective fluid plane between critical structures. This represents a paradigm shift in high-risk foreign-body removal by addressing a fundamental limitation of traditional approaches: preventing iatrogenic nerve injury during instrument manipulation in proximity to neural structures.
When utilizing 5% dextrose in water (D5W) as the injectate, without local anesthetics, HD achieves three protective effects. First, it mechanically separates tissue planes to isolate nerves from implants [
10]. Second, it modulates inflammation by downregulating TRPV1 [
14,
15] channels and mitigating neurogenic inflammation [
16,
17,
18,
19]. Finally, it stabilizes metabolic conditions by correcting perineural glycopenia [
17], reducing nerve hyperexcitability. The biocompatibility and iso-osmolality of D5W (277 mOsm/L) ensure the safety of neural structures while facilitating real-time patient feedback. In this pioneering case, we employed ultrasound-guided D5W HD to establish a safety buffer between the ulnar nerve and a contraceptive implant, achieving the first documented percutaneous extraction utilizing HD to separate the nerve from the implant, even when the nerve was adherent to it, without resorting to open surgery. Additionally, the use of percutaneous needle stabilization for implants that are highly slippery and mobile in nature represents another pioneering technique in the literature, as there are currently no effective treatments for such implants apart from open exploration.
2. Case Presentation
A 38-year-old Asian woman presented with a three-day history of progressively worsening paroxysmal pain characterized by burning, shooting, and electric shock-like sensations, accompanied by persistent tingling, numbness, and paresthesia radiating from the medial left elbow and forearm to the medial hand. Her medical history included a contraceptive etonogestrel implant (Implanon ®) in the left upper arm, which had been asymptomatic for the past three years, coinciding with the end of its intended lifespan. Multiple removal attempts at a local clinic were unsuccessful due to the implant’s significant depth. Each manipulation exacerbated neuropathic symptoms along the ulnar nerve distribution, leading to increasingly severe paresthesia that ultimately precluding further intervention. Despite concerted efforts by three obstetricians over three consecutive days, removal remained unsuccessful (
Figure 1). The patient was discharged home with urgent referral to our orthopedic center three days following the final attempt, without prior radiological investigation.
Within 48–72 h following the last unsuccessful removal attempt, the patient developed new-onset localized tingling and pain at the medial elbow, exacerbated by arm movement. During the nights preceding her initial evaluation, symptoms worsened dramatically, resulting in intolerable nocturnal paresthesia characterized by severe “pins and needles” sensations that disrupted her sleep. As a nail salon proprietor, she became unable to perform occupational or daily activities due to the debilitating symptoms. Progressive nocturnal pain exacerbations prevented sleep. Her medical history was otherwise unremarkable, with no documented trauma accounting for her presentation.
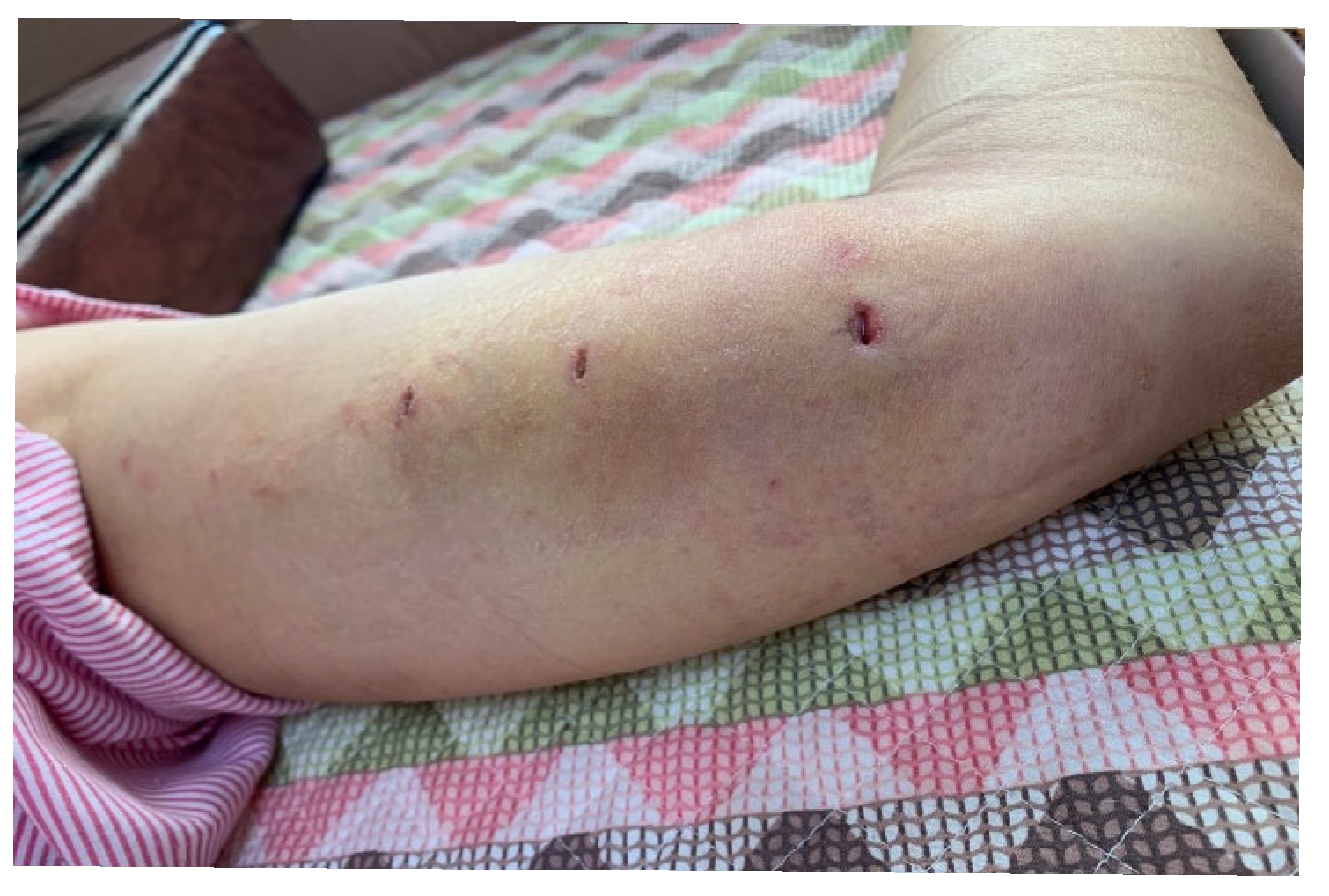
Physical examination revealed three small wounds over the medial left arm from previous removal attempts (
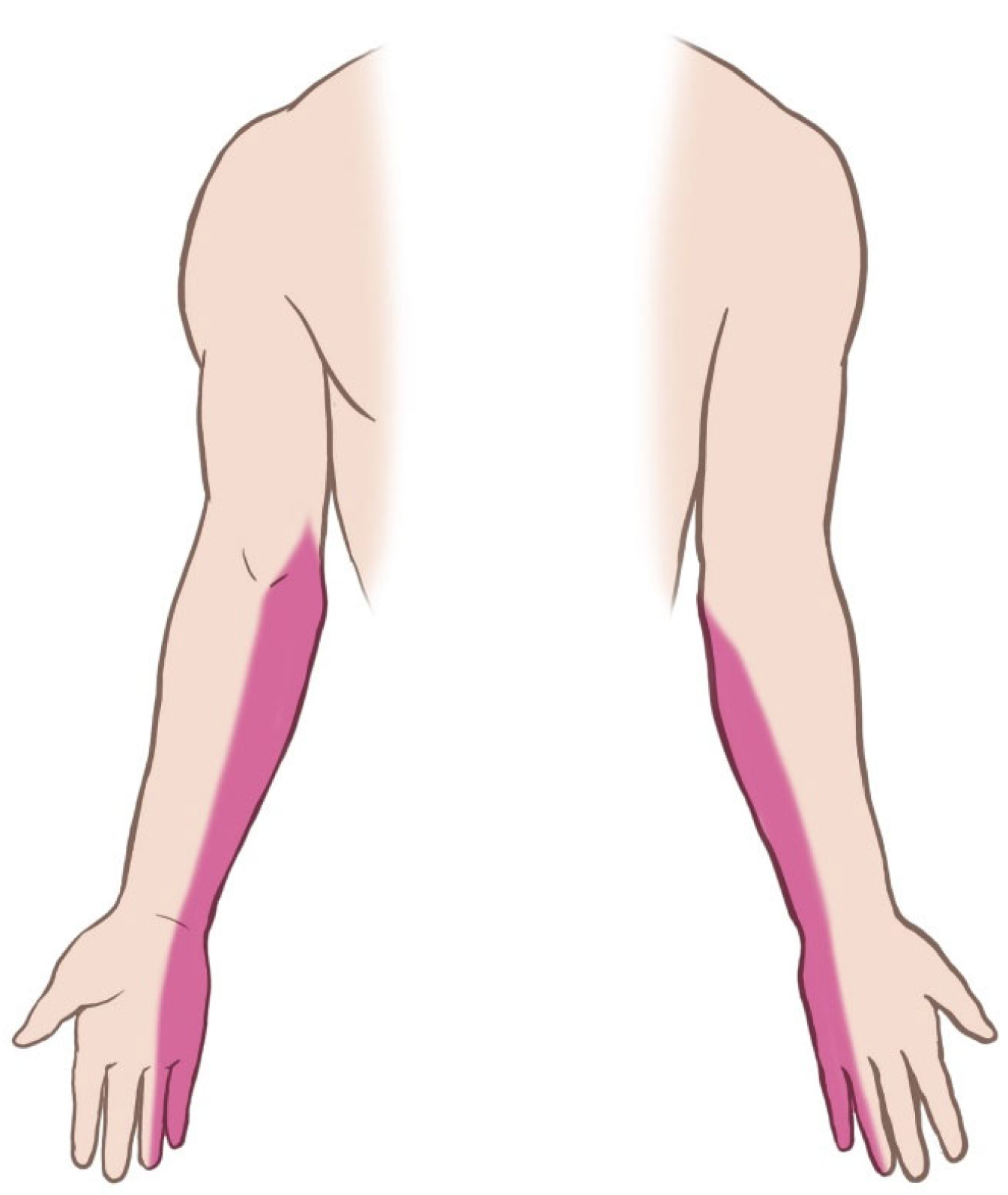
Figure 1). The implant was not palpable. Tinel’s sign was positive at the medial arm, eliciting radiating pain along the ulnar nerve distribution. Sensory examination demonstrated decreased sensation in the medial antebrachial cutaneous and ulnar nerve territories (
Figure 2). Although the patient exhibited guarded arm movement due to pain, a comprehensive neurological assessment showed no motor deficits. Her pain was rated at maximum severity (numeric pain rating scale [NPRS] score: 10/10), significantly imparing her function, as reflected by a Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) score of 55, indicating marked upper extremity dysfunction. Clinical evaluation strongly suggested deep implant migration, with probable neurovascular impingement.
2.1. Prior Imaging Included Radiography (Figure 3) Confirming Implant Location, and Ultrasound (Figure 4) Demonstrating Its Adjacency to the Ulnar Nerve
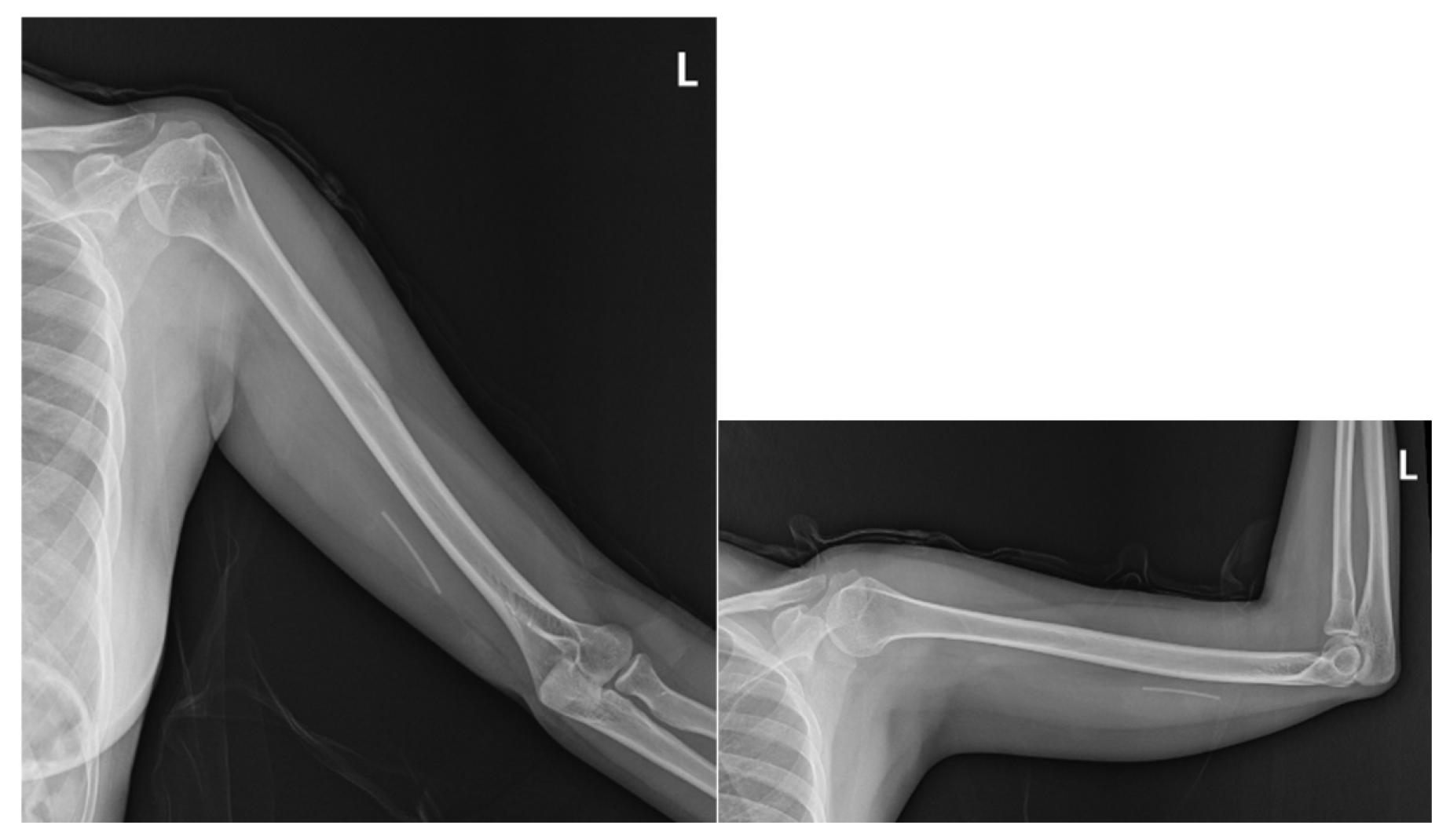
Plain radiography of the left arm revealed no bony deformities or structural abnormalities accounting for her neurological symptoms. However, imaging demonstrated a linear radiopaque structure consistent with the residual Implanon device in the medial aspect of the arm (
Figure 3).
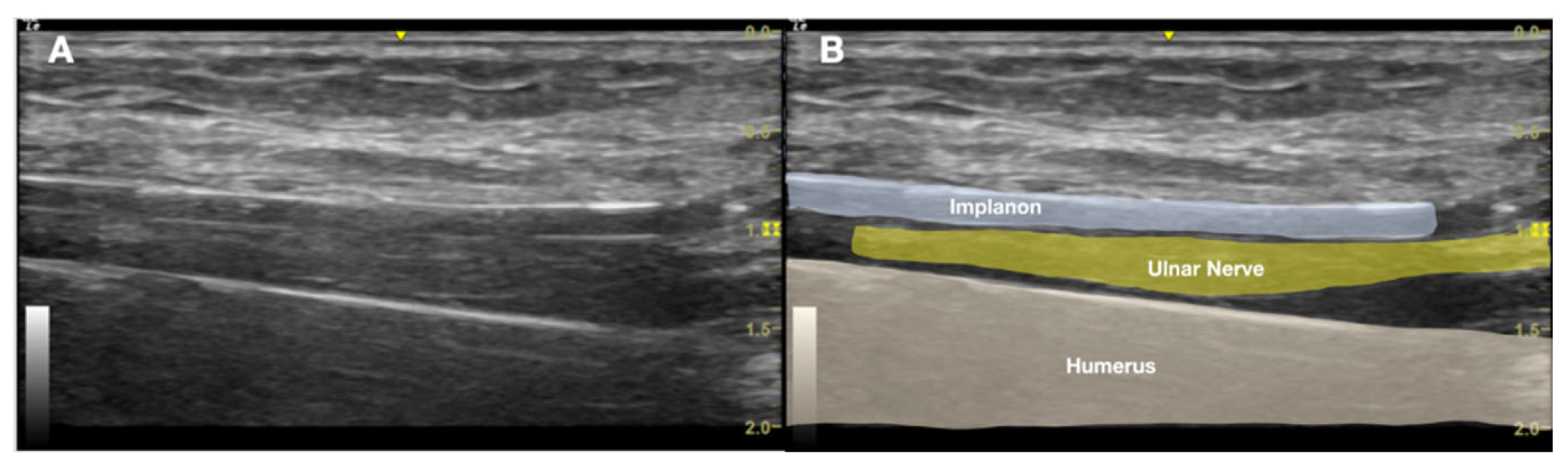
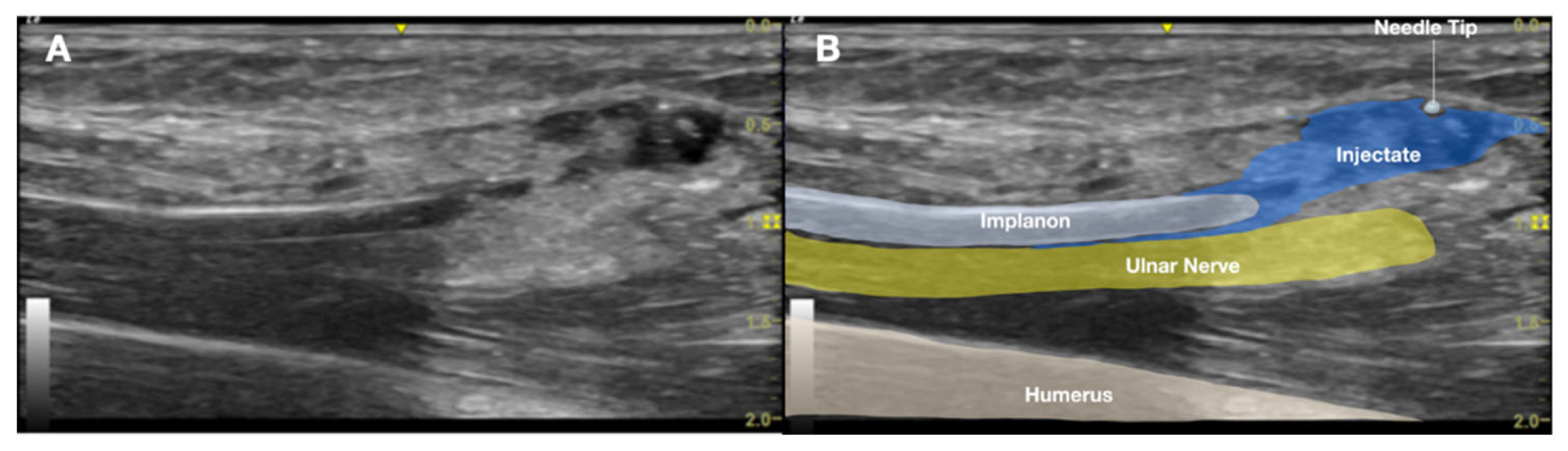
This radiographic confirmation prompted further evaluation with US imaging of the left medial arm due to clinical suspicion of implant-related nerve irritation. Using a high-frequency linear transducer (e.g. GE Linear L4-20t-RS General Electric, Boston, MA, USA), sonographic assessment revealed a linear echogenic structure with a reverberation artifact just above the medial humerus, approximately 5 cm proximal to the medial epicondyle.
The US findings localized the echogenic structure beneath the muscle layer, adjacent to the ulnar nerve, within the neurovascular bundle of the upper arm (
Figure 4,
Figure 5A). No neuroma or nerve enlargement was observed; however, dynamic US demonstrated apparent direct contact between the implant and the nerve sheath. The clinical impression was of a migrated, deeply seated, and unstable implant in close proximity to the ulnar nerve. Intermittent nerve irritation or impingement, likely exacerbated by daily movement and previous failed removal attempts, was considered the probable cause of her neuropathic pain. After obtaining informed consent, the decision was made to pursue minimally invasive, US-guided removal as an alternative to open surgical exploration.
Figure 3.
Plain radiograph of the left arm showing a linear radiopaque structure consistent with the retained Implanon® device. No bony deformities or structural abnormalities were noted that could account for her neurological symptoms.
Figure 3.
Plain radiograph of the left arm showing a linear radiopaque structure consistent with the retained Implanon® device. No bony deformities or structural abnormalities were noted that could account for her neurological symptoms.
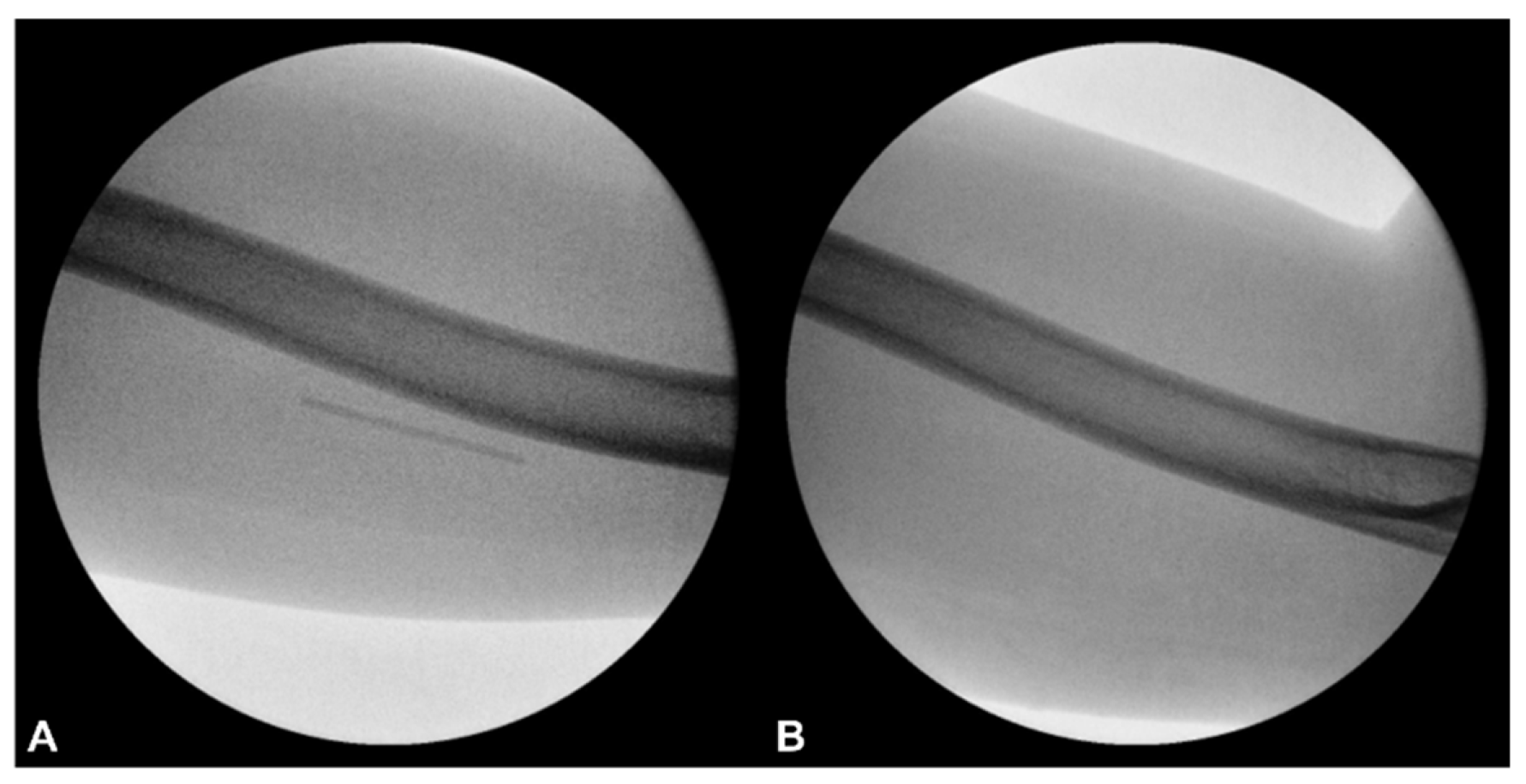
Figure 4.
Long-axis ultrasound image of the medial arm demonstrating the Implanon® device positioned deep into the muscle layer adjacent to the ulnar nerve within the neurovascular bundle. A).Ultrasound image; B). Labeled explanation.
Figure 4.
Long-axis ultrasound image of the medial arm demonstrating the Implanon® device positioned deep into the muscle layer adjacent to the ulnar nerve within the neurovascular bundle. A).Ultrasound image; B). Labeled explanation.
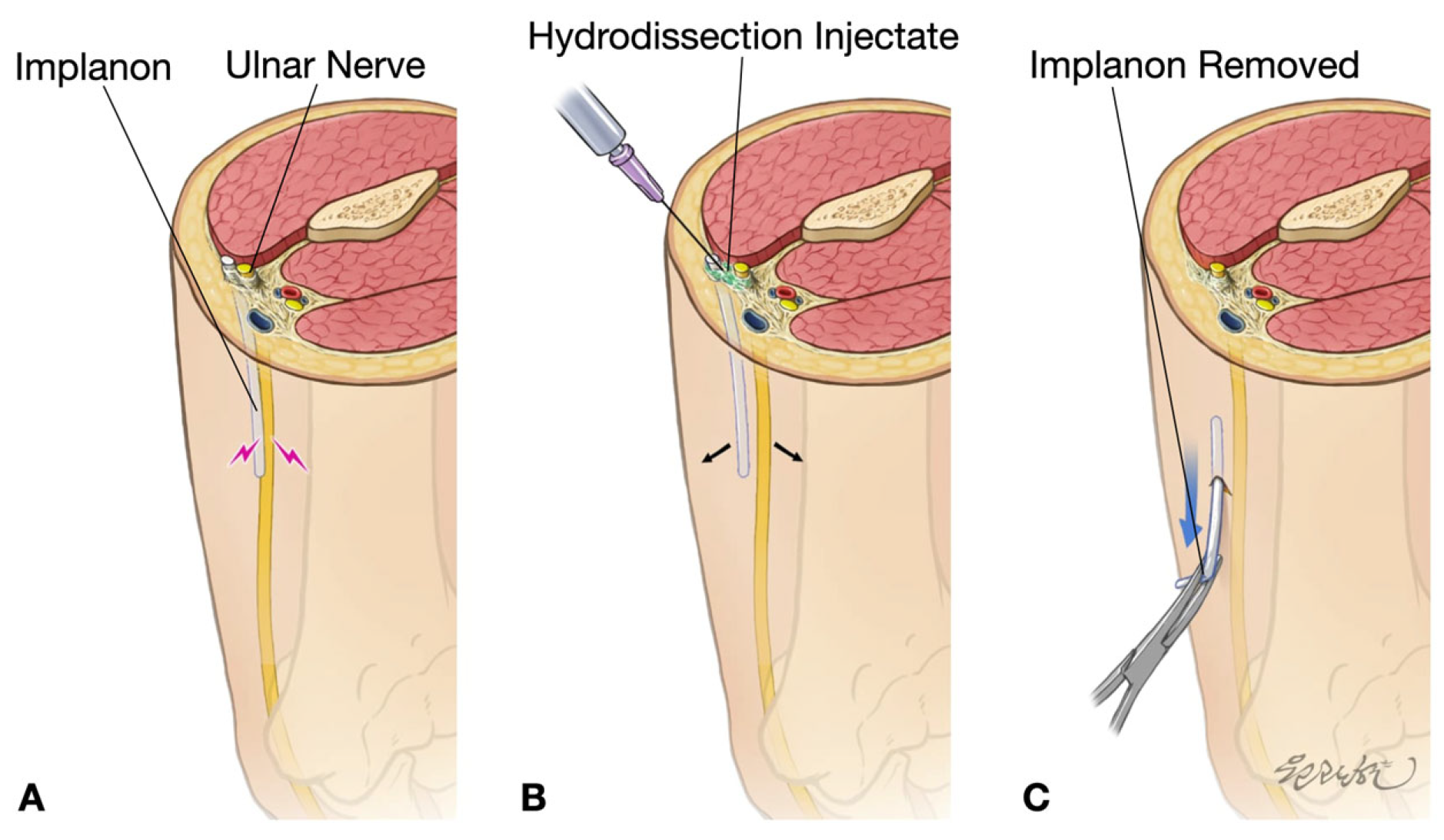
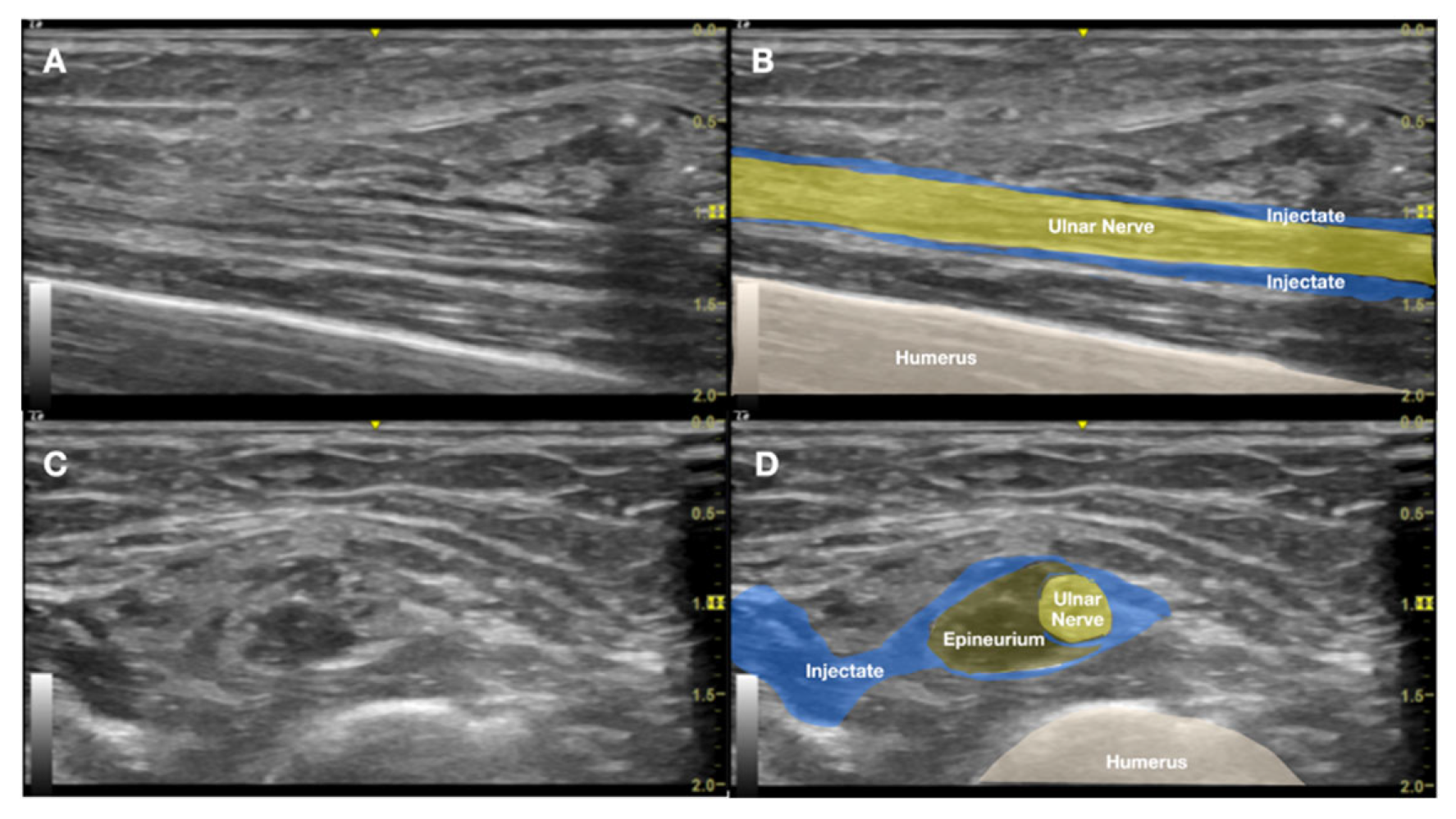
Figure 5.
A: Close relationship between Implanon® and the ulnar nerve. B: Stabilization of one end of the Implanon device with a 25G needle. C: Removal of Implanon.
Figure 5.
A: Close relationship between Implanon® and the ulnar nerve. B: Stabilization of one end of the Implanon device with a 25G needle. C: Removal of Implanon.
2.2. The Procedures:
The procedure was performed on the same first day of the visit, under local anesthesia in an outpatient interventional suite. With the patient positioned supine and the left arm abducted, the team re-explored the previous incision site under sterile conditions. US confirmed the retained Implanon 2cm distal to one of the incision sites, immediately adjacent to the ulnar nerve. Initial direct US-guided removal attempts were unsuccessful due to significant nerve irritation triggered by manipulation. To address this challenge, approximately 50 mL of 5% dextrose in water (D5W) without local anesthetic (LA) was injected via US-guided HD at the distal aspect of the implant. This approach aimed to achieve both a biochemical anti-inflammatory effect and mechanical separation between the implant and the adjacent nerve (
Figure 6). The HD technique successfully created a protective fluid buffer around the neural structures.
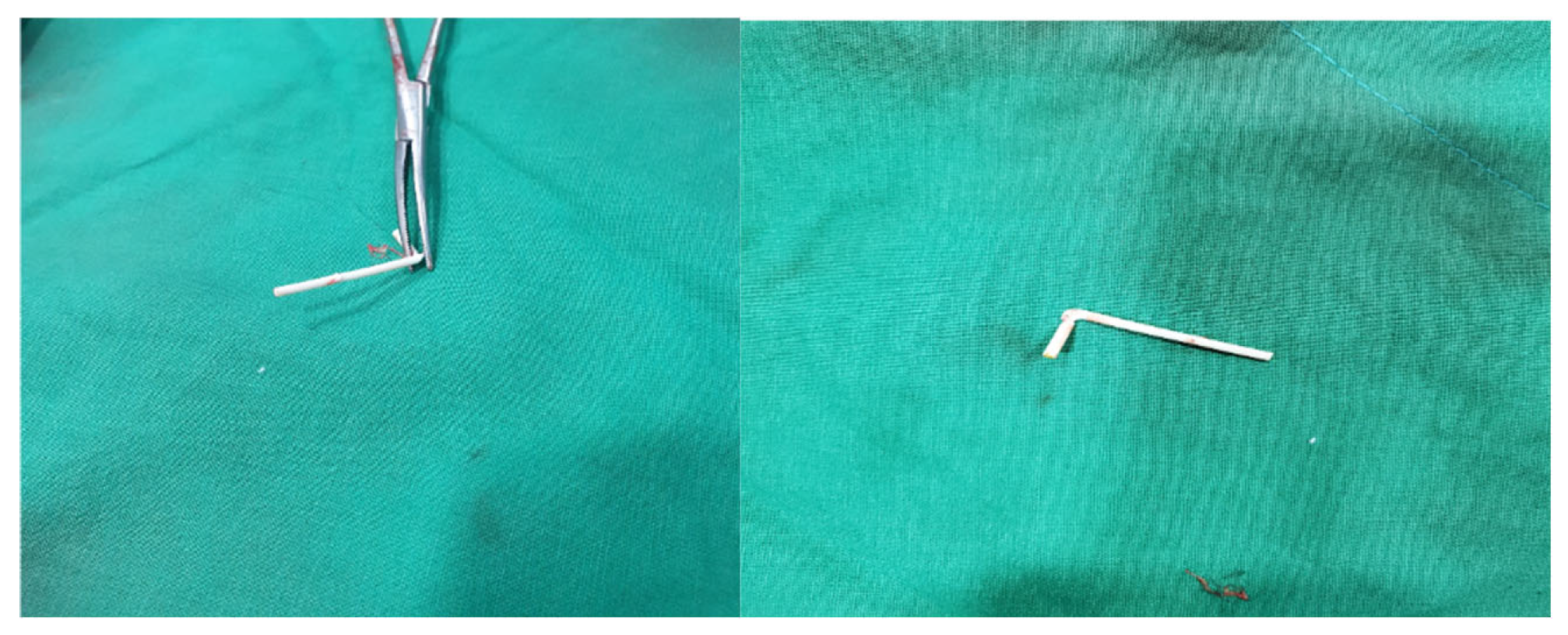
Despite ulnar nerve protection achieved with D5W HD, the implant could not be secured due to its low-friction surface and evaded capture with mosquito forceps under US-guidance. Subsequent fluoroscopic correlation using C-arm imaging confirmed iatrogenic proximal migration of the implant toward the axillary region. Under combined US-fluoroscopic guidance, the implant was re-identified in the proximal upper arm. A second targeted HD session was performed under US guidance, during which an additional 50 mL of D5W without LA was injected from proximal to distal along the anticipated tract to facilitate distal repositioning of the implant back to the distal arm. To overcome the technical challenge of further implant mobility during the second trial, the proximal tip of the implant was percutaneously penetrated and stabilized using a 25-gauge (G) needle under US guidance (
Figure 5B). This innovative stabilization technique enabled secure grasping of the implant with mosquito forceps and successful extraction through the previous incision under real-time US visualization (
Figure 5C).
The entire 4-cm Implanon rod segment was removed intact (
Figure 7). No additional incisions or surgical dissections were required. Hemostasis was achieved via manual compression, and the wound was approximated with adhesive strips. The patient tolerated the procedure well without any immediate complications. Complete removal of the implant was confirmed by concurrent C-arm fluoroscopy and US imaging (
Figure 8 and
Figure 9). Notably, the patient reported immediate substantial symptom relief, with pain severity decreasing to NPRS 2/10 (80% reduction). The entire procedure—including the intial removal attempt, US-guided HD, second attempt, further US-guided HD, percutaneous 25G needle stabilization, and final retrieval—was completed within two hours. Postprocedural monitoring for one hour revealed fluctuations in pain scores. A long-arm splint maintaining elbow extension was applied. Prophylactic antibiotics and one-day NSAID therapy were prescribed due to repeated wound manipulation and local inflammation. The patient was discharged home on the same day.
2.3. Follow-Up and Long-Term Outcomes
The wound was reassessed at our center the day after extraction, revealing no signs of infection or wound exudate. Her pain level was measured at 1-2/10 on the Numeric Pain Rating Scale (NPRS), and she demonstrated full range of motion in both the left elbow and wrist.
During follow-up evaluations one-month post-procedure, the patient exhibited significant clinical improvement. The initial presentation of debilitating radiating forearm pain and tingling had largely resolved, with only minimal residual numbness in the little finger that showed continuous gradual improvement. Quantitative assessment indicated dramatic functional recovery, as evidenced by a decrease in the QuickDASH score to 4.5, reflecting near-complete restoration of upper extremity function. Serial neurological examinations confirmed the absence of motor deficits or new neurological symptoms, supporting the conclusion that neuropathic pain originated from ulnar nerve irritation secondary to the retained implant.
The long-term outcomes were equally impressive, as demonstrated during the one-year follow-up assessment conducted via a structured telephone interview. All clinical indicators confirmed sustained therapeutic success. The patient reported a pain score of 0 on the NPRS, and upper limb function had normalized completely, reflected by a QuickDASH score of 0.
3. Discussion
This case represents a paradigm shift in the management of migrated contraceptive implants through innovative approaches. First, the observed migration pattern —where the implant traveled proximally along the fascial plane or through the epineurium to the axillary region —demonstrates a rare trajectory indicative of a unique pathomechanism of nerve irritation. While the literature documents ectopic implants encasing or penetrating nerve sheaths, these occurrences remain extraordinarily uncommon [
4,
6,
7]. Unlike prior reports, such as that of Saeed et al. [
20], in which neuropathy resulted from spontaneous migration our case highlights iatrogenic displacement during unsuccessful removal attempts.
Second, our percutaneous approach, utilizing ultrasound-guided hydrodissection (HD) with 5% dextrose in water (D5W) without local anesthetics to separate the nerve from the implant prior to extraction, fundamentally surpasses conventional open neurolysis—typically employed when implants are in contact with or adjacent to neural structures—by enabling fluid-mediated nerve liberation under real-time visualization[
4]. Third, our needle-stabilization technique addressed a persistent challenge in US-guided removals: implant mobility during grasping. Percutaneous fixation with a 25G needle enabled secure extraction through existing incisions, representing a technical refinement beyond standard hydrodissection.
While previous studies have described ultrasound-guided removal of deep implants [
6,
8], our technique represents a significant advancement by integrating 5% dextrose in water (D5W) hydrodissection to separate the nerve from the implant, thereby creating a safety zone for ultrasound-guided manipulation of the implant with surgical instruments and preventing further nerve damage. This approach also reduces neurogenic inflammation associated with implant manipulation. Additionally, our technique introduces percutaneous needle stabilization specifically designed for easily migrated or slippery implants.
This is, to our knowledge, the first documented case of successful management of an iatrogenically malpositioned implant, addressed with ultrasound-guided hydrodissection using D5W to mitigate mechanical impingement and neurogenic inflammation. The implant was successfully removed, despite its slippery nature, via ultrasound-guided percutaneous extraction with a single needle stabilization, obviating the need for additional incisions or open surgery.
This exceptional clinical course, from immediate post-procedural improvement (NPRS 10→2/10) to complete long-term resolution (QuickDASH 0 at 1 year), provides compelling evidence that US-guided minimally invasive removal offers both an effective and lasting solution for implant-induced nerve compression.
Management Strategies for Non-Palpable or Deeply Placed Implants
When considering optimal strategies for managing non-palpable or deeply placed implants, current clinical guidelines recommend imaging localization and specialist referral [
3,
7,
21]. Our experience supports US as the first-line imaging modality due to its absence of ionizing radiation and superior real-time spatial resolution [
4,
6,
7,
8]. In cases where US confirms an implant’s precarious location adjacent to neural structures, removal planning must be approached with particular caution.
Although some cases require operative intervention under regional or general anesthesia with wider surgical exposure [
4,
7], our findings demonstrates that implants in close proximity to neural structures can be removed percutaneously. Traditional surgical management of such cases typically involves gaining proximal and distal control of both the implant and nerve via open exploration to prevent traction injury [
4,
7]. However, open surgical approaches carry risks of postoperative perineural adhesions and undesirable scarring, potentially impairing functional and cosmetic outcomes. The US-guided HD technique we employed [
22] directly addresses these challenges by creating a protective fluid cushion around the affected nerve.
D5W achieves a glucose concentration approximately 50-fold higher than physiological plasma and tissue fluid levels while maintaining an osmolality (277 mOsm/L) comparable to normal saline (308 mOsm/L). Clinical evidence indicates that perineural administration of D5W induces significantly less injection-associated discomfort than sterile water [
23]. Additionally, studies confirm an absence of neurotoxic effects on neural tissues following D5W exposure [
23,
24,
25]. To date, no complications attributable to dextrose-based hydrodissection have been reported [
26,
27].
The application of D5W provides immediate mechanical separation between the implant and the ulnar nerve through controlled fluid dissection. Unlike local anesthetics, D5W preserves sensory and motor function by avoiding neural blockade, thereby allowing real-time patient feedback during instrument manipulation and reducing the risk of iatrogenic nerve injury.
D5W demonstrates therapeutic superiority over normal saline in managing neuropathy and neurogenic inflammation. Research indicates that D5W not only offers effective mechanical separation but also exerts beneficial metabolic effects that enhance neural recovery and reduce inflammation more significantly than normal saline[
27,
28,
29]. This is particularly important in conditions characterized by neuropathic pain, as D5W may enhance neuronal cell function[
16,
28]. Furthermore, D5W exhibits multimodal anti-inflammatory effects on compressed neural tissues through distinct biochemical pathways. Specifically, glucose-mediated modulation attenuates the transient receptor potential vanilloid receptor-1 (TRPV1) in sensory neurons[
28,
30,
31] and reduces neurogenic inflammation[
16,
17,
27], contributing to improved outcomes in patients undergoing nerve-adherent implant removal.
The clinical safety and efficacy profile of D5W further enhances its utility. The absence of local anesthetics mitigates several potentially associated risks, including temporary sensory and motor blockade that may obscure nerve traction injuries, chondrotoxicity, and allergic reactions. Additionally, avoiding corticosteroids [
31,
32], eliminates concerns regarding tendon weakening, glycemic dysregulation, and delayed tissue healing.
Moreover, D5W HD enables pain-tolerant extraction while maintaining protective sensation. The immediate post-procedural pain reduction (NPRS 10→2/10) without sensory blockade indicates that symptom resolution derived primarily from implant removal rather than anesthetic effects. The absence of neuropathic sequelae at 1-year follow-up, along with complete functional recovery (QuickDASH 0), possibly, further illustrates the therapeutic superiority over anesthetic-containing solutions.
Ensuring complete implant removal is critical, especially when a fracture is suspected. Our protocol emphasizes meticulous US inspection post-extraction to verify that no residual fragments remain [
6], Our patient successfully underwent this verification, with the intact 4-cm rod retrieved.
While Implanon® removal is conventionally performed by obstetrician-gynecologists, cases with neurological symptoms necessitate referral to clinicians proficient in ultrasound-guided extraction. Operator expertise in ultrasound-guided interventions—particularly nerve hydrodissection and needle placement—is critical, as inadequate visualization during removal can lead to iatrogenic neural injury [
4]. Clinicians employing these advanced techniques must possess specialized ultrasound and procedural training.
The accumulating literature supports US guidance as the preferred method for locating and removing non-palpable contraceptive implants. Early evidence from case series in family planning clinics, such as Singh et al. (2006), demonstrated successful identification and removal of 21 “lost” Implanon devices under US guidance [
3]. Subsequent studies, including Patel et al. (2014) and Persaud et al. (2008) reinforced these findings, achieving high success rates in ultrasound-guided removal[
33,
34]. Recent advancements have further refined percutaneous removal techniques [
35], establishing US-guided localization and removal as a reliable first-line approach for challenging implants, with success rates and safety profiles comparable to other established US-guided procedures [
2].
The comparison of surgical techniques for implant removal has been summarized in table 1 below, Metrics include operative time, incision size, risk of nerve injury, soft tissue damage, potential for conversion to open surgery, learning curve, and complications associated with each method.
Table 1: This table summarizes the key differences between traditional open removal, ultrasound-guided removal, and ultrasound-guided removal with hydrodissection (HD) and stabilization techniques.
Some important limitations must be acknowledged when interpreting the findings of this case report. As a single-patient experience, it does not establish the comparative efficacy of the described technique. While the successful outcome suggests the clinical utility of US-guided HD and removal, more extensive studies or case series are needed for generalizability. The absence of pre- and post-procedure nerve conduction studies limits our ability to rigorously assess neurological recovery; however, the documented sensory deficits in the medial antebrachial cutaneous and ulnar nerve distributions, which completely resolved at both one-month and one-year follow-up, may serve as surrogate markers for neural functional restoration.
The specialized skill set required for this procedure, performed by an orthopedic surgeon with extensive US experience, may not be widely available, potentially limiting broader adoption. This highlights the need for comprehensive training programs and clear referral pathways, especially for primary care providers and gynecologists who frequently encounter these cases [
21].
Technical risks associated with US-guided HD also warrant careful consideration. Iatrogenic nerve injury is a most significant concern, particularly when performed by less experienced operators who may inadvertently damage adjacent neurovascular structures. The success of the procedure relies on operator expertise in US interpretation and interventional techniques, resulting in variability in outcomes across different clinical settings. Although US provides excellent real-time visualization, its limitations in detecting deeply located implants or complex anatomical structures may increase the risk of incomplete removal.
The generalizability of our findings is constrained by the single-case nature of this report, as variations in patient anatomy and clinical presentation may substantially affect outcomes. While the follow-up period demonstrated excellent short-to-mid-term results, it does not address potential long-term complications or recurrences. Standard procedural risks, including infection and inflammatory reactions to injectables, must also be considered in clinical decision-making. These limitations underscore the importance of appropriate patient selection, specialized operator training, and structured postoperative monitoring to optimize outcomes with this technique.
4. Conclusions
This case establishes US-guided percutaneous removal as a first-line strategy for addressing nerve-compromising implant complications, offering distinct advantages over traditional approaches. Compared with blind methods or fluoroscopic guidance, US provides superior soft-tissue resolution, enhancing nerve preservation. In contrast to open surgical exploration, this minimally invasive approach reduces morbidity while maintaining efficacy, as evidenced by the patient’s rapid recovery and sustained symptom resolution.
Three critical practical implications arise from this case:
Early Imaging Evaluation: Essential for non-palpable implants presenting with neurological symptoms.
Specialized Technical Skills: Needle stabilization and HD techniques are crucial for optimal outcomes.
Centralized Referral Pathways: Should be established for complex cases.
As global contraceptive implant use increases, this case illustrates how advanced US techniques can reshape the management of rare but serious complications. Future priorities include standardizing HD protocols, developing simulation-based training for needle stabilization techniques, and establishing multicenter registries to track outcomes. Such initiatives will help ensure that these minimally invasive solutions achieve their potential in improving patient safety worldwide.
Future research should focus on refining minimally invasive techniques, optimizing HD solutions and instrument design, and aggregate outcome data through mulitcenter registries. Given the rarity of implant-related neuropathies, collaborative efforts are essential for developing evidence-based management protocols. Preventive strategies, including correct insertion techniques to ensure true subdermal placement and immediate post-insertion palpation verification, are equally important. A standardized approach that incorporating prompt imaging evaluation for non-palpable implant and early involvement of experienced removal teams should be adopted as a clinical standard to minimize patient morbidity.
Author Contributions
Conceptualization, YHY and KHSL; methodology, Y.S.S., HWL, JHH, CEP, MJL, YHY, HMY, JIC, GSK, DCJS, and KHSL; software, Y.S.S., YHY, DCJS, KDR, TS, AS, and KHSL; validation, Y.S.S., HWL, JHH, CEP, MJL, YHY, HMY, JIC, GSK, DCJS, KDR, TS, AS, and KHSL; resources, Y.S.S., HWL, JHH, CEP, MJL, YHY, HMY, JIC, GSK, and KHSL; writing—original draft preparation, Y.S.S., YHY, and KHSL; writing—review and editing, Y.S.S., HWL, JHH, CEP, MJL, YHY, HMY, JIC, GSK, DCJS, KDR, TS, AS, and KHSL; supervision, JHH, YHY, KDR, and KHSL.; project administration, HWL, JHH, CEP, MJL, YHY, HMY, JIC, GSK, DCJS, TS, AS, and KHSL; funding acquisition, YHY and KHSL.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the research involves the use of collections of information or data from which all personal identifiers have been removed prior to being received by the researchers. This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data related to this study has been included in the manuscript.
Acknowledgments
The authors would like to declare that YSS, YHY, and KHSL are the co-first authors for their equal contribution to this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HD |
Hydrodissection |
| LA |
Local anesthetic |
| NPRS |
Numeric pain rating scale |
| QuickDASH |
Quick Disabilities of the Arm, Shoulder, and Hand |
| US |
Ultrasound |
References
- Adler, R.S.; Sofka, C.M. Percutaneous ultrasound-guided injections in the musculoskeletal system. Ultrasound Q 2003, 19, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gatt, D.L.; Charalambous, C.P. Ultrasound-guided barbotage for calcific tendonitis of the shoulder: a systematic review including 908 patients. Arthroscopy 2014, 30, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Mansour, D.; Richardson, D. Location and removal of non-palpable Implanon implants with the aid of ultrasound guidance. J Fam Plann Reprod Health Care 2006, 32, 153–156. [Google Scholar] [CrossRef]
- Rivera, F.; Bianciotto, A. Contraceptive subcutaneous device migration: what does an orthopaedic surgeon need to know? A case report and literature review. Acta Biomed 2020, 91, 232–237. [Google Scholar] [PubMed]
- United Nations Department of Economic and Social Affairs (UNDESA). *Contraceptive Use by Method, D.B.A.a.; Available from: https://www.un.org/en/development/desa/population/publications/pdf/family/ContraceptiveUseByMethodDataBooklet2019.pdf.
- Soler-Perromat, J.C.; et al. Ultrasound-guided minimally invasive removal of deep contraceptive implants: outcomes and challenges. Quant Imaging Med Surg 2024, 14, 7791–7802. [Google Scholar] [CrossRef]
- Matulich, M.C.; et al. Referral Center Experience With Nonpalpable Contraceptive Implant Removals. Obstet Gynecol 2019, 134, 801–806. [Google Scholar] [CrossRef]
- Jacques, T.; et al. Minimally invasive removal of deep contraceptive implants under continuous ultrasound guidance is effective, quick, and safe. Eur Radiol 2022, 32, 1718–1725. [Google Scholar] [CrossRef]
- Asaad, S.K.; et al. Contraceptive implant migration to the ulnar nerve: A case report with literature review. Clin Case Rep 2024, 12, e9420. [Google Scholar] [CrossRef]
- Lam, K.H.S.; et al. Ultrasound-Guided Nerve Hydrodissection for Pain Management: Rationale, Methods, Current Literature, and Theoretical Mechanisms. Journal of Pain Research 2020, 13, 1957–1968. [Google Scholar] [CrossRef]
- Nugroho, N.; et al. Novel Sonoguided Digital Palpation and Ultrasound-Guided Hydrodissection of the Long Thoracic Nerve for Managing Serratus Anterior Muscle Pain Syndrome: A Case Report with Technical Details. Diagnostics 2025, 15. [Google Scholar] [CrossRef]
- Lee, H.W. Confirming the Presence of Neurapraxia and Its Potential for Immediate Reversal by Novel Diagnostic and Therapeutic Ultrasound-Guided Hydrodissection Using 5% Dextrose in Water Without Local Anesthetics: Application in a Case of Acute Radial Nerve Palsy. Diagnostics 2025, 15. [Google Scholar] [CrossRef]
- Su, D.C.; Chang, K.V.; Lam, S.K.H. Shear Wave Elastography to Guide Perineural Hydrodissection: Two Case Reports. Diagnostics (Basel) 2020, 10. [Google Scholar] [CrossRef]
- Bertrand, H.; et al. Topical mannitol reduces capsaicin-induced pain: Results of a pilot-level, double-blind, randomized controlled trial. PM R 2015, 7, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. Discovery of first-in-class highly selective TRPV1 antagonists with dual analgesic and hypoglycemic effects. Bioorganic & Medicinal Chemistry 2024, 107, 117750. [Google Scholar]
- Cherng, J.H.; et al. The Potential of Glucose Treatment to Reduce Reactive Oxygen Species Production and Apoptosis of Inflamed Neural Cells In Vitro. Biomedicines 2023, 11. [Google Scholar] [CrossRef]
- Wu, Y.T.; et al. Mechanism of Glucose Water as a Neural Injection: A Perspective on Neuroinflammation. Life (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Babaei-Ghazani, A.; et al. Ultrasound-guided 5% dextrose prolotherapy versus corticosteroid injection in carpal tunnel syndrome: a randomized, controlled clinical trial. Pain management 2022, 12, 687–697. [Google Scholar] [CrossRef]
- Topol, G.A.; et al. Dextrose Prolotherapy for Symptomatic Grade IV Knee Osteoarthritis: A Pilot Study of Early and Longer-Term Analgesia and Pain-Specific Cytokine Concentrations. Clin Pract 2022, 12, 926–938. [Google Scholar] [CrossRef]
- Saeed, A.; Narayan, N.; Pandya, A. Contraceptive Implant-Related Acute Ulnar Neuropathy: Prompt Diagnosis, Early Referral, and Management Are Key. Eplasty 2018, 18, e28. [Google Scholar] [PubMed]
- Mansour, D.; et al. Methods of accurate localisation of non-palpable subdermal contraceptive implants. J Fam Plann Reprod Health Care 2008, 34, 9–12. [Google Scholar] [CrossRef]
- Lam, K.H.S.; et al. Ultrasound-Guided Nerve Hydrodissection for Pain Management: Rationale, Methods, Current Literature, and Theoretical Mechanisms. J Pain Res 2020, 13, 1957–1968. [Google Scholar] [CrossRef]
- Tsui, B.C.; Kropelin, B. The electrophysiological effect of dextrose 5% in water on single-shot peripheral nerve stimulation. Anesth Analg 2005, 100, 1837–1839. [Google Scholar] [CrossRef]
- Hashimoto, K.; et al. Comparative toxicity of glucose and lidocaine administered intrathecally in the rat. Reg Anesth Pain Med 1998, 23, 444–450. [Google Scholar] [PubMed]
- Dufour, E.; et al. Ultrasound-guided perineural circumferential median nerve block with and without prior dextrose 5% hydrodissection: a prospective randomized double-blinded noninferiority trial. Anesth Analg 2012, 115, 728–733. [Google Scholar] [CrossRef]
- Buntragulpoontawee, M.; et al. The Effectiveness and Safety of Commonly Used Injectates for Ultrasound-Guided Hydrodissection Treatment of Peripheral Nerve Entrapment Syndromes: A Systematic Review. Front Pharmacol 2020, 11, 621150. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; et al. Efficacy of 5% Dextrose Water Injection for Peripheral Entrapment Neuropathy: A Narrative Review. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Lam, K.H.S.; et al. Ultrasound-Guided Interventions for Carpal Tunnel Syndrome: A Systematic Review and Meta-Analyses. Diagnostics (Basel) 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; et al. Novel Motor-Sparing Ultrasound-Guided Neural Injection in Severe Carpal Tunnel Syndrome: A Comparison of Four Injectates. Biomed Res Int 2022, 2022, 2022, 9745322. [Google Scholar] [CrossRef]
- Lin, C.P.; et al. Utility of ultrasound elastography in evaluation of carpal tunnel syndrome: A systematic review and meta-analysis. Ultrasound Med Biol 2019, 45, 2855–2865. [Google Scholar] [CrossRef]
- Wu, Y.T.; et al. Randomized double-blinded clinical trial of 5% dextrose versus triamcinolone injection for carpal tunnel syndrome patients. Ann Neurol 2018, 84, 601–610. [Google Scholar] [CrossRef]
- Chen, L.C.; et al. Perineural Dextrose and Corticosteroid Injections for Ulnar Neuropathy at the Elbow: a Randomized Double-blind Trial. Archives of physical medicine and rehabilitation 2020, 101, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; et al. The Feasibility of In-Clinic Ultrasound-Guided Removal of Contraceptive Implants: A Case Series. 2014, 2014.
- Persaud, T.; et al. Ultrasound-guided removal of Implanon devices. Eur Radiol 2008, 18, 2582–2585. [Google Scholar] [CrossRef] [PubMed]
- Jacques, T.; et al. Minimally-invasive fully ultrasound-guided removal of nonpalpable single-rod contraceptive implant: Case report and technical description. Contraception 2020, 101, 338–341. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).