Submitted:
13 January 2025
Posted:
13 January 2025
You are already at the latest version
Abstract
The aim of this clinical study was to demonstrate that through a micrograft of viable adipose tissue of cells and its exosomes microfiltered at 35 microns to exclude fibrous shoots and cell debris in a suspension of hyaluronic acid were able to improve symptoms and clinical manifestations of Peyronye's curvature disease or IPP Induratio Penis Plastica with a beneficial effect also in plaques . Background and Objectives: Events leading to Induratio Penis Plastica (PPI) or Peyronie's disease may be discovered in an abnormal healing response within the tunica albuginea. From an anatomopathological point of view, Peyronie's disease derives from an excessive production of extracellular matrix deposited by fibroblasts with the formation of fibrotic tissue that leads to a retractile cicatricial outcome. The clinical course is characterized by an acute phase and a subsequent chronic phase with stabilization of symptoms. Events leading to Induratio Penis Plastica (PPI) or Peyronie's disease may be discovered in an abnormal healing response within the tunica albuginea. Materials and Methods: This study involved 12 patients with a history of symptoms characterized by curvature of the penis and painful erections were analyzed and subjected to this pilot study to verify the improvement of the anatomical area through a suspension containing 1.5 mL of viable micrografts from adipose tissue of cells and its exosomes in a 1.5 mL of hyaluronic acid evaluated by using a modified Vancouver scale. Results: The Modified Vancouver scales showed that with this technique it was possible to obtain excellent results both when the suspension was injected into plaques of Peyronie’s disease when it was injected with the unique pomphs technique, with the intent to revitalize the tissue through progenitors with adult stemness markers. Conclusions: The combination of microfragmented and microfiltered adipose tissue of cells and its exosomes at 35 microns in a scaffold of hyaluronic acid is safe new method to treat Peyronye’s disease or IPP Induratio Penis Plastica.
Keywords:
1. Introduction
2. Materials and Methods
- PATIENTS AND METHODS
- history of symptoms characterized by curvature of the penis
- painful erections.
2.1. Exclusion Criteria
- History of mental disorders or emotional instability;
- History of allergic reaction to HA products;
- Current or previous (within 30 days of enrollment) treatment with an investigational drug and/or medical device or participation in another clinical study;
- History of mental disorders or emotional instability;
2.2. Procedure
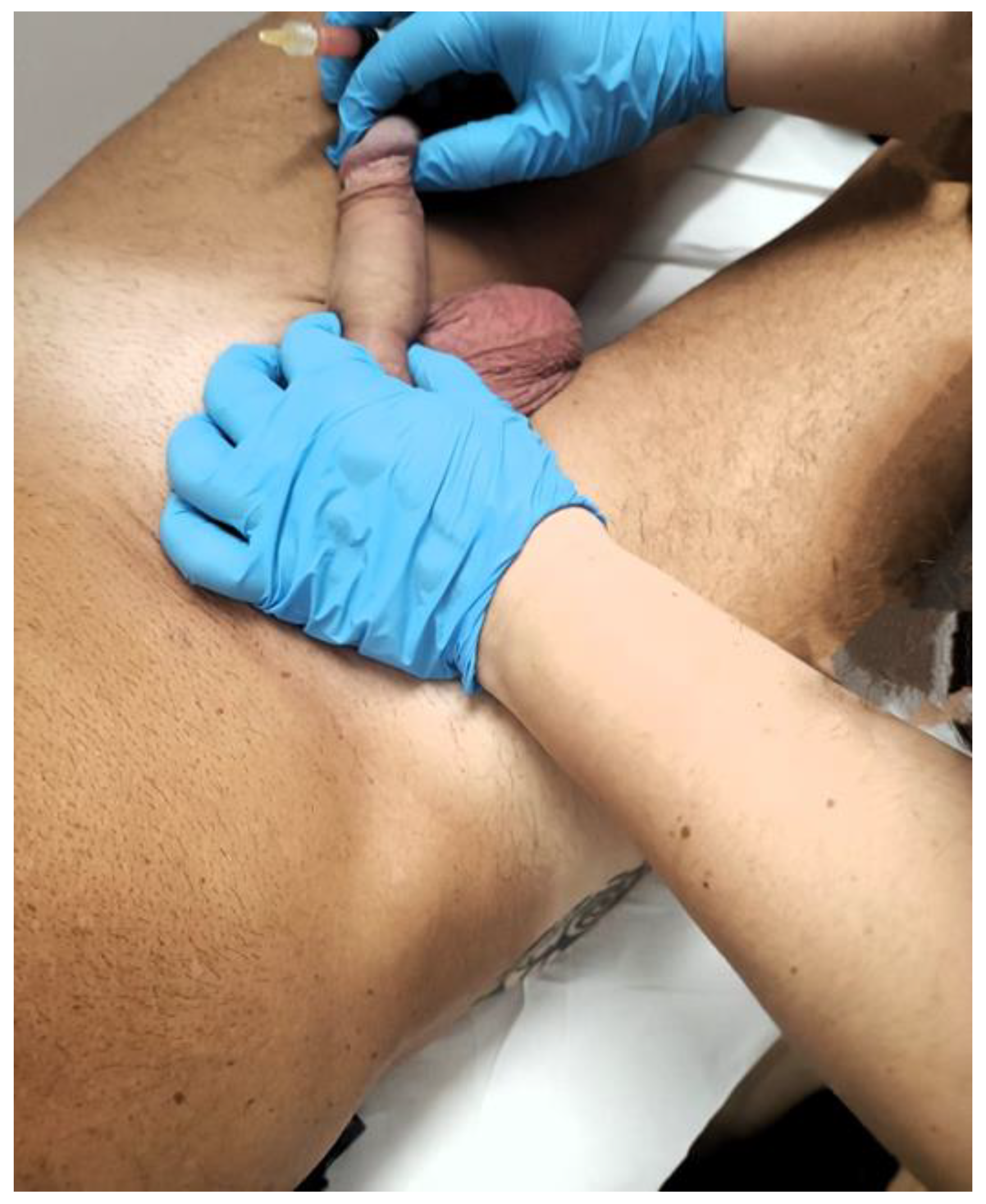
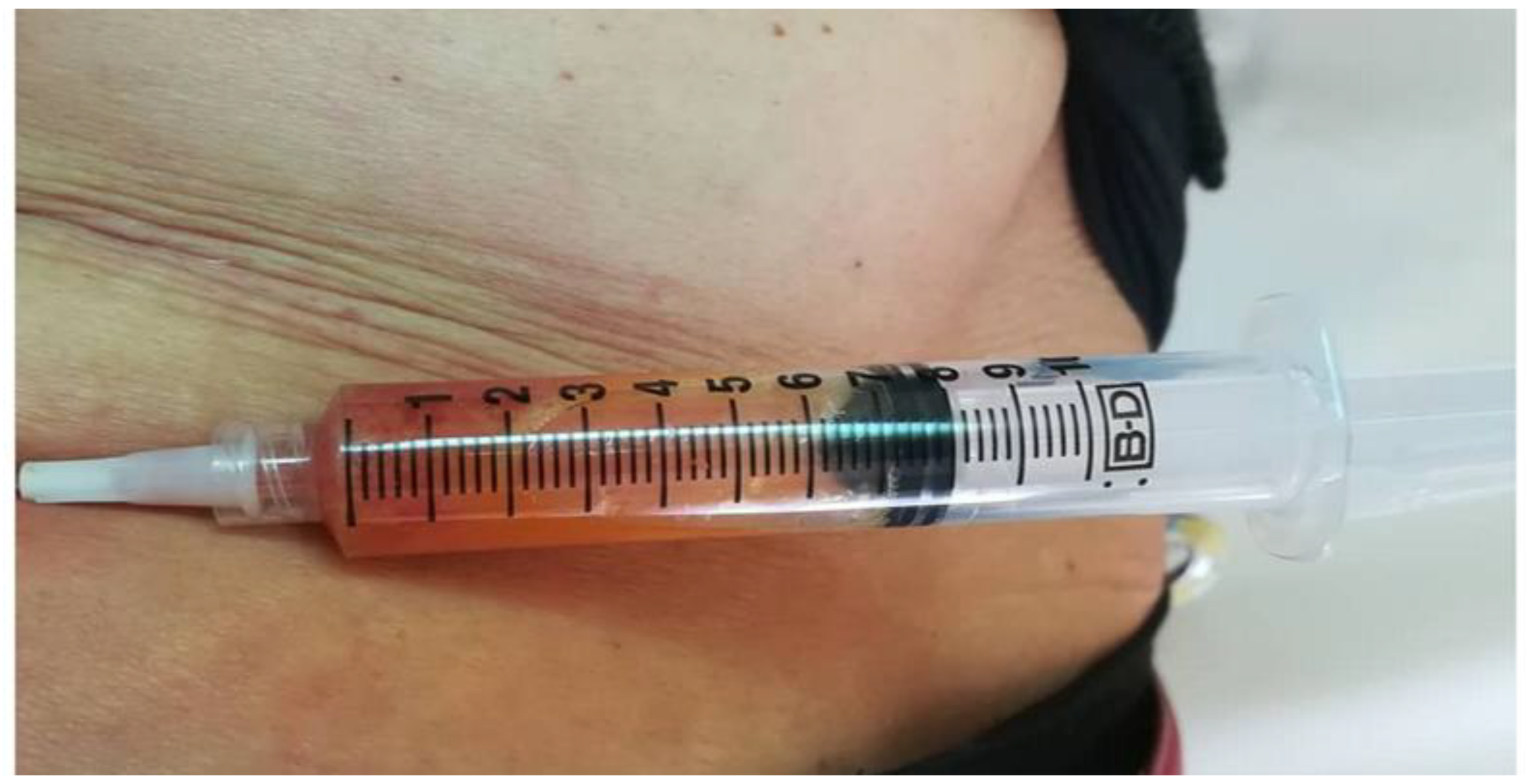
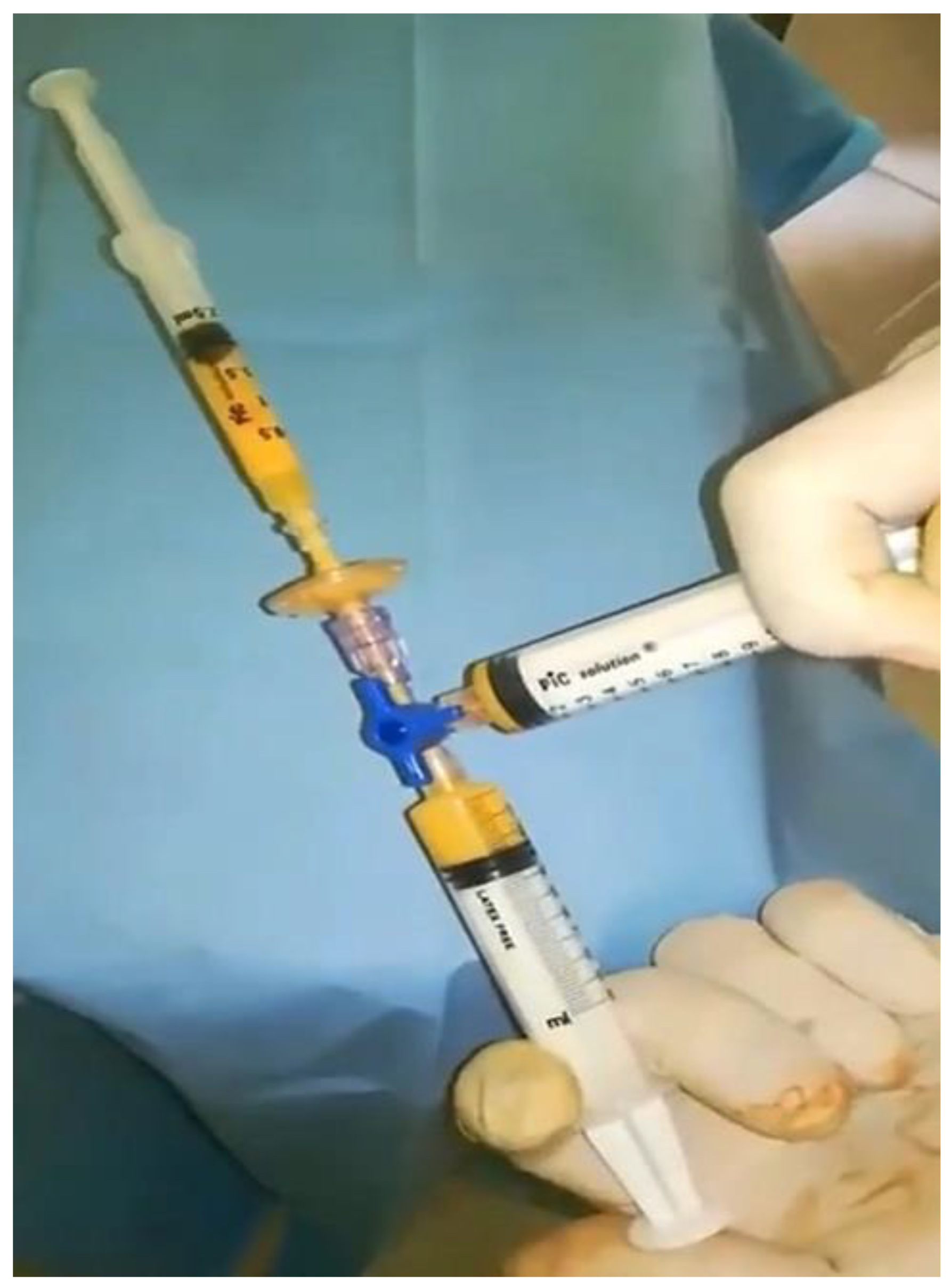
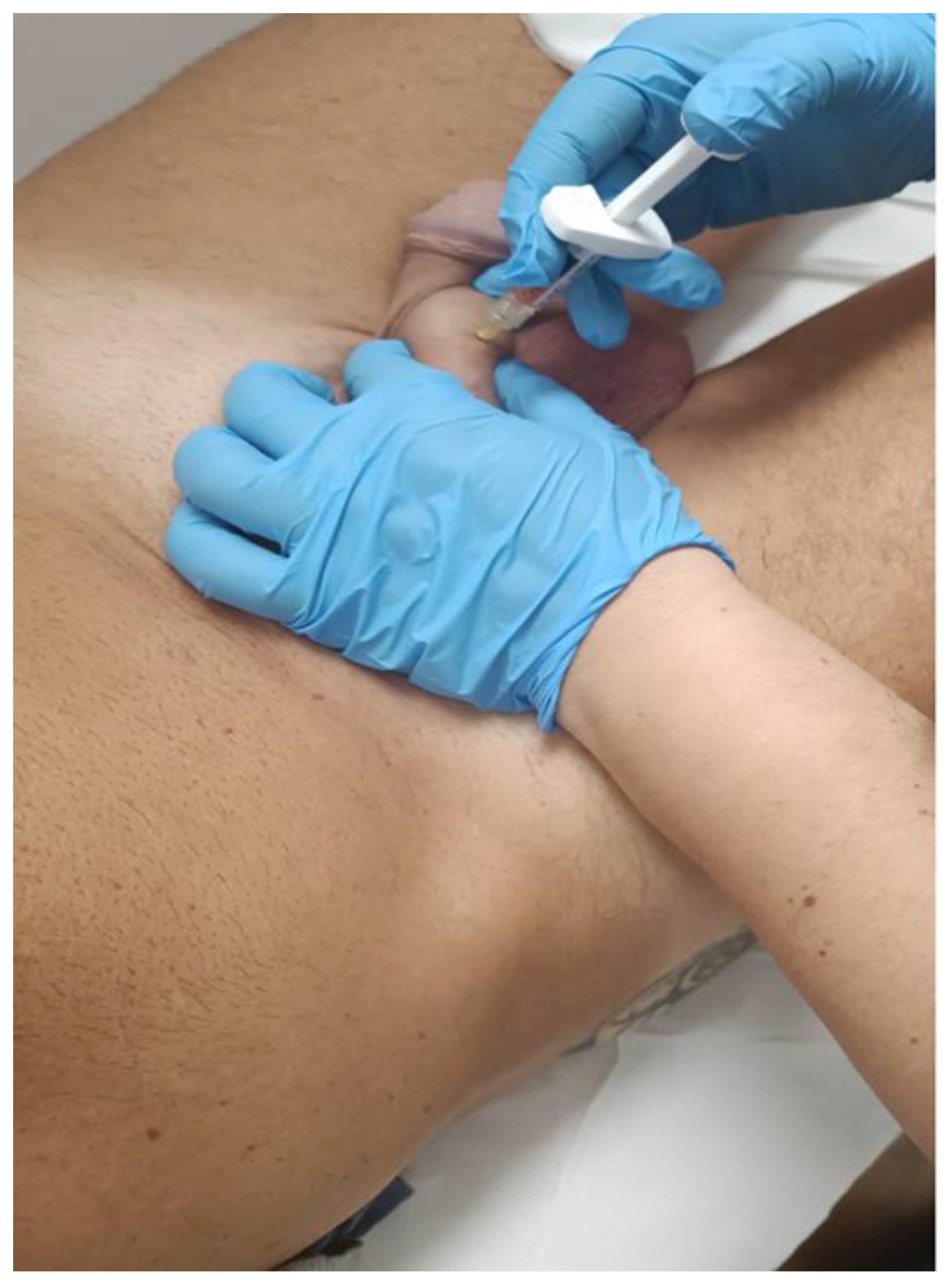
2.3. Detailed Procedure
3. Results
Figures, Tables and Schemes

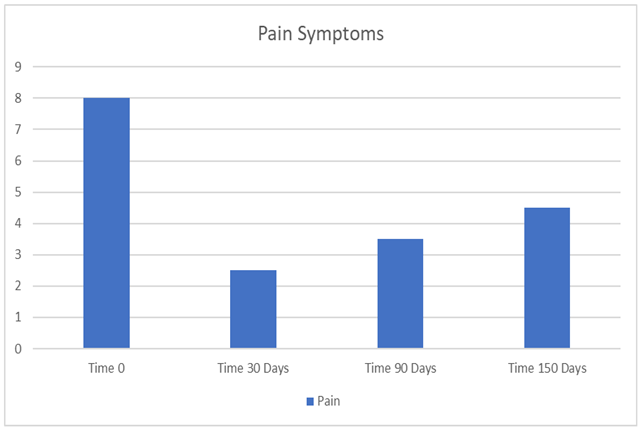 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- De Rose, A.F.; Mantica, G.; Bocca, B.; Szpytko, A.; Van der Merwe, A.; Terrone, C. Supporting the role of penile trauma and micro-trauma in the etiology of Peyronie’s disease. Prospective observational study using the electronic microscope to examine two types of plaques. Aging Male 2019. [CrossRef]
- Bjekic, M.D.; Vlajinac, H.D.; Sipetic, S.B.; Marinkovic, J.M. Risk factors for Peyronie's disease: a case-control study. BJU Int. 2006, 97, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Del Carlo, M.; Cole, A.A.; Levine, L.A. Differential calcium independent regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by interleukin-1beta and transforming growth factor-beta in Peyronie’s plaque fibroblasts. J Urol. 2008, 179, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Mateus, M.; Ilg, M.M.; Stebbeds, W.J.; et al. Understanding the role of adenosine receptors in the myofibroblast transformation in Peyronie's disease. J. Sex. Med. 2018, 15, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Bjekic, M.D.; Vlajinac, H.D.; Sipetic, S.B.; Marinkovic, J.M. Risk factors for Peyronie's disease: a case-control study. BJU Int. 2006, 97, 570–574. [Google Scholar] [CrossRef]
- Gelbard, M.; Goldstein, I.; Hellstrom, W.J.; et al. Clinical efficacy, safety and tolerability of collagenase Clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013, 190, 199–207. [Google Scholar] [CrossRef]
- Nehra, A.; Alterowitz, R.; Culkin, D.J.; et al. Peyronie's disease: AUA guideline. J Urol. 2015, 194, 745–753. [Google Scholar] [CrossRef] [PubMed]
- de la Peyronie, F. Sur quelques obstacles qui s' opposent a l'ejaculation naturelle de la semence. Mem. Acad. R. Chir. 1743, 1, 425–434. [Google Scholar]
- Akkus, E. Historical review of Peyronie’s disease. In: LA Levine (ed.). Peyronie’s Disease. Humana Press, Totowa, 2007; 1–8.
- Tal, R.; Hall, M.S.; Alex, B.; Choi, J.; Mulhall, J.P. Peyronie’s disease in teenagers. J. Sex. Med. 2012, 9, 302–308. [Google Scholar] [CrossRef]
- Nelson, C.J.; Mulhall, J.P. Psychological impact of Peyronie’s disease: a review. J. Sex. Med. 2013, 10, 653–660. [Google Scholar] [CrossRef]
- Wymer, K.; Ziegelmann, M.; Savage, J.; Kohler, T.; Trost, L. Plaque calcification: an important predictor of collagenase Clostridium histolyticum treatment outcomes for men with Peyronie’s disease. Urology 2018, 119, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.; Rybak, J.; Corder, C.; Farrel, M.R. Peyronie’s disease plaque calcification–prevalence, time to identification, and development of a new grading classification. J. Sex. Med. 2013, 10, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, M.G.; Kovanecz, I.; Nolazco, G.; Rajfer, J.; Gonzalez-Cadavid, N.F. Effects of long-term vardenafil treatment on the development of fibrotic plaques in a rat model of Peyronie's disease. BJU Int. 2006, 97, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.G.; Vernet, D.; Ferrini, M.G.; Qian, A.; Rajfer, J.; Gonzalez-Cadavid, N.F. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie’s fibrotic plaque and related fibroblast cultures. Nitric Oxide 2003, 9, 229–244. [Google Scholar] [CrossRef]
- Russo, G.I.; Milenkovic, U.; Hellstrom, W.; Levine, L.A.; Ralph, D.; Albersen, M. Clinical efficacy of injection and mechanical therapy for Peyronie’s disease: a systematic review of the literature. Eur. Urol. 2018, 74, 767–781. [Google Scholar] [CrossRef]
- Abern, M.R.; Larsen, S.; Levine, L.A. Combination of penile traction, intralesional verapamil, and oral therapies for Peyronie’s disease. J. Sex. Med. 2012, 9, 288–295. [Google Scholar] [CrossRef]
- Alom, M.; Sharma, K.L.; Toussi, A.; Kohler, T.; Trost, L. Efficacy of combined collagenase Clostridium histolyticum and RestoreX penile traction therapy in men with Peyronie’s disease. J. Sex. Med. 2019, 16, 891–900. [Google Scholar] [CrossRef]
- Fabiano Svolacchia , Lorenzo Svolacchia “Adult Mesenchymal Stem Cells (MSCa) derived from adipose tissue (ADSCa) in a scaffold of free hyaluronic acid in the regeneration of peri-ocular tissues “ Journal of Applied Cosmetology Vol. 37 , iss. 2 ( July – December 2019) ( Google Scholar ) ( Scopus Index ).
- Lorenzo Svolacchia ,Claudia Prisco ,Federica Giuzio and Fabiano Svolacchia , Adipose Autologous Micrograft and Its Derived Mesenchymal Stem Cells in a Bio Cross-Linked Hyaluronic Acid Scaffold for Correction Deep Wrinkles, Facial Depressions, Scars, Face Dermis and Its Regenerations: A Pilot Study and Cases Report , Medicina 2022, 58, 1692. [CrossRef]
- Isık, S.; Taşkapılıoğlu, M.Ö.; Atalay, F.O.; et al. Effects of cross-linked highmolecular-weight hyaluronic acid on epidural fibrosis: experimental study. J Neurosurg Spine. 2015, 22, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Svolacchia, F.; De Francesco, F.; Trovato, L.; Graziano, A.; Ferraro, G.A. An innovative regenerative treatment of scars with dermal micrografts. J Cosmet Dermatol. [Epub ahead of print]. 2016. [Google Scholar] [CrossRef] [PubMed]
- Svolacchia, F.; Svolacchia, L. Microfiltered vs only disaggregated mesenchymal stem cells from adipose tissue in regenerative medicine. Scr. Med. 2020, 51, 152–157. [Google Scholar] [CrossRef]
- Ettore CITTADINI , Anna M. BRUCCULERI , Fabrizio QUARTARARO ,Roberto VAGLICA , Vitale MICELI , Pier G. CONALDI Stem cell therapy in the treatment of organic and dysfunctional endometrial pathology , Minerva Obstetrics and Gynecology 2022 December;74(6):504-15. [CrossRef]
- Lucie Bacakova, Jana Zarubova, Martina Travnickova, Jana Musilkova, Julia Pajorova, Petr Slepicka, Nikola Slepickova Kasalkova, Vaclav Svorcik, Zdenka Kolska, Hooman Motarjemi, Martin Molitor ; Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review ; Review Biotechnol Adv. 2018, 36, 1111–1126. [CrossRef]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 2013, 132, 1017–1026, Luo, L.; Lucas, R.M.; Liu, L.; Stow, L.J. Signalling, sorting and scaffolding adaptors for Toll-like receptors. J. Cell Sci. 2020,133, 239194. [Google Scholar] [CrossRef]
- Xiangnan Zhao, Yue Liu, Pingping Jia, Hui Cheng, Chen Wang, Shang Chen, Haoyan Huang, Zhibo Han, Zhong-Chao Han, Zongjin Li , Chitosan Hydrogel-loaded MSC-derived Extracellular Vesicles Promote Skin Rejuvenation by Ameliorating the Senescence of Dermal Fibroblasts , Stem Cell Research & Therapy.
- Svolacchia, F.; Svolacchia, L.; Falabella, P.; Scieuzo, C.; Salvia, R.; Giglio, F.; Catalano, A.; Saturnino, C.; Di Lascio, P.; Guarro, G.; Imbriani, G.C.; Ferraro, G.; Giuzio, F. Exosomes and Signaling Nanovesicles from the Nanofiltration of Preconditioned Adipose Tissue with Skin-B® in Tissue Regeneration and Antiaging: A Clinical Study and Case Report. Medicina (Kaunas). 2024, 60, 670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




